Impact of Three-Month Androgen Deprivation Therapy on [68Ga]Ga-PSMA-11 PET/CT Indices in Men with Advanced Prostate Cancer—Results from a Pilot Prospective Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
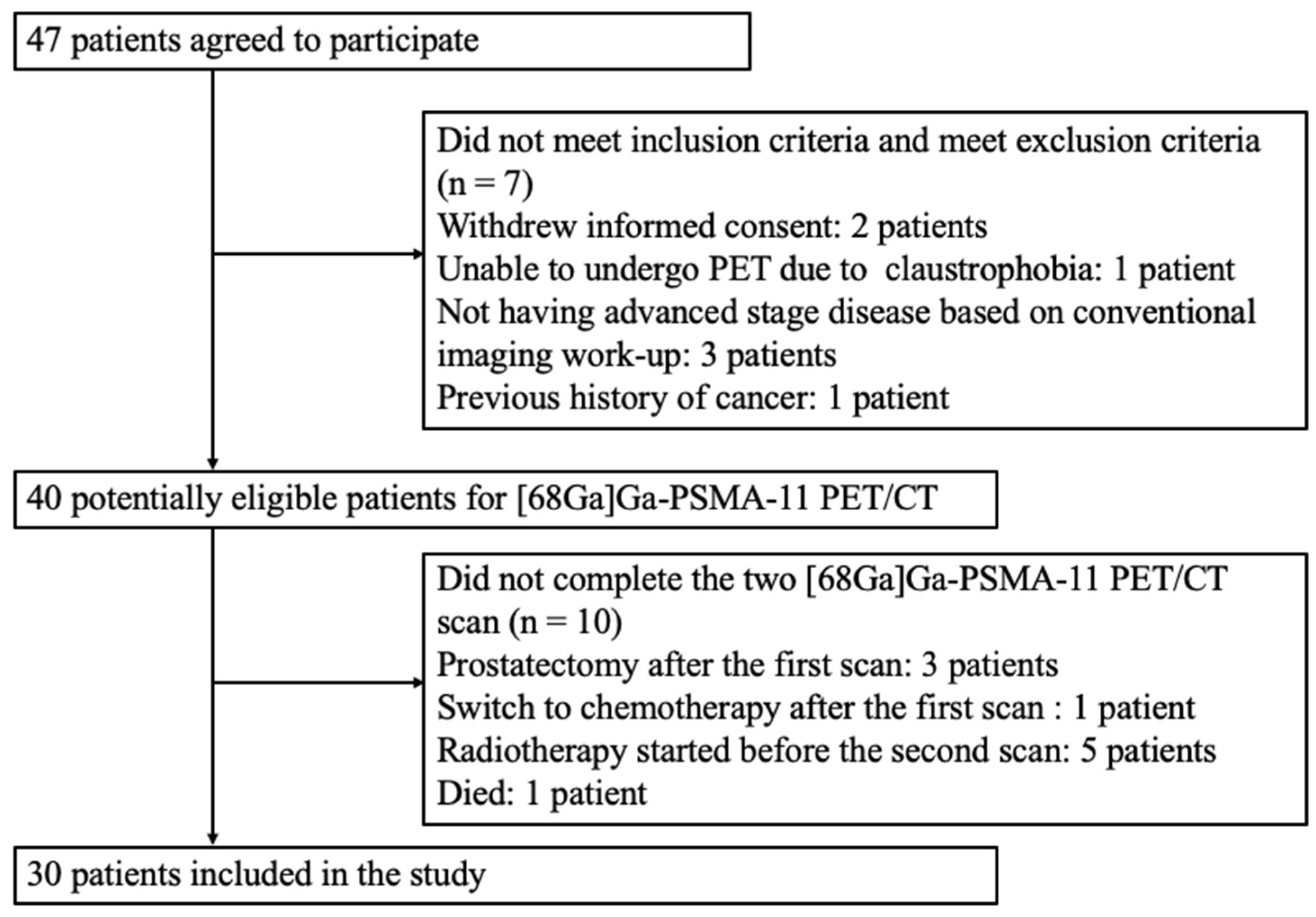
2.2. Patient Selection
2.3. [68Ga]Ga-PSMA-11 PET/CT Acquisition and Reconstruction
2.4. [68Ga]Ga-PSMA-11 PET/CT Indices
2.5. Definition of Response and Reduction Ratio
2.6. Subgrouping of Tumor Aggressiveness with The ISUP Grade Group and ADT Schemes
2.7. Data Analysis
3. Results
3.1. Patient Characteristics and Therapy
3.2. Serum PSA Values and Reduction Ratios
3.3. Baseline [68Ga]Ga-PSMA-11 PET/CT Imaging
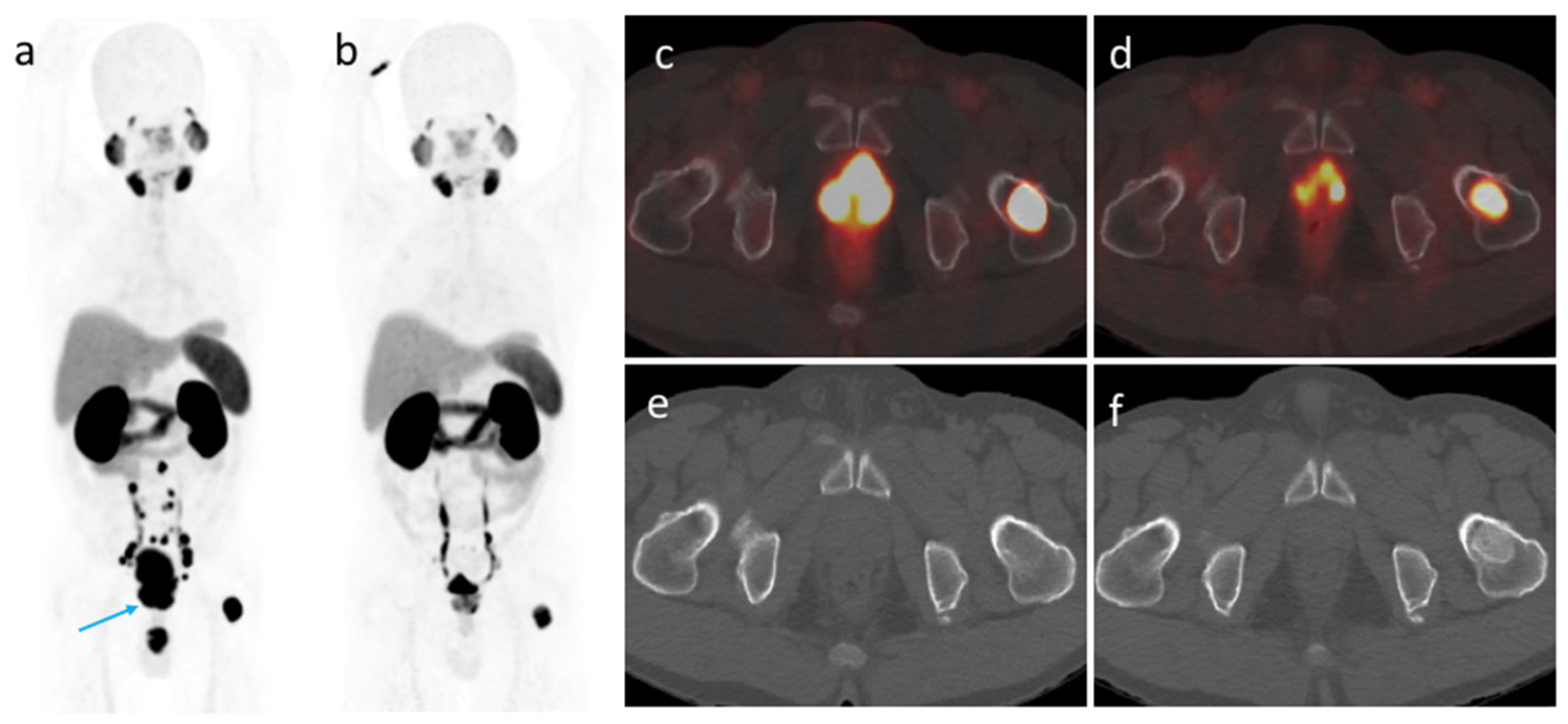
3.4. [68Ga]Ga-PSMA-11 PET/CT Imaging after ADT
3.5. Changes in [68Ga]Ga-PSMA-11 PET/CT Indices According to The ISUP Grade Groups and ADT Schemes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Kim, T.J.; Lee, Y.H.; Koo, K.C. Current Status and Future Perspectives of Androgen Receptor Inhibition Therapy for Prostate Cancer: A Comprehensive Review. Biomolecules 2021, 11, 492. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.S. Overview of prostate-specific membrane antigen. Rev. Urol. 2004, 6, S13–S18. [Google Scholar]
- Hofman, M.S.; Hicks, R.J.; Maurer, T.; Eiber, M. Prostate-specific Membrane Antigen PET: Clinical Utility in Prostate Cancer, Normal Patterns, Pearls, and Pitfalls. Radiographics 2018, 38, 200–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofman, M.S.; Lawrentschuk, N.; Francis, R.J.; Tang, C.; Vela, I.; Thomas, P.; Rutherford, N.; Martin, J.M.; Frydenberg, M.; Shakher, R.; et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): A prospective, randomised, multicentre study. Lancet 2020, 395, 1208–1216. [Google Scholar] [CrossRef]
