Simple Summary
The role of postoperative radiotherapy (PORT) in completely resected non-small cell lung cancer (NSCLC) with ipsilateral mediastinal lymph node involvement (pN2) is controversial. The aim of our review was to study the literature relating to PORT for completely resected NSCLC patients with pN2 involvement. The Lung ART and PORT-C trials indicate better locoregional control with PORT, but this has not yet translated into survival benefits. Given the conflicting results, guidelines do not recommend the use of PORT routinely. Future research should focus on identifying subgroups of patients who might benefit from PORT.
Abstract
Background: For patients with completely resected non-small cell lung cancer (NSCLC) with ipsilateral mediastinal lymph node involvement (pN2), the administration of adjuvant chemotherapy is the standard of care. The role of postoperative radiation therapy (PORT) is controversial. Methods: We describe the current literature focusing on the role of PORT in completely resected NSCLC patients with pN2 involvement and reflect on its role in current guidelines. Results: Based on the results of the recent Lung ART and PORT-C trials, the authors conclude that PORT cannot be generally recommended for all resected pN2 NSCLC patients. A substantial decrease in the locoregional relapse rate without translating into a survival benefit suggests that some patients with risk factors might benefit from PORT. This must be balanced against the risk of cardiopulmonary toxicity with potentially associated mortality. Lung ART has already changed the decision making for the use of PORT in daily practice for many European lung cancer experts, with lower rates of recommendations for PORT overall. Conclusions: PORT is still used, albeit decreasingly, for completely resected NSCLC with pN2 involvement. High-level evidence for its routine use is lacking. Further analyses are required to identify patients who would potentially benefit from PORT.
1. Introduction
Multimodal therapy is the standard of care for patients with locally advanced (LA) non-small cell lung cancer (NSCLC) (stage IIIA-C) [1,2,3,4]. Patients with stage III N2 NSCLC with primarily unresectable disease (IIIA4, IIIB) receive concurrent or sequential chemoradiotherapy (CRT) followed by immunotherapy in some cases [5]. Alternatively, a surgical approach (bi- or trimodal) can be offered to patients with potentially resectable stage III NSCLC (IIIA3) [6,7]. Two meta-analyses confirmed that definitive CRT and a bimodal treatment including surgery and chemotherapy, were comparable options for treating N2 disease [6,7]. Feasible treatment options should be discussed by a multidisciplinary team. In a setting with multiple treatment options, patient preference should play a prominent role in the decision making [8].
After surgical resection, a large proportion of patients develop local recurrence (LR) and distant metastasis [9]. The poor prognosis after surgery has led to the implementation of perioperative treatments. The benefits of chemotherapy in patients with completely resected NSCLC have been demonstrated in many phase 3 studies and a meta-analysis, with an absolute survival benefit of 5% at 5 years [10]. Neoadjuvant or adjuvant chemotherapy is recommended for patients with stage IIB to IIIA and can be considered for patients with stage IIA with a resected primary tumor of >4 cm [1,2,11]. Nodal positive patients have a significant risk of locoregional recurrence even after R0 resection and neoadjuvant or adjuvant chemotherapy [9,12].
First data show that neoadjuvant immune checkpoint inhibitors (ICIs) (e.g., nivolumab) plus chemotherapy increase the rate of pathological complete response compared with chemotherapy alone (Checkmate 816 [13]). Likewise, in the same setting, adjuvant ICIs with anti-PD-L1 agents (e.g., atezolizumab or pembrolizumab) lead to increased disease-free survival (DFS) versus best supportive care for patients with PD-L1-positive tumors (IMpower010 [14], PEARLS [15]). The role of (neo)adjuvant immunotherapy in stage IB–IIIA NSCLC is rapidly developing and several large randomized phase 3 clinical trials are underway [11,13,15]. Osimertinib is indicated for the adjuvant treatment after complete resection with stage IB–IIIA EGFR-mutated NSCLC patients [11,16].
Postoperative radiation therapy (PORT) is often recommended in stage III N2 NSCLC patients, but its role has historically been controversial.
This review focuses on the role of PORT in the multimodal treatment of LA-NSCLC. We discuss how recent results may impact our current treatment approach [5].
2. PORT after Complete Resection
The International Association for the Study of Lung Cancer (IASLC) staging committee defined complete resection as follows: microscopically free resection margins (R0), systematic nodal dissection or lobe-specific systematic nodal dissection, lack of extracapsular nodal extension (ENE) and negativity for tumor infestation at the highest mediastinal node removed [17,18,19].
Between 2017 and 2020, three different studies confirmed the prognostic value of the IASLC definition [17,18,19].
2.1. pN0
A randomized trial from 1980 with 175 completely resected pN0 NSCLC patients showed that PORT was clearly detrimental [20]. The same team highlighted a decade later the potential benefit of modern treatment techniques, although the oncological results were not superior to those of the control group [21]. A more recent randomized trial using linear accelerators was similarly performed for 104 completely resected stage I patients. The 5-year overall survival (OS) showed a positive trend in the treated group (PORT group: 67% vs. non-PORT group: 58%, p = 0.048) [22].
PORT meta-analyses have shown a detrimental effect of PORT for completely resected NSCLC with pN0 and pN1 disease [23,24,25,26].
The ANITA trial was a randomized trial of adjuvant chemotherapy vs. observation in completely resected stage IB to IIIA NSCLC patients, revealing a significant 5-year OS benefit of 8.6% for the chemotherapy group [27]. The post hoc analysis of the ANITA trial study also showed a negative effect of PORT for pN0-1 patients [26].
Based on these data, PORT is not recommended for completely resected pN0 and pN1 NSCLC patients.
2.2. pN+
PORT was evaluated in several trials performed in the 1980s–1990s. An older retrospective study from the Mayo clinic, including 224 pN2 patients resected between 1987 and 1993, found that PORT may improve local control (4-year LR 60% in the non-PORT group vs. 17% in the PORT group, p < 0.0001) and survival (4-year OS 22% in the non-PORT group vs. 43% in the PORT group, p = 0.005) [28]. The largest randomized trial, including 728 stage I-III NSCLC patients, demonstrated that PORT had a detrimental effect on survival without a significant effect on LR [29].
The PORT meta-analyses have shown a deleterious effect of PORT for completely resected stage I–III NSCLC [23,24,25]. In patients with pN2 disease, PORT did not improve survival but did reduce the risk of local recurrence by 24%. Due to these negative results in the whole patient cohort, fewer patients were treated with PORT, even those in pN2 stage. The meta-analyses were criticized due to the use of obsolete two-dimensional (2D) radiation techniques (cobalt-60, not CT planned), suboptimal and outdated radiotherapy volumes and fractionation schemes often using daily fractions of >2 Gy, which might have potentially led to additional toxicities [30,31,32]. A comparison of RT plans used in older vs. current trials showed poor target coverage and excessive heart and lung doses leading to high toxicity with older techniques [33]. The included older studies were performed without 18FDG PET-CT staging or brain MRI imaging. Finally, chemotherapy was not used [34,35].
Since the PORT meta-analysis in 1998, several retrospective population-based cohort studies, database analyses and meta-analyses have found significantly improved OS for PORT in patients with completely resected NSCLC with pN2 involvement (Table 1).

Table 1.
Studies evaluating PORT in stage I–III pN0–pN2 NSCLC patients.
A larger cohort study of the Surveillance, Epidemiology, and End Results (SEER) database with 7465 resected NSCLC patients suggested that PORT in pN2 patients was associated with an increase in cancer-specific survival and 5-year OS [36].
