Application of Artificial Intelligence in Lung Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
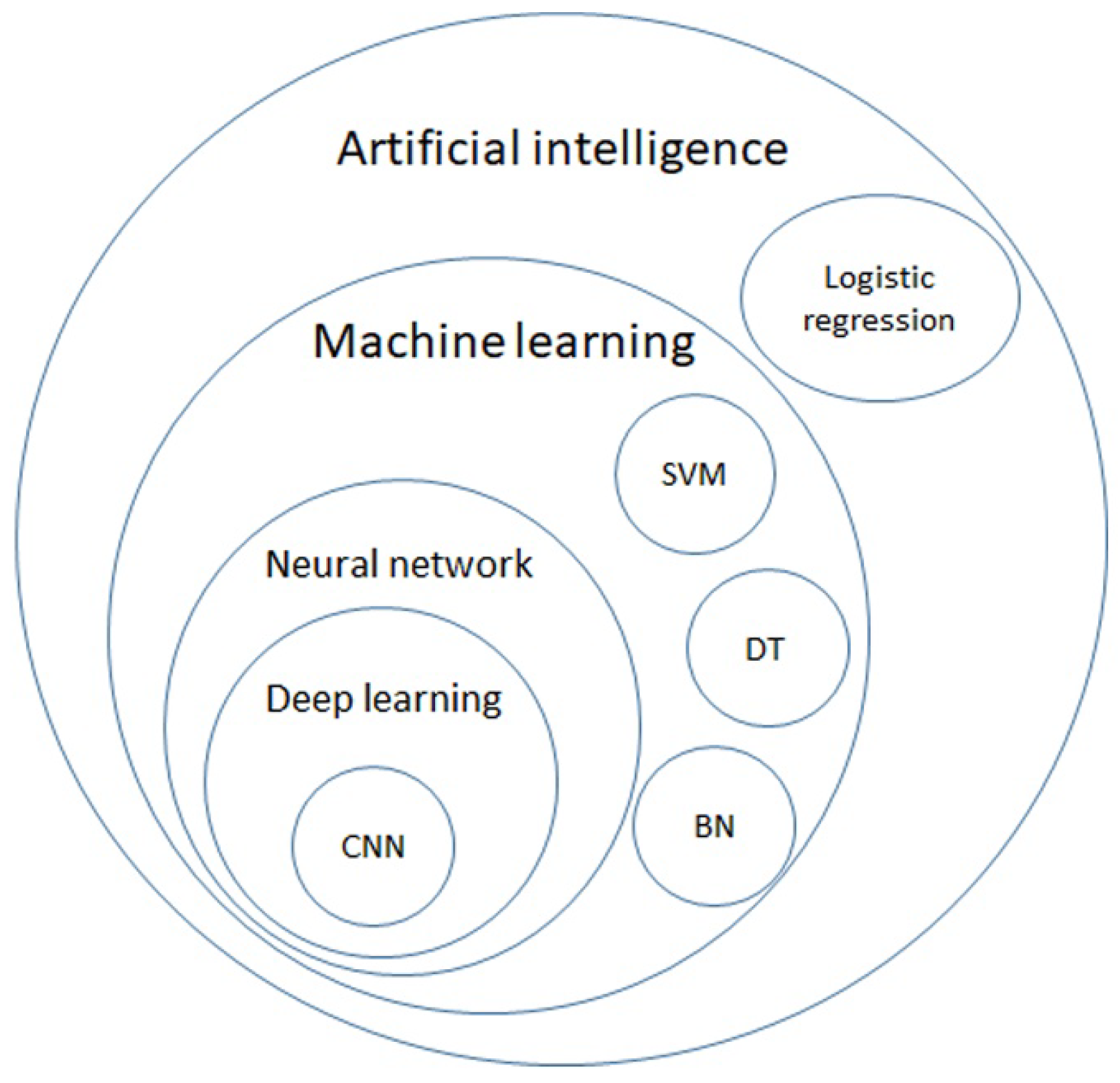
2. AI Models
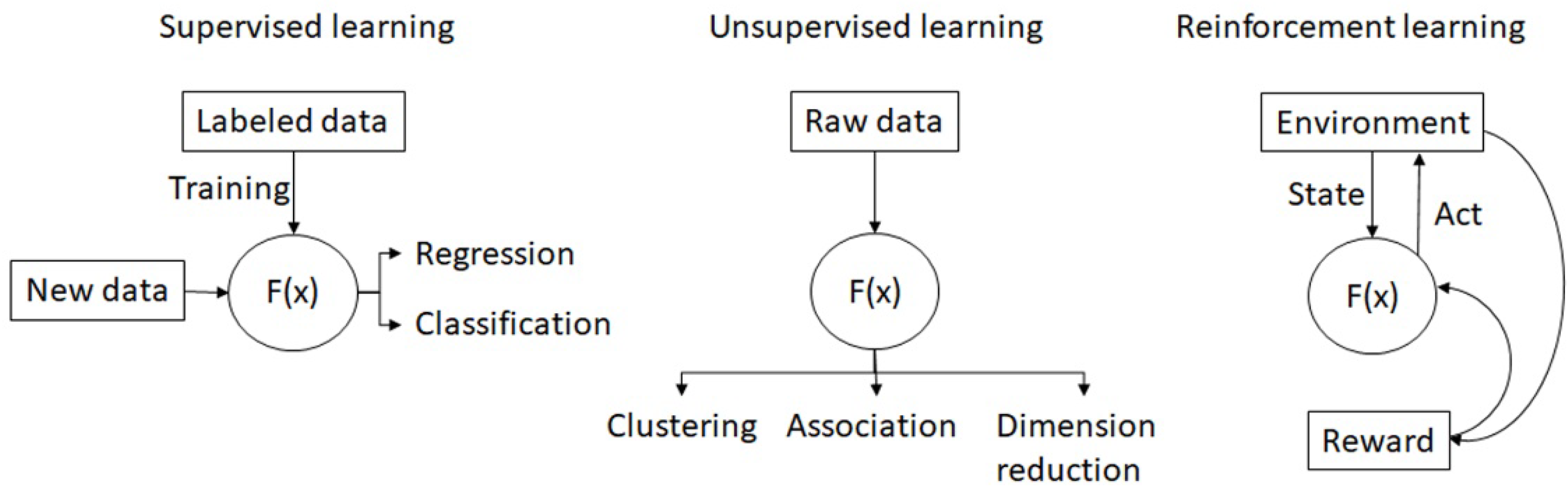
2.1. Supervised Learning
2.2. Unsupervised Learning
2.3. Semi-Supervised Learning
2.4. Reinforcement Learning
3. Screening
3.1. DICOM Format
3.2. CXR
3.3. Chest CT
3.4. Novel Screening Tests
4. Diagnosis
4.1. Radiomics
4.2. WSI
4.3. Histopathology
4.4. Cytology
5. Decision Making and Prognosis Prediction
5.1. Medication Selection
5.2. Surgery
5.3. Radiotherapy
6. Future Development
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 29 November 2021).
- Luo, Y.H.; Chiu, C.H.; Scott Kuo, C.H.; Chou, T.Y.; Yeh, Y.C.; Hsu, H.S.; Yen, S.H.; Wu, Y.H.; Yang, J.C.; Liao, B.C.; et al. Lung Cancer in Republic of China. J. Thorac. Oncol. 2021, 16, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Cause of Death Statistics. Available online: https://www.mohw.gov.tw/lp-4650-2.html (accessed on 1 October 2021).
