Level II Oncoplastic Surgery as an Alternative Option to Mastectomy with Immediate Breast Reconstruction in the Neoadjuvant Setting: A Multidisciplinary Single Center Experience
Abstract
:Simple Summary
Abstract
1. Introduction
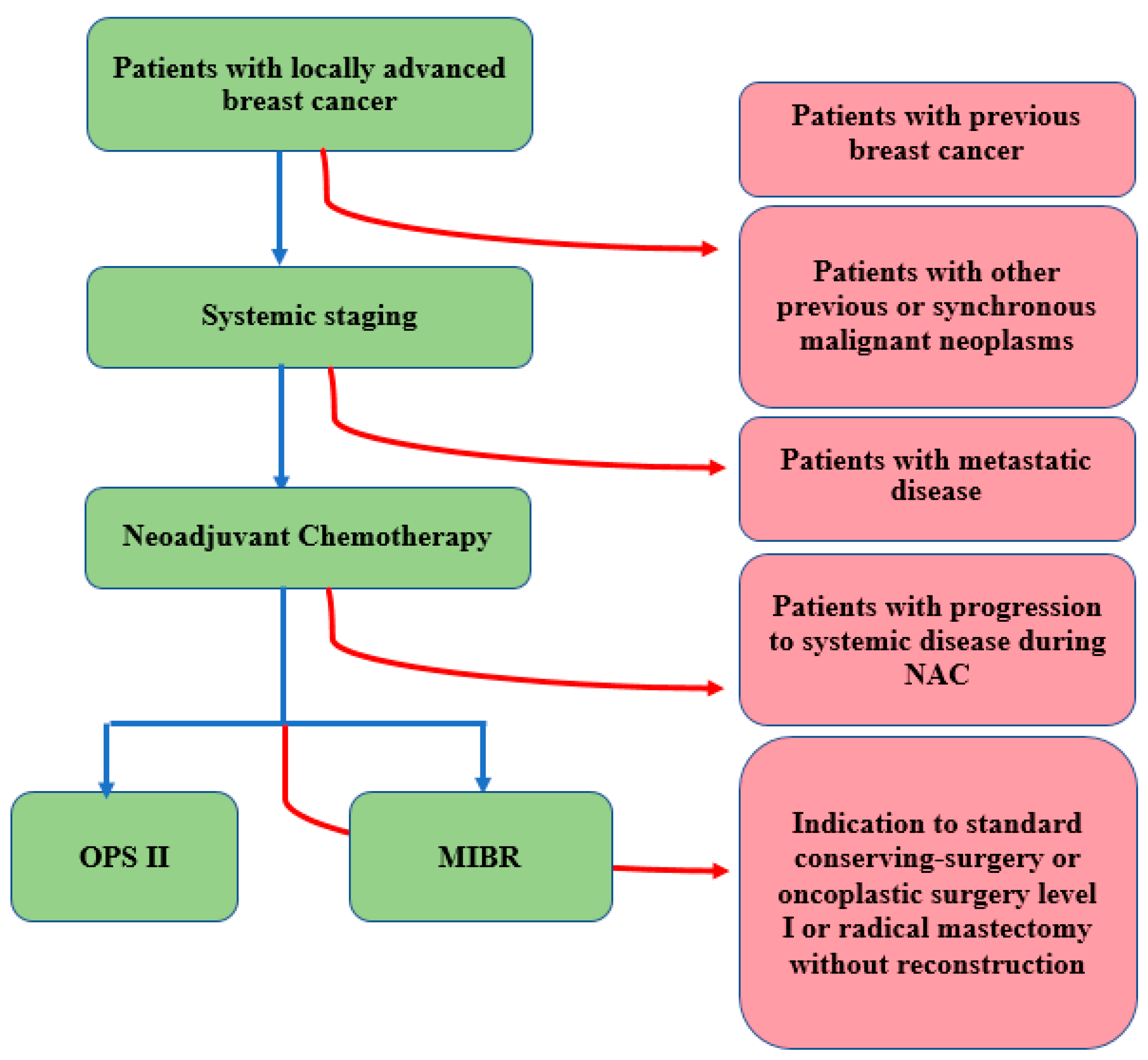
2. Materials and Methods
2.1. Setting and Population
2.2. Study Design
2.2.1. Initial Evaluation of Patients
- −
- Clinical breast examination with the acquisition of two photographs in the frontal and lateral views of the patient’s breasts, with a mark on the skin surface depicting tumor projection and measurements [17];
- −
- Breast and axillary ultrasound (EUS);
- −
- Mammography with tomosynthesis and/or mammography with contrast medium (CESM);
- −
- Magnetic resonance image (MRI) with contrast medium;
- −
- Breast fine needle aspiration biopsy to assess the histotype and biological features; markers were positioned in the breast tissue to ensure pre-surgical localization in the case of pathological complete response (pCR) or regression to a non-palpable lesion [17];
- −
- Suspicious axillary lymph node fine needle aspiration biopsy or cytology; markers were always positioned in pathologic lymph nodes.
2.2.2. Neoadjuvant Chemotherapy
2.2.3. Operative Protocol and Surgical Technique
2.3. Adjuvant Treatments
2.3.1. Adjuvant Chemotherapy
2.3.2. Adjuvant Radiotherapy
2.4. Follow-Up and Endpoints
- −
- “Local disease-free survival” (L-DFS): time from the day of diagnosis to ipsilateral breast recurrence;
- −
- “Regional disease-free survival” (R-DFS): time from the day of diagnosis to ipsilateral regional lymph node recurrence;
- −
- “Distant disease-free survival” (D-DFS): time from the day of diagnosis to distant recurrence;
- −
- “Overall survival” (OS): time from the day of diagnosis to death from any cause or latest follow-up.