- O’Keefe, D.S.; Bacich, D.J.; Huang, S.S.; Heston, W.D.W. A Perspective on the Evolving Story of PSMA Biology, PSMA-Based Imaging, and Endoradiotherapeutic Strategies. J. Nucl. Med. 2018, 59, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Meller, B.; Bremmer, F.; Sahlmann, C.O.; Hijazi, S.; Bouter, C.; Trojan, L.; Meller, J.; Thelenet, P. Alterations in androgen deprivation enhanced prostate-specific membrane antigen (PSMA) expression in prostate cancer cells as a target for diagnostics and therapy. EJNMMI Res. 2015, 5, 66. [Google Scholar] [CrossRef]
- Evans, M.J.; Smith-Jones, P.M.; Wongvipat, J.; Sawyers, C.L. Noninvasive measurement of androgen receptor signaling with a positron-emitting radiopharmaceutical that targets prostate-specific membrane antigen. Proc. Natl. Acad. Sci. USA 2011, 108, 9578–9582. [Google Scholar] [CrossRef] [Green Version]
- Hope, T.A.; Truillet, C.; Ehman, E.C.; Afshar-Oromieh, A.; Aggarwal, R.; Ryan, C.J.; Carroll, P.R.; Small, E.J.; Evans, M.J. 68Ga-PSMA-11 PET Imaging of Response to Androgen Receptor Inhibition: First Human Experience. J. Nucl. Med. 2017, 58, 81–84. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, R.; Wei, X.; Kim, W.; Small, E.J.; Ryan, C.J.; Carroll, P.; Cooperberg, M.; Evans, M.J.; Hope, T. Heterogeneous Flare in Prostate-specific Membrane Antigen Positron Emission Tomography Tracer Uptake with Initiation of Androgen Pathway Blockade in Metastatic Prostate Cancer. Eur. Urol. Oncol. 2018, 1, 78–82. [Google Scholar] [CrossRef]
- Vaz, S.; Hadaschik, B.; Gabriel, M.; Herrmann, K.; Eiber, M.; Costa, D. Influence of androgen deprivation therapy on PSMA expression and PSMA-ligand PET imaging of prostate cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Afshar-Oromieh, A.; Debus, N.; Uhrig, M.; Hope, T.A.; Evans, M.J.; Holland-Letz, T.; Giesel, F.L.; Kopka, K.; Hadaschik, B.; Kratochwil, C.; et al. Impact of long-term androgen deprivation therapy on PSMA ligand PET/CT in patients with castration-sensitive prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2045–2054. [Google Scholar] [CrossRef] [Green Version]
