The Biology of Ocular Adnexal Marginal Zone Lymphomas
Abstract
:Simple Summary
Abstract
1. Definitions
1.1. Ocular Adnexa
1.2. Extranodal Marginal Zone Lymphomas
2. Epidemiology
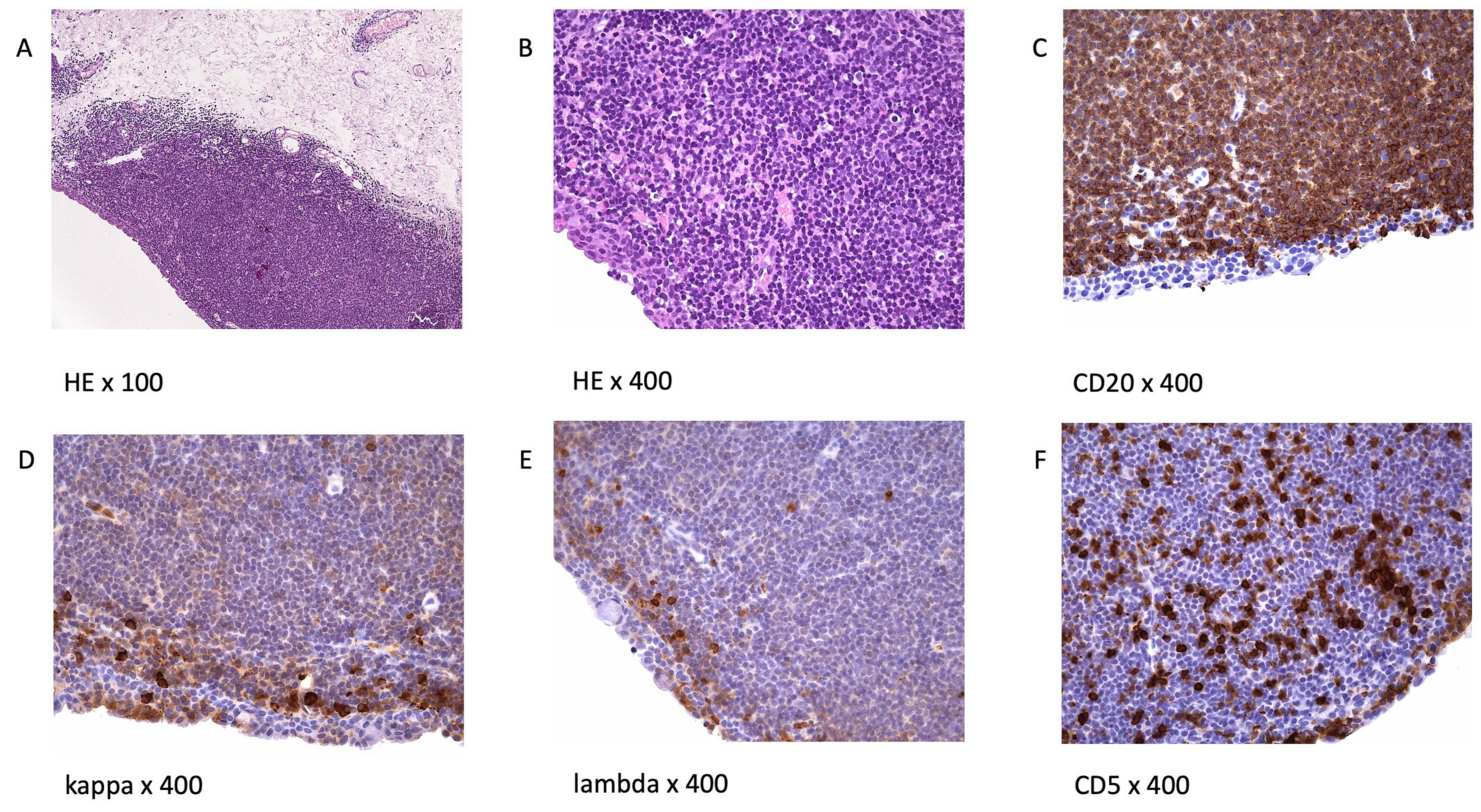
3. Morphology and Immunophenotype
4. Bilateral and Recurrent Disease
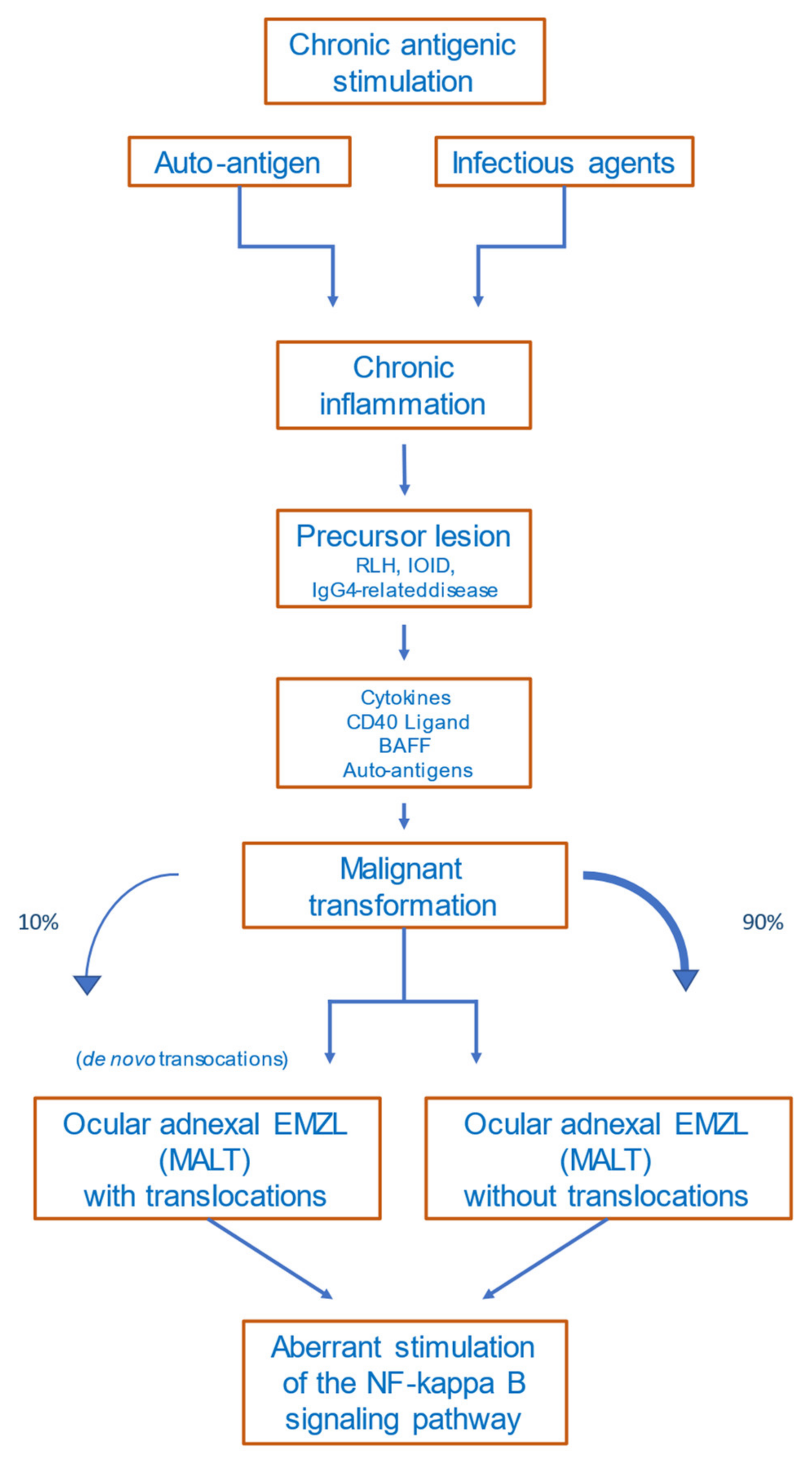
5. Etiology and Pathogenesis
5.1. Precursor Lesions
5.2. Antigen Stimulation
5.2.1. Infectious Agents
Chlamydophila psittaci
- (1)
- Other Bacteria
- (2)
- Viral Pathogens
5.2.2. Autoimmune Diseases
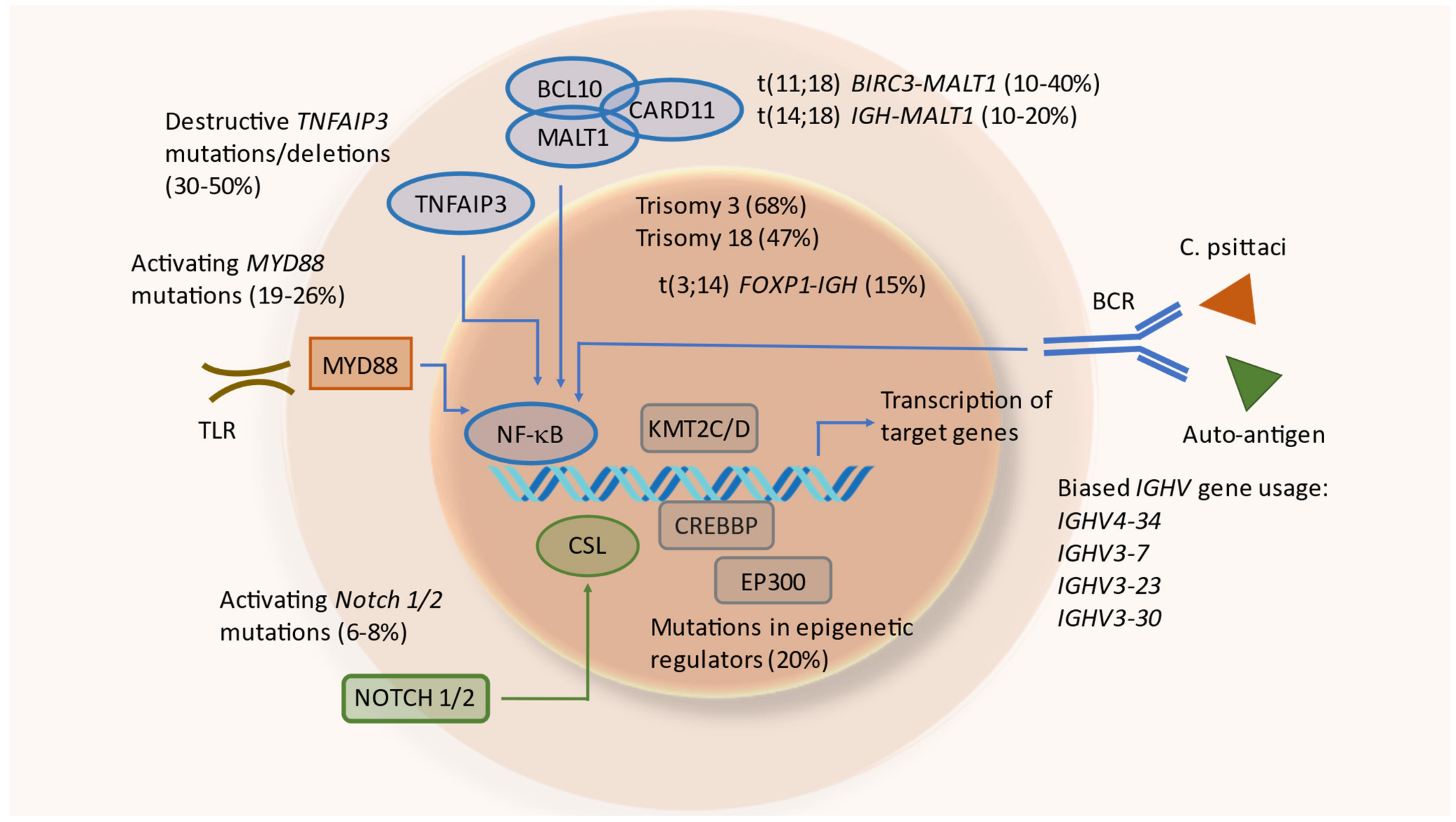
5.3. Chromosomal Aberrations
5.3.1. Translocations
5.3.2. Copy Number Variations
5.4. Genetic Alterations in Particular Signaling Pathways
5.4.1. Nuclear Factor Kappa B (NF-κB) Pathway
5.4.2. NOTCH Pathway
5.4.3. NFAT Signaling
5.5. Epigenetic Regulators
5.6. Additional Mutated Genes
6. B-Cell Receptors of OAL
7. Altered DNA Methylation
8. Altered microRNA Expression
9. Microenvironment
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dutton, J. Atlas of Clinical and Surgical Orbital Anatomy, 2nd ed.; W.B. Saunders Co. Ltd.: Philadelphia, PA, USA; Elsevier: London, UK, 2018. [Google Scholar]
- Freddo, T.C.E. Anatomy of the Eye and Orbit, 1st ed.; Wolters Kluwer Health: Philadelphia, PA, USA, 2018. [Google Scholar]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, H.U.; Son, J.H. Ocular adnexal mucosa-associated lymphoid tissue lymphoma: A narrative review. J. Yeungnam Med. Sci. 2022, 39, 3–11. [Google Scholar] [CrossRef]
- Kalogeropoulos, D.; Papoudou-Bai, A.; Kanavaros, P.; Kalogeropoulos, C. Ocular adnexal marginal zone lymphoma of mucosa-associated lymphoid tissue. Clin. Exp. Med. 2018, 18, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Olsen, T.G.; Holm, F.; Mikkelsen, L.H.; Rasmussen, P.K.; Coupland, S.E.; Esmaeli, B.; Finger, P.T.; Graue, G.F.; Grossniklaus, H.E.; Honavar, S.G.; et al. Orbital Lymphoma-An International Multicenter Retrospective Study. Am. J. Ophthalmol. 2019, 199, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Sassone, M.; Ponzoni, M.; Ferreri, A.J. Ocular adnexal marginal zone lymphoma: Clinical presentation, pathogenesis, diagnosis, prognosis, and treatment. Best Pract. Res. Clin. Haematol. 2017, 30, 118–130. [Google Scholar] [CrossRef]
- Coupland, S.E.; Krause, L.; Delecluse, H.J.; Anagnostopoulos, I.; Foss, H.D.; Hummel, M.; Bornfeld, N.; Lee, W.R.; Stein, H. Lymphoproliferative lesions of the ocular adnexa. Analysis of 112 cases. Ophthalmology 1998, 105, 1430–1441. [Google Scholar] [CrossRef]
- Ferry, J.A.; Fung, C.Y.; Zukerberg, L.; Lucarelli, M.J.; Hasserjian, R.P.; Preffer, F.I.; Harris, N.L. Lymphoma of the ocular adnexa: A study of 353 cases. Am. J. Surg. Pathol. 2007, 31, 170–184. [Google Scholar] [CrossRef]
- Olszewski, A.J.; Castillo, J.J. Survival of patients with marginal zone lymphoma: Analysis of the Surveillance, Epidemiology, and End Results database. Cancer 2013, 119, 629–638. [Google Scholar] [CrossRef]
- Holm, F.; Mikkelsen, L.H.; Kamper, P.; Rasmussen, P.K.; Larsen, T.S.; Sjo, L.D.; Heegaard, S. Ocular adnexal lymphoma in Denmark: A nationwide study of 387 cases from 1980 to 2017. Br. J. Ophthalmol. 2021, 105, 914–920. [Google Scholar] [CrossRef]
- Rootman, D.B.; Mavrikakis, I.; Connors, J.M.; Rootman, J. Primary, unilateral ocular adnexal lymphoma: Disease progression and long-term survival. Ophthalmic Plast. Reconstr. Surg. 2011, 27, 405–409. [Google Scholar] [CrossRef]
- Sjo, L.D. Ophthalmic lymphoma: Epidemiology and pathogenesis. Acta Ophthalmol. 2009, 87, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Svendsen, F.H.; Rasmussen, P.K.; Coupland, S.E.; Esmaeli, B.; Finger, P.T.; Graue, G.F.; Grossniklaus, H.E.; Honavar, S.G.; Khong, J.J.; McKelvie, P.A.; et al. Lymphoma of the Eyelid—An International Multicenter Retrospective Study. Am. J. Ophthalmol. 2017, 177, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Tanenbaum, R.E.; Galor, A.; Dubovy, S.R.; Karp, C.L. Classification, diagnosis, and management of conjunctival lymphoma. Eye Vis. 2019, 6, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, N.G.; Wojno, T.H.; Grossniklaus, H.E. Clinicopathologic findings from lacrimal sac biopsy specimens obtained during dacryocystorhinostomy. Ophthalmic Plast. Reconstr. Surg. 2003, 19, 173–176. [Google Scholar] [CrossRef]
- Vest, S.D.; Mikkelsen, L.H.; Holm, F.; Rasmussen, P.K.; Hindso, T.G.; Knudsen, M.K.H.; Coupland, S.E.; Esmaeli, B.; Finger, P.T.; Graue, G.F.; et al. Lymphoma of the Lacrimal Gland—An International Multicenter Retrospective Study. Am. J. Ophthalmol. 2020, 219, 107–120. [Google Scholar] [CrossRef]
