Circulating Levels of the Cardiovascular Biomarkers ST2 and Adrenomedullin Predict Outcome within a Randomized Phase III Lung Cancer Trial (RASTEN)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. RASTEN Clinical Trial
2.2. Patient Selection and Plasma Sampling
2.3. Clinical Outcome
2.4. Immunoassay Analysis of Vasoactive Peptides
2.5. Proximity Extension Assay
2.6. Statistical Analysis
3. Results
3.1. Study Population
3.2. Cardiovascular Biomarkers at Baseline
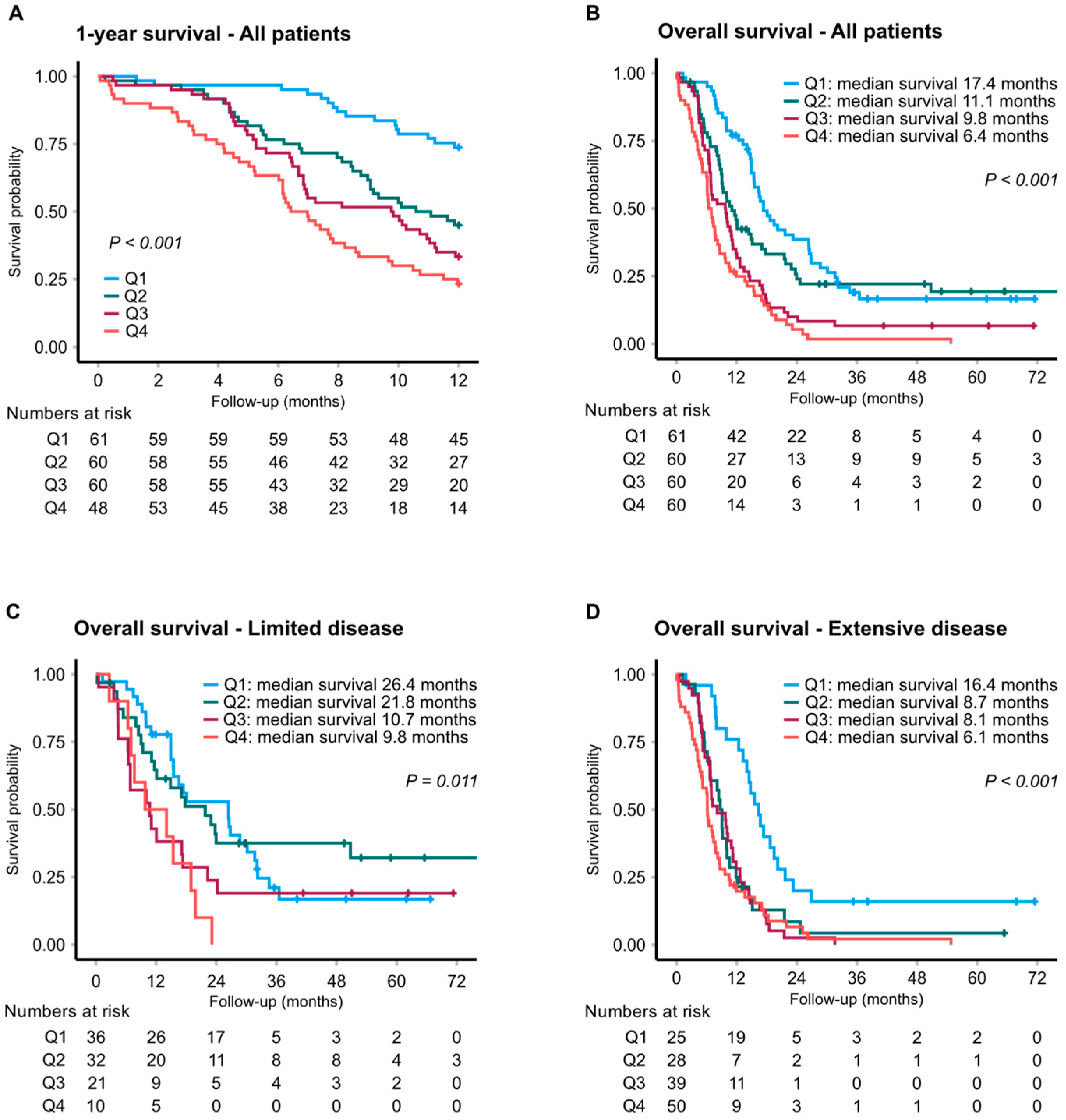
3.3. Cardiovascular Biomarkers and Clinical Outcome
3.4. Prediction Models and Combined Biomarker Score
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Govindan, R.; Page, N.; Morgensztern, D.; Read, W.; Tierney, R.; Vlahiotis, A.; Spitznagel, E.L.; Piccirillo, J. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: Analysis of the surveillance, epidemiologic, and end results database. J. Clin. Oncol. 2006, 24, 4539–4544. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Yang, X.; Huang, Y.; Zhao, M.; Li, M.; Ma, K.; Yin, J.; Zhan, C.; Wang, Q. Trends in the incidence, treatment, and survival of patients with lung cancer in the last four decades. Cancer Manag. Res. 2019, 11, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Waqar, S.N.; Morgensztern, D. Treatment advances in small cell lung cancer (SCLC). Pharmacol. Ther. 2017, 180, 16–23. [Google Scholar] [CrossRef]
- Aarts, M.J.; Aerts, J.G.; van den Borne, B.E.; Biesma, B.; Lemmens, V.E.; Kloover, J.S. Comorbidity in Patients With Small-Cell Lung Cancer: Trends and Prognostic Impact. Clin. Lung Cancer 2015, 16, 282–291. [Google Scholar] [CrossRef]
- Ball, D.; Thursfield, V.; Irving, L.; Mitchell, P.; Richardson, G.; Torn-Broers, Y.; Wright, G.; Giles, G. Evaluation of the Simplified Comorbidity Score (Colinet) as a prognostic indicator for patients with lung cancer: A cancer registry study. Lung Cancer 2013, 82, 358–361. [Google Scholar] [CrossRef]
- Kuo, Y.-W.; Jerng, J.-S.; Shih, J.-Y.; Chen, K.-Y.; Yu, C.-J.; Yang, P.-C. The Prognostic Value of the Simplified Comorbidity Score in the Treatment of Small Cell Lung Carcinoma. J. Thorac. Oncol. 2011, 6, 378–383. [Google Scholar] [CrossRef]
- Sarfati, D.; Koczwara, B.; Jackson, C. The impact of comorbidity on cancer and its treatment. CA Cancer J. Clin. 2016, 66, 337–350. [Google Scholar] [CrossRef]
