Ipilimumab, Pembrolizumab, or Nivolumab in Combination with BBI608 in Patients with Advanced Cancers Treated at MD Anderson Cancer Center
Abstract
:Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Treatment
2.2. Study Design
2.3. Pharmacokinetic Studies
2.4. Study Endpoints and Statistical Analysis
3. Results
3.1. Patients
3.2. Treatment
3.3. Safety
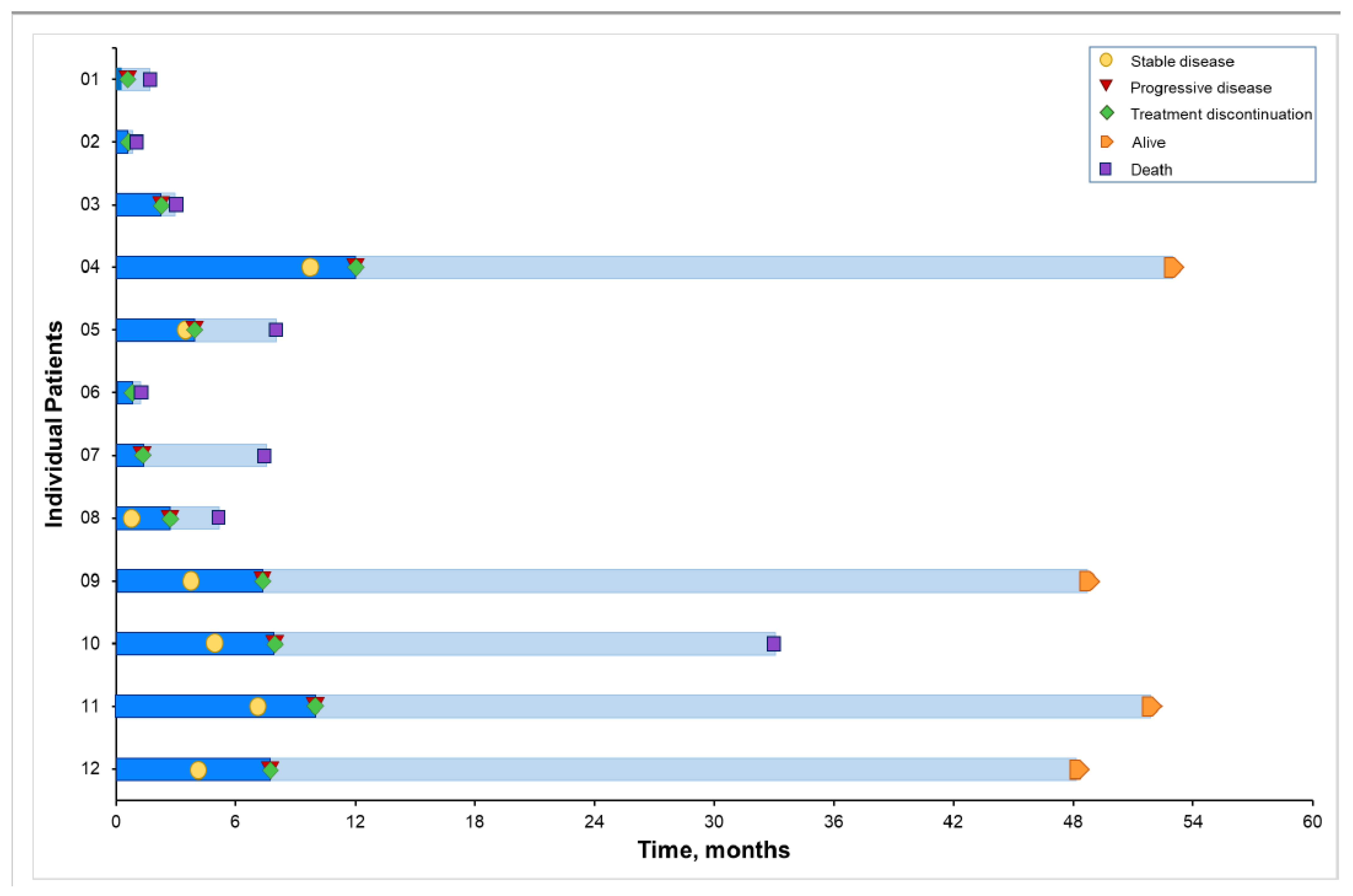
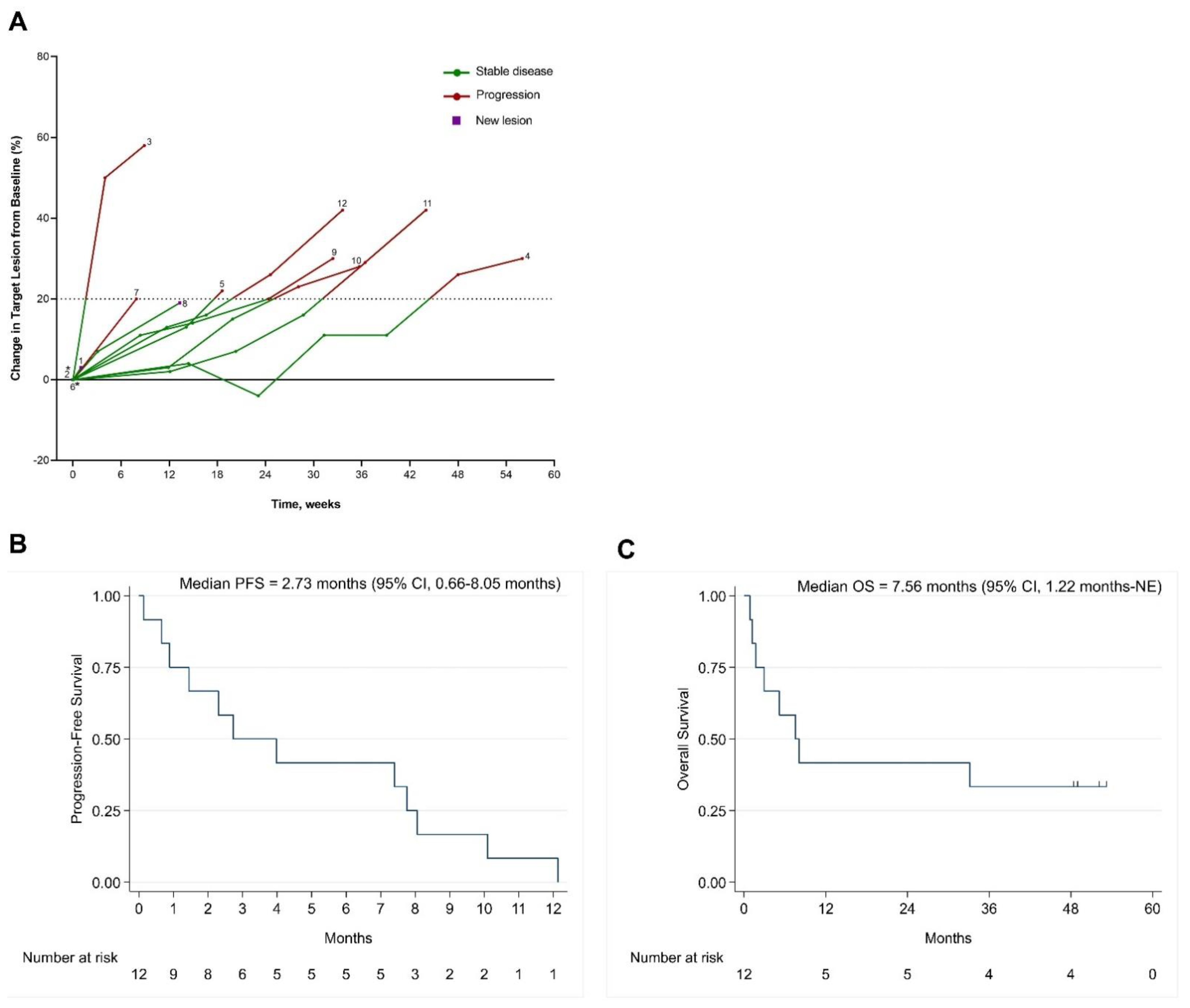
3.4. Clinical Outcomes
3.5. PFS with BBI608 and Immunotherapy Compared with PFS of Previous Systemic Therapy
3.6. Pharmacokinetic Studies
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Froeling, F.E.M.; Swamynathan, M.M.; Deschenes, A.; Chio, I.I.C.; Brosnan, E.; Yao, M.A.; Alagesan, P.; Lucito, M.; Li, J.; Chang, A.Y.; et al. Bioactivation of Napabucasin Triggers Reactive Oxygen Species-Mediated Cancer Cell Death. Clin. Cancer Res. 2019, 25, 7162–7174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, A.Y.; Hsu, E.; Patel, J.; Li, Y.; Zhang, M.; Iguchi, H.; Rogoff, H.A. Evaluation of Tumor Cell-Tumor Microenvironment Component Interactions as Potential Predictors of Patient Response to Napabucasin. Mol. Cancer Res. 2019, 17, 1429–1434. [Google Scholar] [CrossRef] [Green Version]
- Marotta, L.L.; Almendro, V.; Marusyk, A.; Shipitsin, M.; Schemme, J.; Walker, S.R.; Bloushtain-Qimron, N.; Kim, J.J.; Choudhury, S.A.; Maruyama, R.; et al. The JAK2/STAT3 signaling pathway is required for growth of CD44(+)CD24(−) stem cell-like breast cancer cells in human tumors. J. Clin. Investig. 2011, 121, 2723–2735. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, A.; Herrmann, A.; Cherryholmes, G.; Kowolik, C.; Buettner, R.; Pal, S.; Yu, H.; Muller-Newen, G.; Jove, R. Loss of androgen receptor expression promotes a stem-like cell phenotype in prostate cancer through STAT3 signaling. Cancer Res. 2014, 74, 1227–1237. [Google Scholar] [CrossRef] [Green Version]
- Moon, S.H.; Kim, D.K.; Cha, Y.; Jeon, I.; Song, J.; Park, K.S. PI3K/Akt and Stat3 signaling regulated by PTEN control of the cancer stem cell population, proliferation and senescence in a glioblastoma cell line. Int. J. Oncol. 2013, 42, 921–928. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Fahrmann, J.F.; Lee, H.; Li, Y.J.; Tripathi, S.C.; Yue, C.; Zhang, C.; Lifshitz, V.; Song, J.; Yuan, Y.; et al. JAK/STAT3-Regulated Fatty Acid beta-Oxidation Is Critical for Breast Cancer Stem Cell Self-Renewal and Chemoresistance. Cell Metab. 2018, 27, 136–150 e135. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Lee, H.; Herrmann, A.; Buettner, R.; Jove, R. Revisiting STAT3 signalling in cancer: New and unexpected biological functions. Nat. Rev. Cancer 2014, 14, 736–746. [Google Scholar] [CrossRef]
