Biological Characterization and Clinical Relevance of Circulating Tumor Cells: Opening the Pandora’s Box of Multiple Myeloma
Abstract
:Simple Summary
Abstract
1. Introduction
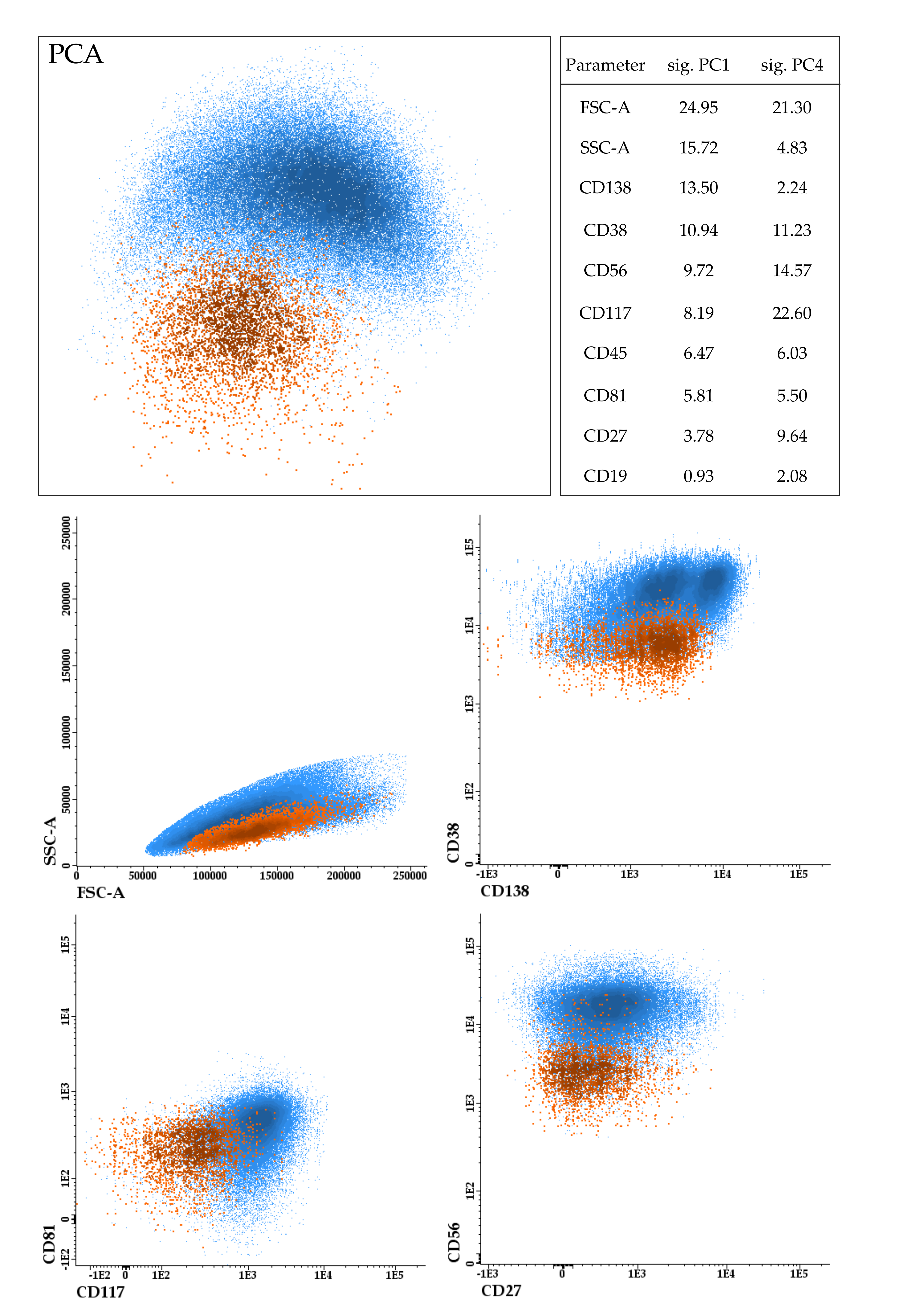
2. Immunophenotypic Characterization of CTCs
3. Genetic Characterization of CTCs
4. Transcriptomic Characterization of CTCs
5. Clinical Utility of CTCs
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Van de Donk, N.W.C.J.; Pawlyn, C.; Yong, K.L. Multiple myeloma. Lancet 2021, 397, 410–427. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Moreau, P.; Terpos, E.; Mateos, M.V.; Zweegman, S.; Cook, G.; Delforge, M.; Hájek, R.; Schjesvold, F.; Cavo, M.; et al. Multiple myeloma: EHA-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Rasche, L.; Chavan, S.S.; Stephens, O.W.; Patel, P.H.; Tytarenko, R.; Ashby, C.; Bauer, M.; Stein, C.; Deshpande, S.; Wardell, C.; et al. Spatial genomic heterogeneity in multiple myeloma revealed by multi-region sequencing. Nat. Commun. 2017, 8, 268. [Google Scholar] [CrossRef] [PubMed]
- Schürch, C.M.; Rasche, L.; Frauenfeld, L.; Weinhold, N.; Fend, F. A review on tumor heterogeneity and evolution in multiple myeloma: Pathological, radiological, molecular genetics, and clinical integration. Virchows Arch. 2019, 476, 337–351. [Google Scholar] [CrossRef]
- Bolli, N.; Avet-Loiseau, H.; Wedge, D.C.; Van Loo, P.; Alexandrov, L.B.; Martincorena, I.; Dawson, K.J.; Iorio, F.; Nik-Zainal, S.; Bignell, G.R.; et al. Heterogeneity of genomic evolution and mutational profiles in multiple myeloma. Nat. Commun. 2014, 5, 2997. [Google Scholar] [CrossRef] [Green Version]
- Ghobrial, I.M. Myeloma as a model for the process of metastasis: Implications for therapy. Blood 2012, 120, 20–30. [Google Scholar] [CrossRef] [Green Version]
- Garcés, J.-J.; Simicek, M.; Vicari, M.; Brozova, L.; Burgos, L.; Bezdekova, R.; Alignani, D.; Calasanz, M.-J.; Growkova, K.; Goicoechea, I.; et al. Transcriptional profiling of circulating tumor cells in multiple myeloma: A new model to understand disease dissemination. Leukemia 2020, 34, 589–603. [Google Scholar] [CrossRef]
- Sanoja-Flores, L.; Flores-Montero, J.; Garcés, J.J.; Paiva, B.; Puig, N.; García-Mateo, A.; García-Sánchez, O.; Corral-Mateos, A.; Burgos, L.; Blanco, E.; et al. Next generation flow for minimally-invasive blood characterization of MGUS and multiple myeloma at diagnosis based on circulating tumor plasma cells (CTPC). Blood Cancer J. 2018, 8, 117. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Rajkumar, S.V.; Kyle, R.A.; Lacy, M.Q.; Dispenzieri, A.; Fonseca, R.; Lust, J.A.; Gertz, M.A.; Greipp, P.R.; Witzig, T.E. Prognostic Value of Circulating Plasma Cells in Monoclonal Gammopathy of Undetermined Significance. J. Clin. Oncol. 2005, 23, 5668–5674. [Google Scholar] [CrossRef]
- Bianchi, G.; Kyle, R.A.; Larson, D.R.; Witzig, T.E.; Kumar, S.; Dispenzieri, A.; Morice, W.G.; Rajkumar, S.V. High levels of peripheral blood circulating plasma cells as a specific risk factor for progression of smoldering multiple myeloma. Leukemia 2013, 27, 680–685. [Google Scholar] [CrossRef]