- Panunzio, A.; Sartori, P. Lung Cancer and Radiological Imaging. Curr. Radiopharm. 2020, 13, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Migliore, M.; Palmucci, S.; Nardini, M.; Basile, A. Imaging patterns of early stage lung cancer for the thoracic surgeon. J. Thorac. Dis. 2020, 12, 3349–3356. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D.; Brambilla, E.; Nicholson, A.G.; Yatabe, Y.; Austin, J.H.M.; Beasley, M.B.; Chirieac, L.R.; Dacic, S.; Duhig, E.; Flieder, D.B.; et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J. Thorac. Oncol. 2015, 10, 1243–1260. [Google Scholar] [CrossRef] [Green Version]
- Nicholson, A.G.; Tsao, M.S.; Beasley, M.B.; Borczuk, A.C.; Brambilla, E.; Cooper, W.A.; Dacic, S.; Jain, D.; Kerr, K.M.; Lantuejoul, S.; et al. The 2021 WHO Classification of Lung Tumors: Impact of advances since 2015. J. Thorac. Oncol. 2021, 17, 362–387. [Google Scholar] [CrossRef]
- Klang, E. Deep learning and medical imaging. J. Thorac. Dis. 2018, 10, 1325–1328. [Google Scholar] [CrossRef]
- Lawson, C.E.; Marti, J.M.; Radivojevic, T.; Jonnalagadda, S.V.R.; Gentz, R.; Hillson, N.J.; Peisert, S.; Kim, J.; Simmons, B.A.; Petzold, C.J.; et al. Machine learning for metabolic engineering: A review. Metab. Eng. 2021, 63, 34–60. [Google Scholar] [CrossRef]
- Leiserson, C.E.; Thompson, N.C.; Emer, J.S.; Kuszmaul, B.C.; Lampson, B.W.; Sanchez, D.; Schardl, T.B. There’s plenty of room at the Top: What will drive computer performance after Moore’s law? Science 2020, 368, eaam9744. [Google Scholar] [CrossRef]
- Shalf, J. The future of computing beyond Moore’s Law. Philos. Trans. A Math. Phys. Eng. Sci. 2020, 378, 20190061. [Google Scholar] [CrossRef] [Green Version]
- Somvanshi, M.; Chavan, P.; Tambade, S.; Shinde, S. A review of machine learning techniques using decision tree and support vector machine. In Proceedings of the 2016 International Conference on Computing Communication Control and Automation (ICCUBEA), Pune, India, 12–13 August 2016; pp. 1–7. [Google Scholar]
- Sesen, M.B.; Nicholson, A.E.; Banares-Alcantara, R.; Kadir, T.; Brady, M. Bayesian networks for clinical decision support in lung cancer care. PLoS ONE 2013, 8, e82349. [Google Scholar] [CrossRef] [Green Version]
- Gao, R.; Huo, Y.; Bao, S.; Tang, Y.; Antic, S.L.; Epstein, E.S.; Balar, A.B.; Deppen, S.; Paulson, A.B.; Sandler, K.L. Distanced LSTM: Time-distanced gates in long short-term memory models for lung cancer detection. In International Workshop on Machine Learning in Medical Imaging; Springer: New York, NY, USA, 2019; pp. 310–318. [Google Scholar]
- Onishi, Y.; Teramoto, A.; Tsujimoto, M.; Tsukamoto, T.; Saito, K.; Toyama, H.; Imaizumi, K.; Fujita, H. Automated pulmonary nodule classification in computed tomography images using a deep convolutional neural network trained by generative adversarial networks. BioMed Res. Int. 2019, 2019, 6051939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kourou, K.; Exarchos, T.P.; Exarchos, K.P.; Karamouzis, M.V.; Fotiadis, D.I. Machine learning applications in cancer prognosis and prediction. Comput. Struct. Biotechnol. J. 2015, 13, 8–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sim, Y.; Chung, M.J.; Kotter, E.; Yune, S.; Kim, M.; Do, S.; Han, K.; Kim, H.; Yang, S.; Lee, D.J.; et al. Deep Convolutional Neural Network-based Software Improves Radiologist Detection of Malignant Lung Nodules on Chest Radiographs. Radiology 2020, 294, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Rajpurkar, P.; Irvin, J.; Zhu, K.; Yang, B.; Mehta, H.; Duan, T.; Ding, D.; Bagul, A.; Langlotz, C.; Shpanskaya, K. Chexnet: Radiologist-level pneumonia detection on chest x-rays with deep learning. arXiv 2017, arXiv:1711.05225. [Google Scholar]
- Kim, Y.-G.; Cho, Y.; Wu, C.-J.; Park, S.; Jung, K.-H.; Seo, J.B.; Lee, H.J.; Hwang, H.J.; Lee, S.M.; Kim, N. Short-term reproducibility of pulmonary nodule and mass detection in chest radiographs: Comparison among radiologists and four different computer-aided detections with convolutional neural net. Sci. Rep. 2019, 9, 18738. [Google Scholar] [CrossRef] [PubMed]
- Tam, M.; Dyer, T.; Dissez, G.; Morgan, T.N.; Hughes, M.; Illes, J.; Rasalingham, R.; Rasalingham, S. Augmenting lung cancer diagnosis on chest radiographs: Positioning artificial intelligence to improve radiologist performance. Clin. Radiol. 2021, 76, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Han, S.G.; Cho, A.; Shin, H.J.; Baek, S.-E. Effect of deep learning-based assistive technology use on chest radiograph interpretation by emergency department physicians: A prospective interventional simulation-based study. BMC Med. Inform. Decis. Mak. 2021, 21, 311. [Google Scholar] [CrossRef]
- Van Ginneken, B.; Armato, S.G., III; de Hoop, B.; van Amelsvoort-van de Vorst, S.; Duindam, T.; Niemeijer, M.; Murphy, K.; Schilham, A.; Retico, A.; Fantacci, M.E. Comparing and combining algorithms for computer-aided detection of pulmonary nodules in computed tomography scans: The ANODE09 study. Med. Image Anal. 2010, 14, 707–722. [Google Scholar] [CrossRef] [Green Version]
- Setio, A.A.A.; Traverso, A.; De Bel, T.; Berens, M.S.; Van Den Bogaard, C.; Cerello, P.; Chen, H.; Dou, Q.; Fantacci, M.E.; Geurts, B. Validation, comparison, and combination of algorithms for automatic detection of pulmonary nodules in computed tomography images: The LUNA16 challenge. Med. Image Anal. 2017, 42, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Roos, J.E.; Paik, D.; Olsen, D.; Liu, E.G.; Chow, L.C.; Leung, A.N.; Mindelzun, R.; Choudhury, K.R.; Naidich, D.P.; Napel, S. Computer-aided detection (CAD) of lung nodules in CT scans: Radiologist performance and reading time with incremental CAD assistance. Eur. Radiol. 2010, 20, 549–557. [Google Scholar] [CrossRef] [Green Version]