- Colinet, B.; Jacot, W.; Bertrand, D.; Lacombe, S.; Bozonnat, M.C.; Daures, J.P.; Pujol, J.L. A new simplified comorbidity score as a prognostic factor in non-small-cell lung cancer patients: Description and comparison with the Charlson’s index. Br. J. Cancer 2005, 93, 1098–1105. [Google Scholar] [CrossRef]
- Savic-Radojevic, A.; Pljesa-Ercegovac, M.; Matic, M.; Simic, D.; Radovanovic, S.; Simic, T. Novel Biomarkers of Heart Failure. Adv. Clin. Chem. 2017, 79, 93–152. [Google Scholar]
- Dudek, M.; Kałużna-Oleksy, M.; Migaj, J.; Straburzyńska-Migaj, E. Clinical value of soluble ST2 in cardiology. Adv. Clin. Exp. Med. 2020, 29, 1205–1210. [Google Scholar] [CrossRef] [PubMed]
- Mueller, T.; Jaffe, A.S. Soluble ST2--analytical considerations. Am. J. Cardiol. 2015, 115 (Suppl. 7), 8B–21B. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Figal, D.A.; Januzzi, J.L. The biology of ST2: The International ST2 Consensus Panel. Am. J. Cardiol. 2015, 115 (Suppl. 7), 3B–7B. [Google Scholar] [CrossRef] [PubMed]
- Hinson, J.P.; Kapas, S.; Smith, D.M. Adrenomedullin, a Multifunctional Regulatory Peptide*. Endocr. Rev. 2000, 21, 138–167. [Google Scholar] [PubMed]
- Robertson, G.L. Antidiuretic hormone. Normal and disordered function. Endocrinol. Metab. Clin. N. Am. 2001, 30, 671–694. [Google Scholar] [CrossRef]
- Vesely, B.A.; Song, S.; Sanchez-Ramos, J.; Fitz, S.R.; Alli, A.A.; Solivan, S.M.; Gower, W.R., Jr.; Vesely, D.L. Five cardiac hormones decrease the number of human small-cell lung cancer cells. Eur. J. Clin. Investig. 2005, 35, 388–398. [Google Scholar] [CrossRef]
- Morgenthaler, N.G.; Struck, J.; Alonso, C.; Bergmann, A. Measurement of Midregional Proadrenomedullin in Plasma with an Immunoluminometric Assay. Clin. Chem. 2005, 51, 1823–1829. [Google Scholar] [CrossRef]
- Morgenthaler, N.G.; Struck, J.; Alonso, C.; Bergmann, A. Assay for the Measurement of Copeptin, a Stable Peptide Derived from the Precursor of Vasopressin. Clin. Chem. 2006, 52, 112–119. [Google Scholar] [CrossRef]
- Morgenthaler, N.G.; Struck, J.; Thomas, B.; Bergmann, A. Immunoluminometric assay for the midregion of pro-atrial natriuretic peptide in human plasma. Clin. Chem. 2004, 50, 234–236. [Google Scholar] [CrossRef]
- Ek, L.; Gezelius, E.; Bergman, B.; Bendahl, P.; Anderson, H.; Sundberg, J.; Wallberg, M.; Falkmer, U.; Verma, S.; Belting, M. Swedish Lung Cancer Study, Randomized phase III trial of low-molecular-weight heparin enoxaparin in addition to standard treatment in small-cell lung cancer: The RASTEN trial. Ann. Oncol. 2017, 29, 398–404. [Google Scholar] [CrossRef]
