Predictive Factors for Resistant Disease with Medical/Radiologic/Liver-Directed Anti-Tumor Treatments in Patients with Advanced Pancreatic Neuroendocrine Neoplasms: Recent Advances and Controversies
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
3. Predictive Factors for Response to SSA in Advanced panNENs
3.1. General: Predictive Factors with Somatostatin
3.2. Clinically-Related Predictive Factors for SSA Efficacy [Clinical, Laboratory, Treatment-Related Factors]
3.3. Pathological Predictive Factors for SSA Efficacy [Histological Factors/Classification/Grading, Molecular Factors]
4. Predictive Factors for Response to Everolimus in Advanced panNENs
4.1. General: Predictive Factors with Everolimus
4.2. Clinically-Related Predictive Factors for Everolimus Efficacy [Clinical, Laboratory, Treatment-Related Factors]
4.3. Pathological Predictive Factors for Everolimus Efficacy [Histological Factors/Classification/Grading, Molecular Factors]
5. Predictive Factors for Response to Sunitinib in Advanced panNENs
5.1. General: Predictive Factors with Sunitinib
5.2. Clinically-Related Predictive Factors for Sunitinib Efficacy [Clinical, Laboratory, Treatment-Related Factors]
5.3. Pathological Predictive Factors for Sunitinib Efficacy [Histological Factors/Classification/Grading, Molecular Factors])
6. Predictive Factors for Response to PRRT in Advanced panNENs
6.1. General: Predictive Factors with PRRT
6.2. Clinical Predictive Factors for PRRT Efficacy
6.3. Laboratory Test/Biomarkers Predictive Factors for PRRT Efficacy
6.4. Imaging Predictive Factors for PRRT Efficacy
6.5. Treatment-Related Predictive Factors for PRRT Efficacy
6.6. Pathological Predictive Factors for PRRT Efficacy [Histological Factors/Classification/Grading, Molecular Factors]
7. Predictive Factors for Response to Chemotherapy in Advanced panNENs
7.1. General: Predictive Factors with Chemotherapy
7.2. Clinically-Related Predictive Factors for Chemotherapy Efficacy [Clinical, Laboratory, Imaging, Treatment-Related Factors]
7.3. Pathological Predictive Factors for Chemotherapy Efficacy [Histological Factors/Classification/Grading, Molecular Factors]
8. Predictive Factors for Response to Liver-Directed Therapies in Advanced panNENs
8.1. General: Predictive Factors with Liver-Directed Therapies
8.2. Clinically-Related Predictive Factors Liver-Directed Therapies Efficacy [Clinical, Laboratory, Imaging, Treatment-Related Factors]
8.3. Pathological Predictive Factors for Liver-Directed Therapies Efficacy [Histological Factors/Classification/Grading, Molecular Factors]
9. Controversies and Uncertainties of Predicting Therapeutic Response in Advanced panNENs
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jensen, R.T. Neuroendocrine tumors of the gastrointestintal tract (GI) and pancreas. In Harrison’s Principles of Internal Medicine-ED.20, 20th ed.; Jamieson, L.L., Fauci, A.S., Kasper, D.L., Hauser, S.L., Longo, D.L., Loscalzo, J., Eds.; McGraw Hill Education Medical Publishing Division: New York, NY, USA, 2018; Volume 1, pp. 596–615. [Google Scholar]
- Chang, A.; Sherman, S.K.; Howe, J.R.; Sahai, V. Progress in the Management of Pancreatic Neuroendocrine Tumors. Annu. Rev Med. 2021, 73, 213–229. [Google Scholar] [CrossRef]
- Falconi, M.; Eriksson, B.; Kaltsas, G.; Bartsch, D.K.; Capdevila, J.; Caplin, M.; Kos-Kudla, B.; Kwekkeboom, D.; Rindi, G.; Kloppel, G.; et al. ENETS Consensus Guidelines Update for the Management of Patients with Functional Pancreatic Neuroendocrine Tumors and Non-Functional Pancreatic Neuroendocrine Tumors. Neuroendocrinology 2016, 103, 153–171. [Google Scholar] [CrossRef] [PubMed]
- Metz, D.C.; Jensen, R.T. Gastrointestinal neuroendocrine tumors: Pancreatic endocrine tumors. Gastroenterology 2008, 135, 1469–1492. [Google Scholar] [CrossRef] [PubMed]
- Pavel, M.; O’Toole, D.; Costa, F.; Capdevila, J.; Gross, D.; Kianmanesh, R.; Krenning, E.; Knigge, U.; Salazar, R.; Pape, U.F.; et al. ENETS Consensus Guidelines Update for the Management of Distant Metastatic Disease of Intestinal, Pancreatic, Bronchial Neuroendocrine Neoplasms (NEN) and NEN of Unknown Primary Site. Neuroendocrinology 2016, 103, 172–185. [Google Scholar] [CrossRef] [PubMed]
- Beyens, M.; Vandamme, T.; Peeters, M.; Van Camp, G.; Op de Beeck, K. Resistance to targeted treatment of gastroenteropancreatic neuroendocrine tumors. Endocr. Relat. Cancer 2019, 26, R109–R130. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.; Ito, T.; Jensen, R.T. Prognostic and predictive factors on overall survival and surgical outcomes in pancreatic neuroendocrine tumors: Recent advances and controversies. Expert Rev. Anticancer Ther. 2019, 19, 1029–1050. [Google Scholar] [CrossRef] [PubMed]
- Fishbeyn, V.A.; Norton, J.A.; Benya, R.V.; Pisegna, J.R.; Venzon, D.J.; Metz, D.C.; Jensen, R.T. Assessment and prediction of long-term cure in patients with Zollinger-Ellison syndrome: The best approach. Ann. Intern. Med. 1993, 119, 199–206. [Google Scholar] [CrossRef]
- Ito, T.; Igarashi, H.; Jensen, R.T. Therapy of metastatic pancreatic neuroendocrine tumors (pNETs): Recent insights and advances. J. Gastroenterol. 2012, 47, 941–960. [Google Scholar] [CrossRef]
- Thom, A.K.; Norton, J.A.; Axiotis, C.A.; Jensen, R.T. Location, incidence and malignant potential of duodenal gastrinomas. Surgery 1991, 110, 1086–1093. [Google Scholar]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients with Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef]
- Yao, J.C.; Hassan, M.; Phan, A.; Dagohoy, C.; Leary, C.; Mares, J.E.; Abdalla, E.K.; Fleming, J.B.; Vauthey, J.N.; Rashid, A.; et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J. Clin. Oncol. 2008, 26, 3063–3072. [Google Scholar] [CrossRef] [PubMed]
- Von Schrenck, T.; Howard, J.M.; Doppman, J.L.; Norton, J.A.; Maton, P.N.; Smith, F.P.; Vinayek, R.; Frucht, H.; Wank, S.A.; Gardner, J.D.; et al. Prospective study of chemotherapy in patients with metastatic gastrinoma. Gastroenterology 1988, 94, 1326–1334. [Google Scholar] [CrossRef]
- Ito, T.; Hijioka, S.; Masui, T.; Kasajima, A.; Nakamoto, Y.; Kobayashi, N.; Komoto, I.; Hijioka, M.; Lee, L.; Igarashi, H.; et al. Advances in the diagnosis and treatment of pancreatic neuroendocrine neoplasms in Japan. J. Gastroenterol. 2017, 52, 9–18. [Google Scholar] [CrossRef]
- Jensen, R.T.; Ito, T. Gastrinoma. Endotext. 2020. Available online: www.endotext.org (accessed on 21 November 2020).
- Cloyd, J.M.; Konda, B.; Shah, M.H.; Pawlik, T.M. The emerging role of targeted therapies for advanced well-differentiated gastroenteropancreatic neuroendocrine tumors. Expert Rev. Clin. Pharm. 2019, 12, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Akirov, A.; Larouche, V.; Alshehri, S.; Asa, S.L.; Ezzat, S. Treatment Options for Pancreatic Neuroendocrine Tumors. Cancers 2019, 11, 828. [Google Scholar] [CrossRef]
- Tsoli, M.; Chatzellis, E.; Koumarianou, A.; Kolomodi, D.; Kaltsas, G. Current best practice in the management of neuroendocrine tumors. Ther. Adv. Endocrinol. Metab. 2018, 10, 2042018818804698. [Google Scholar] [CrossRef]
- Huttner, F.J.; Schneider, L.; Tarantino, I.; Warschkow, R.; Schmied, B.M.; Hackert, T.; Diener, M.K.; Buchler, M.W.; Ulrich, A. Palliative resection of the primary tumor in 442 metastasized neuroendocrine tumors of the pancreas: A population-based, propensity score-matched survival analysis. Langenbecks Arch. Surg. 2015, 400, 715–723. [Google Scholar] [CrossRef]
- Pozas, J.; San, R.M.; Alonso-Gordoa, T.; Pozas, M.; Caracuel, L.; Carrato, A.; Molina-Cerrillo, J. Targeting Angiogenesis in Pancreatic Neuroendocrine Tumors: Resistance Mechanisms. Int. J. Mol. Sci 2019, 20, 4949. [Google Scholar] [CrossRef]
- Maharjan, C.K.; Ear, P.H.; Tran, C.G.; Howe, J.R.; Chandrasekharan, C.; Quelle, D.E. Pancreatic Neuroendocrine Tumors: Molecular Mechanisms and Therapeutic Targets. Cancers 2021, 13, 5117. [Google Scholar] [CrossRef]
- Dawod, M.; Gordoa, T.A.; Cives, M.; de Mestier, L.; Crona, J.; Spada, F.; Oberg, K.; Pavel, M.; Lamarca, A. Antiproliferative Systemic Therapies for Metastatic Small Bowel Neuroendocrine Tumours. Curr. Treat. Opt. Oncol. 2021, 22, 73. [Google Scholar] [CrossRef]
- Yao, J.C.; Shah, M.H.; Ito, T.; Bohas, C.L.; Wolin, E.M.; Van Cutsem, E.; Hobday, T.J.; Okusaka, T.; Capdevila, J.; de Vries, E.G.; et al. Everolimus for Advanced Pancreatic Neuroendocrine Tumors. N. Engl. J. Med. 2011, 364, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Raymond, E.; Dahan, L.; Raoul, J.L.; Bang, Y.J.; Borbath, I.; Lombard-Bohas, C.; Valle, J.; Metrakos, P.; Smith, D.; Vinik, A.; et al. Sunitinib Malate for the Treatment of Pancreatic Neuroendocrine Tumors. N. Engl. J. Med. 2011, 364, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Mujica-Mota, R.; Varley-Campbell, J.; Tikhonova, I.; Cooper, C.; Griffin, E.; Haasova, M.; Peters, J.; Lucherini, S.; Talens-Bou, J.; Long, L.; et al. Everolimus, lutetium-177 DOTATATE and sunitinib for advanced, unresectable or metastatic neuroendocrine tumours with disease progression: A systematic review and cost-effectiveness analysis. Health Technol. Assess. 2018, 22, 1–326. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.; Strosberg, J.R. Medical Management of Gastroenteropancreatic Neuroendocrine Tumors: Current Strategies and Future Advances. J. Nucl. Med. 2019, 60, 721–727. [Google Scholar] [CrossRef]
- Valle, J.W.; Borbath, I.; Rosbrook, B.; Fernandez, K.; Raymond, E. Sunitinib in patients with pancreatic neuroendocrine tumors: Update of safety data. Future. Oncol. 2019, 15, 1219–1230. [Google Scholar] [CrossRef]
- Raymond, E.; Kulke, M.H.; Qin, S.; Yu, X.; Schenker, M.; Cubillo, A.; Lou, W.; Tomasek, J.; Thiis-Evensen, E.; Xu, J.M.; et al. Efficacy and Safety of Sunitinib in Patients with Well-Differentiated Pancreatic Neuroendocrine Tumours. Neuroendocrinology 2018, 107, 237–245. [Google Scholar] [CrossRef]
- Lee, L.; Ito, T.; Jensen, R.T. Everolimus in the treatment of neuroendocrine tumors: Efficacy, side-effects, resistance, and factors affecting its place in the treatment sequence. Expert Opin. Pharmacother. 2018, 19, 909–928. [Google Scholar] [CrossRef]
- Ito, T.; Tori, M.; Hashigaki, S.; Kimura, N.; Sato, K.; Ohki, E.; Sawaki, A.; Okusaka, T. Efficacy and safety of sunitinib in Japanese patients with progressive, advanced/metastatic, well-differentiated, unresectable pancreatic neuroendocrine tumors: Final analyses from a Phase II study. Jpn. J. Clin. Oncol. 2019, 49, 354–360. [Google Scholar] [CrossRef]
- Uri, I.; Avniel-Polak, S.; Gross, D.J.; Grozinsky-Glasberg, S. Update in the Therapy of Advanced Neuroendocrine Tumors. Curr Treat. Options. Oncol. 2017, 18, 72. [Google Scholar] [CrossRef]
- Zhuo, Z.G.; Zhu, Y.K.; Deng, H.Y.; Li, G.; Luo, J.; Alai, G.H.; Lin, Y.D. Role of everolimus in the treatment of advanced neuroendocrine tumor: A meta-analysis of randomized trials. J. BUON 2019, 24, 368–373. [Google Scholar]
- Xu, J. Current treatments and future potential of surufatinib in neuroendocrine tumors (NETs). Ther. Adv. Med. Oncol. 2021, 13, 17588359211042689. [Google Scholar] [CrossRef]
- Xu, J.; Shen, L.; Bai, C.; Wang, W.; Li, J.; Yu, X.; Li, Z.; Li, E.; Yuan, X.; Chi, Y.; et al. Surufatinib in advanced pancreatic neuroendocrine tumours (SANET-p): A randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 1489–1499. [Google Scholar] [CrossRef]
- Xu, J.; Shen, L.; Zhou, Z.; Li, J.; Bai, C.; Chi, Y.; Li, Z.; Xu, N.; Li, E.; Liu, T.; et al. Surufatinib in advanced extrapancreatic neuroendocrine tumours (SANET-ep): A randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 1500–1512. [Google Scholar] [CrossRef]
- Brunner, P.; Jorg, A.C.; Glatz, K.; Bubendorf, L.; Radojewski, P.; Umlauft, M.; Marincek, N.; Spanjol, P.M.; Krause, T.; Dumont, R.A.; et al. The prognostic and predictive value of sstr2-immunohistochemistry and sstr2-targeted imaging in neuroendocrine tumors. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Pusceddu, S.; Prinzi, N.; Raimondi, A.; Corti, F.; Buzzoni, R.; di Bartolomeo, M.; Seregni, E.; Maccauro, M.; Coppa, J.; Milione, M.; et al. Entering the third decade of experience with octreotide LAR in neuroendocrine tumors: A review of current knowledge. Tumori J. 2019, 105, 113–120. [Google Scholar] [CrossRef]
- Enzler, T.; Fojo, T. Long-acting somatostatin analogues in the treatment of unresectable/metastatic neuroendocrine tumors. Semin. Oncol. 2017, 44, 141–156. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Lee, L.; Jensen, R.T. Treatment of symptomatic neuroendocrine tumor syndromes: Recent advances and controversies. Expert Opin. Pharmacother. 2016, 17, 2191–2205. [Google Scholar] [CrossRef]
- Stueven, A.K.; Kayser, A.; Wetz, C.; Amthauer, H.; Wree, A.; Tacke, F.; Wiedenmann, B.; Roderburg, C.; Jann, H. Somatostatin Analogues in the Treatment of Neuroendocrine Tumors: Past, Present and Future. Int. J. Mol. Sci. 2020, 20, 3049. [Google Scholar] [CrossRef]
- Faggiano, A.; Modica, R.; Lo, C.F.; Camera, L.; Napolitano, V.; Altieri, B.; de Cicco, F.; Bottiglieri, F.; Sesti, F.; Badalamenti, G.; et al. Lanreotide therapy vs active surveillance in MEN1-related pancreatic neuroendocrine tumors <2 cm. J. Clin. Endocrinol. Metab. 2019, 105, 78–84. [Google Scholar] [CrossRef]
- Laskaratos, F.M.; Armeni, E.; Shah, H.; Megapanou, M.; Papantoniou, D.; Hayes, A.R.; Navalkissoor, S.; Gnanasegaran, G.; von, S.C.; Phillips, E.; et al. Predictors of antiproliferative effect of lanreotide autogel in advanced gastroenteropancreatic neuroendocrine neoplasms. Endocrine 2020, 67, 233–242. [Google Scholar] [CrossRef]
- Hamiditabar, M.; Ali, M.; Roys, J.; Wolin, E.M.; O’Dorisio, T.M.; Ranganathan, D.; Tworowska, I.; Strosberg, J.R.; Delpassand, E.S. Peptide Receptor Radionuclide Therapy with 177Lu-Octreotate in Patients with Somatostatin Receptor Expressing Neuroendocrine Tumors: Six Years’ Assessment. Clin. Nucl. Med. 2017, 42, 436–443. [Google Scholar] [CrossRef]
- Hu, Y.; Ye, Z.; Wang, F.; Qin, Y.; Xu, X.; Yu, X.; Ji, S. Role of Somatostatin Receptor in Pancreatic Neuroendocrine Tumor Development, Diagnosis, and Therapy. Front. Endocrinol. Lausanne 2021, 12, 679000. [Google Scholar] [CrossRef]
- Rinke, A.; Wittenberg, M.; Schade-Brittinger, C.; Aminossadati, B.; Ronicke, E.; Gress, T.M.; Muller, H.H.; Arnold, R. Placebo-Controlled, Double-Blind, Prospective, Randomized Study on the Effect of Octreotide LAR in the Control of Tumor Growth in Patients with Metastatic Neuroendocrine Midgut Tumors (PROMID): Results of Long-Term Survival. Neuroendocrinology 2017, 104, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Caplin, M.E.; Pavel, M.; Cwikla, J.B.; Phan, A.T.; Raderer, M.; Sedlackova, E.; Cadiot, G.; Wolin, E.M.; Capdevila, J.; Wall, L.; et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N. Engl. J. Med. 2014, 371, 224–233. [Google Scholar] [CrossRef]
- Lee, L.; Ito, T.; Jensen, R.T. Imaging of pancratic neuroendocrine tumors: Recent advances, current status and controversies. Expert Rev. Anticancer Ther. 2018, 18, 837–860. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Jensen, R.T. Molecular imaging in neuroendocrine tumors: Recent advances, controversies, unresolved issues, and roles in management. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Hoppenz, P.; Els-Heindl, S.; Beck-Sickinger, A.G. Peptide-Drug Conjugates and Their Targets in Advanced Cancer Therapies. Front. Chem. 2020, 8, 571. [Google Scholar] [CrossRef]
- Kwekkeboom, D.J.; Krenning, E.P. Peptide Receptor Radionuclide Therapy in the Treatment of Neuroendocrine Tumors. Hematol. Oncol. Clin. 2016, 30, 179–191. [Google Scholar] [CrossRef]
- Moody, T.W.; Ramos-Alvarez, I.; Jensen, R.T. Neuropeptide G Protein-Coupled Receptors as Oncotargets. Front. Endocrinol. 2018, 9, 345. [Google Scholar] [CrossRef]
- Sancho, V.; Di Florio, A.; Moody, T.W.; Jensen, R.T. Bombesin receptor-mediated imaging and cytotoxicity: Review and current status. Curr. Drug Deliv. 2011, 8, 79–134. [Google Scholar] [CrossRef]
- Moreno, P.; Ramos-Alvarez, I.; Moody, T.W.; Jensen, R.T. Bombesin related peptides/receptors and their promising therapeutic roles in cancer imaging, targeting and treatment. Expert Opin. Ther. Targets. 2016, 20, 1055–1073. [Google Scholar] [CrossRef]
- Kim, K.; Kim, S.J. Lu-177-Based Peptide Receptor Radionuclide Therapy for Advanced Neuroendocrine Tumors. Nucl. Med. Mol. Imaging 2018, 52, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Saravana-Bawan, B.; Bajwa, A.; Paterson, J.; McEwan, A.J.B.; McMullen, T.P.W. Efficacy of 177Lu Peptide Receptor Radionuclide Therapy for the Treatment of Neuroendocrine Tumors: A Meta-analysis. Clin. Nucl. Med. 2019, 44, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Al-Toubah, T.; El-Haddad, G.; Strosberg, J. (177) Lu-DOTATATE for the treatment of gastroenteropancreatic neuroendocrine tumors. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Starr, J.S.; Sonbol, M.B.; Hobday, T.J.; Sharma, A.; Kendi, A.T.; Halfdanarson, T.R. Peptide Receptor Radionuclide Therapy for the Treatment of Pancreatic Neuroendocrine Tumors: Recent Insights. Onco. Targets. Ther. 2020, 13, 3545–3555. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of (177)Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef]
- Kwekkeboom, D.J.; de Herder, W.W.; Kam, B.L.; van Eijck, C.H.; Van Essen, M.; Kooij, P.P.; Feelders, R.A.; van Aken, M.O.; Krenning, E.P. Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0, Tyr3] octreotate: Toxicity, efficacy, and survival. J. Clin. Oncol. 2008, 26, 2124–2130. [Google Scholar] [CrossRef]
- Wang, L.F.; Lin, L.; Wang, M.J.; Li, Y. The therapeutic efficacy of 177Lu-DOTATATE/DOTATOC in advanced neuroendocrine tumors: A meta-analysis. Medicine 2020, 99, e19304. [Google Scholar] [CrossRef]
