Simple Summary
The medical care for patients with neuroendocrine tumors arising from the intestines, pancreas, or stomach is frequently limited by the development resistance to common treatment approaches including targeted therapies and chemotherapy. The purpose of this review is to summarize the current treatments for these tumors, the possible cellular changes involved with the development of treatment resistance, and possible ways to overcome or approach this challenge. The goal is to provide an up to date summary of current and upcoming clinical findings regarding therapy-resistant neuroendocrine tumors.
Abstract
Gastroenteropancreatic neuroendocrine tumors (GEP-NETs) are a heterogenous group of malignancies originating from neuroendocrine cells of the gastrointestinal tract, the incidence of which has been increasing for several decades. While there has been significant progress in the development of therapeutic options for patients with advanced or metastatic disease, these remain limited both in quantity and durability of benefit. This review examines the latest research elucidating the mechanisms of both up-front resistance and the eventual development of resistance to the primary systemic therapeutic options including somatostatin analogues, peptide receptor radionuclide therapy with lutetium Lu 177 dotatate, everolimus, sunitinib, and temozolomide-based chemotherapy. Further, potential strategies for overcoming these mechanisms of resistance are reviewed in addition to a comprehensive review of ongoing and planned clinical trials addressing this important challenge.
1. Introduction
This Special Issue of Cancers is focused on identifying and targeting molecular pathways of resistance, reporting original clinical research and reviewing the clinical management of gastroenteropancreatic neuroendocrine tumors ‘beyond the first line’. Neuroendocrine neoplasms (NENs) comprise a diverse group of malignancies that arise from neuroendocrine cells originating from any organ in the human body. As such, NENs are further described by their histologic morphology and primary site of origin. The majority of NENs are well-differentiated and classified as neuroendocrine tumors (NETs), while 10–20% are poorly differentiated and referred to as neuroendocrine carcinomas (NECs) [1]. While the majority of NETs are of embryologic midgut origin, the most common primary tumor sites include those originating from the gastroenteropancreatic organs (GEP-NETs) [2]. Pathologically, NENs demonstrate immunohistochemical markers consistent with neuroendocrine differentiation including chromogranin A, synaptophysin, and/or neuron-specific enolase [3]. While great strides have been made in the diagnosis, management, and treatment of GEP-NETs, treatment resistance remains a critical challenge, particularly considering the limited therapeutic options available for these tumors. Treatment resistance in GEP-NETs may be observed both as primary resistance, or inherent resistance to a treatment manifested as the lack of any response, or secondary resistance, meaning the loss of efficacy in spite of a prior response [4].
2. GEP-NET Epidemiology, Pathology, Grading, and Staging
While frequently considered a rare tumor, the incidence of NETs has rapidly increased over the past few decades, especially in primary sites including the lung, small intestine, stomach, and rectum [2,5]. From 1973 to 2012, cases rose 6.4-fold in the United States, to an estimated annual incidence in 2012 of 6.98 cases per 100,000 people [2]. This rise in incidence has largely been seen in the diagnosis of locoregional rather than metastatic disease. This observation suggests that that this increase in incidence is at least in part due to improved detection of these diseases via increased imaging and endoscopic procedure frequency and improved sensitivity of imaging technologies [2,6,7,8].
While NETs can originate from any organ, 60.9% of primary sites involve the gastrointestinal tract followed by 27.4% from bronchopulmonary sites [3,9]. The most common of these, GEP-NETs, include those originating from the small intestine (30.8%), rectum (26.3%), colon (17.6%), pancreas (12.1%), stomach (8.9%), and appendix (5.7%) [9]. At times, the primary site of origin cannot be identified in spite of a comprehensive workup, in which case these are referred to as NETs of unknown origin.
In 2019, the World Health Organization (WHO) implemented a histologic classification system for GEP-NENs. Per this classification scheme, all poorly differentiated GEP-NENs are neuroendocrine carcinomas (NECs) and classified as high grade [10]. GEP-NETs are all well-differentiated but divided into low, intermediate, and high grade based upon the tumor’s mitotic rate as described by the mitotic or Ki-67 indices. Tumors are low-grade (G1) if they have a mitotic rate < 2 mitoses/2 mm2 or Ki67 index <3%, intermediate-grade (G2) if the mitotic rate is 2–20 mitoses/2 mm2 or Ki-67 index is 3–20%, and high-grade(G3) when the mitotic rate is >20 mitoses/2 mm2 or Ki67 index is >20%, with relatively worse prognoses observed at Ki67 index >55% (Figure 1) [10,11]. While the WHO histologic classification and grading system for GEP-NENs has been widely adopted, studies have suggested intra-grade heterogeneity with regard to prognosis. This appears to be particularly notable within grade 2 disease, in which those patients with Ki-67 index 3–9% may have improved survival compared to those with Ki-67 index 10–20% [12].
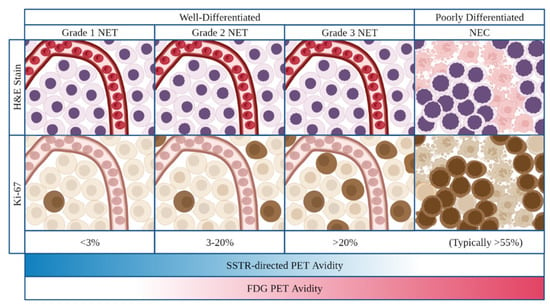
Figure 1.
Classification and grading criteria for neuroendocrine neoplasms of the gastrointestinal tract as per 2019 World Health Organization criteria. On H&E and IHC staining, NETs (well-differentiated) demonstrate preserved organoid pattern and infrequent mitotic activity while NECs (poorly differentiated) demonstrate loss of organoid morphology, necrosis, and typically high levels of mitotic activity. On imaging, increasing tumor grade frequently demonstrates an inverse correlation with SSTR-directed PET activity and a direct correlation with FDG-PET activity. Abrreviations: NET neuroendocrine tumor; NEC neuroenocrine carcinoma, H&E hematoxylin and eosin; PET positron emission tomography, FDG fluorodeoxyglucose.
Imaging of these tumors is now commonly performed with functional positron emission tomography (PET) with either/both radiolabled somatostatin receptor (SSTR) anologues or fluorodeoxyglucose (FDG). Commonly utilized radiolabled somatostatin anologues include the somatostatin anologue octreotate, covalently bonded by the bifunctional chelator tetraxetan (DOTA) to the radioisotopes Gallium-68 (68Ga oxodotreotide or DOTATATE) or Copper-64 (64Cu oxodotreotide or DOTATATE), or the somatostatin anologue Tyr3-octreotide, covalently bound via the DOTA chelator to radioisotopes Gallium-68 (68Ga edotreotide or DOTATOC) or Copper-64 (64Cu edotreotide or DOTATOC) [13].
On functional imaging, low and intermediate grade tumors most commonly demonstrate elevated SSTR-directed and poor FDG PET avidity. Taken together, these findings are thought to be reflective of the more common expression of SSTR and lower metabolic activity observed in these tumors. Conversely, high grade NETs and NECs frequently demonstrate elevated FDG PET and poor SSTR-directed PET avidity, thought to be reflective of the increased metabolic activity and decreased SSTR expression in these tumors (Figure 1) [1,14].
Staging of NETs is performed as per the American Joint Committee on Cancer tumor, node, and metastasis (TNM) staging system and differs based on the primary disease site [1,14]. The most common sites of metastasis are lymph nodes, liver, mesentery, peritoneum, lung, and bone, with rare involvement of the brain [8].
2.1. GEP-NET Prognosis and Clinical Manifestations
The prognosis for GEP-NETs is most directly determined by tumor grade, stage, and primary disease site. Small intestine NETs (siNETs) are classically considered to have a better prognosis than pancreatic NETS (panNETs) or colorectal NETs, although when controlled for tumor grade, this difference is less clear [1]. Higher grade and/or stage tumors are consistently associated with worse prognosis [14].
GEP-NETs may present with a broad diversity of clinical manifestations and are frequently discovered incidentally. Factors contributing to clinical manifestations include mass effect, tumor functionalilty, and the primary site, size, and locations of metastastatic disease [8]. Tumor functionality refers to the rather unique ability of NETs to secrete hormones, a characteristic reflective of their cellular origin. This ability may result in symptoms or clinical syndromes related to the specific hormone secreted. Within siNETs, approximately 19% secrete serotonin or other related vasoactive substances, which may result in the development of carcinoid syndrome, particularly in the setting of bulky liver metastases [3,15]. Carcinoid syndrome presents with wheezing, skin flushing, abdominal cramping, diarrhea and eventual right-sided cardiac valvular fibrosis [8]. Only 10–30% of panNETs are functional, secreting insulin (insulinoma), glucagon (glucagonoma), gastrin (gastrinoma), vasoactive intestinal peptide (VIPoma), or somatostatin (somatostatinoma) [8,16]. Recurrent hypoglycemia is associated with insulinomas; epigastric pain, diarrhea, and ulcer disease with gastrinomas; hyperglycemia, weight loss, and necrolytic migratory erythema with glucagonomas; secretory diarrhea, metabolic acidosis, and hypokalemia with VIPomas; and diabetes mellitus, cholelithiasis and steatorrhea with somatostatinomas [1,3,8,16]. Utilized within the appropriate context, the aforementioned hormones as well as urinary 5-Hydroxyindoleacetic Acid (5-HIAA) for patients with carcinoid syndrome can be utilized for the diagnoses of these tumors and the associated hormonal syndromes. Symptoms from non-functioning GEP-NETs are often a result of mass effect or nonspecific and may present as abdominal pain, nausea, vomiting, jaundice, early satiety, weight loss, and/or gastrointestinal bleeding [8,16].
2.2. Genetics of GEP-NETs
The majority of GEP-NETs occur in the setting of sporadic mutations and exhibit low mutation rates compared to other solid tumor malignancies [8,17]. The genetic mutations identified most commonly involve regulation of the mammalian target of rapamycin (mTOR) pathway, epigenetic changes (including chromatin modification, telomere length regulation, and others), cell cycle, and DNA repair pathways [17,18]. The most common somatic mutations in panNETS include genes involved in the epigenetic machinery including multiple endocrine neoplasia (MEN) 1, followed by Death-associated protein 6 (DAXX) and ATP-dependent helicase (ATRX) [16,19]. While germline MEN1 mutations are pathognomonic to the hereditary syndrome, multiple endocrine neoplasia type 1, most somatic MEN1 mutations in GEP-NETs are sporadic, and are thought to play an important role in tumorigenesis [17]. ATRX and DAXX mutations are frequently observed, particularly in panNETs, and affect the epigenetic alternative lengthening of telomeres (ALT) pathway and chromosome instability [17,18,20,21]. Within siNETs, 20% have cyclin-dependent kinase inhibitor 1B (CDKN1B) mutations that result in cell cycle dysregulation [22,23].
Only 5 to 10% of GEP-NETs are associated with hereditary syndromes and of those, most involve the pancreas [8]. Hereditary syndromes associated with panNETs are often inherited in an autosomal dominant pattern and include multiple endocrine neoplasia type 1 (MEN1), von Hippel-Lindau syndrome (VHL), tuberous sclerosis 1 and 2 (TSC1 and TSC2), and neurofibromatosis type 1 (NF1) [8].
2.3. Current Treatment of GEP-NETs
Treatment of GEP-NETs generally depends upon tumor primary site, grade, and staging. For local or locoregional disease, surgery remains the cornerstone of management, allowing for the greatest likelihood of optimal outcomes in non-metastatic disease [24]. Surgical interventions include enucleation, resection, lymphadenectomy, and/or cytoreductive operations [14]. Other locally targeting modalities include radiofrequency ablation or liver-directed therapies such as chemoembolization, arterial embolization, radioembolization, or ablative therapy [3,14].
For advanced, inoperable or metastatic GEP-NETs that express the somatostatin receptor (SSR+), the first line treatment consists of the somatostatin analogues, octreotide acetate or lanreotide [25,26]. Second-line systemic therapies for both siNETs and panNETs include both peptide radionuclide receptor therapy (PRRT) with Lutetium-177 DOTATATE (177Lu-DOTATATE) for SSTR+ disease and everolimus [27,28]. Additional therapies have been utilized specifically for panNETs in the second and later line setting include sunitinib and temozolamide-based chemotherapy [29,30]. Immune therapies, particularly the programmed death protein 1/programmed death-ligand 1 (PD-1/PD-L1) and cytotoxic T-lymphocyte associated protein 4 (CTLA-4) immune checkpoint inhibitors have demonstrated limited activity which has been primarily demonstrated in high-grade disease (grade 3 NET and NEC) [31]. While treatment options for GEP-NETs remain limited, multiple clinical trials are ongoing investigating novel therapies and therapeutic combinations and comparing current modalities in order to optimize the management of the complex diseases [29,30]. While this research remains ongoing, the development of resistance to existing treatment options for GEP-NETs remains a significant challenge in the management of these patients.
