Inflammation and Prostate Cancer: A Multidisciplinary Approach to Identifying Opportunities for Treatment and Prevention
Abstract
Simple Summary
Abstract
1. Introduction
2. Epidemiological Associations between Inflammation and Prostate Cancer
2.1. Infections in Prostate Cancer Etiology
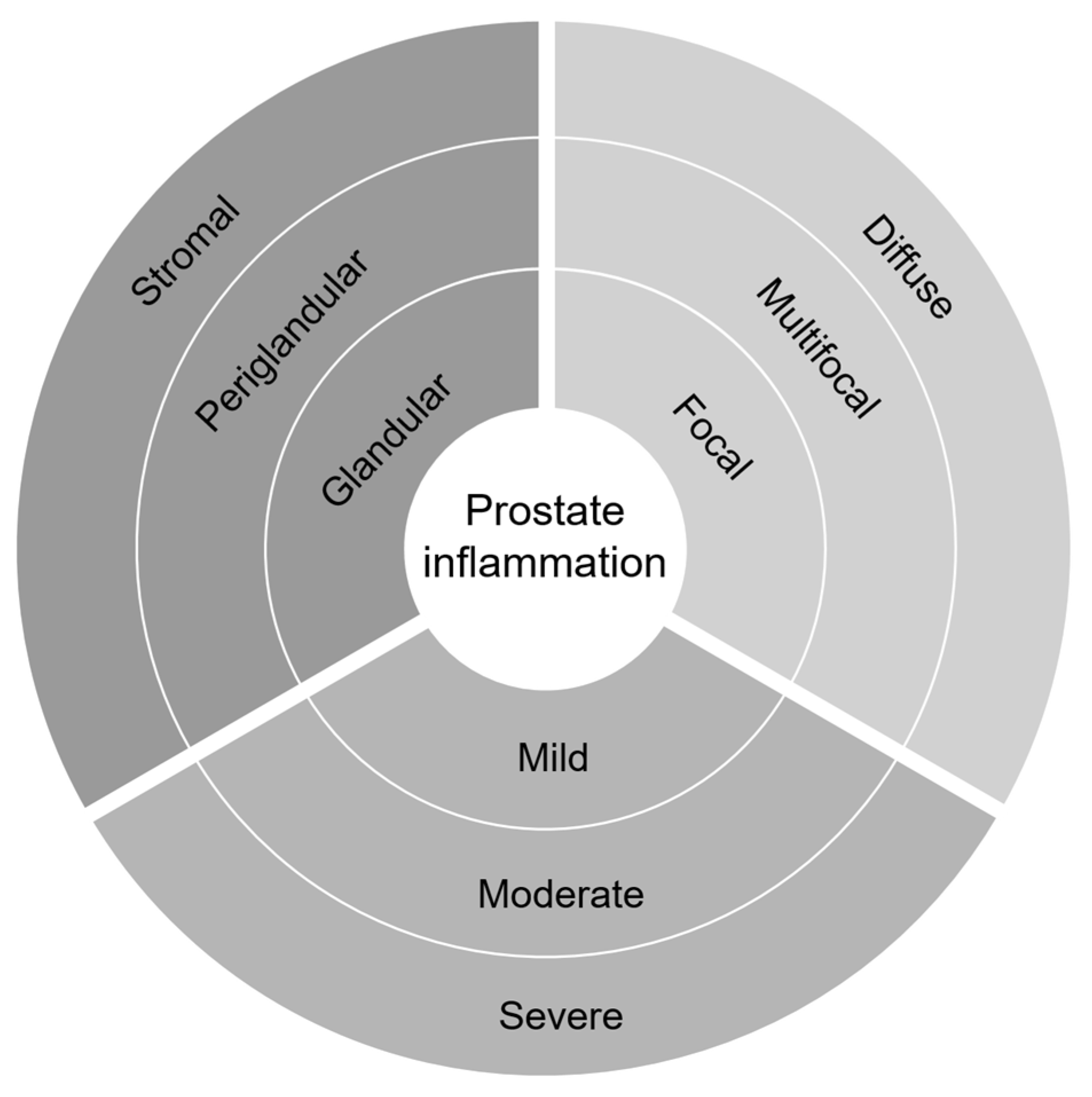
2.2. Histopathological Inflammation in Prostate Cancer
2.3. Lifestyle Factors Altering Prostate Inflammation
3. Immunobiology in Prostate Cancer Microenvironment
3.1. Immune Modulators in Prostate Cancer Progression
3.2. Heterogeneity among Immune Phenotypes
4. Strategies for Measuring Prostatic Inflammation
| Method | Sample Source | Detection Level | Markers Number | Spatial Information | Advanced Analysis Platform |
|---|---|---|---|---|---|
| H&E | FFPE | Cellular structure | NA | Yes | HALO, FIJI/ImageJ, QuPath, CellProfiler, Visiopharm |
| IHC, IF | FFPE | Protein | Up to 60 [104] | Yes | |
| Flow cytometry | Fresh tissue, FF, FFPE | Protein | Up to 28 [105] | No | viSNE, PhenoGraph, SPADE1, FlowSOM, t-SNE |
| Mass cytometry | Fresh tissue, FF, FFPE | Protein | Up to 42 [106] | No | |
| Microarray | Fresh tissue, FF, FFPE | Transcriptomics | High | No | MCP-counter, xCell, TIMER, quanTIseq, EPIC, CIBERSORT |
| RNA-seq | Fresh tissue, FF, FFPE | Transcriptomics | High | No | |
| Digital spatial profiling | FFPE, fresh tissue | Protein, transcriptomics | Up to 50 [107] | Yes | NanoString |
5. Clinical Treatment/Lifestyle Intervention Relevant to Inflammation
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Colotta, F.; Allavena, P.; Sica, A.; Garlanda, C.; Mantovani, A. Cancer-related inflammation, the seventh hallmark of cancer: Links to genetic instability. Carcinogenesis 2009, 30, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Sfanos, K.S.; Yegnasubramanian, S.; Nelson, W.G.; De Marzo, A.M. The inflammatory microenvironment and microbiome in prostate cancer development. Nat. Rev. Urol. 2018, 15, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Platz, E.A.; Kulac, I.; Barber, J.R.; Drake, C.G.; Joshu, C.E.; Nelson, W.G.; Lucia, M.S.; Klein, E.A.; Lippman, S.M.; Parnes, H.L.; et al. A Prospective Study of Chronic Inflammation in Benign Prostate Tissue and Risk of Prostate Cancer: Linked PCPT and SELECT Cohorts. Cancer Epidemiol. Biomark. Prev. 2017, 26, 1549–1557. [Google Scholar] [CrossRef] [PubMed]
- Gurel, B.; Lucia, M.S.; Thompson, I.M., Jr.; Goodman, P.J.; Tangen, C.M.; Kristal, A.R.; Parnes, H.L.; Hoque, A.; Lippman, S.M.; Sutcliffe, S.; et al. Chronic inflammation in benign prostate tissue is associated with high-grade prostate cancer in the placebo arm of the prostate cancer prevention trial. Cancer Epidemiol. Biomark. Prev. 2014, 23, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Moreira, D.M.; Nickel, J.C.; Gerber, L.; Muller, R.L.; Andriole, G.L.; Castro-Santamaria, R.; Freedland, S.J. Baseline prostate inflammation is associated with a reduced risk of prostate cancer in men undergoing repeat prostate biopsy: Results from the REDUCE study. Cancer 2014, 120, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Irani, J.; Goujon, J.M.; Ragni, E.; Peyrat, L.; Hubert, J.; Saint, F.; Mottet, N. Pathologist Multi Center Study Group. High-grade inflammation in prostate cancer as a prognostic factor for biochemical recurrence after radical prostatectomy. Urology 1999, 54, 467–472. [Google Scholar] [CrossRef]
- Davidsson, S.; Fiorentino, M.; Andrén, O.; Fang, F.; Mucci, L.A.; Varenhorst, E.; Fall, K.; Rider, J.R. Inflammation, focal atrophic lesions, and prostatic intraepithelial neoplasia with respect to risk of lethal prostate cancer. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2011, 20, 2280–2287. [Google Scholar] [CrossRef]
- Klink, J.C.; Bañez, L.L.; Gerber, L.; Lark, A.; Vollmer, R.T.; Freedland, S.J. Intratumoral inflammation is associated with more aggressive prostate cancer. World J. Urol. 2013, 31, 1497–1503. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, C.K.; Rencsok, E.M.; Fall, K.; Lotan, T.L.; Loda, M.; Giunchi, F.; Platz, E.A.; De Marzo, A.M.; Mucci, L.A.; et al. A Prospective Study of Intraprostatic Inflammation, Focal Atrophy, and Progression to Lethal Prostate Cancer. Cancer Epidemiol. Biomark. Prev. 2019, 28, 2047–2054. [Google Scholar] [CrossRef]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef] [PubMed]
- Taube, J.M.; Klein, A.; Brahmer, J.R.; Xu, H.; Pan, X.; Kim, J.H.; Chen, L.; Pardoll, D.M.; Topalian, S.L.; Anders, R.A. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2014, 20, 5064–5074. [Google Scholar] [CrossRef] [PubMed]