In the randomized ANITA trial, PORT was recommended for pN+ disease but was not randomized or mandatory [27]. A retrospective post hoc subgroup analysis demonstrated that PORT led to improved OS in patients with resected pN2 NSCLC both in the chemotherapy arm and observation arm [26] (Table 1). This trial was initiated in the era of adjuvant chemotherapy.
The meta-analysis of Billiet et al. [37] (2387 patients) based on phase 3 randomized controlled trials (RCTs), compared the effect of PORT in patients treated on linear accelerators or cobalt machines. PORT significantly decreased LR from 30% to 10% for pN2 patients independently of the radiotherapy machine used. Better OS was only achieved when PORT was delivered with a linear accelerator. Most of the patients in the studies were treated with 2D radiotherapy, which is no longer used in daily practice [37].
Another meta-analysis of 16 trials with 3278 patients indicated that PORT delivered with modern techniques, significantly improved locoregional recurrence-free survival, DFS and OS in patients with stage III N2 NSCLC [38].
A recent meta-analysis by Zhang et al. [12] summarized all studies (three RCTs [39,40,41] with 237 patients and eight retrospective studies with 7748 patients) regarding the effect of PORT on OS and DFS in stage III pN2 NSCLC. The results revealed that the use of PORT tends to prolong OS and significantly improves DFS. The effect of PORT on OS did not differ significantly between RCTs or retrospective studies [12].
A retrospective single center study by Wei et al. [42] with 183 patients from the Hunan Cancer Hospital in China demonstrated a significant improvement in LR-free survival and OS in the postoperative chemoradiotherapy (CRT) versus the postoperative chemotherapy group in stage III pN2 NSCLC, especially in the multiple-station pN2 patients and patients with single-station pN2 combined with multiple-station pN1.
A recently published retrospective study by Wang et al. [43] with 142 pN2 NSCLC patients found that PORT can increase the OS of patients (5-year OS 32% vs. 27%) as well as the local control rate of tumors.
Retrospective studies, large database analyses and meta-analyses on PORT for pN2 completely resected NSCLC patients from the last 20 years (Table 1) show controversial results. Our understanding of the use of PORT in the modern setting with a better selection of patients with 18FDG PET-CT and brain MRIs, improved radiotherapy and thoracic surgery techniques is limited. In the daily routine, PORT was mostly based on the risk of locoregional relapse for each individual patient. Two phase 3 randomized trials, Lung ART and PORT-C [44,45], were published in 2021 and brought more insight to this debate.
The Lung ART trial compared mediastinal PORT with no PORT in a superiority design in patients with completely resected NSCLC with pN2 involvement with modern surgery and 3D conformal radiotherapy [45]. In total, 91% of the patients were staged preoperatively with 18FDG PET-CT in this trial, and 96% of the patients received neoadjuvant or adjuvant chemotherapy. Five hundred and one patients were enrolled and randomized after resection or adjuvant chemotherapy: 252 in the PORT group and 249 in the control arm (no PORT). Only patients who had undergone a complete resection were included. The advisory surgical quality assurance committee reclassified resections based on the IASLC definition into R0, an uncertain resection, or R1 (because of ENE). The trial did not meet the primary endpoint of significantly improved 3-year DFS (47.1% in the PORT arm vs. 43.8% in the control arm, p = 0.18). Three-year OS was 66.5% in the PORT arm vs. 68.5% in the control arm. Twenty-five percent of the PORT patients and 46% of the non-PORT patients had mediastinal relapse at 3 years; this is a reduction of approximately 50% in the risk of locoregional relapse using PORT compared with the control group. Most patients died of recurrence: 85% in the non-PORT group and 69% in the PORT group. Cardiopulmonary toxicity and related death was significantly higher in the PORT group (16 patients, 16%) than in the control group (2 patients, 2%). The Lung ART authors concluded that 3D conformal PORT cannot generally be recommended for all stage III pN2 patients after a (considered) complete resection, although PORT could significantly reduce the risk for mediastinal relapse. In the PORT group there were more toxicities (especially cardiopulmonary) without a statistically significant effect in terms of OS. Further analyses are needed to determine if certain patients could benefit from PORT. It may be noted that the initial target accrual was 700 patients, yet the trial closed with 501 patients. However, it is unlikely that the results would have been significantly different with the initially planned accrual [46].
Two other RCTs comparing PORT versus no PORT were published between 2014 and 2020. One study closed early because of poor accrual [40]. The study of Sun et al. [41] recruited only patients with unsuspected N2 disease.
The Chinese RCT (PORT-C, n = 394) showed no significant difference in 3-year OS (78.3% in the PORT arm vs. 82.8% in the non-PORT arm) for completely resected NSCLC patients with pN2 involvement. The 3-year LR-only rate was significantly lower in the PORT arm (9.5% vs. 18.3%). The authors stated that the low toxic effects in this trial were due to the use of a modern RT technique (89% IMRT) and to the markedly tighter dose restrictions to the organs at risk. Nevertheless, IMRT did not improve OS, with similar death rates in the PORT and non-PORT arms [44].
However, further analysis is needed to identify cohorts that may potentially benefit from PORT without increasing the cardiopulmonary toxicities and potentially related deaths.
Based on all these data, guidelines do not recommend PORT routinely. The NCCN guidelines recommend PORT alone or with chemotherapy for selected pN2 patients only [2]. The updated ESMO Clinical Practice Guidelines see no benefit of PORT for patients with completely resected stage III N2 NSCLC and recommend PORT only in the setting of residual microscopic or macroscopic disease [11]. The recently published ASCO guidelines do not recommend PORT for patients with completely resected NSCLC with mediastinal N2 involvement without extracapsular extension who have received neoadjuvant or adjuvant platinum-based chemotherapy [47].
2.3. Importance of Surgery and Preoperative Staging from the Perspective of Modern PORT
In the last decade there has been major progress in terms of preoperative staging. Patients undergoing multimodality treatments are better selected based on modern staging with 18FDG PET-CT and brain MRI. 18FDG PET-CT is highly sensitive and specific in detecting mediastinal nodal and extracranial metastases. In total, 91% of the patients in the Lung ART trial had undergone 18FDG PET-CT scans. The ESMO guidelines recommend a locoregional lymph node staging with 18FDG PET-CT and, in the case of PET-positive lymph nodes or central or >3 cm tumors, an invasive staging with EBUS/EUS (endoscopic bronchial ultrasound) or VAM (video-assisted mediastinoscopy) [11].
The European Society of Thoracic Surgeons (ESTS) defined adequate intraoperative lymph node staging as: a systematic nodal examination including at least three intrapulmonary and hilar nodes and at least three mediastinal nodal stations depending on the location of the primary tumor [48]. Handa et al. [49] advocate for lobe-specific mediastinal lymph node dissection. One of the most important findings of the study is that nearly 6% of patients in the lobe-specific dissection group might have had their metastatic lymph nodes missed by not sampling stations outside of the lobe-specific stations.
2.4. Sequence of Postoperative Treatment
Sequential chemotherapy and PORT were associated with superior survival compared with concomitant postoperative CRT in two National Cancer Database (NCDB) registry analyses for pN2 NSCLC patients [50,51].
In the randomized trial of the Eastern Cooperative Oncology Group, PORT (50.4 Gy) alone was compared to postoperative CRT (with cisplatin and etoposide). The 3-year OS rates were similar in both groups [52].
