Ag/Au Bimetallic Nanoparticles Trigger Different Cell Death Pathways and Affect Damage Associated Molecular Pattern Release in Human Cell Lines
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. Characterization of NPs
2.2. Cytotoxicity Studies
2.3. Cell Death Pathways Induction
2.4. Extracellular DAMP Levels
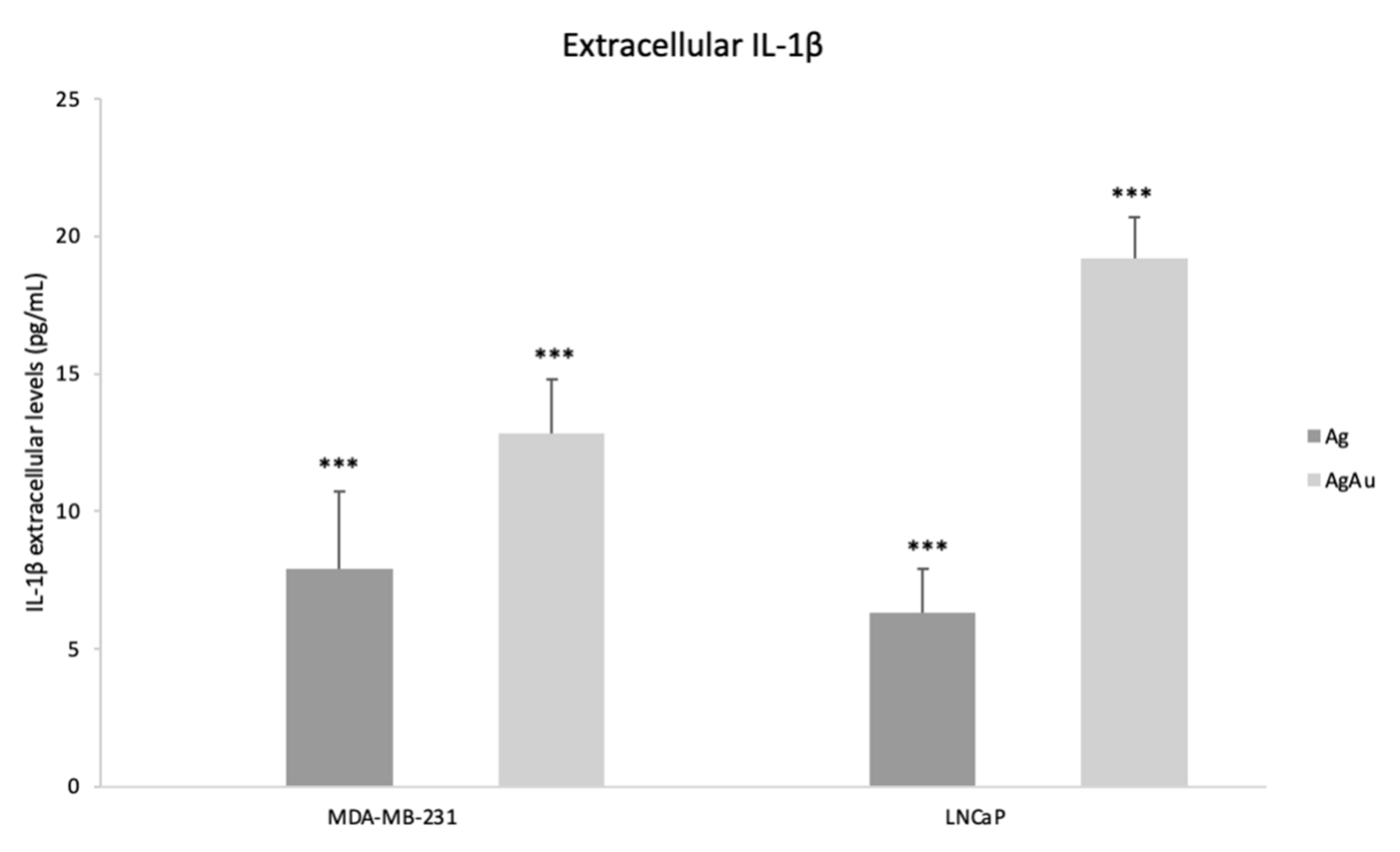
2.5. Extracellular IL-1β Levels
3. Discussion
4. Materials and Methods
4.1. Nanoparticle Preparation and Characterization
4.2. Cell Culture
- for HEK293, HCT 116, MDA-MB-231, MCF-7 and Li-Fraumeni fibroblasts DMEM High Glucose (BioSera, Shanghai, China) was used with 10% FBS (PAN Biotech, Aidenbach, Germany), 100 U/mL penicillin and 100 g/mL streptomycin
- for LNCaP RPMI (Gibco, Waltham, MA, USA) was used with 10% FBS and 100 U/mL penicillin and 100 g/mL streptomycin
- for SJ-GBM2 IMDM 1× (PAN BIOTECH) containing stable Glutamine 25 mM, HEPES (w: 3.024 g/: NaHCO3) with ITS (AOF ITS Supplement, Millipore, Burlingtone, MA, USA) and 20% FBS at 37 °C until reaching 70% confluency.