- Beer, T.M.; Kwon, E.D.; Drake, C.G.; Fizazi, K.; Logothetis, C.; Gravis, G.; Ganju, V.; Polikoff, J.; Saad, F.; Humanski, P.; et al. Randomized, Double-Blind, Phase III Trial of Ipilimumab Versus Placebo in Asymptomatic or Minimally Symptomatic Patients With Metastatic Chemotherapy-Naive Castration-Resistant Prostate Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Galon, J.; Bruni, D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat. Rev. Drug Discov. 2019, 18, 197–218. [Google Scholar] [CrossRef] [PubMed]
- Krieger, J.N.; Nyberg, L., Jr.; Nickel, J.C. NIH consensus definition and classification of prostatitis. JAMA 1999, 282, 236–237. [Google Scholar] [CrossRef] [PubMed]
- Caini, S.; Gandini, S.; Dudas, M.; Bremer, V.; Severi, E.; Gherasim, A. Sexually transmitted infections and prostate cancer risk: A systematic review and meta-analysis. Cancer Epidemiol. 2014, 38, 329–338. [Google Scholar] [CrossRef]
- Vazquez-Salas, R.A.; Torres-Sanchez, L.; Lopez-Carrillo, L.; Romero-Martinez, M.; Manzanilla-Garcia, H.A.; Cruz-Ortiz, C.H.; Mendoza-Pena, F.; Jimenez-Rios, M.A.; Rodriguez-Covarrubias, F.; Hernandez-Toriz, N.; et al. History of gonorrhea and prostate cancer in a population-based case-control study in Mexico. Cancer Epidemiol. 2016, 40, 95–101. [Google Scholar] [CrossRef]
- Sutcliffe, S.; Giovannucci, E.; De Marzo, A.M.; Leitzmann, M.F.; Willett, W.C.; Platz, E.A. Gonorrhea, syphilis, clinical prostatitis, and the risk of prostate cancer. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2006, 15, 2160–2166. [Google Scholar] [CrossRef][Green Version]
- Stark, J.R.; Judson, G.; Alderete, J.F.; Mundodi, V.; Kucknoor, A.S.; Giovannucci, E.L.; Platz, E.A.; Sutcliffe, S.; Fall, K.; Kurth, T.; et al. Prospective study of Trichomonas vaginalis infection and prostate cancer incidence and mortality: Physicians’ Health Study. J. Natl. Cancer Inst. 2009, 101, 1406–1411. [Google Scholar] [CrossRef]
- Sutcliffe, S.; Giovannucci, E.; Alderete, J.F.; Chang, T.H.; Gaydos, C.A.; Zenilman, J.M.; De Marzo, A.M.; Willett, W.C.; Platz, E.A. Plasma antibodies against Trichomonas vaginalis and subsequent risk of prostate cancer. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2006, 15, 939–945. [Google Scholar] [CrossRef]
- Tsang, S.H.; Peisch, S.F.; Rowan, B.; Markt, S.C.; Gonzalez-Feliciano, A.G.; Sutcliffe, S.; Platz, E.A.; Mucci, L.A.; Ebot, E.M. Association between Trichomonas vaginalis and prostate cancer mortality. Int. J. Cancer 2019, 144, 2377–2380. [Google Scholar] [CrossRef] [PubMed]
- Sutcliffe, S.; Alderete, J.F.; Till, C.; Goodman, P.J.; Hsing, A.W.; Zenilman, J.M.; De Marzo, A.M.; Platz, E.A. Trichomonosis and subsequent risk of prostate cancer in the Prostate Cancer Prevention Trial. Int. J. Cancer 2009, 124, 2082–2087. [Google Scholar] [CrossRef]
- Shui, I.M.; Kolb, S.; Hanson, C.; Sutcliffe, S.; Rider, J.R.; Stanford, J.L. Trichomonas vaginalis infection and risk of advanced prostate cancer. Prostate 2016, 76, 620–623. [Google Scholar] [CrossRef] [PubMed]
- Fowke, J.H.; Han, X.; Alderete, J.F.; Moses, K.A.; Signorello, L.B.; Blot, W.J. A prospective study of Trichomonas vaginalis and prostate cancer risk among African American men. BMC Res. Notes 2016, 9, 224. [Google Scholar] [CrossRef] [PubMed]
- Marous, M.; Huang, W.Y.; Rabkin, C.S.; Hayes, R.B.; Alderete, J.F.; Rosner, B.; Grubb, R.L., 3rd; Winter, A.C.; Sutcliffe, S. Trichomonas vaginalis infection and risk of prostate cancer: Associations by disease aggressiveness and race/ethnicity in the PLCO Trial. Cancer Causes Control 2017, 28, 889–898. [Google Scholar] [CrossRef]
- Tantengco, O.A.G.; Aquino, I.M.C.; de Castro Silva, M.; Rojo, R.D.; Abad, C.L.R. Association of mycoplasma with prostate cancer: A systematic review and meta-analysis. Cancer Epidemiol. 2021, 75, 102021. [Google Scholar] [CrossRef]
- Urbanek, C.; Goodison, S.; Chang, M.; Porvasnik, S.; Sakamoto, N.; Li, C.Z.; Boehlein, S.K.; Rosser, C.J. Detection of antibodies directed at M. hyorhinis p37 in the serum of men with newly diagnosed prostate cancer. BMC Cancer 2011, 11, 233. [Google Scholar] [CrossRef]
- Shrestha, E.; White, J.R.; Yu, S.H.; Kulac, I.; Ertunc, O.; De Marzo, A.M.; Yegnasubramanian, S.; Mangold, L.A.; Partin, A.W.; Sfanos, K.S. Profiling the Urinary Microbiome in Men with Positive versus Negative Biopsies for Prostate Cancer. J. Urol. 2018, 199, 161–171. [Google Scholar] [CrossRef]
- Abdul-Wahab, O.M.S.; Al-Shyarba, M.H.; Mardassi, B.B.A.; Sassi, N.; Al Fayi, M.S.S.; Otifi, H.; Al Murea, A.H.; Mlik, B.; Yacoub, E. Molecular detection of urogenital mollicutes in patients with invasive malignant prostate tumor. Infect. Agents Cancer 2021, 16, 6. [Google Scholar] [CrossRef]
- Gorish, B.M.T.; Ournasseir, M.E.H.; Shammat, I.M. A correlation study of BK Polyoma Virus infection and prostate Cancer among Sudanese patients—Immunofluorescence and molecular based case-control study. Infect. Agents Cancer 2019, 14, 25. [Google Scholar] [CrossRef]
