Extranodal Marginal Zone Lymphoma: Pathogenesis, Diagnosis and Treatment
Abstract
Simple Summary
Abstract
1. Introduction
2. Epidemiology
Etiopathogenesis
3. Clinical Presentation and Disease Assessment
4. Prognostic Factors and Disease Features
4.1. EMZL of Gastrointestinal Tract or Gastric MALT
4.2. Bronchus-Associated Lymphoid Tissue (BALT) Lymphoma
4.3. Ocular Adnexal Marginal Zone Lymphoma (OAML)
4.4. Other EMZL Sites
5. Treatment of EMZL
6. Treatment of Particular Entity
6.1. Gastric MALT Lymphoma
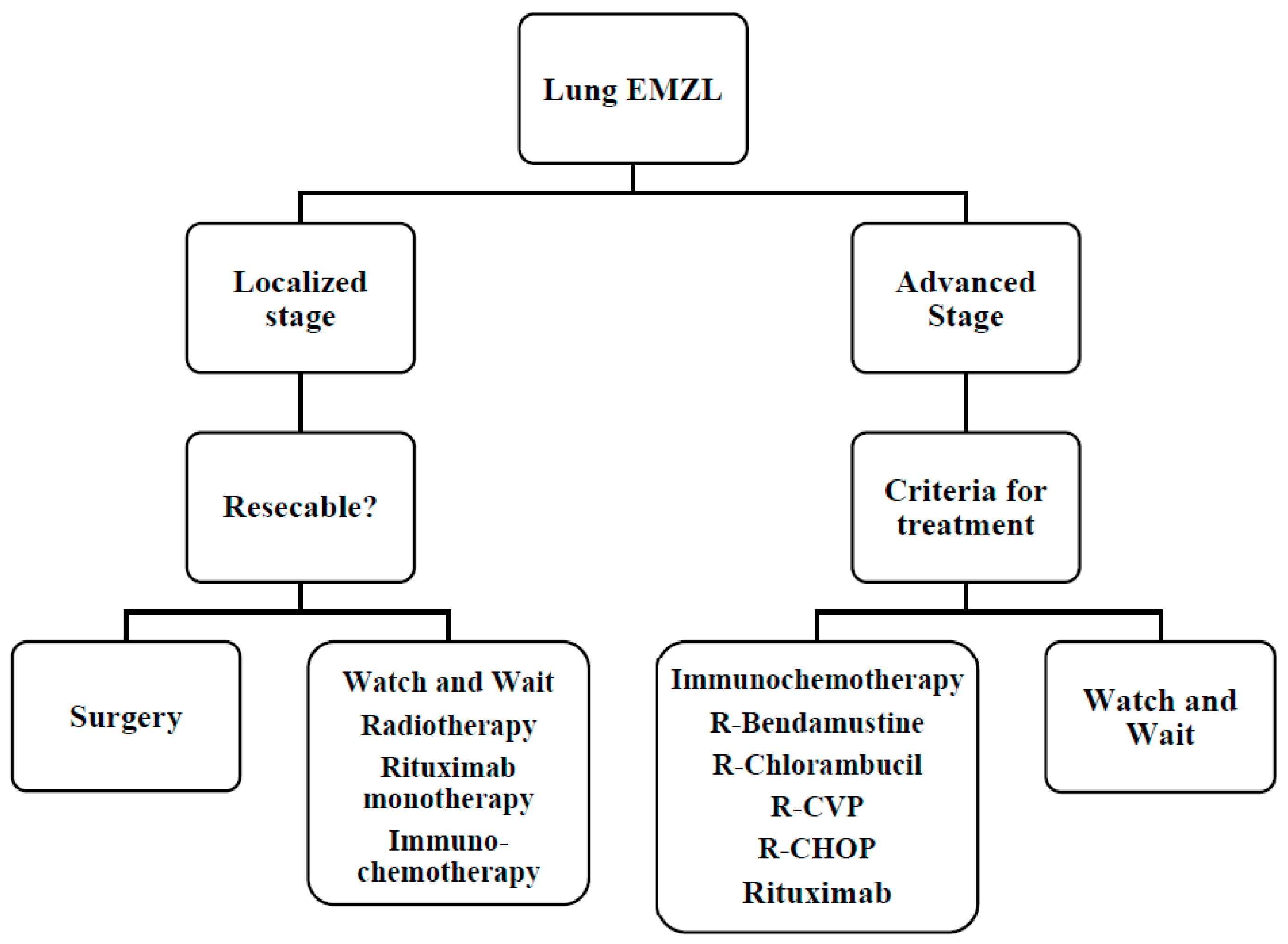
6.2. BALT Lymphoma
6.3. Treatment: OAML
6.4. Other EMZL
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.O.; Morton, L.M.; Devesa, S.S.; Check, D.; Curtis, R.E.; Weisenburger, D.D.; Dores, G. Incidence of marginal zone lymphoma in the United States, 2001–2009 with a focus on primary anatomic site. Br. J. Haematol. 2014, 165, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Craig, V.J.; Arnold, I.; Gerke, C.; Huynh, M.Q.; Wundisch, T.; Neubauer, A.; Renner, C.; Falkow, S.; Muller, A. Gastric MALT lymphoma B cells express polyreactive, somatically mutated immunoglobulins. Blood 2010, 115, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Zucca, E.; Bertoni, F.; Vannata, B.; Cavalli, F. Emerging Role of Infectious Etiologies in the Pathogenesis of Marginal Zone B-cell Lymphomas. Clin. Cancer Res. 2014, 20, 5207–5216. [Google Scholar] [CrossRef] [PubMed]
- Smedby, K.E.; Vajdic, C.M.; Falster, M.; Engels, E.A.; Martínez-Maza, O.; Turner, J.; Hjalgrim, H.; Vineis, P.; Costantini, A.S.; Bracci, P.M.; et al. Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: A pooled analysis within the InterLymph Consortium. Blood 2008, 111, 4029–4038. [Google Scholar] [CrossRef] [PubMed]
- Du, M.Q. MALT lymphoma: A paradigm of NF-kappaB dysregulation. Semin. Cancer Biol. 2016, 39, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Wöhrer, S.; Troch, M.; Streubel, B.; Zwerina, J.; Skrabs, C.; Formanek, M.; Hauff, W.; Hoffmann, M.; Müllauer, L.; Chott, A.; et al. MALT lymphoma in patients with autoimmune diseases: A comparative analysis of characteristics and clinical course. Leukemia 2007, 21, 1812–1818. [Google Scholar] [CrossRef][Green Version]
- Cheah, C.Y.; Zucca, E.; Rossi, D.; Habermann, T.M. Marginal zone lymphoma: Present status and future perspectives. Haematologica 2022, 107, 35–43. [Google Scholar] [CrossRef]
- Zucca, E.; Arcaini, L.; Buske, C.; Johnson, P.; Ponzoni, M.; Raderer, M.; Ricardi, U.; Salar, A.; Stamatopoulos, K.; Thieblemont, C.; et al. Marginal zone lymphomas: ESMO Clinical Practice Guidelines. Ann. Oncol. 2020, 31, 17–29. [Google Scholar] [CrossRef]
- Brynes, R.K.; Almaguer, P.D.; Leathery, K.E.; McCourty, A.; Arber, D.A.; Medeiros, L.J.; Nathwani, B.N. Numerical cytogenetic abnormalities of chromosomes 3, 7, and 12 in marginal zone B-cell lymphomas. Mod. Pathol. 1996, 9, 995–1000. [Google Scholar]
- Wotherspoon, A.C.; Finn, T.M.; Isaacson, P.G. Trisomy 3 in low-grade B-cell lymphomas of mucosa-associated lymphoid tissue. Blood 1995, 85, 2000–2004. [Google Scholar] [CrossRef] [PubMed]
- Ott, G.; Katzenberger, T.; Greiner, A.; Kalla, J.; Rosenwald, A.; Heinrich, U.; Ott, M.M.; Muller-Hermelink, H.K. The t(11;18)(q21;q21) chromosome translocation is a frequent and specific aberration in low-grade but not high-grade malignant non-Hodgkin’s lymphomas of the mucosa-associated lymphoid tissue (MALT-) type. Cancer Res. 1997, 57, 3944–3948. [Google Scholar] [PubMed]