- Satapathy, S.; Mittal, B.R. 177Lu-DOTATATE peptide receptor radionuclide therapy versus Everolimus in advanced pancreatic neuroendocrine tumors: A systematic review and meta-analysis. Nucl. Med. Commun. 2019, 40, 1195–1203. [Google Scholar] [CrossRef]
- Sansovini, M.; Severi, S.; Ianniello, A.; Nicolini, S.; Fantini, L.; Mezzenga, E.; Ferroni, F.; Scarpi, E.; Monti, M.; Bongiovanni, A.; et al. Long-term follow-up and role of FDG PET in advanced pancreatic neuroendocrine patients treated with 177Lu-D OTATATE. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 490–499. [Google Scholar] [CrossRef]
- Paganelli, G.; Sansovini, M.; Nicolini, S.; Grassi, I.; Ibrahim, T.; Amadori, E.; Di Iorio, V.; Monti, M.; Scarpi, E.; Bongiovanni, A.; et al. (177) Lu-PRRT in advanced gastrointestinal neuroendocrine tumors: 10-year follow-up of the IRST phase II prospective study. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhao, Z.; Wang, J.; Lv, W.; Lu, L.; Fu, W.; Li, W. Safety and efficacy of combining capecitabine and temozolomide (CAPTEM) to treat advanced neuroendocrine neoplasms: A meta-analysis. Medicine 2018, 97, e12784. [Google Scholar] [CrossRef] [PubMed]
- Cives, M.; Pelle’, E.; Quaresmini, D.; Mandriani, B.; Tucci, M.; Silvestris, F. The Role of Cytotoxic Chemotherapy in Well-Differentiated Gastroenteropancreatic and Lung Neuroendocrine Tumors. Curr. Treat. Options. Oncol. 2019, 20, 72. [Google Scholar] [CrossRef] [PubMed]
- Coriat, R.; Walter, T.; Terris, B.; Couvelard, A.; Ruszniewski, P. Gastroenteropancreatic Well-Differentiated Grade 3 Neuroendocrine Tumors: Review and Position Statement. Oncologist 2016, 21, 1191–1199. [Google Scholar] [CrossRef]
- Krug, S.; Gress, T.M.; Michl, P.; Rinke, A. The Role of Cytotoxic Chemotherapy in Advanced Pancreatic Neuroendocrine Tumors. Digestion 2017, 96, 67–75. [Google Scholar] [CrossRef]
- Zhai, H.; Li, D.; Feng, Q.; Qian, X.; Li, L.; Yao, J. Pancreatic neuroendocrine tumours: Grade is superior to T, N, or M status in predicting outcome and selecting patients for chemotherapy: A retrospective cohort study in the SEER database. Int. J. Surg. 2019, 66, 103–109. [Google Scholar] [CrossRef]
- Kennedy, A.; Bester, L.; Salem, R.; Sharma, R.A.; Parks, R.W.; Ruszniewski, P. Role of hepatic intra-arterial therapies in metastatic neuroendocrine tumours (NET): Guidelines from the NET-Liver-Metastases Consensus Conference. HPB 2015, 17, 29–37. [Google Scholar] [CrossRef]
- Cazzato, R.L.; Hubele, F.; De Marini, P.; Ouvrard, E.; Salvadori, J.; Addeo, P.; Garnon, J.; Kurtz, J.E.; Greget, M.; Mertz, L.; et al. Liver-Directed Therapy for Neuroendocrine Metastases: From Interventional Radiology to Nuclear Medicine Procedures. Cancers 2021, 13, 6368. [Google Scholar] [CrossRef]
- Clift, A.K.; Frilling, A. Liver-Directed Therapies for Neuroendocrine Neoplasms. Curr. Oncol. Rep. 2021, 23, 44. [Google Scholar] [CrossRef]
- Jia, Z.; Wang, W. Yttrium-90 radioembolization for unresectable metastatic neuroendocrine liver tumor: A systematic review. Eur. J. Radiol. 2018, 100, 23–29. [Google Scholar] [CrossRef]
- Hendifar, A.E.; Dhall, D.; Strosberg, J.R. The Evolving Treatment Algorithm for Advanced Neuroendocrine Neoplasms: Diversity and Commonalities across Tumor Types. Oncologist 2019, 24, 54–61. [Google Scholar] [CrossRef]
- Kanabar, R.; Barriuso, J.; McNamara, M.G.; Mansoor, W.; Hubner, R.A.; Valle, J.W.; Lamarca, A. Liver Embolisation for Patients with Neuroendocrine Neoplasms: Systematic Review. Neuroendocrinology 2021, 111, 354–369. [Google Scholar] [CrossRef] [PubMed]
- Tijeras-Raballand, A.; Neuzillet, C.; Couvelard, A.; Serova, M.; de Gramont, A.; Hammel, P.; Raymond, E.; Faivre, S. Resistance to targeted therapies in pancreatic neuroendocrine tumors (PNETs): Molecular basis, preclinical data, and counteracting strategies. Target Oncol. 2012, 7, 173–181. [Google Scholar] [CrossRef]
- Antonuzzo, L.; Del, R.M.; Barucca, V.; Spada, F.; Meoni, G.; Restante, G.; Danesi, R.; Di Costanzo, F.; Fazio, N. Critical focus on mechanisms of resistance and toxicity of m-TOR inhibitors in pancreatic neuroendocrine tumors. Cancer Treat. Rev. 2017, 57, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Fazio, N.; Cella, C.A.; Del Re, M.; Laffi, A.; Rubino, M.; Zagami, P.; Spada, F. Pharmacodynamics, clinical findings and approval status of current and emerging tyrosine-kinase inhibitors for pancreatic neuroendocrine tumors. Expert Opin. Drug Metab. Toxicol. 2019, 15, 993–1004. [Google Scholar] [CrossRef]
- Zanini, S.; Renzi, S.; Giovinazzo, F.; Bermano, G. mTOR Pathway in Gastroenteropancreatic Neuroendocrine Tumor (GEP-NETs). Front. Endocrinol. Lausanne 2020, 11, 562505. [Google Scholar] [CrossRef]
- Carmona-Bayonas, A.; Jimenez-Fonseca, P.; Custodio, A.; Grande, E.; Capdevila, J.; Lopez, C.; Teule, A.; Garcia-Carbonero, R. Optimizing Somatostatin Analog Use in Well or Moderately Differentiated Gastroenteropancreatic Neuroendocrine Tumors. Curr. Oncol. Rep. 2017, 19, 72. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, H.; Chen, X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist. 2019, 2, 141–160. [Google Scholar] [CrossRef] [PubMed]
- Papaxoinis, G.; Syrigos, K.; Saif, M.W. Novel therapeutic approaches and mechanisms in neuroendocrine tumors: The role of targeted agents. Discov. Med. 2016, 21, 391–402. [Google Scholar]
- Rinke, A.; Muller, H.H.; Schade-Brittinger, C.; Klose, K.J.; Barth, P.; Wied, M.; Mayer, C.; Aminossadati, B.; Pape, U.F.; Blaker, M.; et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: A report from the PROMID Study Group. J. Clin. Oncol. 2009, 27, 4656–4663. [Google Scholar] [CrossRef]
- Strosberg, J.R.; Caplin, M.E.; Kunz, P.L.; Ruszniewski, P.B.; Bodei, L.; Hendifar, A.; Mittra, E.; Wolin, E.M.; Yao, J.C.; Pavel, M.E.; et al. (177) Lu-Dotatate plus long-acting octreotide versus high‒dose long-acting octreotide in patients with midgut neuroendocrine tumours (NETTER-1): Final overall survival and long-term safety results from an open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021, 22, 1752–1763. [Google Scholar] [CrossRef]
- Ducreux, M.; Ruszniewski, P.; Chayvialle, J.A.; Blumberg, J.; Cloarec, D.; Michel, H.; Raymond, J.M.; Dupas, J.L.; Gouerou, H.; Jian, R.; et al. The antitumoral effect of the long-acting somatostatin analog lanreotide in neuroendocrine tumors. Am. J. Gastroenterol. 2000, 95, 3276–3281. [Google Scholar] [CrossRef]
- Arnold, R.; Benning, R.; Neuhaus, C.; Rolwage, M.; Trautmann, M.E. Gastroenteropancreatic endocrine tumors: Effect of Sandostatin on tumor growth. The German Sandostatin Study Group. Metabolism 1992, 41 (Suppl. 2), 116–118. [Google Scholar] [CrossRef]
- Arnold, R.; Trautmann, M.E.; Creutzfeldt, W.; Benning, R.; Benning, M.; Neuhaus, C.; Jurgensen, R.; Stein, K.; Schafer, H.; Bruns, C.; et al. Somatostatin analogue octreotide and inhibition of tumour growth in metastatic endocrine gastroenteropancreatic tumours. Gut 1996, 38, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Kunz, P.L.; Catalano, P.J.; Nimeiri, H.; Fisher, G.A.; Longacre, T.A.; Suarez, C.J.; Kulke, M.H.; Hendifar, A.E.; Shanks, J.C.; Shah, M.H.; et al. A randomized study of temolozomide or temozolomide and capecitabine in patients with advanced pancreatic neuroendocrine tumors: A trial of ECOG-ACRIN Cancer Research Group (E2211). J. Clin. Oncol. 2018, 36 (Suppl. 15), 4004. [Google Scholar] [CrossRef]
- Chan, J.A.; Stuart, K.; Earle, C.C.; Clark, J.W.; Bhargava, P.; Miksad, R.; Blaszkowsky, L.; Enzinger, P.C.; Meyerhardt, J.A.; Zheng, H.; et al. Prospective study of bevacizumab plus temozolomide in patients with advanced neuroendocrine tumors. J. Clin. Oncol. 2012, 30, 2963–2968. [Google Scholar] [CrossRef]
- Mitry, E.; Walter, T.; Baudin, E.; Kurtz, J.E.; Ruszniewski, P.; Dominguez-Tinajero, S.; Begrine-Lefevre, L.; Cadiot, G.; Dromain, C.; Farace, F.; et al. Bevacizumab plus capecitabine in patients with progressive advanced well-differentiated neuroendocrine tumors of the gastro-intestinal (GI-NETs) tract (BETTER trial)—A phase II non-randomised trial. Eur. J. Cancer 2014, 50, 3107–3115. [Google Scholar] [CrossRef]
- Yao, J.C.; Pavel, M.; Lombard-Bohas, C.; Van, C.E.; Voi, M.; Brandt, U.; He, W.; Chen, D.; Capdevila, J.; de Vries, E.G.E.; et al. Everolimus for the Treatment of Advanced Pancreatic Neuroendocrine Tumors: Overall Survival and Circulating Biomarkers from the Randomized, Phase III RADIANT-3 Study. J. Clin. Oncol. 2016, 34, 3906–3913. [Google Scholar] [CrossRef]
- Rogers, S.C.; Garcia, C.A.; Wu, S. Discontinuation of Everolimus Due to Related and Unrelated Adverse Events in Cancer Patients: A Meta-Analysis. Cancer Investig. 2017, 35, 552–561. [Google Scholar] [CrossRef]
- Qi, W.X.; Huang, Y.J.; Yao, Y.; Shen, Z.; Min, D.L. Incidence and risk of treatment-related mortality with mTOR inhibitors everolimus and temsirolimus in cancer patients: A meta-analysis. PLoS ONE 2013, 8, e65166. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Je, Y.; Sonpavde, G.; Richards, C.J.; Galsky, M.D.; Nguyen, P.L.; Schutz, F.; Heng, D.Y.; Kaymakcalan, M.D. Incidence and risk of treatment-related mortality in cancer patients treated with the mammalian target of rapamycin inhibitors. Ann. Oncol. 2013, 24, 2092–2097. [Google Scholar] [CrossRef] [PubMed]
- Panzuto, F.; Rinzivillo, M.; Fazio, N.; de Braud, F.; Luppi, G.; Zatelli, M.C.; Lugli, F.; Tomassetti, P.; Riccardi, F.; Nuzzo, C.; et al. Real-world study of everolimus in advanced progressive neuroendocrine tumors. Oncologist 2014, 19, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Cho, J.H.; Lee, S.H.; Song, S.Y.; Lee, K.H.; Jeong, S.; Ryu, J.K.; Woo, S.M.; Bang, S.; Lee, J.K.; et al. Clinical outcomes of everolimus in patients with advanced, nonfunctioning pancreatic neuroendocrine tumors: A multicenter study in Korea. Cancer Chemother. Pharm. 2017, 80, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Pavel, M.E.; Hainsworth, J.D.; Baudin, E.; Peeters, M.; Horsch, D.; Winkler, R.E.; Klimovsky, J.; Lebwohl, D.; Jehl, V.; Wolin, E.M.; et al. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): A randomised, placebo-controlled, phase 3 study. Lancet 2011, 378, 2005–2012. [Google Scholar] [CrossRef]
- Metzger, J.M.; Gagen, K.; Raustad, K.A.; Yang, L.; White, A.; Wang, S.P.; Craw, S.; Liu, P.; Lanza, T.; Lin, L.S.; et al. Body temperature as a mouse pharmacodynamic response to bombesin receptor subtype-3 agonists and other potential obesity treatments. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E816–E824. [Google Scholar] [CrossRef]
- Yao, J.C.; Fazio, N.; Singh, S.; Buzzoni, R.; Carnaghi, C.; Wolin, E.; Tomasek, J.; Raderer, M.; Lahner, H.; Voi, M.; et al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): A randomised, placebo-controlled, phase 3 study. Lancet 2016, 387, 968–977. [Google Scholar] [CrossRef]
- Kulke, M.H.; Ruszniewski, P.; Van Cutsem, E.; Lombard-Bohas, C.; Valle, J.W.; de Herder, W.W.; Pavel, M.; Degtyarev, E.; Brase, J.C.; Bubuteishvili-Pacaud, L.; et al. A randomized, open-label, phase 2 study of everolimus in combination with pasireotide LAR or everolimus alone in advanced, well-differentiated, progressive pancreatic neuroendocrine tumors: COOPERATE-2 trial. Ann. Oncol. 2017, 28, 1309–1315. [Google Scholar] [CrossRef]
- Lee, L.; Ito, T.; Igarashi, H.; Ueda, K.; Fujiyama, T.; Kawabe, K.; Ogawa, Y. Impact of everolimus on Japanese patients with advanced pancreatic neuroendocrine neoplasms. J. Hepatobiliary Pancreat. Sci. 2017, 24, 95–102. [Google Scholar] [CrossRef]
- Angelousi, A.; Kamp, K.; Kaltsatou, M.; O’Toole, D.; Kaltsas, G.; de Herder, W. Sequential Everolimus and Sunitinib Treatment in Pancreatic Metastatic Well-Differentiated Neuroendocrine Tumours Resistant to Prior Treatments. Neuroendocrinology 2017, 105, 394–402. [Google Scholar] [CrossRef]
- Liu, T.; Liao, J.; Dang, J.; Li, G. Treatments for patients with advanced neuroendocrine tumors: A network meta-analysis. Ther. Adv. Med. Oncol. 2019, 11, 1758835919853673. [Google Scholar] [CrossRef]
- Kaderli, R.M.; Spanjol, M.; Kollar, A.; Butikofer, L.; Gloy, V.; Dumont, R.A.; Seiler, C.A.; Christ, E.R.; Radojewski, P.; Briel, M.; et al. Therapeutic Options for Neuroendocrine Tumors: A Systematic Review and Network Meta-analysis. JAMA Oncol. 2019, 5, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Genc, C.G.; Klumpen, H.J.; van Oijen, M.G.H.; van Eijck, C.H.J.; Nieveen van Dijkum, E.J.M. A Nationwide Population-Based Study on the Survival of Patients with Pancreatic Neuroendocrine Tumors in The Netherlands. World J. Surg. 2018, 42, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Capdevila, J.; Hernando, J.; Perez-Hoyos, S.; Roman-Gonzalez, A.; Grande, E. Meta-Analysis of Randomized Clinical Trials Comparing Active Treatment with Placebo in Metastatic Neuroendocrine Tumors. Oncologist 2019, 24, e1315–e1320. [Google Scholar] [CrossRef]
- Pozzari, M.; Maisonneuve, P.; Spada, F.; Berruti, A.; Amoroso, V.; Cella, C.A.; Laffi, A.; Pellicori, S.; Bertani, E.; Fazio, N. Systemic therapies in patients with advanced well-differentiated pancreatic neuroendocrine tumors (PanNETs): When cytoreduction is the aim. A critical review with meta-analysis. Cancer Treat. Rev. 2018, 71, 39–46. [Google Scholar] [CrossRef]
- Shojamanesh, H.; Gibril, F.; Louie, A.; Ojeaburu, J.V.; Bashir, S.; Abou-Saif, A.; Jensen, R.T. Prospective study of the anti-tumor efficacy of long-term octreotide treatment in patients with progressive metastatic gastrinomas. Cancer 2002, 94, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Olarte, P.; La Salvia, A.; Riesco-Martinez, M.C.; Anton-Pascual, B.; Garcia-Carbonero, R. Chemotherapy in NEN: Still has a role? Rev. Endocr. Metab. Disord. 2021, 22, 595–614. [Google Scholar] [CrossRef]
- Garcia-Carbonero, R.; Rinke, A.; Valle, J.W.; Fazio, N.; Caplin, M.; Gorbounova, V.; Connor, O.; Eriksson, B.; Sorbye, H.; Kulke, M.; et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Neoplasms. Systemic Therapy 2: Chemotherapy. Neuroendocrinology 2017, 105, 281–294. [Google Scholar] [CrossRef]
- Thang, S.P.; Lung, M.S.; Kong, G.; Hofman, M.S.; Callahan, J.; Michael, M.; Hicks, R.J. Peptide receptor radionuclide therapy (PRRT) in European Neuroendocrine Tumour Society (ENETS) grade 3 (G3) neuroendocrine neoplasia (NEN)—A single-institution retrospective analysis. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 262–277. [Google Scholar] [CrossRef]
- Carlsen, E.A.; Fazio, N.; Granberg, D.; Grozinsky-Glasberg, S.; Ahmadzadehfar, H.; Grana, C.M.; Zandee, W.T.; Cwikla, J.; Walter, M.A.; Oturai, P.S.; et al. Peptide receptor radionuclide therapy in gastroenteropancreatic NEN G3: A multicenter cohort study. Endocr. Relat Cancer 2019, 26, 227–239. [Google Scholar] [CrossRef]
- Zhang, J.; Kulkarni, H.R.; Singh, A.; Niepsch, K.; Muller, D.; Baum, R.P. Peptide Receptor Radionuclide Therapy in Grade 3 Neuroendocrine Neoplasms: Safety and Survival Analysis in 69 Patients. J. Nucl. Med. 2019, 60, 377–385. [Google Scholar] [CrossRef]
- Ito, T.; Jensen, R.T. Imaging in multiple endocrine neoplasia type 1: Recent studies show enhanced sensitivities but increased controversies. Int. J. Endocr. Oncol. 2016, 3, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Aalbersberg, E.A.; Huizing, D.M.; Walraven, I.; de Wit-van der Veen, B.; Kulkarni, H.R.; Singh, A.; Stokkel, M.P.; Baum, R.P. Parameters to predict progression free and overall survival after peptide receptor radionuclide therapy: A multivariate analysis in 782 patients. J. Nucl. Med. 2019, 60, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Krudy, A.G.; Doppman, J.L.; Jensen, R.T.; Norton, J.A.; Collen, M.J.; Shawker, T.H.; Gardner, J.D.; McArthur, K.K.; Gorden, P. Localization of islet cell tumors by dynamic CT: Comparison with plain CT, arteriography, sonography and venous sampling. Am. J. Roentgenol. 1984, 143, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Doppman, J.L.; Miller, D.L.; Chang, R.; Maton, P.N.; London, J.F.; Gardner, J.D.; Jensen, R.T.; Norton, J.A. Gastrinomas: Localization by means of selective intraarterial injection of secretin. Radiology 1990, 174, 25–29. [Google Scholar] [CrossRef]
- Frucht, H.; Doppman, J.L.; Norton, J.A.; Miller, D.L.; Dwyer, A.J.; Frank, J.A.; Vinayek, R.; Maton, P.N.; Jensen, R.T. Gastrinomas: Comparison of MR Imaging with CT, angiography and US. Radiology 1989, 171, 713–717. [Google Scholar] [CrossRef]
- Cherner, J.A.; Doppman, J.L.; Norton, J.A.; Miller, D.L.; Krudy, A.G.; Raufman, J.P.; Collen, M.J.; Maton, P.N.; Gardner, J.D.; Jensen, R.T. Selective venous sampling for gastrin to localize gastrinomas. A prospective study. Ann. Intern. Med. 1986, 105, 841–847. [Google Scholar] [CrossRef]
- Norton, J.A.; Alexander, H.R.; Fraker, D.L.; Venzon, D.J.; Jensen, R.T. Does the use of routine duodenotomy (DUODX) affect rate of cure, development of liver metastases or survival in patients with Zollinger-Ellison syndrome (ZES)? Ann. Surg. 2004, 239, 617–626. [Google Scholar] [CrossRef]
- Gibril, F.; Doppman, J.L.; Reynolds, J.C.; Chen, C.C.; Sutliff, V.E.; Yu, F.; Serrano, J.; Venzon, D.J.; Jensen, R.T. Bone metastases in patients with gastrinomas: A prospective study of bone scanning, somatostatin receptor scanning, and MRI in their detection, their frequency, location and effect of their detection on management. J. Clin. Oncol. 1998, 16, 1040–1053. [Google Scholar] [CrossRef]
- Maton, P.N.; Miller, D.L.; Doppman, J.L.; Collen, M.J.; Norton, J.A.; Vinayek, R.; Slaff, J.I.; Wank, S.A.; Gardner, J.D.; Jensen, R.T. Role of selective angiography in the management of Zollinger- Ellison syndrome. Gastroenterology 1987, 92, 913–918. [Google Scholar] [CrossRef]