- Leitsmann, C.; Thelen, P.; Schmid, M.; Meller, J.; Sahlmann, C.-O.; Meller, B.; Trojan, L.; Strauss, A. Enhancing PSMA-uptake with androgen deprivation therapy-a new way to detect prostate cancer metastases? Int. Braz. J. Urol. 2019, 45, 459–467. [Google Scholar] [CrossRef] [Green Version]
- Tseng, J.R.; Yu, K.J.; Liu, F.Y.; Yang, L.-Y.; Hong, J.-H.; Yen, T.-C.; Pang, S.-T.; Wang, L.-J. Comparison between (68)Ga-PSMA-11 PET/CT and multiparametric magnetic resonance imaging in patients with biochemically recurrent prostate cancer following robot-assisted radical prostatectomy. J. Formos. Med. Assoc. 2021, 120, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Gafita, A.; Bieth, M.; Kronke, M.; Tetteh, G.; Navarro, F.; Wang, H.; Guenther, E.; Menze, B.; Weber, W.A.; Eiber, M. qPSMA: Semiautomatic Software for Whole-Body Tumor Burden Assessment in Prostate Cancer Using (68)Ga-PSMA11 PET/CT. J. Nucl. Med. 2019, 60, 1277–1283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmuck, S.; von Klot, C.A.; Henkenberens, C.; Sohns, J.M.; Christiansen, H.; Wester, H.J.; Ross, T.L.; Bengel, F.M.; Derlin, T. Initial Experience with Volumetric (68)Ga-PSMA I&T PET/CT for Assessment of Whole-Body Tumor Burden as a Quantitative Imaging Biomarker in Patients with Prostate Cancer. J. Nucl. Med. 2017, 58, 1962–1968. [Google Scholar] [PubMed] [Green Version]
- Fanti, S.; Goffin, K.; Hadaschik, B.A.; Herrmann, K.; Maurer, T.; MacLennan, S.; Oprea-Lager, D.E.; Oyen, W.J.; Rouvière, O.; Mottet, N.; et al. Consensus statements on PSMA PET/CT response assessment criteria in prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 469–476. [Google Scholar] [CrossRef]
- Scher, H.I.; Halabi, S.; Tannock, I.; Morris, M.J.; Sternberg, C.N.; Carducci, M.A.; Eisenberger, M.A.; Higano, C.; Bubley, G.J.; Dreicer, R.; et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: Recommendations of the Prostate Cancer Clinical Trials Working Group. J. Clin. Oncol. 2008, 26, 1148–1159. [Google Scholar] [CrossRef]