2.5. Statistical Analysis
3. Results
3.1. Adjuvant Treatment
3.2. Oncological Outcomes
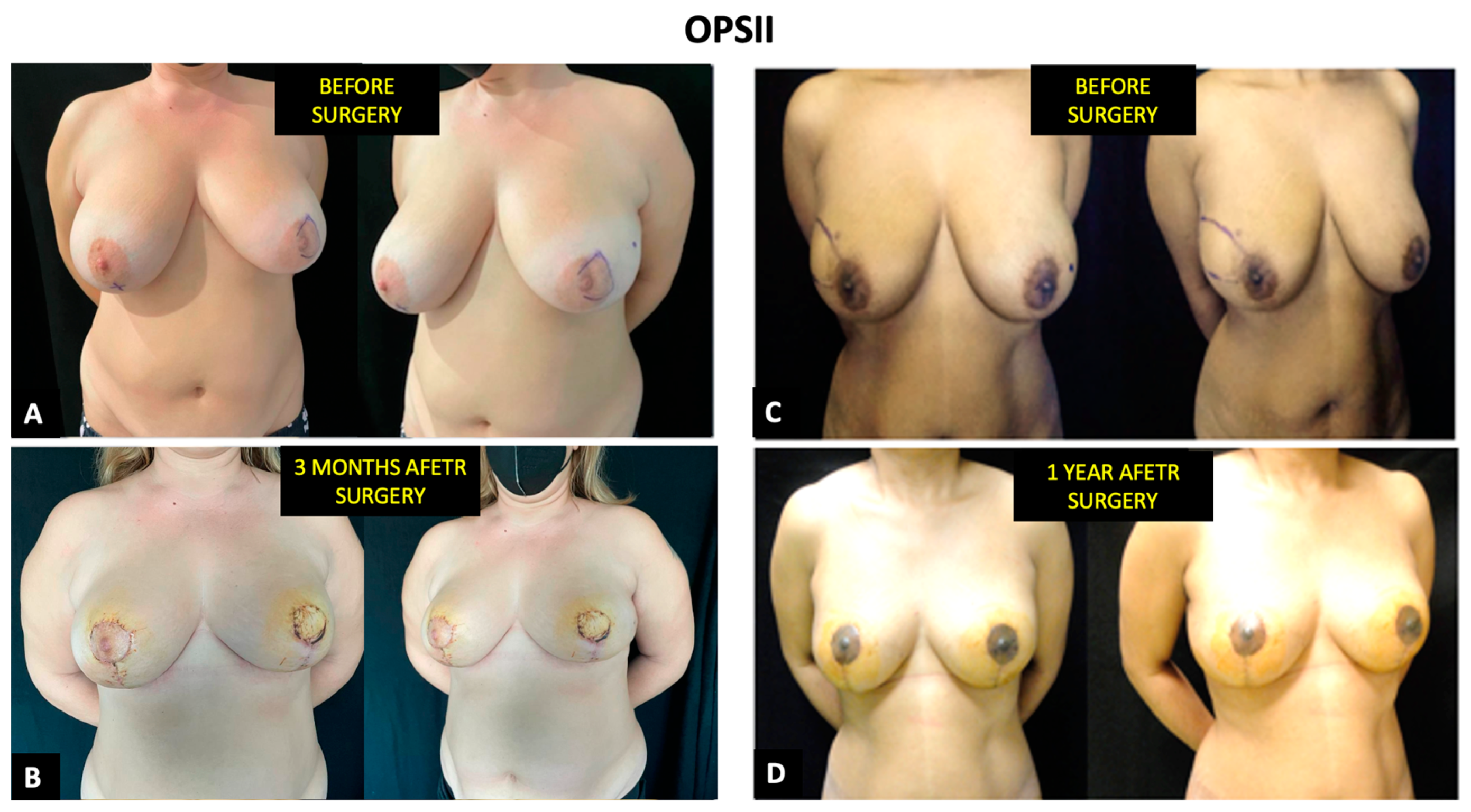
3.3. Aesthetic Outcomes and Health-Related Quality of Life
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benjamin, M.A.; Sinnott, C.; Bawa, S.; Kaufman, D.I.; Guarino, K.; Addona, T. Re-excision rate after partial mastectomy in oncoplastic breast-conserving surgery: A single-institutional experience and review of the literature. Ann. Plast. Surg. 2019, 82 (Suppl. 3), S170–S172. [Google Scholar] [CrossRef]
- Clinical Practice Guidelines in Oncology. Breast Cancer (NCCN Guidelines) Version 1. 2022. Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (accessed on 7 January 2022).