- Lo, S.B.; Freedman, M.T.; Gillis, L.B.; White, C.S.; Mun, S.K. JOURNAL CLUB: Computer-aided detection of lung nodules on CT with a computerized pulmonary vessel suppressed function. Am. J. Roentgenol. 2018, 210, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Tang, W.; Xu, D.M.; Jirapatnakul, A.C.; Reeves, A.P.; Henschke, C.I.; Yankelevitz, D. Low-dose CT screening for lung cancer: Computer-aided detection of missed lung cancers. Radiology 2016, 281, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Ciompi, F.; Chung, K.; Van Riel, S.J.; Setio, A.A.A.; Gerke, P.K.; Jacobs, C.; Scholten, E.T.; Schaefer-Prokop, C.; Wille, M.M.; Marchiano, A. Towards automatic pulmonary nodule management in lung cancer screening with deep learning. Sci. Rep. 2017, 7, 46479. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, C.; Jin, L.; Gao, P.; Zhao, W.; Ma, W.; Tan, M.; Wu, W.; Duan, S.; Shan, Y. Radiomics for lung adenocarcinoma manifesting as pure ground-glass nodules: Invasive prediction. Eur. Radiol. 2020, 30, 3650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawkins, S.; Wang, H.; Liu, Y.; Garcia, A.; Stringfield, O.; Krewer, H.; Li, Q.; Cherezov, D.; Gatenby, R.A.; Balagurunathan, Y. Predicting malignant nodules from screening CT scans. J. Thorac. Oncol. 2016, 11, 2120–2128. [Google Scholar] [CrossRef] [Green Version]
- Tu, S.-J.; Wang, C.-W.; Pan, K.-T.; Wu, Y.-C.; Wu, C.-T. Localized thin-section CT with radiomics feature extraction and machine learning to classify early-detected pulmonary nodules from lung cancer screening. Phys. Med. Biol. 2018, 63, 065005. [Google Scholar] [CrossRef]
- Balagurunathan, Y.; Beers, A.; Mcnitt-Gray, M.; Hadjiiski, L.; Napel, S.; Goldgof, D.; Perez, G.; Arbelaez, P.; Mehrtash, A.; Kapur, T. Lung Nodule Malignancy Prediction in Sequential CT Scans: Summary of ISBI 2018 Challenge. IEEE Trans. Med. Imaging 2021, 40, 3748–3761. [Google Scholar] [CrossRef]
- Xiao, N.; Qiang, Y.; Bilal Zia, M.; Wang, S.; Lian, J. Ensemble classification for predicting the malignancy level of pulmonary nodules on chest computed tomography images. Oncol. Lett. 2020, 20, 401–408. [Google Scholar] [CrossRef]
- Lv, E.; Liu, W.; Wen, P.; Kang, X. Classification of Benign and Malignant Lung Nodules Based on Deep Convolutional Network Feature Extraction. J. Healthc. Eng. 2021, 2021, 8769652. [Google Scholar] [CrossRef]
- Poore, G.D.; Kopylova, E.; Zhu, Q.; Carpenter, C.; Fraraccio, S.; Wandro, S.; Kosciolek, T.; Janssen, S.; Metcalf, J.; Song, S.J. Microbiome analyses of blood and tissues suggest cancer diagnostic approach. Nature 2020, 579, 567–574. [Google Scholar] [CrossRef]
- Hilario, M.; Kalousis, A.; Müller, M.; Pellegrini, C. Machine learning approaches to lung cancer prediction from mass spectra. Proteomics 2003, 3, 1716–1719. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Leng, W.; Sun, C.; Lu, T.; Chen, Z.; Men, X.; Wang, Y.; Wang, G.; Zhen, B.; Qin, J. Urine proteome profiling predicts lung cancer from control cases and other tumors. EBioMedicine 2018, 30, 120–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tirzïte, M.; Bukovskis, M.; Strazda, G.; Jurka, N.; Taivans, I. Detection of lung cancer with electronic nose and logistic regression analysis. J. Breath Res. 2018, 13, 016006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.-H.; Zeng, C.; Wang, Y.-C.; Peng, H.-Y.; Lin, C.-S.; Chang, C.-J.; Yang, H.-Y. A study of diagnostic accuracy using a chemical sensor array and a machine learning technique to detect lung cancer. Sensors 2018, 18, 2845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kort, S.; Brusse-Keizer, M.; Gerritsen, J.W.; Schouwink, H.; Citgez, E.; de Jongh, F.; van der Maten, J.; Samii, S.; van den Bogart, M.; van der Palen, J. Improving lung cancer diagnosis by combining exhaled-breath data and clinical parameters. ERJ Open Res. 2020, 6, 00221–02019. [Google Scholar] [CrossRef]
- Wu, G.; Jochems, A.; Ibrahim, A.; Yan, C.; Sanduleanu, S.; Woodruff, H.C.; Lambin, P. Structural and functional radiomics for lung cancer. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3961–3974. [Google Scholar] [CrossRef]
- Liu, S.; Liu, S.; Zhang, C.; Yu, H.; Liu, X.; Hu, Y.; Xu, W.; Tang, X.; Fu, Q. Exploratory study of a CT Radiomics model for the classification of small cell lung cancer and non-small-cell lung cancer. Front. Oncol. 2020, 10, 1268. [Google Scholar] [CrossRef]
- Zhu, X.; Dong, D.; Chen, Z.; Fang, M.; Zhang, L.; Song, J.; Yu, D.; Zang, Y.; Liu, Z.; Shi, J. Radiomic signature as a diagnostic factor for histologic subtype classification of non-small cell lung cancer. Eur. Radiol. 2018, 28, 2772–2778. [Google Scholar] [CrossRef]
- Gu, Q.; Feng, Z.; Liang, Q.; Li, M.; Deng, J.; Ma, M.; Wang, W.; Liu, J.; Liu, P.; Rong, P. Machine learning-based radiomics strategy for prediction of cell proliferation in non-small cell lung cancer. Eur. J. Radiol. 2019, 118, 32–37. [Google Scholar] [CrossRef]
- Wang, S.; Shi, J.; Ye, Z.; Dong, D.; Yu, D.; Zhou, M.; Liu, Y.; Gevaert, O.; Wang, K.; Zhu, Y. Predicting EGFR mutation status in lung adenocarcinoma on computed tomography image using deep learning. Eur. Respir. J. 2019, 53, 1800986. [Google Scholar] [CrossRef]
- Song, L.; Zhu, Z.; Mao, L.; Li, X.; Han, W.; Du, H.; Wu, H.; Song, W.; Jin, Z. Clinical, conventional CT and radiomic feature-based machine learning models for predicting ALK rearrangement status in lung adenocarcinoma patients. Front. Oncol. 2020, 10, 369. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, Y.-L.; Feng, Y.; Yang, X.-Y.; Zhang, J.; Chang, D.-D.; Wu, X.; Tian, X.; Tang, K.-J.; Xie, C.-M. A CT-derived deep neural network predicts for programmed death ligand-1 expression status in advanced lung adenocarcinomas. Ann. Transl. Med. 2020, 8, 930. [Google Scholar] [CrossRef]
- Šarić, M.; Russo, M.; Stella, M.; Sikora, M. CNN-based method for lung cancer detection in whole slide histopathology images. In Proceedings of the 2019 4th International Conference on Smart and Sustainable Technologies (SpliTech), Split, Croatia, 18–21 June 2019; pp. 1–4. [Google Scholar]
- Wei, J.W.; Tafe, L.J.; Linnik, Y.A.; Vaickus, L.J.; Tomita, N.; Hassanpour, S. Pathologist-level classification of histologic patterns on resected lung adenocarcinoma slides with deep neural networks. Sci. Rep. 2019, 9, 3358. [Google Scholar] [CrossRef] [Green Version]
