Biglycan Interacts with Type I Insulin-like Receptor (IGF-IR) Signaling Pathway to Regulate Osteosarcoma Cell Growth and Response to Chemotherapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Cells and Cell Culture
2.1.2. Transfection with siRNA
2.1.3. Proliferation Assay
2.1.4. RNA Isolation and Reverse Transcription Quantitative PCR (RT qPCR)
2.1.5. Protein Immunoprecipitation
2.1.6. Nuclear and Cytoplasmic Extract Separation
2.1.7. Western Blot Analysis
2.1.8. Immunofluorescence
2.1.9. Statistical Analysis
2.2. Modeling Biglycan-IGF-IR Complexes
2.2.1. Protein Structures
2.2.2. Protein Structure Refinement of Homology Models
2.2.3. Electrostatic Potential Calculations
2.2.4. Docking Using the ClusPro Server
2.2.5. MD of Docked Complexes
3. Results
3.1. Biglycan Forms a Complex with the Activated IGF-IR and Regulates Its Internalization and Nuclear Translocation
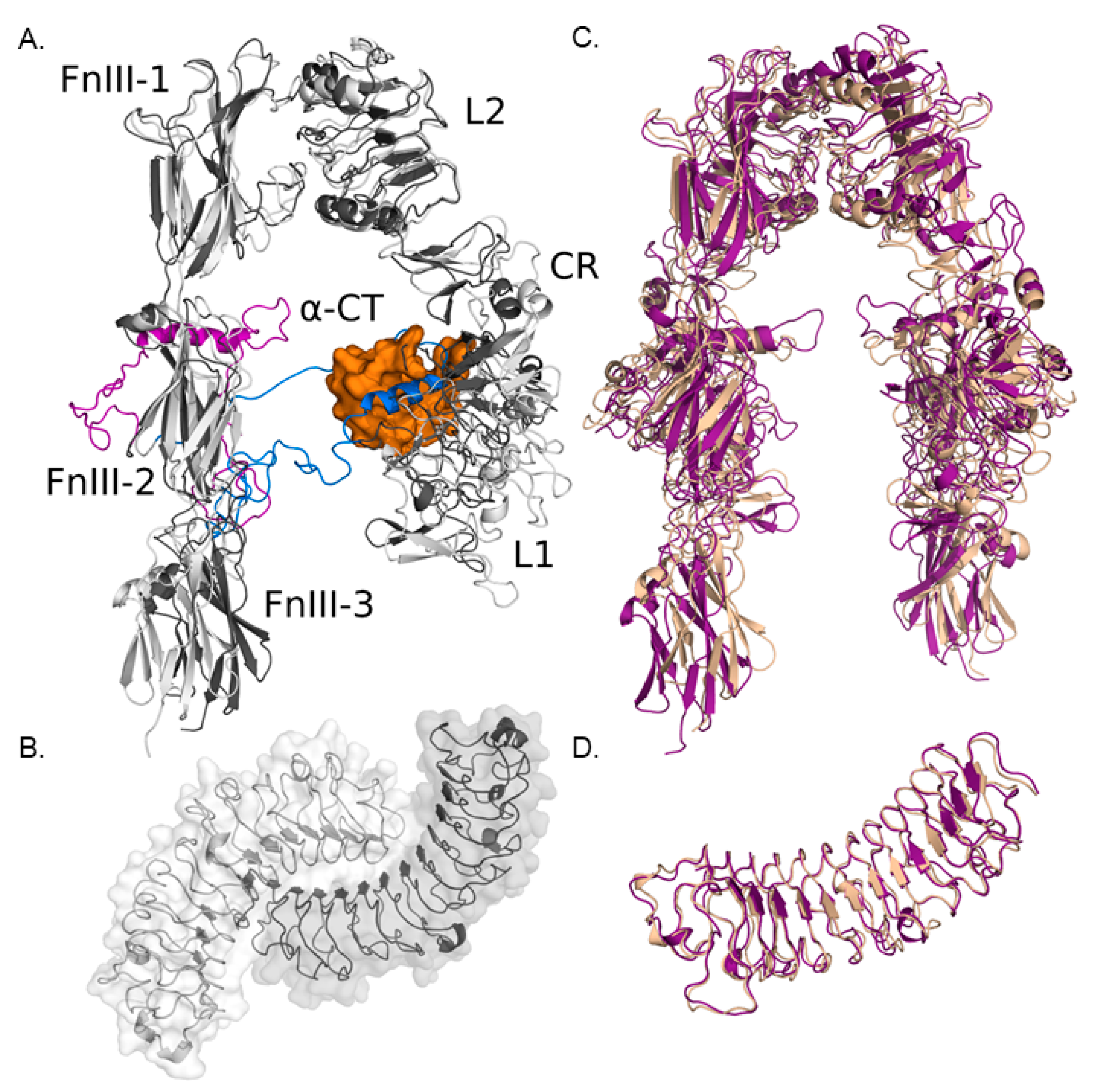
3.1.1. Modeling IGF-IR and Biglycan Structures
3.1.2. Computational Models of IGF-IR and Biglycan Docking
3.1.3. Effect of Biglycan Glycosylation on IGF-IR/Biglycan Docking
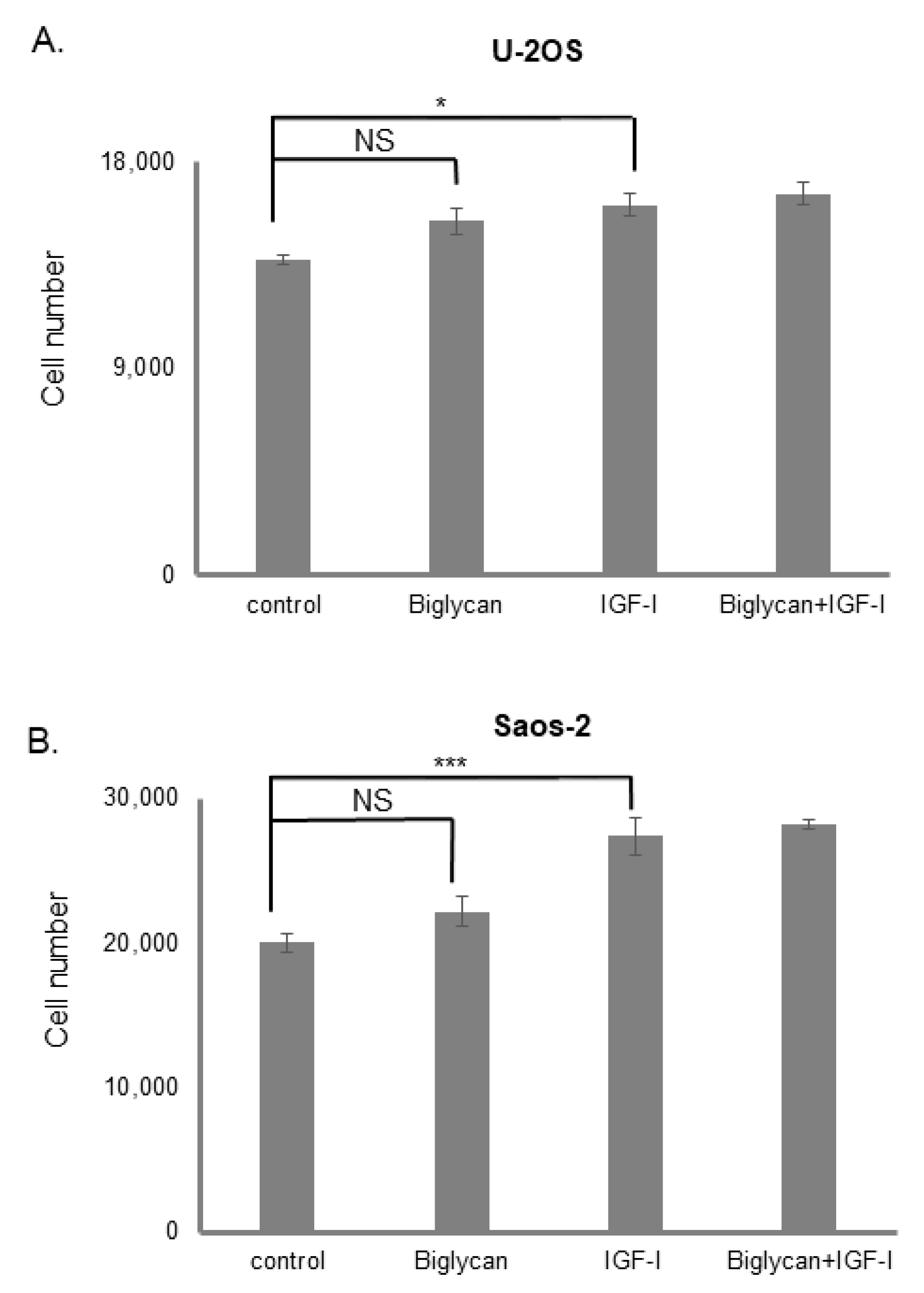
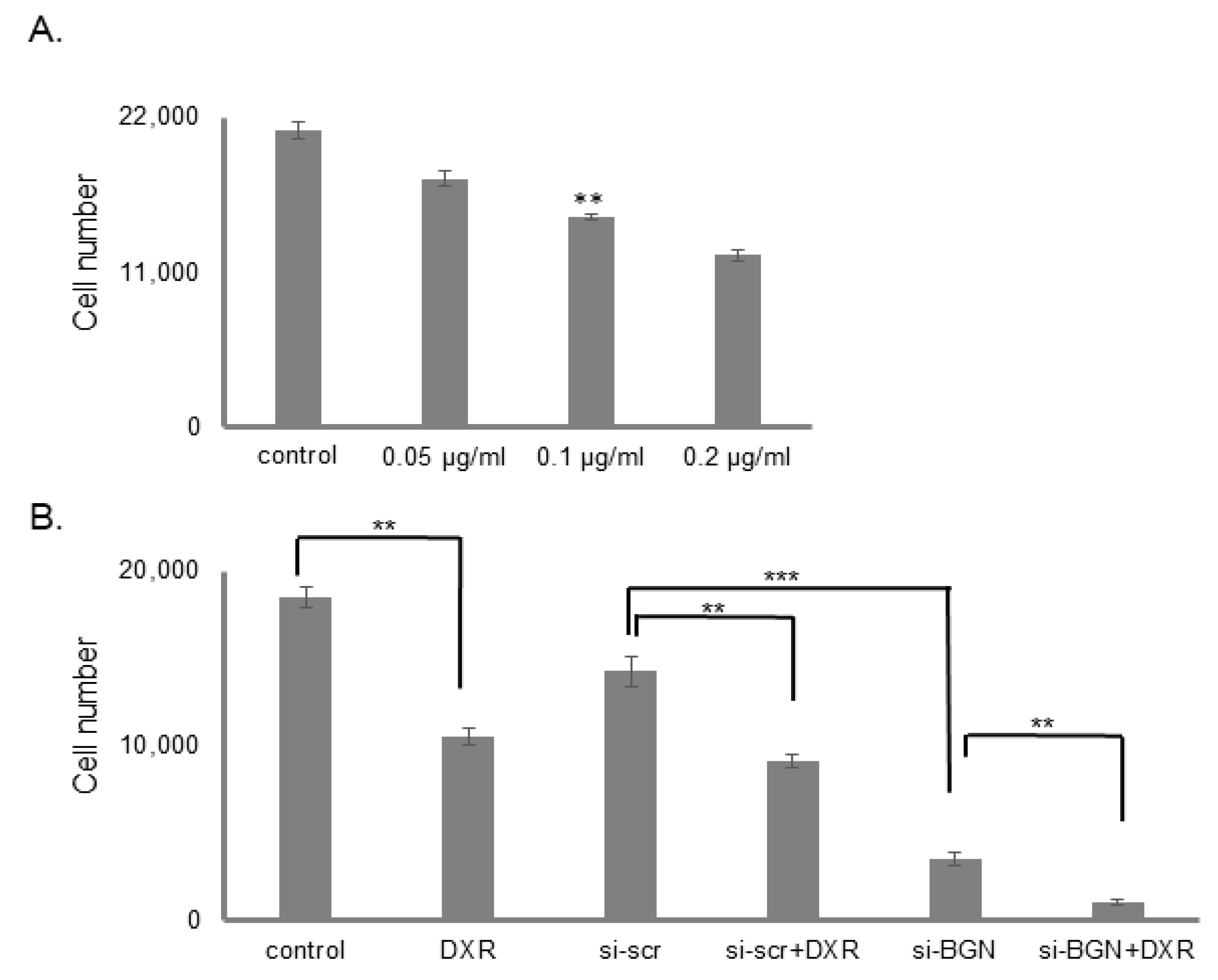
3.2. The Effect of Biglycan on Osteosarcoma Cell Growth Is Differentiation and Biglycan Expression Status Dependent
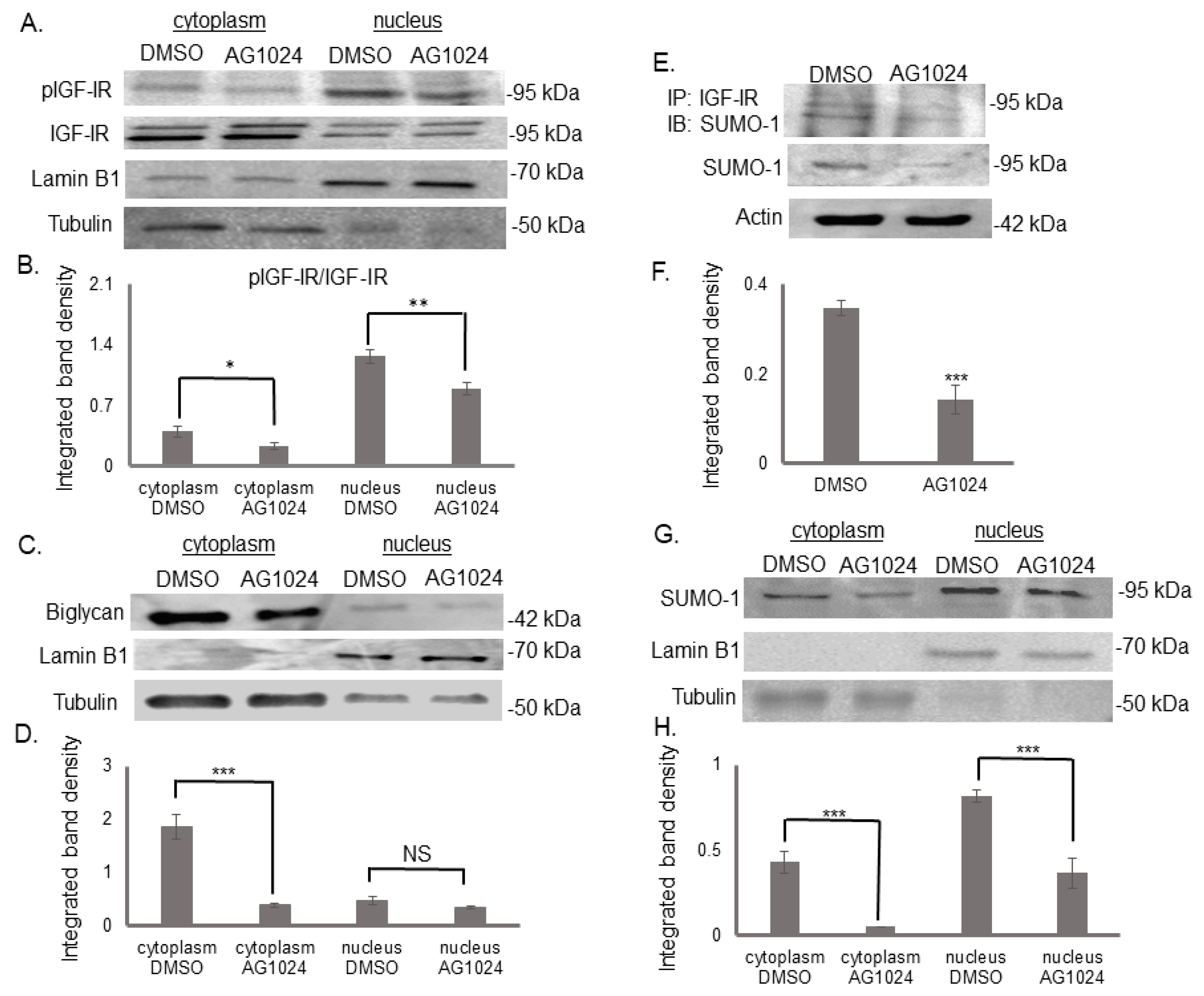
3.3. Biglycan Enhances IGF-IR Sumoylation
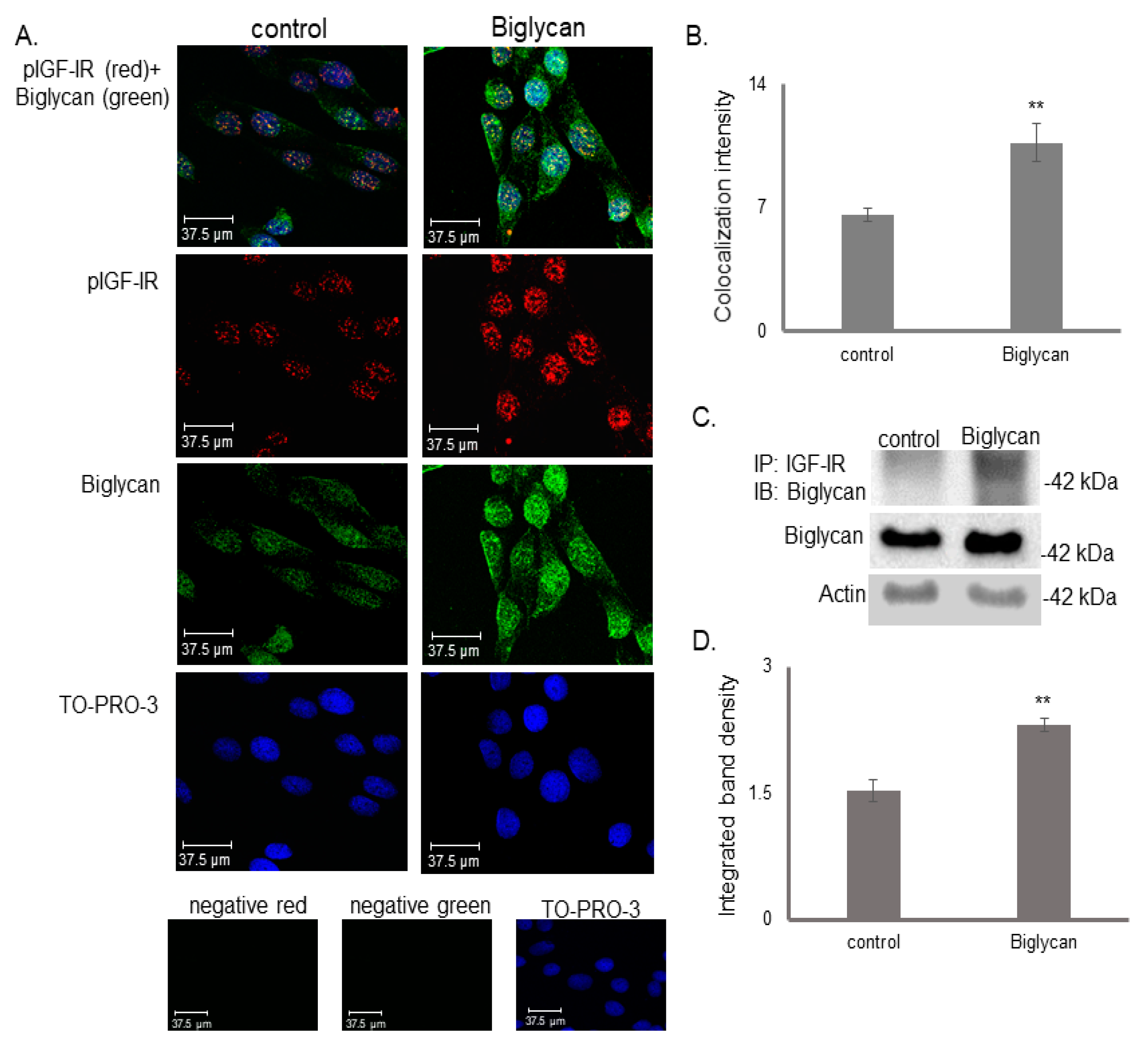
3.4. IGF-IR Colocalizes with DNA in a Biglycan-Dependent Manner
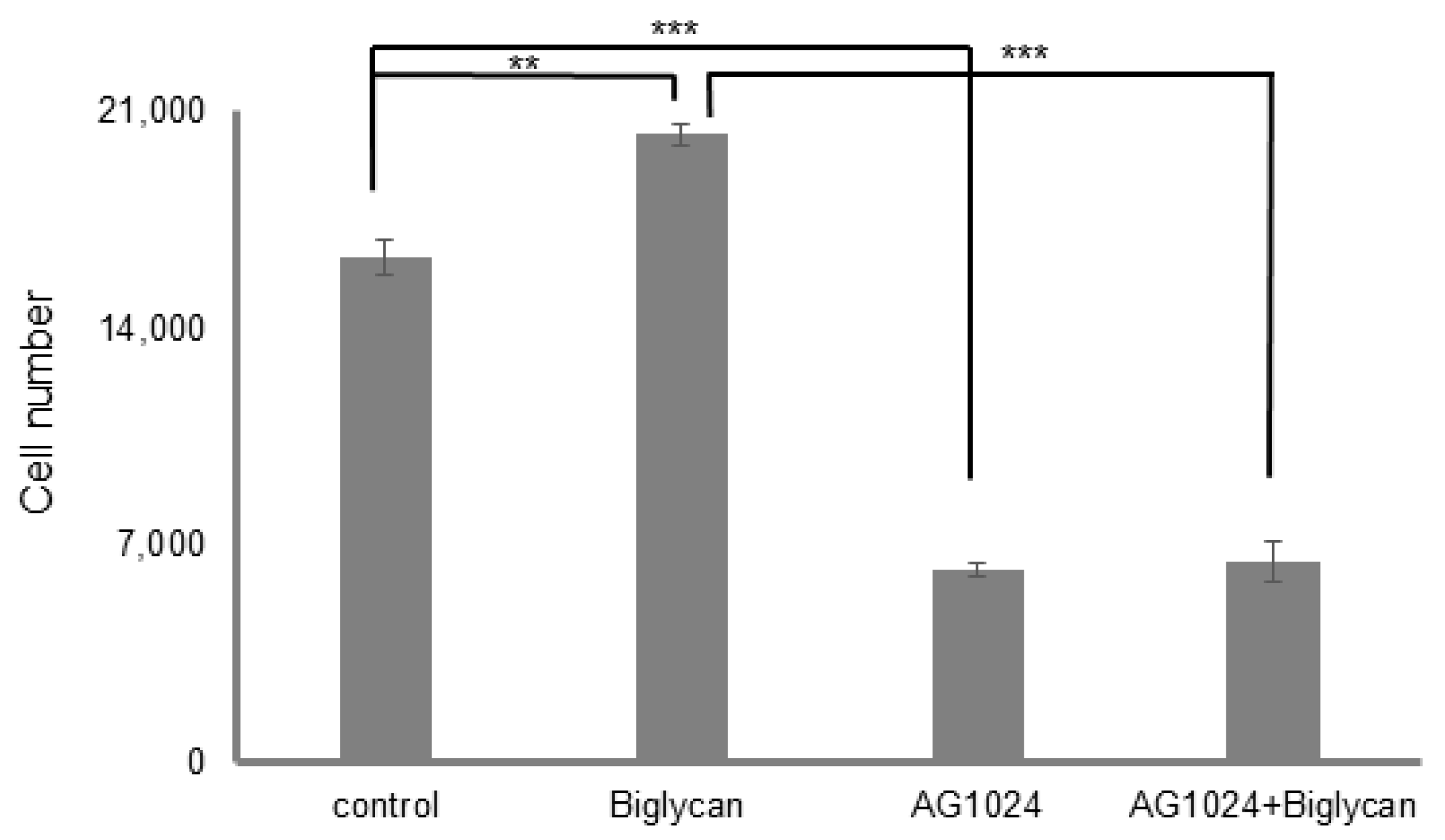
3.5. The Tyrosine Kinase Activity of IGF-IR Affects Its’ Cellular Localization and Sumoylation
3.6. Biglycan Promotes MG63 Osteosarcoma Cells’ Aggressive Phenotype
3.7. Biglycan Controls Chemosensitivity to Doxorubicin in MG63 Osteosarcoma Cells
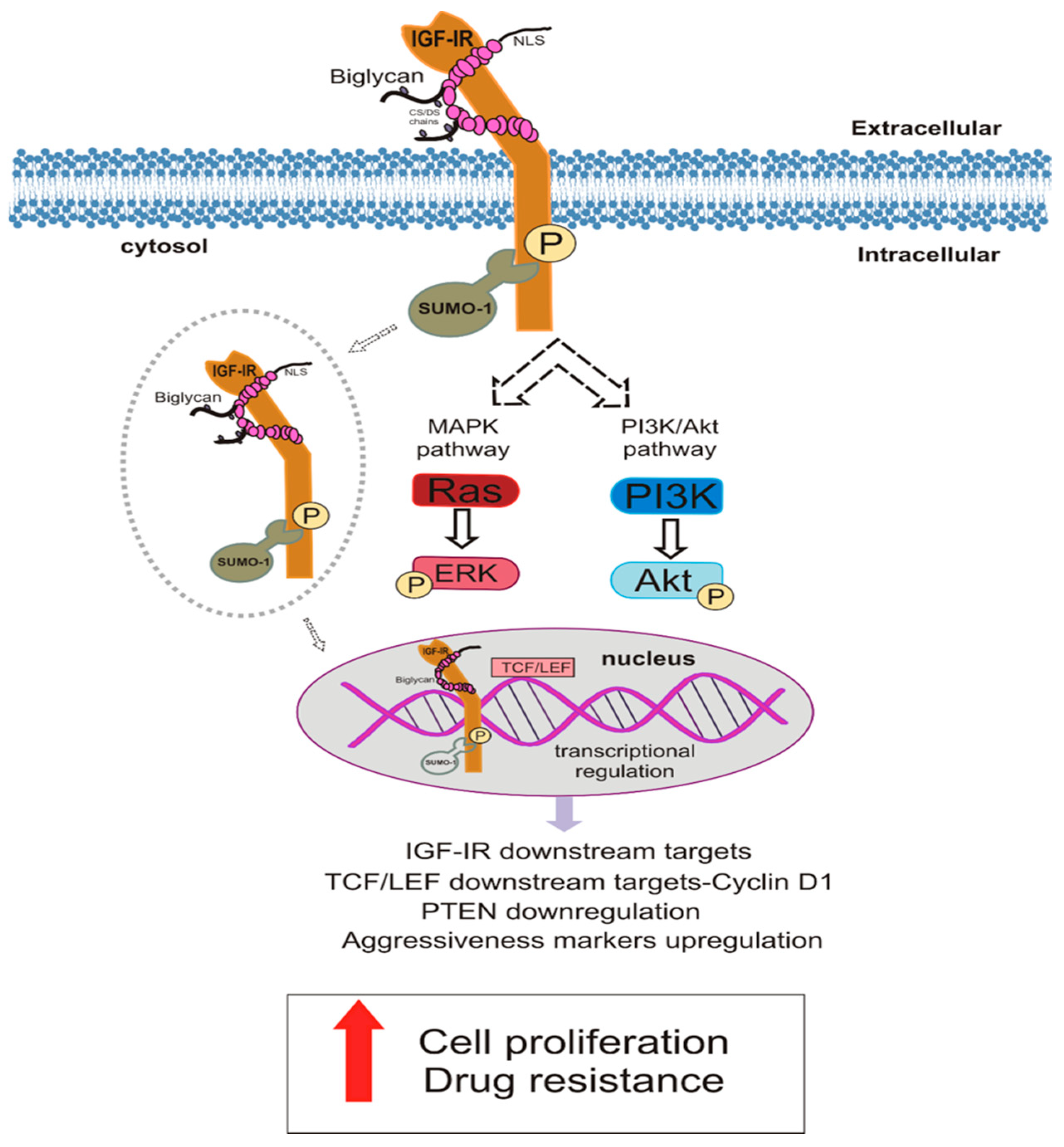
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jaffe, N.; Carrasco, H.; Raymond, K.; Ayala, A.; Eftekhari, F. Can cure in patients with osteosarcoma be achieved exclusively with chemotherapy and abrogation of surgery? Cancer 2002, 95, 2202–2210. [Google Scholar] [CrossRef] [PubMed]
- Griend, R.A.V. Osteosarcoma and its variants. Orthop. Clin. N. Am. 1996, 27, 575–581. [Google Scholar] [CrossRef]
- Benayahu, D.; Shur, I.; Marom, R.; Meller, I.; Issakov, J. Cellular and molecular properties associated with osteosarcoma cells. J. Cell. Biochem. 2002, 84, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Dean, D.; Hornicek, F.J.; Chen, Z.; Duan, Z. The role of extracelluar matrix in osteosarcoma progression and me-tastasis. J. Exp. Clin. Cancer Res. 2020, 39, 178. [Google Scholar] [CrossRef]
- Nikitovic, D.; Aggelidakis, J.; Young, M.F.; Iozzo, R.V.; Karamanos, N.K.; Tzanakakis, G.N. The biology of small leu-cine-rich proteoglycans in bone pathophysiology. J. Biol. Chem. 2012, 287, 33926–33933. [Google Scholar] [CrossRef]
- Couchman, J.R.; Pataki, C.A. An Introduction to Proteoglycans and Their Localization. J. Histochem. Cytochem. 2012, 60, 885–897. [Google Scholar] [CrossRef]
- Iozzo, R.V.; Schaefer, L. Proteoglycans in health and disease: Novel regulatory signaling mechanisms evoked by the small leucine-rich proteoglycans. FEBS J. 2010, 277, 3864–3875. [Google Scholar] [CrossRef]
