Short and Long-Term Toxicity in Pediatric Cancer Treatment: Central Nervous System Damage
Abstract
:Simple Summary
Abstract
1. Introduction
2. CNS Effects of Traditional Chemotherapy
3. CNS Radiation Toxicity
- →
- the important data in the literature. We have divided the consequences of
- →
- radiotherapy treatment according to the period of onset by inserting a simplified
- →
- table and then described in detail the long-term consequences of this
- →
- type of treatment in pediatric cancer patients.
3.1. Neurotoxic Effects of Radiation Therapy
3.2. Neurocognitive Impairment of Radiation Therapy
3.3. Stroke-like Migraine Attacks after Radiation Therapy
3.4. Acute Late-Onset Encephalopathy after Radiotherapy (ALERT Syndrome)
3.5. Secondary Brain Tumors
3.6. Radiation-Induced Cerebrovascular Disease
4. CNS Toxicity of Immunotherapy
4.1. CAR T Cell
4.2. Immune Checkpoint Inhibitors (ICIs)
4.3. Blinatumomab
4.4. Brentuximab Vedotin
4.5. Rituximab
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADEM | Acute disseminated encephalomyelitis |
| CNS | Central nervous system |
| CSA | Cyclosporine |
| Ara-C | Cytosine arabinoside |
| GVHD | Graft versus host disease |
| HSCT | Hematopoietic stem cell transplantation |
| MTX | Methotrexate |
| PRES | Posterior reversible encephalopathy syndrome |
References
- Armstrong, C.; Sun, L.R. Neurological complications of pediatric cancer. Cancer Metastasis Rev. 2020, 39, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Giglio, P.; Gilbert, M.R. Neurologic Complications of Cancer and its Treatment. Curr. Oncol. Rep. 2010, 12, 50–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weaver, L.; Samkari, A. Neurological Complications of Childhood Cancer. Semin. Pediatr. Neurol. 2017, 24, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Cordelli, D.M.; Masetti, R.; Zama, D.; Toni, F.; Castelli, I.; Ricci, E.; Franzoni, E.; Pession, A. Central Nervous System Complications in Children Receiving Chemotherapy or Hematopoietic Stem Cell Transplantation. Front. Pediatr. 2017, 5, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.R.; Cooper, S. Neurological Complications of the Treatment of Pediatric Neoplastic Disorders. Pediatr. Neurol. 2018, 85, 33–42. [Google Scholar] [CrossRef]
- Linares, S.R.; Atienza, J.B.; Rudilla, M.C.; Rull, P.R.; Rodríguez, A.C.; Alonso, J.M. Severe ifosfamide-induced neurotoxicity: A case report. Pharm. Weekbl. Sci. Ed. 2010, 32, 109–111. [Google Scholar] [CrossRef]
- Taupin, D.; Racela, R.; Friedman, D. Ifosfamide Chemotherapy and Nonconvulsive Status Epilepticus: Case Report and Review of the Literature. Clin. EEG Neurosci. 2014, 45, 222–225. [Google Scholar] [CrossRef]
- Di Francia, R.; Crisci, S.; De Monaco, A.; Cafiero, C.; Re, A.; Iaccarino, G.; De Filippi, R.; Frigeri, F.; Corazzelli, G.; Micera, A.; et al. Response and Toxicity to Cytarabine Therapy in Leukemia and Lymphoma: From Dose Puzzle to Pharmacogenomic Biomarkers. Cancers 2021, 13, 966. [Google Scholar] [CrossRef]
- Watanabe, K.; Arakawa, Y.; Oguma, E.; Uehara, T.; Yanagi, M.; Oyama, C.; Ikeda, Y.; Sasaki, K.; Isobe, K.; Mori, M.; et al. Characteristics of methotrexate-induced stroke-like neurotoxicity. Int. J. Hematol. 2018, 108, 630–636. [Google Scholar] [CrossRef]
- Hartrampf, S.; Dudakov, J.A.; Johnson, L.K.; Smith, O.M.; Tsai, J.; Singer, N.V.; West, M.L.; Hanash, A.M.; Albert, M.H.; Liu, B.; et al. The central nervous system is a target of acute graft versus host disease in mice. Blood 2013, 121, 1906–1910. [Google Scholar] [CrossRef] [Green Version]