- Gertych, A.; Swiderska-Chadaj, Z.; Ma, Z.; Ing, N.; Markiewicz, T.; Cierniak, S.; Salemi, H.; Guzman, S.; Walts, A.E.; Knudsen, B.S. Convolutional neural networks can accurately distinguish four histologic growth patterns of lung adenocarcinoma in digital slides. Sci. Rep. 2019, 9, 1483. [Google Scholar] [CrossRef]
- Coudray, N.; Ocampo, P.S.; Sakellaropoulos, T.; Narula, N.; Snuderl, M.; Fenyö, D.; Moreira, A.L.; Razavian, N.; Tsirigos, A. Classification and mutation prediction from non–small cell lung cancer histopathology images using deep learning. Nat. Med. 2018, 24, 1559–1567. [Google Scholar] [CrossRef]
- Wang, X.; Chen, H.; Gan, C.; Lin, H.; Dou, Q.; Tsougenis, E.; Huang, Q.; Cai, M.; Heng, P.-A. Weakly supervised deep learning for whole slide lung cancer image analysis. IEEE Trans. Cybern. 2019, 50, 3950–3962. [Google Scholar] [CrossRef]
- Kapil, A.; Meier, A.; Zuraw, A.; Steele, K.E.; Rebelatto, M.C.; Schmidt, G.; Brieu, N. Deep semi supervised generative learning for automated tumor proportion scoring on NSCLC tissue needle biopsies. Sci. Rep. 2018, 8, 17343. [Google Scholar] [CrossRef] [Green Version]
- Aprupe, L.; Litjens, G.; Brinker, T.J.; van der Laak, J.; Grabe, N. Robust and accurate quantification of biomarkers of immune cells in lung cancer micro-environment using deep convolutional neural networks. PeerJ 2019, 7, e6335. [Google Scholar] [CrossRef]
- Jones, G.D.; Brandt, W.S.; Shen, R.; Sanchez-Vega, F.; Tan, K.S.; Martin, A.; Zhou, J.; Berger, M.; Solit, D.B.; Schultz, N. A genomic-pathologic annotated risk model to predict recurrence in early-stage lung adenocarcinoma. JAMA Surg. 2021, 156, e205601. [Google Scholar] [CrossRef]
- Sha, L.; Osinski, B.L.; Ho, I.Y.; Tan, T.L.; Willis, C.; Weiss, H.; Beaubier, N.; Mahon, B.M.; Taxter, T.J.; Yip, S.S. Multi-field-of-view deep learning model predicts nonsmall cell lung cancer programmed death-ligand 1 status from whole-slide hematoxylin and eosin images. J. Pathol. Inform. 2019, 10, 24. [Google Scholar]
- Jiang, M.; Sun, D.; Guo, Y.; Guo, Y.; Xiao, J.; Wang, L.; Yao, X. Assessing PD-L1 expression level by radiomic features from PET/CT in nonsmall cell lung cancer patients: An initial result. Acad. Radiol. 2020, 27, 171–179. [Google Scholar] [CrossRef]
- Li, S.; Ding, C.; Zhang, H.; Song, J.; Wu, L. Radiomics for the prediction of EGFR mutation subtypes in non-small cell lung cancer. Med. Phys. 2019, 46, 4545–4552. [Google Scholar] [CrossRef]
- Dercle, L.; Fronheiser, M.; Lu, L.; Du, S.; Hayes, W.; Leung, D.K.; Roy, A.; Wilkerson, J.; Guo, P.; Fojo, A.T. Identification of non–small cell lung cancer sensitive to systemic cancer therapies using radiomics. Clin. Cancer Res. 2020, 26, 2151–2162. [Google Scholar] [CrossRef] [Green Version]
- Le, V.-H.; Kha, Q.-H.; Hung, T.N.K.; Le, N.Q.K. Risk score generated from CT-based radiomics signatures for overall survival prediction in non-small cell lung cancer. Cancers 2021, 13, 3616. [Google Scholar] [CrossRef]
- Sun, F.; Chen, Y.; Chen, X.; Sun, X.; Xing, L. CT-based radiomics for predicting brain metastases as the first failure in patients with curatively resected locally advanced non-small cell lung cancer. Eur. J. Radiol. 2021, 134, 109411. [Google Scholar] [CrossRef]
- Yoshiyasu, N.; Kojima, F.; Hayashi, K.; Bando, T. Radiomics technology for identifying early-stage lung adenocarcinomas suitable for sublobar resection. J. Thorac. Cardiovasc. Surg. 2021, 162, 477–485.e1. [Google Scholar] [CrossRef]
- Choi, H.; Kim, H.; Hong, W.; Park, J.; Hwang, E.J.; Park, C.M.; Kim, Y.T.; Goo, J.M. Prediction of visceral pleural invasion in lung cancer on CT: Deep learning model achieves a radiologist-level performance with adaptive sensitivity and specificity to clinical needs. Eur. Radiol. 2021, 31, 2866–2876. [Google Scholar] [CrossRef]
- Mattonen, S.A.; Palma, D.A.; Haasbeek, C.J.; Senan, S.; Ward, A.D. Early prediction of tumor recurrence based on CT texture changes after stereotactic ablative radiotherapy (SABR) for lung cancer. Med. Phys. 2014, 41, 033502. [Google Scholar] [CrossRef]
- Lewis, J.E.; Kemp, M.L. Integration of machine learning and genome-scale metabolic modeling identifies multi-omics biomarkers for radiation resistance. Nat. Commun. 2021, 12, 2700. [Google Scholar] [CrossRef]
- Krafft, S.P.; Rao, A.; Stingo, F.; Briere, T.M.; Court, L.E.; Liao, Z.; Martel, M.K. The utility of quantitative CT radiomics features for improved prediction of radiation pneumonitis. Med. Phys. 2018, 45, 5317–5324. [Google Scholar] [CrossRef]
- Bourbonne, V.; Da-Ano, R.; Jaouen, V.; Lucia, F.; Dissaux, G.; Bert, J.; Pradier, O.; Visvikis, D.; Hatt, M.; Schick, U. Radiomics analysis of 3D dose distributions to predict toxicity of radiotherapy for lung cancer. Radiother. Oncol. 2021, 155, 144–150. [Google Scholar] [CrossRef]
- Girard, L.; Zochbauer-Muller, S.; Virmani, A.K.; Gazdar, A.F.; Minna, J.D. Genome-wide allelotyping of lung cancer identifies new regions of allelic loss, differences between small cell lung cancer and non-small cell lung cancer, and loci clustering. Cancer Res. 2000, 60, 4894–4906. [Google Scholar]
- Shen, R.; Olshen, A.B.; Ladanyi, M. Integrative clustering of multiple genomic data types using a joint latent variable model with application to breast and lung cancer subtype analysis. Bioinformatics 2009, 25, 2906–2912. [Google Scholar] [CrossRef]
- Silver, D.; Hubert, T.; Schrittwieser, J.; Antonoglou, I.; Lai, M.; Guez, A.; Lanctot, M.; Sifre, L.; Kumaran, D.; Graepel, T. A general reinforcement learning algorithm that masters chess, shogi, and Go through self-play. Science 2018, 362, 1140–1144. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; Lu, J.; Zhou, Q. A novel data augmentation method using style-based GAN for robust pulmonary nodule segmentation. In Proceedings of the 2020 Chinese Control and Decision Conference (CCDC), Hefei, China, 22–24 August 2020; pp. 2486–2491. [Google Scholar]
- Ali, I.; Hart, G.R.; Gunabushanam, G.; Liang, Y.; Muhammad, W.; Nartowt, B.; Kane, M.; Ma, X.; Deng, J. Lung Nodule Detection via Deep Reinforcement Learning. Front. Oncol. 2018, 8, 108. [Google Scholar] [CrossRef] [Green Version]
