Stem Cell Theory of Cancer: Implications for Drug Resistance and Chemosensitivity in Cancer Care
Abstract
Simple Summary
Abstract
1. Introduction
2. Brief History
3. Chemotherapy
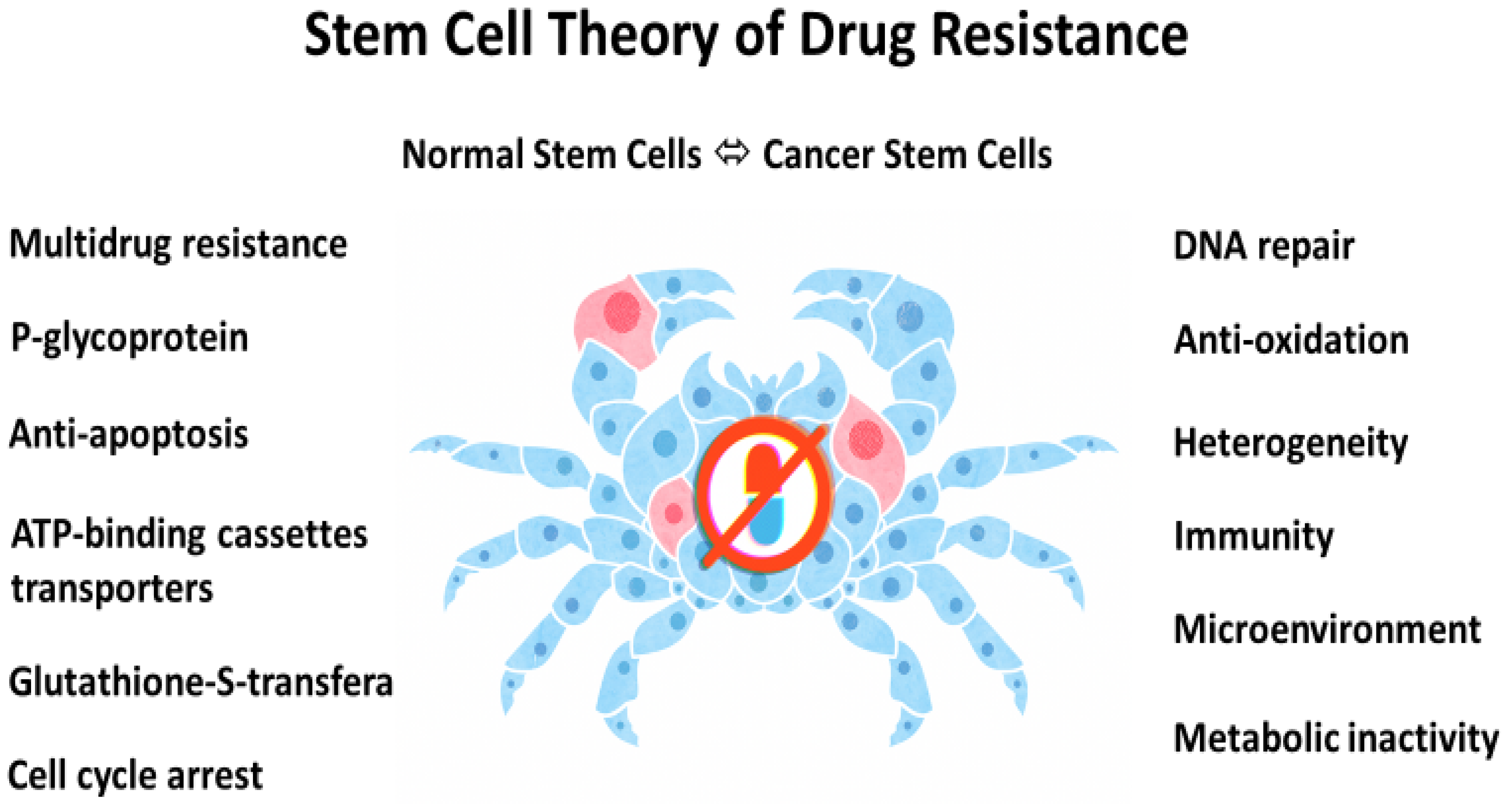
4. Chemoresistance
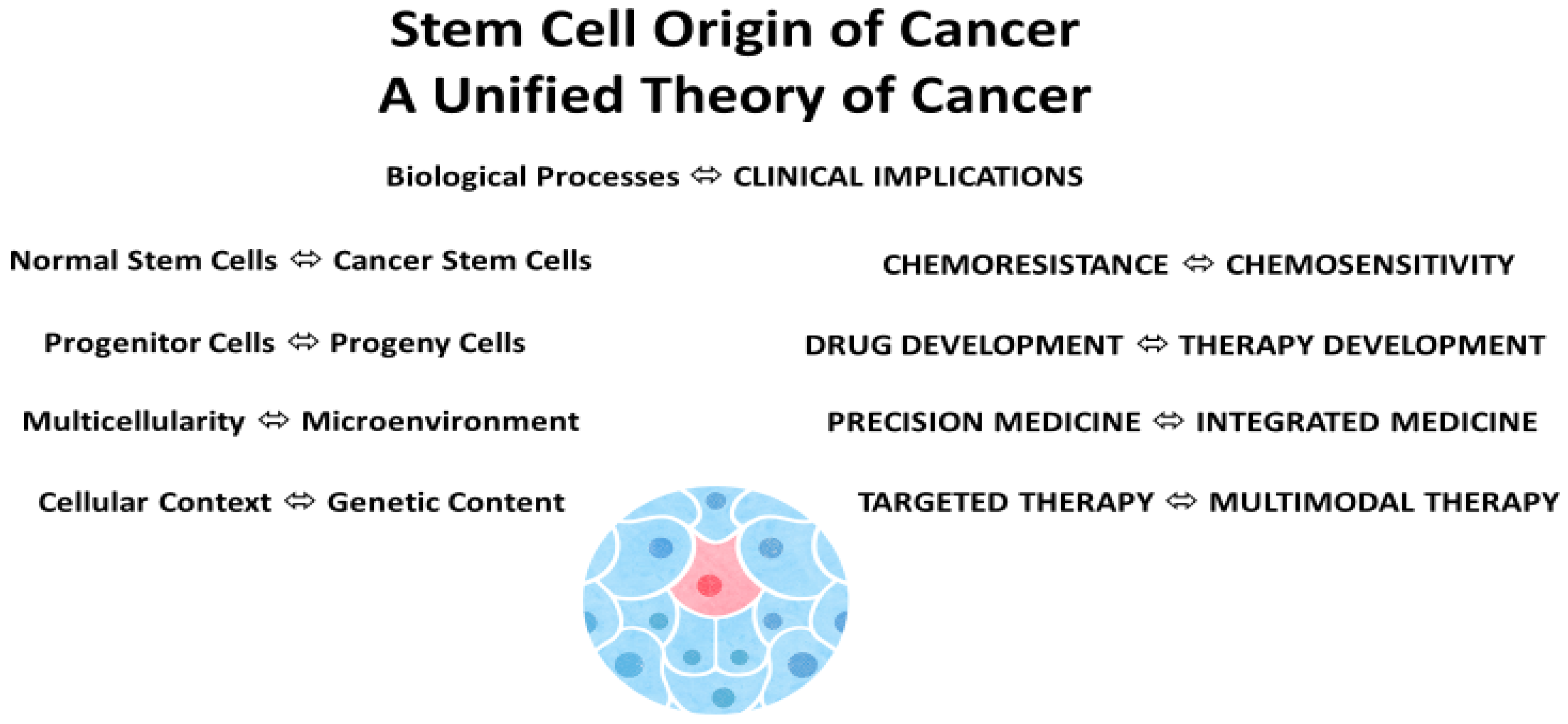
5. Stem Cell Origin
6. Cellular Context
7. DNA Repair
8. Asymmetric Division
9. A Fortuitous Experiment
10. Chronotherapy
11. Drug Development
12. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tu, S.-M.; Lin, S.-H.; Logothetis, C.J. Stem-cell origin of metastasis and heterogeneity in solid tumours. Lancet Oncol. 2002, 3, 508–513. [Google Scholar] [CrossRef]
- Koch, R. Die Atiologic der Tuberkulose. Berl. Klin. Wochenschr. 1882, 15, 221–230. [Google Scholar]
- Iseman, M.D. Tuberculosis therapy: Past, present and future. Eur. Respir. J. 2002, 20, 87S–94S. [Google Scholar] [CrossRef] [PubMed]
- Christakis, P. The birth of chemotherapy at Yale. Bicentennial lecture series: Surgery Grand Round. Yale J. Biol. Med. 2011, 84, 169–172. [Google Scholar] [PubMed]
- Miller, D.R. A tribute to Sidney Farber—The father of modern chemotherapy. Br. J. Haematol. 2006, 134, 20–26. [Google Scholar] [CrossRef]
- Thiebaut, F.; Tsuruo, T.; Hamada, H.; Gottesman, M.M.; Pastan, I.; Willingham, M.C. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc. Natl. Acad. Sci. USA 1987, 84, 7735–7738. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, C.A.; Rosenthal, C.T.; Lowe, S.W. Genetic analysis of chemoresistance in primary murine lymphomas. Nat. Med. 2000, 6, 1029–1035. [Google Scholar] [CrossRef]
- Dean, M. ABC Transporters, Drug Resistance, and Cancer Stem Cells. J. Mammary Gland. Biol. Neoplasia 2009, 14, 3–9. [Google Scholar] [CrossRef]
- Sun, M.; Yang, C.; Zheng, J.; Wang, M.; Chen, M.; Le, D.Q.S.; Kjems, J.; Bünger, C.E. Enhanced efficacy of chemotherapy for breast cancer stem cells by simultaneous suppression of multidrug resistance and antiapoptotic cellular defense. Acta Biomater. 2015, 28, 171–182. [Google Scholar] [CrossRef]
- Leccia, F.; Del Vecchio, L.; Mariotti, E.; Di Noto, R.; Morel, A.-P.; Puisieux, A.; Salvatore, F.; Ansieau, S. ABCG2, a novel antigen to sort luminal progenitors of BRCA1-breast cancer cells. Mol. Cancer 2014, 13, 213. [Google Scholar] [CrossRef]