- Server, A.; Bargalló, N.; Fløisand, Y.; Sponheim, J.; Graus, F.; Hald, J.K. Imaging spectrum of central nervous system complications of hematopoietic stem cell and solid organ transplantation. Neuroradiology 2017, 59, 105–126. [Google Scholar] [CrossRef] [PubMed]
- Aspesberro, F.; Milewski, L.; Brogan, T. Acute central nervous system complications in pediatric hematopoietic stem cell patients. J. Pediatr. Intensiv. Care 2015, 3, 169–181. [Google Scholar] [CrossRef]
- Noè, A.; Cappelli, B.; Biffi, A.; Chiesa, R.; Frugnoli, I.; Biral, E.; Finizio, V.; Baldoli, C.; Vezzulli, P.; Minicucci, F.; et al. High incidence of severe cyclosporine neurotoxicity in children affected by haemoglobinopaties undergoing myeloablative haematopoietic stem cell transplantation: Early diagnosis and prompt intervention ameliorates neurological outcome. Ital. J. Pediatr. 2010, 36, 14–16. [Google Scholar] [CrossRef] [Green Version]
- Straathof, K.; Anoop, P.; Allwood, Z.; Silva, J.; Nikolajeva, O.; Chiesa, R.; Veys, P.; Amrolia, P.J.; Rao, K. Long-term outcome following cyclosporine-related neurotoxicity in paediatric allogeneic haematopoietic stem cell transplantation. Bone Marrow Transplant. 2017, 52, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Smart, D. Radiation Toxicity in the Central Nervous System: Mechanisms and Strategies for Injury Reduction. Semin. Radiat. Oncol. 2017, 27, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Tseng, B.P.; Giedzinski, E.; Izadi, A.; Suarez, T.; Lan, M.L.; Tran, K.K.; Acharya, M.M.; Nelson, G.A.; Raber, J.; Parihar, V.K.; et al. Functional Consequences of Radiation-Induced Oxidative Stress in Cultured Neural Stem Cells and the Brain Exposed to Charged Particle Irradiation. Antioxid. Redox Signal. 2014, 20, 1410–1422. [Google Scholar] [CrossRef] [PubMed]
- Kalapurakal, J.A. Radiation therapy in the management of pediatric craniopharyngiomas—A review. Child Nerv. Syst. 2005, 21, 808–816. [Google Scholar] [CrossRef]
- Lee, W.H.; Sonntag, W.E.; Mitschelen, M.; Yan, H.; Lee, Y.W. Irradiation induces regionally specific alterations in pro-inflammatory environments in rat brain. Int. J. Radiat. Biol. 2010, 86, 132–144. [Google Scholar] [CrossRef] [Green Version]
- Jensterle, M.; Jazbinsek, S.; Bosnjak, R.; Popovic, M.; Zaletel, L.Z.; Vesnaver, T.V.; Kotnik, B.F.; Kotnik, P. Advances in the management of craniopharyngioma in children and adults. Radiol. Oncol. 2019, 53, 388–396. [Google Scholar] [CrossRef] [Green Version]
- Turnquist, C.; Harris, B.T.; Harris, C.C. Radiation-induced brain injury: Current concepts and therapeutic strategies targeting neuroinflammation. Neuro-Oncol. Adv. 2020, 2, vdaa057. [Google Scholar] [CrossRef]
- Wang, Q.Q.; Yin, G.; Huang, J.R.; Xi, S.J.; Qian, F.; Lee, R.X.; Tang, F.R. Ionizing Radiation-Induced Brain Cell Aging and the Potential Underlying Molecular Mechanisms. Cells 2021, 17, 3570. [Google Scholar] [CrossRef] [PubMed]
- Betlazar, C.; Middleton, R.J.; Banati, R.B.; Liu, G.J. The impact of high and low dose ionising radiation on the central nerv-ous system. Redox Biol. 2016, 9, 144–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, J.; Niinimäki, R.; Lähteenmäki, P.; Myrberg, I.H.; Arola, M.; Riikonen, P.; Lönnqvist, T.; Palomäki, M.; Ranta, S.; Harila-Saari, A.; et al. The spectrum of acute central nervous system symptoms during the treatment of childhood acute lymphoblastic leukaemia. Pediatr. Blood Cancer 2020, 67, e27999. [Google Scholar] [CrossRef]