- Atsaves, V.; Tsesmetzis, N.; Chioureas, D.; Kis, L.; Leventaki, V.; Drakos, E.; Panaretakis, T.; Grander, D.; Medeiros, L.J.; Young, K.H.; et al. PD-L1 is commonly expressed and transcriptionally regulated by STAT3 and MYC in ALK-negative anaplastic large-cell lymphoma. Leukemia 2017, 31, 1633–1637. [Google Scholar] [CrossRef]
- Song, T.L.; Nairismagi, M.L.; Laurensia, Y.; Lim, J.Q.; Tan, J.; Li, Z.M.; Pang, W.L.; Kizhakeyil, A.; Wijaya, G.C.; Huang, D.C.; et al. Oncogenic activation of the STAT3 pathway drives PD-L1 expression in natural killer/T-cell lymphoma. Blood 2018, 132, 1146–1158. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Diaz, A.; Shin, D.S.; Moreno, B.H.; Saco, J.; Escuin-Ordinas, H.; Rodriguez, G.A.; Zaretsky, J.M.; Sun, L.; Hugo, W.; Wang, X.; et al. Interferon Receptor Signaling Pathways Regulating PD-L1 and PD-L2 Expression. Cell Rep. 2017, 19, 1189–1201. [Google Scholar] [CrossRef] [Green Version]
- Yoyen-Ermis, D.; Tunali, G.; Tavukcuoglu, E.; Horzum, U.; Ozkazanc, D.; Sutlu, T.; Buyukasik, Y.; Esendagli, G. Myeloid maturation potentiates STAT3-mediated atypical IFN-gamma signaling and upregulation of PD-1 ligands in AML and MDS. Sci. Rep. 2019, 9, 11697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrmann, A.; Lahtz, C.; Nagao, T.; Song, J.Y.; Chan, W.C.; Lee, H.; Yue, C.; Look, T.; Mulfarth, R.; Li, W.; et al. CTLA4 Promotes Tyk2-STAT3-Dependent B-cell Oncogenicity. Cancer Res. 2017, 77, 5118–5128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, P.; Santner-Nanan, B.; Hu, M.; Skarratt, K.; Lee, C.H.; Stormon, M.; Wong, M.; Fuller, S.J.; Nanan, R. IL-10 Potentiates Differentiation of Human Induced Regulatory T Cells via STAT3 and Foxo1. J. Immunol. 2015, 195, 3665–3674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Rogoff, H.A.; Keates, S.; Gao, Y.; Murikipudi, S.; Mikule, K.; Leggett, D.; Li, W.; Pardee, A.B.; Li, C.J. Suppression of cancer relapse and metastasis by inhibiting cancer stemness. Proc. Natl. Acad. Sci. USA 2015, 112, 1839–1844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanmamed, M.F.; Chen, L. A Paradigm Shift in Cancer Immunotherapy: From Enhancement to Normalization. Cell 2018, 175, 313–326. [Google Scholar] [CrossRef] [Green Version]
- Sondak, V.K.; Smalley, K.S.; Kudchadkar, R.; Grippon, S.; Kirkpatrick, P. Ipilimumab. Nat. Rev. Drug Discov. 2011, 10, 411–412. [Google Scholar] [CrossRef]