- Weber, W.P.; Soysal, S.D.; El-Tamer, M.; Sacchini, V.; Knauer, M.; Tausch, C.; Hauser, N.; Günthert, A.; Harder, Y.; Kappos, E.A.; et al. First international consensus conference on standardization of oncoplastic breast conserving surgery. Breast Cancer Res. Treat. 2017, 165, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Clough, K.B.; Van la Parra, R.F.; Thygesen, H.H.; Levy, E.; Russ, E.; Halabi, N.M.; Sarfati, I.; Nos, C. Long-term results after oncoplastic surgery for breast cancer: A 10-year follow-up. Ann. Surg. 2018, 268, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Franceschini, G.; Terribile, D.; Magno, S.; Fabbri, C.; Accetta, C.; Di Leone, A.; Moschella, F.; Barbarino, R.; Scaldaferri, A.; D’Archi, S.; et al. Update on oncoplastic breast surgery. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 1530–1540. [Google Scholar]
- Franceschini, G.; Magno, S.; Fabbri, C.; Chiesa, F.; Di Leone, A.; Moschella, F.; Scafetta, I.; Scaldaferri, A.; Fragomeni, S.M.; Barone, L.A.; et al. Conservative and radical oncoplastic approches in the surgical treatment of breast cancer. Eur. Rev. Med. Pharmacol. Sci. 2009, 12, 387–396. [Google Scholar]
- Masetti, R.; Di Leone, A.; Franceschini, G.; Magno, S.; Terribile, D.; Fabbri, M.C.; Chiesa, F. Oncoplastic techniques in the conservative surgical treatment of breast cancer: An overview. Breast J. 2006, 12 (Suppl. 2), S174–S180. [Google Scholar] [CrossRef] [PubMed]
- Masetti, R.; Pirulli, P.G.; Magno, S.; Franceschini, G.; Chiesa, F.; Antinori, A. Oncoplastic techniques in the conservative surgical treatment of breast cancer. Breast Cancer 2000, 7, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.D. A step-by-step oncoplastic breast conservation surgical atlas of reproducible dissection techniques and anatomically ideal incision placement. Breast Cancer Res. Treat. 2017, 165, 505–516. [Google Scholar] [CrossRef]
- Kopkash, K.; Clark, P. Basic oncoplastic surgery for breast conservation: Tips and techniques. Ann. Surg. Oncol. 2018, 25, 2823–2828. [Google Scholar] [CrossRef] [PubMed]
- Strach, M.C.; Prasanna, T.; Kirova, Y.M.; Alran, S.; O’Toole, S.; Beith, J.M.; Poortmans, P.; McNeil, C.M.; Carroll, S. Optimise not compromise: The importance of a multidisciplinary breast cancer patient pathway in the era of oncoplastic and reconstructive surgery. Crit. Rev. Oncol. 2018, 134, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Crown, A.; Laskin, R.; Rocha, F.G.; Grumley, J. Extreme oncoplasty: Expanding indications for breast conservation. Am. J. Surg. 2019, 217, 851–856. [Google Scholar] [CrossRef]
- Silverstein, M.J.; Savalia, N.; Khan, S.; Ryan, J. Extreme oncoplasty: Breast conservation for patients who need mastectomy. Breast J. 2015, 21, 52–59. [Google Scholar] [CrossRef] [Green Version]
- Silverstein, M.J. Radical mastectomy to radical conservation (extreme oncoplasty): A revolutionary change. J. Am. Coll. Surg. 2016, 222, 1–9. [Google Scholar] [CrossRef] [PubMed]