- Greene-Schloesser, D.; Robbins, M.E.; Peiffer, A.M.; Shaw, E.G.; Wheeler, K.T.; Chan, M.D. Radiation-induced brain injury: A review. Front. Oncol. 2012, 2, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahmathulla, G.; Marko, N.F.; Weil, R.J. Cerebral radiation necrosis: A review of the pathobiology, diagnosis and management considerations. J. Clin. Neurosci. 2013, 20, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Toussaint, L.; Indelicato, D.J.; Stokkevåg, C.H.; Lassen-Ramshad, Y.; Pedro, C.; Mikkelsen, R.; Di Pinto, M.; Li, Z.; Flampouri, S.; Vestergaard, A.; et al. Radiation doses to brain substructures associated with cognition in radiotherapy of pediatric brain tumors. Acta Oncol. 2019, 58, 1457–1462. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, M.; Masera, G.; Brouwers, P.; Valsecchi, M.G.; Veldhuizen, A.V.; Kingma, A.; Huisman, J.; Kamphuis, R.; Mor, W.; Dongen-Melman, J.; et al. Association of 1800 cGy cranial irradiation with intellectual function in children with acute lymphoblastic leukaemia. Lancet 1994, 344, 224–227. [Google Scholar] [CrossRef] [Green Version]
- Meadows, A.; Massari, D.; Fergusson, J.; Gordon, J.; Littman, P.; Moss, K. Declines in IQ scores and cognitive dysfunctions in children with acute lymphocytic leukaemia treated with cranial irradia-tion. Lancet 1981, 318, 1015–1018. [Google Scholar] [CrossRef]
- Castellino, S.M.; Ullrich, N.J.; Whelen, M.J.; Lange, B.J. Developing Interventions for Cancer-Related Cognitive Dysfunction in Childhood Cancer Survivors. JNCI J. Natl. Cancer Inst. 2014, 106, dju186. [Google Scholar] [CrossRef]
- Mabbott, D.J.; Spiegler, B.J.; Greenberg, M.L.; Rutka, J.T.; Hyder, D.J.; Bouffet, E. Serial Evaluation of Academic and Behavioral Outcome After Treatment with Cranial Radiation in Childhood. J. Clin. Oncol. 2005, 23, 2256–2263. [Google Scholar] [CrossRef]
- Brown, W.R.; Thore, C.R.; Moody, D.M.; Robbins, M.E.; Wheeler, K.T. Vascular Damage after Fractionated Whole-Brain Irradiation in Rats. Radiat. Res. 2005, 164, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Kamiryo, T.; Lopes, M.B.S.; Kassell, N.F.; Steiner, L.; Lee, K.S. Radiosurgery-induced Microvascular Alterations Precede Necrosis of the Brain Neuropil. Neurosurgery 2001, 49, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Peiffer, A.M.; Leyrer, C.M.; Greene-Schloesser, D.M.; Shing, E.; Kearns, W.T.; Hinson, W.H.; Tatter, S.B.; Ip, E.H.; Rapp, S.R.; Robbins, M.E.; et al. Neuroanatomical target theory as a predictive model for radiation-induced cognitive decline. Neurology 2013, 80, 747–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baliga, S.; Yock, T.I. Proton beam therapy in pediatric oncology. Curr. Opin. Pediatr. 2019, 31, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Kerklaan, J.P.; Lycklama á Nijeholt, G.J.; Wiggenraad, R.G.J.; Berghuis, B.; Postma, T.J.; Taphoorn, M.J.B. SMART syndrome: A late reversible complication after radiation therapy for brain tumours. J. Neurol. 2011, 258, 1098–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rigamonti, A.; Lauria, G.; Mantero, V.; Filizzolo, M.; Salmaggi, A. SMART (stroke-like migraine attack after radiation therapy) syndrome: A case report with review of the literature. Neurol. Sci. 2016, 37, 157–161. [Google Scholar] [CrossRef]