- Dalianis, T.; Hirsch, H.H. Human polyomaviruses in disease and cancer. Virology 2013, 437, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Mischitelli, M.; Bellizzi, A.; Anzivino, E.; Rodio, D.M.; Sciarra, A.; Gentile, V.; Pietropaolo, V. Results, questions, perspectives of a study on human Polyomavirus BK and molecular actors in prostate cancer development. Cancer Genom. Proteom. 2015, 12, 57–65. [Google Scholar]
- Sutcliffe, S.; Viscidi, R.P.; Till, C.; Goodman, P.J.; Hoque, A.M.; Hsing, A.W.; Thompson, I.M.; Zenilman, J.M.; De Marzo, A.M.; Platz, E.A. Human papillomavirus types 16, 18, and 31 serostatus and prostate cancer risk in the Prostate Cancer Prevention Trial. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2010, 19, 614–618. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moghoofei, M.; Keshavarz, M.; Ghorbani, S.; Babaei, F.; Nahand, J.S.; Tavakoli, A.; Mortazavi, H.S.; Marjani, A.; Mostafaei, S.; Monavari, S.H. Association between human papillomavirus infection and prostate cancer: A global systematic review and meta-analysis. Asia-Pac. J. Clin. Oncol. 2019, 15, e59–e67. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.I.; Calogero, A.E.; Condorelli, R.A.; Scalia, G.; Morgia, G.; La Vignera, S. Human papillomavirus and risk of prostate cancer: A systematic review and meta-analysis. Aging Male Off. J. Int. Soc. Study Aging Male 2020, 23, 132–138. [Google Scholar] [CrossRef]
- Rosenblatt, K.A.; Carter, J.J.; Iwasaki, L.M.; Galloway, D.A.; Stanford, J.L. Serologic evidence of human papillomavirus 16 and 18 infections and risk of prostate cancer. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2003, 12, 763–768. [Google Scholar]
- Langston, M.E.; Pakpahan, R.; Nevin, R.L.; De Marzo, A.M.; Elliott, D.J.; Gaydos, C.A.; Isaacs, W.B.; Nelson, W.G.; Sokoll, L.J.; Zenilman, J.M.; et al. Sustained influence of infections on prostate-specific antigen concentration: An analysis of changes over 10 years of follow-up. Prostate 2018, 78, 1024–1034. [Google Scholar] [CrossRef]
- Sutcliffe, S.; Nevin, R.L.; Pakpahan, R.; Elliott, D.J.; Cole, S.R.; De Marzo, A.M.; Gaydos, C.A.; Isaacs, W.B.; Nelson, W.G.; Sokoll, L.J.; et al. Prostate involvement during sexually transmitted infections as measured by prostate-specific antigen concentration. Br. J. Cancer 2011, 105, 602–605. [Google Scholar] [CrossRef]
- De Marzo, A.M.; Platz, E.A.; Sutcliffe, S.; Xu, J.; Gronberg, H.; Drake, C.G.; Nakai, Y.; Isaacs, W.B.; Nelson, W.G. Inflammation in prostate carcinogenesis. Nat. Rev. Cancer 2007, 7, 256–269. [Google Scholar] [CrossRef]
- De Marzo, A.M.; Marchi, V.L.; Epstein, J.I.; Nelson, W.G. Proliferative inflammatory atrophy of the prostate: Implications for prostatic carcinogenesis. Am. J. Pathol. 1999, 155, 1985–1992. [Google Scholar] [CrossRef]
- Shrestha, E.; Coulter, J.B.; Guzman, W.; Ozbek, B.; Hess, M.M.; Mummert, L.; Ernst, S.E.; Maynard, J.P.; Meeker, A.K.; Heaphy, C.M.; et al. Oncogenic gene fusions in nonneoplastic precursors as evidence that bacterial infection can initiate prostate cancer. Proc. Natl. Acad. Sci. USA 2021, 118, e2018976118. [Google Scholar] [CrossRef] [PubMed]
- Bethel, C.R.; Faith, D.; Li, X.; Guan, B.; Hicks, J.L.; Lan, F.; Jenkins, R.B.; Bieberich, C.J.; De Marzo, A.M. Decreased NKX3.1 protein expression in focal prostatic atrophy, prostatic intraepithelial neoplasia, and adenocarcinoma: Association with gleason score and chromosome 8p deletion. Cancer Res. 2006, 66, 10683–10690. [Google Scholar] [CrossRef] [PubMed]
- Nickel, J.C.; True, L.D.; Krieger, J.N.; Berger, R.E.; Boag, A.H.; Young, I.D. Consensus development of a histopathological classification system for chronic prostatic inflammation. BJU Int. 2001, 87, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Langston, M.E.; Sfanos, K.S.; Khan, S.; Nguyen, T.Q.; De Marzo, A.M.; Platz, E.A.; Sutcliffe, S. Why Do Epidemiologic Studies Find an Inverse Association Between Intraprostatic Inflammation and Prostate Cancer: A Possible Role for Colliding Bias? Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2021, 30, 255–259. [Google Scholar] [CrossRef]
- Stoyanova, R.; Pollack, A.; Takhar, M.; Lynne, C.; Parra, N.; Lam, L.L.; Alshalalfa, M.; Buerki, C.; Castillo, R.; Jorda, M.; et al. Association of multiparametric MRI quantitative imaging features with prostate cancer gene expression in MRI-targeted prostate biopsies. Oncotarget 2016, 7, 53362–53376. [Google Scholar] [CrossRef]
- Pernar, C.H.; Ebot, E.M.; Pettersson, A.; Graff, R.E.; Giunchi, F.; Ahearn, T.U.; Gonzalez-Feliciano, A.G.; Markt, S.C.; Wilson, K.M.; Stopsack, K.H.; et al. A Prospective Study of the Association between Physical Activity and Risk of Prostate Cancer Defined by Clinical Features and TMPRSS2:ERG. Eur. Urol. 2019, 76, 33–40. [Google Scholar] [CrossRef]