- Frucht, H.; Norton, J.A.; London, J.F.; Vinayek, R.; Doppman, J.L.; Gardner, J.D.; Jensen, R.T.; Maton, P.N. Detection of duodenal gastrinomas by operative endoscopic transillumination: A prospective study. Gastroenterology 1990, 99, 1622–1627. [Google Scholar] [CrossRef]
- Sundin, A.; Arnold, R.; Baudin, E.; Cwikla, J.B.; Eriksson, B.; Fanti, S.; Fazio, N.; Giammarile, F.; Hicks, R.J.; Kjaer, A.; et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Radiological, Nuclear Medicine & Hybrid Imaging. Neuroendocrinology 2017, 105, 212–244. [Google Scholar] [PubMed]
- Jang, K.M.; Kim, S.H.; Lee, S.J.; Choi, D. The value of gadoxetic acid-enhanced and diffusion-weighted MRI for prediction of grading of pancreatic neuroendocrine tumors. Acta Radiol. 2014, 55, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Rinzivillo, M.; Partelli, S.; Prosperi, D.; Capurso, G.; Pizzichini, P.; Iannicelli, E.; Merola, E.; Muffatti, F.; Scopinaro, F.; Schillaci, O.; et al. Clinical Usefulness of (18)F-Fluorodeoxyglucose Positron Emission Tomography in the Diagnostic Algorithm of Advanced Entero-Pancreatic Neuroendocrine Neoplasms. Oncologist 2018, 23, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Toriihara, A.; Baratto, L.; Nobashi, T.; Park, S.; Hatami, N.; Davidzon, G.; Kunz, P.L.; Iagaru, A. Prognostic value of somatostatin receptor expressing tumor volume calculated from (68) Ga-DOTATATE PET/CT in patients with well-differentiated neuroendocrine tumors. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2244–2251. [Google Scholar] [CrossRef]
- Lee, D.Y.; Kim, Y.I. Prognostic Value of Maximum Standardized Uptake Value in 68Ga-Somatostatin Receptor Positron Emission Tomography for Neuroendocrine Tumors: A Systematic Review and Meta-analysis. Clin. Nucl. Med. 2019, 44, 777–783. [Google Scholar] [CrossRef]
- Modlin, I.M.; Kidd, M.; Malczewska, A.; Drozdov, I.; Bodei, L.; Matar, S.; Chung, K.M. The NETest: The Clinical Utility of Multigene Blood Analysis in the Diagnosis and Management of Neuroendocrine Tumors. Endocrinol. Metab Clin. N. Am. 2018, 47, 485–504. [Google Scholar] [CrossRef]
- Pavel, M.; Jann, H.; Prasad, V.; Drozdov, I.; Modlin, I.M.; Kidd, M. NET Blood Transcript Analysis Defines the Crossing of the Clinical Rubicon: When Stable Disease Becomes Progressive. Neuroendocrinology 2017, 104, 170–182. [Google Scholar] [CrossRef]
- Malczewska, A.; Kidd, M.; Matar, S.; Kos-Kudla, B.; Modlin, I.M. A Comprehensive Assessment of the Role of miRNAs as Biomarkers in Gastroenteropancreatic Neuroendocrine Tumors. Neuroendocrinology 2018, 107, 73–90. [Google Scholar] [CrossRef]
- Modlin, I.M.; Drozdov, I.; Alaimo, D.; Callahan, S.; Teixiera, N.; Bodei, L.; Kidd, M. A multianalyte PCR blood test outperforms single analyte ELISAs (chromogranin A, pancreastatin, neurokinin A) for neuroendocrine tumor detection. Endocr. Relat Cancer 2014, 21, 615–628. [Google Scholar] [CrossRef]
- Liu, E.; Paulson, S.; Gulati, A.; Freudman, J.; Grosh, W.; Kafer, S.; Wickremesinghe, P.C.; Salem, R.R.; Bodei, L. Assessment of NETest Clinical Utility in a U.S. Registry-Based Study. Oncologist 2019, 24, 783–790. [Google Scholar] [CrossRef]
- Bodei, L.; Kidd, M.S.; Singh, A.; van der Zwan, W.A.; Severi, S.; Drozdov, I.A.; Cwikla, J.; Baum, R.P.; Kwekkeboom, D.J.; Paganelli, G.; et al. PRRT genomic signature in blood for prediction of (177) Lu-octreotate efficacy. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1155–1169. [Google Scholar] [CrossRef] [PubMed]
- Bodei, L.; Kidd, M.; Modlin, I.M.; Severi, S.; Drozdov, I.; Nicolini, S.; Kwekkeboom, D.J.; Krenning, E.P.; Baum, R.P.; Paganelli, G. Measurement of circulating transcripts and gene cluster analysis predicts and defines therapeutic efficacy of peptide receptor radionuclide therapy (PRRT) in neuroendocrine tumors. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Bodei, L.; Kidd, M.; Modlin, I.M.; Prasad, V.; Severi, S.; Ambrosini, V.; Kwekkeboom, D.J.; Krenning, E.P.; Baum, R.P.; Paganelli, G.; et al. Gene transcript analysis blood values correlate with (68) Ga-DOTA-somatostatin analog (SSA) PET/CT imaging in neuroendocrine tumors and can define disease status. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1341–1352. [Google Scholar] [CrossRef] [PubMed]
- Genc, C.G.; Jilesen, A.P.J.; Nieveen van Dijkum, E.J.M.; Klumpen, H.J.; van Eijck, C.H.J.; Drozdov, I.; Malczewska, A.; Kidd, M.; Modlin, I. Measurement of circulating transcript levels (NETest) to detect disease recurrence and improve follow-up after curative surgical resection of well-differentiated pancreatic neuroendocrine tumors. J. Surg. Oncol. 2019, 118, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Kidd, M.; Drozdov, I.A.; Matar, S.; Gurunlian, N.; Ferranti, N.J.; Malczewska, A.; Bennett, P.; Bodei, L.; Modlin, I.M. Utility of a ready-to-use PCR system for neuroendocrine tum.mor diagnosis. PLoS ONE 2019, 14, e0218592. [Google Scholar] [CrossRef] [PubMed]
- Van Treijen, M.J.C.; Korse, C.M.; van Leeuwaarde, R.S.; Saveur, L.J.; Vriens, M.R.; Verbeek, W.H.M.; Tesselaar, M.E.T.; Valk, G.D. Blood Transcript Profiling for the Detection of Neuroendocrine Tumors: Results of a Large Independent Validation Study. Front. Endocrinol. Lausanne 2018, 9, 740. [Google Scholar] [CrossRef]
- Di Giacinto, P.; Rota, F.; Rizza, L.; Campana, D.; Isidori, A.; Lania, A.; Lenzi, A.; Zuppi, P.; Baldelli, R. Chromogranin A: From Laboratory to Clinical Aspects of Patients with Neuroendocrine Tumors. Int. J. Endocrinol. 2018, 2018, 8126087. [Google Scholar] [CrossRef]
- Pulvirenti, A.; Rao, D.; Mcintyre, C.A.; Gonen, M.; Tang, L.H.; Klimstra, D.S.; Fleisher, M.; Ramanathan, L.V.; Reidy-Lagunes, D.; Allen, P.J. Limited role of Chromogranin A as clinical biomarker for pancreatic neuroendocrine tumors. HPB 2019, 21, 612–618. [Google Scholar] [CrossRef]
- Raoof, M.; Jutric, Z.; Melstrom, L.G.; Lee, B.; Li, D.; Warner, S.G.; Fong, Y.; Singh, G. Prognostic significance of Chromogranin A in small pancreatic neuroendocrine tumors. Surgery 2019, 165, 760–766. [Google Scholar] [CrossRef]
- Marotta, V.; Zatelli, M.C.; Sciammarella, C.; Ambrosio, M.R.; Bondanelli, M.; Colao, A.; Faggiano, A. Chromogranin A as circulating marker for diagnosis and management of neuroendocrine neoplasms: More flaws than fame. Endocr. Relat. Cancer 2018, 25, R11–R29. [Google Scholar] [CrossRef]
- Corti, A.; Marcucci, F.; Bachetti, T. Circulating chromogranin A and its fragments as diagnostic and prognostic disease markers. Pflug. Arch. 2018, 470, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.E.; Ciafardini, C.; Sciola, V.; Conte, D.; Massironi, S. Chromogranin A in the Follow-up of Gastroenteropancreatic Neuroendocrine Neoplasms: Is It Really Game Over? A Systematic Review and Meta-analysis. Pancreas 2018, 47, 1249–1255. [Google Scholar] [CrossRef] [PubMed]
- Dam, G.; Gronbaek, H.; Sorbye, H.; Thiis, E.E.; Paulsson, B.; Sundin, A.; Jensen, C.; Ebbesen, D.; Knigge, U.; Tiensuu, J.E. Prospective study of chromogranin A as a predictor of progression in patients with pancreatic, small intestinal and unknown primary neuroendocrine tumors. Neuroendocrinology 2020, 110, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.V.; Lopez-Aguiar, A.G.; Rendell, V.R.; Pokrzywa, C.; Rocha, F.G.; Kanji, Z.S.; Poultsides, G.A.; Makris, E.A.; Dillhoff, M.E.; Beal, E.W.; et al. Predictive Value of Chromogranin A and a Pre-Operative Risk Score to Predict Recurrence After Resection of Pancreatic Neuroendocrine Tumors. J. Gastrointest. Surg. 2019, 23, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Hijioka, M.; Ito, T.; Igarashi, H.; Fujimori, N.; Lee, L.; Nakamura, T.; Jensen, R.T.; Takayanagi, R. Serum chromogranin A is a useful marker for Japanese patients with pancreatic neuroendocrine tumors. Cancer Sci. 2014, 105, 1464–1471. [Google Scholar] [CrossRef]
- Hofland, J.; Zandee, W.T.; de Herder, W.W. Role of biomarker tests for diagnosis of neuroendocrine tumours. Nat. Rev. Endocrinol. 2018, 14, 656–669. [Google Scholar] [CrossRef]
- Kidd, M.; Bodei, L.; Modlin, I.M. Chromogranin A: Any relevance in neuroendocrine tumors? Curr. Opin. Endocrinol. Diabetes Obes. 2016, 23, 28–37. [Google Scholar] [CrossRef]
- Malczewska, A.; Kidd, M.; Matar, S.; Kos-Kudla, B.; Bodei, L.; Oberg, K.; Modlin, I.M. An Assessment of Circulating Chromogranin A as a Biomarker of Bronchopulmonary Neuroendocrine Neoplasia: A Systematic Review and Meta-analysis. Neuroendocrinology 2020, 110, 198–216. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, Y.; Long, J.; Yao, X.; Wang, J.; Zang, S.; Qu, W.; Wang, F. Serum chromogranin A for the diagnosis of gastroenteropancreatic neuroendocrine neoplasms and its association with tumour expression. Oncol. Lett. 2019, 17, 1497–1504. [Google Scholar] [CrossRef]
- Sansone, A.; Lauretta, R.; Vottari, S.; Chiefari, A.; Barnabei, A.; Romanelli, F.; Appetecchia, M. Specific and Non-Specific Biomarkers in Neuroendocrine Gastroenteropancreatic Tumors. Cancers 2019, 11, 1113. [Google Scholar] [CrossRef]
- Genus, T.S.E.; Bouvier, C.; Wong, K.F.; Srirajaskanthan, R.; Rous, B.A.; Talbot, D.C.; Valle, J.W.; Khan, M.; Pearce, N.; Elshafie, M.; et al. Impact of neuroendocrine morphology on cancer outcomes and stage at diagnosis: A UK nationwide cohort study 2013–2015. Br. J. Cancer 2019, 12, 966–972. [Google Scholar] [CrossRef]
- Berna, M.J.; Hoffmann, K.M.; Serrano, J.; Gibril, F.; Jensen, R.T. Serum gastrin in Zollinger-Ellison syndrome: I. Prospective study of fasting serum gastrin in 309 patients from the National Institutes of Health and comparison with 2229 cases from the literature. Medicine 2006, 85, 295–330. [Google Scholar] [CrossRef]
- Yu, F.; Venzon, D.J.; Serrano, J.; Goebel, S.U.; Doppman, J.L.; Gibril, F.; Jensen, R.T. Prospective study of the clinical course, prognostic factors and survival in patients with longstanding Zollinger-Ellison syndrome. J. Clin. Oncol. 1999, 17, 615–630. [Google Scholar] [CrossRef]
- Berna, M.J.; Hoffmann, K.M.; Long, S.H.; Serrano, J.; Gibril, F.; Jensen, R.T. Serum gastrin in Zollinger-Ellison syndrome: II. Prospective study of gastrin provocative testing in 293 patients from the National Institutes of Health and comparison with 537 cases from the literature. evaluation of diagnostic criteria, proposal of new criteria, and correlations with clinical and tumoral features. Medicine 2006, 85, 331–364. [Google Scholar] [PubMed]
- Ito, T.; Igarashi, H.; Uehara, H.; Berna, M.J.; Jensen, R.T. Causes of Death and Prognostic Factors in Multiple Endocrine Neoplasia Type 1: A Prospective Study: Comparison of 106 MEN1/Zollinger-Ellison Syndrome Patients with 1613 Literature MEN1 Patients with or without Pancreatic Endocrine Tumors. Medicine 2013, 92, 135–181. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Igarashi, H.; Jensen, R.T. Pancreatic neuroendocrine tumors: Clinical features, diagnosis and medical treatment: Advances. Best Pract. Res. Clin. Gastroenterol. 2012, 26, 737–753. [Google Scholar] [CrossRef] [PubMed]
- Jensen, R.T.; Gardner, J.D. Gastrinoma. In The Pancreas: Biology, Pathobiology and Disease, 2nd ed.; Go, V.L.W., DiMagno, E.P., Gardner, J.D., Lebenthal, E., Reber, H.A., Scheele, G.A., Eds.; Raven Press Publishing Co.: New York, NY, USA, 1993; pp. 931–978. [Google Scholar]
- Ito, T.; Lee, L.; Jensen, R.T. Carcinoid-syndrome: Recent advances, current status and controversies. Curr. Opin. Endocrinol. Diabetes Obes. 2018, 25, 22–35. [Google Scholar] [CrossRef]
- Sutliff, V.E.; Doppman, J.L.; Gibril, F.; Yu, F.; Serrano, J.; Venzon, D.J.; Jensen, R.T. Growth of newly diagnosed, untreated metastatic gastrinomas and predictors of growth patterns. J. Clin. Oncol. 1997, 15, 2420–2431. [Google Scholar] [CrossRef]
- Weber, H.C.; Venzon, D.J.; Lin, J.T.; Fishbein, V.A.; Orbuch, M.; Strader, D.B.; Gibril, F.; Metz, D.C.; Fraker, D.L.; Norton, J.A.; et al. Determinants of metastatic rate and survival in patients with Zollinger-Ellison syndrome: A prospective long-term study. Gastroenterology 1995, 108, 1637–1649. [Google Scholar] [CrossRef]
- Ito, T.; Igarashi, H.; Jensen, R.T. Serum pancreastatin: The long sought universal, sensitive, specific tumor marker for neuroendocrine tumors? Pancreas 2012, 41, 505–507. [Google Scholar] [CrossRef]
- Lee, L.; Ramos-Alvarez, I.; Ito, T.; Jensen, R.T. Insights into Effects/Risks of Chronic Hypergastrinemia and Lifelong PPI Treatment in Man Based on Studies of Patients with Zollinger-Ellison Syndrome. Int. J. Mol. Sci. 2019, 20, 5128. [Google Scholar] [CrossRef]
- Barriuso, J.; Custodio, A.; Afonso, R.; Alonso, V.; Astudillo, A.; Capdevila, J.; Garcia-Carbonero, R.; Grande, E.; Jimenez-Fonseca, P.; Marazuela, M.; et al. Prognostic and predictive biomarkers for somatostatin analogs, peptide receptor radionuclide therapy and serotonin pathway targets in neuroendocrine tumours. Cancer Treat. Rev. 2018, 70, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Kulke, M.H.; Shah, M.H.; Benson, A.B.; Bergsland, E.; Berlin, J.D.; Besh, S.A.; Blaszkowsky, L.S.; Eads, J.; Emerson, L.; Engstrom, P.F.; et al. Neuroendocrine Tumors: Version 3.2017: NCCN Clinical Practice Guidelines in Oncology. NCCN Clin. Pract. Guidel. Oncol. 2017, 1–116. [Google Scholar]
- Strosberg, J.R.; Halfdanarson, T.R.; Bellizzi, A.M.; Chan, J.A.; Dillon, J.S.; Heaney, A.P.; Kunz, P.L.; O’Dorisio, T.M.; Salem, R.; Segelov, E.; et al. The North American Neuroendocrine Tumor Society Consensus Guidelines for Surveillance and Medical Management of Midgut Neuroendocrine Tumors. Pancreas 2017, 46, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Janson, E.T.; Sorbye, H.; Welin, S.; Federspiel, B.; Gronbaek, H.; Hellman, P.; Ladekarl, M.; Langer, S.W.; Mortensen, J.; Schalin-Jantti, C.; et al. Nordic guidelines 2014 for diagnosis and treatment of gastroenteropancreatic neuroendocrine neoplasms. Acta Oncol. 2014, 53, 1284–1297. [Google Scholar] [CrossRef]
- Oberg, K.; Knigge, U.; Kwekkeboom, D.; Perren, A. Neuroendocrine gastro-entero-pancreatic tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2012, 23 (Suppl. 7), vii124–vii130. [Google Scholar] [CrossRef]
- Brighi, N.; Lamberti, G.; Maggio, I.; Manuzzi, L.; Ricci, C.; Casadei, R.; Santini, D.; Mosconi, C.; Lisotti, A.; Ambrosini, V.; et al. Biliary stone disease in patients receiving somatostatin analogs for neuroendocrine neoplasms. A retrospective observational study. Dig. Liver Dis. 2019, 51, 689–694. [Google Scholar] [CrossRef]
- Godara, A.; Siddiqui, N.S.; Byrne, M.M.; Saif, M.W. The safety of lanreotide for neuroendocrine tumor. Expert Opin. Drug Saf. 2019, 18, 1–10. [Google Scholar] [CrossRef]
- Ozaslan, E.; Karaca, H.; Koca, S.; Sevinc, A.; Hacioglu, B.; Ozkan, M.; Ozcelik, M.; Duran, A.O.; Hacibekiroglu, I.; Yildiz, Y.; et al. Comparison of survival with somatostatin analog and chemotherapy and prognostic factors for treatment in 165 advanced neuroendocrine tumor patients with Ki-67 20% or less. Anticancer Drugs 2017, 28, 222–229. [Google Scholar] [CrossRef]
- Carmona-Bayonas, A.; Jimenez-Fonseca, P.; Lamarca, A.; Barriuso, J.; Castano, A.; Benavent, M.; Alonso, V.; Riesco-Martinez, M.D.C.; Alonso-Gordoa, T.; Custodio, A.; et al. Prediction of Progression-Free Survival in Patients with Advanced, Well-Differentiated, Neuroendocrine Tumors Being Treated with a Somatostatin Analog: The GETNE-TRASGU Study. J. Clin. Oncol. 2019, 37, 2571–2580. [Google Scholar] [CrossRef]
- Dromain, C.; Pavel, M.E.; Ruszniewski, P.; Langley, A.; Massien, C.; Baudin, E.; Caplin, M.E. Tumor growth rate as a metric of progression, response, and prognosis in pancreatic and intestinal neuroendocrine tumors. BMC Cancer 2019, 19, 66. [Google Scholar] [CrossRef] [PubMed]
- Ter-Minassian, M.; Zhang, S.; Brooks, N.V.; Brais, L.K.; Chan, J.A.; Christiani, D.C.; Lin, X.; Gabriel, S.; Dinet, J.; Kulke, M.H. Association Between Tumor Progression Endpoints and Overall Survival in Patients with Advanced Neuroendocrine Tumors. Oncologist 2017, 22, 165–172. [Google Scholar] [CrossRef]
- Pavel, M.E.; Phan, A.T.; Wolin, E.M.; Mirakhur, B.; Liyanage, N.; Pitman, L.S.; Fisher, G.A., Jr.; Vinik, A.I. Effect of Lanreotide Depot/Autogel on Urinary 5-Hydroxyindoleacetic Acid and Plasma Chromogranin A Biomarkers in Nonfunctional Metastatic Enteropancreatic Neuroendocrine Tumors. Oncologist 2019, 24, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Buil-Bruna, N.; Dehez, M.; Manon, A.; Nguyen, T.X.; Troconiz, I.F. Establishing the Quantitative Relationship Between Lanreotide Autogel(R), Chromogranin A, and Progression-Free Survival in Patients with Nonfunctioning Gastroenteropancreatic Neuroendocrine Tumors. AAPS J. 2016, 18, 703–712. [Google Scholar] [CrossRef]
- Cwikla, J.B.; Bodei, L.; Kolasinska-Cwikla, A.; Sankowski, A.; Modlin, I.M.; Kidd, M. Circulating Transcript Analysis (NETest) in GEP-NETs Treated with Somatostatin Analogs Defines Therapy. J. Clin. Endocrinol. Metab 2015, 100, E1437–E1445. [Google Scholar] [CrossRef]
- Kang, J.; Yoo, C.; Hwang, H.S.; Hong, S.M.; Kim, K.P.; Kim, S.Y.; Hong, Y.S.; Kim, T.W.; Ryoo, B.Y. Efficacy and safety of lanreotide in Korean patients with metastatic, well-differentiated gastroenteropancreatic-neuroendocrine tumors: A retrospective analysis. Investig. New Drugs 2019, 37, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Lamberti, G.; Faggiano, A.; Brighi, N.; Tafuto, S.; Ibrahim, T.; Brizzi, M.P.; Pusceddu, S.; Albertelli, M.; Massironi, S.; Panzuto, F.; et al. Nonconventional Doses of Somatostatin Analogs in Patients with Progressing Well-Differentiated Neuroendocrine Tumor. J. Clin. Endocrinol. Metab. 2021, 105. in press. [Google Scholar] [CrossRef]