- Vagnoni, D.; Travaglini, F.; Pezzoni, V.; Ruggieri, M.; Bigazzi, C.; Dalsass, A.; Mestichelli, F.; Troiani, E.; Falcioni, S.; Mazzotta, S.; et al. Circulating plasma cells in newly diagnosed symptomatic multiple myeloma as a possible prognostic marker for patients with standard-risk cytogenetics. Br. J. Haematol. 2015, 170, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Nowakowski, G.S.; Witzig, T.E.; Dingli, D.; Tracz, M.J.; Gertz, M.A.; Lacy, M.Q.; Lust, J.A.; Dispenzieri, A.; Greipp, P.R.; Kyle, R.A.; et al. Circulating plasma cells detected by flow cytometry as a predictor of survival in 302 patients with newly diagnosed multiple myeloma. Blood 2005, 106, 2276–2279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonsalves, W.I.; Rajkumar, S.V.; Dispenzieri, A.; Dingli, D.; Timm, M.M.; Morice, W.G.; Lacy, M.Q.; Buadi, F.K.; Go, R.S.; Leung, N.; et al. Quantification of circulating clonal plasma cells via multiparametric flow cytometry identifies patients with smoldering multiple myeloma at high risk of progression. Leukemia 2016, 31, 130–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonsalves, W.T.; Rajkumar, S.V.; Gupta, V.; Morice, W.G.; Timm, M.M.; Singh, P.P.; Dispenzieri, A.; Buadi, F.K.; Lacy, M.Q.; Kapoor, P.; et al. Quantification of clonal circulating plasma cells in newly diagnosed multiple myeloma: Implications for redefining high-risk myeloma. Leukemia 2014, 28, 2060–2065. [Google Scholar] [CrossRef] [Green Version]
- Kis, O.; Kaedbey, R.; Chow, S.; Danesh, A.; Dowar, M.; Li, T.; Li, Z.; Liu, J.; Mansour, M.; Masih-Khan, E.; et al. Circulating tumour DNA sequence analysis as an alternative to multiple myeloma bone marrow aspirates. Nat. Commun. 2017, 8, 15086. [Google Scholar] [CrossRef]
- Mithraprabhu, S.; Khong, T.; Ramachandran, M.; Chow, A.; Klarica, D.; Mai, L.; Walsh, S.; Broemeling, D.; Marziali, A.; Wiggin, M.; et al. Circulating tumour DNA analysis demonstrates spatial mutational heterogeneity that coincides with disease relapse in myeloma. Leukemia 2017, 31, 1695–1705. [Google Scholar] [CrossRef]
- Gerber, B.; Manzoni, M.; Spina, V.; Bruscaggin, A.; Lionetti, M.; Fabris, S.; Barbieri, M.; Ciceri, G.; Pompa, A.; Forestieri, G.; et al. Circulating tumor DNA as a liquid biopsy in plasma cell dyscrasias. Haematologica 2018, 103, e245–e248. [Google Scholar] [CrossRef] [Green Version]
- Manier, S.; Park, J.; Capelletti, M.; Bustoros, M.; Freeman, S.; Ha, G.; Rhoades, J.; Liu, C.J.; Huynh, D.; Reed, S.; et al. Whole-exome sequencing of cell-free DNA and circulating tumor cells in multiple myeloma. Nat. Commun. 2018, 9, 1691. [Google Scholar] [CrossRef]
- Guo, G.; Raje, N.S.; Seifer, C.; Kloeber, J.; Isenhart, R.; Ha, G.; Yee, A.J.; O’Donnell, E.K.; Tai, Y.-T.; Richardson, P.G.; et al. Genomic discovery and clonal tracking in multiple myeloma by cell-free DNA sequencing. Leukemia 2018, 32, 1838–1841. [Google Scholar] [CrossRef]
- Pantel, K.; Alix-Panabières, C. Liquid biopsy and minimal residual disease—Latest advances and implications for cure. Nat. Rev. Clin. Oncol. 2019, 16, 409–424. [Google Scholar] [CrossRef]
- Heitzer, E.; Haque, I.S.; Roberts, C.E.S.; Speicher, M.R. Current and future perspectives of liquid biopsies in genomics-driven oncology. Nat. Rev. Genet. 2019, 20, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Rushton, A.; Nteliopoulos, G.; Shaw, J.; Coombes, R. A Review of Circulating Tumour Cell Enrichment Technologies. Cancers 2021, 13, 970. [Google Scholar] [CrossRef] [PubMed]
- Sanoja-Flores, L.; Flores-Montero, J.; Pérez-Andrés, M.; Puig, N.; Orfao, A. Detection of Circulating Tumor Plasma Cells in Monoclonal Gammopathies: Methods, Pathogenic Role, and Clinical Implications. Cancers 2020, 12, 1499. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Medina, R.; López-Tarruella, S.; del Monte-Millán, M.; Massarrah, T.; Martín, M. Technical Challenges for CTC Implementation in Breast Cancer. Cancers 2021, 13, 4619. [Google Scholar] [CrossRef] [PubMed]