- Wright, G.L.; Mayer Grob, B.; Haley, C.; Grossman, K.; Newhall, K.; Petrylak, D.; Troyer, J.; Konchuba, A.; Schellhammer, P.F.; Moriarty, R. Upregulation of prostate-specific membrane antigen after androgen-deprivation therapy. Urology 1996, 48, 326–334. [Google Scholar] [CrossRef]
- DiPippo, V.A.; Magargal, W.W.; Moorji, S.M.; Murga, J.D.; Olson, W.C. Antiandrogen modulation of prostate-specific membrane antigen (PSMA): Dynamics and synergy with PSMA-targeted therapy. J. Clin. Oncol. 2013, 31, e16007. [Google Scholar] [CrossRef]
- Murga, J.D.; Moorji, S.M.; Han, A.Q.; Magargal, W.W.; DiPippo, V.A.; Olson, W.C. Synergistic co-targeting of prostate-specific membrane antigen and androgen receptor in prostate cancer. Prostate 2015, 75, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Zacho, H.D.; Petersen, L.J. Bone Flare to Androgen Deprivation Therapy in Metastatic, Hormone-Sensitive Prostate Cancer on 68Ga-Prostate-Specific Membrane Antigen PET/CT. Clin. Nucl. Med. 2018, 43, e404–e406. [Google Scholar] [CrossRef] [PubMed]
- Onal, C.; Guler, O.C.; Torun, N.; Reyhan, M.; Yapar, A.F. The effect of androgen deprivation therapy on (68)Ga-PSMA tracer uptake in non-metastatic prostate cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Emmett, L.; Yin, C.; Crumbaker, M.; Hruby, G.; Kneebone, A.; Epstein, R.; Nguyen, Q.; Hickey, A.; Ihsheish, N.; O’Neill, G.; et al. Rapid Modulation of PSMA Expression by Androgen Deprivation: Serial (68)Ga-PSMA-11 PET in Men with Hormone-Sensitive and Castrate-Resistant Prostate Cancer Commencing Androgen Blockade. J. Nucl. Med. 2019, 60, 950–954. [Google Scholar] [CrossRef] [Green Version]
- Ettala, O.; Malaspina, S.; Tuokkola, T.; Luoto, P.; Löyttyniemi, E.; Boström, P.J.; Kemppainen, J. Prospective study on the effect of short-term androgen deprivation therapy on PSMA uptake evaluated with (68)Ga-PSMA-11 PET/MRI in men with treatment-naive prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 665–673. [Google Scholar] [CrossRef] [Green Version]