- Pernar, C.H.; Parmigiani, G.; Giovannucci, E.L.; Rimm, E.B.; Tyekucheva, S.; Loda, M.; Finn, S.P.; Vander Heiden, M.G.; Fiorentino, M.; Ebot, E.M.; et al. Gene Expression Pathways in Prostate Tissue Associated with Vigorous Physical Activity in Prostate Cancer. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2021, 30, 751–756. [Google Scholar] [CrossRef]
- Fu, B.C.; Tabung, F.K.; Pernar, C.H.; Wang, W.; Gonzalez-Feliciano, A.G.; Chowdhury-Paulino, I.M.; Clinton, S.K.; Folefac, E.; Song, M.; Kibel, A.S.; et al. Insulinemic and Inflammatory Dietary Patterns and Risk of Prostate Cancer. Eur. Urol. 2021, 79, 405–412. [Google Scholar] [CrossRef]
- Aroke, D.; Folefac, E.; Shi, N.; Jin, Q.; Clinton, S.K.; Tabung, F.K. Inflammatory and Insulinemic Dietary Patterns: Influence on Circulating Biomarkers and Prostate Cancer Risk. Cancer Prev. Res. 2020, 13, 841–852. [Google Scholar] [CrossRef]
- Nash, S.H.; Schenk, J.M.; Kristal, A.R.; Goodman, P.J.; Lucia, M.S.; Parnes, H.L.; Thompson, I.M.; Lippman, S.M.; Song, X.; Gurel, B.; et al. Association between Serum Phospholipid Fatty Acids and Intraprostatic Inflammation in the Placebo Arm of the Prostate Cancer Prevention Trial. Cancer Prev. Res. 2015, 8, 590–596. [Google Scholar] [CrossRef]
- Moreira, D.M.; Nickel, J.C.; Gerber, L.; Muller, R.L.; Andriole, G.L.; Castro-Santamaria, R.; Freedland, S.J. Smoking Is Associated with Acute and Chronic Prostatic Inflammation: Results from the REDUCE Study. Cancer Prev. Res. 2015, 8, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Prueitt, R.L.; Wallace, T.A.; Glynn, S.A.; Yi, M.; Tang, W.; Luo, J.; Dorsey, T.H.; Stagliano, K.E.; Gillespie, J.W.; Hudson, R.S.; et al. An Immune-Inflammation Gene Expression Signature in Prostate Tumors of Smokers. Cancer Res. 2016, 76, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- Downer, M.K.; Allard, C.B.; Preston, M.A.; Wilson, K.M.; Kenfield, S.A.; Chan, J.M.; Mucci, L.A.; Giovannucci, E.; Stampfer, M.J. Aspirin Use and Lethal Prostate Cancer in the Health Professionals Follow-up Study. Eur. Urol. Oncol. 2019, 2, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, L.M.; Kulac, I.; Gumuskaya, B.; Valle, J.; Benedetti, I.; Pan, F.; Liu, J.O.; Marrone, M.T.; Arnold, K.B.; Goodman, P.J.; et al. Use of Aspirin and Statins in Relation to Inflammation in Benign Prostate Tissue in the Placebo Arm of the Prostate Cancer Prevention Trial. Cancer Prev. Res. 2020, 13, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Allott, E.H.; Howard, L.E.; Vidal, A.C.; Moreira, D.M.; Castro-Santamaria, R.; Andriole, G.L.; Freedland, S.J. Statin Use, Serum Lipids, and Prostate Inflammation in Men with a Negative Prostate Biopsy: Results from the REDUCE Trial. Cancer Prev. Res. 2017, 10, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Allott, E.H.; Ebot, E.M.; Stopsack, K.H.; Gonzalez-Feliciano, A.G.; Markt, S.C.; Wilson, K.M.; Ahearn, T.U.; Gerke, T.A.; Downer, M.K.; Rider, J.R.; et al. Statin Use Is Associated with Lower Risk of PTEN-Null and Lethal Prostate Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 1086–1093. [Google Scholar] [CrossRef]
- Harrison, S.; Tilling, K.; Turner, E.L.; Martin, R.M.; Lennon, R.; Lane, J.A.; Donovan, J.L.; Hamdy, F.C.; Neal, D.E.; Bosch, J.; et al. Systematic review and meta-analysis of the associations between body mass index, prostate cancer, advanced prostate cancer, and prostate-specific antigen. Cancer Causes Control 2020, 31, 431–449. [Google Scholar] [CrossRef]
- Jochems, S.H.J.; Stattin, P.; Häggström, C.; Järvholm, B.; Orho-Melander, M.; Wood, A.M.; Stocks, T. Height, body mass index and prostate cancer risk and mortality by way of detection and cancer risk category. Int. J. Cancer 2020, 147, 3328–3338. [Google Scholar] [CrossRef]
- Riviere, P.; Kumar, A.; Luterstein, E.; Vitzthum, L.K.; Nalawade, V.; Sarkar, R.R.; Bryant, A.K.; Einck, J.P.; Mundt, A.J.; Murphy, J.D.; et al. Tobacco smoking and death from prostate cancer in US veterans. Prostate Cancer Prostatic Dis. 2020, 23, 252–259. [Google Scholar] [CrossRef]
- Rohrmann, S.; Linseisen, J.; Allen, N.; Bueno-de-Mesquita, H.B.; Johnsen, N.F.; Tjønneland, A.; Overvad, K.; Kaaks, R.; Teucher, B.; Boeing, H.; et al. Smoking and the risk of prostate cancer in the European Prospective Investigation into Cancer and Nutrition. Br. J. Cancer 2013, 108, 708–714. [Google Scholar] [CrossRef]