- Lianidou, E.S.; Markou, A.; Strati, A. Molecular characterization of circulating tumor cells in breast cancer: Challenges and promises for individualized cancer treatment. Cancer Metastasis Rev. 2012, 31, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.-M.; Krebs, M.; Lancashire, L.; Sloane, R.S.; Backen, A.; Swain, R.; Priest, L.J.; Greystoke, A.; Zhou, C.; Morris, K.; et al. Clinical Significance and Molecular Characteristics of Circulating Tumor Cells and Circulating Tumor Microemboli in Patients With Small-Cell Lung Cancer. J. Clin. Oncol. 2012, 30, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Shen, L.; Luo, M.; Zhang, K.; Li, J.; Yang, Q.; Zhu, F.; Zhou, D.; Zheng, S.; Chen, Y.; et al. Circulating tumor cells: Biology and clinical significance. Signal Transduct. Target. Ther. 2021, 6, 404. [Google Scholar] [CrossRef]
- Billadeau, D.; Quam, L.; Thomas, W.; Kay, N.; Greipp, P.; Kyle, R.; Oken, M.M.; Van Ness, B. Detection and quantitation of malignant cells in the peripheral blood of multiple myeloma patients. Blood 1992, 80, 1818–1824. [Google Scholar] [CrossRef]
- Ruiz-Argüelles, G.J.; A Katzmann, J.; Greipp, P.R.; Gonchoroff, N.J.; Garton, J.P.; A Kyle, R. Multiple myeloma: Circulating lymphocytes that express plasma cell antigens. Blood 1984, 64, 352–356. [Google Scholar] [CrossRef] [Green Version]
- Rawstron, A.C.; Owen, R.G.; Davies, F.E.; Johnson, R.J.; Jones, R.A.; Richards, S.J.; Evans, P.A.; Child, J.A.; Smith, G.M.; Jack, A.S.; et al. Circulating plasma cells in multiple myeloma: Characterization and correlation with disease stage. Br. J. Haematol. 1997, 97, 46–55. [Google Scholar] [CrossRef] [Green Version]
- Paiva, B.; Paino, T.; Sayagues, J.-M.; Garayoa, M.; San-Segundo, L.; Martín, M.; Mota, I.; Sanchez, M.-L.; Bárcena, P.; Aires-Mejia, I.; et al. Detailed characterization of multiple myeloma circulating tumor cells shows unique phenotypic, cytogenetic, functional, and circadian distribution profile. Blood 2013, 122, 3591–3598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kraj, M.; Kopeć-Szlęzak, J.; Pogłód, R.; Kruk, B. Flow cytometric immunophenotypic characteristics of 36 cases of plasma cell leukemia. Leuk. Res. 2011, 35, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Klimienė, I.; Radzevičius, M.; Matuzevičienė, R.; Sinkevič-Belliot, K.; Kučinskienė, Z.A.; Pečeliūnas, V. Adhesion molecule immunophenotype of bone marrow multiple myeloma plasma cells impacts the presence of malignant circulating plasma cells in peripheral blood. Int. J. Lab. Hematol. 2021, 43, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Chaidos, A.; Barnes, C.; Cowan, G.; May, P.; Melo, V.; Hatjiharissi, E.; Papaioannou, M.; Harrington, H.; Doolittle, H.; Terpos, E.; et al. Clinical drug resistance linked to interconvertible phenotypic and functional states of tumor-propagating cells in multiple myeloma. Blood 2013, 121, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Kawano, Y.; Fujiwara, S.; Wada, N.; Izaki, M.; Yuki, H.; Okuno, Y.; Iyama, K.; Yamasaki, H.; Sakai, A.; Mitsuya, H.; et al. Multiple myeloma cells expressing low levels of CD138 have an immature phenotype and reduced sensitivity to lenalidomide. Int. J. Oncol. 2012, 41, 876–884. [Google Scholar] [CrossRef] [Green Version]