- Assarsson, E.; Lundberg, M.; Holmquist, G.; Björkesten, J.; Thorsen, S.B.; Ekman, D.; Eriksson, A.; Dickens, E.R.; Ohlsson, S.; Edfeldt, G.; et al. Homogenous 96-Plex PEA Immunoassay Exhibiting High Sensitivity, Specificity, and Excellent Scalability. PLoS ONE 2014, 9, e95192. [Google Scholar]
- Brueckl, W.; Herbst, L.; Lechler, A.; Fuchs, F.; Schoeberl, A.; Zirlik, S.; Klein, P.; Brunner, T.B.; Papadopoulos, T.; Hohenberger, W.; et al. Predictive and prognostic factors in small cell lung carcinoma (SCLC)—Analysis from routine clinical practice. Anticancer Res. 2006, 26, 4825–4832. [Google Scholar] [PubMed]
- Hansen, O.; Sorensen, P.; Hansen, K.H. The occurrence of hyponatremia in SCLC and the influence on prognosis: A retrospective study of 453 patients treated in a single institution in a 10-year period. Lung Cancer 2010, 68, 111–114. [Google Scholar] [CrossRef]
- Johansson, M.; Ricci, F.; Di Martino, G.; Rogmark, C.; Sutton, R.; Hamrefors, V.; Melander, O.; Fedorowski, A. Cardiovascular biomarkers predict fragility fractures in older adults. Heart 2019, 105, 449–454. [Google Scholar] [CrossRef]
- Jónsdóttir, B.; Severinsen, M.Z.; von Wowern, F.; Miguel, C.S.; Goetze, J.P.; Melander, O. ST2 Predicts Mortality In Patients With Acute Hypercapnic Respiratory Failure Treated With Noninvasive Positive Pressure Ventilation. Int. J. Chronic Obstr. Pulm. Dis. 2019, 14, 2385–2393. [Google Scholar] [CrossRef]
- Van Der Jeught, K.; Sun, Y.; Fang, Y.; Zhou, Z.; Jiang, H.; Yu, T.; Yang, J.; Kamocka, M.M.; So, K.M.; Li, Y.; et al. ST2 as checkpoint target for colorectal cancer immunotherapy. JCI Insight 2020, 5, e136073. [Google Scholar] [CrossRef]
- Akimoto, M.; Maruyama, R.; Takamaru, H.; Ochiya, T.; Takenaga, K. Soluble IL-33 receptor sST2 inhibits colorectal cancer malignant growth by modifying the tumour microenvironment. Nat. Commun. 2016, 7, 13589. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.-P.; Zhou, X.-Y.; Yao, L.-T.; Liu, C.; Ma, W.; Jin, F.; Wu, Y.-F. Serum soluble ST2 is associated with ER-positive breast cancer. BMC Cancer 2014, 14, 198. [Google Scholar] [CrossRef]
- Pavo, N.; Raderer, M.; Hülsmann, M.; Neuhold, S.; Adlbrecht, C.; Strunk, G.; Goliasch, G.; Gisslinger, H.; Steger, G.G.; Hejna, M.; et al. Cardiovascular biomarkers in patients with cancer and their association with all-cause mortality. Heart 2015, 101, 1874–1880. [Google Scholar] [CrossRef]