- Tzanakakis, G.; Giatagana, E.-M.; Kuskov, A.; Berdiaki, A.; Tsatsakis, A.; Neagu, M.; Nikitovic, D. Proteoglycans in the Pathogenesis of Hormone-Dependent Cancers: Mediators and Effectors. Cancers 2020, 12, 2401. [Google Scholar] [CrossRef]
- Schaefer, L.; Iozzo, R.V. Biological Functions of the Small Leucine-rich Proteoglycans: From Genetics to Signal Transduction. J. Biol. Chem. 2008, 283, 21305–21309. [Google Scholar] [CrossRef]
- Neill, T.; Schaefer, L.; Iozzo, R.V. Decoding the Matrix: Instructive Roles of Proteoglycan Receptors. Biochemistry 2015, 54, 4583–4598. [Google Scholar] [CrossRef]
- Tzanakakis, G.; Giatagana, E.-M.; Berdiaki, A.; Spyridaki, I.; Hida, K.; Neagu, M.; Tsatsakis, A.; Nikitovic, D. The Role of IGF/IGF-IR-Signaling and Extracellular Matrix Effectors in Bone Sarcoma Pathogenesis. Cancers 2021, 13, 2478. [Google Scholar] [CrossRef] [PubMed]
- Zafiropoulos, A.; Nikitovic, D.; Katonis, P.; Tsatsakis, A.; Karamanos, N.K.; Tzanakakis, G.N. Decorin-Induced Growth Inhibition Is Overcome through Protracted Expression and Activation of Epidermal Growth Factor Receptors in Osteosarcoma Cells. Mol. Cancer Res. 2008, 6, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Papoutsidakis, A.; Giatagana, E.M.; Berdiaki, A.; Spyridaki, I.; Spandidos, D.A.; Tsatsakis, A.; Tzanakakis, G.N.; Nikitovic, D. Lumican mediates HTB94 chondrosarcoma cell growth via an IGFIR/Erk1/2 axis. Int. J. Oncol. 2020, 57, 791–803. [Google Scholar] [CrossRef] [PubMed]
- Kram, V.; Shainer, R.; Jani, P.; Meester, J.; Loeys, B.; Young, M.F. Biglycan in the Skeleton. J. Histochem. Cytochem. 2020, 68, 747–762. [Google Scholar] [CrossRef] [PubMed]
- McEwan, P.A.; Scott, P.G.; Bishop, P.; Bella, J. Structural correlations in the family of small leucine-rich repeat proteins and proteoglycans. J. Struct. Biol. 2006, 155, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Scott, P.G.; Dodd, C.M.; Bergmann, E.M.; Sheehan, J.K.; Bishop, P. Crystal Structure of the Biglycan Dimer and Evidence That Dimerization Is Essential for Folding and Stability of Class I Small Leucine-rich Repeat Proteoglycans. J. Biol. Chem. 2006, 281, 13324–13332. [Google Scholar] [CrossRef]
- Scott, I.C.; Imamura, Y.; Pappano, W.N.; Troedel, J.M.; Recklies, A.D.; Roughley, P.J.; Greenspan, D. Bone Morphogenetic Protein-1 Processes Probiglycan. J. Biol. Chem. 2000, 275, 30504–30511. [Google Scholar] [CrossRef]
- Nastase, M.V.; Young, M.F.; Schaefer, L. Biglycan: A multivalent proteoglycan providing structure and signals. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2012, 60, 963–975. [Google Scholar] [CrossRef]
- Desnoyers, L.; Arnott, D.; Pennica, D. WISP-1 Binds to Decorin and Biglycan. J. Biol. Chem. 2001, 276, 47599–47607. [Google Scholar] [CrossRef]
- Chen, X.D.; Fisher, L.W.; Robey, P.G.; Young, M.F. The small leucine-rich proteoglycan biglycan modulates BMP-4-induced osteoblast differentiation. FASEB J. 2004, 18, 948–958. [Google Scholar] [CrossRef]
- Mochida, Y.; Parisuthiman, D.; Yamauchi, M. Biglycan Is a Positive Modulator of BMP-2 Induced Osteoblast Differentiation. Adv. Exp. Med. Biol. 2006, 585, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, A.; Romaris, M.; Rasmussen, L.M.; Heinegard, D.; Twardzik, D.R.; Border, W.A.; Ruoslahti, E. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor beta. Bio-Chem. J. 1994, 302, 527–534. [Google Scholar]
