Topical Bimiralisib Shows Meaningful Cutaneous Drug Levels in Healthy Volunteers and Mycosis Fungoides Patients but No Clinical Activity in a First-in-Human, Randomized Controlled Trial
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Randomization and Treatments
2.2. Participants
2.3. Clinical Efficacy and Pharmacodynamics
2.4. Safety and Tolerability
2.5. Treatment Compliance and Exposure
2.6. Pharmacokinetics
2.7. Statistics
3. Results
3.1. Patient Characteristics
3.2. Safety and Tolerability
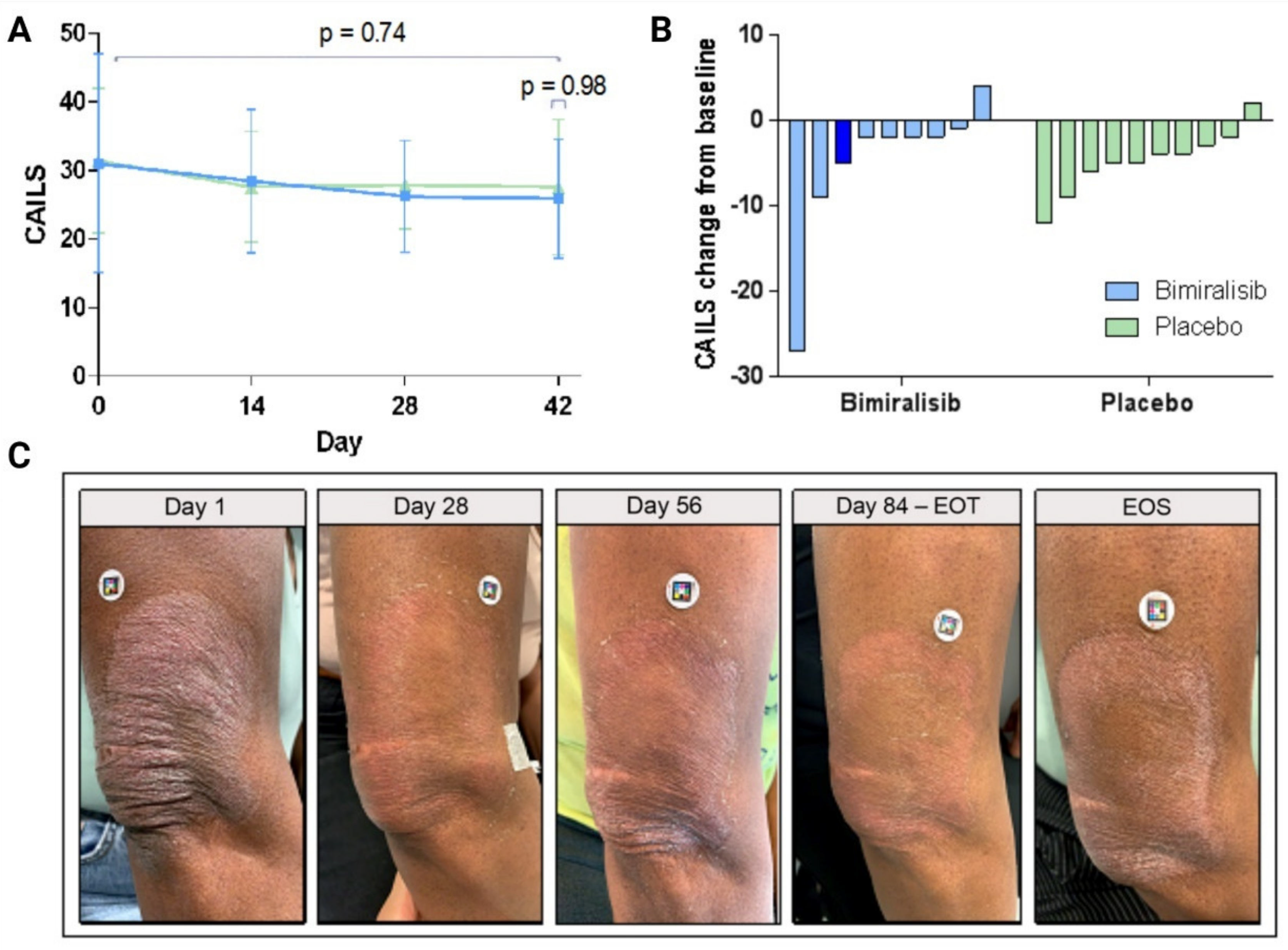
3.3. Clinical Efficacy and Pharmacodynamics
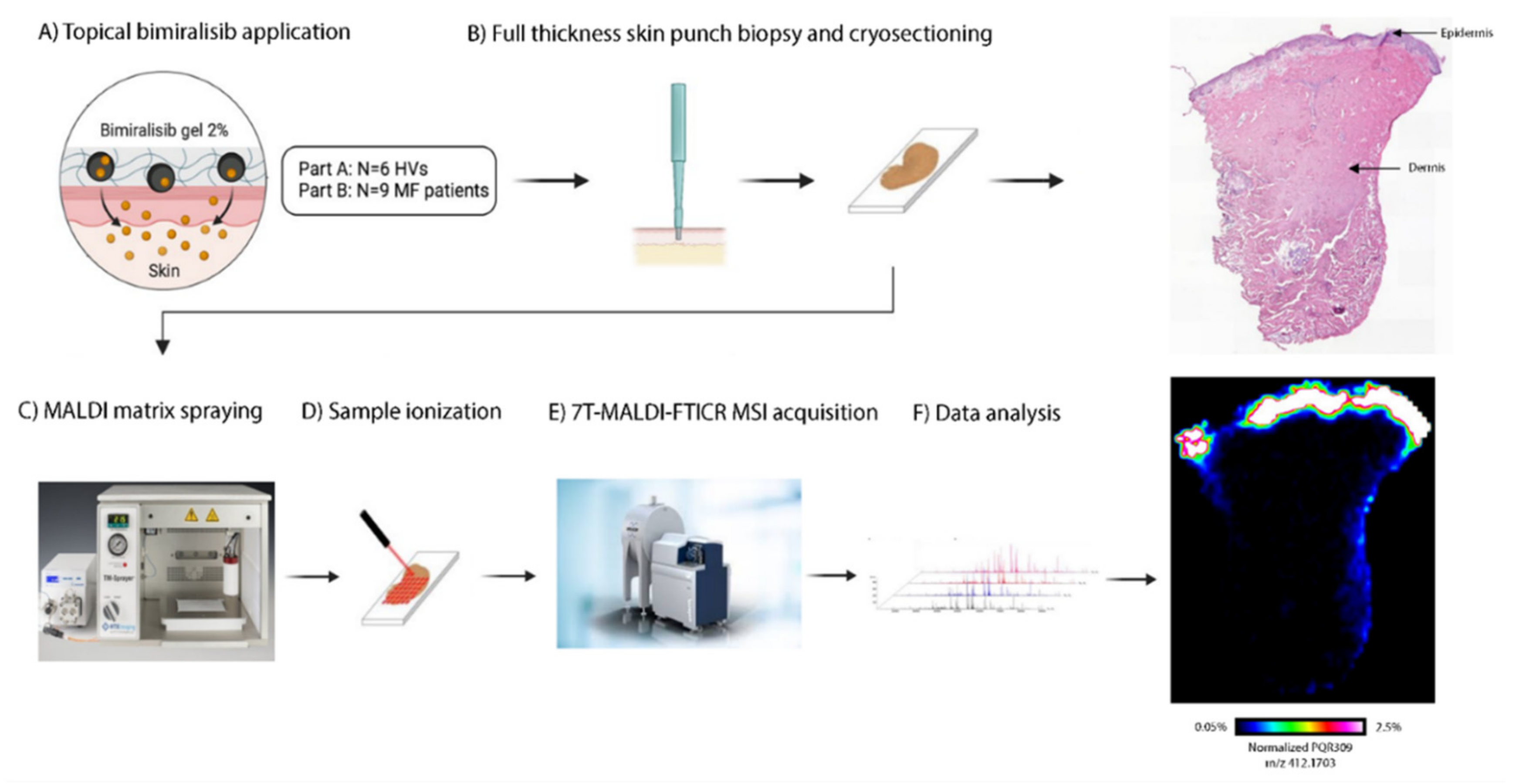
3.4. Systemic and Cutaneous Pharmacokinetics
4. Discussion
4.1. Topical Bimiralisib Is Safe and Has a Favorable Pharmacokinetic Profile
4.2. Potential Reasons for Lack of Efficacy—A Question-Based Approach
4.3. CAILS: Subjected to Subjectivity?
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maguire, A.; Puelles, J.; Raboisson, P.; Chavda, R.; Gabriel, S.; Thornton, S. Early-stage mycosis fungoides: Epidemiology and prognosis. Acta Dermato-Venereol. 2020, 100, adv00013. [Google Scholar] [CrossRef] [PubMed]
- Willemze, R.; Cerroni, L.; Kempf, W.; Berti, E.; Facchetti, F.; Swerdlow, S.H.; Jaffe, E.S. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood 2019, 133, 1703–1714. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, M.M.; Mahurin, H.M.; Tarabadkar, E.; Hippe, D.S.; Lachance, K.; Kim, E.J.; Loggers, E.T. Health-related quality of life in cutaneous T-cell lymphoma: A cross-sectional survey study. Skin Health Dis. 2021, 1, e45. [Google Scholar] [CrossRef]
- Stern, R.S.; Lunder, E.J. Risk of squamous cell carcinoma and methoxsalen (psoralen) and UV-A radiotion (PUVA). A meta-analysis. Arch Dermatol. 1998, 134, 1582–1585. [Google Scholar] [CrossRef]