- De Lorenzi, F.; Borelli, F.; Pagan, E.; Bagnardi, V.; Peradze, N.; Jereczek-Fossa, B.A.; Leonardi, C.; Mazzarol, G.; Favia, G.; Corso, G.; et al. Oncoplastic breast-conserving surgery for synchronous multicentric and multifocal tumors: Is it oncologically safe? A retrospective matched-cohort analysis. Ann. Surg. Oncol. 2022, 29, 427–436. [Google Scholar] [CrossRef]
- Abidi, S.S.; Vohra, L.M.; Javed, M.R.; Khan, N. Oncoplastic surgery: A suitable alternative to conventional breast conserving surgery in low–Middle income countries; a retrospective cohort study. Ann. Med. Surg. 2021, 68, 102618. [Google Scholar] [CrossRef] [PubMed]
- Di Leone, A.; Terribile, D.; Magno, S.; Sanchez, A.M.; Scardina, L.; Mason, E.J.; D’Archi, S.; Maggiore, C.; Rossi, C.; Di Micco, A.; et al. Neoadjuvant chemotherapy in breast cancer: An advanced personalized multidisciplinary prehabilitation model (APMP-M) to optimize outcomes. J. Pers. Med. 2021, 11, 324. [Google Scholar] [CrossRef] [PubMed]
- Man, V.C.; Cheung, P.S. Neoadjuvant chemotherapy increases rates of breast-conserving surgery in early operable breast cancer. Hong Kong Med. J. 2017, 23, 251–257. [Google Scholar] [CrossRef] [Green Version]
- Recht, A.; Comen, E.A.; Fine, R.E.; Fleming, G.F.; Hardenbergh, P.H.; Ho, A.Y.; Hudis, C.A.; Hwang, E.S.; Kirshner, J.J.; Morrow, M.; et al. Postmastectomy radiotherapy: An American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology Focused Guideline Update. Pract. Radiat. Oncol. 2016, 6, e219–e234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franceschini, G.; Scardina, L.; Di Leone, A.; Terribile, D.; Sanchez, A.; Magno, S.; D’Archi, S.; Franco, A.; Mason, E.; Carnassale, B.; et al. Immediate prosthetic breast reconstruction after nipple-sparing mastectomy: Traditional subpectoral technique versus direct-to-implant prepectoral reconstruction without acellular dermal matrix. J. Pers. Med. 2021, 11, 153. [Google Scholar] [CrossRef] [PubMed]
- Nardone, L.; Valentini, V.; Marino, L.; De Santis, M.C.; Terribile, D.; Franceschini, G.; Balducci, M.; Mantini, G.; Mattiucci, G.; Mule, A.; et al. A feasibility study of neo-adjuvant low-dose fractionated radiotherapy with two different concurrent anthracycline-docetaxel schedules in stage IIA/B-IIIA breast cancer. Tumori 2012, 98, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Arlow, R.L.; Paddock, L.E.; Niu, X.; Kirstein, L.; Haffty, B.G.; Goyal, S.; Kearney, T.; Toppmeyer, D.; Stroup, A.M.; Khan, A.J. Breast-conservation therapy after neoadjuvant chemotherapy does not compromise 10-year breast cancer–specific mortality. Am. J. Clin. Oncol. 2018, 41, 1246–1251. [Google Scholar] [CrossRef] [PubMed]
- Mauri, D.; Pavlidis, N.; Ioannidis, J.P. Neoadjuvant versus adjuvant systemic treatment in breast cancer: A meta-analysis. J. Natl. Cancer Inst. 2005, 97, 188–194. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, S.; Pappas, L.; Neumayer, L.; Kokeny, K.; Agarwal, J. Effect of breast conservation therapy vs mastectomy on disease-specific survival for early-stage breast cancer. JAMA Surg. 2014, 149, 267–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.; Laws, A.; Hu, J.; Barry, W.; Golshan, M.; King, T. Margins in breast-conserving surgery after neoadjuvant therapy. Ann. Surg. Oncol. 2018, 25, 3541–3547. [Google Scholar] [CrossRef] [PubMed]