4.3. Cytotoxicity Assay (MTS Assay)
4.4. RNA Extraction, cDNA Synthesis and Real-Time PCR
4.5. Evaluation of Extracellular DAMPs (HMGB1) and IL-1β
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Pfeffer, C.M.; Singh, A.T.K. Apoptosis: A Target for Anticancer Therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neophytou, C.M.; Trougakos, I.P.; Erin, N.; Papageorgis, P. Apoptosis Deregulation and the Development of Cancer Multi-Drug Resistance. Cancers 2021, 13, 4363. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Qin, X.; Liang, J.; Ge, P. Induction of Pyroptosis: A Promising Strategy for Cancer Treatment. Front. Oncol. 2021, 11, 635774. [Google Scholar] [CrossRef] [PubMed]
- Man, S.M.; Karki, R.; Kanneganti, T.D. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol. Rev. 2017, 277, 61–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, Y.; Fan, Z.; Luo, G.; Yang, C.; Huang, Q.; Fan, K.; Cheng, H.; Kaizhou, J.; Ni, Q.; Yu, X.; et al. The role of necroptosis in cancer biology and therapy. Mol. Cancer 2019, 18, 100. [Google Scholar] [CrossRef] [Green Version]
- Jiao, H.; Wachsmuth, L.; Kumari, S.; Schwarzer, R.; Lin, J.; Eren, R.O.; Fisher, A.; Lane, R.; Young, G.R.; Kassiotis, G.; et al. Z-nucleic-acid sensing triggers ZBP1-dependent necroptosis and inflammation. Nature 2020, 580, 391–395. [Google Scholar] [CrossRef]
- Scarpitta, A.; Hacker, U.T.; Büning, H.; Boyer, O. Pyroptotic and Necroptotic Cell Death in the Tumor Microenvironment and Their Potential to Stimulate Anti-Tumor Immune Responses. Front. Oncol. 2021, 11, 731598. [Google Scholar] [CrossRef]
- Bai, D.P.; Zhang, X.F.; Zhang, G.L.; Huang, Y.F.; Gurunathan, S. Zinc oxide nanoparticles induce apoptosis and autophagy in human ovarian cancer cells. Int. J. Nanomed. 2017, 12, 6521–6535. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Du, L.; Feng, Y.; Wu, W.; Yan, Z. Pyroptosis induced by zinc oxide nanoparticles in A549 cells. Wei Sheng Yan Jiu J. Hyg. Res. 2013, 42, 273–276. [Google Scholar] [PubMed]
- Gao, J.; Qiu, X.; Xi, G.; Liu, H.; Zhang, F.; Lv, T.; Song, Y. Downregulation of GSDMD Attenuates Tumor Proliferation via the Intrinsic Mitochondrial Apoptotic Pathway and Inhibition of EGFR/Akt Signaling and Predicts a Good Prognosis in Non-Small Cell Lung Cancer. Oncol. Rep. 2018, 40, 1971–1984. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, S.; Chalk, A.M.; Ng, A.J.M.; Martin, T.J.; Zannettino, A.C.; Purton, L.E.; Lu, J.; Baker, E.K.; Walkley, C.R. In-creased miR-155-5p and Reduced miR-148a-3p Contribute to the Suppression of Osteosarcoma Cell Death. Oncogene 2016, 35, 5282–5294. [Google Scholar] [CrossRef] [PubMed]