- Paiva, B.; Gutiérrez, N.-C.; Chen, X.; Vídriales, M.-B.; Montalbán, M.Á.; Rosiñol, L.; Oriol, A.; Martínez-López, J.; Mateos, M.-V.; López-Corral, L.; et al. Clinical significance of CD81 expression by clonal plasma cells in high-risk smoldering and symptomatic multiple myeloma patients. Leukemia 2012, 26, 1862–1869. [Google Scholar] [CrossRef] [Green Version]
- Bataille, R.; Jégo, G.; Robillard, N.; Barille-Nion, S.; Harousseau, J.-L.; Moreau, P.; Amiot, M.; Pellat-Deceunynck, C. The phenotype of normal, reactive and malignant plasma cells. Identification of “many and multiple myelomas” and of new targets for myeloma therapy. Haematologica 2006, 91, 1234–1240. [Google Scholar]
- Deceunynck, C.; Barille-Nion, S.; Jego, G.; Puthier, D.; Robillard, N.; Pineau, D.; Rapp, M.-J.; Harousseau, J.-L.; Amiot, M.; Bataille, R. The absence of CD56 (NCAM) on malignant plasma cells is a hallmark of plasma cell leukemia and of a special subset of multiple myeloma. Leukemia 1998, 12, 1977–1982. [Google Scholar] [CrossRef] [Green Version]
- Vandyke, K.; Zeissig, M.N.; Hewett, D.R.; Martin, S.K.; Mrozik, K.M.; Cheong, C.M.; Diamond, P.; To, L.B.; Gronthos, S.; Peet, D.J.; et al. HIF-2α Promotes Dissemination of Plasma Cells in Multiple Myeloma by Regulating CXCL12/CXCR4 and CCR1. Cancer Res. 2017, 77, 5452–5463. [Google Scholar] [CrossRef] [Green Version]
- Roccaro, A.M.; Mishima, Y.; Sacco, A.; Moschetta, M.; Tai, Y.-T.; Shi, J.; Zhang, Y.; Reagan, M.R.; Huynh, D.; Kawano, Y.; et al. CXCR4 Regulates Extra-Medullary Myeloma through Epithelial-Mesenchymal-Transition-like Transcriptional Activation. Cell Rep. 2015, 12, 622–635. [Google Scholar] [CrossRef] [Green Version]
- García-Ortiz, A.; Rodríguez-García, Y.; Encinas, J.; Maroto-Martín, E.; Castellano, E.; Teixidó, J.; Martínez-López, J. The Role of Tumor Microenvironment in Multiple Myeloma Development and Progression. Cancers 2021, 13, 217. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-F.; Wu, L.; Liu, S.-P.; Jiang, M.-M.; Hu, B.; Zhou, K.-Q.; Guo, W.; Xu, Y.; Zhong, Y.; Zhou, X.-R.; et al. Dissecting spatial heterogeneity and the immune-evasion mechanism of CTCs by single-cell RNA-seq in hepatocellular carcinoma. Nat. Commun. 2021, 12, 4091. [Google Scholar] [CrossRef] [PubMed]
- Szczerba, B.M.; Castro-Giner, F.; Vetter, M.; Krol, I.; Gkountela, S.; Landin, J.; Scheidmann, M.C.; Donato, C.; Scherrer, R.; Singer, J.; et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature 2019, 566, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Anvari, S.; Osei, E.; Maftoon, N. Interactions of platelets with circulating tumor cells contribute to cancer metastasis. Sci. Rep. 2021, 11, 15477. [Google Scholar] [CrossRef] [PubMed]
- Okegawa, T.; Ninomiya, N.; Masuda, K.; Nakamura, Y.; Tambo, M.; Nutahara, K. AR-V7 in circulating tumor cells cluster as a predictive biomarker of abiraterone acetate and enzalutamide treatment in castration-resistant prostate cancer patients. Prostate 2018, 78, 576–582. [Google Scholar] [CrossRef]
- Aceto, N.; Bardia, A.; Miyamoto, D.T.; Donaldson, M.C.; Wittner, B.S.; Spencer, J.A.; Yu, M.; Pely, A.; Engstrom, A.; Zhu, H.; et al. Circulating Tumor Cell Clusters Are Oligoclonal Precursors of Breast Cancer Metastasis. Cell 2014, 158, 1110–1122. [Google Scholar] [CrossRef] [Green Version]
- Botta, C.; Maia, C.; Garcés, J.-J.; Termini, R.; Perez, C.; Manrique, I.; Burgos, L.; Zabaleta, A.; Alignani, D.; Sarvide, S.; et al. FlowCT for the analysis of large immunophenotypic datasets and biomarker discovery in cancer immunology. Blood Adv. 2022, 2, 690–703. [Google Scholar] [CrossRef]