- Archier, E.; Devaux, S.; Castela, E.; Gallini, A.; Aubin, A.; Le Maître, M.; Aractingi, S.; Bachelez, H.; Cribier, B.; Joly, P.; et al. Carcinogenic risks of psoralen UV-A therapy and narrowband UV-B therapy in chronic plaque psoriasis: A systematic literature review. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 22–31. [Google Scholar] [CrossRef]
- Engelman, J.A. Targeting PI3K signalling in cancer: Opportunities, challenges and limitations. Nat. Rev. Cancer 2009, 9, 550–562. [Google Scholar] [CrossRef]
- Courtney, K.D.; Corcoran, R.B.; Engelman, J.A. The PI3K pathway as drug target in human cancer. J. Clin. Oncol. 2010, 28, 1075–1083. [Google Scholar] [CrossRef]
- Yuan, T.; Cantley, L. PI3K pathway alterations in cancer: Variations on a theme. Oncogene 2008, 27, 5497. [Google Scholar] [CrossRef]
- Rodon, J.; Dienstmann, R.; Serra, V.; Tabernero, J. Development of PI3K inhibitors: Lessons learned from early clinical trials. Nat. Rev. Clin. Oncol. 2013, 10, 143–153. [Google Scholar] [CrossRef]
- Banham-Hall, E.; Clatworthy, M.R.; Okkenhaug, K. The Therapeutic Potential for PI3K Inhibitors in Autoimmune Rheumatic Diseases. Open Rheumatol. J. 2012, 6, 245–258. [Google Scholar] [CrossRef]
- Mishra, R.; Patel, H.; Alanazi, S.; Kilroy, M.K.; Garrett, J.T. PI3K Inhibitors in Cancer: Clinical Implications and Adverse Effects. Int. J. Mol. Sci. 2021, 22, 3464. [Google Scholar] [CrossRef]
- So, L.; Fruman, D.A. PI3K Signaling in B and T Lymphocytes: New Developments and Therapeutic Advances. Biochem. J. 2012, 442, 465–481. [Google Scholar] [CrossRef] [PubMed]
- Witzig, T.E.; Reeder, C.; Han, J.J.; LaPlant, B.; Stenson, M.; Tun, H.W.; Macon, W.; Ansell, S.M.; Habermann, T.M.; Inwards, D.J.; et al. The mTORC1 inhibitor everolimus has antitumor activity in vitro and produces tumor responses in patients with relapsed T-cell lymphoma. Blood 2015, 126, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Papadavid, E.; Korkolopoulou, P.; Levidou, G.; Saetta, A.A.; Papadaki, T.; Siakantaris, M.; Nikolaou, V.; Oikonomidi, A.; Chatziandreou, I.; Marinos, L.; et al. In situ assessment of PI3K and PTEN alterations in mycosis fungoides: Correlation with clinicopathological features. Exp. Dermatol. 