- Binnewies, M.; Roberts, E.W.; Kersten, K.; Chan, V.; Fearon, D.F.; Merad, M.; Coussens, L.M.; Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Hedrick, C.C.; et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018, 24, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Davidsson, S.; Andren, O.; Ohlson, A.L.; Carlsson, J.; Andersson, S.O.; Giunchi, F.; Rider, J.R.; Fiorentino, M. FOXP3+ regulatory T cells in normal prostate tissue, postatrophic hyperplasia, prostatic intraepithelial neoplasia, and tumor histological lesions in men with and without prostate cancer. Prostate 2018, 78, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Davidsson, S.; Ohlson, A.L.; Andersson, S.O.; Fall, K.; Meisner, A.; Fiorentino, M.; Andrén, O.; Rider, J.R. CD4 helper T cells, CD8 cytotoxic T cells, and FOXP3+ regulatory T cells with respect to lethal prostate cancer. Mod. Pathol. 2013, 26, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Kanao, K.; Suzuki, S.; Muramatsu, H.; Morinaga, S.; Kajikawa, K.; Kobayashi, I.; Nishikawa, G.; Kato, Y.; Zennami, K.; et al. Increased infiltration of CCR4-positive regulatory T cells in prostate cancer tissue is associated with a poor prognosis. Prostate 2019, 79, 1658–1665. [Google Scholar] [CrossRef] [PubMed]
- Flammiger, A.; Weisbach, L.; Huland, H.; Tennstedt, P.; Simon, R.; Minner, S.; Bokemeyer, C.; Sauter, G.; Schlomm, T.; Trepel, M. High tissue density of FOXP3+ T cells is associated with clinical outcome in prostate cancer. Eur. J. Cancer 2013, 49, 1273–1279. [Google Scholar] [CrossRef]
- Zhang, B.W.; Li, G.; Ye, J.J.; Li, Z.R. Changes of CD4 + CD25 + Foxp3 + regulatory T cells in the peripheral blood and their correlation with insulin resistance in different stages of prostate cancer. Zhonghua Nan Ke Xue Natl. J. Androl. 2015, 21, 420–423. [Google Scholar]
- Weaver, C.T.; Harrington, L.E.; Mangan, P.R.; Gavrieli, M.; Murphy, K.M. Th17: An effector CD4 T cell lineage with regulatory T cell ties. Immunity 2006, 24, 677–688. [Google Scholar] [CrossRef]
- Sfanos, K.S.; Bruno, T.C.; Maris, C.H.; Xu, L.; Thoburn, C.J.; DeMarzo, A.M.; Meeker, A.K.; Isaacs, W.B.; Drake, C.G. Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2008, 14, 3254–3261. [Google Scholar] [CrossRef]
- Derhovanessian, E.; Adams, V.; Hähnel, K.; Groeger, A.; Pandha, H.; Ward, S.; Pawelec, G. Pretreatment frequency of circulating IL-17+ CD4+ T-cells, but not Tregs, correlates with clinical response to whole-cell vaccination in prostate cancer patients. Int. J. Cancer 2009, 125, 1372–1379. [Google Scholar] [CrossRef]
- Mangiola, S.; McCoy, P.; Modrak, M.; Souza-Fonseca-Guimaraes, F.; Blashki, D.; Stuchbery, R.; Keam, S.P.; Kerger, M.; Chow, K.; Nasa, C.; et al. Transcriptome sequencing and multi-plex imaging of prostate cancer microenvironment reveals a dominant role for monocytic cells in progression. BMC Cancer 2021, 21, 846. [Google Scholar] [CrossRef]
- Lundholm, M.; Hägglöf, C.; Wikberg, M.L.; Stattin, P.; Egevad, L.; Bergh, A.; Wikström, P.; Palmqvist, R.; Edin, S. Secreted Factors from Colorectal and Prostate Cancer Cells Skew the Immune Response in Opposite Directions. Sci. Rep. 2015, 5, 15651. [Google Scholar] [CrossRef] [PubMed]
- Erlandsson, A.; Carlsson, J.; Lundholm, M.; Fält, A.; Andersson, S.O.; Andrén, O.; Davidsson, S. M2 macrophages and regulatory T cells in lethal prostate cancer. Prostate 2019, 79, 363–369. [Google Scholar] [CrossRef]
- Zarif, J.C.; Baena-Del Valle, J.A.; Hicks, J.L.; Heaphy, C.M.; Vidal, I.; Luo, J.; Lotan, T.L.; Hooper, J.E.; Isaacs, W.B.; Pienta, K.J.; et al. Mannose Receptor-positive Macrophage Infiltration Correlates with Prostate Cancer Onset and Metastatic Castration-resistant Disease. Eur. Urol. Oncol. 2019, 2, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research, N. The Molecular Taxonomy of Primary Prostate Cancer. Cell 2015, 163, 1011–1025. [Google Scholar] [CrossRef]
- Mani, R.S.; Amin, M.A.; Li, X.; Kalyana-Sundaram, S.; Veeneman, B.A.; Wang, L.; Ghosh, A.; Aslam, A.; Ramanand, S.G.; Rabquer, B.J.; et al. Inflammation-Induced Oxidative Stress Mediates Gene Fusion Formation in Prostate Cancer. Cell Rep. 2016, 17, 2620–2631. [Google Scholar] [CrossRef]
- Rao, S.R.; Alham, N.K.; Upton, E.; McIntyre, S.; Bryant, R.J.; Cerundolo, L.; Bowes, E.; Jones, S.; Browne, M.; Mills, I.; et al. Detailed Molecular and Immune Marker Profiling of Archival Prostate Cancer Samples Reveals an Inverse Association between TMPRSS2:ERG Fusion Status and Immune Cell Infiltration. J. Mol. Diagn. JMD 2020, 22, 652–669. [Google Scholar] [CrossRef]
- Burdova, A.; Rulisek, P.; Bouchal, J.; Král, M.; Student, V.; Kolar, Z. Infiltration of Prostate Cancer by CD204+ and CD3+ Cells Correlates with ERG Expression and TMPRSS2-ERG Gene Fusion. Klin. Onkol. Cas. Ceske Slov. Onkol. Spol. 2018, 31, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.B.; Guedes, L.B.; Lu, J.; Maldonado, L.; Reitz, L.; Barber, J.R.; De Marzo, A.M.; Tosoian, J.J.; Tomlins, S.A.; Schaeffer, E.M.; et al. Association of tumor-infiltrating T-cell density with molecular subtype, racial ancestry and clinical outcomes in prostate cancer. Mod. Pathol. 2018, 31, 1539–1552. [Google Scholar] [CrossRef]
- Roudier, M.P.; Winters, B.R.; Coleman, I.; Lam, H.M.; Zhang, X.; Coleman, R.; Chéry, L.; True, L.D.; Higano, C.S.; Montgomery, B.; et al. Characterizing the molecular features of ERG-positive tumors in primary and castration resistant prostate cancer. Prostate 2016, 76, 810–822. [Google Scholar] [CrossRef]