3. Treatment Resistance in GEP-NETs
3.1. Somatostatin Analogues: Octreotide Acetate and Lanreotide
The somatostatin analogues (SSAs), octreotide and lanreotide, exert their mechanism of action through binding SSTRs (G protein-coupled receptors) with strong affinity for SSTR2 and moderate affinity for SSTR5 [32,33]. Consistent with this mechanism of action, positive SSTR status by functional imaging or histology is required although not predictive of anti-proliferative and anti-secretory response to SSAs [14,34]. Octreotide acetate and lanreotide have demonstrated benefit in the treatment of NETs through both an anti-proliferative effect (as evidenced by decreased tumor growth) and an anti-secretory effect, resulting in improvement of symptoms of hormone hypersecretion in patients with functional tumors [25,26,30]. The anti-proliferative effect of SSAs is understood to be mediated through SSTR activation of protein tyrosine phosphatases (PTPs) and cGMP-dependent protein kinase, leading to the regulation of multiple downstream signaling pathways, resulting in decreased cell growth, proliferation, and migration/invasion [35,36,37,38,39]. In contrast, the anti-secretory effect is believed to be mediated through SSTR activated G protein-mediated inhibition of voltage-dependent calcium channels and the stimulation of potassium channels, thus inhibiting hormone secretion [40,41]. Both SSAs may be administered as long-acting, monthly intramuscular (octreotide acetate) or subcutaneous (lanreotide) injections [25,30].
The superiority of octreotide acetate over placebo in patients with metastatic, well-differentiated GEP-NETs was demonstrated in the PROMID trial (NCT00171873), published in 2009. This prospective, randomized, double-blinded study demonstrated significantly improved median time to tumor progression with octreotide compared to placebo (14.3 vs. 6 months, respectively, p < 0.001) [26]. Similarly, the CLARINET trial (NCT00353496) published in 2014, was a randomized, double-blind study in patients with metastatic, grade 1 or 2, non-functioning, enteropancreatic NETs. This trial demonstrated improved progression-free survival (PFS) at 18 and 24 months in patients receiving monthly lanreotide compared to those receiving placebo (median not reached vs. median of 18 months, respectively, p < 0.001) [25]. The low objective response rates to octreotide acetate and lanreotide (2.4, 2%, respectively), supports the notion that both agents act through cytostatic rather than cytocidal mechanisms. The anti-secretory effects of both SSAs have similarly been supported in prospective clinical trials utilizing these agents for the treatment of carcinoid syndrome [42,43]. Predictors of decreased time to progression to SSAs include primary pancreatic site of disease, presence of liver metastases and higher grade tumors, while initiation of treatment in the presence of stable disease, male sex, and carcinoid heart disease are predictive of a longer response [44]. The US Food and Drug Administration (FDA) approved octreotide for symptomatic treatment of carcinoid syndrome in 1998 and lanreotide in 2014 for patients with unresectable, grade 1 or 2, metastatic GEP-NETs.
Consistent with the dual anti-proliferative and anti-secretory benefits of SSAs for GEP-NETs, resistance to these agents can be manifested through both tumor progression and recurrence of hormonal symptoms. Within the context of carcinoid syndrome, resistance or insufficient anti-secretory response to SSAs in clinical trials has been defined as experiencing an average of four or more bowel movements per day, in spite of receipt of stable-dose SSAs [45].
Proposed mechanisms for the development of resistance to octreotide or lanreotide include epigenetic changes within tumor cells including histone methylation and/or acetylation changes that impact SSTR expression (Figure 2) [46,47,48,49]. More recently, further epigenetic regulation of SSTR expression in PanNETs has been proposed to occur through the expression of the antisense transcript, SSTR5-AS1. Expression of this antisense transcript appears to be influenced by the methylation of selected CpG islands, and resulted in downregulated SSTR5 expression and decreased SSA response [50]. The importance of epigenetic changes to the development of SSA resistance has been supported by studies demonstrating the effects of tumor exposure to both histone deacetylase inhibitors (HDACi) and DNA methyltransferase inhibitors (DNMTi) resulting in increased SSTR2 expression in NET cells both in vitro and in small animal imaging models [49,51]. The DNMTi, decitabine, and HDACi, tacedinaline, also demonstrated increased SSTR2 expression in the NET cell lines, BON1, H727, and QGP1 [52]. Valproic acid, a HDACi, was found to upregulate SSTR2 expression and inhibit GEP-NET cell growth through regulating MYC signaling, TGF-β1, FOXO3, and p53 [53,54].
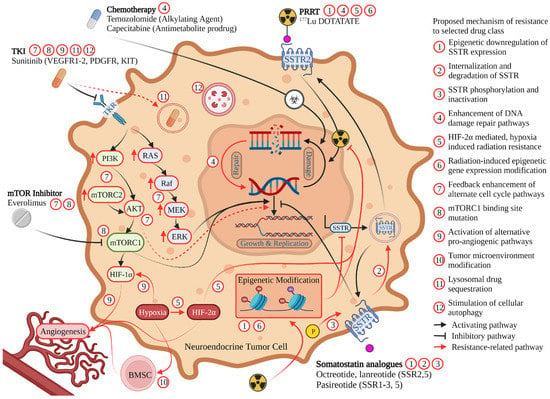
Figure 2.
Mechanism of action for commonly utilized therapies for the treatment of GEP-NETs. Described mechanisms of treatment resistance are denoted by red arrows with specific resistance mechanism to each therapeutic class denoted by a corresponding numbered red circle. Abbreviations: SSTR somatostatin receptor, PRRT peptide receptor radionuclide therapy, mTOR mammalian target of rapamycin, TKI tyrosine kinase inhibitor, VEGFR, vascular endothelial growth factor receptor; PDGFR, platelet-derived growth factor receptor; KIT stem cell growth factor receptor, TKR tyrosine kinase receptor, BMSC bone marrow-derived stem cells.
Overcoming this epigenetically derived resistance could potentially be achieved by combining SSAs with a HDACi and/or DNMTi to potentially augment the uptake and anti-tumor activity of these agents [47,48,49,54]. However, the toxicities of existing HDACi’s and DNMTi’s would provide significant challenges to implementing this approach. Agents from both classes are known to be capable of causing significant myelosuppression and bothersome gastrointestinal symptoms while HDACi’s have been associated with cardiac toxicities and ventricular arrhythmias [55,56].
Beyond epigenetic alterations, there is limited research within NETs regarding alternative resistance mechanisms to SSAs. Mechanisms have been studied in other cancer types that express SSTR [57]. In the neuroblastoma and glioma hybrid cell line, NG108-15, for example, cells exposed to continuous somatostatin become desensitized to the drug through internalization of the SSTR [58]. Both the rate and magnitude of desensitization were dependent on the concentration of somatostatin administered [58]. In these cells, the administration of an inhibitor of receptor sequestration led to reduced desensitization to somatostatin [58]. When pituitary tumor cells that overexpressed the SSTR2A somatostatin receptor were exposed to somatostatin or an SSA, desensitization mechanisms included receptor sequestration as well as receptor phosphorylation (Figure 2) [59]. Increasing the dose of SSAs or intermittent drug holidays may briefly reverse desensitization in cell models, however this approach has not demonstrated efficacy when attempted in NET patients resistant to SSA treatment [57]. Further, considering that resistance to SSAs typically develops over weeks or months, receptor internalization may not represent the predominant mechanism of resistance in these tumors. Rather, this time course may be consistent with the growth of tumor cells clones that do not express the targeted SSTR subtype [60].
Targeting alternative SSTRs present on NETs has been proposed as a potential means of overcoming resistance to existing SSAs. While octreotide acetate and lanreotide have a high affinity for SSTR2 and moderate for SSTR5, pasireotide, is a somatostatin analogue that exhibits a high affinity for binding SSTR1-3, and 5 [32,33]. Neuroendocrine tumors can markedly differ in their expression of somatostatin receptor subtypes, thus suggesting a possible mechanism allowing for pasireotide to overcome SSA resistance [33,60,61]. The efficacy of pasireotide was evaluated in a phase III study by Wolin et al. in patients with metastatic neuroendocrine tumors with carcinoid syndrome refractory to prior SSA. This study found that when compared to octreotide acetate 40 mg, pasireotide 60 mg (both given every 28 days), exhibited similar symptom control rates (20.9% vs. 26.7%, p = 0.53), a trend towards higher tumor control rates (62.7% vs. 42.6%, p = 0.09), and a longer progression-free survival (11.8 months vs. 6.8 months, p = 0.045) [32]. While these findings suggested potential benefit of pasireotide in this patient population, pasireotide has additionally been found to cause significantly more frequent hyperglycemia, nausea, and bradycardia than octreotide acetate, and has not been approved for use in NETs [32,62].
Alternative strategies to overcome the lack of response to the anti-secretory effects of SSAs in functional NETs include the addition of the tryptophan hydroxylase inhibitor telotristat ethyl in carcinoid syndrome as well as the second-line use of PRRT with 177Lu-DOTATATE. Telotristat ethyl was approved in 2017 in combination with SSA therapy for the treatment of adults with carcinoid syndrome diarrhea inadequately controlled by SSA therapy alone [63]. Telotristat ethyl is understood to have synergistic anti-secretory effects when combined with SSAs by inhibiting tryptophan hydroxylase (TPH), the rate-limiting enzyme in serotonin synthesis [45]. The second-line use of PRRT has similarly been shown to improve hormone-related symptoms in metastatic functional NETs refractory to treatment with SSAs. Of those considered resistant to octreotide therapy (as well as multi-kinase inhibitors and chemotherapy), those who received 177Lu-DOTATATE experienced a 54% complete and 35% partial response in clinical symptoms, and 17% complete and 28% partial response in chromogranin A biomarker levels [64]. Although the mechanism of maintained efficacy of a SSTR-directed treatment (PRRT) in patients resistant to somatostatin analogue therapy is unknown, it suggests that therapies targeting the SSTR remain viable targets for future research seeking to overcome resistance to octreotide and lanreotide.
3.2. Peptide Receptor Radionuclide Therapy (PRRT): 177Lu-DOTATATE
PRRT is a general therapeutic modality in which a radionuclide is linked to a peptide capable of targeting a receptor to deliver a localized radiopharmaceutical payload. 177Lu-DOTATATE is the first FDA-approved PRRT and utilizes a somatostatin analogue (DOTATATE) covalently bound to the beta-minus emitting radioisotope 177Lu in order to provide targeted radiation directly to NET cells overexpressing SSTRs (primarily SSTR2) [27,65]. This PRRT is administered intravenously generally every 8 weeks for a total of 4 cycles. Similarly to SSAs, SSTR positivity is mandatory for response to PRRT utilizing 177Lu-DOTATATE [14]. The efficacy of this therapy was confirmed in the NETTER-1 trial (NCT01578239) comparing 177Lu-DOTATATE, combined with the standard dose of 30 mg of octreotide versus 60 mg octreotide alone in patients with metastatic midgut NETs and radiographic progression on first-line SSA therapy [27]. NETTER-1 demonstrated that treatment with 177Lu-DOTATATE and octreotide resulted in a progression free survival (PFS) rate of 65.2% vs. 10.8% in the high-dose octreotide group. The objective response rate was significantly higher in the 177Lu-DOTATATE group at 18% vs. 3% in the octreotide group (p < 0.001) [27]. In 2021, Strosberg et al. provided updated survival results from the NETTER-1 trial, which showed no statistically significant difference in median overall survival in the 177Lu-DOTATATE group and octreotide group vs. the control group (48.0 months vs. 36.3 months, respectively, p = 0.30), a result that was likely impacted by the high rate of crossover (36%) in the investigational arm of the study [66]. 177Lu-DOTATATE was approved for use in advanced GEP-NETs by the FDA in 2018 [65]. Subsequent studies have identified multiple factors associated with unfavorable prognosis or response to PRRT. These include elevated inflammatory markers (including elevated C-reactive protein, neutrophil-to-lymphocyte, and platelet-to-lymphocyte ratio), the presence of ascites, marked liver metastases burden, ≥5 bone metastases, and FDG PET disease avidity [67,68,69,70,71]. In contrast, higher 68Ga-DOTATATE PET avidity and primary pancreatic disease site have been associated with improved response to PRRT [72,73].
While frequently used in the second-line setting following the development of resistance to SSA, resistance to PRRT may also occur. Waldeck et al. proposed that rather than target (SSR) down-regulation or alteration, PRRT failure appears to be related to enhanced DNA damage repair pathways resulting in rapid repair of DNA damage induced by the radionuclide emissions (Figure 2) [74]. In their study, Xenograft SSR2 cell lines (AR42J, H1299-7, and H69) were repeatedly exposed to 177Lu-DOTATATE until they developed resistance and then evaluated for double strand breaks by utilizing gamma H2Ax staining. The initial DNA damage from radiation was not different in the resistant cell lines in immunohistochemical staining, suggesting upregulated DNA repair in these cells [74].