- Bausch-Fluck, D.; Hofmann, A.; Bock, T.; Frei, A.P.; Cerciello, F.; Jacobs, A.; Moest, H.; Omasits, U.; Gundru, R.L.; Yoon, C.; et al. A Mass Spectrometric-Derived Cell Surface Protein Atlas. PLoS ONE 2015, 10, e0121314. [Google Scholar] [CrossRef] [Green Version]
- Palumbo, A.; Avet-Loiseau, H.; Oliva, S.; Lokhorst, H.M.; Goldschmidt, H.; Rosinol, L.; Richardson, P.; Caltagirone, S.; Lahuerta, J.J.; Facon, T.; et al. Revised International Staging System for Multiple Myeloma: A Report From International Myeloma Working Group. J. Clin. Oncol. 2015, 33, 2863–2869. [Google Scholar] [CrossRef]
- Lohr, J.G.; Kim, S.; Gould, J.; Knoechel, B.; Drier, Y.; Cotton, M.J.; Gray, D.; Birrer, N.; Wong, B.; Ha, G.; et al. Genetic interrogation of circulating multiple myeloma cells at single-cell resolution. Sci. Transl. Med. 2016, 8, 363ra147. [Google Scholar] [CrossRef] [Green Version]
- Mishima, Y.; Paiva, B.D.L.; Shi, J.; Park, J.; Manier, S.; Takagi, S.; Massoud, M.; Perilla-Glen, A.; Aljawai, Y.; Huynh, D.; et al. The Mutational Landscape of Circulating Tumor Cells in Multiple Myeloma. Cell Rep. 2017, 19, 218–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcés, J.-J.; Bretones, G.; Burgos, L.; Valdes-Mas, R.; Puig, N.; Cedena, M.-T.; Alignani, D.; Rodriguez, I.; Puente, D.Á.; Álvarez, M.G.; et al. Circulating tumor cells for comprehensive and multiregional non-invasive genetic characterization of multiple myeloma. Leukemia 2020, 34, 3007–3018. [Google Scholar] [CrossRef] [PubMed]
- Paíno, T.; Paiva, B.; Sayagués, J.M.; Mota, I.; Carvalheiro, T.; Corchete, L.A.; Aires-Mejía, I.; Pérez, J.J.; Sanchez, M.L.; Barcena, P.; et al. Phenotypic identification of subclones in multiple myeloma with different chemoresistant, cytogenetic and clonogenic potential. Leukemia 2015, 29, 1186–1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ledergor, G.; Weiner, A.; Zada, M.; Wang, S.-Y.; Cohen, Y.C.; Gatt, M.E.; Snir, N.; Magen, H.; Koren-Michowitz, M.; Herzog-Tzarfati, K.; et al. Single cell dissection of plasma cell heterogeneity in symptomatic and asymptomatic myeloma. Nat. Med. 2018, 24, 1867–1876. [Google Scholar] [CrossRef]
- Fokkema, C.; de Jong, M.M.E.; Tahri, S.; Kellermayer, Z.; den Hollander, C.; Vermeulen, M.; Papzian, N.; van Duin, M.; Wevers, M.J.W.; Sanders, M.A.; et al. Abstract #1566: High Levels of Circulating Tumor Cells Are Associated with Increased Bone Marrow Proliferation in Newly Diagnosed Multiple Myeloma Patients. In Proceedings of the 63rd ASH Annual Meeting & Exposition, Atlanta, GA, USA, 11–14 December 2021. [Google Scholar]
- Foulk, B.; Schaffer, M.; Gross, S.; Rao, C.; Smirnov, D.; Connelly, M.C.; Chaturvedi, S.; Reddy, M.; Brittingham, G.; Mata, M.; et al. Enumeration and characterization of circulating multiple myeloma cells in patients with plasma cell disorders. Br. J. Haematol. 2018, 180, 71–81. [Google Scholar] [CrossRef] [Green Version]
- Hui, H.; Fuller, K.A.; Chuah, H.; Liang, J.; Sidiqi, H.; Radeski, D.; Erber, W.N. Imaging flow cytometry to assess chromosomal abnormalities in chronic lymphocytic leukaemia. Methods 2018, 134–135, 32–40. [Google Scholar] [CrossRef]
- Zhan, F.; Huang, Y.; Colla, S.; Stewart, J.P.; Hanamura, I.; Gupta, S.; Epstein, J.; Yaccoby, S.; Sawyer, J.; Burington, B.; et al. The molecular classification of multiple myeloma. Blood 2006, 108, 2020–2028. [Google Scholar] [CrossRef] [Green Version]