- Plouznikoff, N.; Artigas, C.; Sideris, S.; Chanza, N.M.; Gil, T.; Peltier, A.; Flamen, P. Evaluation of PSMA expression changes on PET/CT before and after initiation of novel antiandrogen drugs (enzalutamide or abiraterone) in metastatic castration-resistant prostate cancer patients. Ann. Nucl. Med. 2019, 33, 945–954. [Google Scholar] [CrossRef]
- Rosar, F.; Dewes, S.; Ries, M.; Schaefer, A.; Khreish, F.; Maus, S.; Bohnenberger, H.; Linxweiler, J.; Bartholomä, M.; Ohlmann, C.; et al. New insights in the paradigm of upregulation of tumoral PSMA expression by androgen receptor blockade: Enzalutamide induces PSMA upregulation in castration-resistant prostate cancer even in patients having previously progressed on enzalutamide. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 687–694. [Google Scholar] [CrossRef]
- Hoberuck, S.; Lock, S.; Winzer, R.; Zöphel, K.; Froehner, M.; Fedders, D.; Kotzerke, J.; Hölscher, T. [(68)Ga]Ga-PSMA-11 PET before and after initial long-term androgen deprivation in patients with newly diagnosed prostate cancer: A retrospective single-center study. EJNMMI Res. 2020, 10, 135. [Google Scholar] [CrossRef]
- Gupta, P.; Murthy, V.; Agarwal, A.; Maitre, M.; Mhatre, N.; Rangarajan, V. 68Ga-prostate-specific membrane antigen PETCT-based response to androgen deprivation therapy in patients with prostate cancer. Nucl. Med. Commun. 2019, 40, 1283–1288. [Google Scholar] [CrossRef]
- Schmidkonz, C.; Cordes, M.; Schmidt, D.; Bäuerle, T.; Goetz, T.I.; Beck, M.; Prante, O.; Cavallaro, A.; Uder, M.; Wullich, B.; et al. (68)Ga-PSMA-11 PET/CT-derived metabolic parameters for determination of whole-body tumor burden and treatment response in prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1862–1872. [Google Scholar] [CrossRef]
- Zhang, H.; Koumna, S.; Pouliot, F.; Beauregard, J.M.; Kolinsky, M. PSMA Theranostics: Current Landscape and Future Outlook. Cancers 2021, 13, 4023. [Google Scholar] [CrossRef] [PubMed]


| Number of Patients (%) | ||||
|---|---|---|---|---|
| Characteristic | Entire Cohort (n = 30) | ISUP Grade < 5 (n = 14) | ISUP Grade 5 (n = 16) | p |