- Afonina, I.S.; Elton, L.; Carpentier, I.; Beyaert, R. MALT1—A universal soldier: Multiple strategies to ensure NF-κB activation and target gene expression. FEBS J. 2015, 282, 3286–3297. [Google Scholar] [CrossRef] [PubMed]
- Du, M.-Q. MALT lymphoma: Many roads lead to nuclear factor-κb activation. Histopathology 2011, 58, 26–38. [Google Scholar] [CrossRef]
- Van Keimpema, M.; Grüneberg, L.J.; Mokry, M.; van Boxtel, R.; Koster, J.; Coffer, P.J.; Pals, S.T.; Spaargaren, M. FOXP1 directly represses transcription of proapoptotic genes and cooperates with NF-κB to promote survival of human B cells. Blood 2014, 124, 3431–3440. [Google Scholar] [CrossRef]
- Schreuder, M.; Van den Brand, M.; Hebeda, K.; Groenen, P.; Van Krieken, J.; Scheijen, B. Novel developments in the pathogenesis and diagnosis of extranodal marginal zone lymphoma. J. Hematopathol. 2017, 10, 91–107. [Google Scholar] [CrossRef]
- Teckie, S.; Qi, S.; Chelius, M.; Lovie, S.; Hsu, M.; Noy, A.; Portlock, C.; Yahalom, J. Long-term outcome of 487 patients with early-stage extra-nodal marginal zone lymphoma. Ann. Oncol. 2017, 28, 1064–1069. [Google Scholar] [CrossRef]
- Albano, D.; Bertoli, M.; Ferro, P.; Fallanca, F.; Gianolli, L.; Picchio, M.; Giubbini, R.; Bertagna, F. 18F-FDG PET/CT in gastric MALT lymphoma. Eur. J. Nucl. Med. Mol. Imaging 2016, 44, 589–597. [Google Scholar] [CrossRef]
- Mayerhoefer, M.E.; Jaeger, U.; Staber, P.; Raderer, M.; Wadsak, W.; Pfaff, S.; Kornauth, C.; Senn, D.; Weber, M.; Wester, H.-J.; et al. [68Ga]Ga-Pentixafor PET/MRI for CXCR4 Imaging of Chronic Lymphocytic Leukemia: Preliminary results. Investig. Radiol. 2018, 53, 403–408. [Google Scholar] [CrossRef]
- Conconi, A.; Martinelli, G.; Thiéblemont, C.; Ferreri, A.J.M.; Devizzi, L.; Peccatori, F.; Ponzoni, M.; Pedrinis, E. Clinical activity of rituximab in extranodal marginal zone B-cell lymphoma of MALT type. Blood 2003, 102, 2741–2745. [Google Scholar] [CrossRef]
- Raderer, M.; Kiesewetter, B.; Ferreri, A.J.M. Clinicopathologic characteristics and treatment of marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma). CA Cancer J. Clin. 2016, 66, 153–171. [Google Scholar] [CrossRef] [PubMed]
- Wotherspoon, A.C.; Ortiz-Hidalgo, C.; Falzon, M.R.; Isaacson, P.G. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet 1991, 338, 1175–1176. [Google Scholar] [CrossRef]
- Wöhrer, S.; Streubel, B.; Bartsch, R.; Chott, A.; Raderer, M. Monoclonal Immunoglobulin Production Is a Frequent Event in Patients with Mucosa-Associated Lymphoid Tissue Lymphoma. Clin. Cancer Res. 2004, 10, 7179–7181. [Google Scholar] [CrossRef]
- Cook, J.R.; Isaacson, P.G.; Chott, A.; Nakamura, S.; Muller-Hermelink, H.K.; Harris, N.L.; Swerdlow, S.H. Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma). In WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th ed.; Swerdlow, S.H., Campo, E., Harris, N.L., Jaffe, E.S., Pileri, S.A., Stein, H., Thiele, J., Eds.; IARC: Lyon, France, 2017; pp. 259–262. [Google Scholar]
- Thieblemont, C.; Berger, F.; Dumontet, C.; Moullet, I.; Bouafia, F.; Felman, P.; Salles, G.; Coiffier, B. Mucosa-associated lymphoid tissue lymphoma is a disseminated disease in one third of 158 patients analyzed. Blood 2000, 95, 802–806. [Google Scholar] [CrossRef] [PubMed]
- Hyeon, J.; Lee, B.; Shin, S.-H.; Yoo, H.Y.; Kim, S.J.; Kim, W.S.; Park, W.-Y.; Ko, Y.-H. Targeted deep sequencing of gastric marginal zone lymphoma identified alterations of TRAF3 and TNFAIP3 that were mutually exclusive for MALT1 rearrangement. Mod. Pathol. 2018, 31, 1418–1428. [Google Scholar] [CrossRef] [PubMed]