- Stefanovic, A.; Lossos, I.S. Extranodal marginal zone lymphoma of the ocular adnexa. Blood 2009, 114, 501–510. [Google Scholar] [CrossRef] [Green Version]
- Freeman, C.; Berg, J.W.; Cutler, S.J. Occurrence and prognosis of extranodal lymphomas. Cancer 1972, 29, 252–260. [Google Scholar] [CrossRef]
- Moslehi, R.; Devesa, S.S.; Schairer, C.; Fraumeni, J.F., Jr. Rapidly increasing incidence of ocular non-hodgkin lymphoma. J. Natl. Cancer Inst. 2006, 98, 936–939. [Google Scholar] [CrossRef]
- Cerhan, J.R.; Habermann, T.M. Epidemiology of Marginal Zone Lymphoma. Ann. Lymphoma 2021, 5. [Google Scholar] [CrossRef]
- Isaacson, P.G.; Du, M.Q. MALT lymphoma: From morphology to molecules. Nat. Rev. Cancer 2004, 4, 644–653. [Google Scholar] [CrossRef]
- Coupland, S.E.; Hellmich, M.; Auw-Haedrich, C.; Lee, W.R.; Anagnostopoulos, I.; Stein, H. Plasmacellular differentiation in extranodal marginal zone B cell lymphomas of the ocular adnexa: An analysis of the neoplastic plasma cell phenotype and its prognostic significance in 136 cases. Br. J. Ophthalmol. 2005, 89, 352–359. [Google Scholar] [CrossRef] [Green Version]
- Coupland, S.E.; Damato, B. Lymphomas involving the eye and the ocular adnexa. Curr. Opin. Ophthalmol. 2006, 17, 523–531. [Google Scholar] [CrossRef] [Green Version]
- Matsuo, T.; Ichimura, K.; Okada, H.; Shinagawa, K.; Fukushima, K.; Okano, M.; Otsuka, M.; Yoshino, T. Clonal analysis of bilateral, recurrent, or systemically multifocal ocular adnexal lymphoma. J. Clin. Exp. Hematop. 2010, 50, 27–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sagiv, O.; Thakar, S.D.; Manning, J.T.; Kandl, T.J.; Fayad, L.E.; Fowler, N.; Hagemeister, F.B.; Fanale, M.A.; Pinnix, C.C.; Samaniego, F.; et al. Prevalence of a Histologic Change of Ocular Adnexal Lymphoma in Patients With a History of Lymphoma. Ophthalmic Plast. Reconstr. Surg. 2019, 35, 243–246. [Google Scholar] [CrossRef]
- Das, D.; Deka, P.; Bhattacharjee, K.; Das, J.K.; Kuri, G.C.; Bhattaacharjee, H.; Deori, N.; Deshmukh, S.; Paidi, R.; Deka, A. Idiopathic inflammatory diseases of orbit and ocular adnexa: Histopathological and immunochemical analysis. Indian J. Ophthalmol. 2019, 67, 1993–1995. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, A.J.; Dolcetti, R.; Du, M.Q.; Doglioni, C.; Resti, A.G.; Politi, L.S.; De Conciliis, C.; Radford, J.; Bertoni, F.; Zucca, E.; et al. Ocular adnexal MALT lymphoma: An intriguing model for antigen-driven lymphomagenesis and microbial-targeted therapy. Ann. Oncol. 2008, 19, 835–846. [Google Scholar] [CrossRef] [PubMed]
- Go, H.; Kim, J.E.; Kim, Y.A.; Chung, H.K.; Khwarg, S.I.; Kim, C.W.; Jeon, Y.K. Ocular adnexal IgG4-related disease: Comparative analysis with mucosa-associated lymphoid tissue lymphoma and other chronic inflammatory conditions. Histopathology 2012, 60, 296–312. [Google Scholar] [CrossRef]
- Kubota, T.; Moritani, S.; Yoshino, T.; Nagai, H.; Terasaki, H. Ocular adnexal marginal zone B cell lymphoma infiltrated by IgG4-positive plasma cells. J. Clin. Pathol. 2010, 63, 1059–1065. [Google Scholar] [CrossRef]
- Nishida, K.; Sogabe, Y.; Makihara, A.; Senoo, A.; Morimoto, H.; Takeuchi, M.; Gion, Y.; Yoshino, T.; Sato, Y. Ocular adnexal marginal zone lymphoma arising in a patient with IgG4-related ophthalmic disease. Mod. Rheumatol. 2019, 29, 383–387. [Google Scholar] [CrossRef]
- Ohno, K.; Sato, Y.; Ohshima, K.; Takata, K.; Miyata-Takata, T.; Takeuchi, M.; Gion, Y.; Tachibana, T.; Orita, Y.; Ito, T.; et al. A subset of ocular adnexal marginal zone lymphomas may arise in association with IgG4-related disease. Sci. Rep. 2015, 5, 13539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sohn, E.J.; Ahn, H.B.; Roh, M.S.; Jung, W.J.; Ryu, W.Y.; Kwon, Y.H. Immunoglobulin G4 (IgG4)-Positive Ocular Adnexal Mucosa-Associated Lymphoid Tissue Lymphoma and Idiopathic Orbital Inflammation. Ophthalmic Plast. Reconstr. Surg. 2018, 34, 313–319. [Google Scholar] [CrossRef]
- Li, K.M.; Xu, M.H.; Wu, X.; He, W.M. The Expression of IgG and IgG4 in Orbital MALT Lymphoma: The Similarities and Differences of IgG4-Related Diseases. OncoTargets Ther. 2020, 13, 5755–5761. [Google Scholar] [CrossRef]
- Karadeniz, H.; Vaglio, A. IgG4-related disease: A contemporary review. Turk. J. Med. Sci. 2020, 50, 1616–1631. [Google Scholar] [CrossRef]
- Nishikori, A.; Nishimura, Y.; Shibata, R.; Ohshima, K.I.; Gion, Y.; Ikeda, T.; Nishimura, M.F.; Yoshino, T.; Sato, Y. Upregulated Expression of Activation-Induced Cytidine Deaminase in Ocular Adnexal Marginal Zone Lymphoma with IgG4-Positive Cells. Int. J. Mol. Sci. 2021, 22, 4083. [Google Scholar] [CrossRef]