2014, 23, 931–933. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, S.M.; Koch, R.; Porcu, P.; Oki, Y.; Moskowitz, A.; Perez, M.; Myskowski, P.; Officer, A.; Jaffe, J.D.; Morrow, S.N.; et al. Activity of the PI3K-δ,γ inhibitor duvelisib in a phase 1 trial and preclinical models of T-cell lymphoma. Blood 2018, 131, 888–898. [Google Scholar] [CrossRef]
- Shah, P.A.; Huang, C.; Li, Q.; Kazi, S.A.; Byers, L.A.; Wang, J.; Johnson, F.M.; Frederick, M.J. NOTCH1 signaling in head and neck squamous cell carcinoma. Cells 2020, 9, 2677. [Google Scholar] [CrossRef] [PubMed]
- Janku, F.; Johnson, F.M.; Opyrchal, M.; Dowlati, A.; Hierro, C.; Forester, M.; Blagden, S.P.; Wicki, A.; Schmitz, D.; Adjei, A.A. Abstract B109: Oral dual PI3K/mTOR inhibitor bimiralisib demonstrates tolerability and a signal of activity in head and neck squamous cell cancer with NOTCH1 loss-of-function mutation. Mol. Cancer Therap. 2019, 18, B109. [Google Scholar]
- ClinicalTrials.gov. Phase 2 Study with PQR309 in Relapsed or Refractory Lymphoma Patients. Available online: https://clinicaltrials.gov/ct2/show/NCT03127020 (accessed on 28 September 2021).
- ClinicalTrials.gov. PQR309 in Patients with Relapsed or Refractory Primary Central Nervous System Lymphoma. Available online: https://www.clinicaltrials.gov/ct2/show/NCT02669511 (accessed on 28 September 2021).
- Wicki, A.; Brown, N.; Xyrafas, A.; Bize, V.; Hawle, H.; Berardi, S.; Cmiljanović, N.; Cmiljanović, V.; Stumm, M.; Dimitrijević, S.; et al. First-in human, phase 1, dose-escalation pharmacokinetic and pharmacodynamic study of the oral dual PI3K and mTORC1/2 inhibitor PQR309 in patients with advanced solid tumors (SAKK 67/13). Eur. J. Cancer 2018, 96, 6–16. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Open-Label, Non Randomized Phase 2 Study with Safety Run-In. Available online: https://clinicaltrials.gov/ct2/show/NCT02249429 (accessed on 28 September 2021).