In the RTOG 9705 trial, 86 patients with completely resected NSCLC underwent PORT (50.4 Gy) and chemotherapy (paclitaxel plus carboplatin) concomitantly. This trial evaluated the efficacy of combining chemotherapy and radiation therapy postoperatively. The trial suggested improved 3-year progression-free and OS rates (50% and 61%, respectively), compared with previously reported trials [53].
ESMO guidelines recommend administering chemotherapy first when both postoperative chemotherapy and PORT have been used [1].
2.5. Risk Factors
Stage III pN2 NSCLC is a very heterogeneous group with different clinicopathologic features, such as lymph node (LN) extent (number of stations or zones involved), LN volume (bulky, non-bulky), primary tumor size and histological subtype.
The volume and extent of N2 disease correlates with the LR rate and prognosis [30,42,54,55]. The recent meta-analysis by Liu et al. [30] showed improved OS for PORT in patients with high LN extent (multiple N2 LN metastases or multiple N2 station involvement) but not for patients with single-station N2 involvement. Lymph node ratio (LNR) (the number of pathologically positive LNs divided by the number of LNs examined) seems to be an important prognostic factor [55,56]. Furthermore, in two analyses, PORT showed a survival benefit only in pN2 patients with an LNR of 50% or more [55,56]. In a retrospective study by Wei et al. [42], PORT reduced LR and improved OS for patients in stage III NSCLC with multi-station pN2, single-station pN2 + multi-station pN1, patients with a high positive LNR > 1/3 and tumors with poor histological differentiation.
In the PORT-C trial, a preplanned exploratory analysis found a significant DFS improvement with PORT in patients with four or more LNs compared to patients with less than four LNs involved [44].
In several studies, the predictive value of absolute tumor size or pT stage in pN2 NSCLC regarding PORT was investigated, mostly with conflicting results [26,57,58,59]. The meta-analysis of Liu et al. [30] showed no significant differences in OS between the PORT and non-PORT groups for either patients with tumors >3.0 cm or those with tumors 3 cm or less.
There is also increasing evidence that there might be a benefit for PORT in persistent pN2 disease (ypN2) after induction chemotherapy (ICT). The phase 2 trial of Betticher et al. [60] found worse OS for patients with persistent N2 compared to patients with a downstaging to N0 or N1 after ICT, suggesting a possible benefit of PORT for persistent disease. Randomized trials evaluating PORT for persistent N2 disease after ICT are lacking. The role of PORT in subjects with and without pathologic complete response after ICT remains unclear [35].
The ADAURA trial found a significantly improved DFS for osimertinib in patients with EGFR-mutated completely resected stage IB to III NSCLC [16]. The benefit of PORT may be lower in patients with actionable mutations [61].
These findings indicate that treatments for stage III pN2 NSCLC should be individualized. In routine clinical practice, many criteria influence the decision-making process [62]. The aim of a recently published decision-making analysis was to identify disease characteristics in current clinical practice among European lung cancer experts for stage III pN+ NSCLC and how they impact decision making in the clinical routine regarding the use of PORT before and after the first results of the Lung ART trial presented at ESMO 2020. The most common risk factors used for decision making in the analysis were ENE and/or capsular rupture of LNs, incomplete mediastinal lymphadenectomy, multi-station LNs, high nodal tumor load and poor response to ICT [63].
The definition of complete resection has evolved over time, with findings of ENE/capsular rupture of LNs automatically being classified as incomplete resections (Rami-Porta et al. [17]). However, in clinical practice, ENE is often considered separately from otherwise completely resected tumors (otherwise R0), as has been shown among European radiation oncology experts [63]. Overall, there is ambiguity in differentiating incomplete resections from other risk factors such as ENE.
3. PORT after Incomplete Resection
Incomplete resection is defined by Rami-Porta et al. [17] as the presence of positive margins (R1: microscopic residual tumor, R2: macroscopic residual tumor), ENE of the tumor in the nodes removed separately or those at the margin of the main lung specimen or positive nodes left in the operative field. The definition of uncertain resection (R(uncertain)) was also created by Rami-Porta et al. [17]: no evidence of remnant tumor, but intraoperative nodal evaluation not meeting the requirements of systematic nodal dissection or lobe-specific systematic nodal dissection or involvement of the highest mediastinal node removed [17].
The justification for the presence of an ENE leading to the definition of an incomplete resection is a matter of debate. A recent analysis on the IASLC database was not able to determine the impact of ENEs on survival rates. The relevance of ENEs remains unclear, as does the interplay between ENEs and PORT. The authors concluded that analyses of a greater number of ENE cases are required to understand the prognostic impact [64].
A retrospective NCDB-based analysis of 3395 patients showed an improved OS across all nodal stages with PORT in patients with incompletely resected (R1/2) stage II-III NSCLC [65]. OS improvement was most pronounced in pN0 disease, with a 5-year OS of 41% vs. 26% with and without PORT, respectively. In another analysis of the same database with 1446 incompletely resected (R1 or R2) patients, there was only a trend in favor of sequential vs. concomitant postoperative CRT [66]. Both studies should be interpreted with caution due to the limitations and biases of registries.
A recent analysis of the NCDB registry found no significant difference in the cohort of 277 incompletely resected patients (R1: 94%, R2: 6%) between those who received chemotherapy followed by PORT alone and those who received concomitant CRT postoperatively. Most patients in this cohort had microscopic positive margins rather than gross residual disease, and it is patients in the latter group who, hypothetically, might derive the most benefit from postoperative concomitant CRT [50].
There is growing recognition that the uncertain resection status (inadequate intraoperative lymph node dissection, positivity of the highest LN, involved LNs removed in fragments, ENE) is associated with worse prognosis than R0 resection. These features might be considered as risk factors for an increased rate of local recurrence and could be potentially improved with PORT [67].
Controversial data are available for an ENE being used as a predictive marker for PORT [68]. The retrospective analysis of Moretti et al. [69] involving 83 bulky pN2 patients showed a significantly lower OS in pN2 patients with an ENE but a higher OS rate in patients without an ENE who were treated with PORT.
In the Lung ART trial, after a review of the surgical and pathological reports in accordance with IASLC, 33% of the reports did not contain any information about the ENE of the resected LNs. All patients were reclassified into R0, uncertain and R1 resection. Patients with an ENE were considered as R1 because of the insufficient description of the ENE and whether these nodes were removed separately or not. A total of 149 patients (74/250 in the PORT group, 75/243 in the control group) were reclassified as R1 (because of the ENE). The authors of the Lung ART trial are expected to report on the effect of the quality of surgery and the certainty of resection margins on the efficacy of PORT in the future.
The NCCN guidelines recommend postoperative CRT (either sequential or concurrent) for R1 resection and concurrent CRT postoperatively for R2. Depending on the stage of disease and site of R+-resection, re-excision may be considered [1,2,35,70]. The ESMO guidelines recommend PORT and adjuvant chemotherapy in patients with R1 resection. In case both chemotherapy and PORT are administered, RT may be administered before chemotherapy [11].
4. PORT Toxicity
Toxicity (mostly cardiopulmonary) is an important concern related to PORT, particularly as several studies could not demonstrate a clear oncologic benefit. Excessive volumes of RT, suboptimal radiation techniques, large doses and fraction sizes and non-CT-based RT planning can probably explain the excess toxicity with non-cancer-related deaths observed in previous trials with PORT.
Several retrospective databases and reviews have examined the hypothesis that more modern radiation techniques do not lead to increased deaths. They found a similar risk of intercurrent death between NSCLC patients with and without PORT [32,34,71].