- Garcés, J.-J.; Puig, N.; Termini, R.; Cedena, M.-T.; Moreno, C.; Pérez, J.J.; Alignani, D.; Sarvide, S.; Oriol, A.; González-García, E.; et al. Abstract #76: Circulating Tumor Cells (CTCs) in Smoldering and Active Multiple Myeloma (MM): Mechanism of Egression, Clinical Significance and Therapeutic Endpoints. In Proceedings of the 63rd ASH Annual Meeting & Exposition, Atlanta, GA, USA, 11–14 December 2021. [Google Scholar]
- Vasco-Mogorrón, M.A.; Campillo, J.A.; Periago, A.; Cabañas, V.; Berenguer, M.; García-Garay, M.C.; Gimeno, L.; Soto-Ramírez, M.F.; Martínez-Hernández, M.D.; Muro, M.; et al. Blood-based risk stratification for pre-malignant and symptomatic plasma cell neoplasms to improve patient management. Am. J. Cancer Res. 2021, 11, 2736–2753. [Google Scholar]
- Garcés, J.-J.; Cedena, M.-T.; Puig, N.; Burgos, L.; Pérez, J.J.; Cordon, L.; Flores-Montero, J.; Sanoja-Flores, L.; Oriol, A.; Blanchard, M.-J.; et al. Abstract S185: Circulating tumor cells (CTCs) are the most relevant diagnostic biomarker in transplant-eligible multiple myeloma (MM). In Proceedings of the The European Hematology Association Congress, Virtual, Den Haag, The Netherlands, 12 June 2021. [Google Scholar]
- Mateos, M.-V.; Lopez, J.M.; Rodríguez-Otero, P.; Gonzalez-Calle, V.; Gonzalez, M.S.; Oriol, A.; Gutierrez, N.C.; Rios, R.; Rosinol, L.; Alvarez, M.A.; et al. Abstract #1829: Curative Strategy (GEM-CESAR) for High-Risk Smoldering Myeloma (SMM): Carfilzomib, Lenalidomide and Dexamethasone (KRd) As Induction Followed By HDT-ASCT, Consolidation with Krd and Maintenance with Rd. In Proceedings of the 63rd ASH Annual Meeting & Exposition, Atlanta, GA, USA, 11–14 December 2021. [Google Scholar]
- Gonsalves, W.I.; Jevremovic, D.; Nandakumar, B.; Dispenzieri, A.; Buadi, F.K.; Dingli, D.; Lacy, M.Q.; Hayman, S.R.; Kapoor, P.; Leung, N.; et al. Enhancing the R-ISS classification of newly diagnosed multiple myeloma by quantifying circulating clonal plasma cells. Am. J. Hematol. 2020, 95, 310–315. [Google Scholar] [CrossRef]
- Paiva, B.; GEM (Grupo Español de MM)/PETHEMA (Programa para el Estudio de la Terapéutica en Hemopatías Malignas) Cooperative Study Group; Vídriales, M.-B.; Rosiñol, L.; Martínez-López, J.; Mateos, M.-V.; Ocio, E.M.; Montalbán, M.-Á.; Cordón, L.; Gutiérrez, N.C.; et al. A multiparameter flow cytometry immunophenotypic algorithm for the identification of newly diagnosed symptomatic myeloma with an MGUS-like signature and long-term disease control. Leukemia 2013, 27, 2056–2061. [Google Scholar] [CrossRef] [Green Version]
- De Larrea, C.F.; Kyle, R.A.; Durie, B.G.M.; Ludwig, H.; Usmani, S.; Vesole, D.H.; Hajek, R.; San-Miguel, J.F.; Sezer, O.; Sonneveld, P.; et al. Plasma cell leukemia: Consensus statement on diagnostic requirements, response criteria and treatment recommendations by the International Myeloma Working Group. Leukemia 2013, 27, 780–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Larrea, C.F.; Kyle, R.; Rosiñol, L.; Paiva, B.; Engelhardt, M.; Usmani, S.; Caers, J.; Gonsalves, W.; Schjesvold, F.; Merlini, G.; et al. Primary plasma cell leukemia: Consensus definition by the International Myeloma Working Group according to peripheral blood plasma cell percentage. Blood Cancer J. 2021, 11, 192. [Google Scholar] [CrossRef] [PubMed]
- Jelinek, T.; Bezděková, R.; Zihala, D.; Sevcikova, T.; Capkova, L.; Polackova, P.; Stork, M.; Knechtova, Z.; Venglar, O.; Jurczyszyn, A.; et al. Abstract #546: Circulating Plasma Cells Are the Most Powerful Prognostic Marker in Transplant Ineligible Multiple Myeloma with 2% as a New Cut-Off for Primary Plasma Cell Leukemia. In Proceedings of the 63rd ASH Annual Meeting & Exposition, Atlanta, GA, USA, 11–14 December 2021. [Google Scholar]