- Prasad, V.; Kaestner, V. Nivolumab and pembrolizumab: Monoclonal antibodies against programmed cell death-1 (PD-1) that are interchangeable. Semin. Oncol. 2017, 44, 132–135. [Google Scholar] [CrossRef]
- Ramos-Casals, M.; Brahmer, J.R.; Callahan, M.K.; Flores-Chavez, A.; Keegan, N.; Khamashta, M.A.; Lambotte, O.; Mariette, X.; Prat, A.; Suarez-Almazor, M.E. Immune-related adverse events of checkpoint inhibitors. Nat. Rev. Dis. Primers 2020, 6, 38. [Google Scholar] [CrossRef]
- Fountzilas, E.; Kurzrock, R.; Hiep Vo, H.; Tsimberidou, A.M. Wedding of Molecular Alterations and Immune Checkpoint Blockade: Genomics as a Matchmaker. J. Natl. Cancer Inst. 2021, 113, 1634–1647. [Google Scholar] [CrossRef]
- Emens, L.A.; Ascierto, P.A.; Darcy, P.K.; Demaria, S.; Eggermont, A.M.M.; Redmond, W.L.; Seliger, B.; Marincola, F.M. Cancer immunotherapy: Opportunities and challenges in the rapidly evolving clinical landscape. Eur. J. Cancer 2017, 81, 116–129. [Google Scholar] [CrossRef]
- Champiat, S.; Dercle, L.; Ammari, S.; Massard, C.; Hollebecque, A.; Postel-Vinay, S.; Chaput, N.; Eggermont, A.; Marabelle, A.; Soria, J.C.; et al. Hyperprogressive Disease Is a New Pattern of Progression in Cancer Patients Treated by Anti-PD-1/PD-L1. Clin. Cancer Res. 2017, 23, 1920–1928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, S.; Goodman, A.; Walavalkar, V.; Barkauskas, D.A.; Sharabi, A.; Kurzrock, R. Hyperprogressors after Immunotherapy: Analysis of Genomic Alterations Associated with Accelerated Growth Rate. Clin. Cancer Res. 2017, 23, 4242–4250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adashek, J.J.; Kato, S.; Ferrara, R.; Lo Russo, G.; Kurzrock, R. Hyperprogression and Immune Checkpoint Inhibitors: Hype or Progress? Oncologist 2019, 25, 94–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hubbard, J.M.; Grothey, A. Napabucasin: An Update on the First-in-Class Cancer Stemness Inhibitor. Drugs 2017, 77, 1091–1103. [Google Scholar] [CrossRef]
- MacDonagh, L.; Gray, S.G.; Breen, E.; Cuffe, S.; Finn, S.P.; O’Byrne, K.J.; Barr, M.P. BBI608 inhibits cancer stemness and reverses cisplatin resistance in NSCLC. Cancer Lett. 2018, 428, 117–126. [Google Scholar] [CrossRef]
- Kawazoe, A.; Kuboki, Y.; Bando, H.; Fukuoka, S.; Kojima, T.; Naito, Y.; Iino, S.; Yodo, Y.; Doi, T.; Shitara, K.; et al. Phase 1 study of napabucasin, a cancer stemness inhibitor, in patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2020, 85, 855–862. [Google Scholar] [CrossRef] [Green Version]