- Du, M.Q. MALT lymphoma: A paradigm of NF-kappaB dysregulation. Semin. Cancer Biol. 2016, 39, 49–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melenotte, C.; Mezouar, S.; Mege, J.L.; Gorvel, J.P.; Kroemer, G.; Raoult, D. Bacterial infection and non-Hodgkin’s lymphoma. Crit. Rev. Microbiol. 2020, 46, 270–287. [Google Scholar] [CrossRef] [PubMed]
- Biernat, M.M.; Wrobel, T. Bacterial Infection and Non-Hodgkin B-Cell Lymphoma: Interactions between Pathogen, Host and the Tumor Environment. Int. J. Mol. Sci. 2021, 22, 7372. [Google Scholar] [CrossRef]
- Collina, F.; De Chiara, A.; De Renzo, A.; De Rosa, G.; Botti, G.; Franco, R. Chlamydia psittaci in ocular adnexa MALT lymphoma: A possible role in lymphomagenesis and a different geographical distribution. Infect. Agents Cancer 2012, 7, 8. [Google Scholar] [CrossRef] [Green Version]
- Ferreri, A.J.; Guidoboni, M.; Ponzoni, M.; De Conciliis, C.; Dell’Oro, S.; Fleischhauer, K.; Caggiari, L.; Lettini, A.A.; Dal Cin, E.; Ieri, R.; et al. Evidence for an association between Chlamydia psittaci and ocular adnexal lymphomas. J. Natl. Cancer Inst. 2004, 96, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Yoo, C.; Ryu, M.H.; Huh, J.; Park, J.H.; Kang, H.J.; Ahn, H.S.; Lee, Y.; Kim, M.J.; Lee, H.; Kim, T.W.; et al. Chlamydia psittaci infection and clinicopathologic analysis of ocular adnexal lymphomas in Korea. Am. J. Hematol. 2007, 82, 821–823. [Google Scholar] [CrossRef] [PubMed]
- Daibata, M.; Nemoto, Y.; Togitani, K.; Fukushima, A.; Ueno, H.; Ouchi, K.; Fukushi, H.; Imai, S.; Taguchi, H. Absence of Chlamydia psittaci in ocular adnexal lymphoma from Japanese patients. Br. J. Haematol. 2006, 132, 651–652. [Google Scholar] [CrossRef]
- De Cremoux, P.; Subtil, A.; Ferreri, A.J.; Vincent-Salomon, A.; Ponzoni, M.; Chaoui, D.; Arnaud, P.; Lumbroso-Le Rouic, L.; Sacchetti, F.; Dendale, R.; et al. Re: Evidence for an association between Chlamydia psittaci and ocular adnexal lymphomas. J. Natl. Cancer Inst. 2006, 98, 365–366. [Google Scholar] [CrossRef] [PubMed]
- Mulder, M.M.; Heddema, E.R.; Pannekoek, Y.; Faridpooya, K.; Oud, M.E.; Schilder-Tol, E.; Saeed, P.; Pals, S.T. No evidence for an association of ocular adnexal lymphoma with Chlamydia psittaci in a cohort of patients from the Netherlands. Leuk. Res. 2006, 30, 1305–1307. [Google Scholar] [CrossRef]
- Rosado, M.F.; Byrne, G.E., Jr.; Ding, F.; Fields, K.A.; Ruiz, P.; Dubovy, S.R.; Walker, G.R.; Markoe, A.; Lossos, I.S. Ocular adnexal lymphoma: A clinicopathologic study of a large cohort of patients with no evidence for an association with Chlamydia psittaci. Blood 2006, 107, 467–472. [Google Scholar] [CrossRef] [Green Version]
- Vargas, R.L.; Fallone, E.; Felgar, R.E.; Friedberg, J.W.; Arbini, A.A.; Andersen, A.A.; Rothberg, P.G. Is there an association between ocular adnexal lymphoma and infection with Chlamydia psittaci? The University of Rochester experience. Leuk. Res. 2006, 30, 547–551. [Google Scholar] [CrossRef]
- Zhang, G.S.; Winter, J.N.; Variakojis, D.; Reich, S.; Lissner, G.S.; Bryar, P.; Regner, M.; Mangold, K.; Kaul, K. Lack of an association between Chlamydia psittaci and ocular adnexal lymphoma. Leuk. Lymphoma 2007, 48, 577–583. [Google Scholar] [CrossRef]
- Gracia, E.; Froesch, P.; Mazzucchelli, L.; Martin, V.; Rodriguez-Abreu, D.; Jimenez, J.; Melgares, M.; Santos, D.; Capo, V.; Cavalli, F.; et al. Low prevalence of Chlamydia psittaci in ocular adnexal lymphomas from Cuban patients. Leuk. Lymphoma 2007, 48, 104–108. [Google Scholar] [CrossRef]
- Matthews, J.M.; Moreno, L.I.; Dennis, J.; Byrne, G.E., Jr.; Ruiz, P.; Dubovy, S.R.; Lossos, I.S. Ocular Adnexal Lymphoma: No evidence for bacterial DNA associated with lymphoma pathogenesis. Br. J. Haematol. 2008, 142, 246–249. [Google Scholar] [CrossRef]
- Johansson, P.; Klein-Hitpass, L.; Budeus, B.; Kuhn, M.; Lauber, C.; Seifert, M.; Roeder, I.; Pfortner, R.; Stuschke, M.; Dührsen, U.; et al. Identifying Genetic Lesions in Ocular Adnexal Extranodal Marginal Zone Lymphomas of the MALT Subtype by Whole Genome, Whole Exome and Targeted Sequencing. Cancers 2020, 12, 986. [Google Scholar] [CrossRef] [PubMed]
- Travaglino, A.; Pace, M.; Varricchio, S.; Russo, D.; Pugliese, N.; Severino, A.; Picardi, M.; Pane, F.; Insabato, L.; Staibano, S.; et al. Involvement of Helicobacter Pylori in Ocular Adnexa Lymphoma. Pathol. Oncol. Res. 2020, 26, 2075–2081. [Google Scholar] [CrossRef] [PubMed]
- Kalin-Hajdu, E.; Bernier-Turmel, F.; Frost, E.; Labbe, A.C.; Couture, S.; Wong, J.; Boulos, P.R.; Codere, F.; Hardy, I. Helicobacter pylori Infection of the Gastric Mucosa and Ocular Adnexa-Lack of Association with Ocular Adnexal Lymphoma. Ophthalmic Plast. Reconstr. Surg. 2021, 37, S1–S5. [Google Scholar] [CrossRef]
