Genomic Instability Is Defined by Specific Tumor Microenvironment in Ovarian Cancer: A Subgroup Analysis of AGO OVAR 12 Trial
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. IHC Analysis
2.2. CGH Analysis
2.3. Statistical Analysis
3. Results
3.1. Patient Population
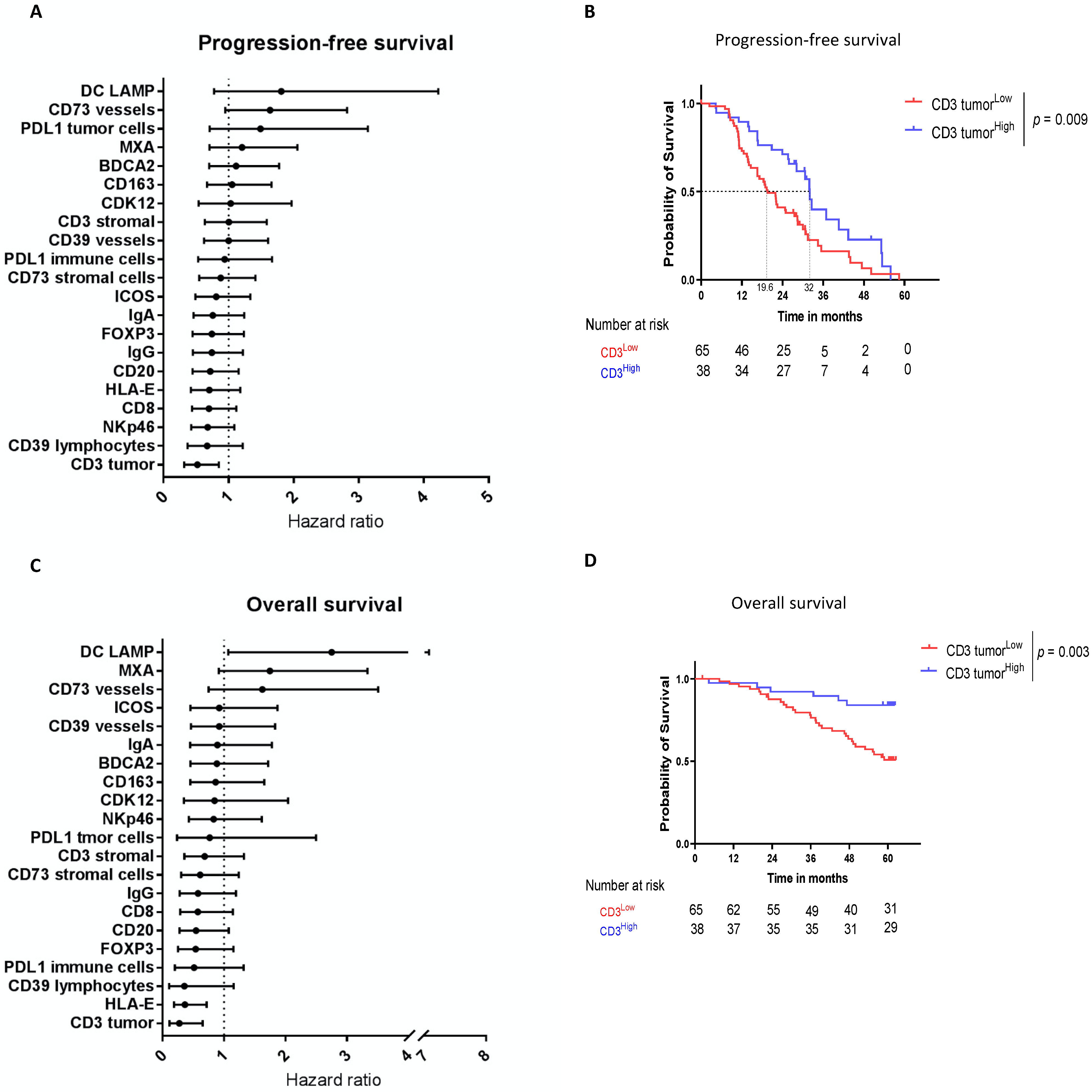
3.2. Intratumoral CD3 Confirmed to Be a Major Prognostic Biomarker in HGSOC
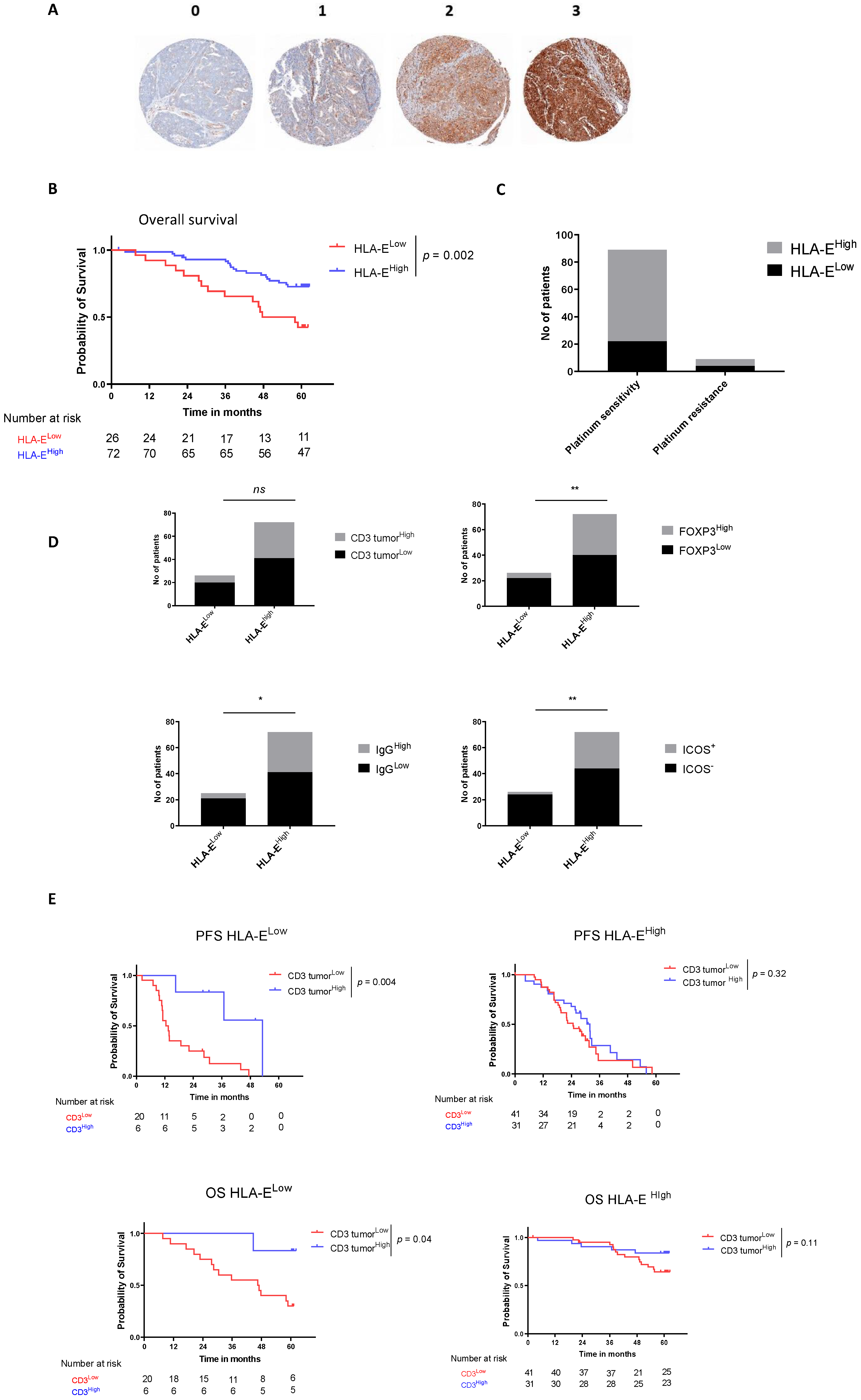