- Katifelis, H.; Lyberopoulou, A.; Mukha, I.; Vityuk, N.; Grodzyuk, G.; Theodoropoulos, G.E.; Efstathopoulos, E.P.; Gazouli, M. Ag/Au bimetallic nanoparticles induce apoptosis in human cancer cell lines via P53, CASPASE-3 and BAX/BCL-2 pathways. Artif. Cells Nanomed. Biotechnol. 2018, 46, S389–S398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernandez, C.; Huebener, P.; Schwabe, R.F. Damage-associated molecular patterns in cancer: A double-edged sword. Oncogene 2016, 35, 5931–5941. [Google Scholar] [CrossRef] [PubMed]
- Krysko, O.; Aaes, T.L.; Bachert, C.; Vandenabeele, P.; Krysko, D.V. Many faces of DAMPs in cancer therapy. Cell Death Dis. 2013, 4, e631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Zhang, Y. HMGB1 in inflammation and cancer. J. Hematol. Oncol. 2020, 13, 116. [Google Scholar] [CrossRef]
- Ren, B.; Luo, S.; Xu, F.; Guoying, Z.; Guofeng, X.; He, J.; Huang, Y.; Zhu, H.; Li, Y. The expression of DAMP proteins HSP70 and cancer-testis antigen SPAG9 in peripheral blood of patients with HCC and lung cancer. Cell Stress Chaperones 2017, 22, 237–244. [Google Scholar] [CrossRef]
- Katifelis, H.; Mukha, I.; Bouziotis, P.; Vityuk, N.; Tsoukalas, C.; Lazaris, A.C.; Lyberopoulou, A.; Theodoropoulos, G.E.; Efstathopoulos, E.P.; Gazouli, M. Ag/Au Bimetallic Nanoparticles Inhibit Tumor Growth and Prevent Metastasis in a Mouse Model. Int. J. Nanomed. 2020, 15, 6019–6032. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Chastain, J., King, R.C., Jr., Eds.; Physical Electronics: Eden Prairie, MN, USA, 1995. [Google Scholar]
- Mukha, I.; Vityuk, N.V.; Eremenko, A.M.; Skoryk, M.A. Stabilization of metal nanoparticles in highly concentrated colloids. Surface 2020, 12, 337–345. [Google Scholar] [CrossRef]
- Ocadlikova, D.; Lecciso, M.; Isidori, A.; Loscocco, F.; Visani, G.; Amadori, S.; Cavo, M.; Curti, A. Chemotherapy-Induced Tumor Cell Death at the Crossroads Between Immunogenicity and Immunotolerance: Focus on Acute Myeloid Leukemia. Front. Oncol. 2019, 9, 1004. [Google Scholar] [CrossRef]
- Tang, D.; Kang, R.; Berghe, T.V.; Vandenabeele, P.; Kroemer, G. The molecular machinery of regulated cell death. Cell Res. 2019, 29, 347–364. [Google Scholar] [CrossRef] [Green Version]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Bin-Jumah, M.; Al-Abdan, M.; Albasher, G.; Alarifi, S. Effects of Green Silver Nanoparticles on Apoptosis and Oxidative Stress in Normal and Cancerous Human Hepatic Cells in vitro. Int. J. Nanomed. 2020, 15, 1537–1548. [Google Scholar] [CrossRef] [Green Version]
- Lagopati, N.; Kotsinas, A.; Veroutis, D.; Evangelou, K.; Papaspyropoulos, A.; Arfanis, M.; Falaras, P.; Kitsiou, P.V.; Pateras, I.; Bergonzini, A.; et al. Biological Effect of Silver-modified Nanostructured Titanium Dioxide in Cancer. Cancer Genom. Proteom. 2021, 18 (Suppl. 3), 425–439. [Google Scholar] [CrossRef]
- Xia, X.; Wang, X.; Cheng, Z.; Qin, W.; Lei, L.; Jiang, J.; Hu, J. The role of pyroptosis in cancer: Pro-cancer or pro-“host”? Cell Death Dis. 2019, 10, 650. [Google Scholar] [CrossRef] [Green Version]