- Mollerup, S.; Mikkelsen, L.H.; Hansen, A.J.; Heegaard, S. High-throughput sequencing reveals no viral pathogens in eight cases of ocular adnexal extranodal marginal zone B-cell lymphoma. Exp. Eye Res. 2019, 185, 107677. [Google Scholar] [CrossRef]
- De Sanjose, S.; Benavente, Y.; Vajdic, C.M.; Engels, E.A.; Morton, L.M.; Bracci, P.M.; Spinelli, J.J.; Zheng, T.; Zhang, Y.; Franceschi, S.; et al. Hepatitis C and non-Hodgkin lymphoma among 4784 cases and 6269 controls from the International Lymphoma Epidemiology Consortium. Clin. Gastroenterol. Hepatol. 2008, 6, 451–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, A.; Hallouch, O.; Chernyak, V.; Kamaya, A.; Sirlin, C.B. Epidemiology of hepatocellular carcinoma: Target population for surveillance and diagnosis. Abdom. Radiol. 2018, 43, 13–25. [Google Scholar] [CrossRef]
- Couronne, L.; Bachy, E.; Roulland, S.; Nadel, B.; Davi, F.; Armand, M.; Canioni, D.; Michot, J.M.; Visco, C.; Arcaini, L.; et al. From hepatitis C virus infection to B-cell lymphoma. Ann. Oncol. 2018, 29, 92–100. [Google Scholar] [CrossRef]
- Gibson, T.M.; Morton, L.M.; Shiels, M.S.; Clarke, C.A.; Engels, E.A. Risk of non-Hodgkin lymphoma subtypes in HIV-infected people during the HAART era: A population-based study. AIDS 2014, 28, 2313–2318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, V.; Shen, D.; Sieving, P.C.; Chan, C.C. The role of infectious agents in the etiology of ocular adnexal neoplasia. Surv. Ophthalmol. 2008, 53, 312–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wohrer, S.; Troch, M.; Streubel, B.; Zwerina, J.; Skrabs, C.; Formanek, M.; Hauff, W.; Hoffmann, M.; Mullauer, L.; Chott, A.; et al. MALT lymphoma in patients with autoimmune diseases: A comparative analysis of characteristics and clinical course. Leukemia 2007, 21, 1812–1818. [Google Scholar] [CrossRef] [Green Version]
- Smedby, K.E.; Vajdic, C.M.; Falster, M.; Engels, E.A.; Martinez-Maza, O.; Turner, J.; Hjalgrim, H.; Vineis, P.; Costantini, A.S.; Bracci, P.M.; et al. Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: A pooled analysis within the InterLymph Consortium. Blood 2008, 111, 4029–4038. [Google Scholar] [CrossRef] [Green Version]
- Zintzaras, E.; Voulgarelis, M.; Moutsopoulos, H.M. The risk of lymphoma development in autoimmune diseases: A meta-analysis. Arch. Intern. Med. 2005, 165, 2337–2344. [Google Scholar] [CrossRef] [Green Version]
- Royer, B.; Cazals-Hatem, D.; Sibilia, J.; Agbalika, F.; Cayuela, J.M.; Soussi, T.; Maloisel, F.; Clauvel, J.P.; Brouet, J.C.; Mariette, X. Lymphomas in patients with Sjogren’s syndrome are marginal zone B-cell neoplasms, arise in diverse extranodal and nodal sites, and are not associated with viruses. Blood 1997, 90, 766–775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nocturne, G.; Virone, A.; Ng, W.F.; Le Guern, V.; Hachulla, E.; Cornec, D.; Daien, C.; Vittecoq, O.; Bienvenu, B.; Marcelli, C.; et al. Rheumatoid Factor and Disease Activity Are Independent Predictors of Lymphoma in Primary Sjogren’s Syndrome. Arthritis Rheumatol. 2016, 68, 977–985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mackay, F.; Woodcock, S.A.; Lawton, P.; Ambrose, C.; Baetscher, M.; Schneider, P.; Tschopp, J.; Browning, J.L. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J. Exp. Med. 1999, 190, 1697–1710. [Google Scholar] [CrossRef] [PubMed]
- Wotherspoon, A.C.; Pan, L.X.; Diss, T.C.; Isaacson, P.G. Cytogenetic study of B-cell lymphoma of mucosa-associated lymphoid tissue. Cancer Genet. Cytogenet. 1992, 58, 35–38. [Google Scholar] [CrossRef]
- Streubel, B.; Simonitsch-Klupp, I.; Mullauer, L.; Lamprecht, A.; Huber, D.; Siebert, R.; Stolte, M.; Trautinger, F.; Lukas, J.; Puspok, A.; et al. Variable frequencies of MALT lymphoma-associated genetic aberrations in MALT lymphomas of different sites. Leukemia 2004, 18, 1722–1726. [Google Scholar] [CrossRef] [Green Version]
- Ye, H.; Liu, H.; Attygalle, A.; Wotherspoon, A.C.; Nicholson, A.G.; Charlotte, F.; Leblond, V.; Speight, P.; Goodlad, J.; Lavergne-Slove, A.; et al. Variable frequencies of t(11;18)(q21;q21) in MALT lymphomas of different sites: Significant association with CagA strains of H pylori in gastric MALT lymphoma. Blood 2003, 102, 1012–1018. [Google Scholar] [CrossRef]
- Hamoudi, R.A.; Appert, A.; Ye, H.; Ruskone-Fourmestraux, A.; Streubel, B.; Chott, A.; Raderer, M.; Gong, L.; Wlodarska, I.; De Wolf-Peeters, C.; et al. Differential expression of NF-kappaB target genes in MALT lymphoma with and without chromosome translocation: Insights into molecular mechanism. Leukemia 2010, 24, 1487–1497. [Google Scholar] [CrossRef]