- Olsen, E.A.; Whittaker, S.; Kim, Y.H.; Duvic, M.; Prince, H.M.; Lessin, S.R.; Wood, G.S.; Willemze, R.; Demierre, M.-F.; Pimpinelli, N.; et al. Clinical end points and response criteria in mycosis fungoides and Sézary syndrome: A consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. J. Clin. Oncol. 2011, 29, 2598–2607. [Google Scholar]
- Ranjan, R.; Partl, R.; Erhart, R.; Kurup, N.; Schnidar, H. The mathematics of erythema: Development of machine learning models for artificial intelligence assisted measurement and severity scoring of radiation induced dermatitis. Comput. Biol. Med. 2021, 139, 104952. [Google Scholar]
- Partl, R.; Jonko, B.; Schnidar, S.; Schöllhammer, M.; Bauer, M.; Singh, S.; Simeckova, J.; Wiesner, K.; Neubauer, A.; Schnidar, H. 128 SHADES of RED: Objective remote assessment of radiation dermatitis by augmented digital skin imaging. Stud. Health Technol. Inform 2017, 236, 363–374. [Google Scholar]
- Rijsbergen, M.; Niemeyer-van der Kolk, T.; Rijneveld, R.; Pinckaers, J.H.F.M.; Meshcheriakov, I.; Bouwes Bavinck, J.N.; van Doorn, M.B.A.; Hogendoorn, G.; Feiss, G.; Cohen, A.F.; et al. Mobile e-diary application facilitates the monitoring of patient-reported outcomes and a high treatment adherence for clinical trials in dermatology. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Rook, A.H.; Gelfand, J.M.; Wysocka, M.; Troxel, A.B.; Benoit, B.; Surber, C.; Elenitsas, R.; Buchanan, M.A.; Leahy, D.S.; Watanabe, R.; et al. Topical resiquimod can induce disease regression and enhance T-cell effector functions in cutaneous T-cell lymphoma. Blood 2015, 126, 1452–1461. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, P.D. Investigator’s Brochure Topical Bimiralisib (PQR309/NCB5), A dual PI3K/mTOR Inhibitor. 2020.