- Miao, E.A.; Rajan, J.V.; Aderem, A. Caspase-1-induced pyroptotic cell death. Immunol. Rev. 2011, 243, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Friedlander, A.M. Macrophages are sensitive to anthrax lethal toxin through an acid-dependent process. J. Biol. Chem. 1986, 261, 7123–7126. [Google Scholar] [CrossRef]
- Ploetz, E.; Zimpel, A.; Cauda, V.; Bauer, D.; Lamb, D.C.; Haisch, C.; Zahler, S.; Vollmar, A.M.; Wuttke, S.; Engelke, H. Metal–Organic Framework Nanoparticles Induce Pyroptosis in Cells Controlled by the Extracellular pH. Adv. Mater. 2020, 32, 1907267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, P.; Wang, M.; Chen, M.; Chen, Z.; Peng, X.; Zhou, F.; Song, J.; Qu, J. Programming cell pyroptosis with biomimetic nanoparticles for solid tumor immunotherapy. Biomaterials 2020, 254, 120142. [Google Scholar] [CrossRef]
- Reisetter, A.C.; Stebounova, L.V.; Baltrusaitis, J.; Powers, L.; Gupta, A.; Grassian, V.H.; Monick, M.M. Induction of inflam-masome-dependent pyroptosis by carbon black nanoparticles. J. Biol. Chem. 2011, 286, 21844–21852. [Google Scholar] [CrossRef] [Green Version]
- Tan, Y.; Chen, Q.; Li, X.; Zeng, Z.; Xiong, W.; Li, G.; Li, X.; Yang, J.; Bo, X.; Yi, M. Pyroptosis: A new paradigm of cell death for fighting against cancer. J. Exp. Clin. Cancer Res. 2021, 40, 153. [Google Scholar] [CrossRef]
- Yan, H.; Luo, B.; Wu, X.; Guan, F.; Yu, X.; Zhao, L.; Ke, X.; Wu, J.; Yuan, J. Cisplatin Induces Pyroptosis via Activation of MEG3/NLRP3/caspase-1/GSDMD Pathway in Triple-Negative Breast Cancer. Int. J. Biol. Sci. 2021, 17, 2606–2621. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.; Vince, J.E. Pyroptosis versus necroptosis: Similarities, differences, and crosstalk. Cell Death Differ. 2019, 26, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Dhuriya, Y.K.; Sharma, D. Necroptosis: A regulated inflammatory mode of cell death. J. Neuroinflamm. 2018, 15, 199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baik, J.Y.; Liu, Z.; Jiao, D.; Kwon, H.J.; Yan, J.; Kadigamuwa, C.; Choe, M.; Lake, R.; Kruhlak, M.; Tandon, M.; et al. ZBP1 not RIPK1 mediates tumor necroptosis in breast cancer. Nat. Commun. 2021, 12, 2666. [Google Scholar] [CrossRef]
- Wu, Y.; Dong, G.; Sheng, C. Targeting necroptosis in anticancer therapy: Mechanisms and modulators. Acta Pharm. Sin. B 2020, 10, 1601–1618. [Google Scholar] [CrossRef]
- Sprooten, J.; De Wijngaert, P.; Vanmeerbeerk, I.; Martin, S.; Vangheluwe, P.; Schlenner, S.; Krysko, D.V.; Parys, J.B.; Bultynck, G.; Vandenabeele, P.; et al. Necroptosis in Immuno-Oncology and Cancer Immunotherapy. Cells 2020, 9, 1823. [Google Scholar] [CrossRef] [PubMed]
- Zielinska, E.; Zauszkiewicz-Pawlak, A.; Wojcik, M.; Inkielewicz-Stepniak, I. Silver nanoparticles of different sizes induce a mixed type of programmed cell death in human pancreatic ductal adenocarcinoma. Oncotarget 2017, 9, 4675–4697. [Google Scholar] [CrossRef] [Green Version]