- Tsimberidou, A.M.; Wen, S.; Hong, D.S.; Wheler, J.J.; Falchook, G.S.; Fu, S.; Piha-Paul, S.; Naing, A.; Janku, F.; Aldape, K.; et al. Personalized medicine for patients with advanced cancer in the phase I program at MD Anderson: Validation and landmark analyses. Clin. Cancer Res. 2014, 20, 4827–4836. [Google Scholar] [CrossRef] [Green Version]
- Tsimberidou, A.M.; Hong, D.S.; Ye, Y.; Cartwright, C.; Wheler, J.J.; Falchook, G.S.; Naing, A.; Fu, S.; Piha-Paul, S.; Janku, F.; et al. Initiative for Molecular Profiling and Advanced Cancer Therapy (IMPACT): An MD Anderson Precision Medicine Study. JCO Precis. Oncol. 2017, 1, 1–18. [Google Scholar] [CrossRef]
- Tsimberidou, A.M.; Hong, D.S.; Wheler, J.J.; Falchook, G.S.; Janku, F.; Naing, A.; Fu, S.; Piha-Paul, S.; Cartwright, C.; Broaddus, R.R.; et al. Long-term overall survival and prognostic score predicting survival: The IMPACT study in precision medicine. J. Hematol. Oncol. 2019, 12, 145. [Google Scholar] [CrossRef] [Green Version]
- Tsimberidou, A.M.; Hong, D.S.; Fu, S.; Karp, D.D.; Piha-Paul, S.; Kies, M.S.; Ravi, V.; Subbiah, V.; Patel, S.M.; Tu, S.M.; et al. Precision medicine: Preliminary results from the Initiative for Molecular Profiling and Advanced Cancer Therapy 2 (IMPACT2) study. NPJ Precis. Oncol. 2021, 5, 21. [Google Scholar] [CrossRef]
- Seymour, L.; Bogaerts, J.; Perrone, A.; Ford, R.; Schwartz, L.H.; Mandrekar, S.; Lin, N.U.; Litière, S.; Dancey, J.; Chen, A.; et al. iRECIST: Guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017, 18, e143–e152. [Google Scholar] [CrossRef] [Green Version]
- Nishino, M.; Tirumani, S.H.; Ramaiya, N.H.; Hodi, F.S. Cancer immunotherapy and immune-related response assessment: The role of radiologists in the new arena of cancer treatment. Eur. J. Radiol. 2015, 84, 1259–1268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, L.J.; Lin, D.Y.; Weissfeld, L. Regression Analysis of Multivariate Incomplete Failure Time Data by Modeling Marginal Distributions. J. Am. Stat. Assoc. 1989, 84, 1065–1073. [Google Scholar] [CrossRef]
- Tsimberidou, A.M.; Iskander, N.G.; Hong, D.S.; Wheler, J.J.; Falchook, G.S.; Fu, S.; Piha-Paul, S.; Naing, A.; Janku, F.; Luthra, R.; et al. Personalized medicine in a phase I clinical trials program: The MD Anderson Cancer Center initiative. Clin. Cancer Res. 2012, 18, 6373–6383. [Google Scholar] [CrossRef] [Green Version]
- Jonker, D.J.; Stephenson, J.; Edenfield, W.J.; Supko, J.G.; Li, Y.; Li, W.; Hitron, M.; Leggett, D.; Kerstein, D.; Li, C. A phase I extension study of BBI608, a first-in-class cancer stem cell (CSC) inhibitor, in patients with advanced solid tumors. J. Clin. Oncol. 2014, 32, 2546. [Google Scholar] [CrossRef]