- Tufvesson, E.; Westergren-Thorsson, G. Tumour necrosis factor-α interacts with biglycan and decorin. FEBS Lett. 2002, 530, 124–128. [Google Scholar] [CrossRef]
- Schaefer, L.; Tredup, C.; Gubbiotti, M.A.; Iozzo, R.V. Proteoglycan neofunctions: Regulation of inflammation and au-tophagy in cancer biology. FEBS J. 2017, 284, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, X. A Review of IGF1 Signaling and IGF1-related Long Noncoding RNAs in Chemoresistance of Cancer. Curr. Cancer Drug Targets 2020, 20, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Adams, T.E.; Epa, V.C.; Garrett, T.P.; Ward, C.W. Structure and function of the type 1 insulin-like growth factor receptor. Cell. Mol. Life Sci. 2000, 57, 1050–1093. [Google Scholar] [CrossRef]
- De Meyts, P. Insulin/receptor binding: The last piece of the puzzle? What recent progress on the structure of the insu-lin/receptor complex tells us (or not) about negative cooperativity and activation. BioEssays News Rev. Mol. Cell. Dev. Biol. 2015, 37, 389–397. [Google Scholar] [CrossRef]
- Taguchi, A.; White, M.F. Insulin-Like Signaling, Nutrient Homeostasis, and Life Span. Annu. Rev. Physiol. 2008, 70, 191–212. [Google Scholar] [CrossRef]
- Mytilinaiou, M.; Nikitovic, D.; Berdiaki, A.; Papoutsidakis, A.; Papachristou, D.J.; Tsatsakis, A.; Tzanakakis, G.N. IGF-I regulates HT1080 fibrosarcoma cell migration through a syndecan-2/Erk/ezrin signaling axis. Exp. Cell Res. 2017, 361, 9–18. [Google Scholar] [CrossRef]
- Aggelidakis, J.; Berdiaki, A.; Nikitovic, D.; Papoutsidakis, A.; Papachristou, D.J.; Tsatsakis, A.M.; Tzanakakis, G.N. Biglycan Regulates MG63 Osteosarcoma Cell Growth Through a LPR6/beta-Catenin/IGFR-IR Signaling Axis. Front. Oncol. 2018, 8, 470. [Google Scholar] [CrossRef]
- Jentzsch, T.; Robl, B.; Husmann, M.; Bode-Lesniewska, B.; Fuchs, B. Worse prognosis of osteosarcoma patients expressing IGF-1 on a tissue microarray. Anticancer Res. 2014, 34, 3881–3889. [Google Scholar] [PubMed]
- Liang, J.; Li, B.; Yuan, L.; Ye, Z. Prognostic value of IGF-1R expression in bone and soft tissue sarcomas: A meta-analysis. OncoTargets Ther. 2015, 8, 1949–1955. [Google Scholar]
- Dhadve, A.C.; Hari, K.; Rekhi, B.; Jolly, M.K.; De, A.; Ray, P. Decoding molecular interplay between RUNX1 and FOXO3a underlying the pulsatile IGF1R expression during acquirement of chemoresistance. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2020, 1866, 165754. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Yin, Z.; Tao, K.; Wang, G.; Gao, J. Function of insulin-like growth factor 1 receptor in cancer resistance to chem-otherapy. Oncol. Lett. 2018, 15, 41–47. [Google Scholar]
- Tian, X.; Hao, K.; Qin, C.; Xie, K.; Xie, X.; Yang, Y. Insulin-Like Growth Factor 1 Receptor Promotes the Growth and Chemoresistance of Pancreatic Cancer. Am. J. Dig. Dis. 2013, 58, 2705–2712. [Google Scholar] [CrossRef]