- Capizzi, G.; Sciuto, G.L.; Napoli, C.; Połap, D.; Woźniak, M. Small lung nodules detection based on fuzzy-logic and probabilistic neural network with bioinspired reinforcement learning. IEEE Trans. Fuzzy Syst. 2019, 28, 1178–1189. [Google Scholar] [CrossRef]
- In, K.-H.; Kwon, Y.-S.; Oh, I.-J.; Kim, K.-S.; Jung, M.-H.; Lee, K.-H.; Kim, S.-Y.; Ryu, J.-S.; Lee, S.-Y.; Jeong, E.-T. Lung cancer patients who are asymptomatic at diagnosis show favorable prognosis: A Korean Lung Cancer Registry Study. Lung. Cancer 2009, 64, 232–237. [Google Scholar] [CrossRef]
- Quadrelli, S.; Lyons, G.; Colt, H.; Chimondeguy, D.; Buero, A. Clinical characteristics and prognosis of incidentally detected lung cancers. Int. J. Surg. Oncol. 2015, 2015, 287604. [Google Scholar] [CrossRef] [Green Version]
- Melamed, M.R.; Flehinger, B.J.; Zaman, M.B.; Heelan, R.T.; Perchick, W.A.; Martini, N. Screening for early lung cancer. Results of the Memorial Sloan-Kettering study in New York. Chest 1984, 86, 44–53. [Google Scholar] [CrossRef]
- Hocking, W.G.; Hu, P.; Oken, M.M.; Winslow, S.D.; Kvale, P.A.; Prorok, P.C.; Ragard, L.R.; Commins, J.; Lynch, D.A.; Andriole, G.L.; et al. Lung cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial. J. Natl. Cancer Inst. 2010, 102, 722–731. [Google Scholar] [CrossRef]
- Chu, G.C.W.; Lazare, K.; Sullivan, F. Serum and blood based biomarkers for lung cancer screening: A systematic review. BMC Cancer 2018, 18, 181. [Google Scholar] [CrossRef] [Green Version]
- Montani, F.; Marzi, M.J.; Dezi, F.; Dama, E.; Carletti, R.M.; Bonizzi, G.; Bertolotti, R.; Bellomi, M.; Rampinelli, C.; Maisonneuve, P.; et al. miR-Test: A blood test for lung cancer early detection. J. Natl. Cancer Inst. 2015, 107, djv063. [Google Scholar] [CrossRef] [Green Version]
- Campanella, A.; De Summa, S.; Tommasi, S. Exhaled breath condensate biomarkers for lung cancer. J. Breath Res. 2019, 13, 044002. [Google Scholar] [CrossRef]
- Lopez-Sanchez, L.M.; Jurado-Gamez, B.; Feu-Collado, N.; Valverde, A.; Canas, A.; Fernandez-Rueda, J.L.; Aranda, E.; Rodriguez-Ariza, A. Exhaled breath condensate biomarkers for the early diagnosis of lung cancer using proteomics. Am. J. Physiol. Lung Cell Mol. Physiol. 2017, 313, L664–L676. [Google Scholar] [CrossRef] [Green Version]
- National Lung Screening Trial Research, T.; Aberle, D.R.; Adams, A.M.; Berg, C.D.; Black, W.C.; Clapp, J.D.; Fagerstrom, R.M.; Gareen, I.F.; Gatsonis, C.; Marcus, P.M.; et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [CrossRef] [Green Version]
- de Koning, H.J.; van der Aalst, C.M.; de Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Lammers, J.J.; Weenink, C.; Yousaf-Khan, U.; Horeweg, N.; et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N. Engl. J. Med. 2020, 382, 503–513. [Google Scholar] [CrossRef]
- Baker, S.R.; Patel, R.H.; Yang, L.; Lelkes, V.M.; Castro, A., 3rd. Malpractice suits in chest radiology: An evaluation of the histories of 8265 radiologists. J. Thorac. Imaging 2013, 28, 388–391. [Google Scholar] [CrossRef]
- Sakai, M.; Kato, A.; Kobayashi, N.; Nakamura, R.; Okawa, S.; Sato, Y. Improved Lung Cancer Detection in Cardiovascular Outpatients by the Pulmonologist-based Interpretation of Chest Radiographs. Intern. Med. 2015, 54, 2991–2997. [Google Scholar] [CrossRef] [Green Version]
- White, C.S.; Salis, A.I.; Meyer, C.A. Missed lung cancer on chest radiography and computed tomography: Imaging and medicolegal issues. J. Thorac. Imaging 1999, 14, 63–68. [Google Scholar] [CrossRef]
- About DICOM: Overview. Available online: https://www.dicomstandard.org/about (accessed on 3 December 2021).
- Shiraishi, J.; Katsuragawa, S.; Ikezoe, J.; Matsumoto, T.; Kobayashi, T.; Komatsu, K.-I.; Matsui, M.; Fujita, H.; Kodera, Y.; Doi, K. Development of a digital image database for chest radiographs with and without a lung nodule: Receiver operating characteristic analysis of radiologists’ detection of pulmonary nodules. Am. J. Roentgenol. 2000, 174, 71–74. [Google Scholar] [CrossRef]
- Jaeger, S.; Candemir, S.; Antani, S.; Wang, Y.X.; Lu, P.X.; Thoma, G. Two public chest X-ray datasets for computer-aided screening of pulmonary diseases. Quant. Imaging Med. Surg. 2014, 4, 475–477. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Peng, Y.; Lu, L.; Lu, Z.; Bagheri, M.; Summers, R.M. Chestx-ray8: Hospital-scale chest x-ray database and benchmarks on weakly-supervised classification and localization of common thorax diseases. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Honolulu, HI, USA, 21–26 July 2017; pp. 2097–2106. [Google Scholar]
- Bustos, A.; Pertusa, A.; Salinas, J.-M.; de la Iglesia-Vayá, M. Padchest: A large chest x-ray image dataset with multi-label annotated reports. Med. Image Anal. 2020, 66, 101797. [Google Scholar] [CrossRef] [PubMed]
- Armato, S.G., III; McLennan, G.; Bidaut, L.; McNitt-Gray, M.F.; Meyer, C.R.; Reeves, A.P.; Zhao, B.; Aberle, D.R.; Henschke, C.I.; Hoffman, E.A. The lung image database consortium (LIDC) and image database resource initiative (IDRI): A completed reference database of lung nodules on CT scans. Med. Phys. 2011, 38, 915–931. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.E.; Pollard, T.J.; Greenbaum, N.R.; Lungren, M.P.; Deng, C.-Y.; Peng, Y.; Lu, Z.; Mark, R.G.; Berkowitz, S.J.; Horng, S. MIMIC-CXR-JPG, a large publicly available database of labeled chest radiographs. arXiv 2019, arXiv:1901.07042. [Google Scholar]
- Irvin, J.; Rajpurkar, P.; Ko, M.; Yu, Y.; Ciurea-Ilcus, S.; Chute, C.; Marklund, H.; Haghgoo, B.; Ball, R.; Shpanskaya, K. Chexpert: A large chest radiograph dataset with uncertainty labels and expert comparison. In Proceedings of the AAAI Conference on Artificial Intelligence, Honolulu, HI, USA, 27 January–1 February 2019; pp. 590–597. [Google Scholar]
- Nguyen, H.C.; Le, T.T.; Pham, H.; Nguyen, H.Q. VinDr-RibCXR: A Benchmark Dataset for Automatic Segmentation and Labeling of Individual Ribs on Chest X-rays. arXiv 2021, arXiv:2107.01327. [Google Scholar]