- Van Vliet, E.I.; van Eijck, C.H.; de Krijger, R.R.; Nieveen van Dijkum, E.J.; Teunissen, J.J.; Kam, B.L.; de Herder, W.W.; Feelders, R.A.; Bonsing, B.A.; Brabander, T.; et al. Neoadjuvant Treatment of Nonfunctioning Pancreatic Neuroendocrine Tumors with [177Lu-DOTA0, Tyr3]Octreotate. J. Nucl. Med. 2015, 56, 1647–1653. [Google Scholar] [CrossRef]
- Baum, R.P.; Kulkarni, H.R.; Singh, A.; Kaemmerer, D.; Mueller, D.; Prasad, V.; Hommann, M.; Robiller, F.C.; Niepsch, K.; Franz, H.; et al. Results and adverse events of personalized peptide receptor radionuclide therapy with (90) Yttrium and (177) Lutetium in 1048 patients with neuroendocrine neoplasms. Oncotarget 2018, 9, 16932–16950. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Q.; Singh, A.; Schuchardt, C.; Kulkarni, H.R.; Baum, R.P. Prognostic Value of (18)F-FDG PET/CT in a Large Cohort of 495 Patients with Advanced Metastatic Neuroendocrine Neoplasms (NEN) Treated with Peptide Receptor Radionuclide Therapy (PRRT). J. Nucl. Med. 2020, 61, 1560–1569. [Google Scholar] [CrossRef]
- Fross-Baron, K.; Garske-Roman, U.; Welin, S.; Granberg, D.; Eriksson, B.; Khan, T.; Sandstrom, M.; Sundin, A. 177Lu-DOTATATE Therapy of Advanced Pancreatic Neuroendocrine Tumors Heavily Pretreated with Chemotherapy: Analysis of Outcome, Safety, and Their Determinants. Neuroendocrinology 2021, 111, 330–343. [Google Scholar] [CrossRef]
- Kim, Y.I.; Yoo, C.; Oh, S.J.; Lee, S.J.; Kang, J.; Hwang, H.S.; Hong, S.M.; Ryoo, B.Y.; Ryu, J.S. Tumour-to-liver ratio determined by [(68)Ga]Ga-DOTA-TOC PET/CT as a prognostic factor of lanreotide efficacy for patients with well-differentiated gastroenteropancreatic-neuroendocrine tumours. EJNMMI Res. 2020, 10, 63. [Google Scholar] [CrossRef]
- Ezziddin, S.; Attassi, M.; Yong-Hing, C.J.; Ahmadzadehfar, H.; Willinek, W.; Grunwald, F.; Guhlke, S.; Biersack, H.J.; Sabet, A. Predictors of long-term outcome in patients with well-differentiated gastroenteropancreatic neuroendocrine tumors after peptide receptor radionuclide therapy with 177Lu-octreotate. J. Nucl. Med. 2014, 55, 183–190. [Google Scholar] [CrossRef]
- Brabander, T.; van der Zwan, W.A.; Teunissen, J.J.M.; Kam, B.L.R.; Feelders, R.A.; de Herder, W.W.; van Eijck, C.H.J.; Franssen, G.J.H.; Krenning, E.P.; Kwekkeboom, D.J. Long-Term Efficacy, Survival, and Safety of [177Lu-DOTA0, Tyr3]octreotate in Patients with Gastroenteropancreatic and Bronchial Neuroendocrine Tumors. Clin. Cancer Res. 2017, 23, 4617–4624. [Google Scholar] [CrossRef] [PubMed]
- Kwekkeboom, D.J.; Teunissen, J.J.; Bakker, W.H.; Kooij, P.P.; de Herder, W.W.; Feelders, R.A.; van Eijck, C.H.; Esser, J.P.; Kam, B.L.; Krenning, E.P. Radiolabeled somatostatin analog [177Lu-DOTA0, Tyr3]octreotate in patients with endocrine gastroenteropancreatic tumors. J. Clin. Oncol. 2005, 23, 2754–2762. [Google Scholar] [CrossRef] [PubMed]
- Ohlendorf, F.; Werner, R.A.; Henkenberens, C.; Ross, T.L.; Christiansen, H.; Bengel, F.M.; Derlin, T. Predictive and Prognostic Impact of Blood-Based Inflammatory Biomarkers in Patients with Gastroenteropancreatic Neuroendocrine Tumors Commencing Peptide Receptor Radionuclide Therapy. Diagnostics 2021, 11, 504. [Google Scholar] [CrossRef]
- Swiha, M.M.; Sutherland, D.E.K.; Sistani, G.; Khatami, A.; Abazid, R.M.; Mujoomdar, A.; Wiseman, D.P.; Romsa, J.G.; Reid, R.H.; Laidley, D.T. Survival predictors of (177) Lu-Dotatate peptide receptor radionuclide therapy (PRRT) in patients with progressive well-differentiated neuroendocrine tumors (NETS). J. Cancer Res. Clin. Oncol. 2022, 148, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Rogowski, W.; Wachula, E.; Lewczuk, A.; Kolasinska-Cwikla, A.; Izycka-Swieszewska, E.; Sulzyc-Bielicka, V.; Cwikla, J.B. Baseline chromogranin A and its dynamics are prognostic markers in gastroenteropancreatic neuroendocrine tumors. Future. Oncol. 2017, 13, 1069–1079. [Google Scholar] [CrossRef]
- Sharma, N.; Naraev, B.G.; Engelman, E.G.; Zimmerman, M.B.; Bushnell, D.L., Jr.; O’Dorisio, T.M.; O’Dorisio, M.S.; Menda, Y.; Muller-Brand, J.; Howe, J.R.; et al. Peptide Receptor Radionuclide Therapy Outcomes in a North American Cohort with Metastatic Well-Differentiated Neuroendocrine Tumors. Pancreas 2017, 46, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Garske-Roman, U.; Sandstrom, M.; Fross Baron, K.; Lundin, L.; Hellman, P.; Welin, S.; Johansson, S.; Khan, T.; Lundqvist, H.; Eriksson, B.; et al. Prospective observational study of (177) Lu-DOTA-octreotate therapy in 200 patients with advanced metastasized neuroendocrine tumours (NETs): Feasibility and impact of a dosimetry-guided study protocol on outcome and toxicity. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 970–988. [Google Scholar] [CrossRef]
- Chen, L.; Navalkissoor, S.; Quigley, A.M.; Gnanasegaran, G.; Mandair, D.; Toumpanakis, C.; Caplin, M.E.; Hayes, A.R. (177) Lu-DOTATATE in older patients with metastatic neuroendocrine tumours: Safety, efficacy and health-related quality of life. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3582–3594. [Google Scholar] [CrossRef] [PubMed]
- Black, J.R.M.; Atkinson, S.R.; Singh, A.; Evans, J.; Sharma, R. The Inflammation-Based Index Can Predict Response and Improve Patient Selection in NETs Treated with PRRT: A Pilot Study. J. Clin. Endocrinol. Metab. 2019, 104, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Satapathy, S.; Mittal, B.R.; Sood, A.; Sood, A.; Kapoor, R.; Gupta, R. Peptide Receptor Radionuclide Therapy as First-Line Systemic Treatment in Advanced Inoperable/Metastatic Neuroendocrine Tumors. Clin. Nucl. Med. 2020, 45, e393–e399. [Google Scholar] [CrossRef]
- Ortega, C.; Wong, R.K.; Schaefferkoetter, J.; Veit-Haibach, P.; Myrehaug, S.; Juergens, R.; Laidley, D.; Anconina, R.; Liu, Z.A.; Metser, U. Quantitative (68)Ga-DOTATATE PET/CT parameters for the prediction of therapy response in patients with progressive metastatic neuroendocrine tumors treated with (177)Lu-DOTATATE. J. Nucl. Med. 2021, 62, 1406–1414. [Google Scholar] [CrossRef]
- Sitani, K.; Parghane, R.V.; Talole, S.; Basu, S. Long-term outcome of indigenous (177) Lu-DOTATATE PRRT in patients with Metastatic Advanced Neuroendocrine Tumours: A single institutional observation in a large tertiary care setting. Br. J. Radiol. 2021, 94, 20201041. [Google Scholar] [CrossRef]
- Sharma, R.; Wang, W.M.; Yusuf, S.; Evans, J.; Ramaswami, R.; Wernig, F.; Frilling, A.; Mauri, F.; Al-Nahhas, A.; Aboagye, E.O.; et al. (68) Ga-DOTATATE PET/CT parameters predict response to peptide receptor radionuclide therapy in neuroendocrine tumours. Radiother. Oncol. 2019, 141, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Teker, F.; Elboga, U. Is SUVmax a useful marker for progression-free survival in patients with metastatic GEP-NET receiving (177) Lu-DOTATATE therapy? Hell. J. Nucl. Med. 2021, 24, 122–131. [Google Scholar]
- Nicolini, S.; Severi, S.; Ianniello, A.; Sansovini, M.; Ambrosetti, A.; Bongiovanni, A.; Scarpi, E.; Di Mauro, F.; Rossi, A.; Matteucci, F.; et al. Investigation of receptor radionuclide therapy with (177) Lu-DOTATATE in patients with GEP-NEN and a high Ki-67 proliferation index. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 923–930. [Google Scholar] [CrossRef]
- Binderup, T.; Knigge, U.; Johnbeck, C.B.; Loft, A.; Berthelsen, A.K.; Oturai, P.; Mortensen, J.; Federspiel, B.; Langer, S.W.; Kjaer, A. (18)F-FDG PET is Superior to WHO Grading as a Prognostic Tool in Neuroendocrine Neoplasms and Useful in Guiding PRRT: A Prospective 10-Year Follow-Up Study. J. Nucl. Med. 2021, 62, 808–815. [Google Scholar] [CrossRef]
- Zemczak, A.; Kolodziej, M.; Gut, P.; Krolicki, L.; Kos-Kuda‚, B.; Kaminski, G.; Ruchala, M.; Pawlak, D.; Kunikowska, J. Effect of peptide receptor radionuclide therapy (PRRT) with tandem isotopes-[90Y]Y/[177Lu]Lu-DOTATATE in patients with disseminated neuroendocrine tumours depending on [18F]FDG PET/CT qualification in Polish multicentre experience-do we need [18F]FDG PET/CT for qualification to PRRT? Endokrynol. Pol. 2020, 71, 240–248. [Google Scholar]
- Rodrigues, M.; Winkler, K.K.; Svirydenka, H.; Nilica, B.; Uprimny, C.; Virgolini, I. Long-Term Survival and Value of (18)F-FDG PET/CT in Patients with Gastroenteropancreatic Neuroendocrine Tumors Treated with Second Peptide Receptor Radionuclide Therapy Course with (177) Lu-DOTATATE. Life 2021, 11, 198. [Google Scholar] [CrossRef] [PubMed]
- Graf, J.; Pape, U.F.; Jann, H.; Denecke, T.; Arsenic, R.; Brenner, W.; Pavel, M.; Prasad, V. Prognostic Significance of Somatostatin Receptor Heterogeneity in Progressive Neuroendocrine Tumor Treated with Lu-177 DOTATOC or Lu-177 DOTATATE. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 881–894. [Google Scholar] [CrossRef]
- Werner, R.A.; Ilhan, H.; Lehner, S.; Papp, L.; Zsoter, N.; Schatka, I.; Muegge, D.O.; Javadi, M.S.; Higuchi, T.; Buck, A.K.; et al. Pre-therapy Somatostatin Receptor-Based Heterogeneity Predicts Overall Survival in Pancreatic Neuroendocrine Tumor Patients Undergoing Peptide Receptor Radionuclide Therapy. Mol. Imaging Biol. 2019, 21, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Werner, R.A.; Lapa, C.; Ilhan, H.; Higuchi, T.; Buck, A.K.; Lehner, S.; Bartenstein, P.; Bengel, F.; Schatka, I.; Muegge, D.O.; et al. Survival prediction in patients undergoing radionuclide therapy based on intratumoral somatostatin-receptor heterogeneity. Oncotarget 2017, 8, 7039–7049. [Google Scholar] [CrossRef] [PubMed]
- Ohlendorf, F.; Henkenberens, C.; Brunkhorst, T.; Ross, T.L.; Christiansen, H.; Bengel, F.M.; Derlin, T. Volumetric 68Ga-DOTA-TATE PET/CT for assessment of whole-body tumor burden as a quantitative imaging biomarker in patients with metastatic gastroenteropancreatic neuroendocrine tumors. Q. J. Nucl. Med. Mol. Imaging 2021, 11, 504. [Google Scholar] [CrossRef]
- Pettersson, O.J.; Fross-Baron, K.; Crona, J.; Sundin, A. Tumor growth rate in pancreatic neuroendocrine tumor patients undergoing PRRT with 177Lu-DOTATATE. Endocr. Connect. 2021, 10, 422–431. [Google Scholar] [CrossRef]
- Dromain, C.; Loaiza-Bonilla, A.; Mirakhur, B.; Beveridge, T.J.R.; Fojo, A.T. Novel Tumor Growth Rate Analysis in the Randomized CLARINET Study Establishes the Efficacy of Lanreotide Depot/Autogel 120 mg with Prolonged Administration in Indolent Neuroendocrine Tumors. Oncologist 2021, 26, e632–e638. [Google Scholar] [CrossRef]
- Campana, D.; Capurso, G.; Partelli, S.; Nori, F.; Panzuto, F.; Tamburrino, D.; Cacciari, G.; Delle Fave, G.; Falconi, M.; Tomassetti, P. Radiolabelled somatostatin analogue treatment in gastroenteropancreatic neuroendocrine tumours: Factors associated with response and suggestions for therapeutic sequence. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 1197–1205. [Google Scholar] [CrossRef]
- Del Prete, M.; Buteau, F.A.; Beauregard, J.M. Personalized (177) Lu-octreotate peptide receptor radionuclide therapy of neuroendocrine tumours: A simulation study. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1490–1500. [Google Scholar] [CrossRef]
- Bertani, E.; Fazio, N.; Radice, D.; Zardini, C.; Grana, C.; Bodei, L.; Funicelli, L.; Ferrari, C.; Spada, F.; Partelli, S.; et al. Resection of the Primary Tumor Followed by Peptide Receptor Radionuclide Therapy as Upfront Strategy for the Treatment of G1-G2 Pancreatic Neuroendocrine Tumors with Unresectable Liver Metastases. Ann. Surg. Oncol. 2016, 23, 981–989. [Google Scholar] [CrossRef]
- Demirci, E.; Kabasakal, L.; Toklu, T.; Ocak, M.; Sahin, O.E.; Alan-Selcuk, N.; Araman, A. 177Lu-DOTATATE therapy in patients with neuroendocrine tumours including high-grade (WHO G3) neuroendocrine tumours: Response to treatment and long-term survival update. Nucl. Med. Commun. 2018, 39, 789–796. [Google Scholar] [CrossRef]
- Huizing, D.M.V.; Aalbersberg, E.A.; Versleijen, M.W.J.; Tesselaar, M.E.T.; Walraven, I.; Lahaye, M.J.; de Wit-van der Veen, B.; Stokkel, M.P.M. Early response assessment and prediction of overall survival after peptide receptor radionuclide therapy. Cancer Imaging 2020, 20, 57. [Google Scholar] [CrossRef]
- Sansovini, M.; Severi, S.; Ambrosetti, A.; Monti, M.; Nanni, O.; Sarnelli, A.; Bodei, L.; Garaboldi, L.; Bartolomei, M.; Paganelli, G. Treatment with the radiolabelled somatostatin analog Lu-DOTATATE for advanced pancreatic neuroendocrine tumors. Neuroendocrinology 2013, 97, 347–354. [Google Scholar] [CrossRef]
- Horsch, D.; Ezziddin, S.; Haug, A.; Gratz, K.F.; Dunkelmann, S.; Miederer, M.; Schreckenberger, M.; Krause, B.J.; Bengel, F.M.; Bartenstein, P.; et al. Effectiveness and side-effects of peptide receptor radionuclide therapy for neuroendocrine neoplasms in Germany: A multi-institutional registry study with prospective follow-up. Eur. J. Cancer 2016, 58, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Katona, B.W.; Roccaro, G.A.; Soulen, M.C.; Yang, Y.X.; Bennett, B.J.; Riff, B.P.; Glynn, R.A.; Wild, D.; Nicolas, G.P.; Pryma, D.A.; et al. Efficacy of Peptide Receptor Radionuclide Therapy in a United States-Based Cohort of Metastatic Neuroendocrine Tumor Patients: Single-Institution Retrospective Analysis. Pancreas 2017, 46, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Sistani, G.; Sutherland, D.E.K.; Mujoomdar, A.; Wiseman, D.P.; Khatami, A.; Tsvetkova, E.; Reid, R.H.; Laidley, D.T. Efficacy of (177) Lu-Dotatate Induction and Maintenance Therapy of Various Types of Neuroendocrine Tumors: A Phase II Registry Study. Curr. Oncol. 2020, 28, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Yordanova, A.; Wicharz, M.M.; Mayer, K.; Brossart, P.; Gonzalez-Carmona, M.A.; Strassburg, C.P.; Fimmers, R.; Essler, M.; Ahmadzadehfar, H. The Role of Adding Somatostatin Analogues to Peptide Receptor Radionuclide Therapy as a Combination and Maintenance Therapy. Clin. Cancer Res. 2018, 24, 4672–4679. [Google Scholar] [CrossRef]
- Prinzi, N.; Raimondi, A.; Maccauro, M.; Milione, M.; Garanzini, E.; Torchio, M.; Corti, F.; Nichetti, F.; Lo, R.G.; Giacomelli, L.; et al. Somatostatin analogs in association with peptide receptor radionucleotide therapy in advanced well-differentiated NETs. Future. Oncol. 2019, 15, 3015–3024. [Google Scholar] [CrossRef]
- Kunikowska, J.; Zemczak, A.; Kolodziej, M.; Gut, P.; Lon, I.; Pawlak, D.; Mikolajczak, R.; Kaminski, G.; Ruchala, M.; Kos-Kudla, B.; et al. Tandem peptide receptor radionuclide therapy using (90)Y/(177)Lu-DOTATATE for neuroendocrine tumors efficacy and side-effects-polish multicenter experience. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 922–933. [Google Scholar] [CrossRef]
- Merola, E.; Alonso, G.T.; Zhang, P.; Al-Toubah, T.; Pelle, E.; Kolasinska-Cwikla, A.; Zandee, W.; Laskaratos, F.; de Mestier, L.; Lamarca, A.; et al. Somatostatin Analogs for Pancreatic Neuroendocrine Tumors: Any Benefit When Ki-67 Is >10%? Oncologist 2021, 26, 294–301. [Google Scholar] [CrossRef]
- Faggiano, A.; Malandrino, P.; Modica, R.; Agrimi, D.; Aversano, M.; Bassi, V.; Giordano, E.A.; Guarnotta, V.; Logoluso, F.A.; Messina, E.; et al. Efficacy and Safety of Everolimus in Extrapancreatic Neuroendocrine Tumor: A Comprehensive Review of Literature. Oncologist 2016, 21, 875–886. [Google Scholar] [CrossRef] [PubMed]
- Saglam, S.; Hacisahinogullari, H.; Ozturk, N.; Kapran, Y.; Gulluoglu, M.; Turkmen, C.; Adalet, I.; Orhan Bilge, A.; Cem Balci, N. Outcomes of first-line long-acting octreotide treatment in non-functional, advanced gastroenteropancreatic neuroendocrine tumors. J. BUON 2015, 20, 1201–1205. [Google Scholar]
- Kasajima, A.; Papotti, M.; Ito, W.; Brizzi, M.P.; La Salvia, A.; Rapa, I.; Tachibana, T.; Yazdani, S.; Sasano, H.; Volante, M. High interlaboratory and interobserver agreement of somatostatin receptor immunohistochemical determination and correlation with response to somatostatin analogs. Hum. Pathol. 2018, 72, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, Y.; Kudo, A.; Akashi, T.; Akahoshi, K.; Ogura, T.; Ogawa, K.; Ono, H.; Mitsunori, Y.; Ban, D.; Tanaka, S.; et al. Sunitinib shrinks NET-G3 pancreatic neuroendocrine neoplasms. J. Cancer Res. Clin. Oncol. 2018, 144, 1155–1163. [Google Scholar] [CrossRef]
- Yao, J.C.; Pavel, M.; Phan, A.T.; Kulke, M.H.; Hoosen, S.; St Peter, J.; Cherfi, A.; Oberg, K.E. Chromogranin A and Neuron-Specific Enolase as Prognostic Markers in Patients with Advanced pNET Treated with Everolimus. J. Clin. Endocrinol. Metab. 2011, 96, 3741–3749. [Google Scholar] [CrossRef] [PubMed]
- Viudez, A.; Crespo, G.; Gomez Dorronsoro, M.L.; Arozarena, I.; Marin-Mendez, J.J.; Custodio, A.; Benavent, M.; Goni, S.; Garcia-Paredes, B.; Hernando, J.; et al. Usefulness of an immunohistochemical score in advanced pancreatic neuroendocrine tumors treated with CAPTEM or everolimus. Pancreatology 2021, 21, 215–223. [Google Scholar] [CrossRef]
- Liu, C.T.; Chen, M.H.; Chen, J.S.; Chen, L.T.; Shan, Y.S.; Lu, C.H.; Su, Y.L.; Ku, F.C.; Chou, W.C.; Chen, Y.Y. The efficacy and safety of everolimus for the treatment of progressive gastroenteropancreatic neuroendocrine tumors: A multi-institution observational study in Taiwan. Asia Pac. J. Clin. Oncol. 2016, 12, 396–402. [Google Scholar] [CrossRef]
- Pusceddu, S.; Vernieri, C.; Di Maio, M.; Marconcini, R.; Spada, F.; Massironi, S.; Ibrahim, T.; Brizzi, M.P.; Campana, D.; Faggiano, A.; et al. Metformin Use Is Associated with Longer Progression-Free Survival of Patients with Diabetes and Pancreatic Neuroendocrine Tumors Receiving Everolimus and/or Somatostatin Analogues. Gastroenterology 2018, 155, 479–489. [Google Scholar] [CrossRef]
- Benslama, N.; Bollard, J.; Vercherat, C.; Massoma, P.; Roche, C.; Hervieu, V.; Peron, J.; Lombard-Bohas, C.; Scoazec, J.Y.; Walter, T. Prediction of response to everolimus in neuroendocrine tumors: Evaluation of clinical, biological and histological factors. Investig. New Drugs 2016, 34, 654–662. [Google Scholar] [CrossRef]