- Imada, E.L.; Sanchez, D.F.; Dinalankara, W.; Vidotto, T.; Ebot, E.M.; Tyekucheva, S.; Franco, G.R.; Mucci, L.A.; Loda, M.; Schaeffer, E.M.; et al. Transcriptional landscape of PTEN loss in primary prostate cancer. BMC Cancer 2021, 21, 856. [Google Scholar] [CrossRef]
- Vidotto, T.; Saggioro, F.P.; Jamaspishvili, T.; Chesca, D.L.; Picanço de Albuquerque, C.G.; Reis, R.B.; Graham, C.H.; Berman, D.M.; Siemens, D.R.; Squire, J.A.; et al. PTEN-deficient prostate cancer is associated with an immunosuppressive tumor microenvironment mediated by increased expression of IDO1 and infiltrating FoxP3+ T regulatory cells. Prostate 2019, 79, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Jenzer, M.; Keß, P.; Nientiedt, C.; Endris, V.; Kippenberger, M.; Leichsenring, J.; Stögbauer, F.; Haimes, J.; Mishkin, S.; Kudlow, B.; et al. The BRCA2 mutation status shapes the immune phenotype of prostate cancer. Cancer Immunol. Immunother. 2019, 68, 1621–1633. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.B.; Lu, J.; Guedes, L.B.; Maldonado, L.; Reitz, L.; Barber, J.R.; De Marzo, A.M.; Tomlins, S.A.; Sfanos, K.S.; Eisenberger, M.; et al. TP53 missense mutation is associated with increased tumor-infiltrating T cells in primary prostate cancer. Hum. Pathol. 2019, 87, 95–102. [Google Scholar] [CrossRef]
- Zhang, J.; Bu, X.; Wang, H.; Zhu, Y.; Geng, Y.; Nihira, N.T.; Tan, Y.; Ci, Y.; Wu, F.; Dai, X.; et al. Cyclin D-CDK4 kinase destabilizes PD-L1 via cullin 3-SPOP to control cancer immune surveillance. Nature 2018, 553, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Abdul Sater, H.; Marté, J.L.; Donahue, R.N.; Walter-Rodriguez, B.; Heery, C.R.; Steinberg, S.M.; Cordes, L.M.; Chun, G.; Karzai, F.; Bilusic, M.; et al. Neoadjuvant PROSTVAC prior to radical prostatectomy enhances T-cell infiltration into the tumor immune microenvironment in men with prostate cancer. J. Immunother. Cancer 2020, 8, NCT02153918. [Google Scholar] [CrossRef]
- Acs, B.; Pelekanou, V.; Bai, Y.; Martinez-Morilla, S.; Toki, M.; Leung, S.C.Y.; Nielsen, T.O.; Rimm, D.L. Ki67 reproducibility using digital image analysis: An inter-platform and inter-operator study. Lab. Investig. J. Tech. Methods Pathol. 2019, 99, 107–117. [Google Scholar] [CrossRef]
- Saltz, J.; Gupta, R.; Hou, L.; Kurc, T.; Singh, P.; Nguyen, V.; Samaras, D.; Shroyer, K.R.; Zhao, T.; Batiste, R.; et al. Spatial Organization and Molecular Correlation of Tumor-Infiltrating Lymphocytes Using Deep Learning on Pathology Images. Cell Rep. 2018, 23, 181–193.e187. [Google Scholar] [CrossRef]
- Swiderska-Chadaj, Z.; Pinckaers, H.; van Rijthoven, M.; Balkenhol, M.; Melnikova, M.; Geessink, O.; Manson, Q.; Sherman, M.; Polonia, A.; Parry, J.; et al. Learning to detect lymphocytes in immunohistochemistry with deep learning. Med. Image Anal. 2019, 58, 101547. [Google Scholar] [CrossRef]
- Viratham Pulsawatdi, A.; Craig, S.G.; Bingham, V.; McCombe, K.; Humphries, M.P.; Senevirathne, S.; Richman, S.D.; Quirke, P.; Campo, L.; Domingo, E.; et al. A robust multiplex immunofluorescence and digital pathology workflow for the characterisation of the tumour immune microenvironment. Mol. Oncol. 2020, 14, 2384–2402. [Google Scholar] [CrossRef]
- Ozbek, B.; Ertunc, O.; Erickson, A.; Vidal, I.D.; Gomes-Alexandre, C.; Guner, G.; Hicks, J.L.; Jones, T.; Taube, J.M.; Sfanos, K.S.; et al. Multiplex immunohistochemical phenotyping of T cells in primary prostate cancer. Prostate 2022. [Google Scholar] [CrossRef]
- Norström, M.M.; Rådestad, E.; Stikvoort, A.; Egevad, L.; Bergqvist, M.; Henningsohn, L.; Mattsson, J.; Levitsky, V.; Uhlin, M. Novel method to characterize immune cells from human prostate tissue. Prostate 2014, 74, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- Nickols, N.G.; Ganapathy, E.; Nguyen, C.; Kane, N.; Lin, L.; Diaz-Perez, S.; Nazarian, R.; Mathis, C.; Felix, C.; Basehart, V.; et al. The intraprostatic immune environment after stereotactic body radiotherapy is dominated by myeloid cells. Prostate Cancer Prostatic Dis. 2021, 24, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, M.H.; Nolan, G.P. Mass Cytometry: Single Cells, Many Features. Cell 2016, 165, 780–791. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.J.; Navarro, H.I.; Hashimoto, T.; Garcia, A.J.; Goldstein, A.S. Mass cytometry reveals species-specific differences and a new level of complexity for immune cells in the prostate. Am. J. Clin. Exp. Urol. 2019, 7, 281–296. [Google Scholar] [PubMed]
- Aran, D.; Hu, Z.; Butte, A.J. xCell: Digitally portraying the tissue cellular heterogeneity landscape. Genome Biol. 2017, 18, 220. [Google Scholar] [CrossRef]