- Jain, S.; Agrawal, A.; Saporta, A.; Truong, S.Q.; Bui, T.; Chambon, P.; Zhang, Y.; Lungren, M.P.; Ng, A.Y.; Langlotz, C. RadGraph: Extracting Clinical Entities and Relations from Radiology Reports. arXiv 2021, arXiv:2106.14463. [Google Scholar]
- Lanfredi, R.B.; Zhang, M.; Auffermann, W.F.; Chan, J.; Duong, P.-A.T.; Srikumar, V.; Drew, T.; Schroeder, J.D.; Tasdizen, T. REFLACX, a dataset of reports and eye-tracking data for localization of abnormalities in chest X-rays. arXiv 2021, arXiv:2109.14187. [Google Scholar]
- Lodwick, G.S.; Keats, T.E.; Dorst, J.P. The Coding of Roentgen Images for Computer Analysis as Applied to Lung Cancer. Radiology 1963, 81, 185–200. [Google Scholar] [CrossRef]
- Munir, K.; Elahi, H.; Ayub, A.; Frezza, F.; Rizzi, A. Cancer diagnosis using deep learning: A bibliographic review. Cancers 2019, 11, 1235. [Google Scholar] [CrossRef] [Green Version]
- Van Riel, S.J.; Jacobs, C.; Scholten, E.T.; Wittenberg, R.; Wille, M.M.W.; de Hoop, B.; Sprengers, R.; Mets, O.M.; Geurts, B.; Prokop, M. Observer variability for Lung-RADS categorisation of lung cancer screening CTs: Impact on patient management. Eur. Radiol. 2019, 29, 924–931. [Google Scholar] [CrossRef] [Green Version]
- Schreuder, A.; Scholten, E.T.; van Ginneken, B.; Jacobs, C. Artificial intelligence for detection and characterization of pulmonary nodules in lung cancer CT screening: Ready for practice? Transl. Lung Cancer Res. 2021, 10, 2378. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Chi, W.; Li, X.; Li, P.; Liang, W.; Liu, H.; Wang, W.; He, J. Evolving the pulmonary nodules diagnosis from classical approaches to deep learning-aided decision support: Three decades’ development course and future prospect. J. Cancer Res. Clin. Oncol. 2020, 146, 153–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.; Mikela Vilmun, B.; Frederik Carlsen, J.; Albrecht-Beste, E.; Ammitzbøl Lauridsen, C.; Bachmann Nielsen, M.; Lindskov Hansen, K. The performance of deep learning algorithms on automatic pulmonary nodule detection and classification tested on different datasets that are not derived from LIDC-IDRI: A systematic review. Diagnostics 2019, 9, 207. [Google Scholar] [CrossRef] [Green Version]
- Pastorino, U.; Rossi, M.; Rosato, V.; Marchianò, A.; Sverzellati, N.; Morosi, C.; Fabbri, A.; Galeone, C.; Negri, E.; Sozzi, G. Annual or biennial CT screening versus observation in heavy smokers: 5-year results of the MILD trial. Eur. J. Cancer Prev. 2012, 21, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, J.H.; Ashraf, H.; Dirksen, A.; Bach, K.; Hansen, H.; Toennesen, P.; Thorsen, H.; Brodersen, J.; Skov, B.G.; Døssing, M. The Danish randomized lung cancer CT screening trial—Overall design and results of the prevalence round. J. Thorac. Oncol. 2009, 4, 608–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, M.D.; Kanne, J.P.; Broderick, L.S.; Kazerooni, E.A.; Meyer, C.A. Lung-RADS: Pushing the limits. Radiographics 2017, 37, 1975–1993. [Google Scholar] [CrossRef] [PubMed]
- MacMahon, H.; Naidich, D.P.; Goo, J.M.; Lee, K.S.; Leung, A.N.; Mayo, J.R.; Mehta, A.C.; Ohno, Y.; Powell, C.A.; Prokop, M. Guidelines for management of incidental pulmonary nodules detected on CT images: From the Fleischner Society 2017. Radiology 2017, 284, 228–243. [Google Scholar] [CrossRef] [Green Version]
- Chabon, J.J.; Hamilton, E.G.; Kurtz, D.M.; Esfahani, M.S.; Moding, E.J.; Stehr, H.; Schroers-Martin, J.; Nabet, B.Y.; Chen, B.; Chaudhuri, A.A. Integrating genomic features for non-invasive early lung cancer detection. Nature 2020, 580, 245–251. [Google Scholar] [CrossRef]
- Cammarota, G.; Ianiro, G.; Ahern, A.; Carbone, C.; Temko, A.; Claesson, M.J.; Gasbarrini, A.; Tortora, G. Gut microbiome, big data and machine learning to promote precision medicine for cancer. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 635–648. [Google Scholar] [CrossRef]
- Peled, N.; Fuchs, V.; Kestenbaum, E.H.; Oscar, E.; Bitran, R. An Update on the Use of Exhaled Breath Analysis for the Early Detection of Lung Cancer. Lung Cancer Targets Ther. 2021, 12, 81. [Google Scholar] [CrossRef]
- Xiang, D.; Zhang, B.; Doll, D.; Shen, K.; Kloecker, G.; Freter, C. Lung cancer screening: From imaging to biomarker. Biomark. Res. 2013, 1, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daniels, M.; Goh, F.; Wright, C.M.; Sriram, K.B.; Relan, V.; Clarke, B.E.; Duhig, E.E.; Bowman, R.V.; Yang, I.A.; Fong, K.M. Whole genome sequencing for lung cancer. J. Thorac. Dis. 2012, 4, 155. [Google Scholar] [PubMed]
- Choi, Y.; Qu, J.; Wu, S.; Hao, Y.; Zhang, J.; Ning, J.; Yang, X.; Lofaro, L.; Pankratz, D.G.; Babiarz, J. Improving lung cancer risk stratification leveraging whole transcriptome RNA sequencing and machine learning across multiple cohorts. BMC Med. Genom. 2020, 13, 151. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.; Creighton, C.; Davis, C.; Donehower, L. The cancer genome atlas pan-cancer analysis project. Nat. Genet. 2013, 45, 1113–1120. [Google Scholar]
- Phillips, M.; Gleeson, K.; Hughes, J.M.B.; Greenberg, J.; Cataneo, R.N.; Baker, L.; McVay, W.P. Volatile organic compounds in breath as markers of lung cancer: A cross-sectional study. Lancet 1999, 353, 1930–1933. [Google Scholar] [CrossRef]
- Evans, A.J.; Bauer, T.W.; Bui, M.M.; Cornish, T.C.; Duncan, H.; Glassy, E.F.; Hipp, J.; McGee, R.S.; Murphy, D.; Myers, C. US Food and Drug Administration approval of whole slide imaging for primary diagnosis: A key milestone is reached and new questions are raised. Arch. Pathol. Lab. Med. 2018, 142, 1383–1387. [Google Scholar] [CrossRef] [Green Version]
- Abels, E.; Pantanowitz, L. Current state of the regulatory trajectory for whole slide imaging devices in the USA. J. Pathol. Inform. 2017, 8, 23. [Google Scholar] [CrossRef]
- Niazi, M.K.K.; Parwani, A.V.; Gurcan, M.N. Digital pathology and artificial intelligence. Lancet Oncol. 2019, 20, e253–e261. [Google Scholar] [CrossRef]
- DICOM Whole Slide Imaging (WSI). Available online: https://dicom.nema.org/Dicom/DICOMWSI/ (accessed on 29 November 2021).