- Koumarianou, A.; Pectasides, D.; Koliou, G.A.; Dionysopoulos, D.; Kolomodi, D.; Poulios, C.; Skondra, M.; Sgouros, J.; Pentheroudakis, G.; Kaltsas, G.; et al. Efficacy and Safety of First-Line Everolimus Therapy Alone or in Combination with Octreotide in Gastroenteropancreatic Neuroendocrine Tumors. A Hellenic Cooperative Oncology Group (HeCOG) Study. Biology 2020, 9, 51. [Google Scholar] [CrossRef]
- Chan, D.L.; Yao, J.C.; Carnaghi, C.; Buzzoni, R.; Herbst, F.; Ridolfi, A.; Strosberg, J.; Kulke, M.H.; Pavel, M.; Singh, S. Markers of Systemic Inflammation in Neuroendocrine Tumors: A Pooled Analysis of the RADIANT-3 and RADIANT-4 Studies. Pancreas 2021, 50, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Zurita, A.J.; Khajavi, M.; Wu, H.K.; Tye, L.; Huang, X.; Kulke, M.H.; Lenz, H.J.; Meropol, N.J.; Carley, W.; DePrimo, S.E.; et al. Circulating cytokines and monocyte subpopulations as biomarkers of outcome and biological activity in sunitinib-treated patients with advanced neuroendocrine tumours. Br. J. Cancer 2015, 112, 1199–1205. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Fonseca, P.; Martin, M.N.; Carmona-Bayonas, A.; Calvo, A.; Fernandez-Mateos, J.; Redrado, M.; Capdevila, J.; Lago, N.M.; Lacasta, A.; Munarriz, J.; et al. Biomarkers and polymorphisms in pancreatic neuroendocrine tumors treated with sunitinib. Oncotarget 2018, 9, 36894–36905. [Google Scholar] [CrossRef] [PubMed]
- Vernieri, C.; Pusceddu, S.; Fuca, G.; Indelicato, P.; Centonze, G.; Castagnoli, L.; Ferrari, E.; Ajazi, A.; Pupa, S.; Casola, S.; et al. Impact of systemic and tumor lipid metabolism on everolimus efficacy in advanced pancreatic neuroendocrine tumors (pNETs). Int. J. Cancer 2019, 144, 1704–1712. [Google Scholar] [CrossRef] [PubMed]
- Wetz, C.; Rogasch, J.; Genseke, P.; Schatka, I.; Furth, C.; Kreissl, M.; Jann, H.; Venerito, M.; Amthauer, H. Asphericity of Somatostatin Receptor Expression in Neuroendocrine Tumors: An Innovative Predictor of Outcome in Everolimus Treatment? Diagnostics 2020, 10, 732. [Google Scholar] [CrossRef]
- Lamarca, A.; Barriuso, J.; Kulke, M.; Borbath, I.; Lenz, H.J.; Raoul, J.L.; Meropol, N.J.; Lombard-Bohas, C.; Posey, J.; Faivre, S.; et al. Determination of an optimal response cut-off able to predict progression-free survival in patients with well-differentiated advanced pancreatic neuroendocrine tumours treated with sunitinib: An alternative to the current RECIST-defined response. Br. J. Cancer 2018, 118, 181–188. [Google Scholar] [CrossRef]
- Luo, Y.; Chen, J.; Huang, K.; Lin, Y.; Chen, M.; Xu, L.; Li, Z.P.; Feng, S.T. Early evaluation of sunitinib for the treatment of advanced gastroenteropancreatic neuroendocrine neoplasms via CT imaging: RECIST 1.1 or Choi Criteria? BMC Cancer 2017, 17, 154. [Google Scholar] [CrossRef]
- Solis-Hernandez, M.P.; del Fernandez, A.F.; Carmona-Bayonas, A.; Garcia-Carbonero, R.; Custodio, A.; Benavent, M.; Alonso, G.T.; Nunez-Valdovino, B.; Sanchez, C.M.; Matos, I.; et al. Evaluating radiological response in pancreatic neuroendocrine tumours treated with sunitinib: Comparison of Choi versus RECIST criteria (CRIPNET_ GETNE1504 study). Br. J. Cancer 2019, 121, 537–544. [Google Scholar] [CrossRef]
- Pellat, A.; Dreyer, C.; Couffignal, C.; Walter, T.; Lombard-Bohas, C.; Niccoli, P.; Seitz, J.F.; Hentic, O.; Andre, T.; Coriat, R.; et al. Clinical and Biomarker Evaluations of Sunitinib in Patients with Grade 3 Digestive Neuroendocrine Neoplasms. Neuroendocrinology 2018, 107, 24–31. [Google Scholar] [CrossRef]
- Berardi, R.; Torniai, M.; Pusceddu, S.; Spada, F.; Ibrahim, T.; Brizzi, M.P.; Antonuzzo, L.; Ferolla, P.; Panzuto, F.; Silvestris, N.; et al. Prognostic impact of the cumulative dose and dose intensity of everolimus in patients with pancreatic neuroendocrine tumors. Cancer Med. 2017, 6, 1493–1499. [Google Scholar] [CrossRef]
- Rugo, H.S.; Hortobagyi, G.N.; Yao, J.; Pavel, M.; Ravaud, A.; Franz, D.; Ringeisen, F.; Gallo, J.; Rouyrre, N.; Anak, O.; et al. Meta-analysis of stomatitis in clinical studies of everolimus: Incidence and relationship with efficacy. Ann. Oncol. 2016, 27, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Fazio, N.; Carnaghi, C.; Buzzoni, R.; Valle, J.W.; Herbst, F.; Ridolfi, A.; Strosberg, J.; Kulke, M.H.; Pavel, M.E.; Yao, J.C. Relationship between metabolic toxicity and efficacy of everolimus in patients with neuroendocrine tumors: A pooled analysis from the randomized, phase 3 RADIANT-3 and RADIANT-4 trials. Cancer 2021, 127, 2674–2682. [Google Scholar] [CrossRef] [PubMed]
- Lombard-Bohas, C.; Yao, J.C.; Hobday, T.; Van, C.E.; Wolin, E.M.; Panneerselvam, A.; Stergiopoulos, S.; Shah, M.H.; Capdevila, J.; Pommier, R. Impact of prior chemotherapy use on the efficacy of everolimus in patients with advanced pancreatic neuroendocrine tumors: A subgroup analysis of the phase III RADIANT-3 trial. Pancreas 2015, 44, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Zatelli, M.C.; Fanciulli, G.; Malandrino, P.; Ramundo, V.; Faggiano, A.; Colao, A. Predictive factors of response to mTOR inhibitors in neuroendocrine tumours. Endocr. Relat. Cancer 2016, 23, R173–R183. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.C.; Garg, A.; Chen, D.; Capdevila, J.; Engstrom, P.; Pommier, R.; Van, C.E.; Singh, S.; Fazio, N.; He, W.; et al. Genomic profiling of NETs: A comprehensive analysis of the RADIANT trials. Endocr. Relat. Cancer 2019, 26, 391–403. [Google Scholar] [CrossRef]
- Jensen, R.T.; Berna, M.J.; Bingham, M.D.; Norton, J.A. Inherited pancreatic endocrine tumor syndromes: Advances in molecular pathogenesis, diagnosis, management and controversies. Cancer 2008, 113 (Suppl. 7), 1807–1843. [Google Scholar] [CrossRef]
- Gibril, F.; Schumann, M.; Pace, A.; Jensen, R.T. Multiple endocrine neoplasia type 1 and Zollinger-Ellison syndrome. A prospective study of 107 cases and comparison with 1009 patients from the literature. Medicine 2004, 83, 43–83. [Google Scholar] [CrossRef]
- Benya, R.V.; Metz, D.C.; Venzon, D.J.; Fishbeyn, V.A.; Strader, D.B.; Orbuch, M.; Jensen, R.T. Zollinger-Ellison syndrome can be the initial endocrine manifestation in patients with multiple endocrine neoplasia-type 1. Am. J. Med. 1994, 97, 436–444. [Google Scholar] [CrossRef]
- McColl, K.E. Helicobacter pylori and acid secretion: Where are we now? Eur. J. Gastroenterol. Hepatol. 1997, 9, 333–335. [Google Scholar] [CrossRef]
- Jensen, R.T.; Norton, J.A. Treatment of Pancreatic Neuroendocrine Tumors in Multiple Endocrine Neoplasia Type 1: Some Clarity but Continued Controversy. Pancreas 2017, 46, 589–594. [Google Scholar] [CrossRef]
- Norton, J.A.; Krampitz, G.; Jensen, R.T. Multiple Endocrine Neoplasia: Genetics and Clinical Management. Surg. Oncol. Clin. N. Am. 2015, 24, 795–832. [Google Scholar] [CrossRef] [PubMed]
- MacFarlane, M.P.; Fraker, D.L.; Alexander, H.R.; Norton, J.A.; Jensen, R.T. A prospective study of surgical resection of duodenal and pancreatic gastrinomas in multiple endocrine neoplasia-Type 1. Surgery 1995, 118, 973–980. [Google Scholar] [CrossRef]
- Nunez, J.E.; Donadio, M.; Filho, D.R.; Rego, J.F.; Barros, M.; Formiga, M.N.; Lopez, R.; Riechelmann, R. The efficacy of everolimus and sunitinib in patients with sporadic or germline mutated metastatic pancreatic neuroendocrine tumors. J. Gastrointest. Oncol. 2019, 10, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Martins, D.; Spada, F.; Lambrescu, I.; Rubino, M.; Cella, C.; Gibelli, B.; Grana, C.; Ribero, D.; Bertani, E.; Ravizza, D.; et al. Predictive Markers of Response to Everolimus and Sunitinib in Neuroendocrine Tumors. Target. Oncol. 2017, 12, 611–622. [Google Scholar] [CrossRef]
- Faivre, S.; Niccoli, P.; Castellano, D.; Valle, J.W.; Hammel, P.; Raoul, J.L.; Vinik, A.; Van Cutsem, E.; Bang, Y.J.; Lee, S.H.; et al. Sunitinib in Pancreatic Neuroendocrine Tumors: Updated Progression-Free Survival and Final Overall Survival from a Phase III Randomized Study. Ann. Oncol. 2017, 28, 339–343. [Google Scholar] [CrossRef]
- Lee, L.; Ito, T.; Igarashi, H.; Miki, M.; Fujimori, N.; Kawabe, K.; Jensen, R.T.; Ogawa, Y. Dose and schedule modification are required for long-term continuation of sunitinib in Japanese patients with advanced pancreatic neuroendocrine tumors. Cancer Chemother. Pharm. 2018, 81, 163–169. [Google Scholar] [CrossRef]
- Matsui, S.; Kudo, A.; Ogura, T.; Ogawa, K.; Ono, H.; Mitsunori, Y.; Ban, D.; Tanaka, S.; Tanabe, M. Does sunitinib have a patient-specific dose without diminishing its antitumor effect on advanced pancreatic neuroendocrine neoplasms? J. Cancer Res. Clin. Oncol. 2019, 145, 2097–2104. [Google Scholar] [CrossRef]
- Fazio, N.; Martini, J.F.; Croitoru, A.E.; Schenker, M.; Li, S.; Rosbrook, B.; Fernandez, K.; Tomasek, J.; Thiis-Evensen, E.; Kulke, M.; et al. Pharmacogenomic analyses of sunitinib in patients with pancreatic neuroendocrine tumors. Future. Oncol. 2019, 2019, 1997–2007. [Google Scholar] [CrossRef]
- Mateo, J.; Heymach, J.V.; Zurita, A.J. Circulating biomarkers of response to sunitinib in gastroenteropancreatic neuroendocrine tumors: Current data and clinical outlook. Mol. Diagn. Ther. 2012, 16, 151–161. [Google Scholar] [CrossRef]
- Lin, E.; Chen, T.; Little, A.; Holliday, L.; Roach, P.; Butler, P.; Hosking, E.; Bailey, E.; Elison, B.; Currow, D. Safety and outcomes of (177) Lu-DOTATATE for neuroendocrine tumours: Experience in New South Wales, Australia. Intern. Med. J. 2019, 49, 1268–1277. [Google Scholar] [CrossRef]
- Kesavan, M.; Claringbold, P.G.; Turner, J.H. Hematological toxicity of combined 177Lu-octreotate radiopeptide chemotherapy of gastroenteropancreatic neuroendocrine tumors in long-term follow-up. Neuroendocrinology 2014, 99, 108–117. [Google Scholar] [CrossRef]
- Kesavan, M.; Turner, J.H. Myelotoxicity of Peptide Receptor Radionuclide Therapy of Neuroendocrine Tumors: A Decade of Experience. Cancer Biother. Radiopharm. 2016, 31, 189–198. [Google Scholar] [CrossRef]
- Cives, M.; Strosberg, J. Radionuclide Therapy for Neuroendocrine Tumors. Curr Oncol. Rep. 2017, 19, 9. [Google Scholar] [CrossRef]
- Goncalves, I.; Burbury, K.; Michael, M.; Iravani, A.; Ravi Kumar, A.S.; Akhurst, T.; Tiong, I.S.; Blombery, P.; Hofman, M.S.; Westerman, D.; et al. Characteristics and outcomes of therapy-related myeloid neoplasms after peptide receptor radionuclide/chemoradionuclide therapy (PRRT/PRCRT) for metastatic neuroendocrine neoplasia: A single-institution series. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1902–1910. [Google Scholar] [CrossRef]
- Valkema, R.; Pauwels, S.A.; Kvols, L.K.; Kwekkeboom, D.J.; Jamar, F.; de, J.M.; Barone, R.; Walrand, S.; Kooij, P.P.; Bakker, W.H.; et al. Long-term follow-up of renal function after peptide receptor radiation therapy with (90) Y-DOTA (0), Tyr (3)-octreotide and (177) Lu-DOTA (0), Tyr (3)-octreotate. J. Nucl. Med. 2005, 46 (Suppl. 1), 83S–91S. [Google Scholar]
- Ramage, J.; Naraev, B.G.; Halfdanarson, T.R. Peptide receptor radionuclide therapy for patients with advanced pancreatic neuroendocrine tumors. Semin. Oncol. 2018, 45, 236–248. [Google Scholar] [CrossRef]
- Al-Toubah, T.; Strosberg, J. Peptide Receptor Radiotherapy Comes of Age. Endocrinol. Metab. Clin. N. Am. 2018, 47, 615–625. [Google Scholar] [CrossRef]
- Ito, T.; Igarashi, H.; Uehara, H.; Jensen, R.T. Pharmacotherapy of Zollinger-Ellison syndrome. Expert Opin. Pharmacother. 2013, 14, 307–321. [Google Scholar] [CrossRef]
- Roy, P.K.; Venzon, D.J.; Feigenbaum, K.M.; Koviack, P.D.; Bashir, S.; Ojeaburu, J.V.; Gibril, F.; Jensen, R.T. Gastric secretion in Zollinger-Ellison syndrome: Correlation with clinical expression, tumor extent and role in diagnosis—A prospective NIH study of 235 patients and review of the literature in 984 cases. Medicine 2001, 80, 189–222. [Google Scholar] [CrossRef]
- Collen, M.J.; Howard, J.M.; McArthur, K.E.; Raufman, J.P.; Cornelius, M.J.; Ciarleglio, C.A.; Gardner, J.D.; Jensen, R.T. Comparison of ranitidine and cimetidine in the treatment of gastric hypersecretion. Ann. Intern. Med. 1984, 100, 52–58. [Google Scholar] [CrossRef]
- Ito, T.; Jensen, R.T. Perspectives on the Current Pharmacotherapeutic Strategies for Management of Functional Neuroendocrine Tumor Syndromes. Expert Opin. Pharmacother. 2021, 22, 685–693. [Google Scholar] [CrossRef]
- Raufman, J.P.; Collins, S.M.; Pandol, S.J.; Korman, L.Y.; Collen, M.J.; Cornelius, M.J.; Feld, M.K.; McCarthy, D.M.; Gardner, J.D.; Jensen, R.T. Reliability of symptoms in assessing control of gastric acid secretion in patients with Zollinger-Ellison syndrome. Gastroenterology 1983, 84, 108–113. [Google Scholar] [CrossRef]
- Maton, P.N.; Vinayek, R.; Frucht, H.; McArthur, K.A.; Miller, L.S.; Saeed, Z.A.; Gardner, J.D.; Jensen, R.T. Long-term efficacy and safety of omeprazole in patients with Zollinger-Ellison syndrome: A prospective study. Gastroenterology 1989, 97, 827–836. [Google Scholar] [CrossRef]
- Kratochwil, C.; Stefanova, M.; Mavriopoulou, E.; Holland-Letz, T.; Dimitrakopoulou-Strauss, A.; Afshar-Oromieh, A.; Mier, W.; Haberkorn, U.; Giesel, F.L. SUV of [68Ga] DOTATOC-PET/CT Predicts Response Probability of PRRT in Neuroendocrine Tumors. Mol. Imaging Biol. 2015, 17, 313–318. [Google Scholar] [CrossRef]
- Evangelista, L.; Ravelli, I.; Bignotto, A.; Cecchin, D.; Zucchetta, P. Ga-68 DOTA-peptides and F-18 FDG PET/CT in patients with neuroendocrine tumor: A review. Clin. Imaging 2020, 67, 113–116. [Google Scholar] [CrossRef]
- Hope, T.A.; Calais, J.; Zhang, L.; Dieckmann, W.; Millo, C. (111) In-pentetreotide scintigraphy vs. (68)Ga-DOTATATE PET: Impact on Krenning Scores and Effect of Tumor Burden. J. Nucl. Med. 2019, 60, 1266–1269. [Google Scholar] [CrossRef]
- Alsadik, S.; Yusuf, S.; Al-Nahhas, A. Peptide Receptor Radionuclide Therapy for Pancreatic Neuroendocrine Tumours. Curr Radiopharm. 2019, 12, 126–134. [Google Scholar] [CrossRef]
- Ilan, E.; Sandstrom, M.; Wassberg, C.; Sundin, A.; Garske-Roman, U.; Eriksson, B.; Granberg, D.; Lubberink, M. Dose response of pancreatic neuroendocrine tumors treated with peptide receptor radionuclide therapy using 177Lu-DOTATATE. J. Nucl. Med. 2015, 56, 177–182. [Google Scholar] [CrossRef]
- Van Essen, M.; Sundin, A.; Krenning, E.P.; Kwekkeboom, D.J. Neuroendocrine tumours: The role of imaging for diagnosis and therapy. Nat. Rev. Endocrinol. 2014, 10, 102–114. [Google Scholar] [CrossRef]
- Yordanova, A.; Ahrens, H.; Feldmann, G.; Brossart, P.; Gaertner, F.C.; Fottner, C.; Weber, M.M.; Ahmadzadehfar, H.; Schreckenberger, M.; Miederer, M.; et al. Peptide Receptor Radionuclide Therapy Combined with Chemotherapy in Patients with Neuroendocrine Tumors. Clin. Nucl. Med. 2019, 44, e329–e335. [Google Scholar] [CrossRef]
- Sonbol, M.B.; Halfdanarson, T.R. Management of Well-Differentiated High-Grade (G3) Neuroendocrine Tumors. Curr Treat. Options. Oncol. 2019, 20, 74. [Google Scholar] [CrossRef]
- Nakayama, Y.; Wada, R.; Yajima, N.; Hakamada, K.; Yagihashi, S. Profiling of somatostatin receptor subtype expression by quantitative PCR and correlation with clinicopathological features in pancreatic endocrine tumors. Pancreas 2010, 39, 1147–1154. [Google Scholar] [CrossRef]
- Mizutani, G.; Nakanishi, Y.; Watanabe, N.; Honma, T.; Obana, Y.; Seki, T.; Ohni, S.; Nemoto, N. Expression of Somatostatin Receptor (SSTR) Subtypes (SSTR-1, 2A, 3, 4 and 5) in Neuroendocrine Tumors Using Real-time RT-PCR Method and Immunohistochemistry. Acta Histochem. Cytochem. 2012, 45, 167–176. [Google Scholar] [CrossRef]
- Gabriel, M.; Nilica, B.; Kaiser, B.; Virgolini, I.J. Twelve-Year Follow-up after Peptide Receptor Radionuclide Therapy. J. Nucl. Med. 2019, 60, 524–529. [Google Scholar] [CrossRef]
- Zamora, V.; Cabanne, A.; Salanova, R.; Bestani, C.; Domenichini, E.; Marmissolle, F.; Giacomi, N.; O’Connor, J.; Mendez, G.; Roca, E. Immunohistochemical expression of somatostatin receptors in digestive endocrine tumours. Dig. Liver Dis. 2010, 42, 220–225. [Google Scholar] [CrossRef]
- Diakatou, E.; Alexandraki, K.I.; Tsolakis, A.V.; Kontogeorgos, G.; Chatzellis, E.; Leonti, A.; Kaltsas, G.A. Somatostatin and dopamine receptor expression in neuroendocrine neoplasms: Correlation of immunohistochemical findings with somatostatin receptor scintigraphy visual scores. Clin. Endocrinol. 2015, 83, 420–428. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Jin, K.; Fang, C.; Lin, Y.; Xue, L.; Feng, S.; Zhou, Z.; Shao, C.; Chen, M.; et al. Somatostatin receptor expression indicates improved prognosis in gastroenteropancreatic neuroendocrine neoplasm, and octreotide long-acting release is effective and safe in Chinese patients with advanced gastroenteropancreatic neuroendocrine tumors. Oncol. Lett. 2017, 13, 1165–1174. [Google Scholar] [CrossRef]
- Kaemmerer, D.; Trager, T.; Hoffmeister, M.; Sipos, B.; Hommann, M.; Sanger, J.; Schulz, S.; Lupp, A. Inverse expression of somatostatin and CXCR4 chemokine receptors in gastroenteropancreatic neuroendocrine neoplasms of different malignancy. Oncotarget 2015, 6, 27566–27579. [Google Scholar] [CrossRef]
- Waseem, N.; Aparici, C.M.; Kunz, P.L. Evaluating the Role of Theranostics in Grade 3 Neuroendocrine Neoplasms. J. Nucl. Med. 2019, 60, 882–891. [Google Scholar] [CrossRef]
- Konukiewitz, B.; Schlitter, A.M.; Jesinghaus, M.; Pfister, D.; Steiger, K.; Segler, A.; Agaimy, A.; Sipos, B.; Zamboni, G.; Weichert, W.; et al. Somatostatin receptor expression related to TP53 and RB1 alterations in pancreatic and extrapancreatic neuroendocrine neoplasms with a Ki67-index above 20. Mod. Pathol. 2017, 30, 587–598. [Google Scholar] [CrossRef]
- WHO Classification of Tumours of Endocrine Organs, 4th ed.; Agency for Research on Cancer: Lyon, France, 2017; Volume 10, pp. 1–355.