- Salisbury, T.B.; Tomblin, J.K. Insulin/Insulin-Like Growth Factors in Cancer: New Roles for the Aryl Hydrocarbon Receptor, Tumor Resistance Mechanisms, and New Blocking Strategies. Front. Endocrinol. 2015, 6, 12. [Google Scholar] [CrossRef][Green Version]
- Burrow, S.; Andrulis, I.L.; Pollak, M.; Bell, R.S. Expression of insulin-like growth factor receptor, IGF-1, and IGF-2 in primary and metastatic osteosarcoma. J. Surg. Oncol. 1998, 69, 21–27. [Google Scholar] [CrossRef]
- Singla, A.; Wang, J.; Yang, R.; Geller, D.S.; Loeb, D.M.; Hoang, B.H. Wnt Signaling in Osteosarcoma. Issues Clin. Epileptol. View Bench 2020, 1258, 125–139. [Google Scholar] [CrossRef]
- Mintz, M.B.; Sowers, R.; Brown, K.M.; Hilmer, S.C.; Mazza, B.; Huvos, A.G.; Meyers, P.; LaFleur, B.J.; McDonough, W.S.; Henry, M.M.; et al. An Expression Signature Classifies Chemotherapy-Resistant Pediatric Osteosarcoma. Cancer Res. 2005, 65, 1748–1754. [Google Scholar] [CrossRef]
- Xu, Y.; Kong, G.K.-W.; Menting, J.G.; Margetts, M.B.; Delaine, C.A.; Jenkin, L.M.; Kiselyov, V.V.; De Meyts, P.; Forbes, B.E.; Lawrence, M.C. How ligand binds to the type 1 insulin-like growth factor receptor. Nat. Commun. 2018, 9, 821. [Google Scholar] [CrossRef]
- Garrett, T.P.J.; McKern, N.M.; Lou, M.; Frenkel, M.J.; Bentley, J.D.; Lovrecz, G.O.; Elleman, T.C.; Cosgrove, L.J.; Ward, C.W. Crystal structure of the first three domains of the type-1 insulin-like growth factor receptor. Nature 1998, 394, 395–399. [Google Scholar] [CrossRef]
- Xu, L.; Tang, L.; Zhang, L. Proteoglycans as miscommunication biomarkers for cancer diagnosis. Prog. Mol. Biol. Transl. Sci. 2019, 162, 59–92. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; De Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Case, D.A.; Betz, R.M.; Cerutti, D.S.; Cheatham III, T.E.; Darden, T.A.; Duke, R.E.; Giese, T.J.; Gohlke, H.; Goetz, A.W.; Homeyer, N.; et al. University of California, San Francisco. 2016. Available online: https://ambermd.org/doc12/Amber16.pdf (accessed on 12 January 2021).
- Case, D.A.; Aktulga, H.M.; Belfon, K.; Ben-Shalom, I.Y.; Brozell, S.R.; Cerutti, D.S.; Cheatham, T.E., III; Cisneros, G.A.; Cruzeiro, V.W.D.; Darden, T.A.; et al. Amber University of California, San Francisco. 2021. Available online: https://ambermd.org/doc12/Amber21.pdf (accessed on 12 January 2021).
- Nguyen, H.; Maier, J.; Huang, H.; Perrone, V.; Simmerling, C. Folding Simulations for Proteins with Diverse Topologies Are Accessible in Days with a Physics-Based Force Field and Implicit Solvent. J. Am. Chem. Soc. 2014, 136, 13959–13962. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Samsonov, S.A.; Pisabarro, M.T. Computational analysis of interactions in structurally available pro-tein-glycosaminoglycan complexes. Glycobiology 2016, 26, 850–861. [Google Scholar] [CrossRef]
- Kozakov, D.; Hall, D.R.; Xia, B.; Porter, K.A.; Padhorny, D.; Yueh, C.; Beglov, D.; Vajda, S. The ClusPro web server for protein–protein docking. Nat. Protoc. 2017, 12, 255–278. [Google Scholar] [CrossRef]
- Onufriev, A.; Bashford, D.; Case, D.A. Modification of the Generalized Born Model Suitable for Macromolecules. J. Phys. Chem. B 2000, 104, 3712–3720. [Google Scholar] [CrossRef]