An analysis of the SEER database investigated the cardiac toxicity and related mortality in 6148 patients treated with or without PORT. PORT was associated with a significantly increased number of deaths from heart disease in patients diagnosed with NSCLC between 1983 and 1988 but not in the latter cohorts [72].
The meta-analysis of the oldest trials using linear accelerators showed that the evolution from conventional 2D to 3D-RT has clearly alleviated radiation toxicity [35,37].
Subsequently, the improvements in RT technologies such as 3D conformal radiation therapy (3D-CRT), intensity-modulated radiotherapy (IMRT) or volumetric modulated arc therapy (VMAT) could have potential benefits [12,32,71,73,74].
A comparison of the dosimetric PORT plans of ten pN2 NSCLC patients using 3D-CRT, IMRT and VMAT was unable to reveal that any technique had absolute dosimetry advantages for all patients [75]. The selection of the technique should be individualized, balancing target coverage and protection of the organs at risk. The use of protons may be another step towards reducing RT-induced toxicity. Small retrospective series showed there were significantly lower RT doses to surrounding organs at risk (heart, lungs) with proton-based PORT [76,77,78,79]. In the monocentric retrospective review of Boyce-Fappiano et al. [78], the improved sparing of the heart and lung with the use of proton beam PORT was associated with improved OS. Multicenter studies randomizing patients to PORT with proton therapy versus photon therapy, including a cardiopulmonary toxicity endpoint, would be a good approach to understanding the potential benefits of proton therapy.
Unfortunately, patients in the Lung ART trial were mainly treated with 3D-CRT (89%), and only 11% of the patients in the study received IMRT, because when accrual started for the study, IMRT was not standard. IMRT has now become a standard RT technique with better dose conformity and organ at risk avoidance such as heart or lungs. In the Lung ART trial, cardiopulmonary toxicity and related death was significantly higher in the PORT group (16 patients, 16%) than in the control group (2 patients, 2%). Eleven percent of the PORT patients and 5% of the non-PORT patients had late grade 3–4 cardiopulmonary toxicities; the most common event was pneumonitis (6% in the PORT group, <1% in the control group). Further analysis will be needed to explore which patient groups might benefit most from PORT with lower toxicity [45].
5. Dose and Fractionation
The total RT dose, fractionation and the treated volume (including organs at risk) should also be taken into consideration in decision making.
The randomized trial by Dautzenberg et al. [30] showed a higher risk for late toxicity and intercurrent deaths using fraction sizes >2 Gy. There was a correlation between fractionation size and morbidity. The risk for non-cancer-related death was 7% in the control group (no PORT), 16–18% among patients treated with PORT with daily fractions <2 Gy and 26% among patients who had >2 Gy doses per fraction [30].
In terms of RT dose, Corso et al. [80] showed a significantly improved OS in completely resected NSCLC patients who received 45 to 54 Gy compared with patients without PORT. With RT doses of over 54 Gy, the survival was equivalent to patients treated without PORT, suggesting that these higher doses were detrimental.
6. Target Volume Delineation
PORT clinical target volume (CTV) must account for the lymph nodes involved according to the surgery and pathology report and should consider preoperative imaging. In cases of neoadjuvant chemotherapy, initially involved lymph node stations should be included, even in cases of downstaging.
Spoelstra et al. [81] summarized which lymph node regions should be included postoperatively for different positive lymph stations [81].
Several studies have investigated the pattern of locoregional relapse according to the tumor location. Billiet et al. [82] showed the greatest LR at LN stations 7 (18%), 4R (16%) and 10R (16%). Among left-sided tumors, LR occurred mostly bilaterally, whereas among right-sided tumors, LR was more unilateral [82].
ESTRO/ACROP guidelines suggested that CTV should include the pathologically involved and resected mediastinal lymph node stations, bronchial stump, ipsilateral hilum and ipsilateral nodal stations 4 and 7. The definition of CTV depends on the lung lobe where the tumor was located, but the bronchial stump, ipsilateral hilum, positive lymph node regions and LN stations 4 and 7 must always be included in the CTV due to their high risk of relapse [83] (Figure 1).
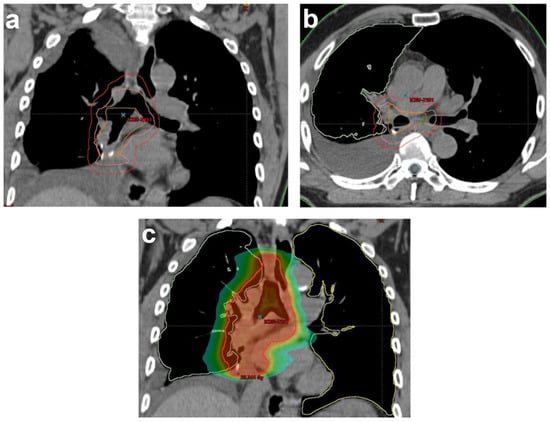
Figure 1.
Illustration of RT planning of PORT for a completely resected NSCLC patient with histologically proven lymph nodes (2/9) in stations 7 and 10R in (a) coronal and (b) sagittal views. Delineation based on the Lung ART protocol of rCTV (orange): bronchial stump, ipsilateral hilar node region (10R) and lymph node station 7. CTV (pink): rCTV+1 cm. In this case, 4R, 7 and 10R had a maximal upper limit to the top of the aortic arc and a maximal lower limit 5 cm below the carina. PTV (red); (c) color wash of dose distribution ranging from 20 Gy to 57.7 Gy (prescribed dose: 54 Gy). rCTV: resected clinical tumor volume, CTV: clinical tumor volume, PTV: planning target volume.
7. Conclusions
The role of PORT in patients with completely resected NSCLC with pN2 involvement remained unclear, based mostly on data from non-randomized studies and large database analyses, until the publication of the Lung ART and PORT-C trials. The older studies suggested for this patient cohort that PORT after adjuvant chemotherapy could improve overall survival providing that a conformal RT technique such as 3D or IMRT would be related with less cardiopulmonary toxicity.
The Lung ART and PORT-C trials represent robust evidence that 3D conformal PORT should not be generally recommended in patients with resected stage III pN2 NSCLC patients. Data indicate a significantly lower locoregional relapse rate with PORT without translating into a survival benefit. It is a matter of ongoing debate as to whether PORT might be beneficial for selected patients with high risk features, such as more than or equal to four LN metastases, multiple-station N2 or persistent N2 disease after ICT [61,67]. It is unclear whether PORT could improve the outcome for patients with ENE. The optimal RT technique for PORT, such as IMRT or intensity-modulated proton therapy, and the role for PORT in selected high-risk patients should be evaluated. Future research should focus on defining the profile of optimal candidates who might benefit from PORT.