- Keleg, S.; Kayed, H.; Jiang, X.; Penzel, R.; Giese, T.; Büchler, M.W.; Friess, H.; Kleeff, J. Adrenomedullin is induced by 427 hypoxia and enhances pancreatic cancer cell invasion. Int. J. Cancer 2007, 121, 21–32. [Google Scholar] [CrossRef]
- Nikitenko, L.L.; Fox, S.B.; Kehoe, S.; Rees, M.C.P.; Bicknell, R. Adrenomedullin and tumour angiogenesis. Br. J. Cancer 2005, 94, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Oehler, M.K.; Hague, S.; Rees, M.C.; Bicknell, R. Adrenomedullin promotes formation of xenografted endometrial tumors by stimulation of autocrine growth and angiogenesis. Oncogene 2002, 21, 2815–2821. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vázquez, R.; Riveiro, M.E.; Berenguer-Daize, C.; O’kane, A.; Gormley, J.; Touzelet, O.; Rezai, K.; Bekradda, M.; Ouafik, L.H. Targeting Adrenomedullin in Oncology: A Feasible Strategy With Potential as Much More Than an Alternative Anti-Angiogenic Therapy. Front. Oncol. 2020, 10, 589218. [Google Scholar] [CrossRef] [PubMed]
- Nouguerède, E.; Berenguer, C.; Garcia, S.; Bennani, B.; Delfino, C.; Nanni, I.; Dahan, L.; Gasmi, M.; Seitz, J.; Martin, P.; et al. Expression of adrenomedullin in human colorectal tumors and its role in cell growth and invasion in vitro and in xenograft growth in vivo. Cancer Med. 2013, 2, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Deville, J.-L.; Bartoli, C.; Berenguer, C.; Fernandez-Sauze, S.; Kaafarani, I.; Delfino, C.; Fina, F.; Salas, S.; Muracciole, X.; Mancini, J.; et al. Expression and role of adrenomedullin in renal tumors and value of its mRNA levels as prognostic factor in clear-cell renal carcinoma. Int. J. Cancer 2009, 125, 2307–2315. [Google Scholar] [CrossRef]
- Harris, A.L. Hypoxia—A key regulatory factor in tumour growth. Nat. Rev. Cancer 2002, 2, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Garayoa, M.; Martínez, A.; Lee, S.; Pío, R.; An, W.G.; Neckers, L.; Trepel, J.; Montuenga, L.M.; Ryan, H.; Johnson, R.; et al. Hypoxia-inducible factor-1 (HIF-1) up-regulates adrenomedullin expression in human tumor cell lines during oxygen deprivation: A possible promotion mechanism of carcinogenesis. Mol. Endocrinol. 2000, 14, 848–862. [Google Scholar] [CrossRef] [PubMed]
- Buyukberber, S.; Sari, I.; Camci, C.; Buyukberber, N.M.; Sevinc, A.; Turk, H.M. Adrenomedullin expression does not correlate with survival in lung cancer. Med. Oncol. 2007, 24, 245–249. [Google Scholar] [CrossRef]