- Tanimoto, K.; Sekiguchi, N.; Yokota, Y.; Kaneko, A.; Watanabe, T.; Maeshima, A.M.; Matsuno, Y.; Harada, M.; Tobinai, K.; Kobayashi, Y. Fluorescence in situ hybridization (FISH) analysis of primary ocular adnexal MALT lymphoma. BMC Cancer 2006, 6, 249. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, H.; Usui, Y.; Ueda, S.; Yamakawa, N.; Sato-Otsubo, A.; Sato, Y.; Ogawa, S.; Goto, H. Genome-Wide Analysis of Ocular Adnexal Lymphoproliferative Disorders Using High-Resolution Single Nucleotide Polymorphism Array. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4156–4165. [Google Scholar] [CrossRef] [Green Version]
- Schiby, G.; Polak-Charcon, S.; Mardoukh, C.; Rosenblatt, K.; Goldberg, I.; Kneller, A.; Rosner, M.; Kopolovic, J. Orbital marginal zone lymphomas: An immunohistochemical, polymerase chain reaction, and fluorescence in situ hybridization study. Hum. Pathol. 2007, 38, 435–442. [Google Scholar] [CrossRef]
- Takada, S.; Yoshino, T.; Taniwaki, M.; Nakamura, N.; Nakamine, H.; Oshima, K.; Sadahira, Y.; Inagaki, H.; Oshima, K.; Tadaatsu, A. Involvement of the chromosomal translocation t(11;18) in some mucosa-associated lymphoid tissue lymphomas and diffuse large B-cell lymphomas of the ocular adnexa: Evidence from multiplex reverse transcriptase-polymerase chain reaction and fluorescence in situ hybridization on using formalin-fixed, paraffin-embedded specimens. Mod. Pathol. 2003, 16, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Ikpatt, O.F.; Dubovy, S.R.; Lossos, C.; Natkunam, Y.; Chapman-Fredricks, J.R.; Fan, Y.S.; Lossos, I.S. Molecular and genomic aberrations in Chlamydophila psittaci negative ocular adnexal marginal zone lymphomas. Am. J. Hematol. 2013, 88, 730–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Streubel, B.; Vinatzer, U.; Lamprecht, A.; Raderer, M.; Chott, A. T(3;14)(p14.1;q32) involving IGH and FOXP1 is a novel recurrent chromosomal aberration in MALT lymphoma. Leukemia 2005, 19, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Adam, P.; Haralambieva, E.; Hartmann, M.; Mao, Z.; Ott, G.; Rosenwald, A. Rare occurrence of IgVH gene translocations and restricted IgVH gene repertoire in ocular MALT-type lymphoma. Haematologica 2008, 93, 319–320. [Google Scholar] [CrossRef] [Green Version]
- Bi, Y.; Zeng, N.; Chanudet, E.; Huang, Y.; Hamoudi, R.A.; Liu, H.; Dong, G.; Watkins, A.J.; Ley, S.C.; Zou, L.; et al. A20 inactivation in ocular adnexal MALT lymphoma. Haematologica 2012, 97, 926–930. [Google Scholar] [CrossRef] [Green Version]
- Johansson, P.; Klein-Hitpass, L.; Grabellus, F.; Arnold, G.; Klapper, W.; Pfortner, R.; Dührsen, U.; Eckstein, A.; Dürig, J.; Küppers, R. Recurrent mutations in NF-kappaB pathway components, KMT2D, and NOTCH1/2 in ocular adnexal MALT-type marginal zone lymphomas. Oncotarget 2016, 7, 62627–62639. [Google Scholar] [CrossRef]
- Chanudet, E.; Huang, Y.; Ichimura, K.; Dong, G.; Hamoudi, R.A.; Radford, J.; Wotherspoon, A.C.; Isaacson, P.G.; Ferry, J.; Du, M.Q. A20 is targeted by promoter methylation, deletion and inactivating mutation in MALT lymphoma. Leukemia 2010, 24, 483–487. [Google Scholar] [CrossRef]
- Jung, H.; Yoo, H.Y.; Lee, S.H.; Shin, S.; Kim, S.C.; Lee, S.; Joung, J.G.; Nam, J.Y.; Ryu, D.; Yun, J.W.; et al. The mutational landscape of ocular marginal zone lymphoma identifies frequent alterations in TNFAIP3 followed by mutations in TBL1XR1 and CREBBP. Oncotarget 2017, 8, 17038–17049. [Google Scholar] [CrossRef] [Green Version]
- Vela, V.; Juskevicius, D.; Dirnhofer, S.; Menter, T.; Tzankov, A. Mutational landscape of marginal zone B-cell lymphomas of various origin: Organotypic alterations and diagnostic potential for assignment of organ origin. Virchows Arch. 2021. [Google Scholar] [CrossRef]
- Behdad, A.; Zhou, X.Y.; Gao, J.; Raparia, K.; Dittman, D.; Green, S.J.; Qi, C.; Betz, B.; Bryar, P.; Chen, Q.; et al. High Frequency of MYD88 L265P Mutation in Primary Ocular Adnexal Marginal Zone Lymphoma and Its Clinicopathologic Correlation: A Study From a Single Institution. Arch. Pathol. Lab. Med. 2019, 143, 483–493. [Google Scholar] [CrossRef] [Green Version]
- Cani, A.K.; Soliman, M.; Hovelson, D.H.; Liu, C.J.; McDaniel, A.S.; Haller, M.J.; Bratley, J.V.; Rahrig, S.E.; Li, Q.; Briceno, C.A.; et al. Comprehensive genomic profiling of orbital and ocular adnexal lymphomas identifies frequent alterations in MYD88 and chromatin modifiers: New routes to targeted therapies. Mod. Pathol. 2016, 29, 685–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, Q.; Wang, M.; Moody, S.; Xue, X.; Huang, Y.; Bi, Y.; Du, M.Q. Distinct involvement of NF-kappaB regulators by somatic mutation in ocular adnexal malt lymphoma. Br. J. Haematol. 2013, 160, 851–854. [Google Scholar] [CrossRef]