- Mancebo, S.E.; Cordova, M.; Myskowski, P.L.; Flores, E.S.; Busam, K.; Jawed, S.I.; Skripnik, A.; Rajadhyaksha, M.; Querfeld, C. Reflectance confocal microscopy features of mycosis fungoides and Sézary syndrome: Correlation with histopathologic and T-cell receptor rearrangement studies. J. Cutan. Pathol. 2016, 43, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Suga, H.; Sugaya, M.; Miyagaki, T.; Ohmatsu, H.; Kawaguchi, M.; Takahashi, N.; Fujita, H.; Asano, Y.; Tada, Y.; Kadono, T.; et al. Skin barrier dysfunction and low antimicrobial peptide expression in cutaneous T-cell lymphoma. Clin. Cancer Res. 2014, 20, 4339–4348. [Google Scholar] [CrossRef] [PubMed]
- Kezic, S.; Jakasa, I. Filaggrin and skin barrier function. Curr. Probl. Dermatol. 2016, 49, 1–7. [Google Scholar]
- Halling-Overgaard, A.S.; Kezic, S.; Jakasa, I.; Engebretsen, K.A.; Maibach, H.; Thyssen, J.P. Skin absorption through atopic dermatitis skin: A systematic review. Br. J. Dermatol. 2017, 177, 84–106. [Google Scholar] [CrossRef]
- Beaufils, F.; Cmiljanovic, N.; Cmiljanovic, V.; Bohnacker, T.; Melone, A.; Marone, R.; Jackson, E.; Zhang, X.; Sele, A.; Borsari, C.; et al. 5-(4,6-Dimorpholino-1,3,5-triazin-2-yl)-4-(trifluoromethyl)pyridin-2-amine (PQR309), a potent, brain-penetrant, orally bioavailable, pan-class I PI3K/mTOR inhibitor as clinical candidate in oncology. J. Med. Chem. 2017, 60, 7524–7538. [Google Scholar] [CrossRef]
- Bohnacker, T.; Prota, A.E.; Beaufils, F.; Burke, J.E.; Melone, A.; Inglis, A.J.; Rageot, D.; Sele, A.M.; Cmiljanovic, V.; Cmiljanovic, N.; et al. Deconvolution of Buparlisib’s mechanism of action defines specific PI3K and tubulin inhibitors for therapeutic intervention. Nat. Commun. 2017, 8, 14683. [Google Scholar] [CrossRef]
- Meininger, G.A.; Davis, M.J. Cellular mechanisms involved in the vascular myogenic response. Am. J. Physiol. 1992, 263, H647–H659. [Google Scholar] [CrossRef]
- Baskurt, O.K.; Yalcin, O.; Meiselman, H.J. Hemorheology and vascular control mechanisms. Clin. Hemorheol. Microcirc. 2004, 30, 169–178. [Google Scholar]
- Santucci, M.; Biggeri, A.; Feller, A.C.; Massi, D.; Burg, G. Efficacy of histologic criteria for diagnosing early mycosis fungoides: An EORTC cutaneous lymphoma study group investigation. European Organization for Research and Treatment of Cancer. Am. J. Surg. Pathol. 2000, 24, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Cerroni, L. Mycosis fungoides-clinical and histopathologic features, differential diagnosis, and treatment. Semin. Cutan. Med. Surg. 2018, 37, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Charli-Joseph, Y.V.; Gatica-Torres, M.; Pincus, L.B. Approach to cutaneous lymphoid infiltrates: When to consider lymphoma? Indian J. Dermatol. 2016, 61, 351–374. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.J.; Choi, G.E.; Ryu, S.; Kwon, S.J.; Kim, S.C.; Booth, C.; Nichols, K.E.; Kim, H.S. Stepwise phosphorylation of p65 promotes NF-κB activation and NK cell responses during target cell recognition. Nat. Commun. 2016, 7, 11686. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, M.C.; Er, E.E.; Blenis, J. The Ras-ERK and PI3K-mTOR pathways: Cross-talk and compensation. Trends Biochem. Sci. 2011, 36, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Dienstmann, R.; Rodon, J.; Serra, V.; Tabernero, J. Picking the point of inhibition: A comparative review of PI3K/AKT/mTOR pathway inhibitors. Mol. Cancer Therap. 2014, 13, 1021–1031. [Google Scholar] [CrossRef]