- Rossi, D.; Bertoni, F.; Zucca, E. Marginal-Zone Lymphomas. N. Engl. J. Med. 2022, 386, 568–581. [Google Scholar] [CrossRef]
- Carrillo, J.; Restrepo, C.S.; de Christenson, M.R.; Leon, P.O.; Rivera, A.L.; Koss, M.N. Lymphoproliferative Lung Disorders: A Radiologic-Pathologic Overview. Part I: Reactive disorders. Semin. Ultrasound CT MRI 2013, 34, 525–534. [Google Scholar] [CrossRef]
- Halle, S.; Dujardin, H.C.; Bakocevic, N.; Fleige, H.; Danzer, H.; Willenzon, S.; Suezer, Y.; Hämmerling, G.; Garbi, N.; Sutter, G.; et al. Induced bronchus-associated lymphoid tissue serves as a general priming site for T cells and is maintained by dendritic cells. J. Exp. Med. 2009, 206, 2593–2601. [Google Scholar] [CrossRef]
- Foo, S.Y.; Phipps, S. Regulation of inducible BALT formation and contribution to immunity and pathology. Mucosal Immunol. 2010, 3, 537–544. [Google Scholar] [CrossRef]
- Travis, W.D.; Brambilla, E.; Burke, A.P.; Marx, A.; Nicholson, A.G. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart; IARC: Lyon, France, 2015. [Google Scholar]
- Sammassimo, S.; Pruneri, G.; Andreola, G.; Montoro, J.; Steffanoni, S.; Nowakowski, G.S.; Gandini, S.; Negri, M.; Habermann, T.M.; Raderer, M.; et al. A retrospective international study on primary extranodal marginal zone lymphoma of the lung (BALT lymphoma) on behalf of International Extranodal Lymphoma Study Group (IELSG). Hematol. Oncol. 2016, 34, 177–183. [Google Scholar] [CrossRef]
- Zinzani, P.L.; Poletti, V.; Zompatori, M.; Tani, M.; Spaggiari, L.; Tomassetti, S.; Broccoli, A.; Derenzini, E.; Baccarani, M. Bronchus associated lymphoid tissue lymphomas: An update of a rare extranodal maltoma. Clin. Lymphoma Myeloma 2007, 7, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Magazine, B.; Shahul, H.A.; Monappa, V.; Chogtu, B. BALToma masquerading as pulmonary tuberculosis. BMJ Case Rep. 2014, 2014, bcr2014206997. [Google Scholar] [CrossRef] [PubMed]
- Borie, R.; Wislez, M.; Antoine, M.; Bergman, C.C.; Thieblemont, C.; Cadranel, J. Pulmonary mucosa-associated lymphoid tissue lymphoma revisited. Eur. Respir. J. 2016, 47, 1244–1260. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, J.-I.; Kohara, M.; Tsuruta, Y.; Nojima, S.; Tahara, S.; Ohshima, K.; Kurashige, M.; Wada, N.; Morii, E. Immunohistochemical analysis of the novel marginal zone B-cell marker IRTA1 in malignant lymphoma. Hum. Pathol. 2017, 59, 70–79. [Google Scholar] [CrossRef]
- Piña-Oviedo, S.; Weissferdt, A.; Kalhor, N.; Moran, C.A. Primary Pulmonary Lymphomas. Adv. Anat. Pathol. 2015, 22, 355–375. [Google Scholar] [CrossRef]
- Du, M.-Q. MALT Lymphoma: Recent Advances in Aetiology and Molecular Genetics. J. Clin. Exp. Hematop. 2007, 47, 31–42. [Google Scholar] [CrossRef]
- Cascione, L.; Rinaldi, A.; Bruscaggin, A.; Tarantelli, C.; Arribas, A.J.; Kwee, I.; Pecciarini, L.; Mensah, A.A.; Spina, V.; Chung, E.Y.; et al. Novel insights into the genetics and epigenetics of MALT lymphoma unveiled by next generation sequencing analyses. Haematologica 2019, 104, e558–e561. [Google Scholar] [CrossRef]
- Kligerman, S.J.; Franks, T.J.; Galvin, J.R. Primary Extranodal Lymphoma of the Thorax. Radiol. Clin. N. Am. 2016, 54, 673–687. [Google Scholar] [CrossRef]
- Cardenas-Garcia, J.; Talwar, A.; Shah, R.; Fein, A. Update in primary pulmonary lymphomas. Curr. Opin. Pulm. Med. 2015, 21, 333–337. [Google Scholar] [CrossRef]