| Age, years | 69.8 ± 11.1 | 71.9 ± 9.8 | 68.1 ± 12.2 | 0.355 |
| ECOG performance status | 1.0 | |||
| 0 | 22 (73) | 10 (71) | 12 (75) | |
| 1 | 8 (27) | 4 (29) | 4 (25) | |
| AJCC stage (8th edition) | 0.068 | |||
| IIIB | 7 (23) | 7 (50) | 0 (0) | |
| IIIC | 2 (7) | 0 (0) | 2 (12) | |
| IVA | 6 (20) | 2 (14) | 4 (25) | |
| IVB | 15 (50) | 5 (36) | 10 (63) | |
| Gleason score | <0.001 | |||
| 7 | 9 (30) | 9 (64) | 0 (0) | |
| 8 | 5 (17) | 5 (36) | 0 (0) | |
| 9 | 13 (43) | 0 (0) | 13 (81) | |
| 10 | 3 (10) | 0 (0) | 3 (19) | |
| ISUP grade | <0.001 | |||
| 2 | 2 (7) | 2 (14) | 0 (0) | |
| 3 | 7 (23) | 7 (50) | 0 (0) | |
| 4 | 5 (17) | 5 (36) | 0 (0) | |
| 5 | 16 (53) | 0 (0) | 16 (100) | |
| ADT regimen | 0.289 | |||
| Leuprorelin + bicalutamide | 7 (23) | 5 (36) | 2 (12) | |
| Goserelin + bicalutamide | 9 (30) | 6 (42) | 3 (19) | |
| Goserelin | 4 (13) | 1 (7) | 3 (19) | |
| Leuprorelin | 2 (7) | 1 (7) | 1 (6) | |
| Leuprorelin + cyproterone | 3 (10) | 1 (7) | 2 (12) | |
| Leuprorelin + abiraterone | 2 (7) | 0 (0) | 2 (12) | |
| Leuprorelin + abiraterone + bicalutamide | 2 (7) | 0 (0) | 2 (12) | |
| Triptorelin + cyproterone | 1 (3) | 0 (0) | 1 (6) | |
| Characteristic | Entire Cohort (n = 30) | ISUP Grade < 5 (n = 14) | ISUP Grade 5 (n = 16) | p |
|---|---|---|---|---|
| Initial serum PSA, ng/mL | 117 ± 200 | 59 ± 101 | 168 ± 250 | 0.208 |
| Serum PSA at 2nd PET/CT scan, ng/mL | 4.5 ± 12.0 | 1.3 ± 1.5 | 7.3 ± 15.6 | 0.240 |
| Reduction ratio of serum PSA | 0.95 ± 0.06 | 0.95 ± 0.07 | 0.96 ± 0.04 | 0.580 |
| Parameter | Entire Cohort (n = 30) | ISUP Grade < 5 (n = 14) | ISUP Grade 5 (n = 16) | p |
|---|---|---|---|---|
| Baseline PET/CT findings | 0.444 | |||
| PT only | 9 (30) | 6 (42) | 3 (19) | |
| PT + LN | 6 (20) | 3 (22) | 3 (19) | |
| PT + BM | 5 (17) | 2 (14) | 3 (19) | |
| PT + LN + BM | 10 (33) | 3 (22) | 7 (43) | |
| Impact on traditional imaging | 0.538 | |||
| Upstaging | 5 (17) | 2 (14) | 3 (19) | |
| Downstaging | 1 (3) | 1 (7) | 0 (0) | |
| No change | 24 (80) | 11 (79) | 13 (81) | |