- Langleben, A.; Supko, J.G.; Hotte, S.J.; Batist, G.; Hirte, H.W.; Rogoff, H.; Li, Y.; Li, W.; Kerstein, D.; Leggett, D.; et al. A dose-escalation phase I study of a first-in-class cancer stemness inhibitor in patients with advanced malignancies. J. Clin. Oncol. 2013, 31, 2542. [Google Scholar] [CrossRef]
- O’Donnell, P.H.; Dolan, M.E. Cancer pharmacoethnicity: Ethnic differences in susceptibility to the effects of chemotherapy. Clin. Cancer Res. 2009, 15, 4806–4814. [Google Scholar] [CrossRef] [Green Version]
- Jonker, D.J.; Nott, L.; Yoshino, T.; Gill, S.; Shapiro, J.; Ohtsu, A.; Zalcberg, J.; Vickers, M.M.; Wei, A.C.; Gao, Y.; et al. Napabucasin versus placebo in refractory advanced colorectal cancer: A randomised phase 3 trial. Lancet. Gastroenterol. Hepatol. 2018, 3, 263–270. [Google Scholar] [CrossRef]
- Lipson, E.J.; Sharfman, W.H.; Drake, C.G.; Wollner, I.; Taube, J.M.; Anders, R.A.; Xu, H.; Yao, S.; Pons, A.; Chen, L.; et al. Durable cancer regression off-treatment and effective reinduction therapy with an anti-PD-1 antibody. Clin. Cancer Res. 2013, 19, 462–468. [Google Scholar] [CrossRef] [Green Version]
- Queirolo, P.; Spagnolo, F. Atypical responses in patients with advanced melanoma, lung cancer, renal-cell carcinoma and other solid tumors treated with anti-PD-1 drugs: A systematic review. Cancer Treat. Rev. 2017, 59, 71–78. [Google Scholar] [CrossRef]
- Gyawali, B.; Hey, S.P.; Kesselheim, A.S. A Comparison of Response Patterns for Progression-Free Survival and Overall Survival Following Treatment for Cancer with PD-1 Inhibitors: A Meta-analysis of Correlation and Differences in Effect Sizes. JAMA Netw. Open 2018, 1, e180416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patnaik, A.; Kang, S.P.; Rasco, D.; Papadopoulos, K.P.; Elassaiss-Schaap, J.; Beeram, M.; Drengler, R.; Chen, C.; Smith, L.; Espino, G.; et al. Phase I Study of Pembrolizumab (MK-3475; Anti-PD-1 Monoclonal Antibody) in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2015, 21, 4286–4293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brahmer, J.R.; Drake, C.G.; Wollner, I.; Powderly, J.D.; Picus, J.; Sharfman, W.H.; Stankevich, E.; Pons, A.; Salay, T.M.; McMiller, T.L.; et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: Safety, clinical activity, pharmacodynamics, and immunologic correlates. J. Clin. Oncol. 2010, 28, 3167–3175. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Highlights of Prescribing Information. YERVOY (Ipilimumab) Injection, for Intravenous Use. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125377s115lbl.pdf. (accessed on 28 October 2021).