- Hijioka, S.; Hosoda, W.; Matsuo, K.; Ueno, M.; Furukawa, M.; Yoshitomi, H.; Kobayashi, N.; Ikeda, M.; Ito, T.; Nakamori, S.; et al. Rb Loss and KRAS Mutation Are Predictors of the Response to Platinum-Based Chemotherapy in Pancreatic Neuroendocrine Neoplasm with Grade 3: A Japanese Multicenter Pancreatic NEN-G3 Study. Clin. Cancer Res. 2017, 23, 4625–4632. [Google Scholar] [CrossRef]
- Tang, L.H.; Basturk, O.; Sue, J.J.; Klimstra, D.S. A Practical Approach to the Classification of WHO Grade 3 (G3) Well-differentiated Neuroendocrine Tumor (WD-NET) and Poorly Differentiated Neuroendocrine Carcinoma (PD-NEC) of the Pancreas. Am. J. Surg. Pathol. 2016, 40, 1192–1202. [Google Scholar] [CrossRef]
- Jiao, Y.; Shi, C.; Edil, B.H.; de Wilde, R.F.; Klimstra, D.S.; Maitra, A.; Schulick, R.D.; Tang, L.H.; Wolfgang, C.L.; Choti, M.A.; et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science 2011, 331, 1199–1203. [Google Scholar] [CrossRef]
- Sorbye, H.; Welin, S.; Langer, S.W.; Vestermark, L.W.; Holt, N.; Osterlund, P.; Dueland, S.; Hofsli, E.; Guren, M.G.; Ohrling, K.; et al. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): The NORDIC NEC study. Ann. Oncol. 2013, 20, 152–160. [Google Scholar] [CrossRef]
- Heetfeld, M.; Chougnet, C.N.; Olsen, I.H.; Rinke, A.; Borbath, I.; Crespo, G.; Barriuso, J.; Pavel, M.; O’Toole, D.; Walter, T. Characteristics and treatment of patients with G3 gastroenteropancreatic neuroendocrine neoplasms. Endocr. Relat Cancer 2015, 22, 657–664. [Google Scholar] [CrossRef]
- Raj, N.; Klimstra, D.S.; Horvat, N.; Zhang, L.; Chou, J.F.; Capanu, M.; Basturk, O.; Do, R.K.G.; Allen, P.J.; Reidy-Lagunes, D. O6-Methylguanine DNA Methyltransferase Status Does Not Predict Response or Resistance to Alkylating Agents in Well-Differentiated Pancreatic Neuroendocrine Tumors. Pancreas 2017, 46, 758–763. [Google Scholar] [CrossRef]
- Rinke, A.; Gress, T.M. Neuroendocrine Cancer, Therapeutic Strategies in G3 Cancers. Digestion 2017, 95, 109–114. [Google Scholar] [CrossRef]
- De Mestier, L.; Walter, T.; Evrard, C.; de Boissieu, P.; Hentic, O.; Cros, J.; Tougeron, D.; Lombard-Bohas, C.; Rebours, V.; Hammel, P.; et al. Temozolomide alone or combined to capecitabine for the treatment of advanced pancreatic NET. Neuroendocrinology 2019, 110, 83–91. [Google Scholar] [CrossRef]
- De Mestier, L.; Walter, T.; Brixi, H.; Evrard, C.; Legoux, J.L.; de Boissieu, P.; Hentic, O.; Cros, J.; Hammel, P.; Tougeron, D.; et al. Comparison of Temozolomide-Capecitabine to 5-Fluorouracile-Dacarbazine in 247 Patients with Advanced Digestive Neuroendocrine Tumors Using Propensity Score Analyses. Neuroendocrinology 2019, 108, 343–353. [Google Scholar] [CrossRef]
- Childs, A.; Kirkwood, A.; Edeline, J.; Luong, T.V.; Watkins, J.; Lamarca, A.; Alrifai, D.; Nsiah-Sarbeng, P.; Gillmore, R.; Mayer, A.; et al. Ki-67 index and response to chemotherapy in patients with neuroendocrine tumours. Endocr. Relat Cancer 2016, 23, 563–570. [Google Scholar] [CrossRef]
- Krug, S.; Boch, M.; Nimphius, W.; Gress, T.M.; Michl, P.; Rinke, A. Relevance of dihydropyrimidine-dehydrogenase and thymidylate-synthase in patients with pancreatic neuroendocrine neoplasms treated with 5-FU-based chemotherapy. Pancreatology 2017, 17, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Krug, S.; Boch, M.; Daniel, H.; Nimphius, W.; Muller, D.; Michl, P.; Rinke, A.; Gress, T.M. Streptozocin-Based Chemotherapy in Patients with Advanced Neuroendocrine Neoplasms—Predictive and Prognostic Markers for Treatment Stratification. PLoS ONE 2015, 10, e0143822. [Google Scholar] [CrossRef]
- Spada, F.; Maisonneuve, P.; Fumagalli, C.; Marconcini, R.; Gelsomino, F.; Antonuzzo, L.; Campana, D.; Puliafito, I.; Rossi, G.; Faviana, P.; et al. Temozolomide alone or in combination with capecitabine in patients with advanced neuroendocrine neoplasms: An Italian multicenter real-world analysis. Endocrine 2021, 72, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.S.; Gronberg, M.; Federspiel, B.; Scoazec, J.Y.; Hjortland, G.O.; Gronbaek, H.; Ladekarl, M.; Langer, S.W.; Welin, S.; Vestermark, L.W.; et al. Expression of p53 protein in high-grade gastroenteropancreatic neuroendocrine carcinoma. PLoS ONE 2017, 12, e0187667. [Google Scholar] [CrossRef] [PubMed]
- Roquin, G.; Baudin, E.; Lombard-Bohas, C.; Cadiot, G.; Dominguez, S.; Guimbaud, R.; Niccoli, P.; Legoux, J.L.; Mitry, E.; Rohmer, V.; et al. Chemotherapy for Well-Differentiated Pancreatic Neuroendocrine Tumours with a Ki-67 Index >/=10%: Is There a More Effective Antitumour Regimen? A Retrospective Multicentre Study of the French Group of Endocrine Tumours (GTE). Neuroendocrinology 2018, 106, 38–46. [Google Scholar] [CrossRef]
- Chatzellis, E.; Angelousi, A.; Daskalakis, K.; Tsoli, M.; Alexandraki, K.I.; Wachula, E.; Meirovitz, A.; Maimon, O.; Grozinsky-Glasberg, S.; Gross, D.; et al. Activity and Safety of Standard and Prolonged Capecitabine/Temozolomide Administration in Patients with Advanced Neuroendocrine Neoplasms. Neuroendocrinology 2019, 109, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.E.; Lam, M.; Halperin, D.M.; Dagohoy, C.G.; Yao, J.C.; Dasari, A. FAS and Subsequent Therapies in Pancreatic Neuroendocrine Tumors. Neuroendocrinology 2022, 112, 34–42. [Google Scholar] [CrossRef]
- Clewemar Antonodimitrakis, P.; Sundin, A.; Wassberg, C.; Granberg, D.; Skogseid, B.; Eriksson, B. Streptozocin and 5-Fluorouracil for the Treatment of Pancreatic Neuroendocrine Tumors: Efficacy, Prognostic Factors and Toxicity. Neuroendocrinology 2016, 103, 345–353. [Google Scholar] [CrossRef]
- Schrader, J.; Henes, F.O.; Blaeker, M.; Zimmermann-Fraedrich, K.; Pace, A.; Perez, D.; Izbicki, J.R.; Lohse, A.W.; Benten, D. Extended cycle streptozotocin/5-FU chemotherapy for maintenance therapy in pancreatic neuroendocrine tumors. Endocrine 2019, 65, 460–467. [Google Scholar] [CrossRef]
- Mueller, D.; Krug, S.; Majumder, M.; Rinke, A.; Gress, T.M. Low dose DTIC is effective and safe in pretreated patients with well differentiated neuroendocrine tumors. BMC Cancer 2016, 16, 645. [Google Scholar] [CrossRef][Green Version]
- Kim, M.; Lee, S.; Lee, J.; Park, S.H.; Park, J.O.; Park, Y.S.; Kang, W.K.; Kim, S.T. The Role of Plasma Chromogranin A as Assessment of Treatment Response in Non-functioning Gastroenteropancreatic Neuroendocrine Tumors. Cancer Res. Treat. 2016, 48, 153–161. [Google Scholar] [CrossRef]
- Salman, T.; Kazaz, S.N.; Varol, U.; Oflazoglu, U.; Unek, I.T.; Kucukzeybek, Y.; Alacacioglu, A.; Atag, E.; Semiz, H.S.; Cengiz, H.; et al. Prognostic Value of the Pretreatment Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio for Patients with Neuroendocrine Tumors: An Izmir Oncology Group Study. Chemotherapy 2016, 61, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Merola, E.; Dal, B.A.; Denecke, T.; Arsenic, R.; Pape, U.F.; Jann, H.; Wiedenmann, B.; Pavel, M.E. Efficacy and Toxicity of 5-Fluorouracil-Oxaliplatin in Gastroenteropancreatic Neuroendocrine Neoplasms. Pancreas 2020, 49, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Campana, D.; Walter, T.; Pusceddu, S.; Gelsomino, F.; Graillot, E.; Prinzi, N.; Spallanzani, A.; Fiorentino, M.; Barritault, M.; Dall’Olio, F.; et al. Correlation between MGMT promoter methylation and response to temozolomide-based therapy in neuroendocrine neoplasms: An observational retrospective multicenter study. Endocrine 2018, 60, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Bongiovanni, A.; Liverani, C.; Foca, F.; Fausti, V.; Di Menna, G.; Mercatali, L.; De Vita, A.; Riva, N.; Calpona, S.; Miserocchi, G.; et al. Temozolomide Alone or Combined with Capecitabine for the Treatment of Metastatic Neuroendocrine Neoplasia: A “Real World” data analysis. Neuroendocrinology 2021, 111, 895–906. [Google Scholar] [CrossRef]
- Spada, F.; Antonuzzo, L.; Marconcini, R.; Radice, D.; Antonuzzo, A.; Ricci, S.; Di Costanzo, F.; Fontana, A.; Gelsomino, F.; Luppi, G.; et al. Oxaliplatin-Based Chemotherapy in Advanced Neuroendocrine Tumors: Clinical Outcomes and Preliminary Correlation with Biological Factors. Neuroendocrinology 2016, 103, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Dilz, L.M.; Denecke, T.; Steffen, I.G.; Prasad, V.; von Weikersthal, L.F.; Pape, U.F.; Wiedenmann, B.; Pavel, M. Streptozocin/5-fluorouracil chemotherapy is associated with durable response in patients with advanced pancreatic neuroendocrine tumours. Eur. J. Cancer 2015, 51, 1253–1262. [Google Scholar] [CrossRef]
- Ostwal, V.; Basu, S.; Bhargava, P.; Shah, M.; Parghane, R.V.; Srinivas, S.; Chaudhari, V.; Bhandare, M.S.; Shrikhande, S.V.; Ramaswamy, A. Capecitabine-Temozolomide (CAPTEM) in advanced Grade 2 and grade 3 Neuroendocrine neoplasms (NENs)-benefits of chemotherapy in NENs with significant 18FDG uptake. Neuroendocrinology 2021, 111, 998–1004. [Google Scholar] [CrossRef]
- Thomas, K.; Voros, B.A.; Meadows-Taylor, M.; Smeltzer, M.P.; Griffin, R.; Boudreaux, J.P.; Thiagarajan, R.; Woltering, E.A.; Ramirez, R.A. Outcomes of Capecitabine and Temozolomide (CAPTEM) in Advanced Neuroendocrine Neoplasms (NENs). Cancers 2020, 12, 206. [Google Scholar] [CrossRef]
- Jeong, H.; Shin, J.; Jeong, J.H.; Kim, K.P.; Hong, S.M.; Kim, Y.I.; Ryu, J.S.; Ryoo, B.Y.; Yoo, C. Capecitabine plus temozolomide in patients with grade 3 unresectable or metastatic gastroenteropancreatic neuroendocrine neoplasms with Ki-67 index >55%: Single-arm phase II study. ESMO Open 2021, 6, 100119. [Google Scholar] [CrossRef]
- Hayes, A.R.; Furnace, M.; Shah, R.; Rundell, C.; Muller, G.; Dehbi, H.M.; Luong, T.V.; Toumpanakis, C.; Caplin, M.E.; Krell, D.; et al. High-Grade Gastroenteropancreatic Neuroendocrine Neoplasms and Improved Prognostic Stratification with the New World Health Organization 2019 Classification: A Validation Study from a Single-Institution Retrospective Analysis. Pancreas 2021, 50, 516–523. [Google Scholar] [CrossRef]
- Pulvirenti, A.; Raj, N.; Cingarlini, S.; Pea, A.; Tang, L.H.; Luchini, C.; Chou, J.F.; Grego, E.; Marinova, I.; Capanu, M.; et al. Platinum-Based Treatment for Well- and Poorly Differentiated Pancreatic Neuroendocrine Neoplasms. Pancreas 2021, 50, 138–146. [Google Scholar] [CrossRef]
- Cives, M.; Ghayouri, M.; Morse, B.; Brelsford, M.; Black, M.; Rizzo, A.; Meeker, A.; Strosberg, J. Analysis of potential response predictors to capecitabine/temozolomide in metastatic pancreatic neuroendocrine tumors. Endocr. Relat. Cancer 2016, 23, 759–767. [Google Scholar] [CrossRef]
- Walter, T.; van Brakel, B.; Vercherat, C.; Hervieu, V.; Forestier, J.; Chayvialle, J.A.; Molin, Y.; Lombard-Bohas, C.; Joly, M.O.; Scoazec, J.Y. O6-Methylguanine-DNA methyltransferase status in neuroendocrine tumours: Prognostic relevance and association with response to alkylating agents. Br. J. Cancer 2015, 112, 523–531. [Google Scholar] [CrossRef]
- Cros, J.; Moati, E.; Raffenne, J.; Hentic, O.; Svrcek, M.; de, M.L.; Sbidian, E.; Guedj, N.; Bedossa, P.; Paradis, V.; et al. Gly388Arg FGFR4 Polymorphism Is Not Predictive of Everolimus Efficacy in Well-Differentiated Digestive Neuroendocrine Tumors. Neuroendocrinology 2016, 103, 495–499. [Google Scholar] [CrossRef]
- Owen, D.H.; Alexander, A.J.; Konda, B.; Wei, L.; Hemminger, J.A.; Schmidt, C.R.; Abdel-Misih, S.R.Z.; Dillhoff, M.E.; Sipos, J.A.; Kirschner, L.S.; et al. Combination therapy with capecitabine and temozolomide in patients with low and high grade neuroendocrine tumors, with an exploratory analysis of O (6)-methylguanine DNA methyltransferase as a biomarker for response. Oncotarget 2017, 8, 104046–104056. [Google Scholar] [CrossRef]
- Hijioka, S.; Sakuma, K.; Aoki, M.; Mizuno, N.; Kuwahara, T.; Okuno, N.; Hara, K.; Yatabe, Y. Clinical and in vitro studies of the correlation between MGMT and the effect of streptozocin in pancreatic NET. Cancer Chemother. Pharm. 2019, 83, 43–52. [Google Scholar] [CrossRef]
- Krug, S.; Boch, M.; Rexin, P.; Gress, T.M.; Michl, P.; Rinke, A. Impact of Therapy Sequence with Alkylating Agents and MGMT Status in Patients with Advanced Neuroendocrine Tumors. Anticancer Res. 2017, 37, 2491–2500. [Google Scholar] [CrossRef]
- Girot, P.; Dumars, C.; Mosnier, J.F.; Muzellec, L.; Senellart, H.; Foubert, F.; Caroli-Bosc, F.X.; Cauchin, E.; Regenet, N.; Matysiak-Budnik, T.; et al. Short article: Evaluation of O6-methylguanine-DNA methyltransferase as a predicting factor of response to temozolomide-based chemotherapy in well-differentiated metastatic pancreatic neuroendocrine tumors. Eur. J. Gastroenterol. Hepatol. 2017, 29, 826–830. [Google Scholar] [CrossRef]
- Fu, D.; Calvo, J.A.; Samson, L.D. Balancing repair and tolerance of DNA damage caused by alkylating agents. Nat. Rev. Cancer 2012, 12, 104–120. [Google Scholar] [CrossRef]
- Lemelin, A.; Barritault, M.; Hervieu, V.; Payen, L.; Peron, J.; Couvelard, A.; Cros, J.; Scoazec, J.Y.; Bin, S.; Villeneuve, L.; et al. O6-methylguanine-DNA methyltransferase (MGMT) status in neuroendocrine tumors: A randomized phase II study (MGMT-NET). Dig. Liver Dis. 2019, 51, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Trillo Aliaga, P.; Spada, F.; Peveri, G.; Bagnardi, V.; Fumagalli, C.; Laffi, A.; Rubino, M.; Gervaso, L.; Guerini, R.E.; Pisa, E.; et al. Should temozolomide be used on the basis of O(6)-methylguanine DNA methyltransferase status in patients with advanced neuroendocrine tumors? A systematic review and meta-analysis. Cancer Treat. Rev. 2021, 99, 102261. [Google Scholar] [CrossRef]
- Lv, Y.; Han, X.; Xu, X.F.; Ji, Y.; Zhou, Y.H.; Sun, H.C.; Zhou, J.; Fan, J.; Lou, W.H.; Huang, C. Risk factors affecting prognosis in metachronous liver metastases from WHO classification G1 and G2 gastroenteropancreatic neuroendocrine tumors after initial R0 surgical resection. BMC. Cancer 2019, 19, 335. [Google Scholar] [CrossRef]
- Han, X.; Zhang, C.; Tang, M.; Xu, X.; Liu, L.; Ji, Y.; Pan, B.; Lou, W. The value of serum chromogranin A as a predictor of tumor burden, therapeutic response, and nomogram-based survival in well-moderate nonfunctional pancreatic neuroendocrine tumors with liver metastases. Eur. J. Gastroenterol. Hepatol. 2015, 27, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Brosnan-Cashman, J.A.; An, S.; Kim, S.J.; Song, K.B.; Kim, M.S.; Kim, M.J.; Hwang, D.W.; Meeker, A.K.; Yu, E.; et al. Alternative Lengthening of Telomeres in Primary Pancreatic Neuroendocrine Tumors Is Associated with Aggressive Clinical Behavior and Poor Survival. Clin. Cancer Res. 2017, 23, 1598–1606. [Google Scholar] [CrossRef] [PubMed]
- Bertani, E.; Fazio, N.; Botteri, E.; Chiappa, A.; Falconi, M.; Grana, C.; Bodei, L.; Papis, D.; Spada, F.; Bazolli, B.; et al. Resection of the primary pancreatic neuroendocrine tumor in patients with unresectable liver metastases: Possible indications for a multimodal approach. Surgery 2014, 155, 607–614. [Google Scholar] [CrossRef]