- Sims-Mourtada, J.; Izzo, J.G.; Ajani, J.; Chao, K.S.C. Sonic Hedgehog promotes multiple drug resistance by regulation of drug transport. Oncogene 2007, 26, 5674–5679. [Google Scholar] [CrossRef]
- Safa, A.R. Resistance to Cell Death and Its Modulation in Cancer Stem Cells. Crit. Rev. Oncog. 2016, 21, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Z.; Ajani, J.A.; Song, S. Drug resistance and Cancer stem cells. Cell Commun. Signal. 2021, 19, 19. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, S.; Das, S.K.; Emdad, L.; Fisher, P.B. Autophagy and senescence: Insights from normal and cancer stem cells. Adv. Cancer Res. 2021, 150, 147–208. [Google Scholar]
- Morrow, C.S.; Cowan, K.H. Glutathione S-transferases and drug resistance. Cancer Cells 1990, 2, 15–22. [Google Scholar] [PubMed]
- Singh, R.R.; Reindl, K.M. Glutathione S-Transferases in Cancer. Antioxidants 2021, 10, 701. [Google Scholar] [CrossRef] [PubMed]
- Townsend, M.; Tew, K.D. The role of glutathione-S-transferase in anti-cancer drug resistance. Oncogene 2003, 22, 7369–7375. [Google Scholar] [CrossRef]
- Tu, S.M. Origin of Cancers. Clinical Perspectives and Implications of a Stem-Cell Theory of Cancer; Rosen, S.T., Ed.; Cancer Treatment and Research; Springer: New York, NY, USA, 2010; Volume 154. [Google Scholar]
- Tu, S.M. Story of Hydra: Portrait of Cancer as a Stem-Cell Disease; Nova: New York, NY, USA, 2019. [Google Scholar]
- Tu, S.M.; Campbell, M.; Shah, A.; Logothetis, C.J. Application of a successful germ cell tumor paradigm to the challenges of common adult solid cancers. J. Cell Sci. Ther. 2021, 12, 301. [Google Scholar]
- Savage, P. Clinical observations on chemotherapy curable malignancies: Unique genetic events, frozen development and enduring apoptotic potential. BMC Cancer 2015, 15, 2707. [Google Scholar] [CrossRef][Green Version]
- Duvoix, A.; Morceau, F.; Delhalle, S.; Schmitz, M.; Schnekenburger, M.; Galteau, M.-M.; Dicato, M.; Diederich, M. Induction of apoptosis by curcumin: Mediation by glutathione S-transferase P1-1 inhibition. Biochem. Pharmacol. 2003, 66, 1475–1483. [Google Scholar] [CrossRef]
- Flinders, C.; Lam, L.; Rubbi, L.; Ferrari, R.; Fitz-Gibbon, S.; Chen, P.-Y.; Thompson, M.; Christofk, H.; Agus, D.B.; Ruderman, D.; et al. Epigenetic changes mediated by polycomb repressive complex 2 and E2a are associated with drug resistance in a mouse model of lymphoma. Genome Med. 2016, 8, 54. [Google Scholar] [CrossRef] [PubMed]