- Farasat, M.; Niazvand, F.; Khorsandi, L. Zinc oxide nanoparticles induce necroptosis and inhibit autophagy in MCF-7 human breast cancer cells. Biologia 2020, 75, 161–174. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhan, X.; Xiong, J.; Peng, S.; Huang, W.; Joshi, R.; Cai, Y.; Liu, Y.; Li, R.; Yuan, K.; et al. Temperature-dependent cell death patterns induced by functionalized gold nanoparticle photothermal therapy in melanoma cells. Sci. Rep. 2018, 8, 8720. [Google Scholar] [CrossRef] [Green Version]
- Zhu, K.; Liang, W.; Ma, Z.; Xu, D.; Cao, S.; Lu, X.; Liu, N.; Shan, B.; Qian, L.; Yuan, J. Necroptosis promotes cell-autonomous activation of proinflammatory cytokine gene expression. Cell Death Dis. 2018, 9, 500. [Google Scholar] [CrossRef]
- McNamee, L.M.; Brodsky, M.H. p53-independent apoptosis limits DNA damage-induced aneuploidy. Genetics 2009, 182, 423–435. [Google Scholar] [CrossRef] [Green Version]
- Alhajala, H.S.; Nguyen, H.S.; Shabani, S.; Best, B.; Kaushal, M.; Al-Gizawiy, M.M.; Erin Ahn, E.Y.; Knipstein, J.A.; Mirza, S.; Schmainda, K.M.; et al. Irradiation of pediatric glioblastoma cells promotes radioresistance and enhances glioma malignancy via genome-wide transcriptome changes. Oncotarget 2018, 9, 34122–34131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, S.; Cheng, J.; Sun, L.; Wang, Y.; Wang, C.; Liu, X.; Zhang, Z.; Zhao, M.; Luo, Y.; Tian, L.; et al. HMGB1 re-leased by irradiated tumor cells promotes living tumor cell proliferation via paracrine effect. Cell Death Dis. 2018, 9, 648. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Zhang, Q.; Zeh, H.J., 3rd; Lotze, M.T.; Tang, D. HMGB1 in cancer: Good, bad, or both? Clin. Cancer Res. 2013, 19, 4046–4057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albakova, Z.; Armeev, G.A.; Kanevskiy, L.M.; Kovalenko, E.I.; Sapozhnikov, A.M. HSP70 Multi-Functionality in Cancer. Cells 2020, 9, 587. [Google Scholar] [CrossRef] [Green Version]
- Murao, A.; Aziz, M.; Wang, H.; Brenner, M.; Wang, P. Release mechanisms of major DAMPs. Apoptosis 2021, 26, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, F.Z.; Yim, S.O.; Pathak, S.; Grant, G.; Siciliano, M.J.; Giovanella, B.C.; Strong, L.C.; Tainsky, M.A. Spontaneous abnormalities in normal fibroblasts from patients with Li-Fraumeni cancer syndrome: Aneuploidy and immortalization. Cancer Res. 1990, 50, 7979–7984. [Google Scholar] [PubMed]
- Children’s Oncology Group, Cell line and Xenograft Repository. SJ-GBM2 Cell Line Data Sheet. Texas Tech University, HEalth Sciences Center, School of Medicine, Cancer Center. Available online: https://www.cccells.org/dl/BrainTumorDataSheets/SJ-GBM2_Cell_Line_Data_Sheet_COGcell_org.pdf (accessed on 17 November 2021).