- Islam, M.; Gor, J.; Perkins, S.J.; Ishikawa, Y.; Bächinger, H.P.; Hohenester, E. The Concave Face of Decorin Mediates Reversible Dimerization and Collagen Binding. J. Biol. Chem. 2013, 288, 35526–35533. [Google Scholar] [CrossRef]
- Gubbiotti, M.A.; Vallet, S.D.; Ricard-Blum, S.; Iozzo, R.V. Decorin interacting network: A comprehensive analysis of decorin-binding partners and their versatile functions. Matrix Biol. 2016, 55, 7–21. [Google Scholar] [CrossRef]
- Goldoni, S.; Owens, R.T.; McQuillan, D.J.; Shriver, Z.; Sasisekharan, R.; Birk, D.E.; Campbell, S.; Iozzo, R.; Brill, J.; Klocke, R.; et al. Biologically Active Decorin Is a Monomer in Solution. J. Biol. Chem. 2004, 279, 6606–6612. [Google Scholar] [CrossRef]
- Qi, B.; Zhang, R.; Sun, R.; Guo, M.; Zhang, M.; Wei, G.; Zhang, L.; Yu, S.; Huang, H. IGF-1R inhibitor PQ401 inhibits oste-osarcoma cell proliferation, migration and colony formation. Int. J. Clin. Exp. Pathol. 2019, 12, 1589–1598. [Google Scholar] [PubMed]
- Mac Ewen, E.G.; Pastor, J.; Kutzke, J.; Tsan, R.; Kurzman, I.D.; Thamm, D.H.; Wilson, M.; Radinsky, R. IGF-1 receptor contributes to the malignant phenotype in human and canine osteosarcoma. J. Cell. Biochem. 2004, 92, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Jamwal, G.; Singh, G.; Dar, M.S.; Singh, P.; Bano, N.; Syed, S.H.; Sandhu, P.; Akhter, Y.; Monga, S.P.; Dar, M.J. Identification of a unique loss-of-function mutation in IGF1R and a crosstalk between IGF1R and Wnt/beta-catenin signaling pathways. Biochimica et biophysica acta. Mol. Cell Res. 2018, 1865, 920–931. [Google Scholar]
- Sehat, B.; Tofigh, A.; Lin, Y.; Trocme, E.; Liljedahl, U.; Lagergren, J.; Larsson, O. SUMOylation mediates the nuclear trans-location and signaling of the IGF-1 receptor. Sci. Signal. 2010, 3, ra10. [Google Scholar] [CrossRef]
- Warsito, D.; Sjostrom, S.; Andersson, S.; Larsson, O.; Sehat, B. Nuclear IGF1R is a transcriptional co-activator of LEF1/TCF. EMBO Rep. 2012, 13, 244–250. [Google Scholar] [CrossRef]
- Zheng, C.; Tang, F.; Min, L.; Hornicek, F.; Duan, Z.; Tu, C. PTEN in osteosarcoma: Recent advances and the therapeutic potential. Biochim. Biophys. Acta 2020, 1874, 188405. [Google Scholar] [CrossRef]
- Tian, K.; Di, R.; Wang, L. RETRACTED ARTICLE: MicroRNA-23a enhances migration and invasion through PTEN in osteosarcoma. Cancer Gene Ther. 2015, 22, 351–359. [Google Scholar] [CrossRef]
- Carrle, D.; Bielack, S.S. Current strategies of chemotherapy in osteosarcoma. Int. Orthop. 2006, 30, 445–451. [Google Scholar] [CrossRef]
- Hattinger, C.M.; Fanelli, M.; Tavanti, E.; Vella, S.; Riganti, C.; Picci, P.; Serra, M. Doxorubicin-resistant osteosarcoma: Novel therapeutic approaches in sight? Future Oncol. 2017, 13, 673–677. [Google Scholar] [CrossRef]
- Pan, S.; Cheng, L.; White, J.T.; Lu, W.; Utleg, A.G.; Yan, X.; Urban, N.D.; Drescher, C.W.; Hood, L.; Lin, B. Quantitative Proteomics Analysis Integrated with Microarray Data Reveals That Extracellular Matrix Proteins, Catenins, and P53 Binding Protein 1 Are Important for Chemotherapy Response in Ovarian Cancers. OMICS J. Integr. Biol. 2009, 13, 345–354. [Google Scholar] [CrossRef]
- Roedig, H.; Damiescu, R.; Zeng-Brouwers, J.; Kutija, I.; Trebicka, J.; Wygrecka, M.; Schaefer, L. Danger matrix molecules orchestrate CD14/CD44 signaling in cancer development. Semin. Cancer Biol. 2020, 62, 31–47. [Google Scholar] [CrossRef]