- Lüönd, F.; Sugiyama, N.; Bill, R.; Bornes, L.; Hager, C.; Tang, F.; Santacroce, N.; Beisel, C.; Ivanek, R.; Bürglin, T.; et al. Distinct contributions of partial and full EMT to breast cancer malignancy. Dev. Cell 2021, 56, 3203–3221.e11. [Google Scholar] [CrossRef]
- Fennell, K.A.; Vassiliadis, D.; Lam, E.Y.N.; Martelotto, L.G.; Balic, J.J.; Hollizeck, S.; Weber, T.S.; Semple, T.; Wang, Q.; Miles, D.C.; et al. Non-genetic determinants of malignant clonal fitness at single-cell resolution. Nature 2021, 601, 125–131. [Google Scholar] [CrossRef]
- Turati, V.A.; Guerra-Assunção, J.A.; Potter, N.E.; Gupta, R.; Ecker, S.; Daneviciute, A.; Tarabichi, M.; Webster, A.P.; Ding, C.; May, G.; et al. Chemotherapy induces canalization of cell state in childhood B-cell precursor acute lymphoblastic leukemia. Nat. Cancer 2021, 2, 835–852. [Google Scholar] [CrossRef] [PubMed]
- Marine, J.-C.; Dawson, S.-J.; Dawson, M.A. Non-genetic mechanisms of therapeutic resistance in cancer. Nat. Cancer 2020, 20, 743–756. [Google Scholar] [CrossRef]
- Kernek, K.M.; Ulbright, T.M.; Zhang, S.; Billings, S.D.; Cummings, O.W.; Henley, J.D.; Michael, H.; Brunelli, M.; Martignoni, G.; Foster, R.S.; et al. Identical Allelic Losses in Mature Teratoma and Other Histologic Components of Malignant Mixed Germ Cell Tumors of the Testis. Am. J. Pathol. 2003, 163, 2477–2484. [Google Scholar] [CrossRef]
- Jones, T.D.; Wang, M.; Sung, M.-T.; Zhang, S.; Ulbright, T.M.; Eble, J.N.; Beck, S.D.; Foster, R.S.; Anagnostou, J.J.; Conner, C.; et al. Clonal Origin of Metastatic Testicular Teratomas. Clin. Cancer Res. 2006, 12, 5377–5383. [Google Scholar] [CrossRef] [PubMed]
- Umbreit, E.C.; Siddiqui, B.A.; Hwang, M.J.; Joon, A.Y.; Maity, T.; Westerman, M.E.; Merriman, K.W.; Alhasson, H.; Uthup, J.; Guo, T.; et al. Origin of Subsequent Malignant Neoplasms in Patients with History of Testicular Germ Cell Tumor. Cancers 2020, 12, 3755. [Google Scholar] [CrossRef] [PubMed]
- Casorelli, I.; Pelosi, E.; Biffoni, M.; Cerio, A.M.; Peschle, C.; Testa, U.; Bignami, M. Methylation damage response in hematpoietic progenitor cells. DNA Repair. 2007, 6, 1170–1178. [Google Scholar] [CrossRef]
- Bracker, T.U.; Giebel, B.; Spanholtz, J.; Sorg, U.R.; Klein-Hitpass, L.; Moritz, T.; Thomale, J. Stringent Regulation of DNA Repair During Human Hematopoietic Differentiation: A Gene Expression and Functional Analysis. Stem Cells 2006, 24, 722–730. [Google Scholar] [CrossRef]