- Becht, E.; Giraldo, N.A.; Lacroix, L.; Buttard, B.; Elarouci, N.; Petitprez, F.; Selves, J.; Laurent-Puig, P.; Sautès-Fridman, C.; Fridman, W.H.; et al. Estimating the population abundance of tissue-infiltrating immune and stromal cell populations using gene expression. Genome Biol. 2016, 17, 218. [Google Scholar] [CrossRef]
- Chen, B.; Khodadoust, M.S.; Liu, C.L.; Newman, A.M.; Alizadeh, A.A. Profiling Tumor Infiltrating Immune Cells with CIBERSORT. Methods Mol. Biol. 2018, 1711, 243–259. [Google Scholar] [CrossRef]
- Li, B.; Li, T.; Liu, J.S.; Liu, X.S. Computational Deconvolution of Tumor-Infiltrating Immune Components with Bulk Tumor Gene Expression Data. Methods Mol. Biol. 2020, 2120, 249–262. [Google Scholar] [CrossRef]
- Brady, L.; Kriner, M.; Coleman, I.; Morrissey, C.; Roudier, M.; True, L.D.; Gulati, R.; Plymate, S.R.; Zhou, Z.; Birditt, B.; et al. Inter- and intra-tumor heterogeneity of metastatic prostate cancer determined by digital spatial gene expression profiling. Nat. Commun. 2021, 12, 1426. [Google Scholar] [CrossRef]
- Meng, J.; Zhou, Y.; Lu, X.; Bian, Z.; Chen, Y.; Zhou, J.; Zhang, L.; Hao, Z.; Zhang, M.; Liang, C. Immune response drives outcomes in prostate cancer: Implications for immunotherapy. Mol. Oncol. 2020, 15, 1358–1375. [Google Scholar] [CrossRef]
- Liu, Z.; Zhong, J.; Cai, C.; Lu, J.; Wu, W.; Zeng, G. Immune-related biomarker risk score predicts prognosis in prostate cancer. Aging 2020, 12, 22776–22793. [Google Scholar] [CrossRef] [PubMed]
- Jairath, N.K.; Farha, M.W.; Srinivasan, S.; Jairath, R.; Green, M.D.; Dess, R.T.; Jackson, W.C.; Weiner, A.B.; Schaeffer, E.M.; Zhao, S.G.; et al. Tumor Immune Microenvironment Clusters in Localized Prostate Adenocarcinoma: Prognostic Impact of Macrophage Enriched/Plasma Cell Non-Enriched Subtypes. J. Clin. Med. 2020, 9, 1973. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.G.; Lehrer, J.; Chang, S.L.; Das, R.; Erho, N.; Liu, Y.; Sjöström, M.; Den, R.B.; Freedland, S.J.; Klein, E.A.; et al. The Immune Landscape of Prostate Cancer and Nomination of PD-L2 as a Potential Therapeutic Target. J. Natl. Cancer Inst. 2019, 111, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.R.; Izar, B.; Wang, S.; Yapp, C.; Mei, S.; Shah, P.M.; Santagata, S.; Sorger, P.K. Highly multiplexed immunofluorescence imaging of human tissues and tumors using t-CyCIF and conventional optical microscopes. eLife 2018, 7, e31657. [Google Scholar] [CrossRef]
- Liechti, T.; Roederer, M. OMIP-051—28-color flow cytometry panel to characterize B cells and myeloid cells. Cytom. A 2019, 95, 150–155. [Google Scholar] [CrossRef]
- Brodie, T.M.; Tosevski, V.; Medová, M. OMIP-045: Characterizing human head and neck tumors and cancer cell lines with mass cytometry. Cytom. A 2018, 93, 406–410. [Google Scholar] [CrossRef]
- Taube, J.M.; Akturk, G.; Angelo, M.; Engle, E.L.; Gnjatic, S.; Greenbaum, S.; Greenwald, N.F.; Hedvat, C.V.; Hollmann, T.J.; Juco, J.; et al. The Society for Immunotherapy of Cancer statement on best practices for multiplex immunohistochemistry (IHC) and immunofluorescence (IF) staining and validation. J. Immunother. Cancer 2020, 8, e000155. [Google Scholar] [CrossRef]
- Kantoff, P.W.; Higano, C.S.; Shore, N.D.; Berger, E.R.; Small, E.J.; Penson, D.F.; Redfern, C.H.; Ferrari, A.C.; Dreicer, R.; Sims, R.B.; et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 2010, 363, 411–422. [Google Scholar] [CrossRef]
- Hansen, A.R.; Massard, C.; Ott, P.A.; Haas, N.B.; Lopez, J.S.; Ejadi, S.; Wallmark, J.M.; Keam, B.; Delord, J.P.; Aggarwal, R.; et al. Pembrolizumab for advanced prostate adenocarcinoma: Findings of the KEYNOTE-028 study. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2018, 29, 1807–1813. [Google Scholar] [CrossRef]
- Obradovic, A.Z.; Dallos, M.C.; Zahurak, M.L.; Partin, A.W.; Schaeffer, E.M.; Ross, A.E.; Allaf, M.E.; Nirschl, T.R.; Liu, D.; Chapman, C.G.; et al. T-Cell Infiltration and Adaptive Treg Resistance in Response to Androgen Deprivation With or Without Vaccination in Localized Prostate Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 3182–3192. [Google Scholar] [CrossRef]
- Ruiz de Porras, V.; Pardo, J.C.; Notario, L.; Etxaniz, O.; Font, A. Immune Checkpoint Inhibitors: A Promising Treatment Option for Metastatic Castration-Resistant Prostate Cancer? Int. J. Mol. Sci. 2021, 22, 4712. [Google Scholar] [CrossRef] [PubMed]
- Thompson, I., Jr.; Kristal, A.; Platz, E.A. Prevention of prostate cancer: Outcomes of clinical trials and future opportunities. Am. Soc. Clin. Oncol. Educ. Book 2014, 34, e76–e80. [Google Scholar] [CrossRef] [PubMed]