- Xing, X.; Gu, X.; Ma, T.; Ye, H. Biglycan up-regulated vascular endothelial growth factor (VEGF) expression and promoted angiogenesis in colon cancer. Tumor Biol. 2014, 36, 1773–1780. [Google Scholar] [CrossRef]
- Hatano, S.; Watanabe, H. Regulation of Macrophage and Dendritic Cell Function by Chondroitin Sulfate in Innate to An-tigen-Specific Adaptive Immunity. Front. Immunol. 2020, 11, 232. [Google Scholar] [CrossRef]
- Miguez, P.A. Evidence of biglycan structure-function in bone homeostasis and aging. Connect. Tissue Res. 2019, 61, 19–33. [Google Scholar] [CrossRef]
- Datsis, G.A.; Berdiaki, A.; Nikitovic, D.; Mytilineou, M.; Katonis, P.; Karamanos, N.K.; Tzanakakis, G.N. Parathyroid hormone affects the fibroblast growth factor-proteoglycan signaling axis to regulate osteosarcoma cell migration. FEBS J. 2011, 278, 3782–3792. [Google Scholar] [CrossRef]
- Ungefroren, H.; Cikós, T.; Krull, N.B.; Kalthoff, H. Biglycan Gene Promoter Activity in Osteosarcoma Cells Is Regulated by Cyclic AMP. Biochem. Biophys. Res. Commun. 1997, 235, 413–417. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Han, X.-D.; Qiu, Y.; Xiong, J.; Yu, Y.; Wang, B.; Zhu, Z.-Z.; Qian, B.-P.; Chen, Y.-X.; Wang, S.-F.; et al. Increased expression of insulin-like growth factor-1 receptor is correlated with tumor metastasis and prognosis in patients with osteosarcoma. J. Surg. Oncol. 2012, 105, 235–243. [Google Scholar] [CrossRef]
- Fisher, L.W.; Termine, J.D.; Young, M.F. Deduced protein sequence of bone small proteoglycan I (biglycan) shows ho-mology with proteoglycan II (decorin) and several nonconnective tissue proteins in a variety of species. J. Bio-Log. Chem. 1989, 264, 4571–4576. [Google Scholar] [CrossRef]
- Roedig, H.; Nastase, M.V.; Frey, H.; Moreth, K.; Zeng-Brouwers, J.; Poluzzi, C.; Hsieh, L.T.-H.; Brandts, C.; Fulda, S.; Wygrecka, M.; et al. Biglycan is a new high-affinity ligand for CD14 in macrophages. Matrix Biol. J. Int. Soc. Matrix Biol. 2019, 77, 4–22. [Google Scholar] [CrossRef]
- Chughtai, S. The nuclear translocation of insulin-like growth factor receptor and its significance in cancer cell survival. Cell Biochem. Funct. 2019, 38, 347–351. [Google Scholar] [CrossRef]
- Chen, C.-W.; Oberley, T.D.; Roy, D. Inhibition of stilbene estrogen-induced cell proliferation of renal epithelial cells through the modulation of insulin-like growth factor-I receptor expression. Cancer Lett. 1996, 105, 51–59. [Google Scholar] [CrossRef]
- Aleksic, T.; Gray, N.; Wu, X.; Rieunier, G.; Osher, E.; Mills, J.; Verrill, C.; Bryant, R.J.; Han, C.; Hutchinson, K.; et al. Nuclear IGF1R Interacts with Regulatory Regions of Chromatin to Promote RNA Polymerase II Recruitment and Gene Expression Associated with Advanced Tumor Stage. Cancer Res. 2018, 78, 3497–3509. [Google Scholar] [CrossRef]
- Aleksic, T.; Chitnis, M.M.; Perestenko, O.V.; Gao, S.; Thomas, P.H.; Turner, G.D.; Protheroe, A.S.; Howarth, M.; Macaulay, V.M. Type 1 Insulin-like Growth Factor Receptor Translocates to the Nucleus of Human Tumor Cells. Cancer Res. 2010, 70, 6412–6419. [Google Scholar] [CrossRef]