- Guinney, J.; Dienstmann, R.; Wang, X.; De Reyniès, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Hoevenaar, W.H.M.; Janssen, A.; Quirindongo, A.I.; Ma, H.; Klaasen, S.J.; Teixeira, A.; van Gerwen, B.; Lansu, N.; Morsink, F.H.M.; Offerhaus, G.J.A.; et al. Degree and site of chromosomal instability define its oncogenic potential. Nat. Commun. 2020, 11, 1501. [Google Scholar] [CrossRef] [PubMed]
- Gryfe, R.; Kim, H.; Hsieh, E.T.; Aronson, M.D.; Holowaty, E.J.; Bull, S.B.; Redston, M.; Gallinger, S. Tumor Microsatellite Instability and Clinical Outcome in Young Patients with Colorectal Cancer. N. Engl. J. Med. 2000, 342, 69–77. [Google Scholar] [CrossRef]
- Samowitz, W.S.; Curtin, K.; Ma, K.N.; Schaffer, D.; Coleman, L.W.; Leppert, M.; Slattery, M.L. Microsatellite instability in sporadic colon cancer is associated with an improved prognosis at the population level. Cancer Epidemiol. Biomark. Prev. 2001, 10, 917–923. [Google Scholar]
- Gore, A.; Li, Z.; Fung, H.-L.; Young, J.E.; Agarwal, S.; Antosiewicz-Bourget, J.; Canto, I.; Giorgetti, A.; Israel, M.A.; Kiskinis, E.; et al. Somatic coding mutations in human induced pluripotent stem cells. Nature 2011, 471, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.Z.; Gopalakrishna-Pillai, S.; Nay, S.L.; Park, S.W.; Bates, S.E.; Zeng, X.; Iverson, L.E.; O’Connor, T.R. DNA repair in human pluripotent stem cells is distinct from that in non-pluripotent human cells. PLoS ONE 2012, 7, e30541. [Google Scholar] [CrossRef]
- Abad, M.; Mosteiro, L.; Pantoja, C.; Canamero, M.; Rayon, T.; Ors, I.; Grana, O.; Megias, D.; Dominguez, O.; Martinez, D.; et al. Reprogramming in vivo produces teratomas and iPS cells with totipotency features. Nature 2013, 502, 340–345. [Google Scholar] [CrossRef]
- Ohnishi, K.; Semi, K.; Yamamoto, T.; Shimizu, M.; Tanaka, A.; Mitsunaga, K.; Okita, K.; Osafune, K.; Arioka, Y.; Maeda, T.; et al. Premature Termination of Reprogramming In Vivo Leads to Cancer Development through Altered Epigenetic Regulation. Cell 2014, 156, 663–677. [Google Scholar] [CrossRef]
- Nori, S.; Okada, Y.; Nishimura, S.; Sasaki, T.; Itakura, G.; Kobayashi, Y.; Renault-Mihara, F.; Shimizu, A.; Koya, I.; Yoshida, R.; et al. Long-term safety issues of IPSC-based cell therapy in a spinal cord injury model: Oncogenic transformation with epithelial-mesenchymal transition. Stem Cell Rep. 2015, 4, 360–373. [Google Scholar] [CrossRef]