- Martinez, A.; Miller, M.J.; Unsworth, E.J.; Siegfried, J.M.; Cuttitta, F. Expression of adrenomedullin in normal human lung and in pulmonary tumors. Endocrinology 1995, 136, 4099–4105. [Google Scholar] [CrossRef]
- Pavel, M.E.; Hoppe, S.; Papadopoulos, T.; Linder, V.; Mohr, B.; Hahn, E.G.; Lohmann, T.; Schuppan, D. Adrenomedullin is a Novel Marker of Tumor Progression in Neuroendocrine Carcinomas. Horm. Metab. Res. 2006, 38, 112–118. [Google Scholar] [CrossRef]
- Umemura, S.; Segawa, Y.; Ueoka, H.; Hotta, K.; Kiura, K.; Takigawa, N.; Tabata, M.; Bessho, A.; Shinkai, T.; Tanimoto, M. Serum level of arginine-vasopressin influences the prognosis of extensive-disease small-cell lung cancer. J. Cancer Res. Clin. Oncol. 2007, 133, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Tahara, A.; Tsukada, J.; Tomura, Y.; Yatsu, T.; Shibasaki, M. Vasopressin induces human mesangial cell growth via induction of vascular endothelial growth factor secretion. Neuropeptides 2011, 45, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Imai, T.; Hirata, Y.; Emori, T.; Yanagisawa, M.; Masaki, T.; Marumo, F. Induction of endothelin-1 gene by angiotensin and vasopressin in endothelial cells. Hypertension 1992, 19 Pt 2, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Garona, J.; Sobol, N.T.; Pifano, M.; Segatori, V.I.; Gomez, D.E.; Ripoll, G.V.; Alonso, D.F. Preclinical Efficacy of [V4 Q5 ] dDAVP, a Second Generation Vasopressin Analog, on Metastatic Spread and Tumor-Associated Angiogenesis in Colorectal Cancer. Cancer Res. Treat. 2019, 51, 438–450. [Google Scholar] [CrossRef]
- Thomas, A.; Mian, I.; Tlemsani, C.; Pongor, L.; Takahashi, N.; Maignan, K.; Snider, J.; Li, G.; Frampton, G.; Ali, S.; et al. Clinical and Genomic Characteristics of Small Cell Lung Cancer in Never Smokers: Results From a Retrospective Multicenter Cohort Study. Chest 2020, 158, 1723–1733. [Google Scholar] [CrossRef]
- Bergman, B.; Sörenson, S. Smoking and effect of chemotherapy in small cell lung cancer. Eur. Respir. J. 1988, 1, 932–937. [Google Scholar]
- Chen, J.; Jiang, R.; Garces, Y.I.; Jatoi, A.; Stoddard, S.M.; Sun, Z.; Marks, R.S.; Liu, Y.; Yang, P. Prognostic factors for limited-stage small cell lung cancer: A study of 284 patients. Lung Cancer 2010, 67, 221–226. [Google Scholar] [CrossRef]
- Videtic, G.M.; Stitt, L.W.; Dar, A.R.; Kocha, W.I.; Tomiak, A.T.; Truong, P.T.; Vincent, M.D.; Yu, E.W. Continued cigarette smoking by patients receiving concurrent chemoradiotherapy for limited-stage small-cell lung cancer is associated with decreased survival. J. Clin. Oncol. 2003, 21, 1544–1549. [Google Scholar] [CrossRef]