| Response assessment at 2nd PET/CT scan | 0.467 | |||
| Complete response | 2 (7) | 2 (14) | 0 (0) | |
| Partial response | 24 (80) | 10 (72) | 14 (88) | |
| Stable disease | 2 (7) | 1 (7) | 1 (6) | |
| Disease progression | 2 (7) | 1 (7) | 1 (6) |
| Index | Before ADT | After ADT | Reduction Ratio | p | |
|---|---|---|---|---|---|
| Prostate tumor (n = 30) | |||||
| SUVmax | 30.1 ± 21.4 | 14.4 ± 17.6 | 0.49 ± 0.52 | <0.001 | |
| PSMA-TV | 28.2 ± 34.3 | 7.6 ± 13.3 | 0.50 ± 0.94 | 0.001 | |
| PSMA-TL | 346 ± 519 | 69 ± 159 | 0.70 ± 0.49 | <0.001 | |
| Metastatic lymph nodes (n = 16) | |||||
| SUVmax | 27.2 ± 20.6 | 13.0 ± 24.6 | 0.74 ± 0.37 | 0.001 | |
| PSMA-TV | 52.2 ± 114.3 | 10.1 ± 26.9 | 0.89 ± 0.19 | 0.001 | |
| PSMA-TL | 671 ± 1494 | 120 ± 322 | 0.92 ± 0.14 | 0.001 | |
| Bone metastases (n = 15) | |||||
| SUVmax | 28.5 ± 22.4 | 12.0 ± 14.6 | 0.63 ± 0.34 | 0.001 | |
| PSMA-TV | 180 ± 454 | 121 ± 341 | 0.65 ± 0.37 | 0.001 | |
| PSMA-TL | 1202 ± 2886 | 663 ± 1795 | 0.73 ± 0.35 | 0.001 | |
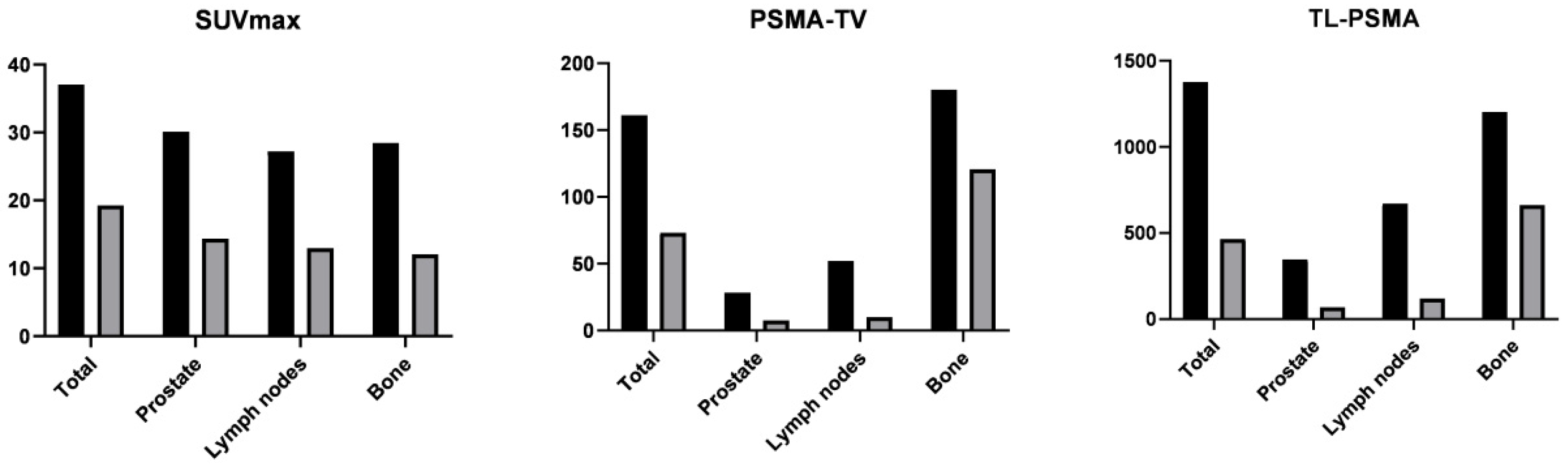
| Whole-body (n = 30) | |||||
| wbSUVmax | 37.1 ± 22.3 | 19.3 ± 22.3 | 0.47 ± 0.43 | <0.001 | |
| wbPSMA-TV | 161 ± 431 | 73 ± 248 | 0.54 ± 0.80 | <0.001 | |
| wbPSMA-TL | 1375 ± 2945 | 464 ± 1374 | 0.72 ± 0.37 | <0.001 | |
| Before ADT | After ADT | Reduction Ratio | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Index | G < 5 | G = 5 | p | G < 5 | G = 5 | p | G < 5 | G = 5 | p | |
| Prostate | ||||||||||
| SUVmax | 27.83 | 32.16 | 1.000 | 8.79 | 19.22 | 0.153 | 0.61 | 0.39 | 0.141 | |
| PSMA-TV | 20.35 | 35.04 | 0.448 | 2.81 | 11.74 | 0.042 | 0.51 | 0.48 | 0.256 | |
| PSMA-TL | 205 | 469.5 | 0.637 | 20.51 | 110.6 | 0.038 | 0.76 | 0.64 | 0.239 | |
| Metastatic lymph nodes | ||||||||||
| SUVmax | 35.18 | 23.58 | 0.320 | 5.38 | 16.44 | 0.923 | 0.80 | 0.62 | 0.678 | |
| PSMA-TV | 10.81 | 70.95 | 0.891 | 0.38 | 14.53 | 0.756 | 0.86 | 0.80 | 0.317 | |
| PSMA-TL | 160.80 | 903.2 | 0.827 | 2.29 | 173.6 | 0.665 | 0.91 | 0.76 | 0.317 | |