An additional potential mechanism for the development of both primary and secondary resistance to PRRT is the activation of hypoxia signal pathways through true tissue hypoxia or by genetic influences that promote the activation of similar hypoxia-related pathways [75]. Demonstrated in mouse pheochromocytoma cells, the expression of hypoxia-inducible factor 2 alpha, or HIF-2α, was associated with increased resistance to 177Lu-DOTATATE (Figure 2) [75]. HIF-2α expression has been associated with the development of a radioresistant phenotype in other cancers including renal cell carcinoma, the mechanism of which is hypothesized to occur through interaction with the tumor suppressor, p53 [75]. While its use in combination with radiotherapies has not yet been studied, a HIF-2α inhibitor, belzutifan, has been demonstrated to be clinically effective in a phase 2 study of patients with von Hippel-Lindau syndrome and renal cell carcinoma (RCC). This study demonstrated that the use of belzutifan resulted in a 49% objective response for their RCC as well as a 77% response in those patients with synchronous with panNETs [76]. Future therapies combining a HIF-2α inhibitor such as belzutifan, could offer a potential treatment augmentation strategy to address hypoxia and HIF-2α mediated resistance to PRRT.
Another potential mechanism to improve the efficacy of PRRT in GEP-NETs and potentially overcome observed resistance would be the use of higher energy alpha-emitting radionuclides in place of currently utilized beta-emitting PRRT. Alpha-emitting PRRT would have the potential to cause significantly higher (estimated 20 fold) amounts of double strand DNA breaks due to the more localized, higher linear energy delivery provided by alpha particles to the targeted tissues [77]. Double strand DNA breaks effectively and rapidly induce cell apoptosis, which may allow for the prevention and circumventing of hypoxia signaling pathways and upregulated DNA repair mechanisms that could otherwise to lead to radiation resistance as is seen with 177Lu-DOTATATE [77]. A retrospective study of 7 patients with neuroendocrine tumors resistant to the beta-emitting radiopharmaceuticals, Yttrium-90 (90Y)/177Lu-DOTATOC, were treated with the alpha-emitting peptide receptor therapy, Bismuth-213 (213Bi)-DOTATOC. Of the 7 patients, objective responses were complete in 1, partial in 2, and stable in 3 patients as determined by RECIST (Response Evaluation Criteria in Solid Tumors) imaging criteria [78]. These results suggest a promising approach for the treatment of GEP-NETs resistant to beta-emitting PRRT. Current challenges to this potential strategy include cost, limited supplies for production, a short half-life, and hematological and renal toxicity [77,78]. Clinical trials are currently ongoing evaluating the safety and efficacy of the alpha emitting radiopharmaceuticals, Lead-212 (212Pb)-DOTAMTATE (octreotate covalently bound to the chelating agent DOTAM) and Actinium-225 (225Ac)-DOTATATE, in patients with metastatic and unresectable NETS, including in those patients who have progressed after initial PRRT treatment with 177Lu-DOTATATE (NCT05153772, NCT03466216, NCT05477576) (Table 1).

Table 1.
Active or planned trials to overcome resistance or optimize anti-tumor response in GEP-NETs.
While the use of alpha emitting PRRT represents a particularly exciting area of clinical research to improve upon the clinical activity observed with 177Lu-DOTATATE, other PRRT modalities have also been evaluated including the use of Copper-64 sarcophagine octreotate (64Cu SARTATE), octreotate linked with a more stable radionuclide isotope 64Cu. In a prospective trial of 10 patients, the imaging of 64Cu-SARTATE exhibited tumor uptake with a statistically significant higher max lesion SUVmax at 1 h (41.9 vs. 31.65, respectively, p = 0.01) and 4 h (50.5 vs. 31.65, p = 0.004) when compared to 68-Ga-DOTATATE. 64Cu-SARTATE also demonstrated a safe profile with no grade 3 or 4 adverse events [79]. This more stable radionuclide isotope could serve as a promising PRRT therapy by prolonging activity at the site of pathology in patients with NETs, although no current clinical trials are evaluating this approach [79].
Improving NET radiation sensitivity through the use of adjunct therapies has been the goal of multiple clinical studies to date. These include studies demonstrating that capecitabine, capecitabine with temozolomide, everolimus, and 5-fluorouracil, can be safely administered with PRRT, although none have been powered to demonstrate superiority in therapeutic effect to PRRT alone [80,81,82,83,84,85,86]. More recent interest has developed in targeting the epigenetics of NETs in order to enhance radiotherapy sensitivity in these tumors. Epigenetic changes resulting in radiotherapy resistance are thought to occur either through the epigenetic changes already involved in NET carcinogenesis or the acquired changes from the effects of radiation itself as has been demonstrated to occur in other malignancies treated with radiation [87,88,89]. Within the context of mid-gut NETs, Pollard et al. demonstrated that patients undergoing PET 68Ga-DOTATOC imaging before and after treatment with the HDACi vorinostat demonstrated significantly increased DOTATOC avidity at metastatic hepatic sites, suggesting that vorinostat pretreatment may result in increased SSTR expression [90]. While further mechanistic and confirmatory studies are needed, these findings suggest that epigenetic modifiers could potentially be used to increase SSTR expression, opening a future avenue for addressing epigenetically derived PRRT resistance.
Other radiosensitizers being studied for potential synergistic use along with PRRT include mTOR inhibitors and poly (ADP-ribose) polymerase (PARP) inhibitors. A study by Exner et al. found pre-treatment of five different neuroendocrine neoplasm cell lines (BON, QGP-1, LCC-18, H727, UMC-11) with an mTOR inhibitor, temsirolimus, provided improved in vitro sensitivity to irradiation [91]. Combined mTOR inhibitor and irradiation decreased cell numbers and survival in all cell lines when compared to temsirolimus and irradiation alone [91]. Similarly, potential anti-tumor synergy was demonstrated in a study by Cullinane et al. in which PRRT was combined with the PARP1 inhibitor, talazoparib. This combination was tested in vitro against SSR2 expressing AR42J cell lines compared to PRRT alone and was found to result in a significant increase in DNA double strand breaks and improved overall anti-tumor efficacy [92]. Similar results using the PARP1,5 inhibitor dihydroxyisoquinoline demonstrated that combining PRRT with this agent improved cytotoxicity in both gastroenteropancreatic (BON-1) and bronchopulmonary (NCI-H727) NET cell models through potentiating cell cycle arrest and preventing DNA damage repair [93].
Similar to improving radiation delivery or the use of radiotherapy-sensitizing agents, a novel avenue of research addressing existing PRRT resistance involves the use of an alternative therapeutic approach by modifying the targeted component of PRRT itself. This has been attempted through the use of a SSTR antagonist rather than an agonist. The use of such an agent is based upon the concept that antagonists are able to bind more effectively to SSTR-expressing tumors, as they are able to bind the SSTR in both the active and inactive state, while agonists can only do so in the active state [94,95,96]. While it is believed that somatostatin antagonists may undergo less tumor internalization with binding, Wild et al. demonstrated that not only could a somatostatin antagonist be utilized for PRRT targeting, but it also had a higher uptake and prolonged duration of radiation delivery than 177Lu-DOTATATE therapy as measured by SPECT/CT 3-dimensional voxel-based dosimetry [94]. Reidy-Lagunes et al. were able to determine safe doses of SSTR antagonist PRRT in patients with NETs to avoid the main observed adverse effect, hematologic toxicity [96]. Of the 20 patients, PFS was 21 months with an ORR of 45% with 5% complete response, 40% partial response, and 40% with stable disease [96]. Unfortunately, a clinical trial examining the effect of the PRRT utilizing a somatostatin antagonist, 177Lu-OPS201, in NETs was terminated due to a low patient accrual to study. Overall, while PRRT remains an exciting frontier for the targeted treatment of NETs, many studies are already ongoing seeking to improve tumor uptake and response while minimizing toxicities and the addressing observed radioresistance.
3.3. Mammalian Target of Rapamycin Inhibitor: Everolimus
Everolimus is a mTOR inhibitor administered as a daily oral pill, approved by the US FDA in 2016 for the treatment of progressive, nonfunctional, well-differentiated NETs of gastrointestinal (GI) or lung origin [28,97]. This approval was based upon the results of the RADIANT-4 trial, demonstrating that treatment with everolimus prolonged median PFS compared to placebo (11.0 months vs. 3.9 months with placebo) in patients in the NET population described above [28]. With regard to overall survival, everolimus reduced risk of death by 36% compared to placebo (HR 0.64, p = 0.037), however this benefit was not statistically significant at a second interim analysis of the study [28,98]. Although radiologic responses were infrequently seen (2% in the everolimus arm vs. 1% in the placebo arm), disease stabilization occurred in 81% of patients taking everolimus compared to 64% in the placebo group [28].
Everolimus resistance is believed to largely result from feedback on pathways impacted by its mechanism of action, mTOR inhibition. This can occur at multiple points within the mTOR pathway, including inhibition of mTORC1 shifting the balance towards mTORC2 signaling, thus increasing Akt production, elevation of which is associated with everolimus resistance (Figure 2) [99,100]. Newer-generation mTOR kinase inhibitors including PP242, AZD2014, and OSI-027 exert their effect on both mTORC1 and mTORC2 and could potentially be used to overcome this mechanism of escape [101].
Along with the use of broader-spectrum mTOR inhibitors, synergistic targeting of pathways known to be associated with the mTOR pathway has been considered a potential means of addressing everolimus resistance. This includes combining everolimus therapy with the commonly used anti-hyperglycemic agent metformin, known to inhibit Akt signaling through the IGFR-1/IRS-1/PI3K/Akt pathway which can further dampen mTOR signaling [102]. Metformin and everolimus suppressed cell proliferation in panNET cell lines significantly more than everolimus alone (−71% ± 13%, p < 0.0001) [102]. The combination of everolimus, metformin, and octreotide is currently being studied in patients with well-differentiated panNETs (NCT02294006) (Table 1). Cixutumumab, an anti-IGF-1R monoclonal antibody, was studied by Dasari et al. in 19 patients with NETs receiving everolimus and octreotide. Seventeen patients had stable disease with RECIST tumor measurements reduced by 1 to 22% [103]. Median PFS was 43.6 months and OS was 25.5 months [103]. However, use of cixutumumab was limited by toxicity as patients experienced adverse effects of fatigue, weight loss, hyperlipidemia, hyperglycemia, and mucositis [103]. Additionally, anti-IGF-1R monoclonal antibodies have been studied as monotherapy and have not demonstrated improved tumor responses in larger studies in NETs [103,104,105]. The dual mTOR and PI3K inhibitor, BEZ235, was studied in patients with everolimus-resistant panNETs. Though there was modest anti-tumor activity with a PFS rate of 51.6% at 16 weeks, the PFS rate was not high enough to continue to stage 2 of the study [106]. Additionally, tolerability of the drug was limited with 16 of the 31 patients experiencing grade 3/4 adverse effects that included hyperglycemia, diarrhea, and vomiting [106].
Feedback from mTOR inhibition can also activate the RAF/MEK/ERK pathway resulting in upregulated ERK2 that can reduce the response to everolimus therapy [107,108]. Therefore, combining an mTOR inhibitor with MEK inhibition presents a possible means of combating this feedback response [109,110]. Although this has not yet been well studied within NETs, MEK inhibitors are already approved for other indications including melanoma, thyroid cancer, neurofibroma, and non-small cell lung cancer [111].
Genetic mutations at FKB-12 or the FRB domain can significantly impede the action of mTOR inhibitors. FKB-12 is the site where everolimus binds and the FRB domain is the site where the FKB-12 and everolimus complex bind to evoke their action (Figure 2) [101,112]. Since next generation mTOR kinase inhibitors do not bind the FRB domain or FKB-12, they provide a therapeutic approach for overcoming this challenge [101]. In panNETs, mutations in the growth signaling receptor, FGFR4, has been associated with everolimus treatment resistance [113]. While not yet tested within the context of neuroendocrine tumors, selective and pan-FGFR inhibitors are an active area of research and are approved in other types of cancer with the potential to be a target for NETs alone or in synergistic combination with everolimus in patients with everolimus-resistant disease [114].
An additional potential approach to overcoming everolimus resistance in NETs could involve a drug interruption (holiday) allowing for restoring of sensitivity. Vandamme et al. demonstrated that in everolimus-resistant panNET cell lines QGP-1 and BON-1, a drug holiday resulted in restored sensitivity to everolimus after 10-12 weeks [107]. This study further demonstrated that in these same cells, utilization of drugs that target the PI3K-AKT-mTOR pathway, such as OSI-027, AZD2014, and NVP-BEZ235, inhibited cell proliferation in everolimus-resistant cells [107]. Although this has not previously been studied, a possible strategy to overcome everolimus resistance could involve an alternating therapy regimen allowing for re-sensitization of the mTOR pathway or targeting another related pathway such as PI3K. More clinical research is needed in the various approaches for overcoming everolimus-resistance in order to optimize its disease stabilizing effect.
3.4. Receptor Tyrosine Kinase Inhibitors: Sunitinib
Sunitinib is a small-molecule, multi-targeted receptor tyrosine kinase (RTK) with activity against multiple RTKs including vascular endothelial growth factor receptors (VEGFR type 1 and 2), platelet-derived growth factor receptors (PDGFR-alpha and PDGFR-beta), stem cell factor receptor (KIT) [115]. Sunitinib was approved in 2011 by the US FDA for the treatment of progressive, well-differentiated pancreatic neuroendocrine tumors based upon the results of a pivotal phase III clinical trial (A6181111, NCT00428597) which was terminated early based upon early indications of PFS benefit favoring sunitinib over placebo [115,116,117]. In a retrospective, blinded analysis of the trial data performed by independent third-party radiologists, PFS was assessed as 12.6 (95% CI 11.1–20.6) months and 5.8 (95% CI 3.8–7.2) months for the sunitinib and placebo arms, respectively (HR 0.32; 95% CI 0.16–0.55; p < 0.0001) [118]. The activity of sunitinib in panNETs is thought to be attributable to the highly vascularized nature of these tumors which express a many pro-angiogenic molecules, including VEGF, fibroblast growth factor (FGF), and PDGF [3].