- Clough, K.B.; Acosta-Marín, V.; Nos, C.; Alran, S.; Rouanet, P.; Garbay, J.-R.; Giard, S.; Verhaeghe, J.-L.; Houvenaeghel, G.; Flipo, B.; et al. Rates of neoadjuvant chemotherapy and oncoplastic surgery for breast cancer surgery: A French national survey. Ann. Surg. Oncol. 2015, 22, 3504–3511. [Google Scholar] [CrossRef] [PubMed]
- Regaño, S.; Hernanz, F.; Ortega, E.; Redondo-Figuero, C.; Gómez-Fleitas, M. Oncoplastic techniques extend breast-conserving surgery to patients with neoadjuvant chemotherapy response unfit for conventional techniques. World J. Surg. 2009, 33, 2082–2086. [Google Scholar] [CrossRef] [PubMed]
- Van la Parra, R.F.; Clough, K.B.; Thygesen, H.H.; Levy, E.; Poulet, B.; Sarfati, I.; Nos, C. Oncological safety of oncoplastic level II mammoplasties after neoadjuvant chemotherapy for large breast cancers: A matched-cohort analysis. Ann. Surg. Oncol. 2021, 28, 5920–5928. [Google Scholar] [CrossRef] [PubMed]
- Gulcelik, M.A.; Dogan, L. Unplanned breast-conserving surgery after systemic therapy in locally advanced breast cancer: The results of level II oncoplastic techniques. Int. J. Clin. Pract. 2021, 75, e14268. [Google Scholar] [CrossRef]
- Franceschini, G.; Di Leone, A.; Natale, M.; Sanchez, M.A.; Masett, R. Conservative surgery after neoadjuvant chemotherapy in patients with operable breast cancer. Ann. Ital. Chir. 2018, 89, 290. [Google Scholar]


| Characteristics | All Patients | OPSII | MIBR | p-Value |
|---|---|---|---|---|
| 297 | 87 (29.3%) | 210 (70.7%) | ||
| Age (y) | 46.3 (44.8; 39.7–52) | 50.1 (48.5; 42.7–55.8) | 44.6 (43.5; 38.6–50.5) | 0.000 |
| Postmenopausal status - Yes - No | 100 (33.3%) 197 (65.7%) | 43 (49.4%) 44 (50.6%) | 57 (27.1%) 153 (72.9%) | 0.000 |
| BMI (Kg/m2) | 23.7 (23.4; 21.2–25.1) | 27.1 (26.6; 23.9–30.1) | 23.7 (23.4; 21.2–25.1) | 0.006 |
| BMI classes - BMI < 18 - BMI 18–24 - BMI 24–30 - BMI > 30 | 3 (1.3%) 155 (52.1%) 103 (34.6%) 36 (12%) | 0 (0%) 27 (29.9%) 38 (44.8%) 22 (25.3%) | 3 (1.4%) 128 (60.9%) 65 (31%) 14 (6.7%) | 0.000 |
| Breast related cancer antigens (BRCA) mutations | 53 (17.8%) | 4 (4.6%) | 49 (23.3%) | 0.000 |
| Histotype - Ductal invasive carcinoma - Lobular invasive carcinoma - Other | 198 (66.7%) 22 (7.4%) 77 (25.9%) | 61 (70.1%) 6 (6.9%) 20 (23%) | 137 (65.2%) 16 (7.6%) 57 (27.1%) | 0.715 |
| Tumor subtype - Luminal A - Luminal B - HER2+ - TN | 14 (4.7%) 133 (44.8%) 98 (33%) 52 (17.5%) | 8 (9.2%) 40 (46%) 24 (27.6%) 15 (17.2%) | 6 (2.9%) 93 (44.3%) 74 (35.2%) 37 (17.6%) | 0.095 |
| Grading - G1 - G2 - G3 | 4 (1.3%) 94 (31.7%) 199 (67%) | 3 (3.4%) 26 (29.9%) 58 (66.7%) | 1 (0.5%) 68 (32.4%) 141 (67.1%) | 0.125 |
| Tumor diameter (mm) | 41.6 (35; 27–50) | 44.2 (38; 27–56) | 40.5 (35; 26.7–50) | 0.038 |
| Clinical T - cT2 - cT3 - cT4 | 215 (72.4%) 64 (21.5%) 18 (6.1%) | 59 (67.8%) 20 (23%) 8 (9.2%) | 156 (74.3%) 44 (21%) 10 (4.8%) | 0.291 |
| Multifocality/multicentricity | 159 (53.5%) | 49 (56.3%) | 110 (52.4%) | 0.609 |
| Clinical N - cN0 - cN1 - cN2 - cN3 | 111 (37.4%) 131 (44.1%) 44 (14.8%) 11 (3.7%) | 29 (33.3%) 43 (49.4%) 14 (16.2%) 1 (1.1%) | 82 (39%) 88 (41.9%) 30 (14.3%) 10 (4.8%) | 0.304 |