- Kolarski, D.; Miro-Vinyals, C.; Sugiyama, A.; Srivastava, A.; Ono, D.; Nagai, Y.; Lida, M.; Itami, K.; Tama, F.; Szymanski, W.; et al. Reversible modulation of circadian time with chronophotopharmacology. Nat. Commun. 2021, 12, 3164. [Google Scholar] [CrossRef]
- Allada, R.; Bass, J. Circadian Mechanisms in Medicine. N. Engl. J. Med. 2021, 384, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Levi, F.; Zidani, R.; Misset, J.L. Randomised multicenter trial of chronotherapy with oxaliplatin, fluorouracil, and folinic acid in metastatic colorectal cancer. Lancet 1997, 350, 681–686. [Google Scholar] [CrossRef]
- Qian, D.C.; Kleber, T.; Brammer, B.; Xu, K.M.; Switchenko, J.M.; Janopaul-Naylor, J.R.; Zhong, J.; Yushak, M.L.; Harvey, R.D.; Paulos, C.M.; et al. Effect of immunotherapy time-of-day infusion on overall survival among patients with advanced melanoma in the USA (MEMOIR): A propensity score-matched analysis of a single-centre, longitudinal study. Lancet Oncol. 2021, 22, 1777–1786. [Google Scholar] [CrossRef]
- Weger, M.; Diotel, N.; Dorsemans, A.-C.; Dickmeis, T.; Weger, B.D. Stem cells and the circadian clock. Dev. Biol. 2017, 431, 111–123. [Google Scholar] [CrossRef]
- Dierickx, P.; Van Laake, L.W.; Geijsen, N. Circadian clocks: From stem cells to tissue homeostasis and regeneration. EMBO Rep. 2018, 19, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Benitah, S.A.; Welz, P.-S. Circadian Regulation of Adult Stem Cell Homeostasis and Aging. Cell Stem Cell 2020, 26, 817–831. [Google Scholar] [CrossRef]
- Bilen, M.A.; Hess, K.R.; Campbell, M.T.; Wang, J.; Broaddus, R.R.; Karam, J.A.; Ward, J.F.; Wood, C.G.; Choi, S.L.; Rao, P.; et al. Intratumoral heterogeneity and chemoresistance in nonseminomatous germ cell tumor of the testis. Oncotarget 2016, 7, 86280–86289. [Google Scholar] [CrossRef]
- Bilen, M.A.; Lin, S.-H.; Tang, D.; Parikh, K.; Lee, M.-H.; Yeung, S.-C.J.; Tu, S.-M. Maintenance Therapy Containing Metformin and/or Zyflamend for Advanced Prostate Cancer: A Case Series. Case Rep. Oncol. Med. 2015, 2015, 471861. [Google Scholar] [CrossRef]
| Target | Drugs (Date First Approved by FDA) | Cancer Type |
|---|---|---|