- Sakamoto, T.; Furukawa, T.; Lami, K.; Pham, H.H.N.; Uegami, W.; Kuroda, K.; Kawai, M.; Sakanashi, H.; Cooper, L.A.D.; Bychkov, A. A narrative review of digital pathology and artificial intelligence: Focusing on lung cancer. Transl. Lung Cancer Res. 2020, 9, 2255. [Google Scholar] [CrossRef]
- Giovagnoli, M.R.; Giansanti, D. Artificial Intelligence in Digital Pathology: What Is the Future? Part 1: From the Digital Slide Onwards. Healthc. Multidiscip. Digit. Publ. Inst. 2021, 9, 858. [Google Scholar] [CrossRef]
- Bejnordi, B.E.; Veta, M.; Van Diest, P.J.; Van Ginneken, B.; Karssemeijer, N.; Litjens, G.; Van Der Laak, J.A.; Hermsen, M.; Manson, Q.F.; Balkenhol, M. Diagnostic assessment of deep learning algorithms for detection of lymph node metastases in women with breast cancer. Jama 2017, 318, 2199–2210. [Google Scholar] [CrossRef] [PubMed]
- Biermann, J.; Adkins, D.; Agulnik, M.; Benjamin, R.; Brigman, B.; Butrynski, J.; Cheong, D.; Chow, W.; Curry, W.; Frassica, D. National comprehensive cancer network. Bone cancer. J. Natl. Compr. Cancer Netw. 2013, 11, 688–723. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, T.; Kuroda, K.; Bychkov, A.; Pham, H.; Kashima, Y.; Fukuoka, J. Verification of Deep Learning Model to Measure Tumor Cellularity in Transbronchial Biopsies of Lung Adenocarcinoma; Laboratory Investigation, Nature Publishing Group: New York, NY, USA, 2019. [Google Scholar]
- Sakamoto, T.; Furukawa, T.; Pham, H.H.; Kuroda, K.; Tabata, K.; Kashima, Y.; Okoshi, E.N.; Morimoto, S.; Bychkov, A.; Fukuoka, J. Collaborative workflow between pathologists and deep learning for evaluation of tumor cellularity in lung adenocarcinoma. bioRxiv 2022. [Google Scholar] [CrossRef]
- Hondelink, L.M.; Hüyük, M.; Postmus, P.E.; Smit, V.T.; Blom, S.; von der Thüsen, J.H.; Cohen, D. Development and validation of a supervised deep learning algorithm for automated whole-slide programmed death-ligand 1 tumour proportion score assessment in non-small cell lung cancer. Histopathology 2021, 80, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lin, D. A Review of Artificial Intelligence in Precise Assessment of Programmed Cell Death-ligand 1 and Tumor-infiltrating Lymphocytes in Non− Small Cell Lung Cancer. Adv. Anat. Pathol. 2021, 28, 439–445. [Google Scholar] [CrossRef]
- Campanella, G.; Hanna, M.G.; Geneslaw, L.; Miraflor, A.; Silva, V.W.K.; Busam, K.J.; Brogi, E.; Reuter, V.E.; Klimstra, D.S.; Fuchs, T.J. Clinical-grade computational pathology using weakly supervised deep learning on whole slide images. Nat. Med. 2019, 25, 1301–1309. [Google Scholar] [CrossRef]
- Giansanti, D.; Grigioni, M.; D’Avenio, G.; Morelli, S.; Maccioni, G.; Bondi, A.; Giovagnoli, M.R. Virtual microscopy and digital cytology: State of the art. Ann. Dell’istituto Super. Di Sanità 2010, 46, 115–122. [Google Scholar]
- Boschetto, A.; Pochini, M.; Bottini, L.; Giovagnoli, M.R.; Giansanti, D. The focus emulation and image enhancement in digital cytology: An experience using the software Mathematica. Comput. Methods Biomech. Biomed. Eng. Imaging Vis. 2015, 3, 110–116. [Google Scholar] [CrossRef]
- Lin, Y.-J.; Chao, T.-K.; Khalil, M.-A.; Lee, Y.-C.; Hong, D.-Z.; Wu, J.-J.; Wang, C.-W. Deep Learning Fast Screening Approach on Cytological Whole Slides for Thyroid Cancer Diagnosis. Cancers 2021, 13, 3891. [Google Scholar] [CrossRef]
- Echle, A.; Rindtorff, N.T.; Brinker, T.J.; Luedde, T.; Pearson, A.T.; Kather, J.N. Deep learning in cancer pathology: A new generation of clinical biomarkers. Br. J. Cancer 2021, 124, 686–696. [Google Scholar] [CrossRef]
- Predicting Response to Immunotherapy Using Computer Extracted Featuresof Cancer Nuclei from Hematoxylin and Eosin (H&E) Stained Images of Non-Small Cell Lung Cancer (NSCLC). Available online: https://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=HITOFF&p=1&u=%2Fnetahtml%2FPTO%2Fsearchbool.html&r=1&f=G&l=50&co1=AND&d=PTXT&s1=11,055,844.PN.&OS=PN/11,055,844&RS=PN/11,055,844 (accessed on 6 March 2022).
- Wulczyn, E.; Steiner, D.F.; Xu, Z.; Sadhwani, A.; Wang, H.; Flament-Auvigne, I.; Mermel, C.H.; Chen, P.-H.C.; Liu, Y.; Stumpe, M.C. Deep learning-based survival prediction for multiple cancer types using histopathology images. PLoS ONE 2020, 15, e0233678. [Google Scholar] [CrossRef] [PubMed]
- D’Antonoli, T.A.; Farchione, A.; Lenkowicz, J.; Chiappetta, M.; Cicchetti, G.; Martino, A.; Ottavianelli, A.; Manfredi, R.; Margaritora, S.; Bonomo, L. CT radiomics signature of tumor and peritumoral lung parenchyma to predict nonsmall cell lung cancer postsurgical recurrence risk. Acad. Radiol. 2020, 27, 497–507. [Google Scholar]
- Hosny, A.; Parmar, C.; Coroller, T.P.; Grossmann, P.; Zeleznik, R.; Kumar, A.; Bussink, J.; Gillies, R.J.; Mak, R.H.; Aerts, H.J. Deep learning for lung cancer prognostication: A retrospective multi-cohort radiomics study. PLoS Med. 2018, 15, e1002711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
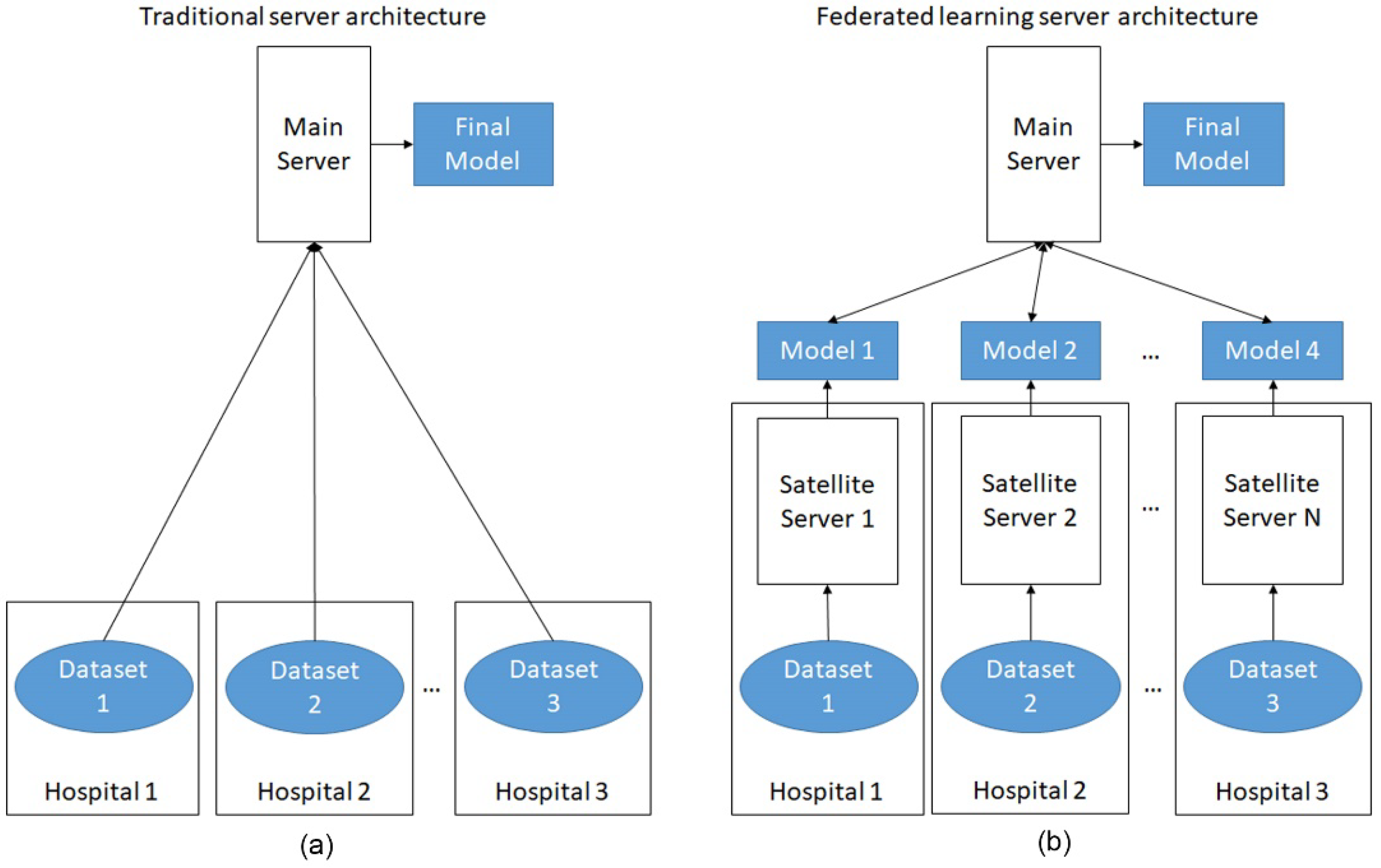