| Characteristics | All Patients | OPSII | MIBR | p-Value |
|---|---|---|---|---|
| 297 | 87 (29.3%) | 210 (70.7%) | ||
| Neoadjuvant chemotherapy - Anthracycline and/or taxanes - Anthracycline + taxanes - Other schemes | 5 (1.7%) 235 (79.1%) 57 (19.2%) | 0 (0%) 73 (83.9%) 14 (16.1%) | 5 (2.3%) 162 (77.2%) 43 (20.5%) | 0.385 |
| Regimens with trastuzumab | 98 (33%) | 24 (27.6%) | 74 (35.2%) | 0.096 |
| Clinical response on T - Clinical complete response - Clinical partial response - No response - Progression | 92 (30.9%) 189 (63.7%) 8 (2.7%) 8 (2.7%) | 23 (26.4%) 61 (70.1%) 2 (2.3%) 1 (1.1%) | 69 (32.9%) 128 (61%) 6 (2.9%) 7 (3.3%) | 0.425 |
| ycT - 0 - 1 - 2 - 3 | 92 (31%) 111 (37.4%) 79 (26.5%) 15 (5.1%) | 23 (26.4%) 37 (42.5%) 25 (28.7%) 2 (2.3%) | 69 (32.9%) 74 (35.2%) 54 (25.7%) 13 (6.2%) | 0.290 |
| Clinical Response on N - ycN0 - ycN+ | 231 (77.8%) 66 (22.2%) | 66 (75.9%) 21 (24.1%) | 165 (78.6%) 45 (21.4%) | 0.646 |
| Characteristics | All Patients | OPSII | MIBR | p-Value |
|---|---|---|---|---|
| 297 | 87 (29.3%) | 210 (70.7%) | ||
| ypT - ypT0 - ypT0i+ 1 - ypTmic 2 - ypT1 - ypT2 - ypT3 - ypT4 | 87 (29.3%) 6 (2%) 24 (8%) 113 (38.1%) 56 (18.9%) 9 (3%) 2 (0.7%) | 19 (21.8%) 0 (0%) 4 (4.6%) 39 (44.9%) 20 (23%) 4 (4.6%) 1 (1.1%) | 68 (32.4%) 6 (2.9%) 20 (9.5%) 74 (35.2%) 36 (17.1%) 5 (2.4%) 1 (0.5%) | 0.074 |
| Mean residual tumor size (mm) | 11.2 (6; 0–18) | 14.2 (10; 1–23) | 10 (4.5; 0–15.3) | 0.168 |
| Pathological response on T - Pathological complete response - Pathological partial response - Pathological progression or no response | 87 (29.3%) 185 (62.3%) 25 (8.4%) | 19 (21.8%) 57 (65.5%) 11 (12.7%) | 68 (32.3%) 128 (61%) 14 (6.7%) | 0.074 |
| Multifocality/multicentricity | 91 (30.3%) | 29 (33.3%) | 62 (29.5%) | 0.383 |
| Histotype in residual disease - Ductal invasive carcinoma - Lobular invasive carcinoma - Others - Not evaluable 3 | 159 (53.5%) 18 (6.1%) 31 (10.4%) 89 (30%) | 53 (60.9%) 6 (6.9%) 9 (10.4%) 19 (21.8%) | 106 (50.3%) 12 (5.7%) 22 (10.5%) 70 (33.3%) | 0.233 |
| ER - Positive - Negative - Not evaluable 3 | 138 (46.5%) 49 (16.5%) 110 (37%) | 44 (50.6%) 17 (19.5%) 26 (29.9%) | 97 (46.2%) 31 (14.8%) 82 (39%) | 0.281 |
| PR - Positive - Negative - Not evaluable 3 | 97 (32.7%) 90 (30.3%) 110 (37%) | 31 (35.6%) 30 (34.5%) 26 (29.9%) | 68 (32.4%) 60 (28.6%) 82 (39%) | 0.313 |
| Ki67 - ≥25 - <24 - Not evaluable 3 | 80 (26.9%) 107 (36.1%) 110 (37%) | 25 (28.7%) 36 (41.4%) 26 (29.9%) | 55 (26.2%) 73 (34.8%) 82 (39%) | 0.227 |
| Tumor subtype - Luminal A - Luminal B - HER2+ - TN - Not evaluable 3 | 92 (31%) 38 (12.8%) 28 (9.4%) 36 (12.1%) 103 (34.7%) | 29 (33.3%) 14 (16.1%) 5 (5.7%) 16 (18.5%) 23 (26.4%) | 61 (29.1%) 24 (11.4%) 23 (11%) 20 (9.5%) 82 (39%) | 0.052 |
| N patients who underwent SLNB Mean number of lymph nodes removed (range) | 217 (73.1%) 2.86 (3; 2–4) | 64 (73.6%) 2.8 (3; 1.25–4) | 153 (72.9%) 2.88 (3; 2–4) | 0.492 |
| ypN (sn) - ypN0 - presence of ITC - presence of mic - 1 lymph node positive - 2 lymph nodes positive - 3 lymph nodes positive | 131 (60.4%) 14 (6.5%) 16 (7.4%) 42 (19.4%) 7 (3.2%) 7 (3.2%%) | 32 (50%) 3 (4.7%) 5 (7.8%) 16 (25%) 3 (4.7%) 5 (7.8%) | 99 (64.7%) 11 (7.2%) 11 (7.2%) 26 (17%) 4 (2.6%) 2 (1.3%) | 0.073 |