- Hojan, K.; Kwiatkowska-Borowczyk, E.; Leporowska, E.; Milecki, P. Inflammation, cardiometabolic markers, and functional changes in men with prostate cancer. A randomized controlled trial of a 12-month exercise program. Pol. Arch. Intern. Med. 2017, 127, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Schenk, A.; Esser, T.; Knoop, A.; Thevis, M.; Herden, J.; Heidenreich, A.; Bloch, W.; Joisten, N.; Zimmer, P. Effect of a Single Bout of Aerobic Exercise on Kynurenine Pathway Metabolites and Inflammatory Markers in Prostate Cancer Patients-A Pilot Randomized Controlled Trial. Metabolites 2020, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Hanson, E.D.; Sakkal, S.; Que, S.; Cho, E.; Spielmann, G.; Kadife, E.; Violet, J.A.; Battaglini, C.L.; Stoner, L.; Bartlett, D.B.; et al. Natural killer cell mobilization and egress following acute exercise in men with prostate cancer. Exp. Physiol. 2020, 105, 1524–1539. [Google Scholar] [CrossRef]
- Penedo, F.J.; Fox, R.S.; Walsh, E.A.; Yanez, B.; Miller, G.E.; Oswald, L.B.; Estabrook, R.; Chatterton, R.T.; Mohr, D.C.; Begale, M.J.; et al. Effects of web-based cognitive behavioral stress management and health promotion interventions on neuroendocrine and inflammatory markers in men with advanced prostate cancer: A randomized controlled trial. Brain Behav. Immun. 2021, 95, 168–177. [Google Scholar] [CrossRef]
- Kaushik, D.; Shah, P.K.; Mukherjee, N.; Ji, N.; Dursun, F.; Kumar, A.P.; Thompson, I.M., Jr.; Mansour, A.M.; Jha, R.; Yang, X.; et al. Effects of yoga in men with prostate cancer on quality of life and immune response: A pilot randomized controlled trial. Prostate Cancer Prostatic Dis. 2021. [Google Scholar] [CrossRef]
- Heymach, J.V.; Shackleford, T.J.; Tran, H.T.; Yoo, S.Y.; Do, K.A.; Wergin, M.; Saintigny, P.; Vollmer, R.T.; Polascik, T.J.; Snyder, D.C.; et al. Effect of low-fat diets on plasma levels of NF-κB-regulated inflammatory cytokines and angiogenic factors in men with prostate cancer. Cancer Prev. Res. 2011, 4, 1590–1598. [Google Scholar] [CrossRef]
- Traka, M.H.; Melchini, A.; Coode-Bate, J.; Al Kadhi, O.; Saha, S.; Defernez, M.; Troncoso-Rey, P.; Kibblewhite, H.; O’Neill, C.M.; Bernuzzi, F.; et al. Transcriptional changes in prostate of men on active surveillance after a 12-mo glucoraphanin-rich broccoli intervention-results from the Effect of Sulforaphane on prostate CAncer PrEvention (ESCAPE) randomized controlled trial. Am. J. Clin. Nutr. 2019, 109, 1133–1144. [Google Scholar] [CrossRef]
- Murtola, T.J.; Syvälä, H.; Tolonen, T.; Helminen, M.; Riikonen, J.; Koskimäki, J.; Pakarainen, T.; Kaipia, A.; Isotalo, T.; Kujala, P.; et al. Atorvastatin Versus Placebo for Prostate Cancer Before Radical Prostatectomy-A Randomized, Double-blind, Placebo-controlled Clinical Trial. Eur. Urol. 2018, 74, 697–701. [Google Scholar] [CrossRef]
| Lifestyle Factor | Assessment | HR (95% CI) | Study Design | Author (Year) | |
|---|---|---|---|---|---|
| Advanced Prostate Cancer | Lethal Prostate Cancer | ||||
| Vigorous exercise | Men in the highest quintile of vigorous activity compared to the lowest quintile | 0.70 (0.53–0.92) | 0.75 (0.59–0.94) | Prospective cohort | Pernar (2019) [46] |
| Obesity | Each 5 kg/m2 increase in BMI | 1.06 (1.01–1.12) | NA | Meta-analysis | Harrison (2020) [57] |
| NA | 1.13 (1.08–1.20) | Meta-analysis | Jochems (2020) [58] | ||
| Inflammatory diet | Each SD increase in inflammatory diet score among men under 65 yrs of age | 1.13 (0.99–1.28) | 1.16 (1.00–1.35) | Prospective cohort | Fu (2021) [48] |
| Cigarette smoking | Current smoking compared to never smoked | NA | 1.14 (1.05–1.24) | Retrospective cohort | Riviere (2020) [59] |
| 1.05 (0.87–1.27) | 1.27 (0.98–1.65) | Prospective cohort | Rohrmann (2013) [60] | ||
| Aspirin | Current aspirin use compared to never used | 1.16 (0.96–1.41) | 0.80 (0.66–0.96) | Prospective cohort | Downer (2019) [53] |
| Statin | Current statin use compared to never/past used | 0.98 (0.73–1.31) | 0.76 (0.60–0.96) | Prospective cohort | Allott (2020) [56] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.; LaBonte, M.J.; Craig, S.G.; Finn, S.P.; Allott, E.H. Inflammation and Prostate Cancer: A Multidisciplinary Approach to Identifying Opportunities for Treatment and Prevention. Cancers 2022, 14, 1367. https://doi.org/10.3390/cancers14061367
Huang L, LaBonte MJ, Craig SG, Finn SP, Allott EH. Inflammation and Prostate Cancer: A Multidisciplinary Approach to Identifying Opportunities for Treatment and Prevention. Cancers. 2022; 14(6):1367. https://doi.org/10.3390/cancers14061367
Chicago/Turabian StyleHuang, Lanshan, Melissa J. LaBonte, Stephanie G. Craig, Stephen P. Finn, and Emma H. Allott. 2022. "Inflammation and Prostate Cancer: A Multidisciplinary Approach to Identifying Opportunities for Treatment and Prevention" Cancers 14, no. 6: 1367. https://doi.org/10.3390/cancers14061367
APA StyleHuang, L., LaBonte, M. J., Craig, S. G., Finn, S. P., & Allott, E. H. (2022). Inflammation and Prostate Cancer: A Multidisciplinary Approach to Identifying Opportunities for Treatment and Prevention. Cancers, 14(6), 1367. https://doi.org/10.3390/cancers14061367