Author Contributions
The concept for this review was developed by K.S. with help from P.M.P. The manuscript was written by K.S. with help from P.M.P., T.I., G.F.F., P.L. and L.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Postmus, P.; Kerr, K.; Oudkerk, M.; Senan, S.; Waller, D.; Vansteenkiste, J.; Escriu, C.; Peters, S. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv1–iv21. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; D’Amico, T.A. NCCN Guidelines Insights: Non–Small Cell Lung Cancer, Version 2.2021: Featured Updates to the NCCN Guidelines. J. Natl. Compr. Cancer Netw. 2021, 19, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Maconachie, R.; Mercer, T.; Navani, N.; McVeigh, G. Lung cancer: Diagnosis and management: Summary of updated NICE guidance. BMJ Br. Med. J. 2019, 364. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.; Vella, E.T.; Ellis, P.M.; Goffin, R.; Hanna, W.; Maziak, D.; Swaminath, A.; Ung, Y.C. Recommendations for the Treatment of Patients with Clinical Stage III Non-Small Cell Lung Cancer: Endorsement of the 2019 National Institute for Health and Care Excellence Guidance and the 2018 Society for Immunotherapy of Cancer Guidance; Program in Evidence-Based Care Guideline No.: 7-3 Version 4; Ontario Health (Cancer Care Ontario): Toronto, ON, Canada, 2020. [Google Scholar]
- Putora, P.M.; Leskow, P.; McDonald, F.; Batchelor, T.; Evison, M. International guidelines on stage III N2 nonsmall cell lung cancer: Surgery or radiotherapy? ERJ Open Res. 2020, 6, 00159–02019. [Google Scholar] [CrossRef] [PubMed]
- McElnay, P.J.; Choong, A.; Jordan, E.; Song, F.; Lim, E. Outcome of surgery versus radiotherapy after induction treatment in patients with N2 disease: Systematic review and meta-analysis of randomised trials. Thorax 2015, 70, 764–768. [Google Scholar] [CrossRef]
- Pottgen, C.; Eberhardt, W.; Stamatis, G.; Stuschke, M. Definitive radiochemotherapy versus surgery within multimodality treatment in stage III non-small cell lung cancer (NSCLC)—A cumulative meta-analysis of the randomized evidence. Oncotarget 2017, 8, 41670–41678. [Google Scholar] [CrossRef] [PubMed]
- Iseli, T.; Süveg, K.; Fischer, G.F.; Glatzer, M.; Putora, P.M. Management of stage IIIA NSCLC: The role of radiotherapy—A narrative review. Curr. Chall. Thorac. Surg. 2021. Online ahead of print. [Google Scholar] [CrossRef]
- Le Péchoux, C. Role of postoperative radiotherapy in resected non-small cell lung cancer: A reassessment based on new data. Oncologist 2011, 16, 672–681. [Google Scholar] [CrossRef]
- Pignon, J.P.; Tribodet, H.; Scagliotti, G.V.; Douillard, J.-Y.; Shepherd, F.A.; Stephens, R.J.; Dunant, A.; Torri, V.; Rosell, R.; Seymour, L.; et al. Lung adjuvant cisplatin evaluation: A pooled analysis by the LACE Collaborative Group. In Database of Abstracts of Reviews of Effects (DARE): Quality-Assessed Reviews; Centre for Reviews and Dissemination (UK): York, UK, 2008. [Google Scholar]
- Remon, J.; Soria, J.-C.; Peters, S. Early and locally advanced non-small-cell lung cancer: An update of the ESMO Clinical Practice Guidelines focusing on diagnosis, staging, systemic and local therapy. Ann. Oncol. 2021, 32, 1637–1642. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, D.X.; Ju, T.; Zhou, J. The effect of postoperative radiotherapy on the survival of patients with resectable stage III-N2 non-small-cell lung cancer: A systematic review and meta-analysis. Neoplasma 2019, 66, 717–726. [Google Scholar] [CrossRef]
- Forde, P.M.; Spicer, J.; Lu, S.; Provencio, M.; Mitsudomi, T.; Awad, M.M.; Felip, E.; Broderick, S.; Brahmer, J.; Swanson, S.J. Nivolumab (NIVO) plus platinum-doublet chemotherapy (chemo) vs chemo as neoadjuvant treatment (tx) for resectable (IB-IIIA) non-small cell lung cancer (NSCLC) in the phase 3 CheckMate 816 trial. In Proceedings of the 112th Annual Meeting of the American Association for Cancer Research, Philadelphia, PA, USA, 10–15 April 2021. [Google Scholar]
- Felip, E.; Altorki, N.; Zhou, C.; Csőszi, T.; Vynnychenko, I.; Goloborodko, O.; Luft, A.; Akopov, A.; Martinez-Marti, A.; Kenmotsu, H. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB–IIIA non-small-cell lung cancer (IMpower010): A randomised, multicentre, open-label, phase 3 trial. Lancet 2021, 398, 1344–1357. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Hasan, B.; Dafni, U.; Menis, J.; De Maio, E.; Oselin, K.; Albert, I.; Faehling, M.; Van Schil, P.; O’Brien, M. A randomized, phase 3 trial with anti-PD-1 monoclonal antibody pembrolizumab (MK-3475) versus placebo for patients with early stage NSCLC after resection and completion of standard adjuvant therapy (EORTC/ETOP 1416-PEARLS). Ann. Oncol. 2017, 28, ii23. [Google Scholar] [CrossRef][Green Version]
- Herbst, R.S.; Tsuboi, M.; John, T.; Grohé, C.; Majem, M.; Goldman, J.W.; Kim, S.-W.; Marmol, D.; Rukazenkov, Y.; Wu, Y.-L. Osimertinib as adjuvant therapy in patients with stage IB-IIIA EGFR mutation positive NSCLC after complete tumor resection: ADAURA. J. Clin. Oncol. 2020, 38, LBA5. [Google Scholar] [CrossRef]
- Rami-Porta, R.; Wittekind, C.; Goldstraw, P.; International Association for the Study of Lung Cancer Staging Committee. Complete resection in lung cancer surgery: Proposed definition. Lung Cancer 2005, 49, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Rami-Porta, R.; Wittekind, C.; Goldstraw, P. Complete resection in lung cancer surgery: From definition to validation and beyond. J. Thorac. Oncol. 2020, 15, 1815–1818. [Google Scholar] [CrossRef]
- Wittekind, C.; Brierley, J.D.; Lee, A.; van Eycken, E. TNM Supplement: A Commentary on Uniform Use; John Wiley & Sons Ltd: Oxford, UK, 2019. [Google Scholar]
- Van Houtte, P.; Rocmans, P.; Smets, P.; Goffin, J.-C.; Lustman-maréchal, J.; Vanderhoeft, P.; Henry, J. Postoperative radiation therapy in lung cancer: A controlled trial after resection of curative design. Int. J. Radiat. Oncol. Biol. Phys. 1980, 6, 983–986. [Google Scholar] [CrossRef]
- Phlips, P.; Rocmans, P.; Vanderhoeft, P.; Van Houtte, P. Postoperative radiotherapy after pneumonectomy: Impact of modern treatment facilities. Int. J. Radiat. Oncol. Biol. Phys. 1993, 27, 525–529. [Google Scholar] [CrossRef]
- Trodella, L.; Granone, P.; Valente, S.; Valentini, V.; Balducci, M.; Mantini, G.; Turriziani, A.; Margaritora, S.; Cesario, A.; Ramella, S. Adjuvant radiotherapy in non-small cell lung cancer with pathological stage I: Definitive results of a phase III randomized trial. Radiother. Oncol. 2002, 62, 11–19. [Google Scholar] [CrossRef]
- Stewart, L.; Burdett, S.; Parmar, M. PORT Meta-analysis Trialists Group. Postoperative radiotherapy in non-small-cell lung cancer: Systematic review and meta-analysis of individual patient data from nine randomised controlled trials. Lancet 1998, 352, 257–263. [Google Scholar] [CrossRef]
- Burdett, S.; Stewart, L.; On behalf of the PORT Meta-analysis Group. Postoperative radiotherapy in non-small-cell lung cancer: Update of an individual patient data meta-analysis. Lung Cancer 2005, 47, 81–83. [Google Scholar] [CrossRef]