- Yardley, D.A.; Noguchi, S.; Pritchard, K.I.; Burris, H.A.; Baselga, J.; Gnant, M.; Hortobagyi, G.N.; Campone, M.; Pistilli, B.; Piccart, M.; et al. Everolimus plus exemestane in postmenopausal patients with HR(+) breast cancer: BOLERO-2 final progression-free survival analysis. Adv. Ther. 2013, 30, 870–884. [Google Scholar] [CrossRef]
- Bresin, A.; Cristofoletti, C.; Caprini, E.; Cantonetti, M.; Monopoli, A.; Russo, G.; Narducci, M.G. Preclinical evidence for targeting PI3K/mTOR signaling with dual-inhibitors as a therapeutic strategy against cutaneous T-Cell lymphoma. J. Investig. Dermatol. 2020, 140, 1045–1053.e6. [Google Scholar] [CrossRef]
- Tarantelli, C.; Gaudio, E.; Arribas, A.J.; Kwee, I.; Hillmann, P.; Rinaldi, A.; Cascione, L.; Spriano, F.; Bernasconi, E.; Guidetti, F.; et al. PQR309 Is a Novel Dual PI3K/mTOR Inhibitor with Preclinical Antitumor Activity in Lymphomas as a Single Agent and in Combination Therapy. Clin. Cancer Res. 2018, 24, 120–129. [Google Scholar] [CrossRef]
- García-Díaz, N.; Piris, M.Á.; Ortiz-Romero, P.L.; Vaqué, J.P. Mycosis fungoides and Sézary syndrome: An integrative review of the pathophysiology, molecular drivers, and targeted therapy. Cancers 2021, 13, 1931. [Google Scholar] [CrossRef]
- McGirt, L.Y.; Jia, P.; Baerenwald, D.A.; Duszynski, R.J.; Dahlman, K.B.; Zic, J.A.; Zwerner, J.P.; Hucks, D.; Dave, U.; Zhao, Z.; et al. Whole-genome sequencing reveals oncogenic mutations in mycosis fungoides. Blood 2015, 126, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Goh, G.; Walradt, T.; Hong, B.S.; Bunick, C.G.; Chen, K.; Bjornson, R.D.; Maman, Y.; Wang, T.; Tordoff, J.; et al. Genomic landscape of cutaneous T cell lymphoma. Nat. Genet. 2015, 47, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Vaqué, J.P.; Gómez-López, G.; Monsálvez, V.; Varela, I.; Martínez, N.; Perez, C.; Domínguez, O.; Grana, O.; Rodríguez-Peralto, J.L.; Rodriguez-Pinilla, S.M.; et al. PLCG1 mutations in cutaneous T-cell lymphomas. Blood 2014, 123, 2034–2043. [Google Scholar] [CrossRef] [PubMed]
- Pérez, C.; González-Rincón, J.; Onaindia, A.; Almaráz, C.; García-Díaz, N.; Pisonero, H.; Curiel-Olmo, S.; Gómez, S.; Cereceda, L.; Madureira, R.; et al. Mutated JAK kinases and deregulated STAT activity are potential therapeutic targets in cutaneous T-cell lymphoma. Haematologica 2015, 100, e450–e453. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Almeida, A.C.; Abate, F.; Khiabanian, H.; Martinez-Escala, E.; Guitart, J.; Tensen, C.P.; Vermeer, M.H.; Rabadan, R.; Ferrando, A.; Palomero, T. The mutational landscape of cutaneous T cell lymphoma and Sézary syndrome. Nat. Genet. 2015, 47, 1465–1470. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Yang, J.; Wenzel, A.T.; Ramachandran, A.; Lee, W.J.; Daniels, J.C.; Kim, J.; Martinez-Escala, E.; Amankulor, N.; Pro, B.; et al. Genomic analysis of 220 CTCLs identifies a novel recurrent gain-of-function alteration in RLTPR (p.Q575E). Blood 2017, 130, 1430–1440. [Google Scholar] [CrossRef]