- Moody, S.; Thompson, J.S.; Chuang, S.S.; Liu, H.; Raderer, M.; Vassiliou, G.; Wlodarska, I.; Wu, F.; Cogliatti, S.; Robson, A.; et al. Novel GPR34 and CCR6 mutation and distinct genetic profiles in MALT lymphomas of different sites. Haematologica 2018, 103, 1329–1336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cascione, L.; Rinaldi, A.; Bruscaggin, A.; Tarantelli, C.; Arribas, A.J.; Kwee, I.; Pecciarini, L.; Mensah, A.A.; Spina, V.; Chung, E.Y.L.; et al. Novel insights into the genetics and epigenetics of MALT lymphoma unveiled by next generation sequencing analyses. Haematologica 2019, 104, e558–e561. [Google Scholar] [CrossRef]
- Magistri, M.; Happ, L.E.; Ramdial, J.; Lu, X.; Stathias, V.; Kunkalla, K.; Agarwal, N.; Jiang, X.; Schürer, S.C.; Dubovy, S.R.; et al. The Genetic Landscape of Ocular Adnexa MALT Lymphoma Reveals Frequent Aberrations in NFAT and MEF2B Signaling Pathways. Cancer Res. Commun. 2021, 1, 1–16. [Google Scholar] [CrossRef]
- Du, M.Q. MALT lymphoma: Many roads lead to nuclear factor-kappab activation. Histopathology 2011, 58, 26–38. [Google Scholar] [CrossRef]
- Van Keimpema, M.; Gruneberg, L.J.; Mokry, M.; van Boxtel, R.; Koster, J.; Coffer, P.J.; Pals, S.T.; Spaargaren, M. FOXP1 directly represses transcription of proapoptotic genes and cooperates with NF-kappaB to promote survival of human B cells. Blood 2014, 124, 3431–3440. [Google Scholar] [CrossRef] [Green Version]
- Clement, C.G.; Potluri, V.R.; Gonzales, J.; Qian, Y.W. Translocation (5; 11) in a conjunctival MALT lymphoma. Int. J. Clin. Exp. Pathol. 2011, 4, 722–726. [Google Scholar]
- Kim, W.S.; Honma, K.; Karnan, S.; Tagawa, H.; Kim, Y.D.; Oh, Y.L.; Seto, M.; Ko, Y.H. Genome-wide array-based comparative genomic hybridization of ocular marginal zone B cell lymphoma: Comparison with pulmonary and nodal marginal zone B cell lymphoma. Genes Chromosomes Cancer 2007, 46, 776–783. [Google Scholar] [CrossRef]
- Sasaki, Y.; Iwai, K. Roles of the NF-kappaB Pathway in B-Lymphocyte Biology. Curr. Top. Microbiol. Immunol. 2016, 393, 177–209. [Google Scholar] [CrossRef]
- Liu, F.; Karube, K.; Kato, H.; Arita, K.; Yoshida, N.; Yamamoto, K.; Tsuzuki, S.; Kim, W.; Ko, Y.H.; Seto, M. Mutation analysis of NF-kappaB signal pathway-related genes in ocular MALT lymphoma. Int. J. Clin. Exp. Pathol. 2012, 5, 436–441. [Google Scholar]
- Fabbri, G.; Rasi, S.; Rossi, D.; Trifonov, V.; Khiabanian, H.; Ma, J.; Grunn, A.; Fangazio, M.; Capello, D.; Monti, S.; et al. Analysis of the chronic lymphocytic leukemia coding genome: Role of NOTCH1 mutational activation. J. Exp. Med. 2011, 208, 1389–1401. [Google Scholar] [CrossRef] [Green Version]
- Kiel, M.J.; Velusamy, T.; Betz, B.L.; Zhao, L.; Weigelin, H.G.; Chiang, M.Y.; Huebner-Chan, D.R.; Bailey, N.G.; Yang, D.T.; Bhagat, G.; et al. Whole-genome sequencing identifies recurrent somatic NOTCH2 mutations in splenic marginal zone lymphoma. J. Exp. Med. 2012, 209, 1553–1565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, D.; Trifonov, V.; Fangazio, M.; Bruscaggin, A.; Rasi, S.; Spina, V.; Monti, S.; Vaisitti, T.; Arruga, F.; Fama, R.; et al. The coding genome of splenic marginal zone lymphoma: Activation of NOTCH2 and other pathways regulating marginal zone development. J. Exp. Med. 2012, 209, 1537–1551. [Google Scholar] [CrossRef] [Green Version]
- Osipo, C.; Golde, T.E.; Osborne, B.A.; Miele, L.A. Off the beaten pathway: The complex cross talk between Notch and NF-kappaB. Lab. Investig. 2008, 88, 11–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwarzer, R.; Dorken, B.; Jundt, F. Notch is an essential upstream regulator of NF-kappaB and is relevant for survival of Hodgkin and Reed-Sternberg cells. Leukemia 2012, 26, 806–813. [Google Scholar] [CrossRef]
- Wang, J.; Shelly, L.; Miele, L.; Boykins, R.; Norcross, M.A.; Guan, E. Human Notch-1 inhibits NF-kappa B activity in the nucleus through a direct interaction involving a novel domain. J. Immunol. 2001, 167, 289–295. [Google Scholar] [CrossRef] [Green Version]
- De Decker, M.; Lavaert, M.; Roels, J.; Tilleman, L.; Vandekerckhove, B.; Leclercq, G.; Van Nieuwerburgh, F.; Van Vlierberghe, P.; Taghon, T. HES1 and HES4 have non-redundant roles downstream of Notch during early human T-cell development. Haematologica 2021, 106, 130–141. [Google Scholar] [CrossRef]
- Morin, R.D.; Mendez-Lago, M.; Mungall, A.J.; Goya, R.; Mungall, K.L.; Corbett, R.D.; Johnson, N.A.; Severson, T.M.; Chiu, R.; Field, M.; et al. Frequent mutation of histone-modifying genes in non-Hodgkin lymphoma. Nature 2011, 476, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Dominguez-Sola, D.; Hussein, S.; Lee, J.E.; Holmes, A.B.; Bansal, M.; Vlasevska, S.; Mo, T.; Tang, H.; Basso, K.; et al. Disruption of KMT2D perturbs germinal center B cell development and promotes lymphomagenesis. Nat. Med. 2015, 21, 1190–1198. [Google Scholar] [CrossRef]