- Armstrong, A.E.; Gillan, E.; Di Mario, F.J. SMART Syndrome (Stroke-Like Migraine Attacks After Radiation Therapy) in Adult and Pediatric Patients. J. Child Neurol. 2014, 29, 336–341. [Google Scholar] [CrossRef]
- Maloney, P.R.; Rabinstein, A.A.; Daniels, D.J.; Link, M.J. Surgically Induced SMART Syndrome: Case Report and Review of the Literature. World Neurosurg. 2014, 82, 240.e7–240.e12. [Google Scholar] [CrossRef]
- Farid, K.; Meissner, W.G.; Samier-Foubert, A.; Barret, O.; Menegon, P.; Rouanet, F.; Fernandez, P.; Orgogozo, J.M.; Allard, M.; Tison, F.; et al. Normal Cerebrovascular Reactivity in Stroke-Like Migraine Attacks After Radiation Therapy Syndrome. Clin. Nucl. Med. 2010, 35, 583–585. [Google Scholar] [CrossRef]
- Black, D.F.; Morris, J.M.; Lindell, E.P.; Krecke, K.N.; Worrell, G.A.; Bartleson, J.D.; Lachance, D.H. Stroke-Like Migraine Attacks after Radiation Therapy (SMART) Syndrome Is Not Always Completely Reversible: A Case Series. Am. J. Neuroradiol. 2013, 34, 2298–2303. [Google Scholar] [CrossRef] [Green Version]
- Alnahhas, I.; Rayi, A.; Palmer, J.D.; Raval, R.; Folefac, E.; Ong, S.; Giglio, P.; Puduvalli, V. The role of VEGF receptor inhibitors in preventing cerebral radiation necrosis: A retrospective cohort study. Neuro-Oncol. Pract. 2021, 8, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, A.L.; Berzero, G.; Vitali, P.; Galimberti, C.A.; Ducray, F.; Ceroni, M.; Bastianello, S.; Colombo, A.A.; Simoncelli, A.; Brunelli, M.C.; et al. Acute late-onset encephalopathy after radiotherapy: An unusual life-threatening complication. Neurology 2013, 81, 1014–1017. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Mori, K.; Kagami, S. Neuroimaging of stroke-like episodes in MELAS. Brain Dev. 2011, 33, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Brada, M.; Ashley, S.; Ford, D.; Traish, D.; Burchell, L.; Rajan, B. Cerebrovascular mortality in patients with pituitary adenoma: Cerebrovascular Mortality in Patients with Pituitary Adenoma. Clin. Endocrinol. 2002, 57, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Remes, T.M.; Suo-Palosaari, M.H.; Heikkilä, V.-P.; Sutela, A.K.; Koskenkorva, P.K.T.; Toiviainen-Salo, S.-M.; Porra, L.; Arikoski, P.M.; Lähteenmäki, P.M.; Pokka, T.M.-L.; et al. Radiation-Induced Meningiomas After Childhood Brain Tumor: A Magnetic Resonance Imaging Screening Study. J. Adolesc. Young Adult Oncol. 2019, 8, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Chojnacka, M.; Pędziwiatr, K.; Skowrońska-Gardas, A.; Perek-Polnik, M.; Perek, D.; Olasek, P. Second brain tumors following central nervous system radiotherapy in childhood. Br. J. Radiol. 2014, 87, 20140211. [Google Scholar] [CrossRef] [Green Version]
- Cleeland, C.S.; Allen, J.D.; Roberts, S.A.; Brell, J.M.; Giralt, S.A.; Khakoo, A.Y.; Kirch, R.A.; Kwitkowski, V.E.; Liao, Z.; Skillings, J. Reducing the toxicity of cancer therapy: Recognizing needs, taking action. Nat. Rev. Clin. Oncol. 2012, 9, 471–478. [Google Scholar] [CrossRef]
- Roongpiboonsopit, D.; Kuijf, H.J.; Charidimou, A.; Xiong, L.; Vashkevich, A.; Martinez-Ramirez, S.; Shih, H.A.; Gill, C.M.; Viswanathan, A.; Dietrich, J. Evolution of cerebral microbleeds after cranial irradiation in medulloblastoma patients. Neurology 2017, 88, 789–796. [Google Scholar] [CrossRef] [Green Version]