| N patients who underwent AD Mean number of lymph node removed (range) | 194 (65.3%) 13.8 (13; 10–16.25) | 61 (70.1%) 14.6 (13; 10–17) | 133 (63.3%) 13.4 (12; 10–16) | 0.289 |
| ypN - ypN0 - ypN0i+ - ypNmic - ypN1 - ypN2 - ypN3 | 59 (30.4%) 10 (5.1%) 12 (6.2%) 67 (34.6%) 24 (12.4%) 22 (11.3%) | 17 (27.8%) 2 (3.2%) 2 (3.2%) 23 (37.8%) 10 (16.5%) 7 (11.5%) | 42 (31.6%) 8 (6%) 10 (7.5%) 44 (33.1%) 14 (10.5%) 15 (11.3%) | 0.388 |
| Characteristics | All Patients | OPSII | MIBR | Long-Rank |
|---|---|---|---|---|
| 297 | 87 (29.3%) | 210 (70.7%) | ||
| Surgical margins - Clear margins - “ink on tumor” | 293 (98.7%) 4 (1.3%) | 86 (98.9%) 1 (1.1%) | 207 (98.6%) 3 (1.4%) | 0.949 |
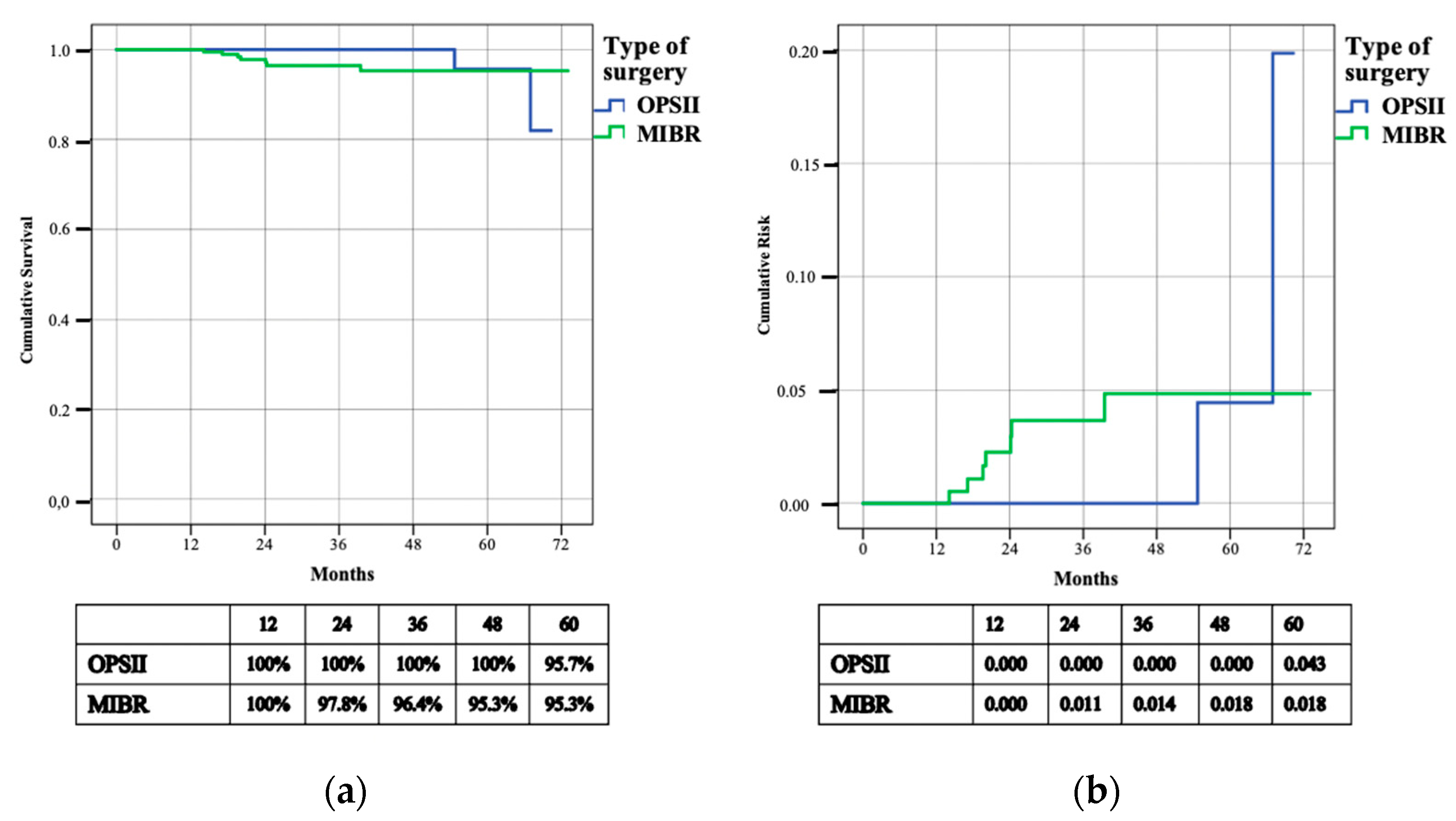
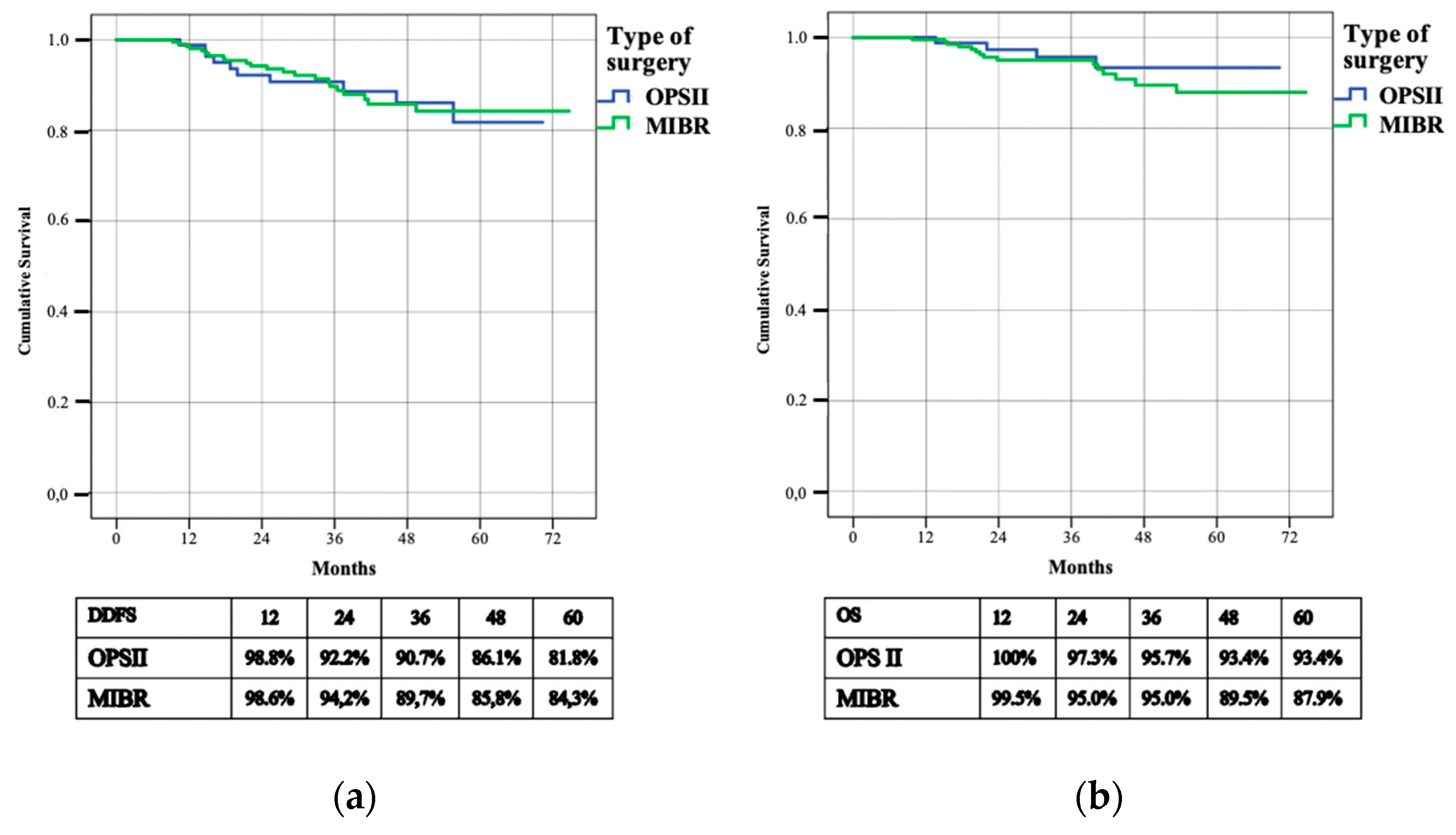
| Local disease free survival - N. patients with breast recurrence - 3 years—L-DFS - 3 years cumulative risk | 16 (5.4%) 95.8% 0.014 | 3 (3.4%) 95.1% 0.028 | 13 (6.1%) 96.2% 0.016 | 0.286 |
| Regional disease free survival - N. patients with axillary recurrence - 3 years—R-DFS - 3 years cumulative risk | 9 (3%) 97.5% 0.010 | 2 (2.3%) 100% 0.000 | 7 (3.3%) 96.4% 0.014 | 0.559 |
| Distant disease free survival - N. patients with systemic recurrence - 3 years—D-DFS | 32 (10.8%) 90% | 10 (11.5%) 90.7% | 22 (10.5%) 89.7% | 0.849 |
| Overall survival - N. patients deceased - 3 years—OS | 19 (6.4%) 95.2% | 4 (4.6%) 95.7% | 15 (7.1%) 95% | 0.394 |
| Characteristics | Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|---|
| p-Value | OR | 95% CI | p-Value | OR | 95% CI | B-Coefficient | |
| Postmenopausal status | 0.607 | 1.221 | 0.571–2.611 | ||||
| BRCA pathological mutations | 0.271 | 1.622 | 0.685–3.841 | ||||
| Grading 3 (G3) | 0.813 | 1.100 | 0.499–2.424 | ||||
| cT (3 or 4) | 0.193 | 1.663 | 0.773–3.557 | ||||
| cN+ | 0.064 | 2.285 | 0.954–5.475 | ||||
| HER2+ | 0.005 | 0.056 | 0.007–0.413 | 0.015 | 0.082 | 0.011–0.617 | −2.506 |
| TN | 0.089 | 2.068 | 0.895–4.782 | ||||
| pCR on breast | 0.009 | 0.068 | 0.009–0.506 | 0.054 | 0.135 | 0.018–1.037 | −2.004 |
| pCR on axilla | 0.007 | 1.685 | 1.156–2.455 | 0.163 | 1.308 | 0.897–1.909 | 0.269 |
| Ink on tumor | 0.999 | 0.000 | 0.000 | ||||
| ypT (3 or 4) | 0.089 | 3.310 | 0.832–13.176 | ||||
| ypN (2 or 3) | 0.574 | 1.249 | 0.575–2.714 | ||||
| Radiotherapy 1 | 0.009 | 2.705 | 1.289–5.675 | 0.133 | 1.568 | 0.872–2.820 | 0.450 |
| Characteristisc | All Patients | OPSII | MIBR | p-Value |
|---|---|---|---|---|
| 297 | 87 (29.3%) | 210 (70.7%) | ||
| Number of answers | 194 (65.3%) | 55 (28.4%) | 139 (71.6%) | |
| Q.1 Satisfaction with breasts | ||||
| Average score (median score) 1 | 54.3 (53) | 61 (58) | 51.6 (53) | 0.656 |
| Score | ||||
| - 0–34 | 36 (18.6%) | 5 (9%) | 31 (22.3%) | |
| - 39–58 | 88 (45.4%) | 25 (45.5%) | 63 (45.3%) | 0.052 |
| - 63–100 | 70 (36.1%) | 25 (45.5%) | 45 (32.4%) | |
| Q.2 Psychosocial well-being | ||||
| Average score (median score) 1 | 59.8 (56) | 64.2 (64) | 58.1 (55) | 0.444 |
| Score | ||||
| - 0–39 | 28 (14.4%) | 4 (7.3%) | 24 (17.3%) | |
| - 41–58 | 73 (37.6%) | 19 (34.5%) | 54 (38.8%) | 0.105 |
| - 60–100 | 93 (47.9%) | 32 (58.2%) | 61 (43.9%) | |