- Burdett, S.; Rydzewska, L.; Tierney, J.; Fisher, D.; Parmar, M.K.B.; Arriagada, R.; Pignon, J.P.; Le Pechoux, C. Postoperative radiotherapy for non-small cell lung cancer. Cochrane Database Syst. Rev. 2016, 10, CD002142. [Google Scholar] [CrossRef] [PubMed]
- Douillard, J.-Y.; Rosell, R.; De Lena, M.; Riggi, M.; Hurteloup, P.; Mahe, M.-A.; Adjuvant Navelbine International Trialist Association. Impact of postoperative radiation therapy on survival in patients with complete resection and stage I, II, or IIIA non–small-cell lung cancer treated with adjuvant chemotherapy: The adjuvant Navelbine International Trialist Association (ANITA) Randomized Trial. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Douillard, J.-Y.; Rosell, R.; De Lena, M.; Carpagnano, F.; Ramlau, R.; Gonzáles-Larriba, J.L.; Grodzki, T.; Pereira, J.R.; Le Groumellec, A.; Lorusso, V. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB–IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): A randomised controlled trial. Lancet Oncol. 2006, 7, 719–727. [Google Scholar] [CrossRef]
- Sawyer, T.E.; Bonner, J.A.; Gould, P.M.; Foote, R.L.; Deschamps, C.; Trastek, V.F.; Pairolero, P.C.; Allen, M.S.; Shaw, E.G.; Marks, R.S. The impact of surgical adjuvant thoracic radiation therapy for patients with nonsmall cell lung carcinoma with ipsilateral mediastinal lymph node involvement. Cancer 1997, 80, 1399–1408. [Google Scholar] [CrossRef]
- Dautzenberg, B.; Arriagada, R.; Boyer Chammard, A.; Jarema, A.; Mezzetti, M.; Mattson, K.; Lagrange, J.L.; Le Pechoux, C.; Lebeau, B.; Chastang, C. A controlled study of postoperative radiotherapy for patients with completely resected nonsmall cell lung carcinoma. Cancer 1999, 86, 265–273. [Google Scholar] [CrossRef]
- Liu, T.; Mu, Y.; Dang, J.; Li, G. The role of postoperative radiotherapy for completely resected pIIIA-N2 non-small cell lung cancer patients with different clinicopathological features: A systemic review and meta-analysis. J. Cancer 2019, 10, 3941–3949. [Google Scholar] [CrossRef] [PubMed]
- Munro, A.J. What now for postoperative radiotherapy for lung cancer? Lancet 1998, 352, 250–251. [Google Scholar] [CrossRef]
- Machtay, M.; Lee, J.H.; Shrager, J.B.; Kaiser, L.R.; Glatstein, E. Risk of death from intercurrent disease is not excessively increased by modern postoperative radiotherapy for high-risk resected non–small-cell lung carcinoma. J. Clin. Oncol. 2001, 19, 3912–3917. [Google Scholar] [CrossRef]
- Abuodeh, Y.; Naghavi, A.O.; Echevarria, M.; DeMarco, M.; Tonner, B.; Feygelman, V.; Stevens, C.W.; Perez, B.A.; Dilling, T.J. Quantitatively excessive normal tissue toxicity and poor target coverage in postoperative lung cancer radiotherapy meta-analysis. Clin. Lung Cancer 2018, 19, e123–e130. [Google Scholar] [CrossRef]
- Billiet, C.; Peeters, S.; Decaluwe, H.; Vansteenkiste, J.; Mebis, J.; De Ruysscher, D. Postoperative radiotherapy for lung cancer: Is it worth the controversy? Cancer Treat. Rev. 2016, 51, 10–18. [Google Scholar] [CrossRef]
- Van Houtte, P.; Moretti, L.; Charlier, F.; Roelandts, M.; Van Gestel, D. Preoperative and postoperative radiotherapy (RT) for non-small cell lung cancer: Still an open question. Transl. Lung Cancer Res. 2021, 10, 1950. [Google Scholar] [CrossRef] [PubMed]
- Lally, B.E.; Zelterman, D.; Colasanto, J.M.; Haffty, B.G.; Detterbeck, F.C.; Wilson, L.D. Postoperative radiotherapy for stage II or III non–small-cell lung cancer using the surveillance, epidemiology, and end results database. J. Clin. Oncol. 2006, 24, 2998–3006. [Google Scholar] [CrossRef] [PubMed]
- Billiet, C.; Decaluwé, H.; Peeters, S.; Vansteenkiste, J.; Dooms, C.; Haustermans, K.; De Leyn, P.; De Ruysscher, D. Modern post-operative radiotherapy for stage III non-small cell lung cancer may improve local control and survival: A meta-analysis. Radiother. Oncol. 2014, 110, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sakib, N.; Li, N.; Zhu, X.; Li, D.; Li, Y.; Wang, H. Effect of postoperative radiotherapy on outcome in resectable stage IIIA-N2 non-small-cell lung cancer: An updated meta-analysis. Nucl. Med. Commun. 2018, 39, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Perry, M.C.; Kohman, L.J.; Bonner, J.A.; Gu, L.; Wang, X.; Vokes, E.E.; Green, M.R. A phase III study of surgical resection and paclitaxel/carboplatin chemotherapy with or without adjuvant radiation therapy for resected stage III non–small-cell lung cancer: Cancer and Leukemia Group B 9734. Clin. Lung Cancer 2007, 8, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.Y.; Ji, J.; Zuo, Y.S.; Pu, J.; Xu, Y.M.; Zong, C.D.; Tao, G.Z.; Chen, X.F.; Ji, F.Z.; Zhou, X.L. Comparison of efficacy for postoperative chemotherapy and concurrent radiochemotherapy in patients with IIIA-pN2 non-small cell lung cancer: An early closed randomized controlled trial. Radiother. Oncol. 2014, 110, 120–125. [Google Scholar] [CrossRef]
- Sun, J.M.; Noh, J.M.; Oh, D.; Kim, H.K.; Lee, S.H.; Choi, Y.S.; Pyo, H.; Ahn, J.S.; Jung, S.H.; Ahn, Y.C. Randomized phase II trial comparing chemoradiotherapy with chemotherapy for completely resected unsuspected N2-positive non–small cell lung cancer. J. Thorac. Oncol. 2017, 12, 1806–1813. [Google Scholar] [CrossRef]
- Wei, W.; Zhou, J.; Zhang, Q.; Liao, D.H.; Liu, Q.D.; Zhong, B.L.; Liang, Z.B.; Zhang, Y.C.; Jiang, R.; Liu, G.Y. Postoperative intensity-modulated radiation therapy reduces local recurrence and improves overall survival in III-N2 non-small-cell lung cancer: A single-center, retrospective study. Cancer Med. 2020, 9, 2820–2832. [Google Scholar] [CrossRef]
- Wang, T.; Jiang, T.; Han, Y.; Zhu, A.; Xin, S.; Xue, M.; Xin, X.; Lu, Q. Effects of postoperative adjuvant radiotherapy on stage IIIA-N2 non-small cell lung cancer and prognostic analysis. Off. J. Balk. Union Oncol. 2021, 26, 328–335. [Google Scholar]
- Hui, Z.; Men, Y.; Hu, C.; Kang, J.; Sun, X.; Bi, N.; Zhou, Z.; Liang, J.; Lv, J.; Feng, Q.; et al. Effect of Postoperative Radiotherapy for Patients With pIIIA-N2 Non–Small Cell Lung Cancer After Complete Resection and Adjuvant Chemotherapy: The Phase 3 PORT-C Randomized Clinical Trial. JAMA Oncol. 2021, 7, 1178–1185. [Google Scholar] [CrossRef]
- Le Pechoux, C.; Pourel, N.; Barlesi, F.; Lerouge, D.; Antoni, D.; Lamezec, B.; Nestle, U.; Boisselier, P.; Dansin, E.; Paumier, A. Postoperative radiotherapy versus no postoperative radiotherapy in patients with completely resected non-small-cell lung cancer and proven mediastinal N2 involvement (Lung ART): An open-label, randomised, phase 3 trial. Lancet Oncol. 2022, 23, 104–114. [Google Scholar] [CrossRef]
- Postoperative Radiotherapy versus No Postoperative Radiotherapy in Patients with Completely Resected Non-Small-Cell Lung Cancer and Proven Mediastinal N2 Involvement (Lung ART). Available online: https://clinicaltrials.gov/ct2/show/NCT00410683 (accessed on 10 January 2022).