- Fagan, R.J.; Dingwall, A.K. COMPASS Ascending: Emerging clues regarding the roles of MLL3/KMT2C and MLL2/KMT2D proteins in cancer. Cancer Lett. 2019, 458, 56–65. [Google Scholar] [CrossRef]
- Perissi, V.; Aggarwal, A.; Glass, C.K.; Rose, D.W.; Rosenfeld, M.G. A corepressor/coactivator exchange complex required for transcriptional activation by nuclear receptors and other regulated transcription factors. Cell 2004, 116, 511–526. [Google Scholar] [CrossRef] [Green Version]
- Li, J.Y.; Daniels, G.; Wang, J.; Zhang, X. TBL1XR1 in physiological and pathological states. Am. J. Clin. Exp. Urol. 2015, 3, 13–23. [Google Scholar] [PubMed]
- Venturutti, L.; Teater, M.; Zhai, A.; Chadburn, A.; Babiker, L.; Kim, D.; Beguelin, W.; Lee, T.C.; Kim, Y.; Chin, C.R.; et al. TBL1XR1 Mutations Drive Extranodal Lymphoma by Inducing a Pro-tumorigenic Memory Fate. Cell 2020, 182, 297–316.e227. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, X.; Cai, W.; Bao, H.; Huang, H.; Liu, Y.; Yang, X.; Ruan, C.; Wu, D.; Shen, H.; et al. TBL1XR1 mutation predicts poor outcome in primary testicular diffuse large B-cell lymphoma patients. Biomark. Res. 2020, 8, 10. [Google Scholar] [CrossRef]
- Jangam, D.; Sridhar, K.; Butzmann, A.; Samghabadi, P.; Plowey, E.D.; Ohgami, R.S. TBL1XR1 Mutations in Primary Marginal Zone Lymphomas of Ocular Adnexa are Associated with Unique Morphometric Phenotypes. Curr. Eye Res. 2020, 45, 1583–1589. [Google Scholar] [CrossRef]
- O’Shea, J.J.; Holland, S.M.; Staudt, L.M. JAKs and STATs in immunity, immunodeficiency, and cancer. N. Engl. J. Med. 2013, 368, 161–170. [Google Scholar] [CrossRef]
- Thurner, L.; Hartmann, S.; Neumann, F.; Hoth, M.; Stilgenbauer, S.; Küppers, R.; Preuss, K.D.; Bewarder, M. Role of Specific B-Cell Receptor Antigens in Lymphomagenesis. Front. Oncol. 2020, 10, 604685. [Google Scholar] [CrossRef]
- Coupland, S.E.; Foss, H.D.; Anagnostopoulos, I.; Hummel, M.; Stein, H. Immunoglobulin VH gene expression among extranodal marginal zone B-cell lymphomas of the ocular adnexa. Investig. Ophthalmol. Vis. Sci. 1999, 40, 555–562. [Google Scholar]
- Dagklis, A.; Ponzoni, M.; Govi, S.; Cangi, M.G.; Pasini, E.; Charlotte, F.; Vino, A.; Doglioni, C.; Davi, F.; Lossos, I.S.; et al. Immunoglobulin gene repertoire in ocular adnexal lymphomas: Hints on the nature of the antigenic stimulation. Leukemia 2012, 26, 814–821. [Google Scholar] [CrossRef] [Green Version]
- Hara, Y.; Nakamura, N.; Kuze, T.; Hashimoto, Y.; Sasaki, Y.; Shirakawa, A.; Furuta, M.; Yago, K.; Kato, K.; Abe, M. Immunoglobulin heavy chain gene analysis of ocular adnexal extranodal marginal zone B-cell lymphoma. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2450–2457. [Google Scholar]
- Mannami, T.; Yoshino, T.; Oshima, K.; Takase, S.; Kondo, E.; Ohara, N.; Nakagawa, H.; Ohtsuki, H.; Harada, M.; Akagi, T. Clinical, histopathological, and immunogenetic analysis of ocular adnexal lymphoproliferative disorders: Characterization of malt lymphoma and reactive lymphoid hyperplasia. Mod. Pathol. 2001, 14, 641–649. [Google Scholar] [CrossRef] [Green Version]
- Van Maldegem, F.; Wormhoudt, T.A.; Mulder, M.M.; Oud, M.E.; Schilder-Tol, E.; Musler, A.R.; Aten, J.; Saeed, P.; Kersten, M.J.; Pals, S.T.; et al. Chlamydia psittaci-negative ocular adnexal marginal zone B-cell lymphomas have biased VH4-34 immunoglobulin gene expression and proliferate in a distinct inflammatory environment. Leukemia 2012, 26, 1647–1653. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Lossos, C.; Chapman-Fredricks, J.R.; Matthews, J.M.; Ikpatt, O.F.; Ruiz, P.; Lossos, I.S. Biased use of the IGHV4 family and evidence for antigen selection in Chlamydophila psittaci-negative ocular adnexal extranodal marginal zone lymphomas. PLoS ONE 2011, 6, e29114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seifert, M.; Küppers, R. Human memory B cells. Leukemia 2016, 30, 2283–2292. [Google Scholar] [CrossRef] [PubMed]
- Bahler, D.W.; Szankasi, P.; Kulkarni, S.; Tubbs, R.R.; Cook, J.R.; Swerdlow, S.H. Use of similar immunoglobulin VH gene segments by MALT lymphomas of the ocular adnexa. Mod. Pathol. 2009, 22, 833–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, D.; Bhatt, S.; Lu, X.; Guo, F.; Veelken, H.; Hsu, D.K.; Liu, F.T.; Cubela, S.A.; Kunkalla, K.; Vega, F.; et al. Chlamydophila psittaci-negative ocular adnexal marginal zone lymphomas express self polyreactive B-cell receptors. Leukemia 2015, 29, 1587–1599. [Google Scholar] [CrossRef]
- Arribas, A.J.; Bertoni, F. Methylation patterns in marginal zone lymphoma. Best Pract. Res. Clin. Haematol. 2017, 30, 24–31. [Google Scholar] [CrossRef]