- Dermine, S.; Palmieri, L.J.; Lavole, J.; Barre, A.; Dohan, A.; Abou, A.E.; Cottereau, A.S.; Gaujoux, S.; Brezault, C.; Chaussade, S.; et al. Non-Pharmacological Therapeutic Options for Liver Metastases in Advanced Neuroendocrine Tumors. J. Clin. Med. 2019, 8, 1907. [Google Scholar] [CrossRef]
- Strosberg, D.; Schneider, E.B.; Onesti, J.; Saunders, N.; Konda, B.; Shah, M.; Dillhoff, M.; Schmidt, C.R.; Shirley, L.A. Prognostic Impact of Serum Pancreastatin Following Chemoembolization for Neuroendocrine Tumors. Ann. Surg. Oncol. 2018, 25, 3613–3620. [Google Scholar] [CrossRef]
- Al-Toubah, T.; Partelli, S.; Cives, M.; Andreasi, V.; Silvestris, F.; Falconi, M.; Anaya Saenz, D.; Strosberg, J. Local treatment for focal progression in metastatic neuroendocrine tumors. Endocr. Relat Cancer 2019, 26, 405–409. [Google Scholar] [CrossRef]
- Zubiri, L.; Bilbao, J.I.; Rodriguez, J.; Sangro, B. Selective internal radiation therapy: An effective treatment for hormonal syndromes in pancreatic neuroendocrine tumors. Hepat. Oncol. 2018, 5, HEP09. [Google Scholar] [CrossRef]
- Frilling, A.; Clift, A.K.; Braat, A.J.A.T.; Alsafi, A.; Wasan, H.S.; Al-Nahhas, A.; Thomas, R.; Drymousis, P.; Habib, N.; Tait, P.N. Radioembolisation with 90Y microspheres for neuroendocrine liver metastases: An institutional case series, systematic review and meta-analysis. HPB 2019, 21, 773–783. [Google Scholar] [CrossRef] [PubMed]
- De Mestier, L.; Zappa, M.; Hentic, O.; Vilgrain, V.; Ruszniewski, P. Liver transarterial embolizations in metastatic neuroendocrine tumors. Rev. Endocr. Metab. Disord. 2017, 18, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Dhir, M.; Shrestha, R.; Steel, J.L.; Marsh, J.W.; Tsung, A.; Tublin, M.E.; Amesur, N.B.; Orons, P.D.; Santos, E.; Geller, D.A. Initial Treatment of Unresectable Neuroendocrine Tumor Liver Metastases with Transarterial Chemoembolization using Streptozotocin: A 20-Year Experience. Ann. Surg. Oncol. 2017, 24, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Touloupas, C.; Faron, M.; Hadoux, J.; Deschamps, F.; Roux, C.; Ronot, M.; Yevich, S.; Joskin, J.; Gelli, M.; Barbé, R.; et al. Long Term Efficacy and Assessment of Tumor Response of Transarterial Chemoembolization in Neuroendocrine Liver Metastases: A 15-Year Monocentric Experience. Cancers 2021, 13, 5366. [Google Scholar] [CrossRef]
- Chen, J.X.; Rose, S.; White, S.B.; El-Haddad, G.; Fidelman, N.; Yarmohammadi, H.; Hwang, W.; Sze, D.Y.; Kothary, N.; Stashek, K.; et al. Embolotherapy for Neuroendocrine Tumor Liver Metastases: Prognostic Factors for Hepatic Progression-Free Survival and Overall Survival. Cardiovasc. Intervent. Radiol. 2017, 40, 69–80. [Google Scholar] [CrossRef]
- Sahu, S.; Schernthaner, R.; Ardon, R.; Chapiro, J.; Zhao, Y.; Sohn, J.H.; Fleckenstein, F.; Lin, M.; Geschwind, J.F.; Duran, R. Imaging Biomarkers of Tumor Response in Neuroendocrine Liver Metastases Treated with Transarterial Chemoembolization: Can Enhancing Tumor Burden of the Whole Liver Help Predict Patient Survival? Radiology 2017, 283, 883–894. [Google Scholar] [CrossRef]
- Jia, Z.; Paz-Fumagalli, R.; Frey, G.; Sella, D.M.; McKinney, J.M.; Wang, W. Single-institution Experience of Radioembolization with Yttrium-90 Microspheres for Unresectable Metastatic Neuroendocrine Liver Tumors. J. Gastroenterol. Hepatol. 2017, 32, 1617–1623. [Google Scholar] [CrossRef]
- Kitano, M.; Davidson, G.W.; Shirley, L.A.; Schmidt, C.R.; Guy, G.E.; Khabiri, H.; Dowell, J.D.; Shah, M.H.; Bloomston, M. Transarterial Chemoembolization for Metastatic Neuroendocrine Tumors with Massive Hepatic Tumor Burden: Is the Benefit Worth the Risk? Ann. Surg. Oncol. 2016, 23, 4008–4015. [Google Scholar] [CrossRef]
- Ludwig, J.M.; Ambinder, E.M.; Ghodadra, A.; Xing, M.; Prajapati, H.J.; Kim, H.S. Lung Shunt Fraction prior to Yttrium-90 Radioembolization Predicts Survival in Patients with Neuroendocrine Liver Metastases: Single-Center Prospective Analysis. Cardiovasc. Intervent. Radiol. 2016, 39, 1007–1014. [Google Scholar] [CrossRef]
- Onesti, J.K.; Shirley, L.A.; Saunders, N.D.; Davidson, G.W.; Dillhoff, M.E.; Khabiri, H.; Guy, G.E.; Dowell, J.D.; Schmidt, C.R.; Shah, M.H.; et al. Elevated Alkaline Phosphatase Prior to Transarterial Chemoembolization for Neuroendocrine Tumors Predicts Worse Outcomes. J. Gastrointest. Surg. 2016, 20, 580–586. [Google Scholar] [CrossRef]
- Tomozawa, Y.; Jahangiri, Y.; Pathak, P.; Kolbeck, K.J.; Schenning, R.C.; Kaufman, J.A.; Farsad, K. Long-Term Toxicity after Transarterial Radioembolization with Yttrium-90 Using Resin Microspheres for Neuroendocrine Tumor Liver Metastases. J. Vasc. Interv. Radiol. 2018, 29, 858–865. [Google Scholar] [CrossRef]
- Pericleous, M.; Caplin, M.E.; Tsochatzis, E.; Yu, D.; Morgan-Rowe, L.; Toumpanakis, C. Hepatic artery embolization in advanced neuroendocrine tumors: Efficacy and long-term outcomes. Asia Pac. J. Clin. Oncol. 2016, 12, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Braat, A.J.A.T.; Kappadath, S.C.; Ahmadzadehfar, H.; Stothers, C.L.; Frilling, A.; Deroose, C.M.; Flamen, P.; Brown, D.B.; Sze, D.Y.; Mahvash, A.; et al. Radioembolization with (90)Y Resin Microspheres of Neuroendocrine Liver Metastases: International Multicenter Study on Efficacy and Toxicity. Cardiovasc. Intervent. Radiol. 2019, 42, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Hur, S.; Chung, J.W.; Kim, H.C.; Oh, D.Y.; Lee, S.H.; Bang, Y.J.; Kim, W.H. Survival outcomes and prognostic factors of transcatheter arterial chemoembolization for hepatic neuroendocrine metastases. J. Vasc. Interv. Radiol. 2013, 24, 947–956. [Google Scholar] [CrossRef]
- Ziv, E.; Rice, S.L.; Filtes, J.; Yarmohammadi, H.; Boas, F.E.; Erinjeri, J.P.; Petre, E.N.; Brody, L.A.; Brown, K.T.; Covey, A.M.; et al. DAXX Mutation Status of Embolization-Treated Neuroendocrine Tumors Predicts Shorter Time to Hepatic Progression. J. Vasc. Interv. Radiol. 2018, 29, 1519–1526. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.R.; Weber, J.M.; Choi, J.; Campos, T.L.; Valone, T.L.; Han, G.; Schell, M.J.; Kvols, L.K. A phase II clinical trial of sunitinib following hepatic transarterial embolization for metastatic neuroendocrine tumors. Ann. Oncol. 2012, 23, 2335–2341. [Google Scholar] [CrossRef] [PubMed]
- Zener, R.; Yoon, H.; Ziv, E.; Covey, A.; Brown, K.T.; Sofocleous, C.T.; Thornton, R.H.; Boas, F.E. Outcomes After Transarterial Embolization of Neuroendocrine Tumor Liver Metastases Using Spherical Particles of Different Sizes. Cardiovasc. Intervent. Radiol. 2019, 42, 569–576. [Google Scholar] [CrossRef]
- Grozinsky-Glasberg, S.; Kaltsas, G.; Kaltsatou, M.; Lev-Cohain, N.; Klimov, A.; Vergadis, V.; Uri, I.; Bloom, A.I.; Gross, D.J. Hepatic intra-arterial therapies in metastatic neuroendocrine tumors: Lessons from clinical practice. Endocrine 2018, 60, 499–509. [Google Scholar] [CrossRef]
- Tsang, E.S.; Loree, J.M.; Davies, J.M.; Gill, S.; Liu, D.; Ho, S.; Renouf, D.J.; Lim, H.J.; Kennecke, H.F. Efficacy and Prognostic Factors for Y-90 Radioembolization (Y-90) in Metastatic Neuroendocrine Tumors with Liver Metastases. Can. J. Gastroenterol. Hepatol. 2020, 2020, 5104082. [Google Scholar] [CrossRef]
- Sartori, S.; Tombesi, P.; Di, V.F.; Bianchi, L.; Ambrosio, R. Percutaneous Laser Ablation of Liver Metastases from Neuroendocrine Neoplasm. A Retrospective Study for Safety and Effectiveness. Cardiovasc. Intervent. Radiol. 2019, 42, 1571–1578. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Ameli, S.; Pandey, A.; Khoshpouri, P.; Ghasabeh, M.A.; Pandey, P.; Li, Z.; Hu, D.; Kamel, I.R. Semi-quantitative visual assessment of hepatic tumor burden can reliably predict survival in neuroendocrine liver metastases treated with transarterial chemoembolization. Eur. Radiol. 2019, 29, 5804–5812. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.S.; Kim, H.Y.; Lee, Y.J.; Kim, E. Subcellular localization of Daxx determines its opposing functions in ischemic cell death. FEBS Lett. 2007, 581, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Marinoni, I.; Kurrer, A.S.; Vassella, E.; Dettmer, M.; Rudolph, T.; Banz, V.; Hunger, F.; Pasquinelli, S.; Speel, E.J.; Perren, A. Loss of DAXX and ATRX are associated with chromosome instability and reduced survival of patients with pancreatic neuroendocrine tumors. Gastroenterology 2014, 146, 453–460. [Google Scholar] [CrossRef]
- Opalinska, M.; Sowa-Staszczak, A.; Grochowska, A.; Olearska, H.; Hubalewska-Dydejczyk, A. Value of Peptide Receptor Radionuclide Therapy as Neoadjuvant Treatment in the Management of Primary Inoperable Neuroendocrine Tumors. Front. Oncol. 2021, 11, 687925. [Google Scholar] [CrossRef] [PubMed]
- Sowa-Staszczak, A.; Pach, D.; Chrzan, R.; Trofimiuk, M.; Stefanska, A.; Tomaszuk, M.; Kolodziej, M.; Mikolajczak, R.; Pawlak, D.; Hubalewska-Dydejczyk, A. Peptide receptor radionuclide therapy as a potential tool for neoadjuvant therapy in patients with inoperable neuroendocrine tumours (NETs). Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1669–1674. [Google Scholar] [CrossRef]
- Parghane, R.V.; Bhandare, M.; Chaudhari, V.; Shrikhande, S.V.; Ostwal, V.; Ramaswamy, A.; Talole, S.; Basu, S. Surgical feasibility, Determinants and overall Efficacy assessment of Neoadjuvant PRRT with 177Lu-DOTATATE for locally Advanced Unresectable Gastroenteropancreatic Neuroendocrine Tumors. J. Nucl. Med. 2021, 62, 1558–1563. [Google Scholar] [CrossRef] [PubMed]
- Schiavo Lena, M.; Partelli, S.; Castelli, P.; Andreasi, V.; Smart, C.E.; Pisa, E.; Bartolomei, M.; Bertani, E.; Zamboni, G.; Falconi, M.; et al. Histopathological and Immunophenotypic Changes of Pancreatic Neuroendocrine Tumors after Neoadjuvant Peptide Receptor Radionuclide Therapy (PRRT). Endocr. Pathol. 2020, 31, 119–131. [Google Scholar] [CrossRef]
- Lamarca, A.; Crona, J.; Ronot, M.; Opalinska, M.; Lopez Lopez, C.; Pezzutti, D.; Najran, P.; Carvhalo, L.; Franca Bezerra, R.O.; Borg, P.; et al. Value of Tumor Growth Rate (TGR) as an Early Biomarker Predictor of Patients’ Outcome in Neuroendocrine Tumors (NET)-The GREPONET Study. Oncologist 2019, 24, e1082–e1090. [Google Scholar] [CrossRef]
- Lamarca, A.; Ronot, M.; Moalla, S.; Crona, J.; Opalinska, M.; Lopez Lopez, C.; Pezzutti, D.; Najran, P.; Carvhalo, L.; Bezerra, R.O.F.; et al. Tumor Growth Rate as a Validated Early Radiological Biomarker Able to Reflect Treatment-Induced Changes in Neuroendocrine Tumors: The GREPONET-2 Study. Clin. Cancer Res. 2019, 25, 6692–6699. [Google Scholar] [CrossRef]
- Malczewska, A.; Witkowska, M.; Makulik, K.; Bocian, A.; Walter, A.; Pilch-Kowalczyk, J.; Zajecki, W.; Bodei, L.; Oberg, K.E.; Kos-Kudla, B. NETest liquid biopsy is diagnostic of small intestine and pancreatic neuroendocrine tumors and correlates with imaging. Endocr. Connect. 2019, 8, 442–453. [Google Scholar] [CrossRef]
- Malczewska, A.; Bodei, L.; Kidd, M.; Modlin, I.M. Blood mRNA Measurement (NETest) for Neuroendocrine Tumor Diagnosis of Image-Negative Liver Metastatic Disease. J. Clin. Endocrinol. Metab. 2019, 104, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Yordanova, A.; Ahmadzadehfar, H. Combination Therapies with PRRT. Pharmaceuticals 2021, 14, 1005. [Google Scholar] [CrossRef] [PubMed]
- Adant, S.; Shah, G.M.; Beauregard, J.M. Combination treatments to enhance peptide receptor radionuclide therapy of neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 907–921. [Google Scholar] [CrossRef]
- Claringbold, P.G.; Turner, J.H. NeuroEndocrine Tumor Therapy with Lutetium-177-octreotate and Everolimus (NETTLE): A Phase I Study. Cancer Biother. Radiopharm. 2015, 30, 261–269. [Google Scholar] [CrossRef]
- Claringbold, P.G.; Turner, J.H. Pancreatic Neuroendocrine Tumor Control: Durable Objective Response to Combination 177Lu-Octreotate-Capecitabine-Temozolomide Radiopeptide Chemotherapy. Neuroendocrinology 2016, 103, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Braat, A.J.A.T.; Kwekkeboom, D.J.; Kam, B.L.R.; Teunissen, J.J.M.; de Herder, W.W.; Dreijerink, K.M.A.; van Rooij, R.; Krijger, G.C.; de Jong, H.W.A.M.; van den Bosch, M.A.A.J.; et al. Additional hepatic (166)Ho-radioembolization in patients with neuroendocrine tumours treated with (177)Lu-DOTATATE; a single center, interventional, non-randomized, non-comparative, open label, phase II study (HEPAR PLUS trial). BMC. Gastroenterol. 2018, 18, 84. [Google Scholar] [CrossRef]
- Taelman, V.F.; Radojewski, P.; Marincek, N.; Ben-Shlomo, A.; Grotzky, A.; Olariu, C.I.; Perren, A.; Stettler, C.; Krause, T.; Meier, L.P.; et al. Upregulation of Key Molecules for Targeted Imaging and Therapy. J. Nucl. Med. 2016, 57, 1805–1810. [Google Scholar] [CrossRef]
- Veenstra, M.J.; van Koetsveld, P.M.; Dogan, F.; Farrell, W.E.; Feelders, R.A.; Lamberts, S.W.J.; de Herder, W.W.; Vitale, G.; Hofland, L.J. Epidrug-induced upregulation of functional somatostatin type 2 receptors in human pancreatic neuroendocrine tumor cells. Oncotarget 2018, 9, 14791–14802. [Google Scholar] [CrossRef]
- Jensen, R.T.; Bodei, L.; Capdevila, J.; Couvelard, A.; Falconi, M.; Glasberg, S.; Kloppel, G.; Lamberts, S.; Peeters, M.; Rindi, G.; et al. Unmet Needs in Functional and Nonfunctional Pancreatic Neuroendocrine Neoplasms. Neuroendocrinology 2019, 108, 26–36. [Google Scholar] [CrossRef]
- Boons, G.; Vandamme, T.; Marien, L.; Lybaert, W.; Roeyen, G.; Rondou, T.; Papadimitriou, K.; Janssens, K.; Op de Beeck, B.; Simoens, M.; et al. Longitudinal Copy Number Alteration Analysis in Plasma Cell-Free DNA of Neuroendocrine Neoplasms is a Novel Specific Biomarker for Diagnosis, Prognosis and Follow-Up. Clin. Cancer Res. 2021, in press. [Google Scholar] [CrossRef]
- Herrera-Martinez, A.D.; Hofland, L.J.; Galvez Moreno, M.A.; Castano, J.P.; de Herder, W.W.; Feelders, R.A. Neuroendocrine neoplasms: Current and potential diagnostic, predictive and prognostic markers. Endocr. Relat Cancer 2019, 26, R157–R179. [Google Scholar] [CrossRef] [PubMed]
- Kong, G.; Hicks, R.J. Peptide Receptor Radiotherapy: Current Approaches and Future Directions. Curr. Treat. Options. Oncol. 2019, 20, 77. [Google Scholar] [CrossRef] [PubMed]
- Pavel, M.; Baudin, E.; Couvelard, A.; Krenning, E.; Oberg, K.; Steinmuller, T.; Anlauf, M.; Wiedenmann, B.; Salazar, R. ENETS Consensus Guidelines for the Management of Patients with Liver and Other Distant Metastases from Neuroendocrine Neoplasms of Foregut, Midgut, Hindgut, and Unknown Primary. Neuroendocrinology 2012, 95, 157–176. [Google Scholar] [CrossRef]
- Fidelman, N.; Kerlan, R.K., Jr.; Hawkins, R.A.; Pampaloni, M.; Taylor, A.G.; Kohi, M.P.; Kolli, K.P.; Atreya, C.E.; Bergsland, E.K.; Kelley, R.K.; et al. Radioembolization with (90)Y glass microspheres for the treatment of unresectable metastatic liver disease from chemotherapy-refractory gastrointestinal cancers: Final report of a prospective pilot study. J. Gastrointest Oncol. 2016, 7, 860–874. [Google Scholar] [CrossRef]