- Fung, C.Y.; Tarbell, N.J.; Lucarelli, M.J.; Goldberg, I.S.; Linggood, R.M.; Harris, N.L.; Ferry, A.J. Ocular adnexal lymphoma: Clinical behavior of distinct World Health Organization classification subtypes. Int. J. Radiat. Oncol. 2003, 57, 1382–1391. [Google Scholar] [CrossRef]
- Ferreri, A.J.; Dolcetti, R.; Du, M.Q.; Doglioni, C.; Resti, A.G.; Politi, L.S.; De Conciliis, C.; Radford, J.; Bertoni, F.; Zucca, E.; et al. OAML: An intriguing model for antigen-driven lymphomagenesis and microbial targeted therapy. Ann. Oncol. 2008, 19, 835–846. [Google Scholar] [CrossRef] [PubMed]
- Coupland, S.E.; Foss, H.D.; Anagnostopoulos, I.; Hummel, M.; Stein, H. Immunoglobulin VH gene expression among extranodal marginal zone B cell lymphomas of the ocular adnexa. Investig. Ophthalmol. Vis. Sci. 1999, 40, 555–562. [Google Scholar]
- Streubel, B.; Simonitschklupp, I.; Mullauer, L.; Lamprecht, A.; Huber, D.H.; Siebert, R.; Stolte, M.; Trautinger, F.; Lukas, J.; Puspok, A.; et al. Variable frequencies of MALT lymphoma-associated genetic aberrations in MALT lymphomas of different sites. Leukemia 2004, 18, 1722–1726. [Google Scholar] [CrossRef] [PubMed]
- Bertoni, F.; Zucca, E. Delving deeper into MALT lymphoma biology. J. Clin. Investig. 2006, 116, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Johansson, P.; Klein-Hitpass, L.; Budeus, B.; Kuhn, M.; Lauber, C.; Seifert, M.; Roeder, I.; Pförtner, R.; Stuschke, M.; Dührsen, U.; et al. Identifying Genetic Lesions in Ocular Adnexal Extranodal Marginal Zone Lymphomas of the MALT Subtype by Whole Genome, Whole Exome and Targeted Sequencing. Cancers 2020, 12, 986. [Google Scholar] [CrossRef]
- Jung, H.; Yoo, H.Y.; Lee, S.H.; Shin, S.; Kim, S.C.; Lee, S.; Joung, J.-G.; Nam, J.-Y.; Ryu, D.; Yun, J.W.; et al. The mutational landscape of ocular marginal zone lymphoma identifies frequent alterations in TNFAIP3 followed by mutations in TBL1XR1 and CREBBP. Oncotarget 2017, 8, 17038–17049, Erratum in Oncotarget 2018, 9, 32882. [Google Scholar] [CrossRef] [PubMed]
- Johansson, P.; Klein-Hitpass, L.; Grabellus, F.; Arnold, G.; Klapper, W.; Pförtner, R.; Dührsen, U.; Eckstein, A.; Dürig, J.; Küppers, R. Recurrent mutations in NF-κB pathway components, KMT2D, and NOTCH1/2 in ocular adnexal MALT-type marginal zone lymphomas. Oncotarget 2016, 7, 62627–62639. [Google Scholar] [CrossRef] [PubMed]
- Raderer, M.; Vorbeck, F.; Formanek, M.; Osterreicher, C.H.; Valencak, J.B.; Penz, M.; Kornek, G.V.; Hamilton, G.S.; Dragosics, B.; Chott, A. Importance of extensive staging in patients with mucosa-associated lymphoid tissue (MALT)-type lymphoma. Br. J. Cancer 2000, 83, 454–457. [Google Scholar] [CrossRef] [PubMed]
- Adam, P.; Czapiewski, P.; Colak, S.; Kosmidis, P.; Tousseyn, T.; Sagaert, X.; Boudova, L.; Okoń, K.; Morresi-Hauf, A.; Agostinelli, C.; et al. Prevalence ofAchromobacter xylosoxidansin pulmonary mucosa-associated lymphoid tissue lymphoma in different regions of Europe. Br. J. Haematol. 2014, 164, 804–810. [Google Scholar] [CrossRef]
- Aoyama, S.; Masaki, A.; Sakamoto, Y.; Takino, H.; Murase, T.; Ohshima, K.; Yoshino, T.; Kato, S.; Inagaki, H. Achromobacter Infection Is Rare in Japanese Patients with Pulmonary B-cell Lymphoma. Intern. Med. 2018, 57, 789–794. [Google Scholar] [CrossRef]