Development of secondary resistance to sunitinib as evidenced by progression after initial disease stabilization or response to this agent is frequently seen in pancreatic neuroendocrine tumors [119]. Multiple molecular mechanisms are thought to account for the development of this resistance including the activation of alternative pro-angiogenic pathways by panNET cells, hypoxia-induced changes to the tumor micro-environment, lysosomal sequestration of sunitinib, and the induction by sunitinib of tumor autophagy, thus promoting tumor cell survival and treatment resistance (Figure 2) [120,121,122,123,124].
Activation of alternative pro-angiogenic pathways is thought to be mediated through the hypoxia-induced activation of HIF-1α, resulting in activation of pathways associated with the expression of proangiogenic factors, such as FGFs, ephrins, angiopoietins, c-MET (hepatocyte growth factor receptor) or epithelial-mesenchymal transition (EMT) induction [125,126,127]. Alterations in the local tumor microenvironment leading to sunitinib resistance are multiple and include the hypoxia-induced recruitment of bone marrow-derived cells including vascular progenitors and pro-angiogenic myeloid cells and the recruitment of inflammatory tumor-associated macrophages. The latter may result in the secretion of multiple angiogenic and pro-inflammatory cytokines including IL-6, TNF-α, and IL-1 [128,129,130,131]. Lysosomal sequestration of sunitinib occurs as a result of the hydrophobic and weakly basic nature of the drug, allowing it to cross the liposomal membrane freely where it is protonated and thus sequestered and eventually degraded [132]. Finally, treatment with sunitinib has been demonstrated in panNET cell lines to induce autophagy, a process that may favor the development treatment resistance and more aggressive tumor behavior [120,121,133].
Addressing the activation of alternative pro-angiogenic pathways through the use of alternative TKIs with distinct and in some cases broader targets represents a particularly promising area of research. Surufatinib, is a TKI with activity against VEGFR1, VEGFR2, VEGFR3, FGFR1 and CSF-1R was demonstrated in the SANET-p and SANET-ep trials to have significant PFS benefit in both pancreatic and extrapancreatic GEP NETs [134,135]. Other TKIs with broad activity are also under active study at this time including cabozantinib (targeted against VEGFR1-3, MET, AXL) and lenvatinib (targeted against multiple VEGFR1-3, KIT and RET tyrosine kinases) (Table 1) [136,137]. Results of lenvatinib in grade 1 and 2 GEP-NETS, including those previously treated with sunitinib, have been particularly promising as reported in the TALENT trial in which ORR of 44.2% (panNET) and 16.4% (GI-NET) and median duration of response of 19.9 (8.4–30.8) and 33.9 (10.6–38.3) months in the panNET and GI-NET groups, respectively, was seen [138].
Disrupting cellular autophagy and lysosomal function have additionally been researched in the preclinical setting for the purpose of addressing TKI resistance and improving efficacy of these agents. In Wiedmer et al., addition of the autophagy inhibitor chloroquine to sunitinib treatment decreased tumor viability and reduced tumor burden in the Rip1Tag2 transgenic panNET mouse models compared to either treatment alone [120]. Lysosomal sequestration of sunitinib has been successfully targeted in vitro in renal and colon cancer cell lines utilizing bafilomycin A1, a specific inhibitor of vacuolar type-H+-ATPase and in breast cancer cell lines utilizing SB02024, a highly potent and selective inhibitor of vacuolar protein sorting 34 (Vps34) [139,140]. Further in vitro studies in panNET models have demonstrated that autophagic cell death can be promoted through epigenetic modification utilizing the combination of the pan-deacetylase inhibitor Panobinostat and bafilomycin [141].
3.5. Chemotherapy
Temozolomide is an orally active alkylating agent prodrug. Its cytotoxic mechanism of action involves delivering methyl groups to purine bases of DNA [142]. Temozolomide, and temozolomide-based chemotherapy combinations (particularly in combination with capecitabine), have demonstrated significant activity in panNETs. In a prospective, randomized phase II trial of temozolomide compared to temozolomide and capecitabine in patients with advanced panNETs, a median PFS benefit was seen with the combination at 22.7 months versus 14.4 months for temozolomide alone (HR = 0.58, p = 0.023). With regard to ORR, there were similar at 33.3% for the combination versus 27.8% for temozolomide alone (p = 0.47) [143].
Development of resistance by panNETs to treatment with temozolomide-based chemotherapy is well documented, however the mechanism of this is poorly understood [144]. In other tumors, particularly glioblastoma multiforme (GBM), expression of O6-methylguanine-DNA-methyltransferase (MGMT) by tumor cells allows for repair of temozolomide-mediated DNA damage, thus conferring treatment resistance [145]. As MGMT expression is suppressed by methylation of the MGMT promoter, de-methylation of the MGMT promoter signifies an epigenetically derived resistance mechanism, linked to decreased survival and responsiveness to temozolomide in patients with GBM [146]. MGMT promoter methylation status is of uncertain significance with regard to alkylating agent treatment response in panNETs, with conflicting results demonstrated in several retrospective studies [147,148].
While the role of MGMT in the development of resistance to temozolomide-based chemotherapy remains unclear, multiple clinical trials attempting to augment the effect of this treatment are ongoing. Several of these studies are focused on combining temozolomide with other DNA-damaging agents including Lutetium Lu 177 Dotatate (NCT05247905), the poly ADP ribose polymerase (PARP) inhibitors talazoparib (NCT05142241), and Yttrium-90 radioisotope treatment (NCT04339036) (Table 1).
3.6. Immune Therapies for GEP-NETs
Immune therapies utilizing immune checkpoint inhibitors (PD-1/PD-L1, CTLA-4), cellular and acellular vaccines, and chimeric antigen receptor (CAR) T-cell therapies have recently revolutionized the treatment of multiple hematologic and solid tumor malignancies. Unfortunately, these same promising results have yet to be reproduced for the treatment of GEP-NETs. In a subgroup analysis of the CA209-538 prospective clinical trial evaluating the combination of the CTLA-4/PD-1 inhibitors ipilimumab/nivolumab in patients with advanced rare cancers, treatment with these immune checkpoint inhibitors demonstrated an encouraging ORR of 24% in 29 patients with neuroendocrine neoplasms [149]. Further studies have yet to replicate these promising results and a retrospective analysis conducted by Al-Toubah et al. reported a more modest ORR of 14.7%, with responses entirely limited to those patients with poorly differentiated NECs [150]. While the monotherapy activity of immune checkpoint inhibitors has thus far been disappointing, the potential synergistic combination of TKI agents with immune checkpoint inhibitors for the treatment of NETs is a highly active area of clinical research. Combinations under active study include the addition of cabozantinib to ipilimumab and nivolumab (NCT04079712), lenvatinib to pembrolizumab (NCT03290079), and surufatinib to tislelizumab (NCT04579757) (Table 1).
Bi-specific T-cell engagers (BiTEs) are a novel immune therapy consisting of a synthetic bispecific monoclonal antibody with two single-chain variable fragments, one binding to a T cell via the CD3 receptor, and the other to a tumor cell via a tumor specific molecule. Through the co-localization of cytotoxic T cells to neoplastic cells, these therapies have demonstrated exciting immune activation and anti-tumor activity in both hematologic and solid tumor malignancies [151,152]. Considering the limited efficacy of immune therapies thus far observed in GEP-NETs, BiTEs represent an encouraging avenue of research to overcome the immune-evading nature of these diseases. One such BiTE, XmAb18087 (tidutamab), a humanized anti–SSTR2, anti-CD3 bispecific antibody, demonstrated significant T cell activation and SSTR2+ cell depletion within in vitro and in vivo NET models [153]. The results of this preclinical study informed the development of a phase 1 clinical trial evaluating XmAb18087 in advanced grade 1 and 2 NETs (NCT03411915). Preliminary results of this study were presented at the 2020 North American Neuroendocrine Tumor Society Annual Symposium, where it was reported that XmAb18087 treatment resulted in a best response of stable disease in 6/14 (43%) evaluable patients, and treatment was accompanied by an acceptable adverse event profile and sustained T-cell activation in peripheral blood [154].
Similarly to BiTEs, CAR-T cell therapies have shown remarkable efficacy in the treatment of multiple hematologic malignancies [155,156,157,158]. While CAR-T cells have yet to be evaluated in clinical trials for patients with NETs, CDH17-targeted CAR-T cells have shown excellent preclinical in vitro and in vivo activity against gastrointestinal cancer xenografts and autochthonous mouse models including NETs with minimal toxicity to non-malignant tissues [159]. While still early in development, the potential use of CDH17-targeted CAR-T cells represents an exciting potential avenue to enhance immune therapies for GEP-NETs in the future.
4. Conclusions
GEP-NETs are a heterogenous group of rare neoplasms with an increasing incidence and few effective therapeutic options for patients with advanced or metastatic disease. This lack of available therapies is only made more challenging in these difficult to treat malignancies by the frequent and sometimes rapid development of resistance to the common therapies utilized. This article summarizes the current research landscape to elucidate the various molecular mechanisms GEP-NETs utilized for the development of treatment resistance.
Notable mechanisms include epigenetic modification and protein expression alteration, SSTR internalization or phosphorylation, alteration of alternative cellular feedback or DNA repair pathways, mutations to drug binding sites, lysosomal sequestration of drugs, and drug-induced cellular autophagy. While these mechanisms result in significant challenges in treatment GEP-NET patients, they also provide important information for possible means of optimizing the treatment effect or re-inducing sensitivity of existing therapies while also providing a potential guidance in the development of novel therapeutic agents.
Further research into these various mechanisms of treatment resistance and means of overcoming them could allow for the development of improved and personalized treatment approaches to patient’s unique tumors. Several clinical trials are underway to optimize tumor response to each of the common therapies, offering promising improvements in the future management of GEP-NETs.
Author Contributions
Conceptualization, G.J.P.; writing—manuscript preparation, K.M. and G.J.P.; writing—review and editing, K.M., G.J.P., E.Y.C., A.K., C.D.L., J.D.R., N.M., F.G.R., Y.K., R.P. and E.M.; visualization, K.M. and G.J.P.; supervision, G.J.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pavel, M.; Oberg, K.; Falconi, M.; Krenning, E.P.; Sundin, A.; Perren, A.; Berruti, A. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 844–860. [Google Scholar] [CrossRef] [PubMed]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Cives, M.; Strosberg, J.R. Gastroenteropancreatic Neuroendocrine Tumors. CA Cancer J. Clin. 2018, 68, 471–487. [Google Scholar] [CrossRef] [PubMed]
- Simasi, J.; Schubert, A.; Oelkrug, C.; Gillissen, A.; Nieber, K. Primary and Secondary Resistance to Tyrosine Kinase Inhibitors in Lung Cancer. Anticancer. Res. 2014, 34, 2841. [Google Scholar] [PubMed]
- Leoncini, E.; Boffetta, P.; Shafir, M.; Aleksovska, K.; Boccia, S.; Rindi, G. Increased incidence trend of low-grade and high-grade neuroendocrine neoplasms. Endocrine 2017, 58, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Fraenkel, M.; Kim, M.; Faggiano, A.; de Herder, W.W.; Valk, G.D.; Knowledge, N. Incidence of gastroenteropancreatic neuroendocrine tumours: A systematic review of the literature. Endocr. Relat. Cancer 2014, 21, R153–R163. [Google Scholar] [CrossRef]
- Hallet, J.; Law, C.H.; Cukier, M.; Saskin, R.; Liu, N.; Singh, S. Exploring the rising incidence of neuroendocrine tumors: A population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer 2015, 121, 589–597. [Google Scholar] [CrossRef]
- Dillon, J.S. Workup of Gastroenteropancreatic Neuroendocrine Tumors. Surg. Oncol. Clin. N. Am. 2020, 29, 165–183. [Google Scholar] [CrossRef]
- Frilling, A.; Akerström, G.; Falconi, M.; Pavel, M.; Ramos, J.; Kidd, M.; Modlin, I.M. Neuroendocrine tumor disease: An evolving landscape. Endocr. Relat. Cancer 2012, 19, R163–R185. [Google Scholar] [CrossRef]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef]
- Shi, H.; Chen, L.; Zhang, Q.; Lin, Y.; Jiang, C.; Yao, H.; Hou, X.; Chen, M.; Lin, R.; Chen, J. Concordance Between the Ki-67 Index Cutoff Value of 55% and Differentiation in Neuroendocrine Tumor and Neuroendocrine Carcinoma in Grade 3 Pancreatic Neuroendocrine Neoplasms. Pancreas 2020, 49, 1378–1382. [Google Scholar] [CrossRef] [PubMed]
- Hauck, L.; Bitzer, M.; Malek, N.; Plentz, R.R. Subgroup analysis of patients with G2 gastroenteropancreatic neuroendocrine tumors. Scand. J. Gastroenterol. 2016, 51, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Poeppel, T.D.; Binse, I.; Petersenn, S.; Lahner, H.; Schott, M.; Antoch, G.; Brandau, W.; Bockisch, A.; Boy, C. 68Ga-DOTATOC Versus 68Ga-DOTATATE PET/CT in Functional Imaging of Neuroendocrine Tumors. J. Nucl. Med. 2011, 52, 1864. [Google Scholar] [CrossRef] [PubMed]
- Network, N.C.C. Neuroendocrine and Adrenal Tumors (Version 3.2021). Available online: https://www.nccn.org/professionals/physician_gls/pdf/neuroendocrine.pdf (accessed on 1 June 2022).