- Merchant, M.S.; Wright, M.; Baird, K.; Wexler, L.H.; Rodriguez-Galindo, C.; Bernstein, D.; Delbrook, C.; Lodish, M.; Bishop, R.; Wolchok, J.D.; et al. Phase I Clinical Trial of Ipilimumab in Pediatric Patients with Advanced Solid Tumors. Clin. Cancer Res. 2016, 22, 1364–1370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawazoe, A.; Kuboki, Y.; Shinozaki, E.; Hara, H.; Nishina, T.; Komatsu, Y.; Yuki, S.; Wakabayashi, M.; Nomura, S.; Sato, A.; et al. Multicenter Phase I/II Trial of Napabucasin and Pembrolizumab in Patients with Metastatic Colorectal Cancer (EPOC1503/SCOOP Trial). Clin. Cancer Res. 2020, 26, 5887–5894. [Google Scholar] [CrossRef]

| BBI608 | BBI608, mg | Ipilimumab, 3 mg/kg | Nivolumab, 3 mg/kg | Pembrolizumab, 2 mg/kg |
|---|---|---|---|---|
| (1C = 21D) | (1C = 28D) | (1C = 21D) | ||
| Twice daily | D1 | D1, 15 | D1 | |
| Dose level | ||||
| 1 | 240 | |||
| 2 | 480 |
| Pt. ID | Age, Yrs | Sex | Tumor Dx | ECOG PS | No. of Prior Rx | Prior Rx | No. of Metastatic sites | Metastatic Sites | Liver Metastasis | PLT, ×109/L | LDH, U/L | Alb., g/dL | Cr, mg/dL | ALT/AST, U/L |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 01 | Late 60s | F | Neuroendocrine carcinoma of the small bowel | 1 | 2 | Carboplatin-etoposide; topotecan | 1 | Liver | Yes | 201 | 501 | 3.7 | 0.87 | 32/34 |
| 02 | Late 70s | M | Mesothelioma of lung | 1 | 1 | Pemetrexed-carboplatin | 1 | Lung | No | 219 | 383 | 3.3 | 0.95 | 36/23 |
| 03 | Early 60s | M | Adenocarcinoma of the esophagus | 1 | 4 | Cisplatin-5-fluorouracil; docetaxel-oxaliplatin-5-fluorouracil; modified leucovorin calcium (calcium folinate)-5-fluorouracil-irinotecan; paclitaxel-ramucirumab; radiation | 6 | Left adrenal, peritoneum/retroperitoneum, pancreas, lymph nodes, abdominal wall musculature and bones, paraspinal | No | 164 | 486 | 3.9 | 0.8 | 20/22 |
| 04 | Early 50s | F | Adenoid cystic carcinoma of the Bartholin gland | 1 | 3 | Adjuvant pelvic radiation; vaginal brachytherapy; vulvar radiation. | 6 | Peritoneum, retroperitoneum, lung, lymph node, liver, spleen | No | 225 | 409 | 4.1 | 0.62 | 46/33 |
| 05 | Early 50s | M | Squamous cell carcinoma of the right anterior tongue and floor of the mouth | 1 | 4 | Docetaxel-carboplatin-5-fluorouracil; chemo-radiation with carboplatin; docetaxel, cisplatin/carboplatin +/− erlotinib; investigational pan-fibroblast growth factor receptor [FGFR] kinase inhibitor | Right perihilar mass | No | 153 | 518 | 4 | 0.81 | 24/25 | |
| 06 | Early 60s | M | Adenocarcinoma of the pancreas | 1 | 2 | Gemcitabine-nab-paclitaxel; leucovorin calcium-fluorouracil-irinotecan hydrochloride-oxaliplatin | 1 | Liver | Yes | 160 | 1007 | 3.9 | 0.69 | 35/17 |
| 07 | Late 60s | F | Adenocarcinoma of the lung | 1 | 3 | Carboplatin-pemetrexed; pemetrexed-bevacizumab; pemetrexed-bevacizumab-carboplatin | 3 | Lung, pleural space, bone | No | 153 | 663 | 4 | 0.91 | 27/33 |
| 08 | Early 50s | M | Adenocarcinoma of the distal esophagus | 1 | 4 | Concurrent chemoradiation with 5-fluorouracil-docetaxel-cisplatin; folinic acid-5-fluorouracil-oxaliplatin; ramucirumab; 5-fluorouracil | 3 | Esophagus, lung, lymph nodes | No | 279 | 385 | 4.1 | 0.96 | 79/86 |
| 09 | Early 40s | F | Adenocarcinoma of the ovary | 1 | 5 | Folinic acid-fluorouracil-oxaliplatin; capecitabine-oxaliplatin-bevacizumab; investigational FGFR inhibitor; investigational micellar formulation of oxaliplatin; investigational pan-RAF inhibitor | 1 | Lung | No | 350 | 354 | 4.9 | 0.57 | 38/30 |