- Benson, A.B., III; Geschwind, J.F.; Mulcahy, M.F.; Rilling, W.; Siskin, G.; Wiseman, G.; Cunningham, J.; Houghton, B.; Ross, M.; Memon, K.; et al. Radioembolisation for liver metastases: Results from a prospective 151 patient multi-institutional phase II study. Eur. J. Cancer 2013, 49, 3122–3130. [Google Scholar] [CrossRef] [PubMed]
- Singhi, A.D.; Klimstra, D.S. Well-differentiated pancreatic neuroendocrine tumours (PanNETs) and poorly differentiated pancreatic neuroendocrine carcinomas (PanNECs): Concepts, issues and a practical diagnostic approach to high-grade (G3) cases. Histopathology 2018, 72, 168–177. [Google Scholar] [CrossRef]
- Matsumoto, T.; Okabe, H.; Yamashita, Y.I.; Yusa, T.; Itoyama, R.; Nakao, Y.; Yamao, T.; Umzaki, N.; Tsukamoto, M.; Kitano, Y.; et al. Clinical role of fludeoxyglucose (18F) positron emission tomography/computed tomography ((18)F-FDG PET/CT) in patients with pancreatic neuroendocrine tumors. Surg. Today 2019, 49, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.H.; Untch, B.R.; Reidy, D.L.; O’Reilly, E.; Dhall, D.; Jih, L.; Basturk, O.; Allen, P.J.; Klimstra, D.S. Well-Differentiated Neuroendocrine Tumors with a Morphologically Apparent High-Grade Component: A Pathway Distinct from Poorly Differentiated Neuroendocrine Carcinomas. Clin. Cancer Res. 2016, 22, 1011–1017. [Google Scholar] [CrossRef]
- Alevroudis, E.; Spei, M.E.; Chatziioannou, S.N.; Tsoli, M.; Wallin, G.; Kaltsas, G.; Daskalakis, K. Clinical Utility of (18)F-FDG PET in Neuroendocrine Tumors Prior to Peptide Receptor Radionuclide Therapy: A Systematic Review and Meta-Analysis. Cancers 2021, 13, 1813. [Google Scholar] [CrossRef]
- Rindi, G.; Klersy, C.; Albarello, L.; Baudin, E.; Bianchi, A.; Buchler, M.W.; Caplin, M.; Couvelard, A.; Cros, J.; de Herder, W.W.; et al. Competitive Testing of the WHO 2010 versus the WHO 2017 Grading of Pancreatic Neuroendocrine Neoplasms: Data from a Large International Cohort Study. Neuroendocrinology 2018, 107, 375–386. [Google Scholar] [CrossRef]
- Raj, N.; Valentino, E.; Capanu, M.; Tang, L.H.; Basturk, O.; Untch, B.R.; Allen, P.J.; Klimstra, D.S.; Reidy-Lagunes, D. Treatment Response and Outcomes of Grade 3 Pancreatic Neuroendocrine Neoplasms Based on Morphology: Well Differentiated Versus Poorly Differentiated. Pancreas 2017, 46, 296–301. [Google Scholar] [CrossRef]
- Chan, D.L.; Clarke, S.J.; Diakos, C.I.; Roach, P.J.; Bailey, D.L.; Singh, S.; Pavlakis, N. Prognostic and predictive biomarkers in neuroendocrine tumours. Crit. Rev. Oncol. Hematol. 2017, 113, 268–282. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, F.M.; Vesely, C.; Childs, A.; Marafioti, T.; Khan, M.S.; Mandair, D.; Cives, M.; Ensell, L.; Lowe, H.; Akarca, A.U.; et al. Circulating tumour cells and their association with bone metastases in patients with neuroendocrine tumours. Br. J. Cancer 2019, 120, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Kirkwood, A.; Tsigani, T.; Garcia-Hernandez, J.; Hartley, J.A.; Caplin, M.E.; Meyer, T. Circulating tumor cells as prognostic markers in neuroendocrine tumors. J. Clin. Oncol. 2013, 31, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Klinge, C.M. Non-coding RNAs: Long non-coding RNAs and microRNAs in endocrine-related cancers. Endocr. Relat Cancer 2018, 25, R259–R282. [Google Scholar] [CrossRef] [PubMed]
- Zatelli, M.C.; Grossrubatscher, E.M.; Guadagno, E.; Sciammarella, C.; Faggiano, A.; Colao, A. Circulating tumor cells and miRNAs as prognostic markers in neuroendocrine neoplasms. Endocr. Relat Cancer 2017, 24, R223–R237. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, F.M.; Meyer, T. Liquid Biopsies for Neuroendocrine Tumors: Circulating Tumor Cells, DNA, and MicroRNAs. Endocrinol. Metab. Clin. N. Am. 2018, 47, 471–483. [Google Scholar] [CrossRef]
- Park, J.K.; Paik, W.H.; Lee, K.; Ryu, J.K.; Lee, S.H.; Kim, Y.T. DAXX/ATRX and MEN1 genes are strong prognostic markers in pancreatic neuroendocrine tumors. Oncotarget 2017, 8, 49796–49806. [Google Scholar] [CrossRef] [PubMed]
- Singhi, A.D.; Liu, T.C.; Roncaioli, J.L.; Cao, D.; Zeh, H.J.; Zureikat, A.H.; Tsung, A.; Marsh, J.W.; Lee, K.K.; Hogg, M.E.; et al. Alternative Lengthening of Telomeres and Loss of DAXX/ATRX Expression Predicts Metastatic Disease and Poor Survival in Patients with Pancreatic Neuroendocrine Tumors. Clin. Cancer Res. 2017, 23, 600–609. [Google Scholar] [CrossRef]
- Uemura, J.; Okano, K.; Oshima, M.; Suto, H.; Ando, Y.; Kumamoto, K.; Kadota, K.; Ichihara, S.; Kokudo, Y.; Maeba, T.; et al. Immunohistochemically Detected Expression of ATRX, TSC2, and PTEN Predicts Clinical Outcomes in Patients with Grade 1 and 2 Pancreatic Neuroendocrine Tumors. Ann. Surg. 2020, 274, e949–e956. [Google Scholar] [CrossRef]
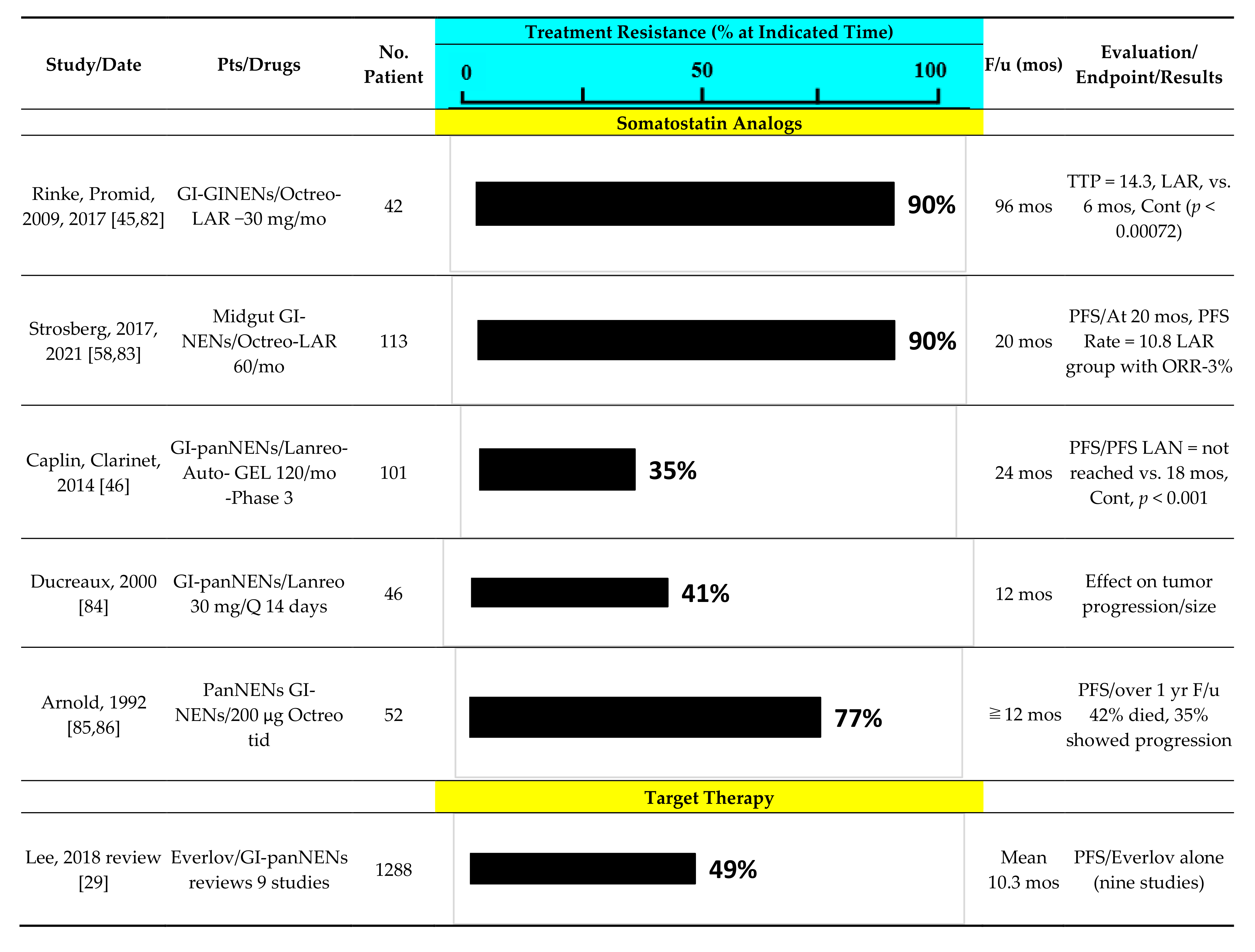
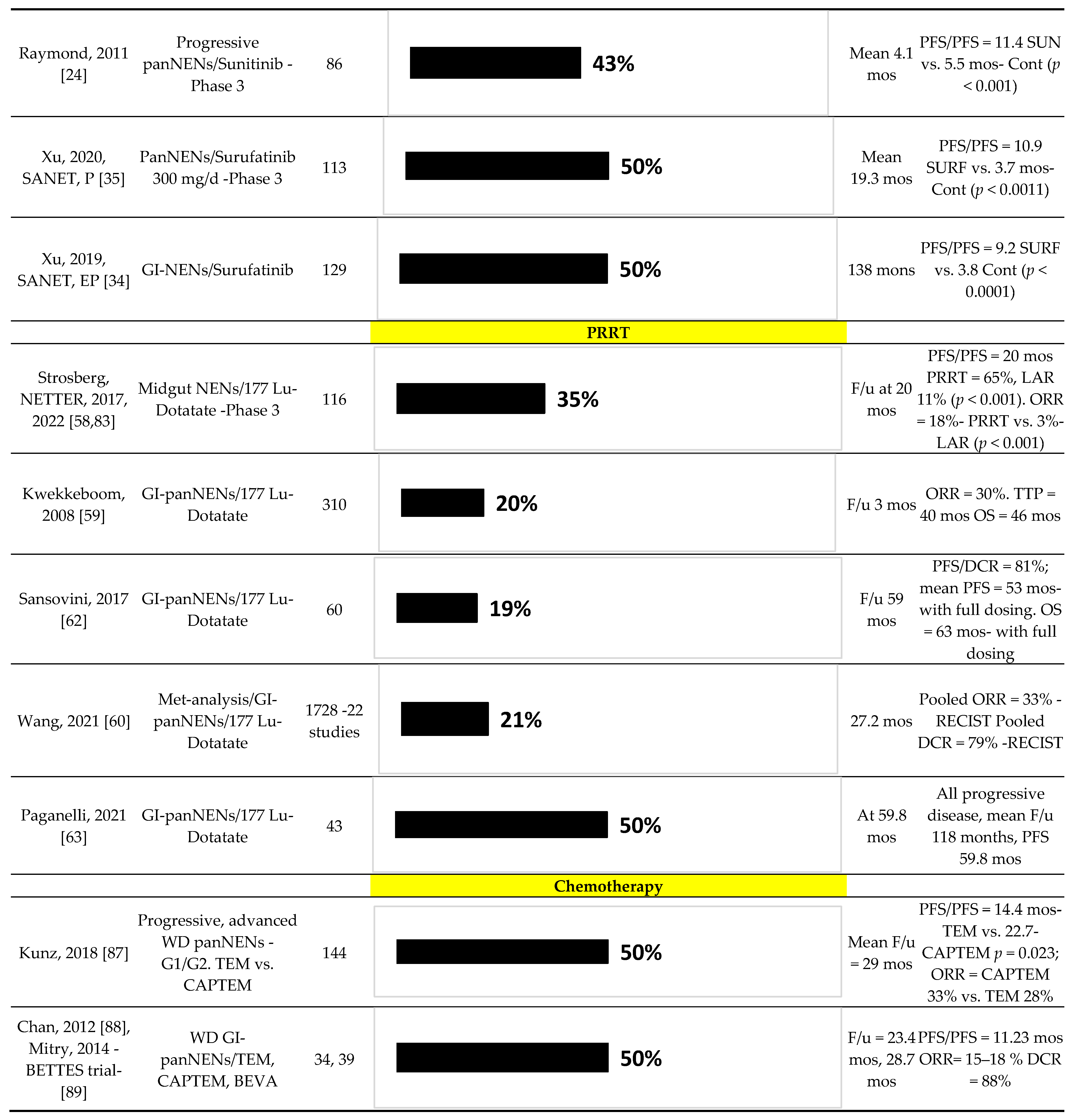
1. Which patients will benefit from each of the available therapeutic options?
|
2. What is the exact order of various therapeutic options for each individual patient?
|
3. Difference in anti-tumor activity/toxicity between different therapeutic agents.
|
4. When to stop or alter the treatment?
|
5. How to follow-up during/after treatment?
|
6. Which patient will benefit from combination therapies?
|
| Factors | PRRT | SSA | ||
|---|---|---|---|---|
| PFS | OS | PFS | OS | |
| Clinical factor | ||||
| Age | [63,111]/[133,181] | [63,182]/[183] | [175] | |
| Gender, male | [182,183] | [175] | ||
| Performance status | [111]/[114,133,184,185] | [59,111,114,184,186]/[187] | [172] | |
| Functioning tumor | [188] | [59]/[186] | ||
| Symptomatic | [188] | [59]/[186] | [173] | |
| Progression at baseline | [184] | [42,173,174] | ||
| Body weight loss | [59] | |||
| Diabetes | [114] | [114] | ||
| Laboratory test/Biological marker | ||||
| CgA, high | [114,184]/[189,190] | [114]/[184,190,191,192] | [174]/[172,175] | [175] |
| CgA response, no | [184] | [184,191,193] | [176,177] | |
| NSE, high | [186] | |||
| Pancreastatin, high | [192] | |||
| 5-HIAA, non-responder | [176] | |||
| Quotient, NETest and Ki-67 | [133] | |||
| LDH, high | [111] | [111] | ||
| ALP, high | [111,184] | [111,184]/[187,194] | [173]/[172] | |
| CRP, high | [189]/[195] | |||
| Somatostatin, high | [195] | [195] | ||
| Albumin, low | [195] | [195] | ||
| Inflammation-based index score | [195] | [195] | ||
| WBC, high | [189] | |||
| Abnormal blood count | [114] | |||
| NLR, high | [173] | |||
| PLR high | [196] | |||
| Imaging factor | ||||
| SUV, low (SSTR-PET) | [197,198]/[112,185,199,200] | [112,186,198] | ||
| SUV, high (FDG-PET) | [62,183,198]/[112,134,201,202,203] | [62,183]/[112,202,203,204] | ||
| SSTR heterogeneity | [197,205] | [205] | ||
| SSTR-PET textural parameters | [185,189,197]/[206,207,208] | [207] | ||
| Tumor growth rate, high | [209] | [210] | ||
| Treatment-related factor | ||||
| Non-responder/shorter PFS | [211]/[184,199,212] | [59]/ [184,187,193,212,213,214,215] | [175] | |
| Lower cumulative dose/reduced dose | [207]/[198,216] | [62,183,207]/[198,206] | ||
| Absorbed dose to the kidney, <23 Gy | [193] | [193] | ||
| Isotope, 90Y/117Lu alone | [182]/[114,217] | [182]/[217] | ||
| No primary tumor resection | [184,213] | [184]/[114,213] | [172] | |
| Prior systemic therapy | [114,184,198,218]/[182,219] | [114,182,184,218] | [179,180] | [179] |
| Prior TACE | [211] | |||
| No SSA use (combination/maintenance) | [220] | [220] | ||
| Concomitant use of prior-refracted SSA | [221] | [221] | ||
| Histological factor/classification/grading | ||||
| Grade | [182,183,195,211,218]/ [114,222] | [134,182,183,218]/ [36,114,214,217,222] | [223]/[42,172] | [179] |
| Ki-67 | [114,209]/[111,112,186,193,201,206] | [114,186,213]/[111,112,201] | [172,173]/[224] | |
| Differentiation, poorly | [111] | [111] | [175] | [175] |
| Distant metastasis | [211] | [173]/[224] | ||
| Liver metastasis | [63] | [63,187] | [42] | |
| Bone metastasis | [183,184,188] | [59,190,198]/ [183,184,187,214] | [173] | |
| Disease extent | [62] | [187] | ||
| Hepatic tumor burden | [184]/[62,183] | [59,183,184,186]/[62,190,213] | [173,174,179,223]/ [177,225] | |
| Ascites | [190] | [190] | ||
| Molecular factor | ||||
| SSTR2 low | [36] | |||
| Factors | Everolimus | Sunitinib | ||
|---|---|---|---|---|
| PFS | OS | PFS | OS | |
| Clinical factor | ||||
| Age | [227] | |||
| Performance status | [228]/[229] | [228]/[229] | ||
| Functioning tumor | [230] | |||
| Non-functioning tumor | ||||
| No Diabetes | [231] | |||
| No concomitant metformin use | [231] | |||
| Laboratory test/Biological marker | ||||
| CgA, high | [228,232,233] | [90,228]/[232] | ||
| No early CgA response | [228] | [228] | ||
| NSE, high | [228] | [90]/[228] | ||
| No early CgA or NSE response | [228] | |||
| NLR, high | [234] | |||
| LMR, low | [234] | |||
| PlGF, high | [90] | |||
| sVEGFR1, high | [90] | |||
| sVEGFR2, low | [235] | |||
| VEGFR3 SNP | [236] | |||
| SDF-1α, high | [235] | [235] | ||
| IL-6, high | [236] | |||
| Osteopontin, high | [236] | |||
| Triglyceride, high (During first 3 mo.) | [237] | |||
| Hypercholesterolemia (Grade2), no | [232] | [232] | ||
| Imaging factor | ||||
| SRS, negative | ||||
| SRS, asphericity | [238] | |||
| Treatment-related factor | ||||
| Non-responder | [94] | [239]/[240,241] | [242] | |
| Disease-progression (at 3/6/12 month) | [175] | |||
| No primary tumor resection | [231] | [232] | ||
| No resection, post-treatment | ||||
| Prior systemic therapy | [229] | [229]/[243] | ||
| No prior PRRT | [238] | |||
| Dose intensity, low | [243] | |||
| Cumulative dose, low | [243] | [243] | ||
| Stomatitis (within 8 week), no | [244] | |||
| Histological factor/classification/grading | ||||
| Grade | [95,231]/[230,232] | |||
| Ki-67 index | [236]/[227] | [236]/[227] | ||
| Mitosis | [227] | [227] | ||
| Differentiation (non-well/poorly) | [228] | [227] | [227] | |
| Number of metastatic sites | [232,238] | [232] | ||
| Lymph node metastasis | ||||
| Liver metastasis | [231] | |||
| Lung metastasis | ||||
| Bone metastasis | [232,238] | [232] | ||
| Hepatic tumor burden | ||||
| Molecular factor | ||||
| ACC1 high | [237] | |||
| PHLDA-3, positive | [229] | [229] | ||
| phospho-p70S6K, high | [232] | [232] | ||
| Synaptophysin, negative | [227] |
| Factors | PFS | OS |
|---|---|---|
| Clinical factor | ||
| Age | [309] | [304,310,311]/[305] |
| Male | [312] | |
| Performance status | [302,303,307,308] | [298,304,307,308]/[305,306] |
| Functioning tumor | [302]/[312] | |
| Non-functioning tumor | [311] | |
| Laboratory test/Biological marker | ||
| CgA response, no | [305,306,314] | [315] |
| 5-HIAA, high | [305] | |
| NLR, high | [316] | |
| PLR, high | [316] | |
| LDH, high | [298]/[308] | |
| ALP high | [317] | |
| CRP, high | [317] | |
| Platelet, high | [298] | |
| Albumin, low | [317] | |
| Imaging factor | ||
| SRS, negative | [304] | |
| Tumor growth rate, high | [319] | [319] |
| Treatment-related factor | ||
| Non-responder | [306,313,314] | [306] |
| No primary tumor resection | [302]/[313] | [306,312] |
| Prior Chemotherapy | [229,302,310,318]/[303,311,317] | [229,302,310,311,318]/[229] |
| Treatment cycles | [317] | |
| Histological factor/classification/grading | ||
| Grade | [309,312,319]/[303,316,320] | [312,319]/[318] |
| Ki-67 | [302,303,305,306,318,321,322,323]/ [310,324] | [304,305,312,318,321]/ [295,298,310,323,324] |
| Differentiation, poorly | [317]/[299,319,323,324] | [307]/[295,299,319,323,324,325,326] |
| Stage | [312]/[316] | |
| Size | [308] | |
| Number of organs involve | [321] | |
| Hepatic tumor burden | [321] | |
| Extrahepatic metastasis | [302,303] | [307] |
| Molecular factor | ||
| ALT negative | [327] | |
| KRAS mutant | [295] | |
| MGMT methylation, low | [318,328]/[229,329] | [318,328]/[229] |
| MGMT expression, high | [328]/[329] | [328]/[330] |
| Rb loss | [295] | |
| Thymidylate synthase, deficient | [305] |
| Factors | PFS | OS |
|---|---|---|
| Clinical factor | ||
| Age | [347]/[348] | |
| Performance status | [349,350]/[351] | |
| Laboratory test/Biological marker | ||
| CgA, high | [347] | |
| CgA increase after TACE | [356] | [356] |
| Pancreastatin, high | [342]/[352] | |
| Bilirubin, high | [353] | |
| ALP, high | [354]/[355] | |
| Child-Pugh class, B or C | [351]/[355] | |
| Imaging factor | ||
| No ETB response | [350] | |
| Lung shunt fraction >10% | [353] | |
| Arteriovenous shunt | [361] | |
| Treatment-related factor | ||
| Non-responder | [348,357]/[347,351] | |
| Number of TACE sessions | [347]/[352] | |
| No primary tumor resection | [347,362,363] | |
| Enterobiliary communication | [358] | |
| Prior systemic therapy | [348]/[358,359] | [348,353,355,358] |
| No concomitant SSA use | [348] | [348]/[356] |
| No adjuvant therapy | [349] | |
| No surgery for metastasis | [347] | |
| Histological factor/classification/grading | ||
| Grade | [348,349,359]/[356] | [342,347,348,349,357]/[351,354,356] |
| Ki-67 | [363,364] | |
| Differentiation, poorly | [361] | [363,365] |
| Size | [365] | |
| Hepatic tumor burden | [348,349,361] | [342,347,348,349,357,358,361,365]/[352,354] |
| Extrahepatic metastasis | [358] | [355,357,358]/[347,348,350,361,362,365] |
| Lymph node metastasis | [351] | |
| Ascites | [355] | |
| Portal vein thrombosis | [350] | |
| Molecular factor | ||
| DAXX, mutant | [359] | |
| MEN1, mutant | [359] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, L.; Ramos-Alvarez, I.; Jensen, R.T. Predictive Factors for Resistant Disease with Medical/Radiologic/Liver-Directed Anti-Tumor Treatments in Patients with Advanced Pancreatic Neuroendocrine Neoplasms: Recent Advances and Controversies. Cancers 2022, 14, 1250. https://doi.org/10.3390/cancers14051250
Lee L, Ramos-Alvarez I, Jensen RT. Predictive Factors for Resistant Disease with Medical/Radiologic/Liver-Directed Anti-Tumor Treatments in Patients with Advanced Pancreatic Neuroendocrine Neoplasms: Recent Advances and Controversies. Cancers. 2022; 14(5):1250. https://doi.org/10.3390/cancers14051250
Chicago/Turabian StyleLee, Lingaku, Irene Ramos-Alvarez, and Robert T. Jensen. 2022. "Predictive Factors for Resistant Disease with Medical/Radiologic/Liver-Directed Anti-Tumor Treatments in Patients with Advanced Pancreatic Neuroendocrine Neoplasms: Recent Advances and Controversies" Cancers 14, no. 5: 1250. https://doi.org/10.3390/cancers14051250
APA StyleLee, L., Ramos-Alvarez, I., & Jensen, R. T. (2022). Predictive Factors for Resistant Disease with Medical/Radiologic/Liver-Directed Anti-Tumor Treatments in Patients with Advanced Pancreatic Neuroendocrine Neoplasms: Recent Advances and Controversies. Cancers, 14(5), 1250. https://doi.org/10.3390/cancers14051250