- Bastidas Torres, A.N.; Cats, D.; Mei, H.; Szuhai, K.; Willemze, R.; Vermeer, M.H.; Tensen, C.P. Genomic analysis reveals recurrent deletion of JAK-STAT signaling inhibitors HNRNPK and SOCS1 in mycosis fungoides. Genes Chromosomes Cancer 2018, 57, 653–664. [Google Scholar] [CrossRef]
- Oh, H.; Ghosh, S. NF-κB: Roles and regulation in different CD4(+) T-cell subsets. Immunol. Rev. 2013, 252, 41–51. [Google Scholar] [CrossRef]
- Sommer, K.; Guo, B.; Pomerantz, J.L.; Bandaranayake, A.D.; Moreno-García, M.E.; Ovechkina, Y.L.; Rawlings, D.J. Phosphorylation of the CARMA1 linker controls NF-kappaB activation. Immunity 2005, 23, 561–574. [Google Scholar] [CrossRef]
- Waldmann, T.A.; Chen, J. Disorders of the JAK/STAT Pathway in T Cell Lymphoma Pathogenesis: Implications for Immunotherapy. Annu. Rev. Immunol. 2017, 35, 533–550. [Google Scholar] [CrossRef]
- Gaydosik, A.M.; Tabib, T.; Geskin, L.J.; Bayan, C.A.; Conway, J.F.; Lafyatis, R.; Fuschiotti, P. Single-cell lymphocyte heterogeneity in advanced cutaneous T-cell lymphoma skin tumors. Clin. Cancer Res. 2019, 25, 4443–4454. [Google Scholar] [CrossRef] [PubMed]
- Iyer, A.; Hennessey, D.; O’Keefe, S.; Patterson, J.; Wang, W.; Wong, G.K.S.; Gniadecki, R. Branched evolution and genomic intratumor heterogeneity in the pathogenesis of cutaneous T-cell lymphoma. Blood Adv. 2020, 4, 2489–2500. [Google Scholar] [CrossRef] [PubMed]
- Bożek, A.; Reich, A. Assessment of intra- and inter-rater reliability of three methods for measuring atopic dermatitis severity: EASI, Objective SCORAD, and IGA. Dermatology 2017, 233, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Puzenat, E.; Bronsard, V.; Prey, S.; Gourraud, P.A.; Aractingi, S.; Bagot, M.; Cribier, B.; Joly, P.; Jullien, D.; Le Maitre, M.; et al. What are the best outcome measures for assessing plaque psoriasis severity? A systematic review of the literature. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Bożek, A.; Reich, A. The reliability of three psoriasis assessment tools: Psoriasis area and severity index, body surface area and physician global assessment. Adv. Clin. Exp. Med. 2017, 26, 851–856. [Google Scholar] [CrossRef]
- Kalay Yildizhan, I.; Sanli, H.; Akay, B.N.; Sürgün, E.; Heper, A. CD8 + cytotoxic mycosis fungoides: A retrospective analysis of clinical features and follow-up results of 29 patients. Int. J. Dermatol. 2020, 59, 127–133. [Google Scholar] [CrossRef]



| Part A |  | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Day | 0–4 | 5–6 | 7 | 8–13 | 14 | 15–20 | 21 | 35 | |
| Outpatient visit | X | X | X | X | X | X | X | X | |
| Vital signs | X | X | X | X | X | X | X | X | |
| Safety assessments | X | X | X | X | X | ||||
| Systemic PK assessment | X | X | X | X | |||||
| Biopsy | X | ||||||||
| Part B |  | ||||||||
| Day | 0 | 14 | 28 | 42 | 56 | 70 | 84 | 98 | 112 |
| Outpatient visit | X | X | X | X | X | X | X | X | X |
| Safety assessments | X | X | X | X | X | X | X | X | |
| Efficacy assessment | X | X | X | X | X | X | X | X | X |
| Systemic PK assessment | X | X | X | ||||||