- Halperin, D.M.; Shen, C.; Dasari, A.; Xu, Y.; Chu, Y.; Zhou, S.; Shih, Y.T.; Yao, J.C. Frequency of carcinoid syndrome at neuroendocrine tumour diagnosis: A population-based study. Lancet Oncol. 2017, 18, 525–534. [Google Scholar] [CrossRef]
- Gorelik, M.; Ahmad, M.; Grossman, D.; Grossman, M.; Cooperman, A.M. Nonfunctioning Incidental Pancreatic Neuroendocrine Tumors: Who, When, and How to Treat? Surg. Clin. N. Am. 2018, 98, 157–167. [Google Scholar] [CrossRef]
- Di Domenico, A.; Wiedmer, T.; Marinoni, I.; Perren, A. Genetic and epigenetic drivers of neuroendocrine tumours (NET). Endocr. Relat. Cancer 2017, 24, R315–R334. [Google Scholar] [CrossRef]
- Mafficini, A.; Scarpa, A. Genetics and Epigenetics of Gastroenteropancreatic Neuroendocrine Neoplasms. Endocr. Rev. 2019, 40, 506–536. [Google Scholar] [CrossRef]
- Jiao, Y.; Shi, C.; Edil, B.H.; de Wilde, R.F.; Klimstra, D.S.; Maitra, A.; Schulick, R.D.; Tang, L.H.; Wolfgang, C.L.; Choti, M.A.; et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science 2011, 331, 1199–1203. [Google Scholar] [CrossRef]
- Marinoni, I.; Kurrer, A.S.; Vassella, E.; Dettmer, M.; Rudolph, T.; Banz, V.; Hunger, F.; Pasquinelli, S.; Speel, E.J.; Perren, A. Loss of DAXX and ATRX are associated with chromosome instability and reduced survival of patients with pancreatic neuroendocrine tumors. Gastroenterology 2014, 146, 453–460.e455. [Google Scholar] [CrossRef]
- Singhi, A.D.; Liu, T.C.; Roncaioli, J.L.; Cao, D.; Zeh, H.J.; Zureikat, A.H.; Tsung, A.; Marsh, J.W.; Lee, K.K.; Hogg, M.E.; et al. Alternative Lengthening of Telomeres and Loss of DAXX/ATRX Expression Predicts Metastatic Disease and Poor Survival in Patients with Pancreatic Neuroendocrine Tumors. Clin. Cancer Res. 2017, 23, 600–609. [Google Scholar] [CrossRef]
- Francis, J.M.; Kiezun, A.; Ramos, A.H.; Serra, S.; Pedamallu, C.S.; Qian, Z.R.; Banck, M.S.; Kanwar, R.; Kulkarni, A.A.; Karpathakis, A.; et al. Somatic mutation of CDKN1B in small intestine neuroendocrine tumors. Nat. Genet. 2013, 45, 1483–1486. [Google Scholar] [CrossRef] [PubMed]
- Banck, M.S.; Kanwar, R.; Kulkarni, A.A.; Boora, G.K.; Metge, F.; Kipp, B.R.; Zhang, L.; Thorland, E.C.; Minn, K.T.; Tentu, R.; et al. The genomic landscape of small intestine neuroendocrine tumors. J. Clin. Investig. 2013, 123, 2502–2508. [Google Scholar] [CrossRef] [PubMed]
- Partelli, S.; Bartsch, D.K.; Capdevila, J.; Chen, J.; Knigge, U.; Niederle, B.; Nieveen van Dijkum, E.J.M.; Pape, U.F.; Pascher, A.; Ramage, J.; et al. ENETS Consensus Guidelines for Standard of Care in Neuroendocrine Tumours: Surgery for Small Intestinal and Pancreatic Neuroendocrine Tumours. Neuroendocrinology 2017, 105, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Caplin, M.E.; Pavel, M.; Ćwikła, J.B.; Phan, A.T.; Raderer, M.; Sedláčková, E.; Cadiot, G.; Wolin, E.M.; Capdevila, J.; Wall, L.; et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N. Engl. J. Med. 2014, 371, 224–233. [Google Scholar] [CrossRef]
- Rinke, A.; Müller, H.-H.; Schade-Brittinger, C.; Klose, K.-J.; Barth, P.; Wied, M.; Mayer, C.; Aminossadati, B.; Pape, U.-F.; Bläker, M.; et al. Placebo-Controlled, Double-Blind, Prospective, Randomized Study on the Effect of Octreotide LAR in the Control of Tumor Growth in Patients With Metastatic Neuroendocrine Midgut Tumors: A Report From the PROMID Study Group. J. Clin. Oncol. 2009, 27, 4656–4663. [Google Scholar] [CrossRef]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef]
- Yao, J.C.; Fazio, N.; Singh, S.; Buzzoni, R.; Carnaghi, C.; Wolin, E.; Tomasek, J.; Raderer, M.; Lahner, H.; Voi, M.; et al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): A randomised, placebo-controlled, phase 3 study. Lancet 2016, 387, 968–977. [Google Scholar] [CrossRef]
- Cives, M.; Pelle, E.; Strosberg, J. Emerging Treatment Options for Gastroenteropancreatic Neuroendocrine Tumors. J. Clin. Med. 2020, 9, 3655. [Google Scholar] [CrossRef]
- Perez, K.; Chan, J. Treatment of Gastroenteropancreatic Neuroendocrine Tumors. Surg. Pathol. Clin. 2019, 12, 1045–1053. [Google Scholar] [CrossRef]
- Al-Toubah, T.E.; Gile, J.; Strosberg, J.R.; Halfdanarson, T.R. Efficacy of ipilimumab and nivolumab in patients with high-grade neuroendocrine neoplasms. J. Clin. Oncol. 2021, 39, 370. [Google Scholar] [CrossRef]
- Wolin, E.M.; Jarzab, B.; Eriksson, B.; Walter, T.; Toumpanakis, C.; Morse, M.A.; Tomassetti, P.; Weber, M.M.; Fogelman, D.R.; Ramage, J.; et al. Phase III study of pasireotide long-acting release in patients with metastatic neuroendocrine tumors and carcinoid symptoms refractory to available somatostatin analogues. Drug. Des. Devel. Ther. 2015, 9, 5075–5086. [Google Scholar] [CrossRef] [PubMed]
- Schmid, H.A. Pasireotide (SOM230): Development, mechanism of action and potential applications. Mol. Cell. Endocrinol. 2008, 286, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Appetecchia, M.; Baldelli, R. Somatostatin analogues in the treatment of gastroenteropancreatic neuroendocrine tumours, current aspects and new perspectives. J. Exp. Clin. Cancer Res. 2010, 29, 19. [Google Scholar] [CrossRef]
- Bousquet, C.; Lasfargues, C.; Chalabi, M.; Billah, S.M.; Susini, C.; Vezzosi, D.; Caron, P.; Pyronnet, S. Current Scientific Rationale for the Use of Somatostatin Analogs and mTOR Inhibitors in Neuroendocrine Tumor Therapy. J. Clin. Endocrinol. Metab. 2012, 97, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Buchan, A.M.J.; Lin, C.-Y.; Choi, J.; Barber, D.L. Somatostatin, Acting at Receptor Subtype 1, Inhibits Rho Activity, the Assembly of Actin Stress Fibers, and Cell Migration. J. Biol. Chem. 2002, 277, 28431–28438. [Google Scholar] [CrossRef] [PubMed]
- Pola, S.; Cattaneo, M.G.; Vicentini, L.M. Anti-migratory and anti-invasive effect of somatostatin in human neuroblastoma cells: Involvement of Rac and MAP kinase activity. J. Biol. Chem. 2003, 278, 40601–40606. [Google Scholar] [CrossRef] [PubMed]
- Pyronnet, S.; Bousquet, C.; Najib, S.; Azar, R.; Laklai, H.; Susini, C. Antitumor effects of somatostatin. Mol. Cell. Endocrinol. 2008, 286, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Takekoshi, S.; Johbu, I.; Umemura, S.; Shoji, S.; Terachi, T.; Osamura, R.Y. Somatostatin analogue inhibits the mobility of prostate carcinoma cells: A new therapeutic method for advanced prostate carcinoma. Int. J. Oncol. 2010, 37, 1077–1083. [Google Scholar] [CrossRef][Green Version]
- Laklai, H.; Laval, S.; Dumartin, L.; Rochaix, P.; Hagedorn, M.; Bikfalvi, A.; Le Guellec, S.; Delisle, M.-B.; Schally, A.V.; Susini, C.; et al. Thrombospondin-1 is a critical effector of oncosuppressive activity of sst2 somatostatin receptor on pancreatic cancer. Proc. Natl. Acad. Sci. USA 2009, 106, 17769–17774. [Google Scholar] [CrossRef]
- Mei, S.; Cammalleri, M.; Azara, D.; Casini, G.; Bagnoli, P.; Dal Monte, M. Mechanisms underlying somatostatin receptor 2 down-regulation of vascular endothelial growth factor expression in response to hypoxia in mouse retinal explants. J. Pathol. 2012, 226, 519–533. [Google Scholar] [CrossRef]
- Rubin, J.; Ajani, J.; Schirmer, W.; Venook, A.P.; Bukowski, R.; Pommier, R.; Saltz, L.; Dandona, P.; Anthony, L. Octreotide acetate long-acting formulation versus open-label subcutaneous octreotide acetate in malignant carcinoid syndrome. J. Clin. Oncol. 1999, 17, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Ruszniewski, P.; Ducreux, M.; Chayvialle, J.A.; Blumberg, J.; Cloarec, D.; Michel, H.; Raymond, J.M.; Dupas, J.L.; Gouerou, H.; Jian, R.; et al. Treatment of the carcinoid syndrome with the longacting somatostatin analogue lanreotide: A prospective study in 39 patients. Gut 1996, 39, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Laskaratos, F.-M.; Walker, M.; Naik, K.; Maragkoudakis, E.; Oikonomopoulos, N.; Grant, L.; Meyer, T.; Caplin, M.; Toumpanakis, C. Predictive factors of antiproliferative activity of octreotide LAR as first-line therapy for advanced neuroendocrine tumours. Br. J. Cancer 2016, 115, 1321–1327. [Google Scholar] [CrossRef] [PubMed]
- Kulke, M.H.; Hörsch, D.; Caplin, M.E.; Anthony, L.B.; Bergsland, E.; Öberg, K.; Welin, S.; Warner, R.R.; Lombard-Bohas, C.; Kunz, P.L.; et al. Telotristat Ethyl, a Tryptophan Hydroxylase Inhibitor for the Treatment of Carcinoid Syndrome. J. Clin. Oncol. 2017, 35, 14–23. [Google Scholar] [CrossRef]
- Torrisani, J.; Hanoun, N.; Laurell, H.; Lopez, F.; Maoret, J.J.; Souque, A.; Susini, C.; Cordelier, P.; Buscail, L. Identification of an upstream promoter of the human somatostatin receptor, hSSTR2, which is controlled by epigenetic modifications. Endocrinology 2008, 149, 3137–3147. [Google Scholar] [CrossRef]
- Taelman, V.F.; Radojewski, P.; Marincek, N.; Ben-Shlomo, A.; Grotzky, A.; Olariu, C.I.; Perren, A.; Stettler, C.; Krause, T.; Meier, L.P.; et al. Upregulation of Key Molecules for Targeted Imaging and Therapy. J. Nucl. Med. 2016, 57, 1805–1810. [Google Scholar] [CrossRef]
- Veenstra, M.J.; van Koetsveld, P.M.; Dogan, F.; Farrell, W.E.; Feelders, R.A.; Lamberts, S.W.J.; de Herder, W.W.; Vitale, G.; Hofland, L.J. Epidrug-induced upregulation of functional somatostatin type 2 receptors in human pancreatic neuroendocrine tumor cells. Oncotarget 2016, 9, 14791–14802. [Google Scholar] [CrossRef]
- Wanek, J.; Gaisberger, M.; Beyreis, M.; Mayr, C.; Helm, K.; Primavesi, F.; Jäger, T.; Di Fazio, P.; Jakab, M.; Wagner, A.; et al. Pharmacological Inhibition of Class IIA HDACs by LMK-235 in Pancreatic Neuroendocrine Tumor Cells. Int. J. Mol. Sci. 2018, 19, 3128. [Google Scholar] [CrossRef]
- Pedraza-Arevalo, S.; Ibáñez-Costa, A.; Blázquez-Encinas, R.; Branco, M.R.; Vázquez-Borrego, M.C.; Herrera-Martínez, A.D.; Venegas-Moreno, E.; Serrano-Blanch, R.; Arjona-Sánchez, Á.; Gálvez-Moreno, M.A.; et al. Epigenetic and post-transcriptional regulation of somatostatin receptor subtype 5 (SST5) in pituitary and pancreatic neuroendocrine tumors. Mol. Oncol. 2022, 16, 764–779. [Google Scholar] [CrossRef]
- Guenter, R.; Aweda, T.; Carmona Matos, D.M.; Jang, S.; Whitt, J.; Cheng, Y.Q.; Liu, X.M.; Chen, H.; Lapi, S.E.; Jaskula-Sztul, R. Overexpression of somatostatin receptor type 2 in neuroendocrine tumors for improved Ga68-DOTATATE imaging and treatment. Surgery 2020, 167, 189–196. [Google Scholar] [CrossRef]