- Portal-Nuñez, S.; Shankavaram, U.T.; Rao, M.; Datrice, N.; Atay, S.; Aparicio, M.; Camphausen, K.A.; Fernández-Salguero, P.M.; Chang, H.; Lin, P.; et al. Aryl hydrocarbon receptor-induced adrenomedullin mediates cigarette smoke carcinogenicity in humans and mice. Cancer Res. 2012, 72, 5790–5800. [Google Scholar] [CrossRef]
- Kearley, J.; Silver, J.S.; Sanden, C.; Liu, Z.; Berlin, A.A.; White, N.; Mori, M.; Pham, T.H.; Ward, C.K.; Criner, G.J.; et al. Cigarette smoke silences innate lymphoid cell function and facilitates an exacerbated type I interleukin-33-dependent response to infection. Immunity 2015, 42, 566–579. [Google Scholar] [CrossRef]


| Limited Disease N = 104 | Extensive Disease N = 148 | |
|---|---|---|
| Age, years | ||
| Mean ± SD | 67 ± 7.9 | 66 ± 8.6 |
| Gender, N (%) | ||
| Female | 61 (59) | 85 (57) |
| Male | 43 (41) | 63 (43) |
| Performance status, N (%) | ||
| 0–1 | 88 (85) | 92 (62) |
| 2–3 | 16 (15) | 56 (38) |
| Study arm | ||
| LMWH | 50 (48) | 74 (50) |
| Control | 54 (52) | 74 (50) |
| Biochemistry, median (IQR) | ||
| Hemoglobin, g/L | 135 (123–143) | 133 (121–142) |
| Leukocyte count, ×109/L | 9.1 (7.2–12.3) | 10.2 (7.3–12.8) |
| Platelet count, ×109/L | 314 (261–412) | 325 (265–443) |
| Sodium, mmol/L | 139 (135–141) | 138 (135–140) |
| Potassium, mmol/L | 4.1 (3.9–4.5) | 4.2 (4.0–4.5) |
| Serum creatinine, µmol/L | 65 (57–74) | 64 (54–80) |
| aPTT, s | 32 (30–36) | 32 (28–35) |
| Chemotherapy cycles, N (%) | ||
| <4 cycles | 14 (13) | 26 (18) |
| ≥4 cycles | 90 (87) | 122 (82) |
| Additional chemotherapy, N (%) | ||
| Second line | 31 (30) | 55 (37) |
| Third line | 9 (9) | 6 (4) |
| No additional chemotherapy | 73 (70) | 93 (63) |
| Radiotherapy, N (%) a | ||
| Prophylactic cranial | 71 (68) | 44 (30) |
| Thoracic | 71 (68) | 41 (28) |
| Metastatic lesion | 15 (14) | 46 (31) |
| No radiotherapy | 11 (11) | 41 (30) |
| Missing | 5 | 12 |
| VTE events, N (%) | 10 (10) | 5 (3) |
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |||||
|---|---|---|---|---|---|---|---|---|
| All Patients | HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value |
| MR-proADM | 1.00 (ref.) | <0.001 | 1.07 (0.73–1.56) | 0.730 | 1.59 (1.08–2.33) | 0.018 | 2.35 (1.61–3.43) | <0.001 |
| MR-proANP | 1.00 (ref.) | 0.090 | 1.06 (0.73–1.55) | 0.753 | 1.32 (0.91–1.92) | 0.142 | 1.54 (1.06–2.24) | 0.024 |
| Copeptin | 1.00 (ref.) | 0.007 | 1.48 (0.95–2.31) | 0.083 | 1.42 (0.91–2.23) | 0.124 | 2.17 (1.40–3.35) | 0.001 |