| 10 | Early 30s | M | Ex-pleomorphic adenoma of the right parotid | 0 | 4 | Adjuvant chemoradiation; cisplatin-proton radiation; carboplatin-docetaxel; investigational FGFR inhibitor | 1 | Lung | No | 215 | 402 | 4.2 | 0.98 | 33/28 |
| 11 | Late 30s | F | Adenoid cystic carcinoma of parotid gland | 1 | 1 | Surgery; adjuvant radiation therapy; investigational FGFR inhibitor | 3 | Lung, kidney, lymph nodes | No | 255 | 409 | 4.1 | 0.69 | 40/25 |
| 12 | Late 30s | F | Adenoid cystic carcinoma of the left parotid gland | 1 | 0 | Parotidectomy; postoperative radiotherapy; radiation therapy | 5 | Kidney, renal pelvis, liver, lung, bone | Yes | 237 | 431 | 4.4 | 0.7 | 28/20 |
| Adverse Event | All Grades | Grade 1 | Grade 2 | Grade 3 | Grade 4 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| (N = 12) * | No. | % | No. | % | No. | % | No. | % | No. | % |
| Gastrointestinal | ||||||||||
| Diarrhea | 5 | 41.7 | 3 | 25.0 | 2 | 16.7 | ||||
| Nausea | 4 | 33.3 | 3 | 25.0 | 1 | 8.3 | ||||
| Abdominal pain | 4 | 33.3 | 2 | 16.7 | 1 | 8.3 | 1 | 8.3 | ||
| Colitis | 2 | 16.7 | 2 | 16.7 | ||||||
| Constipation | 1 | 8.3 | 1 | 8.3 | ||||||
| Musculoskeletal and connective tissue symptoms | ||||||||||
| Back pain | 2 | 16.7 | 2 | 16.7 | ||||||
| Cancer-related pain | 1 | 8.3 | 1 | 8.3 | ||||||
| Pain right side of face and jaw | 1 | 8.3 | 1 | 8.3 | ||||||
| Trismus | 1 | 8.3 | 1 | 8.3 | ||||||
| Leg swelling | 1 | 8.3 | 1 | 8.3 | ||||||
| Psychiatric symptoms | ||||||||||
| Anxiety | 1 | 8.3 | 1 | 8.3 | ||||||
| Skin and subcutaneous tissue symptoms | ||||||||||
| Dry skin/pruritus | 2 | 16.7 | 2 | 16.7 | ||||||
| Laboratory abnormalities | ||||||||||
| Elevated alkaline phosphatase | 1 | 8.3 | 1 | 8.3 | ||||||
| Elevated ALT | 1 | 8.3 | 1 | 8.3 | ||||||
| General disorders and administration site conditions | ||||||||||
| Fatigue | 3 | 25.0 | 1 | 8.3 | 1 | 8.3 | 1 | 8.3 | ||
| Gait instability | 1 | 8.3 | 1 | 8.3 | ||||||
| General weakness | 1 | 8.3 | 1 | 8.3 | ||||||
| Metabolism and nutrition symptoms | ||||||||||
| Dehydration | 1 | 8.3 | 1 | 8.3 | ||||||
| Hyperkalemia | 1 | 8.3 | 1 | 8.3 | ||||||
| Hypokalemia | 1 | 8.3 | 1 | 8.3 | ||||||
| Hyponatremia | 1 | 8.3 | 1 | 8.3 | ||||||
| Vascular symptoms | ||||||||||
| Hypertension | 1 | 8.3 | 1 | 8.3 | ||||||
| Endocrine symptoms | ||||||||||
| Hypothyroidism | 2 | 16.7 | 1 | 8.3 | 1 | 8.3 | ||||
| Blood and lymphatic system symptoms | ||||||||||
| Anemia | 2 | 16.7 | 1 | 8.3 | 1 | 8.3 | ||||
| Respiratory, thoracic, and mediastinal symptoms | ||||||||||
| Dyspnea | 3 | 25.0 | 3 | 25.0 | ||||||
| Cough | 2 | 16.7 | 2 | 16.7 | ||||||
| Hemoptysis | 1 | 8.3 | 1 | 8.3 | ||||||
| Bronchitis | 1 | 8.3 | 1 | 8.3 | ||||||
| Pulmonary embolism | 2 | 16.7 | 1 | 8.3 | 1 | 8.3 | ||||
| Infectious complications | ||||||||||
| Pneumonia | 1 | 8.3 | 1 | 8.3 | ||||||
| Urinary tract infection | 1 | 8.3 | 1 | 8.3 | ||||||
| Others | ||||||||||
| Orange urine | 1 | 8.3 | 1 | 8.3 | ||||||
| Pt. ID | Cohort | Treatment Arm | BBI608 Dose Level, mg, PO BID | Tumor Type | No. of Cycles | Best RECIST Response | PFS *, Months | Progression Status | Subsequent Therapy | Survival Status | OS †, Months |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 01 | 1 | Ipilimumab | 240 | Neuroendocrine carcinoma of the small bowel | <1 ‡ | PD | 0.1 | Yes | None | Deceased | 1.7 |