| Viability of MDA-MB-231 (%) | Ag NPs | Au NPs | AgAu NPs | Untreated |
|---|---|---|---|---|
| 30 μg/mL | 80 ± 5 | 88 ± 3 | 81 ± 4 | |
| 40 μg/mL | 75 ± 5 | 85 ± 9 | 70 ± 5 | 99 ± 3 |
| 50 μg/mL | 70 ± 8 | 81 ± 4 | 67 ± 8 | |
| Viability of MCF-7 (%) | ||||
| 30 μg/mL | 95 ± 6 | 96 ± 6 | 97 ± 5 | |
| 40 μg/mL | 88 ± 5 | 89 ± 8 | 88 ± 5 | 99 ± 1 |
| 50 μg/mL | 75 ± 3 *** | 82 ± 5 | 68 ± 4 *** | |
| Viability of Li-Fraumeni fibroblasts (%) | ||||
| 30 μg/mL | 100 ± 4 | 100 ± 2 | 100 ± 2 | |
| 40 μg/mL | 99 ± 2 | 97 ± 3 | 98 ± 1 | 100 ± 4 |
| 50 μg/mL | 96 ± 3 | 97 ± 2 | 97 ± 1 | |
| Viability LNCaP (%) | ||||
| 30 μg/mL | 76 ± 7 | 93 ± 4 | 90 ± 3 | |
| 40 μg/mL | 72 ± 3 *** | 86 ± 5 | 81 ± 5 *** | 99 ± 1 |
| 50 μg/mL | 63 ± 4 *** | 79 ± 8 | 75 ± 4 *** | |
| Viability of C4-2B (%) | ||||
| 30 μg/mL | 100 ± 1 | 100 ± 6 | 100 ± 4 | |
| 40 μg/mL | 92 ± 6 | 95 ± 4 | 95 ± 5 | 99 ± 4 |
| 50 μg/mL | 88 ± 4 | 93 ± 5 | 84 ± 6 | |
| Viability of SJ-GBM2 (%) | ||||
| 30 μg/mL | 100 ± 7 | 100 ± 5 | 100 ± 4 | |
| 40 μg/mL | 95 ± 4 | 95 ± 4 | 96 ± 5 | 99 ± 2 |
| 50 μg/mL | 91 ± 6 | 93 ± 4 | 92 ± 4 | |
| Viability of HCT116 (%) | ||||
| 30 μg/mL | 45 ± 2 *** | 65 ± 3 *** | 49 ± 8 *** | |
| 40 μg/mL | 21 ± 7 *** | 41 ± 5 *** | 38 ± 4 *** | 100 ± 5 |
| 50 μg/mL | 14 ± 1 *** | 12 ± 1 *** | 12 ± 13 *** |
| A (Ag NPs) | HEK293 | MDA-MB-231 | MCF-7 | Li-Fraumeni Fibroblasts | LNCaP | C4-2B | SJ-GBM2 | HCT116 |
| CASP1 | −1.11 | 4.92 | −1.62 | −1.23 | 1.74 | −1.63 | −2.29 | −1.58 |
| CASP3 | 1.22 | 2.00 | −1.62 | −1.14 | −1.23 | −1.74 | −6.09 *** | 1.50 |
| BCL-2 | −1.10 | −2.86 | −1.23 | 1.00 | 1.00 | −1.33 | 1.36 | −1.80 |
| ZPB1 | −1.11 | 3.60 | −1.32 | −3.57 | −2.46 | −1.86 | −3.03 | 1.00 |
| HMGB1 | −1.23 | 1.27 | −1.41 | −1.23 | 1.14 | −1.14 | −2.83 | 1.68 |
| HSP70 | 1.00 | −1.75 | −1.51 | 1.14 | 1.31 | −4.00 | −1.51 | 1.23 |
| CXCL8 | 1.07 | 1.15 | 1.15 | −7.60 | −1.20 | −1.53 | −1.24 | 1.07 |
| CSF1 | 1.23 | 1.40 | 1.62 | −5.5 | −2.50 | 1.20 | −1.33 | −1.14 |
| CCL20 | −1.23 | 1.10 | 1.31 | 1.15 | −1.15 | 1.23 | 1.50 | 1.23 |
| NLRP3 | 1.14 | 2.29 | 1.30 | −1.07 | 1.30 | 1.07 | 1.15 | −1.75 |
| IL-1β | 1.62 | 2.46 | 1.07 | −1.07 | 2.82 | 1.23 | 1.23 | 1.87 |