- Choung, H.K.; Kim, Y.A.; Lee, M.J.; Kim, N.; Khwarg, S.I. Multigene methylation analysis of ocular adnexal MALT lymphoma and their relationship to Chlamydophila psittaci infection and clinical characteristics in South Korea. Investig. Ophthalmol. Vis. Sci. 2012, 53, 1928–1935. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.J.; Min, B.J.; Choung, H.K.; Kim, N.; Kim, Y.A.; Khwarg, S.I. Genome-wide DNA methylation profiles according to Chlamydophila psittaci infection and the response to doxycycline treatment in ocular adnexal lymphoma. Mol. Vis. 2014, 20, 1037–1047. [Google Scholar]
- Hother, C.; Rasmussen, P.K.; Joshi, T.; Reker, D.; Ralfkiaer, U.; Workman, C.T.; Heegaard, S.; Ralfkiaer, E.; Gronbaek, K. MicroRNA profiling in ocular adnexal lymphoma: A role for MYC and NFKB1 mediated dysregulation of microRNA expression in aggressive disease. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5169–5175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Middle, S.; Coupland, S.E.; Taktak, A.; Kidgell, V.; Slupsky, J.R.; Pettitt, A.R.; Till, K.J. Immunohistochemical analysis indicates that the anatomical location of B-cell non-Hodgkin’s lymphoma is determined by differentially expressed chemokine receptors, sphingosine-1-phosphate receptors and integrins. Exp. Hematol. Oncol. 2015, 4, 10. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.H.; Kim, T.M.; Go, H.; Kim, W.Y.; Jeon, Y.K.; Lee, S.H.; Kim, D.W.; Khwarg, S.I.; Kim, C.W.; Heo, D.S. Clinical significance of tumor-infiltrating FOXP3+ T cells in patients with ocular adnexal mucosa-associated lymphoid tissue lymphoma. Cancer Sci. 2011, 102, 1972–1976. [Google Scholar] [CrossRef]
- Kinoshita, S.; Kase, S.; Ando, R.; Dong, Z.; Fukuhara, J.; Dong, Y.; Inafuku, S.; Noda, K.; Noda, M.; Kanda, A.; et al. Expression of vascular endothelial growth factor in human ocular adnexal lymphoma. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3461–3467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seifert, M.; Scholtysik, R.; Küppers, R. Origin and Pathogenesis of B Cell Lymphomas. Methods Mol. Biol. 2019, 1956, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Küppers, R. Mechanisms of B-cell lymphoma pathogenesis. Nat. Rev. Cancer 2005, 5, 251–262. [Google Scholar] [CrossRef]
- Krappmann, D.; Vincendeau, M. Mechanisms of NF-kappaB deregulation in lymphoid malignancies. Semin. Cancer Biol. 2016, 39, 3–14. [Google Scholar] [CrossRef]
| Chromosomes or Genes Affected | Type of Genetic Alteration | Pathway or Main Function | Approximate Frequency (%) | References |
|---|---|---|---|---|
| Chromosomal alterations | ||||
| Trisomy 3 | Chromosomal gain | unclear (FOXP1?) | 30–60 | [70,71,72] |
| Trisomy 18 | Chromosomal gain | unclear | 20–55 | [70,71,72] |
| t(11;18)(q21;q21) | BIRC3-MALT1 translocation | NF-κB pathway | 10–15 | [68,73] |
| t(14;18)(q32;q21) | IGH-MALT1 translocation | NF-κB pathway | 5–10 | [67,70,74] |
| t(3;14)(p14.1;q32) | FOXP1-IGH translocation | B-cell development and survival (NF-κB pathway) | 5–15 | [75,76] |
| Gene mutations | ||||
| TNFAIP3 | Deletions, non-synonymous mutations | NF-κB pathway | 30–50 | [77,78,79,80,81] |
| MYD88 | Non-synonymous mutations (mostly p.L265P) | NF-κB pathway | 5–35 | [78,81,82,83,84] |
| NOTCH1 | Non-synonymous mutations (mostly HD and PEST domains) | NOTCH pathway | 2–10 | [78,80,85] |
| NOTCH2 | Non-synonymous mutations (mostly TAD and PEST domains) | NOTCH pathway | 5–10 | [78,86] |
| KMT2D | Non-synonymous mutations | Epigenetic regulation | 5–20 | [78,80,81,86] |
| CREBBP | Non-synonymous mutations | Epigenetic regulation | 15 | [51,80] |
| TBL1XR1 | Non-synonymous mutations (mostly WD40 domain) | Regulation of nuclear receptor activity (NF-κB and AP1 pathway) | 10–20 | [51,80,81,85] |
| JAK3 | Non-synonymous mutations | JAK/STAT signaling | 5–10 | [51,81] |
| CABIN1 | deletions, Non-synonymous mutations | NFAT signaling | 30% | [87] |
| RHOA | deletions, Non-synonymous mutations | Rho signaling | 26% | [87] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johansson, P.; Eckstein, A.; Küppers, R. The Biology of Ocular Adnexal Marginal Zone Lymphomas. Cancers 2022, 14, 1264. https://doi.org/10.3390/cancers14051264
Johansson P, Eckstein A, Küppers R. The Biology of Ocular Adnexal Marginal Zone Lymphomas. Cancers. 2022; 14(5):1264. https://doi.org/10.3390/cancers14051264
Chicago/Turabian StyleJohansson, Patricia, Anja Eckstein, and Ralf Küppers. 2022. "The Biology of Ocular Adnexal Marginal Zone Lymphomas" Cancers 14, no. 5: 1264. https://doi.org/10.3390/cancers14051264
APA StyleJohansson, P., Eckstein, A., & Küppers, R. (2022). The Biology of Ocular Adnexal Marginal Zone Lymphomas. Cancers, 14(5), 1264. https://doi.org/10.3390/cancers14051264