| Bone | ||||||||||
| SUVmax | 29.30 | 28.02 | 0.768 | 6.36 | 14.88 | 0.361 | 0.67 | 0.60 | 0.762 | |
| PSMA-TV | 15.60 | 262.7 | 0.679 | 1.78 | 180.2 | 0.361 | 0.70 | 0.63 | 0.762 | |
| TL-PSMA | 130.73 | 1737 | 0.679 | 8.69 | 989.7 | 0.361 | 0.74 | 0.72 | 0.762 | |
| Whole-body | ||||||||||
| wbSUVmax | 32.40 | 41.13 | 0.473 | 9.87 | 27.46 | 0.021 | 0.60 | 0.35 | 0.006 | |
| wbPSMA-TV | 29.46 | 276.5 | 0.028 | 3.58 | 134.3 | 0.001 | 0.50 | 0.58 | 0.057 | |
| wbPSMA-TL | 302.3 | 2314 | 0.028 | 24.43 | 848.5 | <0.001 | 0.75 | 0.68 | 0.057 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tseng, J.-R.; Chang, S.-H.; Wu, Y.-Y.; Fan, K.-H.; Yu, K.-J.; Yang, L.-Y.; Hsiao, I.-T.; Liu, F.-Y.; Pang, S.-T. Impact of Three-Month Androgen Deprivation Therapy on [68Ga]Ga-PSMA-11 PET/CT Indices in Men with Advanced Prostate Cancer—Results from a Pilot Prospective Study. Cancers 2022, 14, 1329. https://doi.org/10.3390/cancers14051329
Tseng J-R, Chang S-H, Wu Y-Y, Fan K-H, Yu K-J, Yang L-Y, Hsiao I-T, Liu F-Y, Pang S-T. Impact of Three-Month Androgen Deprivation Therapy on [68Ga]Ga-PSMA-11 PET/CT Indices in Men with Advanced Prostate Cancer—Results from a Pilot Prospective Study. Cancers. 2022; 14(5):1329. https://doi.org/10.3390/cancers14051329
Chicago/Turabian StyleTseng, Jing-Ren, Szu-Han Chang, Yao-Yu Wu, Kang-Hsing Fan, Kai-Jie Yu, Lan-Yan Yang, Ing-Tsung Hsiao, Feng-Yuan Liu, and See-Tong Pang. 2022. "Impact of Three-Month Androgen Deprivation Therapy on [68Ga]Ga-PSMA-11 PET/CT Indices in Men with Advanced Prostate Cancer—Results from a Pilot Prospective Study" Cancers 14, no. 5: 1329. https://doi.org/10.3390/cancers14051329
APA StyleTseng, J.-R., Chang, S.-H., Wu, Y.-Y., Fan, K.-H., Yu, K.-J., Yang, L.-Y., Hsiao, I.-T., Liu, F.-Y., & Pang, S.-T. (2022). Impact of Three-Month Androgen Deprivation Therapy on [68Ga]Ga-PSMA-11 PET/CT Indices in Men with Advanced Prostate Cancer—Results from a Pilot Prospective Study. Cancers, 14(5), 1329. https://doi.org/10.3390/cancers14051329