| IL-18 | 1.75 | 1.15 | 1.86 | 1.31 | 1.00 | 1.50 | 1.10 | 1.23 |
| B (Au NPs) | HEK293 | MDA-MB-231 | MCF-7 | Li-Fraumeni Fibroblasts | LNCaP | C4-2B | SJ-GBM2 | HCT116 |
| CASP1 | −1.33 | 2.14 | −4.92 | 1.07 | 6.96 *** | −2.29 | −2.00 | 1.31 |
| CASP3 | 1.00 | 2.14 | −1.41 | 1.00 | −1.20 | −2.29 | −6.08 *** | 1.70 |
| BCL-2 | 1.01 | −1.78 | 1.62 | −1.33 | −1.13 | 1.36 | 1.10 | −1.31 |
| ZPB1 | 2.21 | 1.93 | 1.31 | −1.41 | −1.74 | −2.00 | −3.48 | 1.56 |
| HMGB1 | −1.20 | 1.14 | −1.51 | 1.00 | 1.74 | 1.40 | −1.86 | 2.21 |
| HSP70 | −1.47 | 1.18 | 1.31 | −3.03 | 1.30 | −1.86 | −2.83 | 1.31 |
| CXCL8 | 1.31 | −1.75 | −1.88 | −2.17 | 1.31 | 1.86 | −1.66 | 1.62 |
| CSF1 | 1.29 | 1.15 | −2.17 | −1.33 | −1.64 | −1.25 | 1.10 | 1.23 |
| CCL20 | 1.03 | 1.74 | −2.86 | −1.53 | 1.41 | −1.43 | 1.10 | 1.10 |
| NLRP3 | 1.42 | 1.86 | 1.62 | −1.54 | −1.42 | 1.00 | −1.15 | 1.74 |
| IL-1β | 1.41 | 1.23 | 1.23 | 1.23 | 1.60 | 1.39 | −1.43 | 1.41 |
| IL-18 | 1.23 | 1.41 | 1.32 | −1.07 | 1.00 | 1.30 | −1.66 | 1.87 |
| C (AgAu NPs) | HEK293 | MDA-MB-231 | MCF-7 | Li-Fraumeni Fibroblasts | LNCaP | C4-2B | SJ-GBM2 | HCT116 |
| CASP1 | 2.46 | 3.03 | 1.51 | −1.75 | 6.96 *** | −1.33 | 1.14 | −6.66 *** |
| CASP3 | 1.00 | 2.07 | 1.07 | −1.75 | −1.23 | −1.07 | −2.46 | 2.00 |
| BCL-2 | 1.12 | −2.38 | 1.40 | −1.30 | 1.00 | −1.60 | 1.80 | −1.90 |
| ZPB1 | −1.11 | 3.03 | −2.00 | −2.29 | −2.29 | −2.29 | −2.64 | 1.14 |
| HMGB1 | 2.46 | 1.27 | −1.07 | 1.23 | 1.74 | 3.24 | −1.41 | −1.00 |
| HSP70 | −1.96 | −1.36 | −1.07 | −3.03 | −1.51 | −2.46 | −2.00 | −1.19 |
| CXCL8 | −1.33 | 1.32 | 1.32 | −1.08 | −1.15 | −1.15 | 1.50 | 1.00 |
| CSF1 | 1.23 | 1.74 | −1.42 | −1.23 | 1.10 | 1.41 | −2.00 | 1.10 |
| CCL20 | 1.10 | 1.00 | 1.75 | 1.22 | 1.41 | 1.23 | 1.29 | 1.62 |
| NLRP3 | 1.08 | 1.63 | 1.29 | 1.00 | 1.00 | −2.00 | 1.11 | −2.70 |
| IL-1β | 1.14 | 2.83 | 1.75 | 1.00 | 2.64 | 1.32 | −1.10 | 2.29 |
| IL-18 | 1.75 | 1.74 | −1.53 | −1.89 | −1.43 | 1.67 | 1.15 | 1.32 |
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| ZPB1 | 5′-TGGTCATCGCCCAAGCACTG-3′ | 5′-GGCGGTAAATCGTCCATGCT-3′ |
| CASP1 | 5′-GCCTGTTCCTGTGATGTGGAG-3′ | 5′-TGCCCACAGACATTCATACAGTTTC-3′ |
| CASP3 | 5′-TGGTTCATCCAGTCGCTTTG-3′ | 5′-CATTCTGTTGCCACCTTTCG-3′ |