- Engels, E.A.; Cerhan, J.; Linet, M.S.; Cozen, W.; Colt, J.S.; Davis, S.; Gridley, G.; Severson, R.K.; Hartge, P. Immune-Related Conditions and Immune-Modulating Medications as Risk Factors for Non-Hodgkin’s Lymphoma: A Case-Control Study. Am. J. Epidemiol. 2005, 162, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Stott, I.D.; Hiepe, F.; Hummel, M.; Steinhauser, G.; Berek, C. Antigen-driven clonal proliferation of B cells within the target tissue of an autoimmune disease. The salivary glands of patients with Sjögren’s syndrome. J. Clin. Investig. 1998, 102, 938–946. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, A.J.M.; Dolcetti, R.; Magnino, S.; Doglioni, C.; Ponzoni, M. Chlamydial infection: The link with ocular adnexal lymphomas. Nat. Rev. Clin. Oncol. 2009, 6, 658–669. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, A.J.M.; Guidoboni, M.; Ponzoni, M.; De Conciliis, C.; Dell’Oro, S.; Fleischhauer, K.; Caggiari, L.; Lettini, A.A.; Dal Cin, E.; Ieri, R.; et al. Evidence for an Association Between Chlamydia psittaci and Ocular Adnexal Lymphomas. J. Natl. Cancer Inst. 2004, 96, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, A.J.; Dolcetti, R.; Dognini, G.P.; Malabarba, L.; Vicari, N.; Pasini, E.; Ponzoni, M.; Cangi, M.G.; Pecciarini, L.; Resti, A.G.; et al. Chlamydophila psittaciis viable and infectious in the conjunctiva and peripheral blood of patients with ocular adnexal lymphoma: Results of a single-center prospective case–control study. Int. J. Cancer 2008, 123, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, A.J.; Ponzoni, M.; Guidoboni, M.; De Conciliis, C.; Resti, A.G.; Mazzi, B.; Lettini, A.A.; Demeter, J.; Dell’Oro, S.; Doglioni, C.; et al. Regression of Ocular Adnexal Lymphoma after Chlamydia Psittaci–Eradicating Antibiotic Therapy. J. Clin. Oncol. 2005, 23, 5067–5073. [Google Scholar] [CrossRef]
- Treglia, G.; Zucca, E.; Sadeghi, R.; Cavalli, F.; Giovanella, L.; Ceriani, L. Detection rate of fluorine-18-fluorodeoxyglucose positron emission tomography in patients with marginal zone lymphoma of MALT type: A meta-analysis. Hematol. Oncol. 2015, 33, 113–124. [Google Scholar] [CrossRef]
- Cerrato, M.; Orlandi, E.; Vella, A.; Bartoncini, S.; Iorio, G.C.; Bongiovanni, D. Efficacy of low-dose radiotherapy (2 Gy × 2) in the treatment of marginal zone and mucosa-associated lymphoid tissue lymphomas. Br. J. Radiol. 2021, 94, 20210012. [Google Scholar] [CrossRef]
- Wöhrer, S.; Drach, J.; Hejna, M.; Scheithauer, W.; Dirisamer, A.; Püspök, A.; Chott, A.; Raderer, M. Treatment of extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) with mitoxantrone, chlorambucil and prednisone (MC. PSITTACI). Ann. Oncol. 2003, 14, 1758–1761. [Google Scholar] [CrossRef]
- Zucca, E.; Conconi, A.; Laszlo, D.; López-Guillermo, A.; Bouabdallah, R.; Coiffier, B.; Sebban, C.; Jardin, F.; Vitolo, U.; Morschhauser, F.; et al. Addition of Rituximab to Chlorambucil Produces Superior Event-Free Survival in the Treatment of Patients with Extranodal Marginal-Zone B-Cell Lymphoma: 5-Year Analysis of the IELSG-19 Randomized Study. J. Clin. Oncol. 2013, 31, 565–572. [Google Scholar] [CrossRef]
- Salar, A.; Domingo-Domenech, E.; Panizo, C.; Nicolás, C.; Bargay, J.; Muntañola, A.; Canales, M.; Bello, J.L.; Sancho, J.M.; Tomás, J.F.; et al. First-line response-adapted treatment with the combination of bendamustine and rituximab in patients with mucosa-associated lymphoid tissue lymphoma (MALT2008-01): A multicentre, single-arm, phase 2 trial. Lancet Haematol. 2014, 1, e104–e111. [Google Scholar] [CrossRef]