| ADM | 1.00 (ref.) | 0.001 | 1.19 (0.80–1.76) | 0.384 | 1.61 (1.09–2.36) | 0.016 | 2.14 (1.46–3.15) | <0.001 |
| ST2 | 1.00 (ref.) | <0.001 | 1.34 (0.90–2.02) | 0.153 | 2.23 (1.51–3.29) | <0.001 | 3.16 (2.14–4.66) | <0.001 |
| Limited disease | ||||||||
| MR-proADM | 1.00 (ref.) | 0.089 | 0.78 (0.42–1.43) | 0.416 | 1.58 (0.87–2.89) | 0.135 | 1.50 (0.77–2.90) | 0.231 |
| MR-proANP | 1.00 (ref.) | 0.425 | 0.72 (0.38–1.37) | 0.312 | 1.04 (0.56–1.94) | 0.898 | 1.25 (0.70–2.24) | 0.444 |
| Copeptin | 1.00 (ref.) | 0.052 | 1.58 (0.75–3.32) | 0.226 | 0.84 (0.37–1.91) | 0.677 | 2.24 (1.06–4.77) | 0.036 |
| ADM | 1.00 (ref.) | 0.339 | 0.98 (0.52–1.83) | 0.939 | 1.46 (0.76–2.79) | 0.253 | 1.57 (0.83–2.98) | 0.169 |
| ST2 | 1.00 (ref.) | 0.028 | 0.92 (0.52–1.64) | 0.777 | 1.61 (0.87–2.96) | 0.127 | 2.57 (1.23–5.40) | 0.013 |
| Extensive disease | ||||||||
| MR-proADM | 1.00 (ref.) | <0.001 | 1.51 (0.93–2.46) | 0.099 | 1.65 (1.00–2.72) | 0.051 | 3.24 (1.99–5.26) | <0.001 |
| MR-proANP | 1.00 (ref.) | 0.050 | 1.28 (0.79–2.07) | 0.322 | 1.41 (0.88–2.27) | 0.156 | 1.99 (1.22–3.26) | 0.006 |
| Copeptin | 1.00 (ref.) | 0.009 | 1.66 (0.95–2.91) | 0.077 | 2.23 (1.29–3.83) | 0.004 | 2.34 (1.36–4.01) | 0.002 |
| ADM | 1.00 (ref.) | <0.001 | 1.55 (0.94–2.58) | 0.088 | 1.70 (1.04–2.75) | 0.033 | 3.03 (1.85–4.95) | <0.001 |
| ST2 | 1.00 (ref.) | 0.001 | 2.24 (1.25–4.02) | 0.007 | 2.51 (1.46–4.31) | 0.001 | 2.99 (1.78–5.03) | <0.001 |
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |||||
|---|---|---|---|---|---|---|---|---|
| All Patients a | Adj HR (95% CI) | p-Value | Adj HR (95% CI) | p-Value | Adj HR (95% CI) | p-Value | Adj HR (95% CI) | p-Value |
| MR-proADM | 1.00 (ref.) | 0.007 | 1.12 (0.74–1.69) | 0.603 | 1.39 (0.89–2.16) | 0.144 | 2.18 (1.35–3.51) | 0.001 |
| MR-proANP | 1.00 (ref.) | 0.295 | 0.83 (0.53–1.29) | 0.403 | 0.91 (0.59–1.41) | 0.680 | 1.23 (0.76–2.00) | 0.392 |
| Copeptin | 1.00 (ref.) | 0.036 | 1.64 (1.00–2.69) | 0.049 | 1.53 (0.92–2.56) | 0.103 | 2.20 (1.28–3.78) | 0.004 |
| ADM | 1.00 (ref.) | 0.041 | 1.24 (0.81–1.90) | 0.317 | 1.41 (0.91–2.18) | 0.126 | 2.00 (1.23–3.24) | 0.005 |
| ST2 | 1.00 (ref.) | 0.004 | 1.47 (0.93–2.33) | 0.101 | 2.19 (1.37–3.52) | 0.001 | 2.40 (1.44–3.98) | 0.001 |
| Limited disease b | ||||||||
| MR-proADM | 1.00 (ref.) | 0.405 | 0.69 (0.36–1.32) | 0.261 | 1.27 (0.65–2.48) | 0.487 | 1.01 (0.46–2.24) | 0.974 |