- Murphy, E.S.; Xie, H.; Merchant, T.E.; Yu, J.S.; Chao, S.T.; Suh, J.H. Review of cranial radiotherapy-induced vasculopathy. J. Neuro-Oncol. 2015, 122, 421–429. [Google Scholar] [CrossRef]
- Bavle, A.; Srinivasan, A.; Choudhry, F.; Anderson, M.; Confer, M.; Simpson, H.; Gavula, T.; Thompson, J.S.; Clifton, S.; Gross, N.L.; et al. Systematic review of the incidence and risk factors for cerebral vasculopathy and stroke after cranial proton and photon radiation for childhood brain tumors. Neuro-Oncol. Pract. 2021, 8, 31–39. [Google Scholar] [CrossRef]
- Locatelli, F.; Schrappe, M.; Bernardo, M.E.; Rutella, S. How I treat relapsed childhood acute lymphoblastic leukemia. Blood 2012, 120, 2807–2816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Stackelberg, A.; Locatelli, F.; Zugmaier, G.; Handgretinger, R.; Trippett, T.M.; Rizzari, C.; Bader, P.; O’Brien, M.M.; Brethon, B.; Bhojwani, D.; et al. Phase I/Phase II Study of Blinatumomab in Pediatric Patients with Relapsed/Refractory Acute Lymphoblastic Leukemia. J. Clin. Oncol. 2016, 34, 4381–4389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, J.S.; Yang, J.C.; Atkins, M.B.; Disis, M.L. Toxicities of Immunotherapy for the Practitioner. J. Clin. Oncol. 2015, 33, 2092–2099. [Google Scholar] [CrossRef] [PubMed]
- Karschnia, P.; Jordan, J.T.; Forst, D.A.; Arrillaga-Romany, I.C.; Batchelor, T.T.; Baehring, J.M.; Clement, N.F.; Castro, L.N.G.; Herlopian, A.; Maus, M.V.; et al. Clinical presentation, management, and biomarkers of neurotoxicity after adoptive immunotherapy with CAR T cells. Blood 2019, 133, 2212–2221. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Kochenderfer, J.N.; Stetler-Stevenson, M.; Cui, Y.K.; Delbrook, C.; Feldman, S.A.; Fry, T.J.; Orentas, R.; Sabatino, M.; Shah, N.N.; et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: A phase 1 dose-escalation trial. Lancet 2015, 385, 517–528. [Google Scholar] [CrossRef]
- Grupp, S.A.; Kalos, M.; Barrett, D.; Aplenc, R.; Porter, D.L.; Rheingold, S.R.; Teachey, D.T.; Chew, A.; Hauck, B.; Wright, J.F.; et al. Chimeric Antigen Receptor–Modified T Cells for Acute Lymphoid Leukemia. N. Engl. J. Med. 2013, 368, 1509–1518. [Google Scholar] [CrossRef] [Green Version]
- Maude, S.L.; Frey, N.; Shaw, P.A.; Aplenc, R.; Barrett, D.M.; Bunin, N.J.; Chew, A.; Gonzalez, V.E.; Zheng, Z.; Lacey, S.F.; et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014, 371, 1507–1517. [Google Scholar] [CrossRef] [Green Version]
- Park, J.H.; Rivière, I.; Gonen, M.; Wang, X.; Sénéchal, B.; Curran, K.J.; Sauter, C.; Wang, Y.; Santomasso, B.; Mead, E.; et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 449–459. [Google Scholar] [CrossRef]
- Neelapu, S.S.; Tummala, S.; Kebriaei, P.; Wierda, W.; Gutierrez, C.; Locke, F.L.; Komanduri, K.V.; Lin, Y.; Jain, N.; Daver, N.; et al. Chimeric antigen receptor T-cell therapy—assessment and management of toxicities. Nat. Rev. Clin. Oncol. 2018, 15, 47–62. [Google Scholar] [CrossRef]
- Norelli, M.; Camisa, B.; Barbiera, G.; Falcone, L.; Purevdorj, A.; Genua, M.; Sanvito, F.; Ponzoni, M.; Doglioni, C.; Cristofori, P.; et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat. Med. 2018, 24, 739–748. [Google Scholar] [CrossRef]