- Jochems, A.; Deist, T.M.; Van Soest, J.; Eble, M.; Bulens, P.; Coucke, P.; Dries, W.; Lambin, P.; Dekker, A. Distributed learning: Developing a predictive model based on data from multiple hospitals without data leaving the hospital–a real life proof of concept. Radiother. Oncol. 2016, 121, 459–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jochems, A.; Deist, T.M.; El Naqa, I.; Kessler, M.; Mayo, C.; Reeves, J.; Jolly, S.; Matuszak, M.; Ten Haken, R.; van Soest, J. Developing and validating a survival prediction model for NSCLC patients through distributed learning across 3 countries. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 344–352. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.D.; Zhou, W.; Yan, H.; Wong, M.; Lee, V. Personalized prediction of EGFR mutation-induced drug resistance in lung cancer. Sci. Rep. 2013, 3, 2855. [Google Scholar] [CrossRef] [Green Version]
- Giang, T.-T.; Nguyen, T.-P.; Tran, D.-H. Stratifying patients using fast multiple kernel learning framework: Case studies of Alzheimer’s disease and cancers. BMC Med. Inform. Decis. Mak. 2020, 20, 108. [Google Scholar] [CrossRef]
- Gao, Y.; Zhou, R.; Lyu, Q. Multiomics and machine learning in lung cancer prognosis. J. Thorac. Dis. 2020, 12, 4531. [Google Scholar] [CrossRef]
- Wissel, D.; Rowson, D.; Boeva, V. Hierarchical autoencoder-based integration improves performance in multi-omics cancer survival models through soft modality selection. bioRxiv 2022. [Google Scholar] [CrossRef]
- Coory, M.; Gkolia, P.; Yang, I.A.; Bowman, R.V.; Fong, K.M. Systematic review of multidisciplinary teams in the management of lung cancer. Lung Cancer 2008, 60, 14–21. [Google Scholar] [CrossRef]
- Denton, E.; Conron, M. Improving outcomes in lung cancer: The value of the multidisciplinary health care team. J. Multidiscip. Healthc. 2016, 9, 137–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wichmann, J.L.; Willemink, M.J.; De Cecco, C.N. Artificial Intelligence and Machine Learning in Radiology: Current State and Considerations for Routine Clinical Implementation. Investig. Radiol. 2020, 55, 619–627. [Google Scholar] [CrossRef] [PubMed]
| Screening | Diagnosis | Treatment |
|---|---|---|
| Radiology: CXR [17,18,19,20,21] CXR [17,18,19,20,21] LDCT [22,23,24,25,26,27,28,29,30,31,32,33] Novel tools: Genomics [34] Genomics [34] Proteomics [35,36] Exhaled breath [37,38,39] | Risk prediction: Radiomics [40,41,42,43,44,45,46] WSI [47,48,49,50,51,52,53] WSI [47,48,49,50,51,52,53] Genomics [50,54] | Tumor property classification: Drug selection [44,45,46,50,55,56,57] Prognosis prediction: Drug treatment response [58,59,60] Post-Surgery recurrence [54,61,62] Radiotherapy response [63,64] Side effect estimation: Radiation pneumonitis [65,66] |
| Database | Year | Material | Volume | Features |
|---|---|---|---|---|
| JSRT [87] | 1998 | CXR | 154 | Contains 100 CXRs with malignant nodule, 54 CXRs with benigh nodule, and 93 normal CXRs |
| Shenzhen CXR set [88] | 2012 | CXR | 662 | Contains 326 normal CXRs, and 336 CXRs with tuberculosis. Ribs were labeled. |
| Montgomery CXR set [88] | 2014 | CXR | 138 | Contains 80 normal CXRs, and 58 CXRs with tuberculosis. Ribs were labeled. |
| ChestXray8 [89] | 1992–2015 | CXR | 108,948 | Classified into 8 features: atelectasis, cardiomegaly, effusion, infiltration, mass, nodule, normal, pneumonia, and pneumothorax |
| ChestXray14 [89] | 1992–2015 | CXR | Classified into 14 features: atelectasis, cardiomegaly, consolidation, edema, effusion, emphysema, fibrosis, hernia, infiltration, mass, nodule, pleural thickening, pneumonia, pneumothorax. | |
| PadChest [90] | 2009–2017 | CXR | >160,000 | Labeled with 174 different radiographic findings, 19 differential diagnoses and 104 anatomic locations |
| LIDC [91] | 2011 | LDCT | 1018 | Nodules were annotated and labeled with nodule sizes |
| LUNA16 [23] | 2016 | LDCT | 888 | Adapted from LIDC, with additional nodules found during model training. 1186 lung nodules annotated in 888 CT scans |
| MIMIC-CXR [92] | 2011–2016 | CXR | 377,110 | Classified into 14 labels derived from two natural language processing tools. |
| ChestXpert [93] | 2019 | CXR | 224,316 | Labeled with 14 features: no finding, enlarged cardiom, cardiomegaly, lung opacity, lung lesion, edema, consolidation, pneumonia, atelectasis, pneumothorax, pleural effusion, pleural other, fracture, support devices |
| VinDr-RibCXR [94] | 2020 | CXR | 18,000 | Rib suppression images |
| RadGraph [95] | 2021 | CXR | 500 | Inference dataset of MMIC-CXR and reports |
| REFLACX [96] | 2021 | CXR | 3032 | Labeled by 5 radiologists and synchronized sets of eye-tracking data and timestamped report transcriptions |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiu, H.-Y.; Chao, H.-S.; Chen, Y.-M. Application of Artificial Intelligence in Lung Cancer. Cancers 2022, 14, 1370. https://doi.org/10.3390/cancers14061370
Chiu H-Y, Chao H-S, Chen Y-M. Application of Artificial Intelligence in Lung Cancer. Cancers. 2022; 14(6):1370. https://doi.org/10.3390/cancers14061370
Chicago/Turabian StyleChiu, Hwa-Yen, Heng-Sheng Chao, and Yuh-Min Chen. 2022. "Application of Artificial Intelligence in Lung Cancer" Cancers 14, no. 6: 1370. https://doi.org/10.3390/cancers14061370
APA StyleChiu, H.-Y., Chao, H.-S., & Chen, Y.-M. (2022). Application of Artificial Intelligence in Lung Cancer. Cancers, 14(6), 1370. https://doi.org/10.3390/cancers14061370