| Q.3 Physical well-being: chest | ||||
| Average score (median score) 1 | 37 (32) | 28.6 (28) | 40.3 (3) | 0.007 |
| Score | ||||
| - 0–32 | 103 (53.1%) | 35 (63.6%) | 68 (48.9%) | |
| - 36–68 | 66 (34%) | 18 (32.7%) | 48 (34.5%) | 0.027 |
| - 72–100 | 25 (12.9%) | 2 (3.6%) | 23 (16.5%) | |
| Q.4 Loss of sensitivity | ||||
| - No | 46 (23.7%) | 28 (50.9%) | 18 (12.9%) | 0.000 |
| - Yes | 148 (76.3%) | 27 (49.1%) | 121 (87.1%) | |
| Q.4.1 Percentage of sensitivity loss | ||||
| - Mean percentage loss | 7.49 (8) | 6.44 (7) | 7.73 (8) | 0.631 |
| Score | ||||
| - 10–30 | 7 (4.7%) | 4 (14.8%) | 3 (2.5%) | |
| - 40–70 | 53 (35.8%) | 12 (44.4%) | 41 (33.9%) | 0.011 |
| - 80–100 | 88 (59.5%) | 11 (40.7%) | 77 (63.6%) | |
| Q.4.2 Influence of sensitivity loss on ordinary life | ||||
| - Mean influence | 4.15 (5) | 3.56 (3) | 4.28 (5) | 0.784 |
| Score | ||||
| - 0–30 | 62 (41.9%) | 14 (51.9%) | 48 (39.7%) | |
| - 40–70 | 60 (40.5%) | 9 (33.3%) | 51 (42.1%) | 0.621 |
| - 80–100 | 26 (17.6%) | 4 (40.7%) | 22 (18.2%) | |
| Q.4.3 Influence of sensitivity loss on sex life | ||||
| - Mean influence | 6.06 (7) | 6.11 (7) | 6.05 (7) | 0.260 |
| Score | ||||
| - 0–30 | 37 (25%) | 5 (18.5%) | 32 (26.4%) | |
| - 40–70 | 39 (26.4%) | 10 (37.1%) | 29 (24%) | 0.499 |
| - 80–100 | 72 (48.6%) | 12 (44.4%) | 60 (49.6%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Leone, A.; Franco, A.; Terribile, D.A.; Magno, S.; Fabi, A.; Sanchez, A.M.; D’Archi, S.; Scardina, L.; Natale, M.; Mason, E.J.; et al. Level II Oncoplastic Surgery as an Alternative Option to Mastectomy with Immediate Breast Reconstruction in the Neoadjuvant Setting: A Multidisciplinary Single Center Experience. Cancers 2022, 14, 1275. https://doi.org/10.3390/cancers14051275
Di Leone A, Franco A, Terribile DA, Magno S, Fabi A, Sanchez AM, D’Archi S, Scardina L, Natale M, Mason EJ, et al. Level II Oncoplastic Surgery as an Alternative Option to Mastectomy with Immediate Breast Reconstruction in the Neoadjuvant Setting: A Multidisciplinary Single Center Experience. Cancers. 2022; 14(5):1275. https://doi.org/10.3390/cancers14051275
Chicago/Turabian StyleDi Leone, Alba, Antonio Franco, Daniela Andreina Terribile, Stefano Magno, Alessandra Fabi, Alejandro Martin Sanchez, Sabatino D’Archi, Lorenzo Scardina, Maria Natale, Elena Jane Mason, and et al. 2022. "Level II Oncoplastic Surgery as an Alternative Option to Mastectomy with Immediate Breast Reconstruction in the Neoadjuvant Setting: A Multidisciplinary Single Center Experience" Cancers 14, no. 5: 1275. https://doi.org/10.3390/cancers14051275
APA StyleDi Leone, A., Franco, A., Terribile, D. A., Magno, S., Fabi, A., Sanchez, A. M., D’Archi, S., Scardina, L., Natale, M., Mason, E. J., Murando, F., Marazzi, F., Orlandi, A., Paris, I., Visconti, G., Palazzo, A., Masiello, V., Barone Adesi, L., Salgarello, M., ... Franceschini, G. (2022). Level II Oncoplastic Surgery as an Alternative Option to Mastectomy with Immediate Breast Reconstruction in the Neoadjuvant Setting: A Multidisciplinary Single Center Experience. Cancers, 14(5), 1275. https://doi.org/10.3390/cancers14051275

