- Daly, M.E.; Singh, N.; Ismaila, N.; Antonoff, M.B.; Arenberg, D.A.; Bradley, J.; David, E.; Detterbeck, F.; Früh, M.; Gubens, M.A. Management of Stage III Non–Small-Cell Lung Cancer: ASCO Guideline. J. Clin. Oncol. 2021. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Lardinois, D.; De Leyn, P.; Van Schil, P.; Porta, R.R.; Waller, D.; Passlick, B.; Zielinski, M.; Junker, K.; Rendina, E.A.; Ris, H.-B. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur. J. Cardio Thorac. Surg. 2006, 30, 787–792. [Google Scholar] [CrossRef]
- Gooseman, M.R.; Brunelli, A. Intraoperative lymph node management during non-small cell lung cancer surgery. Ann. Surg. Oncol. 2021, 28, 6925–6926. [Google Scholar] [CrossRef] [PubMed]
- Francis, S.; Orton, A.; Stoddard, G.; Tao, R.; Hitchcock, Y.J.; Akerley, W.; Kokeny, K.E. Sequencing of postoperative radiotherapy and chemotherapy for locally advanced or incompletely resected non–small-cell lung cancer. J. Clin. Oncol. 2018, 36, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.C.; Haque, W.; Verma, V.; Fang, P.; Lin, S.H. Concurrent versus sequential chemoradiation therapy in completely resected pathologic N2 non-small cell lung cancer: Propensity-matched analysis of the National Cancer Data Base. Ann. Surg. Oncol. 2018, 25, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- Keller, S.M.; Adak, S.; Wagner, H.; Herskovic, A.; Komaki, R.; Brooks, B.J.; Perry, M.C.; Livingston, R.B.; Johnson, D.H. A randomized trial of postoperative adjuvant therapy in patients with completely resected stage II or IIIA non–small-cell lung cancer. N. Engl. J. Med. 2000, 343, 1217–1222. [Google Scholar] [CrossRef]
- Bradley, J.D.; Paulus, R.; Graham, M.V.; Ettinger, D.S.; Johnstone, D.W.; Pilepich, M.V.; Machtay, M.; Komaki, R.; Atkins, J.; Curran, W.J. Phase II trial of postoperative adjuvant paclitaxel/carboplatin and thoracic radiotherapy in resected stage II and IIIa non–small-cell lung cancer: Promising long-term results of the Radiation Therapy Oncology Group—RTOG 9705. J. Clin. Oncol. 2005, 23, 3480–3487. [Google Scholar] [CrossRef]
- Yuan, C.; Tao, X.; Zheng, D.; Pan, Y.; Ye, T.; Hu, H.; Xiang, J.; Zhang, Y.; Chen, H.; Sun, Y. The lymph node status and histologic subtypes influenced the effect of postoperative radiotherapy on patients with N2 positive IIIA non–small cell lung cancer. J. Surg. Oncol. 2019, 119, 379–387. [Google Scholar] [CrossRef]
- Urban, D.; Bar, J.; Solomon, B.; Ball, D. Lymph node ratio may predict the benefit of postoperative radiotherapy in non–small-cell lung cancer. J. Thorac. Oncol. 2013, 8, 940–946. [Google Scholar] [CrossRef]
- Zeng, W.Q.; Feng, W.; Xie, L.; Zhang, C.C.; Yu, W.; Cai, X.W.; Fu, X.L. Postoperative radiotherapy for resected stage IIIA-N2 non-small-cell lung cancer: A population-based time-trend study. Lung 2019, 197, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Xie, M.; Tian, J.; Song, X.; Wu, B.; Liu, L. Propensity score-matching analysis of postoperative radiotherapy for stage IIIA-N2 non-small cell lung cancer using the Surveillance, Epidemiology, and End Results database. Radiat. Oncol. 2017, 12, 96. [Google Scholar] [CrossRef]
- Kou, P.; Wang, H.; Lin, J.; Zhang, Y.; Yu, J. Male patients with resected IIIA-N2 non-small-cell lung cancer may benefit from postoperative radiotherapy: A population-based survival analysis. Future Oncol. 2018, 14, 2371–2381. [Google Scholar] [CrossRef] [PubMed]
- Hui, Z.; Dai, H.; Liang, J.; Lv, J.; Zhou, Z.; Feng, Q.; Xiao, Z.; Chen, D.; Zhang, H.; Yin, W. Selection of proper candidates with resected pathological stage IIIA-N2 non-small cell lung cancer for postoperative radiotherapy. Thorac. Cancer 2015, 6, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Betticher, D.C.; Hsu Schmitz, S.-F.; Totsch, M.; Hansen, E.; Joss, C.; Von Briel, C.; Schmid, R.A.; Pless, M.; Habicht, J.; Roth, A.D. Mediastinal lymph node clearance after docetaxel-cisplatin neoadjuvant chemotherapy is prognostic of survival in patients with stage IIIA pN2 non–small-cell lung cancer: A multicenter phase II trial. J. Clin. Oncol. 2003, 21, 1752–1759. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ladbury, C.; Kim, J.; Raz, D.; Erhunmwunsee, L.; West, H.J.; Williams, T.; Salgia, R.; Massarelli, E.; Amini, A. Postoperative Radiation Therapy Should Be Used for Completely Resected Stage III-N2 NSCLC in Select Patients. J. Thorac. Oncol. 2022, 17, 194–196. [Google Scholar] [CrossRef] [PubMed]
- Glatzer, M.; Panje, C.M.; Siren, C.; Cihoric, N.; Putora, P.M. Decision making criteria in oncology. Oncology 2020, 98, 370–378. [Google Scholar] [CrossRef]
- Süveg, K.; Le Pechoux, C.; Faivre-Finn, C.; Putora, P.M.; De Ruysscher, D.; Widder, J.; Van Houtte, P.; Troost, E.G.C.; Slotman, B.J.; Ramella, S.; et al. Role of postoperative radiotherapy in the management for resected NSCLC—Decision criteria in clinical routine pre- and post-LungART. Clin. Lung Cancer 2021, 22, 579–586. [Google Scholar] [CrossRef]
- Edwards, J.G.; Chansky, K.; Van Schil, P.; Nicholson, A.G.; Boubia, S.; Brambilla, E.; Donington, J.; Galateau-Salle, F.; Hoffmann, H.; Infante, M.; et al. The IASLC lung cancer staging project: Analysis of resection margin status and proposals for residual tumor descriptors for non-small cell lung cancer. J. Thorac. Oncol. 2020, 15, 344–359. [Google Scholar] [CrossRef]
- Wang, E.H.; Corso, C.D.; Rutter, C.E.; Park, H.S.; Chen, A.B.; Kim, A.W.; Wilson, L.D.; Decker, R.H.; Yu, J.B. Postoperative radiation therapy is associated with improved overall survival in incompletely resected stage II and III non-small-cell lung cancer. J. Clin. Oncol. 2015, 33, 2727–2734. [Google Scholar] [CrossRef]