| Multiple/CD30 | Mechlorethamine * (1949), vincristine (1963), vinblastine (1965), procarbazine (1969), bleomycin (1973), doxorubicin (1974), dacarbazine (1975), brentuximab (2018) | Hodgkin lymphoma 1,2 |
| Multiple | Vinblastine (1965), bleomycin (1973), cisplatin (1978), etoposide (1983), ifosfamide (1988), paclitaxel (1992) | Testis 3–5 |
| AR | Flutamide (1989), bicalutamide (2008), abiraterone (2011), enzalutamide (2012), apalutamide (2018), darolutamide (2019) | Prostate |
| CD20 | Rituximab (1997), ofatumumab (2009), obinutuzumab (2013) | NHL/CLL |
| HER-2 | Trastuzumab (1998), pertuzumab (2012), T-DM1 (2013), neratinib (2017), tucatinib (2020), margetuximab (2020) | Breast |
| BCR-ABL | Imatinib (2001), dasatinib (2006), nilotinib (2007), bosutinib (2012), ponatinib (2012) | CML |
| EGFR | Erlotinib (2004), afatinib (2013), gefitinib (2015), osimertinib (2015), dacomitinib (2018), amivantamab (2021) | NSCLC |
| VEGFR/PDGFR/C-KIT | Sorafenib (2005), sunitinib (2006), pazopanib (2009), axitinib (2012), cabozantinib (2016), lenvatinib (2016), tivozanib (2021) | RCC |
| ALK | Crizotinib (2011), ceritinib (2014), alectinib (2015), brigatinib (2017), lorlatinib (2018) | NSCLC |
| PARP | Olaparib (2014), rucaparib (2016), niraparib (2019) | Ovary |
| CDK4/6 | Palbociclib (2015), ribociclib (2017), abemaciclib (2017) | Breast |
| PD-1/PD-L1 | Atezolumab (2016), nivolumab (2017), pembrolizumab (2017), durvalumab (2017), avelumab (2017) | Bladder |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, S.-M.; Guo, C.C.; Chow, D.S.-L.; Zacharias, N.M. Stem Cell Theory of Cancer: Implications for Drug Resistance and Chemosensitivity in Cancer Care. Cancers 2022, 14, 1548. https://doi.org/10.3390/cancers14061548
Tu S-M, Guo CC, Chow DS-L, Zacharias NM. Stem Cell Theory of Cancer: Implications for Drug Resistance and Chemosensitivity in Cancer Care. Cancers. 2022; 14(6):1548. https://doi.org/10.3390/cancers14061548
Chicago/Turabian StyleTu, Shi-Ming, Charles C. Guo, Diana S. -L. Chow, and Niki M. Zacharias. 2022. "Stem Cell Theory of Cancer: Implications for Drug Resistance and Chemosensitivity in Cancer Care" Cancers 14, no. 6: 1548. https://doi.org/10.3390/cancers14061548
APA StyleTu, S.-M., Guo, C. C., Chow, D. S.-L., & Zacharias, N. M. (2022). Stem Cell Theory of Cancer: Implications for Drug Resistance and Chemosensitivity in Cancer Care. Cancers, 14(6), 1548. https://doi.org/10.3390/cancers14061548