- An, G.; Qin, X.; Acharya, C.; Xu, Y.; Deng, S.; Shi, L.; Zang, M.; Sui, W.; Yi, S.; Li, Z.; et al. Multiple myeloma patients with low proportion of circulating plasma cells had similar survival with primary plasma cell leukemia patients. Ann. Hematol. 2015, 94, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.A.; Jevremovic, D.; Nandakumar, B.; Dispenzieri, A.; Buadi, F.K.; Dingli, D.; Lacy, M.Q.; Hayman, S.R.; Kapoor, P.; Leung, N.; et al. Utilizing multiparametric flow cytometry in the diagnosis of patients with primary plasma cell leukemia. Am. J. Hematol. 2020, 95, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Sanoja-Flores, L.; Flores-Montero, J.; Puig, N.; Contreras-Sanfeliciano, T.; Pontes, R.; Corral-Mateos, A.; García-Sánchez, O.; Díez-Campelo, M.; De Magalhães, R.J.P.; García-Martín, L.; et al. Blood monitoring of circulating tumor plasma cells by next generation flow in multiple myeloma after therapy. Blood 2019, 134, 2218–2222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.-M.; Lee, Y.; Jeong, D.; Yun, J.; Yoon, S.-S.; Hwang, S.M.; Lee, N.; Lee, D.S. Significance of analyzing circulating plasma cells in multiple myeloma: Differences from measuring minimal residual diseases in bone marrow. Leuk. Lymphoma 2021, 63, 487–490. [Google Scholar] [CrossRef]
- Chakraborty, R.; Muchtar, E.; Kumar, S.K.; Jevremovic, D.; Buadi, F.K.; Dingli, D.; Dispenzieri, A.; Hayman, S.R.; Hogan, W.J.; Kapoor, P.; et al. Serial measurements of circulating plasma cells before and after induction therapy have an independent prognostic impact in patients with multiple myeloma undergoing upfront autologous transplantation. Haematologica 2017, 102, 1439–1445. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Ni, X.; Guo, H.; Su, Z.; Ba, Y.; Tong, Z.; Guo, Z.; Yao, X.; Chen, X.; Yin, J.; et al. Single-cell sequencing deciphers a convergent evolution of copy number alterations from primary to circulating tumor cells. Genome Res. 2017, 27, 1312–1322. [Google Scholar] [CrossRef]
- Chang, B.Y.; Francesco, M.; De Rooij, M.F.M.; Magadala, P.; Steggerda, S.M.; Huang, M.M.; Kuil, A.; Herman, S.E.M.; Chang, S.; Pals, S.T.; et al. Egress of CD19+CD5+ cells into peripheral blood following treatment with the Bruton tyrosine kinase inhibitor ibrutinib in mantle cell lymphoma patients. Blood 2013, 122, 2412–2424. [Google Scholar] [CrossRef]
- Termini, R.; Zihala, D.; Botta, C.; Maia, C.; Garcés, J.-J.; Terpos, E.; Pérez Montaña, A.; Jelinek, T.; Bargay, J.; Ocio, E.; et al. Abstract OAB-035: Minimally invasive profiling of tumor and immune cells to stratify risk in smoldering multiple myeloma (SMM): The iMMunocell study. In Proceedings of the The European Hematology Association Congress, Vienna, Austria, 11–21 June 2020. [Google Scholar]
| # Samples | #CTCs | Enrichment Technology | Sequencing Approach | Key Ideas | Ref. |
|---|---|---|---|---|---|
| 7 | 155 | RosetteSep™ + manual selection (CD138 + CD45-) | (single-cell) Targeted panel (n = 13) | - All mutations clinically detected by bulk BM genotyping are also detected in single CTCs. - There are mutations with greater frequency in CTCs compared to BM tumor cells. - CTCs analysis allows the detection of LOH in tumor suppressor-associated genes. | [50] |