- Verma, V.; Moreno, A.C.; Haque, W.; Fang, P.; Lin, S.H. Sequential versus concurrent chemoradiation therapy by surgical margin status in resected non–small cell lung cancer. J. Natl. Compr. Cancer Netw. 2018, 16, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Faivre-Finn, C.; Edwards, J.G.; Hatton, M. Postoperative Radiation Therapy Should Not Be Used for the Therapy of Stage III-N2 NSCLC. J. Thorac. Oncol. 2022, 17, 197–199. [Google Scholar] [CrossRef] [PubMed]
- Vansteenkiste, J.F.; De Leyn, P.R.; Deneffe, G.J.; Lerut, T.E.; Demedts, M.G. Clinical prognostic factors in surgically treated stage IIIA-N2 non-small cell lung cancer: Analysis of the literature. Lung Cancer 1998, 19, 3–13. [Google Scholar] [CrossRef]
- Moretti, L.; David, S.Y.; Chen, H.; Carbone, D.P.; Johnson, D.H.; Keedy, V.L.; Putnam Jr, J.B.; Sandler, A.B.; Shyr, Y.; Lu, B. Prognostic factors for resected non-small cell lung cancer with pN2 status: Implications for use of postoperative radiotherapy. Oncologist 2009, 14, 1106. [Google Scholar] [CrossRef]
- Engels, E.A. Epidemiology of thymoma and associated malignancies. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2010, 5, S260–S265. [Google Scholar] [CrossRef]
- Wakelee, H.A.; Stephenson, P.; Keller, S.M.; Wagner, H.; Herskovic, A.; Komaki, R.; Marks, R.S.; Perry, M.C.; Livingston, R.B.; Johnson, D.H. Post-operative radiotherapy (PORT) or chemoradiotherapy (CPORT) following resection of stages II and IIIA non-small cell lung cancer (NSCLC) does not increase the expected risk of death from intercurrent disease (DID) in Eastern Cooperative Oncology Group (ECOG) trial E3590. Lung Cancer 2005, 48, 389–397. [Google Scholar] [CrossRef]
- Lally, B.E.; Detterbeck, F.C.; Geiger, A.M.; Thomas Jr, C.R.; Machtay, M.; Miller, A.A.; Wilson, L.D.; Oaks, T.E.; Petty, W.J.; Robbins, M.E. The risk of death from heart disease in patients with nonsmall cell lung cancer who receive postoperative radiotherapy: Analysis of the Surveillance, Epidemiology, and End Results database. Cancer 2007, 110, 911–917. [Google Scholar] [CrossRef]
- Deng, J.Y.; Wang, C.; Shi, X.H.; Jiang, G.L.; Wang, Y.; Liu, Y.; Zhao, K.L. Reduced toxicity with three-dimensional conformal radiotherapy or intensity-modulated radiotherapy compared with conventional two-dimensional radiotherapy for esophageal squamous cell carcinoma: A secondary analysis of data from four prospective clinical trials. Dis. Esophagus 2016, 29, 1121–1127. [Google Scholar] [CrossRef]
- Chun, S.G.; Hu, C.; Choy, H.; Komaki, R.U.; Timmerman, R.D.; Schild, S.E.; Bogart, J.A.; Dobelbower, M.C.; Bosch, W.; Galvin, J.M. Impact of intensity-modulated radiation therapy technique for locally advanced non–small-cell lung cancer: A secondary analysis of the NRG oncology RTOG 0617 randomized clinical trial. J. Clin. Oncol. 2017, 35, 56–62. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, Z.; Xu, S.; Zhang, Z. The dosimetric comparisons of CRT, IMRT, ARC, CRT+ IMRT, and CRT+ ARC of postoperative radiotherapy in IIIA-N2 stage non-small-cell lung cancer patients. BioMed Res. Int. 2019, 2019, 8989241. [Google Scholar] [CrossRef]
- Vyfhuis, M.A.; Onyeuku, N.; Diwanji, T.; Mossahebi, S.; Amin, N.P.; Badiyan, S.N.; Mohindra, P.; Simone, C.B. Advances in proton therapy in lung cancer. Ther. Adv. Respir. Dis. 2018, 12, 1753466618783878. [Google Scholar] [CrossRef] [PubMed]
- Remick, J.S.; Schonewolf, C.; Gabriel, P.; Doucette, A.; Levin, W.P.; Kucharczuk, J.C.; Singhal, S.; Pechet, T.T.; Rengan, R.; Simone II, C.B. First clinical report of proton beam therapy for postoperative radiotherapy for non–small-cell lung cancer. Clin. Lung Cancer 2017, 18, 364–371. [Google Scholar] [CrossRef]
- Boyce-Fappiano, D.; Nguyen, Q.-N.; Chapman, B.V.; Allen, P.K.; Gjyshi, O.; Pezzi, T.A.; De, B.; Gomez, D.; Lin, S.H.; Chang, J.Y. Single Institution Experience of Proton and Photon-based Postoperative Radiation Therapy for Non–small-cell Lung Cancer. Clin. Lung Cancer 2021, 22, e745–e755. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, A.F. Proton therapy for post-operative radiation therapy of non-small cell lung cancer. Transl. Lung Cancer Res. 2018, 7, 205. [Google Scholar] [CrossRef] [PubMed]
- Corso, C.D.; Rutter, C.E.; Wilson, L.D.; Kim, A.W.; Decker, R.H.; Husain, Z.A. Re-evaluation of the role of postoperative radiotherapy and the impact of radiation dose for non–small-cell lung cancer using the national cancer database. J. Thorac. Oncol. 2015, 10, 148–155. [Google Scholar] [CrossRef]
- Spoelstra, F.O.; Senan, S.; Le Péchoux, C.; Ishikura, S.; Casas, F.; Ball, D.; Price, A.; De Ruysscher, D.; de Koste, J.R.V.S.; Lung Adjuvant Radiotherapy Trial Investigators Group. Variations in target volume definition for postoperative radiotherapy in stage III Non–Small-Cell lung cancer: Analysis of an international contouring study. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 1106–1113. [Google Scholar] [CrossRef]
- Billiet, C.; De Ruysscher, D.; Peeters, S.; Decaluwé, H.; Vansteenkiste, J.; Dooms, C.; Deroose, C.M.; De Leyn, P.; Hendrikx, M.; Bulens, P. Patterns of locoregional relapses in patients with contemporarily staged stage III-N2 NSCLC treated with induction chemotherapy and resection: Implications for postoperative radiotherapy target volumes. J. Thorac. Oncol. 2016, 11, 1538–1549. [Google Scholar] [CrossRef]
- Nestle, U.; De Ruysscher, D.; Ricardi, U.; Geets, X.; Belderbos, J.; Pöttgen, C.; Dziadiuszko, R.; Peeters, S.; Lievens, Y.; Hurkmans, C. ESTRO ACROP guidelines for target volume definition in the treatment of locally advanced non-small cell lung cancer. Radiother. Oncol. 2018, 127, 1–5. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).