- Raderer, M.; Wöhrer, S.; Streubel, B.; Drach, J.; Jäger, U.; Turetschek, K.; Troch, M.; Püspök, A.; Zielinski, C.C.; Chott, A. Activity of Rituximab plus Cyclophosphamide, Doxorubicin/Mitoxantrone, Vincristine and Prednisone in Patients with Relapsed MALT Lymphoma. Oncology 2006, 70, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Lossos, I.S.; Fabregas, J.C.; Koru-Sengul, T.; Miao, F.; Goodman, D.; Serafini, A.N.; Hosein, P.J.; Stefanovic, A.; Rosenblatt, J.D.; Hoffman, J.E. Phase II study of 90Y Ibritumomab tiuxetan (Zevalin) in patients with previously untreated marginal zone lymphoma. Leuk. Lymphoma 2015, 56, 1750–1755. [Google Scholar] [CrossRef] [PubMed]
- Fowler, N.H.; Davis, R.E.; Rawal, S.; Nastoupil, L.; Hagemeister, F.B.; McLaughlin, P.; Kwak, L.W.; Romaguera, E.J.; Fanale, A.M.; Fayad, E.L.; et al. Safety and activity of lenalidomide and rituximab in untreated indolent lymphoma: An open-label, phase 2 trial. Lancet Oncol. 2014, 15, 1311–1318. [Google Scholar] [CrossRef]
- Becnel, M.R.; Nastoupil, L.J.; Samaniego, F.; Davis, R.E.; You, M.J.; Green, M.; Hagemeister, F.B.; Fanale, M.A.; Fayad, L.E.; Westin, J.R.; et al. Lenalidomide plus rituximab (R2) in previously untreated marginal zone lymphoma: Subgroup analysis and long-term follow-up of an open-label phase 2 trial. Br. J. Haematol. 2019, 185, 874–882. [Google Scholar] [CrossRef]
- Noy, A.; De Vos, S.; Coleman, M.; Martin, P.; Flowers, C.R.; Thieblemont, C.; Morschhauser, F.; Collins, G.P.; Ma, S.; Peles, S.; et al. Durable ibrutinib responses in relapsed/refractory marginal zone lymphoma: Long-term follow-up and biomarker analysis. Blood Adv. 2020, 4, 5773–5784. [Google Scholar] [CrossRef]
- Raderer, M.; Wöhrer, S.; Streubel, B.; Troch, M.; Turetschek, K.; Jäger, U.; Skrabs, C.; Gaiger, A.; Drach, J.; Puespoek, A.; et al. Assessment of Disease Dissemination in Gastric Compared with Extragastric Mucosa-Associated Lymphoid Tissue Lymphoma Using Extensive Staging: A Single-Center Experience. J. Clin. Oncol. 2006, 24, 3136–3141. [Google Scholar] [CrossRef]
- Fischbach, W.; Goebeler, E.M.; Ruskone-Fourmestraux, A.; Wundisch, T.; Neubauer, A.; Raderer, M.; Savio, A.; EGILS (European Gastro-Intestinal Lymphoma Study) Group. Most patients with minimal histological residuals of gastric MALT lymphoma after successful eradication of Helicobacter pylori can be managed safely by a watch and wait strategy: Experience from a large international series. Gut 2007, 56, 1685–1687. [Google Scholar] [CrossRef]
- Parsonnet, J.; Hansen, S.; Rodriguez, L.; Gelb, A.B.; Warnke, R.A.; Jellum, E.; Orentreich, N.; Vogelman, J.H.; Friedman, G.D. Helicobacter pylori Infection and Gastric Lymphoma. N. Engl. J. Med. 1994, 330, 1267–1271. [Google Scholar] [CrossRef]
- Wotherspoon, A.; Diss, T.; Pan, L.; Isaacson, P.; Doglioni, C.; Moschini, A.; De Boni, M. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet 1993, 342, 575–577. [Google Scholar] [CrossRef]
- Thieblemont, C.; Zucca, E. Clinical aspects and therapy of gastrointestinal MALT lymphoma. Best Pract. Res. Clin. Haematol. 2017, 30, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, A.J.M.; Cecchetti, C.; Kiesewetter, B.; Sassone, M.; Calimeri, T.; Perrone, S.; Ponzoni, M.; Raderer, M. Clarithromycin as a “repurposing drug” against MALT lymphoma. Br. J. Haematol. 2017, 182, 913–915. [Google Scholar] [CrossRef] [PubMed]
- Hoskin, P.; Popova, B.; Schofield, O.; Brammer, C.; Robinson, M.; Brunt, A.M.; Madhavan, K.; Illidge, T.; Gallop-Evans, E.; Syndikus, I.; et al. 4 Gy versus 24 Gy radiotherapy for follicular and marginal zone lymphoma (FoRT): Long-term follow-up of a multicentre, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2021, 22, 332–340. [Google Scholar] [CrossRef]