- Santomasso, B.D.; Park, J.H.; Salloum, D.; Riviere, I.; Flynn, J.; Mead, E.; Halton, E.; Wang, X.; Senechal, B.; Purdon, T.; et al. Clinical and Biological Correlates of Neurotoxicity Associated with CAR T-cell Therapy in Patients with B-cell Acute Lymphoblastic Leukemia. Cancer Discov. 2018, 8, 958–971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.W.; Santomasso, B.D.; Locke, F.L.; Ghobadi, A.; Turtle, C.J.; Brudno, J.N.; Maus, M.V.; Park, J.H.; Mead, E.; Pavletic, S.; et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol. Blood Marrow Transplant. 2019, 25, 625–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubin, D.B.; Danish, H.H.; Ali, A.B.; Li, K.; LaRose, S.; Monk, A.D.; Cote, D.J.; Spendley, L.; Kim, A.H.; Robertson, M.S.; et al. Neurological toxicities associated with chimeric antigen receptor T-cell therapy. Brain 2019, 142, 1334–1348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neelapu, S.S.; Locke, F.L.; Bartlett, N.L.; Lekakis, L.J.; Miklos, D.B.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y.; et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017, 377, 2531–2544. [Google Scholar] [CrossRef]
- Taraseviciute, A.; Tkachev, V.; Ponce, R.; Turtle, C.J.; Snyder, J.M.; Liggitt, H.D.; Myerson, D.; Gonzalez-Cuyar, L.; Baldessari, A.; English, C.; et al. Chimeric Antigen Receptor T Cell–Mediated Neurotoxicity in Nonhuman Primates. Cancer Discov. 2018, 8, 750–763. [Google Scholar] [CrossRef] [Green Version]
- Locke, F.L.; Neelapu, S.S.; Bartlett, N.L.; Lekakis, L.J.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y.; Timmerman, J.M.; et al. Preliminary Results of Prophylactic Tocilizumab after Axicabtageneciloleucel (Axi-Cel; KTE-C19) Treatment for Patients with Refractory, Aggressive Non-Hodgkin Lymphoma (NHL). Blood 2017, 130, 1547. [Google Scholar] [CrossRef]
- Maude, S.L.; Barrett, D.; Teachey, D.T.; Grupp, S.A. Managing Cytokine Release Syndrome Associated with Novel T Cell-Engaging Therapies. Cancer J. 2014, 20, 119–122. [Google Scholar] [CrossRef]
- Wei, S.C.; Duffy, C.R.; Allison, J.P. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018, 8, 1069–1086. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, L.B.; Salama, A.K.S. A review of cancer immunotherapy toxicity. CA Cancer J. Clin. 2020, 70, 86–104. [Google Scholar] [CrossRef] [Green Version]
- Johnson, D.B.; Manouchehri, A.; Haugh, A.M.; Quach, H.T.; Balko, J.M.; Lebrun-Vignes, B.; Mammen, A.; Moslehi, J.J.; Salem, J.-E. Neurologic toxicity associated with immune checkpoint inhibitors: A pharmacovigilance study. J. Immunother. Cancer 2019, 7, 134. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Nylander, A.; Ramanan, S.; Goods, B.A.; Ponath, G.; Zabad, R.; Chiang, V.L.; Vortmeyer, A.O.; Hafler, D.A.; Pitt, D. CNS demyelination and enhanced myelin-reactive responses after ipilimumab treatment. Neurology 2016, 86, 1553–1556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tambaro, F.P.; Khazal, S.; Nunez, C.; Ragoonanan, D.; Tewari, P.; Petropoulos, D.; Kebriaei, P.; Wierda, W.G.; Mahadeo, K.M. Complete remission in refractory acute lymphoblastic leukemia using blinatumomab after failure of response to CD-19 chimeric antigen receptor T-cell therapy. Clin. Case Rep. 2020, 8, 1678–1681. [Google Scholar] [CrossRef] [PubMed]
- Karnell, J.L.; DiMasi, N.; Karnell, F.G.; Fleming, R.; Kuta, E.; Wilson, M.; Wu, H.; Gao, C.; Herbst, R.; Ettinger, R. CD19 and CD32b Differentially Regulate Human B Cell Responsiveness. J. Immunol. 2014, 192, 1480–1490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, M.; Wu, B.; Brandl, C.; Johnson, J.; Wolf, A.; Chow, A.; Doshi, S. Blinatumomab, a Bispecific T-cell Engager (BiTE®) for CD-19 Targeted Cancer Immunotherapy: Clinical Pharmacology and Its Implications. Clin. Pharmacokinet. 2016, 55, 1271–1288. [Google Scholar] [CrossRef]