- Jin, X.F.; Auernhammer, C.J.; Ilhan, H.; Lindner, S.; Nolting, S.; Maurer, J.; Spottl, G.; Orth, M. Combination of 5-Fluorouracil with Epigenetic Modifiers Induces Radiosensitization, Somatostatin Receptor 2 Expression, and Radioligand Binding in Neuroendocrine Tumor Cells In Vitro. J. Nucl. Med. 2019, 60, 1240–1246. [Google Scholar] [CrossRef] [PubMed]
- Arvidsson, Y.; Johanson, V.; Pfragner, R.; Wängberg, B.; Nilsson, O. Cytotoxic Effects of Valproic Acid on Neuroendocrine Tumour Cells. Neuroendocrinology 2016, 103, 578–591. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Qian, Q.; Sun, G.; Mackey, L.V.; Fuselier, J.A.; Coy, D.H.; Yu, C.Y. Valproic acid induces NET cell growth arrest and enhances tumor suppression of the receptor-targeted peptide-drug conjugate via activating somatostatin receptor type II. J. Drug. Target 2016, 24, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Suraweera, A.; O’Byrne, K.J.; Richard, D.J. Combination Therapy With Histone Deacetylase Inhibitors (HDACi) for the Treatment of Cancer: Achieving the Full Therapeutic Potential of HDACi. Front. Oncol. 2018, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Christman, J. 5-Azacytidine and 5-aza-2′-deoxycytidine as inhibitors of DNA methylation: Mechanistic studies and their implications for cancer therapy. Oncogene 2002, 21, 5483–5495. [Google Scholar] [CrossRef]
- Lamberts, S.W.; Pieters, G.F.; Metselaar, H.J.; Ong, G.L.; Tan, H.S.; Reubi, J.C. Development of resistance to a long-acting somatostatin analogue during treatment of two patients with metastatic endocrine pancreatic tumours. Acta Endocrinol. 1988, 119, 561–566. [Google Scholar] [CrossRef]
- Beaumont, V.; Hepworth, M.B.; Luty, J.S.; Kelly, E.; Henderson, G. Somatostatin receptor desensitization in NG108-15 cells. A consequence of receptor sequestration. J. Biol. Chem. 1998, 273, 33174–33183. [Google Scholar] [CrossRef]
- Hipkin, R.W.; Friedman, J.; Clark, R.B.; Eppler, C.M.; Schonbrunn, A. Agonist-induced desensitization, internalization, and phosphorylation of the sst2A somatostatin receptor. J. Biol. Chem. 1997, 272, 13869–13876. [Google Scholar] [CrossRef]
- Hofland, L.J.; Lamberts, S.W. The pathophysiological consequences of somatostatin receptor internalization and resistance. Endocr. Rev. 2003, 24, 28–47. [Google Scholar] [CrossRef]
- Kvols, L.K.; Oberg, K.E.; O’Dorisio, T.M.; Mohideen, P.; de Herder, W.W.; Arnold, R.; Hu, K.; Zhang, Y.; Hughes, G.; Anthony, L.; et al. Pasireotide (SOM230) shows efficacy and tolerability in the treatment of patients with advanced neuroendocrine tumors refractory or resistant to octreotide LAR: Results from a phase II study. Endocr. Relat. Cancer 2012, 19, 657–666. [Google Scholar] [CrossRef]
- Yao, J.C.; Chan, J.A.; Mita, A.C.; Kundu, M.G.; Hermosillo Reséndiz, K.; Hu, K.; Ravichandran, S.; Strosberg, J.R.; Wolin, E.M. Phase I dose-escalation study of long-acting pasireotide in patients with neuroendocrine tumors. Onco. Targets Ther. 2017, 10, 3177–3186. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Markham, A. Telotristat Ethyl: First Global Approval. Drugs 2017, 77, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Kalshetty, A.; Ramaswamy, A.; Ostwal, V.; Basu, S. Resistant functioning and/or progressive symptomatic metastatic gastroenteropancreatic neuroendocrine tumors: Efficacy of 177Lu-DOTATATE peptide receptor radionuclide therapy in this setting. Nucl. Med. Commun. 2018, 39, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Hennrich, U.; Kopka, K. Lutathera(®): The First FDA- and EMA-Approved Radiopharmaceutical for Peptide Receptor Radionuclide Therapy. Pharmaceuticals 2019, 12, 114. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.R.; Caplin, M.E.; Kunz, P.L.; Ruszniewski, P.B.; Bodei, L.; Hendifar, A.; Mittra, E.; Wolin, E.M.; Yao, J.C.; Pavel, M.E.; et al. 177Lu-Dotatate plus long-acting octreotide versus high-dose long-acting octreotide in patients with midgut neuroendocrine tumours (NETTER-1): Final overall survival and long-term safety results from an open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021, 22, 1752–1763. [Google Scholar] [CrossRef]
- Binderup, T.; Knigge, U.; Johnbeck, C.B.; Loft, A.; Berthelsen, A.K.; Oturai, P.; Mortensen, J.; Federspiel, B.; Langer, S.W.; Kjaer, A. (18)F-FDG PET is Superior to WHO Grading as a Prognostic Tool in Neuroendocrine Neoplasms and Useful in Guiding PRRT: A Prospective 10-Year Follow-up Study. J. Nucl. Med. 2021, 62, 808–815. [Google Scholar] [CrossRef]
- Ohlendorf, F.; Werner, R.A.; Henkenberens, C.; Ross, T.L.; Christiansen, H.; Bengel, F.M.; Derlin, T. Predictive and Prognostic Impact of Blood-Based Inflammatory Biomarkers in Patients with Gastroenteropancreatic Neuroendocrine Tumors Commencing Peptide Receptor Radionuclide Therapy. Diagnostics 2021, 11, 504. [Google Scholar] [CrossRef]
- Ortega, C.; Wong, R.K.S.; Schaefferkoetter, J.; Veit-Haibach, P.; Myrehaug, S.; Juergens, R.; Laidley, D.; Anconina, R.; Liu, A.; Metser, U. Quantitative (68)Ga-DOTATATE PET/CT Parameters for the Prediction of Therapy Response in Patients with Progressive Metastatic Neuroendocrine Tumors Treated with (177)Lu-DOTATATE. J. Nucl. Med. 2021, 62, 1406–1414. [Google Scholar] [CrossRef]
- Satapathy, S.; Bhattacharya, A.; Sood, A.; Kapoor, R.; Gupta, R.; Sood, A.; Sharma, P.; Khosla, D.; Mittal, B.R. Hematological Markers as Predictors of Treatment Outcomes with Lutetium 177 ((177)Lu)-DOTATATE in Patients with Advanced Neuroendocrine Tumors. Cancer Biother. Radiopharm. 2022, 37, 23–29. [Google Scholar] [CrossRef]
- Swiha, M.M.; Sutherland, D.E.K.; Sistani, G.; Khatami, A.; Abazid, R.M.; Mujoomdar, A.; Wiseman, D.P.; Romsa, J.G.; Reid, R.H.; Laidley, D.T. Survival predictors of (177)Lu-Dotatate peptide receptor radionuclide therapy (PRRT) in patients with progressive well-differentiated neuroendocrine tumors (NETS). J. Cancer Res. Clin. Oncol. 2022, 148, 225–236. [Google Scholar] [CrossRef]
- Teker, F.; Elboga, U. Is SUVmax a useful marker for progression-free survival in patients with metastatic GEP-NET receiving (177)Lu-DOTATATE therapy? Hell. J. Nucl. Med. 2021, 24, 122–131. [Google Scholar] [CrossRef]
- Vaghaiwalla, T.; Ruhle, B.; Memeh, K.; Angelos, P.; Kaplan, E.; Liao, C.Y.; Polite, B.; Keutgen, X. Response rates in metastatic neuroendocrine tumors receiving peptide receptor radionuclide therapy and implications for future treatment strategies. Surgery 2021, 169, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Waldeck, K.; Van Zuylekom, J.; Blyth, B.; Cullinane, C.; Pattison, A.; Flynn, A.; Tothill, R.; Hicks, R. Modelling resistance and sensitivity to PRRT. In Proceedings of the 2020 NETRF Virtual Research Symposium, 19–20 November 2020. [Google Scholar]
- Seifert, V.; Richter, S.; Bechmann, N.; Bachmann, M.; Ziegler, C.G.; Pietzsch, J.; Ullrich, M. HIF2alpha-Associated Pseudohypoxia Promotes Radioresistance in Pheochromocytoma: Insights from 3D Models. Cancers 2021, 13, 385. [Google Scholar] [CrossRef] [PubMed]
- Jonasch, E.; Donskov, F.; Iliopoulos, O.; Rathmell, W.K.; Narayan, V.K.; Maughan, B.L.; Oudard, S.; Else, T.; Maranchie, J.K.; Welsh, S.J.; et al. Belzutifan for Renal Cell Carcinoma in von Hippel-Lindau Disease. N. Engl. J. Med. 2021, 385, 2036–2046. [Google Scholar] [CrossRef] [PubMed]
- Kunikowska, J.; Krolicki, L. Targeted alpha-Emitter Therapy of Neuroendocrine Tumors. Semin. Nucl. Med. 2020, 50, 171–176. [Google Scholar] [CrossRef]
- Kratochwil, C.; Giesel, F.L.; Bruchertseifer, F.; Mier, W.; Apostolidis, C.; Boll, R.; Murphy, K.; Haberkorn, U.; Morgenstern, A. (2)(1)(3)Bi-DOTATOC receptor-targeted alpha-radionuclide therapy induces remission in neuroendocrine tumours refractory to beta radiation: A first-in-human experience. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 2106–2119. [Google Scholar] [CrossRef]
- Hicks, R.J.; Jackson, P.; Kong, G.; Ware, R.E.; Hofman, M.S.; Pattison, D.A.; Akhurst, T.A.; Drummond, E.; Roselt, P.; Callahan, J.; et al. (64)Cu-SARTATE PET Imaging of Patients with Neuroendocrine Tumors Demonstrates High Tumor Uptake and Retention, Potentially Allowing Prospective Dosimetry for Peptide Receptor Radionuclide Therapy. J. Nucl. Med. 2019, 60, 777–785. [Google Scholar] [CrossRef]
- Claringbold, P.G.; Brayshaw, P.A.; Price, R.A.; Turner, J.H. Phase II study of radiopeptide 177Lu-octreotate and capecitabine therapy of progressive disseminated neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 302–311. [Google Scholar] [CrossRef]
- Van Essen, M.; Krenning, E.P.; Kam, B.L.; de Herder, W.W.; van Aken, M.O.; Kwekkeboom, D.J. Report on short-term side effects of treatments with 177Lu-octreotate in combination with capecitabine in seven patients with gastroenteropancreatic neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 743–748. [Google Scholar] [CrossRef]
- Claringbold, P.G.; Price, R.A.; Turner, J.H. Phase I-II study of radiopeptide 177Lu-octreotate in combination with capecitabine and temozolomide in advanced low-grade neuroendocrine tumors. Cancer Biother. Radiopharm. 2012, 27, 561–569. [Google Scholar] [CrossRef]
- Yordanova, A.; Ahrens, H.; Feldmann, G.; Brossart, P.; Gaertner, F.C.; Fottner, C.; Weber, M.M.; Ahmadzadehfar, H.; Schreckenberger, M.; Miederer, M.; et al. Peptide Receptor Radionuclide Therapy Combined With Chemotherapy in Patients With Neuroendocrine Tumors. Clin. Nucl. Med. 2019, 44, e329–e335. [Google Scholar] [CrossRef] [PubMed]
- Aljubran, A.H.; Alrowaili, M.; Raef, H.; Bazarbashi, S.; Alzahrani, A.M.; Almuhaideb, A.; Almanea, H.; Badran, A.a.; Al-Dalee, A.; Tuli, M. Combination of everolimus and lu-177 PRRT in treatment of G1-2 neuroendocrine tumors (NET): Phase 1-2 study. J. Clin. Oncol. 2019, 37, 386. [Google Scholar] [CrossRef]
- Claringbold, P.G.; Turner, J.H. NeuroEndocrine Tumor Therapy with Lutetium-177-octreotate and Everolimus (NETTLE): A Phase I Study. Cancer Biother. Radiopharm. 2015, 30, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, R.; Hofman, M.S.; Michael, M.; Kong, G.; Akhurst, T.; Eu, P.; Zannino, D.; Hicks, R.J. Favourable outcomes of (177)Lu-octreotate peptide receptor chemoradionuclide therapy in patients with FDG-avid neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 176–185. [Google Scholar] [CrossRef]