- Pinnix, C.C.; Gunther, J.R.; Milgrom, S.A.; Cruz-Chamorro, R.; Medeiros, L.J.; Khoury, J.D.; Amini, B.; Neelapu, S.; Lee, H.J.; Westin, J.; et al. Outcomes After Reduced-Dose Intensity Modulated Radiation Therapy for Gastric Mucosa-Associated Lymphoid Tissue (MALT) Lymphoma. Int. J. Radiat. Oncol. 2019, 104, 447–455. [Google Scholar] [CrossRef]
- Kiesewetter, B.; Troch, M.; Dolak, W.; Mullauer, L.; Lukas, J.; Zielinski, C.C.; Raderer, M. A phase II study of lenalidomide in patients with extranodal marginal zone B-cell lymphoma of the mucosa associated lymphoid tissue (MALT lymphoma). Haematologica 2013, 98, 353–356. [Google Scholar] [CrossRef]
- Borie, R.; Wislez, M.; Thabut, G.; Antoine, M.; Rabbat, A.; Couderc, L.-J.; Monnet, I.; Nunes, H.; Blanc, F.; Mal, H.; et al. Clinical characteristics and prognostic factors of pulmonary MALT lymphoma. Eur. Respir. J. 2009, 34, 1408–1416. [Google Scholar] [CrossRef]
- Okamura-Shiki, I.; Imai, H.; Morio, M.; Ogura, K.; Isoda, A.; Mihara, K.; Matsumoto, M.; Saito, R.; Takahashi, T.; Ikeda, T. Rituximab monotherapy as a first-line treatment for pulmonary mucosa-associated lymphoid tissue lymphoma. Int. J. Hematol. 2015, 101, 46–51. [Google Scholar] [CrossRef]
- Sindel, A.; Al-Juhaishi, T.; Yazbeck, V. Marginal Zone Lymphoma: State-of-the-Art Treatment. Curr. Treat. Options Oncol. 2019, 20, 90. [Google Scholar] [CrossRef]
- Martinet, S.; Ozsahin, M.; Belkacémi, Y.; Landmann, C.; Poortmans, P.; Oehlere, C.; Scandolaro, L.; Krengli, M.; Maingon, P.; Miralbell, R.; et al. Outcome and prognostic factors in orbital lymphoma: A Rare Cancer Network study on 90 consecutive patients treated with radiotherapy. Int. J. Radiat. Oncol. 2003, 55, 892–898. [Google Scholar] [CrossRef]
- Tanimoto, K.; Kaneko, A.; Suzuki, S.; Sekiguchi, N.; Maruyama, D.; Kim, S.W.; Watanabe, T.; Kobayashi, Y.; Kagami, Y.; Maeshima, A.; et al. Long-term follow-up results of no initial therapy for ocular adnexal MALT lymphoma. Ann. Oncol. 2006, 17, 135–140. [Google Scholar] [CrossRef]
- Pfeffer, M.R.; Rabin, T.; Tsvang, L.; Goffman, J.; Rosen, N.; Symon, Z. Orbital lymphoma: Is it necessary to treat the entire orbit? Int. J. Radiat. Oncol. Biol. Phys. 2004, 60, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ye, G.; Liu, Z.; Shi, L.; Zhan, C.; Gu, J.; Luo, R.; Lin, Z.; Ge, D.; Wang, Q. Clinical characteristics, diagnosis, treatment, and prognostic factors of pulmonary mucosa-associated lymphoid tissue-derived lymphoma. Cancer Med. 2019, 8, 7660–7668. [Google Scholar] [CrossRef] [PubMed]
- Ejima, Y.; Sasaki, R.; Okamoto, Y.; Maruta, T.; Azumi, A.; Hayashi, Y.; Demizu, Y.; Ota, Y.; Soejima, T.; Sugimura, K. Ocular adnexal mucosa-associated lymphoid tissue lymphoma treated with radiotherapy. Radiother. Oncol. 2006, 78, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Fasola, C.E.; Jones, J.; Huang, D.D.; Le, Q.-T.; Hoppe, R.T.; Donaldson, S.S. Low-Dose Radiation Therapy (2 Gy × 2) in the Treatment of Orbital Lymphoma. Int. J. Radiat. Oncol. 2013, 86, 930–935. [Google Scholar] [CrossRef]
- Ferreri, A.J.M.; Ponzoni, M.; Guidoboni, M.; Resti, A.G.; Politi, L.S.; Cortelazzo, S.; Demeter, J.; Zallio, F.; Palmas, A.; Muti, G.; et al. Bacteria-Eradicating Therapy with Doxycycline in Ocular Adnexal MALT Lymphoma: A Multicenter Prospective Trial. JNCI J. Natl. Cancer Inst. 2006, 98, 1375–1382. [Google Scholar] [CrossRef] [PubMed]