- Goebeler, M.-E.; Knop, S.; Viardot, A.; Kufer, P.; Topp, M.S.; Einsele, H.; Noppeney, R.; Hess, G.; Kallert, S.; Mackensen, A.; et al. Bispecific T-Cell Engager (BiTE) Antibody Construct Blinatumomab for the Treatment of Patients With Relapsed/Refractory Non-Hodgkin Lymphoma: Final Results From a Phase I Study. J. Clin. Oncol. 2016, 34, 1104–1111. [Google Scholar] [CrossRef]
- Mahadeo, K.M.; Khazal, S.J.; Abdel-Azim, H.; Fitzgerald, J.C.; Taraseviciute, A.; Bollard, C.M.; Tewari, P.; Duncan, C.; Traube, C.; McCall, D.; et al. Management guidelines for paediatric patients receiving chimeric antigen receptor T cell therapy. Nat. Rev. Clin. Oncol. 2019, 16, 45–63. [Google Scholar] [CrossRef] [Green Version]
- Benjamin, J.E.; Stein, A.S. The role of blinatumomab in patients with relapsed/refractory acute lymphoblastic leukemia. Ther. Adv. Hematol. 2016, 7, 142–156. [Google Scholar] [CrossRef] [Green Version]
- Teachey, D.T.; Rheingold, S.R.; Maude, S.L.; Zugmaier, G.; Barrett, D.M.; Seif, A.; Nichols, K.E.; Suppa, E.K.; Kalos, M.; Berg, R.A.; et al. Cytokine release syndrome after blinatumomab treatment related to abnormal macrophage activation and ameliorated with cytokine-directed therapy. Blood 2013, 121, 5154–5157. [Google Scholar] [CrossRef]
- Davila, M.L.; Riviere, I.; Wang, X.; Bartido, S.; Park, J.; Curran, K.; Chung, S.S.; Stefanski, J.; Borquez-Ojeda, O.; Olszewska, M.; et al. Efficacy and Toxicity Management of 19-28z CAR T Cell Therapy in B Cell Acute Lymphoblastic Leukemia. Sci. Transl. Med. 2014, 6, 224ra25. [Google Scholar] [CrossRef] [Green Version]
- Topp, M.S.; Gökbuget, N.; Stein, A.S.; Zugmaier, G.; O’Brien, S.; Bargou, R.C.; Dombret, H.; Fielding, A.K.; Heffner, L.; Larson, R.A.; et al. Safety and Activity of Blinatumomab for Adult Patients with Relapsed or Refractory B-Precursor Acute Lympho-blastic Leukaemia: A Multicentre, Single-Arm, Phase 2 Study. Lancet Oncol. 2015, 16, 57–66. [Google Scholar] [CrossRef]
- Stein, A.S.; Schiller, G.; Benjamin, R.; Jia, C.; Zhang, A.; Zhu, M.; Zimmerman, Z.; Topp, M.S. Neurologic adverse events in patients with relapsed/refractory acute lymphoblastic leukemia treated with blinatumomab: Management and mitigating factors. Ann. Hematol. 2019, 98, 159–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, L.J. Brentuximab Vedotin: A Review in CD30-Positive Hodgkin Lymphoma. Drugs 2017, 77, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Younes, A.; Bartlett, N.L.; Leonard, J.P.; Kennedy, D.A.; Lynch, C.M.; Sievers, E.L.; Forero-Torres, A. Brentuximab Vedotin (SGN-35) for Relapsed CD30-Positive Lymphomas. N. Engl. J. Med. 2010, 363, 1812–1821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suri, A.; Mould, D.R.; Song, G.; Collins, G.; Endres, C.J.; Gomez-Navarro, J.; Venkatakrishnan, K. Population Pharmacokinetic Modeling and Exposure–Response Assessment for the Antibody-Drug Conjugate Brentuximab Vedotin in Hodgkin’s Lymphoma in the Phase III ECHELON-1 Study. Clin. Pharmacol. Ther. 2019, 106, 1268–1279. [Google Scholar] [CrossRef]
- Carson, K.R.; Do, S.D.N.; Kim, E.J.; Wagner-Johnston, N.D.; Von Geldern, G.; Moskowitz, C.H.; Moskowitz, A.J.; Rook, A.H.; Jalan, P.; Loren, A.W.; et al. Progressive multifocal leukoencephalopathy associated with brentuximab vedotin therapy: A report of 5 cases from the Southern Network on Adverse Reactions (SONAR) project. Cancer 2014, 120, 2464–2471. [Google Scholar] [CrossRef]