- Bae, J.H.; Kim, J.G.; Heo, K.; Yang, K.; Kim, T.O.; Yi, J.M. Identification of radiation-induced aberrant hypomethylation in colon cancer. BMC Genom. 2015, 16, 56. [Google Scholar] [CrossRef]
- Cabrera-Licona, A.; Perez-Anorve, I.X.; Flores-Fortis, M.; Moral-Hernandez, O.D.; Gonzalez-de la Rosa, C.H.; Suarez-Sanchez, R.; Chavez-Saldana, M.; Arechaga-Ocampo, E. Deciphering the epigenetic network in cancer radioresistance. Radiother. Oncol. 2021, 159, 48–59. [Google Scholar] [CrossRef]
- Kim, E.H.; Park, A.K.; Dong, S.M.; Ahn, J.H.; Park, W.Y. Global analysis of CpG methylation reveals epigenetic control of the radiosensitivity in lung cancer cell lines. Oncogene 2010, 29, 4725–4731. [Google Scholar] [CrossRef]
- Pollard, J.H.; Menda, Y.; Zamba, K.D.; Madsen, M.; O’Dorisio, M.S.; O’Dorisio, T.; Bushnell, D. Potential for Increasing Uptake of Radiolabeled (68)Ga-DOTATOC and (123)I-MIBG in Patients with Midgut Neuroendocrine Tumors Using a Histone Deacetylase Inhibitor Vorinostat. Cancer Biother. Radiopharm. 2021, 36, 632–641. [Google Scholar] [CrossRef]
- Exner, S.; Arrey, G.; Prasad, V.; Grotzinger, C. mTOR Inhibitors as Radiosensitizers in Neuroendocrine Neoplasms. Front. Oncol. 2020, 10, 578380. [Google Scholar] [CrossRef]
- Cullinane, C.; Waldeck, K.; Kirby, L.; Rogers, B.E.; Eu, P.; Tothill, R.W.; Hicks, R.J. Enhancing the anti-tumour activity of (177)Lu-DOTA-octreotate radionuclide therapy in somatostatin receptor-2 expressing tumour models by targeting PARP. Sci. Rep. 2020, 10, 10196. [Google Scholar] [CrossRef]
- Purohit, N.K.; Shah, R.G.; Adant, S.; Hoepfner, M.; Shah, G.M.; Beauregard, J.M. Potentiation of (177)Lu-octreotate peptide receptor radionuclide therapy of human neuroendocrine tumor cells by PARP inhibitor. Oncotarget 2018, 9, 24693–24706. [Google Scholar] [CrossRef] [PubMed]
- Wild, D.; Fani, M.; Fischer, R.; Del Pozzo, L.; Kaul, F.; Krebs, S.; Fischer, R.; Rivier, J.E.; Reubi, J.C.; Maecke, H.R.; et al. Comparison of somatostatin receptor agonist and antagonist for peptide receptor radionuclide therapy: A pilot study. J. Nucl. Med. 2014, 55, 1248–1252. [Google Scholar] [CrossRef] [PubMed]
- Ginj, M.; Zhang, H.; Waser, B.; Cescato, R.; Wild, D.; Wang, X.; Erchegyi, J.; Rivier, J.; Macke, H.R.; Reubi, J.C. Radiolabeled somatostatin receptor antagonists are preferable to agonists for in vivo peptide receptor targeting of tumors. Proc. Natl. Acad. Sci. USA 2006, 103, 16436–16441. [Google Scholar] [CrossRef] [PubMed]
- Reidy-Lagunes, D.; Pandit-Taskar, N.; O’Donoghue, J.A.; Krebs, S.; Staton, K.D.; Lyashchenko, S.K.; Lewis, J.S.; Raj, N.; Gonen, M.; Lohrmann, C.; et al. Phase I Trial of Well-Differentiated Neuroendocrine Tumors (NETs) with Radiolabeled Somatostatin Antagonist (177)Lu-Satoreotide Tetraxetan. Clin. Cancer Res. 2019, 25, 6939–6947. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.C.; Shah, M.H.; Ito, T.; Bohas, C.L.; Wolin, E.M.; Van Cutsem, E.; Hobday, T.J.; Okusaka, T.; Capdevila, J.; de Vries, E.G.; et al. Everolimus for advanced pancreatic neuroendocrine tumors. N. Engl. J. Med. 2011, 364, 514–523. [Google Scholar] [CrossRef]
- Yao, J.C.; Fazio, N.; Singh, S.; Buzzoni, R.; Carnaghi, C.; Wolin, E.M.; Tomasek, J.; Raderer, M.; Lahner, H.; Voi, M.; et al. Everolimus (EVE) in advanced, nonfunctional, well-differentiated neuroendocrine tumors (NET) of gastrointestinal (GI) or lung origin: Second interim overall survival (OS) results from the RADIANT-4 study. J. Clin. Oncol. 2016, 34, 4090. [Google Scholar] [CrossRef]
- Wang, X.; Yue, P.; Kim, Y.A.; Fu, H.; Khuri, F.R.; Sun, S.Y. Enhancing mammalian target of rapamycin (mTOR)-targeted cancer therapy by preventing mTOR/raptor inhibition-initiated, mTOR/rictor-independent Akt activation. Cancer Res. 2008, 68, 7409–7418. [Google Scholar] [CrossRef]
- O’Reilly, K.E.; Rojo, F.; She, Q.B.; Solit, D.; Mills, G.B.; Smith, D.; Lane, H.; Hofmann, F.; Hicklin, D.J.; Ludwig, D.L.; et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006, 66, 1500–1508. [Google Scholar] [CrossRef]
- Beyens, M.; Vandamme, T.; Peeters, M.; Van Camp, G.; Op de Beeck, K. Resistance to targeted treatment of gastroenteropancreatic neuroendocrine tumors. Endocr. Relat. Cancer 2019, 26, R109–R130. [Google Scholar] [CrossRef]
- Vitali, E.; Boemi, I.; Tarantola, G.; Piccini, S.; Zerbi, A.; Veronesi, G.; Baldelli, R.; Mazziotti, G.; Smiroldo, V.; Lavezzi, E.; et al. Metformin and Everolimus: A Promising Combination for Neuroendocrine Tumors Treatment. Cancers 2020, 12, 2143. [Google Scholar] [CrossRef]
- Dasari, A.; Phan, A.; Gupta, S.; Rashid, A.; Yeung, S.C.; Hess, K.; Chen, H.; Tarco, E.; Chen, H.; Wei, C.; et al. Phase I study of the anti-IGF1R antibody cixutumumab with everolimus and octreotide in advanced well-differentiated neuroendocrine tumors. Endocr. Relat. Cancer 2015, 22, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Reidy-Lagunes, D.L.; Vakiani, E.; Segal, M.F.; Hollywood, E.M.; Tang, L.H.; Solit, D.B.; Pietanza, M.C.; Capanu, M.; Saltz, L.B. A phase 2 study of the insulin-like growth factor-1 receptor inhibitor MK-0646 in patients with metastatic, well-differentiated neuroendocrine tumors. Cancer 2012, 118, 4795–4800. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.R.; Chan, J.A.; Ryan, D.P.; Meyerhardt, J.A.; Fuchs, C.S.; Abrams, T.; Regan, E.; Brady, R.; Weber, J.; Campos, T.; et al. A multi-institutional, phase II open-label study of ganitumab (AMG 479) in advanced carcinoid and pancreatic neuroendocrine tumors. Endocr. Relat. Cancer 2013, 20, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Fazio, N.; Buzzoni, R.; Baudin, E.; Antonuzzo, L.; Hubner, R.A.; Lahner, H.; WW, D.E.H.; Raderer, M.; Teulé, A.; Capdevila, J.; et al. A Phase II Study of BEZ235 in Patients with Everolimus-resistant, Advanced Pancreatic Neuroendocrine Tumours. Anticancer. Res. 2016, 36, 713–719. [Google Scholar] [PubMed]
- Vandamme, T.; Beyens, M.; de Beeck, K.O.; Dogan, F.; van Koetsveld, P.M.; Pauwels, P.; Mortier, G.; Vangestel, C.; de Herder, W.; Van Camp, G.; et al. Long-term acquired everolimus resistance in pancreatic neuroendocrine tumours can be overcome with novel PI3K-AKT-mTOR inhibitors. Br. J. Cancer 2016, 114, 650–658. [Google Scholar] [CrossRef]
- Svejda, B.; Kidd, M.; Kazberouk, A.; Lawrence, B.; Pfragner, R.; Modlin, I.M. Limitations in small intestinal neuroendocrine tumor therapy by mTor kinase inhibition reflect growth factor-mediated PI3K feedback loop activation via ERK1/2 and AKT. Cancer 2011, 117, 4141–4154. [Google Scholar] [CrossRef]
- Carew, J.S.; Kelly, K.R.; Nawrocki, S.T. Mechanisms of mTOR inhibitor resistance in cancer therapy. Target. Oncol. 2011, 6, 17–27. [Google Scholar] [CrossRef]
- Mi, R.; Ma, J.; Zhang, D.; Li, L.; Zhang, H. Efficacy of combined inhibition of mTOR and ERK/MAPK pathways in treating a tuberous sclerosis complex cell model. J. Genet. Genom. 2009, 36, 355–361. [Google Scholar] [CrossRef]
- Han, J.; Liu, Y.; Yang, S.; Wu, X.; Li, H.; Wang, Q. MEK inhibitors for the treatment of non-small cell lung cancer. J. Hematol. Oncol. 2021, 14, 1. [Google Scholar] [CrossRef]
- Dumont, F.J.; Staruch, M.J.; Grammer, T.; Blenis, J.; Kastner, C.A.; Rupprecht, K.M. Dominant mutations confer resistance to the immunosuppressant, rapamycin, in variants of a T cell lymphoma. Cell. Immunol. 1995, 163, 70–79. [Google Scholar] [CrossRef]
- Serra, S.; Zheng, L.; Hassan, M.; Phan, A.T.; Woodhouse, L.J.; Yao, J.C.; Ezzat, S.; Asa, S.L. The FGFR4-G388R single-nucleotide polymorphism alters pancreatic neuroendocrine tumor progression and response to mTOR inhibition therapy. Cancer Res. 2012, 72, 5683–5691. [Google Scholar] [CrossRef] [PubMed]
- Perera, T.P.S.; Jovcheva, E.; Mevellec, L.; Vialard, J.; De Lange, D.; Verhulst, T.; Paulussen, C.; Van De Ven, K.; King, P.; Freyne, E.; et al. Discovery and Pharmacological Characterization of JNJ-42756493 (Erdafitinib), a Functionally Selective Small-Molecule FGFR Family Inhibitor. Mol. Cancer Ther. 2017, 16, 1010–1020. [Google Scholar] [CrossRef] [PubMed]
- Papaetis, G.S.; Syrigos, K.N. Sunitinib: A multitargeted receptor tyrosine kinase inhibitor in the era of molecular cancer therapies. BioDrugs 2009, 23, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, G.M.; Cortazar, P.; Zhang, J.J.; Tang, S.; Sridhara, R.; Murgo, A.; Justice, R.; Pazdur, R. FDA approval summary: Sunitinib for the treatment of progressive well-differentiated locally advanced or metastatic pancreatic neuroendocrine tumors. Oncologist 2012, 17, 1108–1113. [Google Scholar] [CrossRef] [PubMed]
- Raymond, E.; Dahan, L.; Raoul, J.L.; Bang, Y.J.; Borbath, I.; Lombard-Bohas, C.; Valle, J.; Metrakos, P.; Smith, D.; Vinik, A.; et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N. Engl. J. Med. 2011, 364, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Faivre, S.; Niccoli, P.; Castellano, D.; Valle, J.W.; Hammel, P.; Raoul, J.L.; Vinik, A.; Van Cutsem, E.; Bang, Y.J.; Lee, S.H.; et al. Sunitinib in pancreatic neuroendocrine tumors: Updated progression-free survival and final overall survival from a phase III randomized study. Ann. Oncol. 2017, 28, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Fazio, N.; Kulke, M.; Rosbrook, B.; Fernandez, K.; Raymond, E. Updated Efficacy and Safety Outcomes for Patients with Well-Differentiated Pancreatic Neuroendocrine Tumors Treated with Sunitinib. Target. Oncol. 2021, 16, 27–35. [Google Scholar] [CrossRef]
- Wiedmer, T.; Blank, A.; Pantasis, S.; Normand, L.; Bill, R.; Krebs, P.; Tschan, M.P.; Marinoni, I.; Perren, A. Autophagy Inhibition Improves Sunitinib Efficacy in Pancreatic Neuroendocrine Tumors via a Lysosome-dependent Mechanism. Mol. Cancer Ther. 2017, 16, 2502–2515. [Google Scholar] [CrossRef]
- Galluzzi, L.; Pietrocola, F.; Bravo-San Pedro, J.M.; Amaravadi, R.K.; Baehrecke, E.H.; Cecconi, F.; Codogno, P.; Debnath, J.; Gewirtz, D.A.; Karantza, V.; et al. Autophagy in malignant transformation and cancer progression. Embo J. 2015, 34, 856–880. [Google Scholar] [CrossRef]