- Romanelli, R.J.; LeBeau, A.P.; Fulmer, C.G.; Lazzarino, D.A.; Hochberg, A.; Wood, T. Insulin-like Growth Factor Type-I Receptor Internalization and Recycling Mediate the Sustained Phosphorylation of Akt. J. Biol. Chem. 2007, 282, 22513–22524. [Google Scholar] [CrossRef]
- Sarfstein, R.; Werner, H. Minireview: Nuclear Insulin and Insulin-like Growth Factor-1 Receptors: A Novel Paradigm in Signal Transduction. Endocrinology 2013, 154, 1672–1679. [Google Scholar] [CrossRef]
- Liang, Y.; Häring, M.; Roughley, P.J.; Margolis, R.K.; Margolis, R.U. Glypican and Biglycan in the Nuclei of Neurons and Glioma Cells: Presence of Functional Nuclear Localization Signals and Dynamic Changes in Glypican During the Cell Cycle. J. Cell Biol. 1997, 139, 851–864. [Google Scholar] [CrossRef]
- Hellewell, A.L.; Adams, J.C. Insider trading: Extracellular matrix proteins and their non-canonical intracellular roles. BioEssays 2015, 38, 77–88. [Google Scholar] [CrossRef]
- Favre, N.; Camps, M.; Arod, C.; Chabert, C.; Rommel, C.; Pasquali, C. Chemokine receptor CCR2 undergoes trans-portin1-dependent nuclear translocation. Proteomics 2008, 8, 4560–4576. [Google Scholar] [CrossRef]
- Janssen, J.A. New Insights from IGF-IR Stimulating Activity Analyses: Pathological Considerations. Cells 2020, 9, 862. [Google Scholar] [CrossRef]
- Freeman, S.S.; Allen, S.W.; Ganti, R.; Wu, J.; Ma, J.; Su, X.; Neale, G.; Dome, J.S.; Daw, N.C.; Khoury, J.D. Copy number gains in EGFR and copy number losses in PTEN are common events in osteosarcoma tumors. Cancer 2008, 113, 1453–1461. [Google Scholar] [CrossRef]
- Zhao, H.; Dupont, J.; Yakar, S.; Karas, M.; LeRoith, D. PTEN inhibits cell proliferation and induces apoptosis by down-regulating cell surface IGF-IR expression in prostate cancer cells. Oncogene 2004, 23, 786–794. [Google Scholar] [CrossRef]
- Zu, K.; Martin, N.E.; Fiorentino, M.; Flavin, R.; Lis, R.T.; Sinnott, J.A.; Finn, S.; Penney, K.L.; Ma, J.; Fazli, L.; et al. Protein Expression of PTEN, Insulin-Like Growth Factor I Receptor (IGF-IR), and Lethal Prostate Cancer: A Prospective Study. Cancer Epidemiology Biomarkers Prev. 2013, 22, 1984–1993. [Google Scholar] [CrossRef]
- Chen, J.; Yan, D.; Wu, W.; Zhu, J.; Ye, W.; Shu, Q. MicroRNA-130a promotes the metastasis and epithelial-mesenchymal transition of osteosarcoma by targeting PTEN. Oncol. Rep. 2016, 35, 3285–3292. [Google Scholar] [CrossRef] [PubMed]
- Beech, D.J.; Perer, E.; Helms, J.; Gratzer, A.; Deng, N. Insulin-like growth factor-I receptor activation blocks doxorubicin cytotoxicity in sarcoma cells. Oncol. Rep. 2003, 10, 181–184. [Google Scholar] [CrossRef]
- Dallas, N.A.; Xia, L.; Fan, F.; Gray, M.J.; Gaur, P.; van Buren, G., 2nd; Samuel, S.; Kim, M.P.; Lim, S.J.; Ellis, L.M. Chemoresistant colorectal cancer cells, the cancer stem cell phenotype, and increased sensitivity to insulin-like growth factor-I receptor inhibition. Cancer Res. 2009, 69, 1951–1957. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, S.; Torossian, A.; Speirs, C.K.; Schleicher, S.; Giacalone, N.J.; Carbone, D.P.; Zhao, Z.; Lu, B. Role of insu-lin-like growth factor-1 signaling pathway in cisplatin-resistant lung cancer cells. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, e563-72. [Google Scholar] [CrossRef]