- Tilly, H.; da Silva, M.G.; Vitolo, U.; Jack, A.; Meignan, M.; Lopez-Guillermo, A.; Walewski, J.; André, M.; Johnson, P.W.; Pfreundschuh, M.; et al. Diffuse large B-cell lymphoma (DLBCL): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26, v116–v125. [Google Scholar] [CrossRef]
- Dreyling, M.; Ghielmini, M.; Rule, S.; Salles, G.; Vitolo, U.; Ladetto, M. Newly diagnosed and relapsed follicular lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013, 27, v83–v90. [Google Scholar] [CrossRef]
- Auweiler, P.W.P.; Müller, D.; Stock, S.; Gerber, A. Cost Effectiveness of Rituximab for Non-Hodgkinʼs Lymphoma: A Systematic Review. PharmacoEconomics 2012, 30, 537–549. [Google Scholar] [CrossRef]
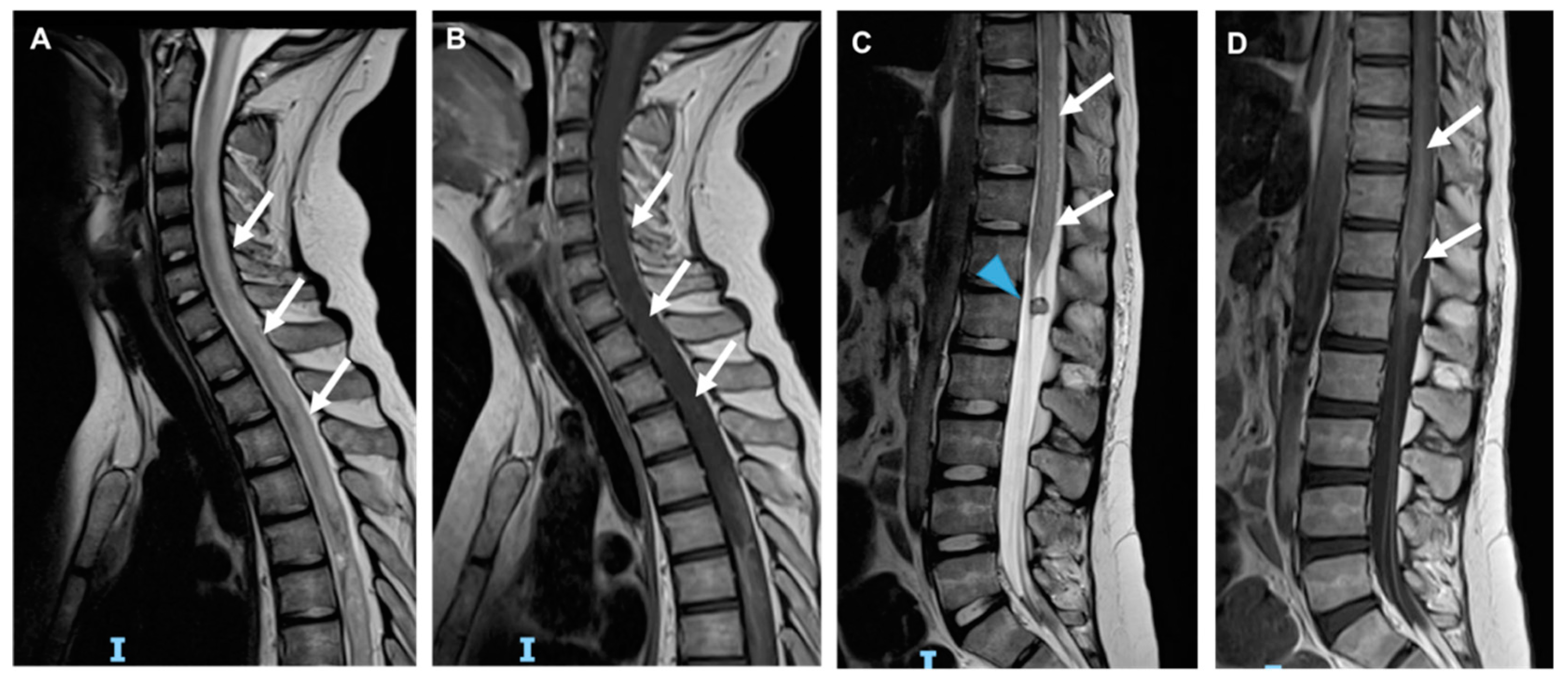
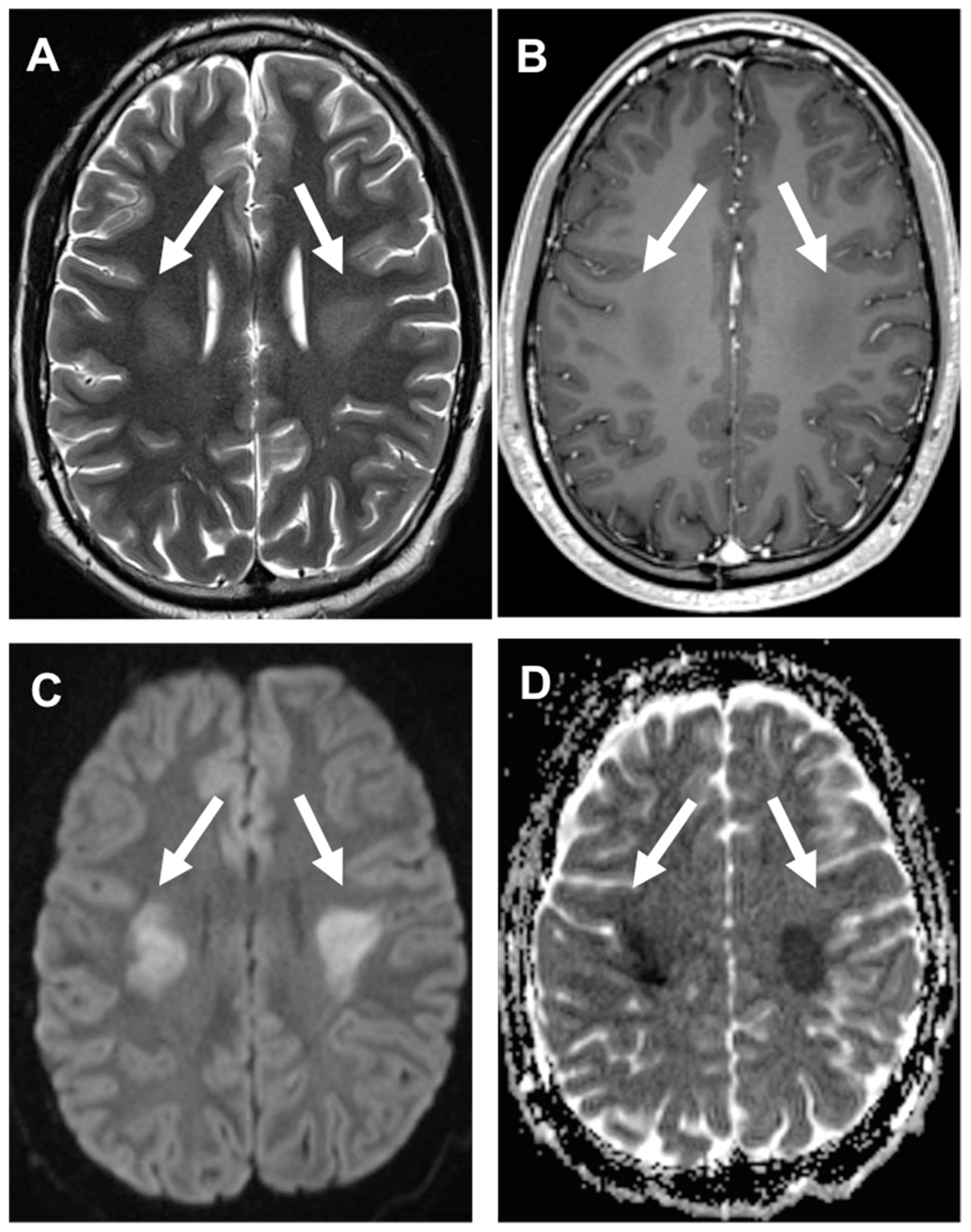
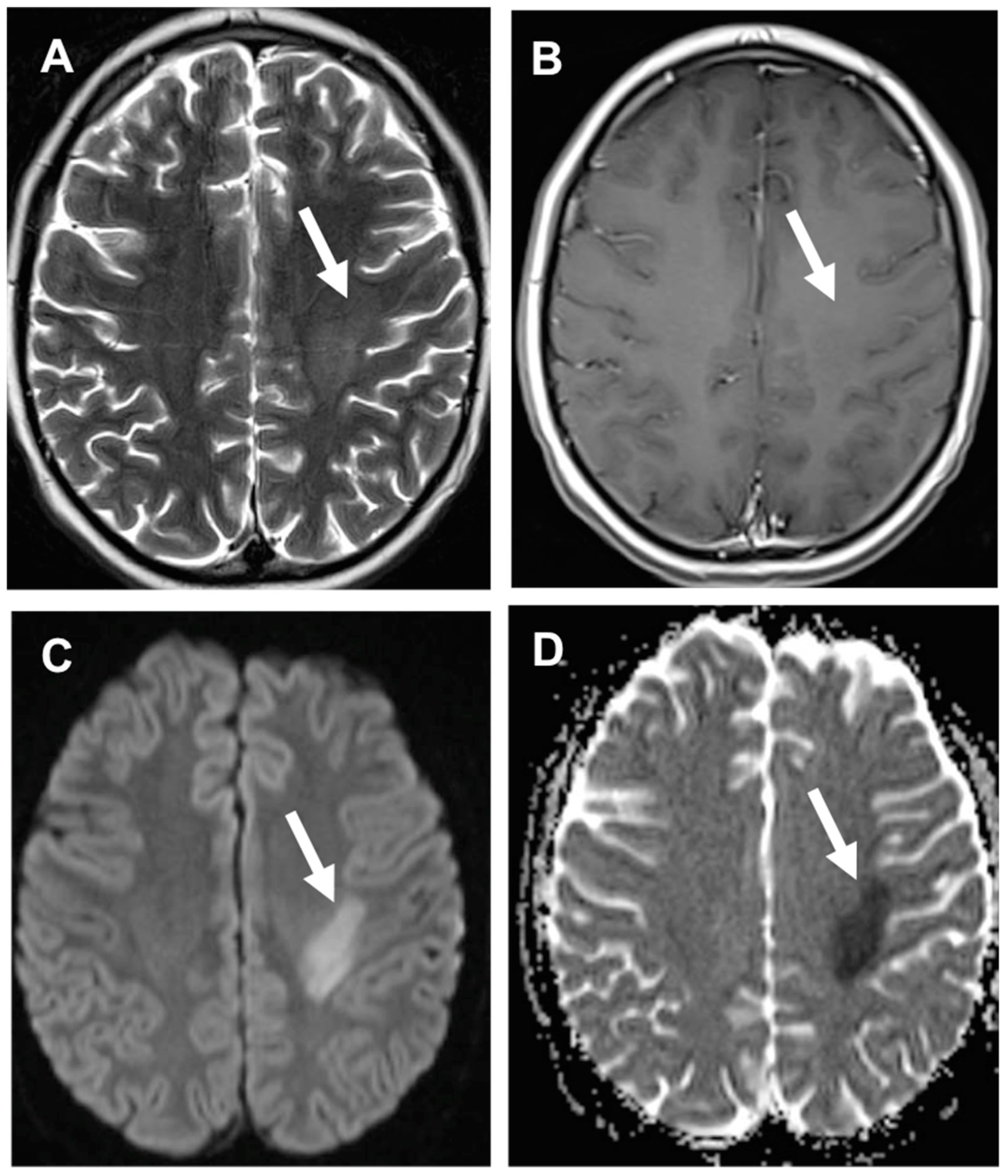
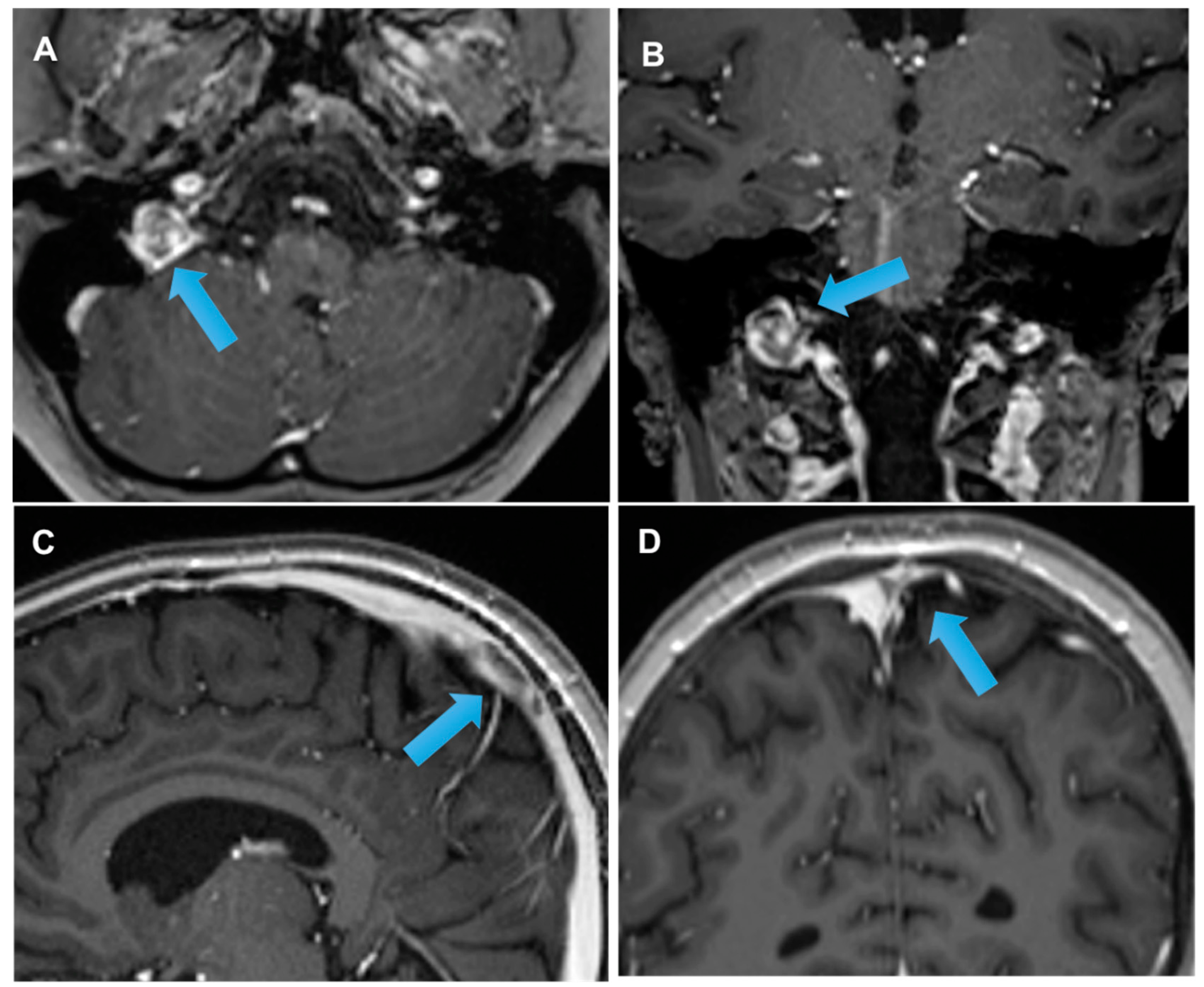
| DRUG | NEUROLOGIC SYMPTOMS |
|---|---|
| ALKYLATING AGENTS | |
| Cyclophosphamide |
|
| Ifosfamide |
|
| Busulfan |
|
| Platinum compounds (cisplatin, carboplatin, and oxaliplatin) |
|
| ANTIMETABOLITES | |
| Cytarabine |
|
| Fludarabine |
|
| Methotrexate |
|
| NATURAL PRODUCTS | |
| Asparaginase |
|
| IMMUNOSUPPRESSANTS | |
| Cyclosporine A (CSA) |
|
| Tacrolimus |
|
| OTHERS | |
| Dimethyl sulfoxide (DMSO) |
|
| ACUTE (Few Days after Radiation) | EARLY DELAYED (Weeks/Months after Radiation) | LATE DELAYED (Several Months/Years after Radiation) |
|---|---|---|
| Neurologic changes, cerebral edema, seizures, altered level of consciousness, persistent headache, hemiplegic symptoms, hallucinations, and visual disturbances [24,25] | Radiation somnolence syndrome (prolonged periods of sleep, irritability, fever, nausea, vomiting, cerebellar ataxia, anorexia, dysphagia and dysarthria, and headaches) [3] | Vascular abnormalities, demyelination [25,26] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alessi, I.; Caroleo, A.M.; de Palma, L.; Mastronuzzi, A.; Pro, S.; Colafati, G.S.; Boni, A.; Della Vecchia, N.; Velardi, M.; Evangelisti, M.; et al. Short and Long-Term Toxicity in Pediatric Cancer Treatment: Central Nervous System Damage. Cancers 2022, 14, 1540. https://doi.org/10.3390/cancers14061540
Alessi I, Caroleo AM, de Palma L, Mastronuzzi A, Pro S, Colafati GS, Boni A, Della Vecchia N, Velardi M, Evangelisti M, et al. Short and Long-Term Toxicity in Pediatric Cancer Treatment: Central Nervous System Damage. Cancers. 2022; 14(6):1540. https://doi.org/10.3390/cancers14061540
Chicago/Turabian StyleAlessi, Iside, Anna Maria Caroleo, Luca de Palma, Angela Mastronuzzi, Stefano Pro, Giovanna Stefania Colafati, Alessandra Boni, Nicoletta Della Vecchia, Margherita Velardi, Melania Evangelisti, and et al. 2022. "Short and Long-Term Toxicity in Pediatric Cancer Treatment: Central Nervous System Damage" Cancers 14, no. 6: 1540. https://doi.org/10.3390/cancers14061540
APA StyleAlessi, I., Caroleo, A. M., de Palma, L., Mastronuzzi, A., Pro, S., Colafati, G. S., Boni, A., Della Vecchia, N., Velardi, M., Evangelisti, M., Carboni, A., Carai, A., Vinti, L., Valeriani, M., Reale, A., Parisi, P., & Raucci, U. (2022). Short and Long-Term Toxicity in Pediatric Cancer Treatment: Central Nervous System Damage. Cancers, 14(6), 1540. https://doi.org/10.3390/cancers14061540