- Han, J.J.; Kim, T.M.; Jeon, Y.K.; Kim, M.K.; Khwarg, S.I.; Kim, C.-W.; Kim, I.H.; Heo, D.S. Long-term outcomes of first-line treatment with doxycycline in patients with previously untreated ocular adnexal marginal zone B cell lymphoma. Ann. Hematol. 2014, 94, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Mino, T.; Mihara, K.; Yoshida, T.; Takihara, Y.; Ichinohe, T. Monthly administration of rituximab is useful for patients with ocular adnexal mucosa-associated lymphoid tissue lymphoma. Blood Cancer J. 2014, 4, e245. [Google Scholar] [CrossRef]
- Tuncer, S.; Tanyildiz, B.; Basaran, M.; Buyukbabani, N.; Dogan, O. Systemic Rituximab Immunotherapy in the Management of Primary Ocular Adnexal Lymphoma: Single Institution Experience. Curr. Eye Res. 2015, 40, 780–785. [Google Scholar] [CrossRef]
- Savino, G.; Battendieri, R.; Gari, M.; Caputo, C.G.; Laurenti, L.; Blasi, M.A. Long-term outcomes of primary ocular adnexal lymphoma treatment with intraorbital rituximab injections. J. Cancer Res. Clin. Oncol. 2013, 139, 1251–1255. [Google Scholar] [CrossRef]
- Hindsø, T.G.; Esmaeli, B.; Holm, F.; Mikkelsen, L.H.; Rasmussen, P.K.; Coupland, E.S.; Finger, P.T.; Graue, G.F.; Grossniklaus, E.H.; Honavar, S.G.; et al. International multicentre retrospective cohort study of ocular adnexal marginal zone B-cell lymphoma. Br. J. Ophthalmol. 2020, 104, 357–362. [Google Scholar] [CrossRef]
- Luminari, S.; Merli, M.; Rattotti, S.; Tarantino, V.; Marcheselli, L.; Cavallo, F.; Varettoni, M.; Bianchi, B.; Merli, F.; Tedeschi, A.; et al. Early progression as a predictor of survival in marginal zone lymphomas: An analysis from the FIL-NF10 study. Blood 2019, 134, 798–801. [Google Scholar] [CrossRef] [PubMed]



| EMZL Site-Related Genomic Aberrations, Pathogens, Autoimmune Disorders | ||
|---|---|---|
| Localization | Genomic Aberrations | Pathogens/Autoimmune Disorders |
| Gastric MALT | Trisomy: +3, +18 | Helicobacter pylori Helicobacter Heimanni |
| IGH/MALT1 t(14;18)(q32;q21) BIRC3/MALT1 t(11;18)(q21;q21) BCL10/IGH t(1;14)(p22;q32) | ||
| Intestine | Trisomy: +3, +18 | Campylobacter jejuni |
| BIRC3/MALT1 t(11;18)(q21;q21) BCL10/IGH t(1;14)(p22;q32) | ||
| Skin | Trisomy: +3, +18 | Borrelia burgdorferi |
| IGH/MALT1 t(14;18)(q32;q21) | ||
| FOXP1/IGH t(3;14)(p14.1;q32) | ||
| Ocular Adnexa | Trisomy: +3, +18 | Chlamydia psittaci Sjogren’s syndrome |
| IGH/MALT1 t(14;18)(q32;q21) | ||
| FOXP1/IGH t(3;14)(p14.1;q32) | ||
| TNFAIP3 target mutation | ||
| Lung | Trisomy: +3, +18 | Achromobacter xylosoxidans Lymphocytic interstitial pneumonia |
| BIRC3/MALT1 t(11;18)(q21;q21) | ||
| Salivary Gland | Trisomy: +3, +18 IGH/MALT1 t(14;18)(q32;q21) | Sjogren’s syndrome Hepatitis C virus |
| TBL1XR1 target mutation | ||
| GPR34 target mutation | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Rocco, A.; Petrucci, L.; Assanto, G.M.; Martelli, M.; Pulsoni, A. Extranodal Marginal Zone Lymphoma: Pathogenesis, Diagnosis and Treatment. Cancers 2022, 14, 1742. https://doi.org/10.3390/cancers14071742
Di Rocco A, Petrucci L, Assanto GM, Martelli M, Pulsoni A. Extranodal Marginal Zone Lymphoma: Pathogenesis, Diagnosis and Treatment. Cancers. 2022; 14(7):1742. https://doi.org/10.3390/cancers14071742
Chicago/Turabian StyleDi Rocco, Alice, Luigi Petrucci, Giovanni Manfredi Assanto, Maurizio Martelli, and Alessandro Pulsoni. 2022. "Extranodal Marginal Zone Lymphoma: Pathogenesis, Diagnosis and Treatment" Cancers 14, no. 7: 1742. https://doi.org/10.3390/cancers14071742
APA StyleDi Rocco, A., Petrucci, L., Assanto, G. M., Martelli, M., & Pulsoni, A. (2022). Extranodal Marginal Zone Lymphoma: Pathogenesis, Diagnosis and Treatment. Cancers, 14(7), 1742. https://doi.org/10.3390/cancers14071742