| Biopsy | X | X | |||||||
| HV (n = 6) | MF Bimiralisib 2% (n = 9) | MF Vehicle Gel (n = 10) | |
|---|---|---|---|
| Age, years (SD, range) | 31.7 (13.3, 21–50) | 52.0 (12.2, 31–70) | 55.2 (16.4, 21–78) |
| Sex, n (%) | |||
| Female | 0 | 4 (44.4) | 3 (30.0) |
| Male | 6 (100) | 5 (55.6) | 7 (70.0) |
| Weight, kg (SD, range) | 80.8 (13.3, 61.2–94.7) | 94.5 (9.3, 79.4–109.2) | 93.24 (16.2, 71.5–124.8) |
| BMI, kg/m2 (SD, range) | 23.7 (2.5, 20.7–27.4) | 29.9 (3.0, 26.7–35.7) | 29.6 (4.6, 25.0–39.2) |
| Fitzpatrick skin type (%) | |||
| I | 0 | 0 | 1 (10.0) |
| II | 2 (33.3) | 3 (33.3) | 5 (50.0) |
| III | 3 (50) | 4 (44.4) | 3 (30.0) |
| IV | 1 (16.7) | 0 | 1 (10.0) |
| V | 0 | 1 (11.1) | 0 |
| VI | 0 | 1 (11.1) | 0 |
| Lesion, n (%) | |||
| 1 | 3 (33.3) | 4 (40.0) | |
| 2 | 4 (44.4) | 2 (20.0) | |
| 3 | 2 (22.2) | 4 (40.0) | |
| mSWAT (SD) | |||
| Patch sum (%BSA*1) | 11.4 (7.3) | 16.2 (9.7) | |
| Plaque (%BSA*2) | 4.0 (9.2) | 3.0 (4.9) | |
| Total mSWAT score | 15.4 (10.6) | 19.2 (12.8) | |
| Treatment history, n (%) | |||
| Topical corticosteroids | 9 (100.0) | 9 (90.0) | |
| UVB | 0 | 1 (10.0) | |
| PUVA | 5 (55.6) | 3 (30.0) | |
| Interferon-α | 1 (11.1) | 0 | |
| Radiotherapy | 0 | 1 (10.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wind, S.S.; Jansen, M.A.A.; Rijsbergen, M.; van Esdonk, M.J.; Ziagkos, D.; Cheng, W.C.; Niemeyer-van der Kolk, T.; Korsten, J.; Gruszka, A.; Schmitz-Rohmer, D.; et al. Topical Bimiralisib Shows Meaningful Cutaneous Drug Levels in Healthy Volunteers and Mycosis Fungoides Patients but No Clinical Activity in a First-in-Human, Randomized Controlled Trial. Cancers 2022, 14, 1510. https://doi.org/10.3390/cancers14061510
Wind SS, Jansen MAA, Rijsbergen M, van Esdonk MJ, Ziagkos D, Cheng WC, Niemeyer-van der Kolk T, Korsten J, Gruszka A, Schmitz-Rohmer D, et al. Topical Bimiralisib Shows Meaningful Cutaneous Drug Levels in Healthy Volunteers and Mycosis Fungoides Patients but No Clinical Activity in a First-in-Human, Randomized Controlled Trial. Cancers. 2022; 14(6):1510. https://doi.org/10.3390/cancers14061510
Chicago/Turabian StyleWind, Selinde S., Manon A. A. Jansen, Melanie Rijsbergen, Michiel J. van Esdonk, Dimitrios Ziagkos, Wing C. Cheng, Tessa Niemeyer-van der Kolk, John Korsten, Agnieszka Gruszka, Debora Schmitz-Rohmer, and et al. 2022. "Topical Bimiralisib Shows Meaningful Cutaneous Drug Levels in Healthy Volunteers and Mycosis Fungoides Patients but No Clinical Activity in a First-in-Human, Randomized Controlled Trial" Cancers 14, no. 6: 1510. https://doi.org/10.3390/cancers14061510
APA StyleWind, S. S., Jansen, M. A. A., Rijsbergen, M., van Esdonk, M. J., Ziagkos, D., Cheng, W. C., Niemeyer-van der Kolk, T., Korsten, J., Gruszka, A., Schmitz-Rohmer, D., Bonnel, D., Legouffe, R., Barré, F., Bekkenk, M. W., de Haas, E. R. M., Quint, K. D., Schnidar, H., Rolli, M., Streefkerk, H. J., ... Rissmann, R. (2022). Topical Bimiralisib Shows Meaningful Cutaneous Drug Levels in Healthy Volunteers and Mycosis Fungoides Patients but No Clinical Activity in a First-in-Human, Randomized Controlled Trial. Cancers, 14(6), 1510. https://doi.org/10.3390/cancers14061510