| MR-proANP | 1.00 (ref.) | 0.160 | 0.42 (0.20–0.90) | 0.025 | 0.70 (0.35–1.40) | 0.312 | 0.54 (0.23–1.26) | 0.153 |
| Copeptin | 1.00 (ref.) | 0.331 | 1.56 (0.65–3.77) | 0.320 | 0.85 (0.33–2.19) | 0.728 | 1.69 (0.62–4.62) | 0.308 |
| ADM | 1.00 (ref.) | 0.521 | 0.79 (0.40–1.54) | 0.486 | 1.34 (0.67–2.69) | 0.403 | 0.95 (0.42–2.13) | 0.893 |
| ST2 | 1.00 (ref.) | 0.128 | 0.86 (0.45–1.64) | 0.641 | 1.45 (0.70–3.00) | 0.318 | 2.39 (0.96–5.98) | 0.063 |
| Extensive disease b | ||||||||
| MR-proADM | 1.00 (ref.) | 0.001 | 1.59 (0.89–2.84) | 0.115 | 1.57 (0.86–2.89) | 0.145 | 3.49 (1.84–6.60) | <0.001 |
| MR-proANP | 1.00 (ref.) | 0.313 | 1.16 (0.65–2.05) | 0.622 | 1.09 (0.61–1.93) | 0.775 | 1.69 (0.89–3.21) | 0.110 |
| Copeptin | 1.00 (ref.) | 0.054 | 1.73 (0.92–3.23) | 0.088 | 2.08 (1.11–3.87) | 0.022 | 2.42 (1.23–4.77) | 0.010 |
| ADM | 1.00 (ref.) | 0.012 | 1.68 (0.94–2.99) | 0.080 | 1.53 (0.86–2.70) | 0.145 | 2.88 (1.52–5.47) | 0.001 |
| ST2 | 1.00 (ref.) | 0.002 | 3.05 (1.54–6.02) | 0.001 | 3.03 (1.56–5.91) | 0.001 | 3.43 (1.73–6.79) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gezelius, E.; Bendahl, P.-O.; Gallo, W.; de Oliveira, K.G.; Ek, L.; Bergman, B.; Sundberg, J.; Melander, O.; Belting, M. Circulating Levels of the Cardiovascular Biomarkers ST2 and Adrenomedullin Predict Outcome within a Randomized Phase III Lung Cancer Trial (RASTEN). Cancers 2022, 14, 1307. https://doi.org/10.3390/cancers14051307
Gezelius E, Bendahl P-O, Gallo W, de Oliveira KG, Ek L, Bergman B, Sundberg J, Melander O, Belting M. Circulating Levels of the Cardiovascular Biomarkers ST2 and Adrenomedullin Predict Outcome within a Randomized Phase III Lung Cancer Trial (RASTEN). Cancers. 2022; 14(5):1307. https://doi.org/10.3390/cancers14051307
Chicago/Turabian StyleGezelius, Emelie, Pär-Ola Bendahl, Widet Gallo, Kelin Gonçalves de Oliveira, Lars Ek, Bengt Bergman, Jan Sundberg, Olle Melander, and Mattias Belting. 2022. "Circulating Levels of the Cardiovascular Biomarkers ST2 and Adrenomedullin Predict Outcome within a Randomized Phase III Lung Cancer Trial (RASTEN)" Cancers 14, no. 5: 1307. https://doi.org/10.3390/cancers14051307
APA StyleGezelius, E., Bendahl, P.-O., Gallo, W., de Oliveira, K. G., Ek, L., Bergman, B., Sundberg, J., Melander, O., & Belting, M. (2022). Circulating Levels of the Cardiovascular Biomarkers ST2 and Adrenomedullin Predict Outcome within a Randomized Phase III Lung Cancer Trial (RASTEN). Cancers, 14(5), 1307. https://doi.org/10.3390/cancers14051307