| BCL-2 | 5′-GATGTGGATGCCTCTGCGAAG-3′ | 5′-CTGCTGATGTCTCTGGATCT-3′ |
| HSP70 | 5′-ATGTCGGTGGTGGGCATAGA-3′ | 5′-CACAGCGACGTAGCAGCTCT-3′ |
| HMGB1 | 5′-ATATGGCAAAGCGGACAAG-3′ | 5′-AGGCCAGGATGTTCTCCTTT-3′ |
| CXCL8 | 5′-CAGTTTGCCAAGGAGTGCT-3′ | 5′-ACTTCTCCACAACCCTCTGC-3′ |
| CSF1 | 5′-TGGCGAGCAGGAGTATCAC-3′ | 5′-AGGTCTCCATCTGACTGTCAAT-3′ |
| CCL20 | 5′-GTGCTGCTACTCCACCTCTG-3′ | 5′-GCATTGATGTCACAGCCTTCA-3′ |
| NLRP3 | 5′-TTCAATGGCGAGGAGAAGGC-3′ | 5′-ACGTGTCATTCCACTCTGGC-3′ |
| IL-1β | 5′-CCTTGTCGAGAATGGGCAGT-3′ | 5′-TCCTGTCGACAATGCTGCCT-3′ |
| IL-18 | 5′-TCTTCATTGACCAAGGAAATCGG-3′ | 5′-TCCGGGGTGCATTATCTCTAC-3′ |
| GAPDH | 5′-CATCTCTGCCCCCTCTGCTG-3′ | 5′-GCCTGCTTCACCACCTTGTTG-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katifelis, H.; Nikou, M.-P.; Mukha, I.; Vityuk, N.; Lagopati, N.; Piperi, C.; Farooqi, A.A.; Pippa, N.; Efstathopoulos, E.P.; Gazouli, M. Ag/Au Bimetallic Nanoparticles Trigger Different Cell Death Pathways and Affect Damage Associated Molecular Pattern Release in Human Cell Lines. Cancers 2022, 14, 1546. https://doi.org/10.3390/cancers14061546
Katifelis H, Nikou M-P, Mukha I, Vityuk N, Lagopati N, Piperi C, Farooqi AA, Pippa N, Efstathopoulos EP, Gazouli M. Ag/Au Bimetallic Nanoparticles Trigger Different Cell Death Pathways and Affect Damage Associated Molecular Pattern Release in Human Cell Lines. Cancers. 2022; 14(6):1546. https://doi.org/10.3390/cancers14061546
Chicago/Turabian StyleKatifelis, Hector, Maria-Paraskevi Nikou, Iuliia Mukha, Nadiia Vityuk, Nefeli Lagopati, Christina Piperi, Ammad Ahmad Farooqi, Natassa Pippa, Efstathios P. Efstathopoulos, and Maria Gazouli. 2022. "Ag/Au Bimetallic Nanoparticles Trigger Different Cell Death Pathways and Affect Damage Associated Molecular Pattern Release in Human Cell Lines" Cancers 14, no. 6: 1546. https://doi.org/10.3390/cancers14061546
APA StyleKatifelis, H., Nikou, M.-P., Mukha, I., Vityuk, N., Lagopati, N., Piperi, C., Farooqi, A. A., Pippa, N., Efstathopoulos, E. P., & Gazouli, M. (2022). Ag/Au Bimetallic Nanoparticles Trigger Different Cell Death Pathways and Affect Damage Associated Molecular Pattern Release in Human Cell Lines. Cancers, 14(6), 1546. https://doi.org/10.3390/cancers14061546