- Azam, F.; Mehta, S.; Harris, A.L. Mechanisms of resistance to antiangiogenesis therapy. Eur. J. Cancer 2010, 46, 1323–1332. [Google Scholar] [CrossRef]
- Pozas, J.; San Román, M.; Alonso-Gordoa, T.; Pozas, M.; Caracuel, L.; Carrato, A.; Molina-Cerrillo, J. Targeting Angiogenesis in Pancreatic Neuroendocrine Tumors: Resistance Mechanisms. Int. J. Mol. Sci. 2019, 20, 4949. [Google Scholar] [CrossRef] [PubMed]
- Casanovas, O.; Hicklin, D.J.; Bergers, G.; Hanahan, D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005, 8, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Tijeras-Raballand, A.; Neuzillet, C.; Couvelard, A.; Serova, M.; de Gramont, A.; Hammel, P.; Raymond, E.; Faivre, S. Resistance to targeted therapies in pancreatic neuroendocrine tumors (PNETs): Molecular basis, preclinical data, and counteracting strategies. Target Oncol. 2012, 7, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Bergers, G.; Song, S.; Meyer-Morse, N.; Bergsland, E.; Hanahan, D. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J. Clin. Investig. 2003, 111, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Bergers, G.; Javaherian, K.; Lo, K.M.; Folkman, J.; Hanahan, D. Effects of angiogenesis inhibitors on multistage carcinogenesis in mice. Science 1999, 284, 808–812. [Google Scholar] [CrossRef] [PubMed]
- Wei, I.H.; Harmon, C.M.; Arcerito, M.; Cheng, D.F.; Minter, R.M.; Simeone, D.M. Tumor-associated macrophages are a useful biomarker to predict recurrence after surgical resection of nonfunctional pancreatic neuroendocrine tumors. Ann. Surg. 2014, 260, 1088–1094. [Google Scholar] [CrossRef]
- Krug, S.; Abbassi, R.; Griesmann, H.; Sipos, B.; Wiese, D.; Rexin, P.; Blank, A.; Perren, A.; Haybaeck, J.; Hüttelmaier, S.; et al. Therapeutic targeting of tumor-associated macrophages in pancreatic neuroendocrine tumors. Int. J. Cancer 2018, 143, 1806–1816. [Google Scholar] [CrossRef]
- Biswas, S.K.; Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat. Immunol. 2010, 11, 889–896. [Google Scholar] [CrossRef]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Azijli, K.; Gotink, K.J.; Verheul, H.M. The Potential Role of Lysosomal Sequestration in Sunitinib Resistance of Renal Cell Cancer. J. Kidney Cancer VHL 2015, 2, 195–203. [Google Scholar] [CrossRef]
- Daskalakis, K.; Alexandraki, K.I.; Kloukina, I.; Kassi, E.; Felekouras, E.; Xingi, E.; Pagakis, S.N.; Tsolakis, A.V.; Andreakos, E.; Kaltsas, G.; et al. Increased autophagy/mitophagy levels in primary tumours of patients with pancreatic neuroendocrine neoplasms. Endocrine 2020, 68, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Shen, L.; Bai, C.; Wang, W.; Li, J.; Yu, X.; Li, Z.; Li, E.; Yuan, X.; Chi, Y.; et al. Surufatinib in advanced pancreatic neuroendocrine tumours (SANET-p): A randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 1489–1499. [Google Scholar] [CrossRef]
- Xu, J.; Shen, L.; Zhou, Z.; Li, J.; Bai, C.; Chi, Y.; Li, Z.; Xu, N.; Li, E.; Liu, T.; et al. Surufatinib in advanced extrapancreatic neuroendocrine tumours (SANET-ep): A randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 1500–1512. [Google Scholar] [CrossRef]
- Grüllich, C. Cabozantinib: Multi-kinase Inhibitor of MET, AXL, RET, and VEGFR2. Recent Results Cancer Res. 2018, 211, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Suyama, K.; Iwase, H. Lenvatinib: A Promising Molecular Targeted Agent for Multiple Cancers. Cancer Control. 2018, 25, 1073274818789361. [Google Scholar] [CrossRef]
- Capdevila, J.; Fazio, N.; Lopez, C.; Teulé, A.; Valle, J.W.; Tafuto, S.; Custodio, A.; Reed, N.; Raderer, M.; Grande, E.; et al. Lenvatinib in Patients With Advanced Grade 1/2 Pancreatic and Gastrointestinal Neuroendocrine Tumors: Results of the Phase II TALENT Trial (GETNE1509). J. Clin. Oncol. 2021, 39, 2304–2312. [Google Scholar] [CrossRef]
- Gotink, K.J.; Broxterman, H.J.; Labots, M.; De Haas, R.R.; Dekker, H.; Honeywell, R.J.; Rudek, M.A.; Beerepoot, L.V.; Musters, R.J.; Jansen, G.; et al. Lysosomal Sequestration of Sunitinib: A Novel Mechanism of Drug Resistance. Clin. Cancer Res. 2011, 17, 7337–7346. [Google Scholar] [CrossRef]
- Dyczynski, M.; Yu, Y.; Otrocka, M.; Parpal, S.; Braga, T.; Henley, A.B.; Zazzi, H.; Lerner, M.; Wennerberg, K.; Viklund, J.; et al. Targeting autophagy by small molecule inhibitors of vacuolar protein sorting 34 (Vps34) improves the sensitivity of breast cancer cells to Sunitinib. Cancer Lett. 2018, 435, 32–43. [Google Scholar] [CrossRef]
- Matrood, S.; de Prisco, N.; Wissniowski, T.T.; Wiese, D.; Jabari, S.; Griesmann, H.; Wanzel, M.; Stiewe, T.; Neureiter, D.; Klieser, E.; et al. Modulation of Pancreatic Neuroendocrine Neoplastic Cell Fate by Autophagy-Mediated Death. Neuroendocrinology 2021, 111, 965–985. [Google Scholar] [CrossRef]
- Zhang, J.; Stevens, M.F.; Bradshaw, T.D. Temozolomide: Mechanisms of action, repair and resistance. Curr. Mol. Pharmacol. 2012, 5, 102–114. [Google Scholar] [CrossRef]
- Kunz, P.L.; Catalano, P.J.; Nimeiri, H.; Fisher, G.A.; Longacre, T.A.; Suarez, C.J.; Yao, J.C.; Kulke, M.H.; Hendifar, A.E.; Shanks, J.C.; et al. A randomized study of temozolomide or temozolomide and capecitabine in patients with advanced pancreatic neuroendocrine tumors: A trial of the ECOG-ACRIN Cancer Research Group (E2211). J. Clin. Oncol. 2018, 36, 4004. [Google Scholar] [CrossRef]
- Chatzellis, E.; Angelousi, A.; Daskalakis, K.; Tsoli, M.; Alexandraki, K.I.; Wachuła, E.; Meirovitz, A.; Maimon, O.; Grozinsky-Glasberg, S.; Gross, D.; et al. Activity and Safety of Standard and Prolonged Capecitabine/Temozolomide Administration in Patients with Advanced Neuroendocrine Neoplasms. Neuroendocrinology 2019, 109, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Parker, N.R.; Khong, P.; Parkinson, J.F.; Howell, V.M.; Wheeler, H.R. Molecular heterogeneity in glioblastoma: Potential clinical implications. Front. Oncol. 2015, 5, 55. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, S.; Song, C.; Zha, Y.; Li, L. The prognostic value of MGMT promoter status by pyrosequencing assay for glioblastoma patients’ survival: A meta-analysis. World J. Surg. Oncol. 2016, 14, 261. [Google Scholar] [CrossRef] [PubMed]
- Walter, T.; van Brakel, B.; Vercherat, C.; Hervieu, V.; Forestier, J.; Chayvialle, J.A.; Molin, Y.; Lombard-Bohas, C.; Joly, M.O.; Scoazec, J.Y. O6-Methylguanine-DNA methyltransferase status in neuroendocrine tumours: Prognostic relevance and association with response to alkylating agents. Br. J. Cancer 2015, 112, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Cives, M.; Ghayouri, M.; Morse, B.; Brelsford, M.; Black, M.; Rizzo, A.; Meeker, A.; Strosberg, J. Analysis of potential response predictors to capecitabine/temozolomide in metastatic pancreatic neuroendocrine tumors. Endocr. Relat. Cancer 2016, 23, 759–767. [Google Scholar] [CrossRef]
- Klein, O.; Kee, D.; Markman, B.; Michael, M.; Underhill, C.; Carlino, M.S.; Jackett, L.; Lum, C.; Scott, C.; Nagrial, A.; et al. Immunotherapy of Ipilimumab and Nivolumab in Patients with Advanced Neuroendocrine Tumors: A Subgroup Analysis of the CA209-538 Clinical Trial for Rare Cancers. Clin. Cancer Res. 2020, 26, 4454–4459. [Google Scholar] [CrossRef]
- Al-Toubah, T.; Halfdanarson, T.; Gile, J.; Morse, B.; Sommerer, K.; Strosberg, J. Efficacy of ipilimumab and nivolumab in patients with high-grade neuroendocrine neoplasms. ESMO Open 2022, 7, 100364. [Google Scholar] [CrossRef]
- Kantarjian, H.; Stein, A.; Gökbuget, N.; Fielding, A.K.; Schuh, A.C.; Ribera, J.-M.; Wei, A.; Dombret, H.; Foà, R.; Bassan, R.; et al. Blinatumomab versus Chemotherapy for Advanced Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2017, 376, 836–847. [Google Scholar] [CrossRef]
- Nathan, P.; Hassel, J.C.; Rutkowski, P.; Baurain, J.-F.; Butler, M.O.; Schlaak, M.; Sullivan, R.J.; Ochsenreither, S.; Dummer, R.; Kirkwood, J.M.; et al. Overall Survival Benefit with Tebentafusp in Metastatic Uveal Melanoma. N. Engl. J. Med. 2021, 385, 1196–1206. [Google Scholar] [CrossRef]
- Lee, S.-H.; Chu, S.Y.; Rashid, R.; Phung, S.; Leung, I.W.; Muchhal, U.S.; Moore, G.L.; Bernett, M.J.; Schubbert, S.; Ardila, C.; et al. Abstract 3633: Anti-SSTR2 × anti-CD3 bispecific antibody induces potent killing of human tumor cells in vitro and in mice, and stimulates target-dependent T cell activation in monkeys: A potential immunotherapy for neuroendocrine tumors. Cancer Res. 2017, 77, 3633. [Google Scholar] [CrossRef]
- El-Rayes, B.; Pant, S.; Villalobos, V.; Hendifar, A.; Chow, W.; Konda, B.; Reilley, M.; Benson, A.; Fisher, G.; Starr, J.; et al. Preliminary safety, PK/PD, and antitumor activity of XmAb18087, an SSTR2 x CD3 bispecific antibody, in patients with advanced neuroendocrine tumors. In Proceedings of the North American Neuroendocrine Tumor Society. 2020 NANETs Annual Symposium Virtual, 2–3 October 2020. [Google Scholar]
- Abramson, J.S.; Palomba, M.L.; Gordon, L.I.; Lunning, M.A.; Wang, M.; Arnason, J.; Mehta, A.; Purev, E.; Maloney, D.G.; Andreadis, C.; et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): A multicentre seamless design study. Lancet 2020, 396, 839–852. [Google Scholar] [CrossRef]
- Munshi, N.C.; Anderson, L.D.; Shah, N.; Madduri, D.; Berdeja, J.; Lonial, S.; Raje, N.; Lin, Y.; Siegel, D.; Oriol, A.; et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. N. Engl. J. Med. 2021, 384, 705–716. [Google Scholar] [CrossRef]
- Neelapu, S.S.; Locke, F.L.; Bartlett, N.L.; Lekakis, L.J.; Miklos, D.B.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y.; et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017, 377, 2531–2544. [Google Scholar] [CrossRef]
- Bishop, M.R.; Dickinson, M.; Purtill, D.; Barba, P.; Santoro, A.; Hamad, N.; Kato, K.; Sureda, A.; Greil, R.; Thieblemont, C.; et al. Second-Line Tisagenlecleucel or Standard Care in Aggressive B-Cell Lymphoma. N. Engl. J. Med. 2021, 386, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; He, X.; Zhang, X.; Wu, Y.; Xing, B.; Knowles, A.; Shan, Q.; Miller, S.; Hojnacki, T.; Ma, J.; et al. Potent suppression of neuroendocrine tumors and gastrointestinal cancers by CDH17CAR T cells without toxicity to normal tissues. Nat. Cancer 2022, 3, 581–594. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).