| 8 | 31,700 (median) | multiparametric FC | WES | - CTCs are detectable in 61% of MGUS and 100% of smoldering and active MM. - Around 15–20 mL of PB would suffice to sort 30,000 CTCs in a significant fraction of MM patients. - Non-recurrent but potential driver mutations and copy-number alterations are detected on CTCs. | [51] |
| 4 | - | CD138 positive selection | WES | - Tumor fraction in enriched CTCs (and ctDNA) correlates with MM progression. - Combined WES of CTCs (and ctDNA) reflects a high concordance in clonal somatic mutations (99%) and copy number alterations (81%) with BM aspirates. | [18] |
| 8 + 10 + 35 | 11,090 (median) | multiparametric FC | WES + barcoded WES + CGH arrays | - Most mutations (≥82%) are simultaneously present in medullar or extra-medullar clones and can be readily monitored through the genetic characterization of CTCs (both by WES and CGH arrays). - Other abnormalities with prognostic value (e.g., amp1q, or TP53) or potential role in progression but not routinely tested (e.g., MYC) are detectable in CTCs whenever present in BM tumor cells. | [52] |
| 2 | 21 | RosetteSep™ + manual selection (CD138 + CD45-) | scRNAseq (Smart-seq2) | - Transcriptomic profiling of CTCs reproduces gene expression of BM tumor cells and can be used to detect targetable antigens (e.g., CD38, SLAMF7, and BCMA). - CTCs are feasible for inferring single-cell expression for translocation-based classification. | [50] |
| 29 + 3 | 5200 (median) + 266 | multiparametric FC | Expression arrays + scRNAseq (Precise WTA Single Cell Assay, BD) | - There is a significant correlation in gene expression between paired CTCs and BM tumor cells (both at single-cell and bulk levels). - Subtle differences between CTCs and BM tumor cells display prognostic potential. - It is suggested that a hypoxic and pro-inflammatory BM microenvironment induces an arrest in proliferation, forcing tumor cells to circulate. | [7] |
| 15 | 2299 (median) | FACS (CD138+ CD38+) (in plate) | scRNAseq (MARS-seq) | - CTC signatures highly resemble the BM transcriptional state(s), with few changes likely resulting from the different environments. - The tumor load in the BM and the PB differs by several orders of magnitude. | [54] |
| 5 | 44,779 (median) | - | scRNAseq | - The absence of specific clusters and the transcriptional similarity suggest that CTC levels are not driven by a transcriptionally-primed migratory clone. - BM tumor cell proliferation is a significant differential factor between high and low levels of CTCs. | [55] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcés, J.-J.; San-Miguel, J.; Paiva, B. Biological Characterization and Clinical Relevance of Circulating Tumor Cells: Opening the Pandora’s Box of Multiple Myeloma. Cancers 2022, 14, 1430. https://doi.org/10.3390/cancers14061430
Garcés J-J, San-Miguel J, Paiva B. Biological Characterization and Clinical Relevance of Circulating Tumor Cells: Opening the Pandora’s Box of Multiple Myeloma. Cancers. 2022; 14(6):1430. https://doi.org/10.3390/cancers14061430
Chicago/Turabian StyleGarcés, Juan-José, Jesús San-Miguel, and Bruno Paiva. 2022. "Biological Characterization and Clinical Relevance of Circulating Tumor Cells: Opening the Pandora’s Box of Multiple Myeloma" Cancers 14, no. 6: 1430. https://doi.org/10.3390/cancers14061430
APA StyleGarcés, J.-J., San-Miguel, J., & Paiva, B. (2022). Biological Characterization and Clinical Relevance of Circulating Tumor Cells: Opening the Pandora’s Box of Multiple Myeloma. Cancers, 14(6), 1430. https://doi.org/10.3390/cancers14061430