| 02 | 2 | Pembrolizumab | 240 | Mesothelioma of lung | <1 § | PD | 0.7 | No | None | Deceased | 0.9 |
| 03 | 2 | Pembrolizumab | 240 | Adenocarcinoma of the esophagus | 2 | PD | 2.3 | Yes | None | Deceased | 3.0 |
| 04 | 3 | Nivolumab | 240 | Adenoid cystic carcinoma of the Bartholin gland | 13 | SD | 12.1 | Yes | Investigational therapy: bevacizumab-temsirolimus-valproic acid; Anti-Globo H mAb; HDAC6 inhibitor | Alive | 53.0 |
| 05 | 3 | Nivolumab | 240 | Squamous cell carcinoma of the right anterior tongue and floor of the mouth | 4 | SD | 4.0 | Yes | None | Deceased | 8.1 |
| 06 | 3 | Nivolumab | 240 | Adenocarcinoma of the pancreas | <1 ‖ | Clinical progression | 0.9 | No | None | Deceased | 1.2 |
| 07 | 3 | Nivolumab | 240 | Adenocarcinoma of the lung | 2 | PD | 1.4 | Yes | Radiation therapy; poziotinib | Deceased | 7.5 |
| 08 | 2 | Pembrolizumab | 240 | Adenocarcinoma of the distal esophagus | 3 | PD | 2.7 | Yes | None | Deceased | 5.2 |
| 09 | 3 | Nivolumab | 240 | Adenocarcinoma of the ovary | 7 | SD | 7.4 | Yes | Radiation therapy; palbociclib | Alive | 48.7 |
| 10 | 3 | Nivolumab | 240 | Ex-pleomorphic adenoma of the right parotid | 9 | SD | 8.0 | Yes | Investigational therapy: FGFR inhibitor; nivolumab/ipilimumab; capecitabine | Deceased | 33.0 |
| 11 | 3 | Nivolumab | 480 | Adenoid cystic carcinoma of parotid gland | 11 | SD | 10.1 | Yes | Investigational therapy: HDAC-6 inhibitor; MoAb Globo H inhibitor; PI3K inhibitor-nivolumab; nitro-benzene-aldo-keto reductase 1C3-activated prodrug; fludarabine-cyclophosphamide-T-cell therapy; MoAbs targeting LAG3 and TIM3; radiation therapy | Alive | 51.9 |
| 12 | 3 | Nivolumab | 240 | Adenoid cystic carcinoma of the left parotid gland | 8 | SD | 7.7 | Yes | ERK 1/2 inhibitor; radiation therapy; lenvatinib | Alive | 48.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vo, H.H.; Cartwright, C.; Song, I.-W.; Karp, D.D.; Nogueras Gonzalez, G.M.; Xie, Y.; Karol, M.; Hitron, M.; Vining, D.; Tsimberidou, A.-M. Ipilimumab, Pembrolizumab, or Nivolumab in Combination with BBI608 in Patients with Advanced Cancers Treated at MD Anderson Cancer Center. Cancers 2022, 14, 1330. https://doi.org/10.3390/cancers14051330
Vo HH, Cartwright C, Song I-W, Karp DD, Nogueras Gonzalez GM, Xie Y, Karol M, Hitron M, Vining D, Tsimberidou A-M. Ipilimumab, Pembrolizumab, or Nivolumab in Combination with BBI608 in Patients with Advanced Cancers Treated at MD Anderson Cancer Center. Cancers. 2022; 14(5):1330. https://doi.org/10.3390/cancers14051330
Chicago/Turabian StyleVo, Henry Hiep, Carrie Cartwright, I-Wen Song, Daniel D. Karp, Graciela M. Nogueras Gonzalez, Yuran Xie, Michael Karol, Matthew Hitron, David Vining, and Apostolia-Maria Tsimberidou. 2022. "Ipilimumab, Pembrolizumab, or Nivolumab in Combination with BBI608 in Patients with Advanced Cancers Treated at MD Anderson Cancer Center" Cancers 14, no. 5: 1330. https://doi.org/10.3390/cancers14051330
APA StyleVo, H. H., Cartwright, C., Song, I.-W., Karp, D. D., Nogueras Gonzalez, G. M., Xie, Y., Karol, M., Hitron, M., Vining, D., & Tsimberidou, A.-M. (2022). Ipilimumab, Pembrolizumab, or Nivolumab in Combination with BBI608 in Patients with Advanced Cancers Treated at MD Anderson Cancer Center. Cancers, 14(5), 1330. https://doi.org/10.3390/cancers14051330