- Worrall, C.; Nedelcu, D.; Serly, J.; Suleymanova, N.; Oprea, I.; Girnita, A.; Girnita, L. Novel mechanisms of regulation of IGF-1R action: Functional and therapeutic implications. Pediatr Endocrinol Rev 2013, 10, 473–484. [Google Scholar]
- Sui, P.; Cao, H.; Meng, L.; Hu, P.; Ma, H.; Du, J. The synergistic effect of humanized monoclonal antibodies targeting in-sulin-like growth factor 1 receptor (IGF-1R) and chemotherapy. Curr. Drug Targets 2014, 15, 674–680. [Google Scholar] [CrossRef]
- Codony-Servat, J.; Cuatrecasas, M.; Asensio, E.; Montironi, C.; Martinez-Cardus, A.; Marin-Aguilera, M.; Horndler, C.; Martinez-Balibrea, E.; Rubini, M.; Jares, P.; et al. Nuclear IGF-1R predicts chemotherapy and targeted therapy resistance in meta-static colorectal cancer. Br. J. Cancer 2017, 117, 1777–1786. [Google Scholar] [CrossRef]
- Zhu, K.P.; Zhang, C.L.; Shen, G.Q.; Zhu, Z.S. Long noncoding RNA expression profiles of the doxorubicin-resistant human osteosarcoma cell line MG63/DXR and its parental cell line MG63 as ascertained by microarray analysis. Int. J. Clin. Exp. Pathol. 2015, 8, 8754–8773. [Google Scholar] [PubMed]
- Ma, L.; Xu, Y.; Xu, X. Targeted MEK inhibition by cobimetinib enhances doxorubicin’s efficacy in osteosarcoma models. Biochem. Biophys. Res. Commun. 2020, 529, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Kreahling, J.M.; Gemmer, J.Y.; Reed, D.; Letson, D.; Bui, M.; Altiok, S. MK1775, a selective Wee1 inhibitor, shows sin-gle-agent antitumor activity against sarcoma cells. Mol. Cancer Ther. 2012, 11, 174–182. [Google Scholar] [CrossRef]
- Chen, X.; Ko, L.J.; Jayaraman, L.; Prives, C. p53 levels, functional domains, and DNA damage determine the extent of the apoptotic response of tumor cells. Genes Dev. 1996, 10, 2438–2451. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giatagana, E.-M.; Berdiaki, A.; Gaardløs, M.; Samsonov, S.A.; Tzanakakis, G.N.; Nikitovic, D. Biglycan Interacts with Type I Insulin-like Receptor (IGF-IR) Signaling Pathway to Regulate Osteosarcoma Cell Growth and Response to Chemotherapy. Cancers 2022, 14, 1196. https://doi.org/10.3390/cancers14051196
Giatagana E-M, Berdiaki A, Gaardløs M, Samsonov SA, Tzanakakis GN, Nikitovic D. Biglycan Interacts with Type I Insulin-like Receptor (IGF-IR) Signaling Pathway to Regulate Osteosarcoma Cell Growth and Response to Chemotherapy. Cancers. 2022; 14(5):1196. https://doi.org/10.3390/cancers14051196
Chicago/Turabian StyleGiatagana, Eirini-Maria, Aikaterini Berdiaki, Margrethe Gaardløs, Sergey A. Samsonov, George N. Tzanakakis, and Dragana Nikitovic. 2022. "Biglycan Interacts with Type I Insulin-like Receptor (IGF-IR) Signaling Pathway to Regulate Osteosarcoma Cell Growth and Response to Chemotherapy" Cancers 14, no. 5: 1196. https://doi.org/10.3390/cancers14051196
APA StyleGiatagana, E.-M., Berdiaki, A., Gaardløs, M., Samsonov, S. A., Tzanakakis, G. N., & Nikitovic, D. (2022). Biglycan Interacts with Type I Insulin-like Receptor (IGF-IR) Signaling Pathway to Regulate Osteosarcoma Cell Growth and Response to Chemotherapy. Cancers, 14(5), 1196. https://doi.org/10.3390/cancers14051196