3.3. HLA-E on Tumor Cells Is an Emergent Prognostic Biomarker in HGSOC
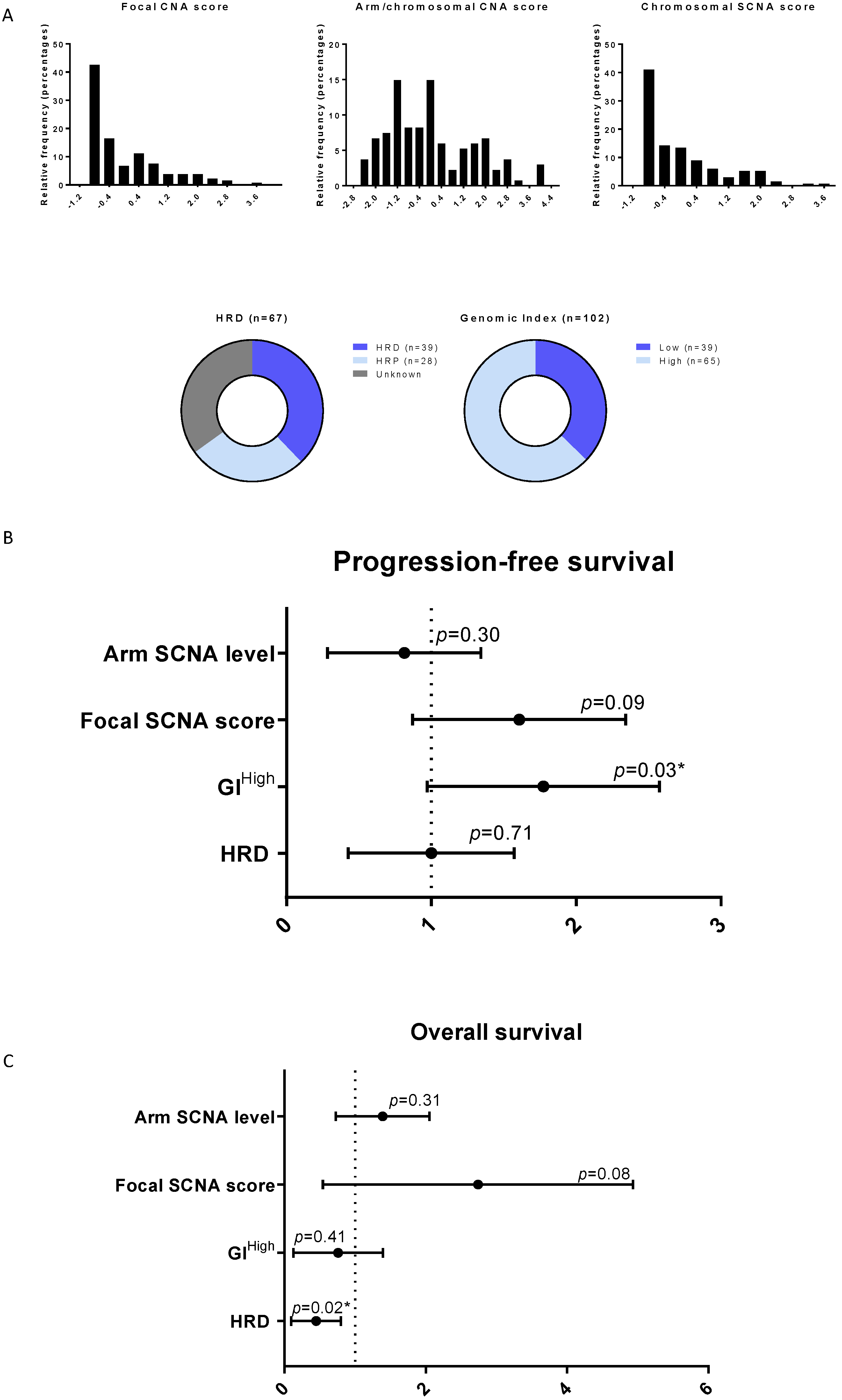
3.4. Genomic Instability Confirmed to Be Correlated to Survival in HGSOC
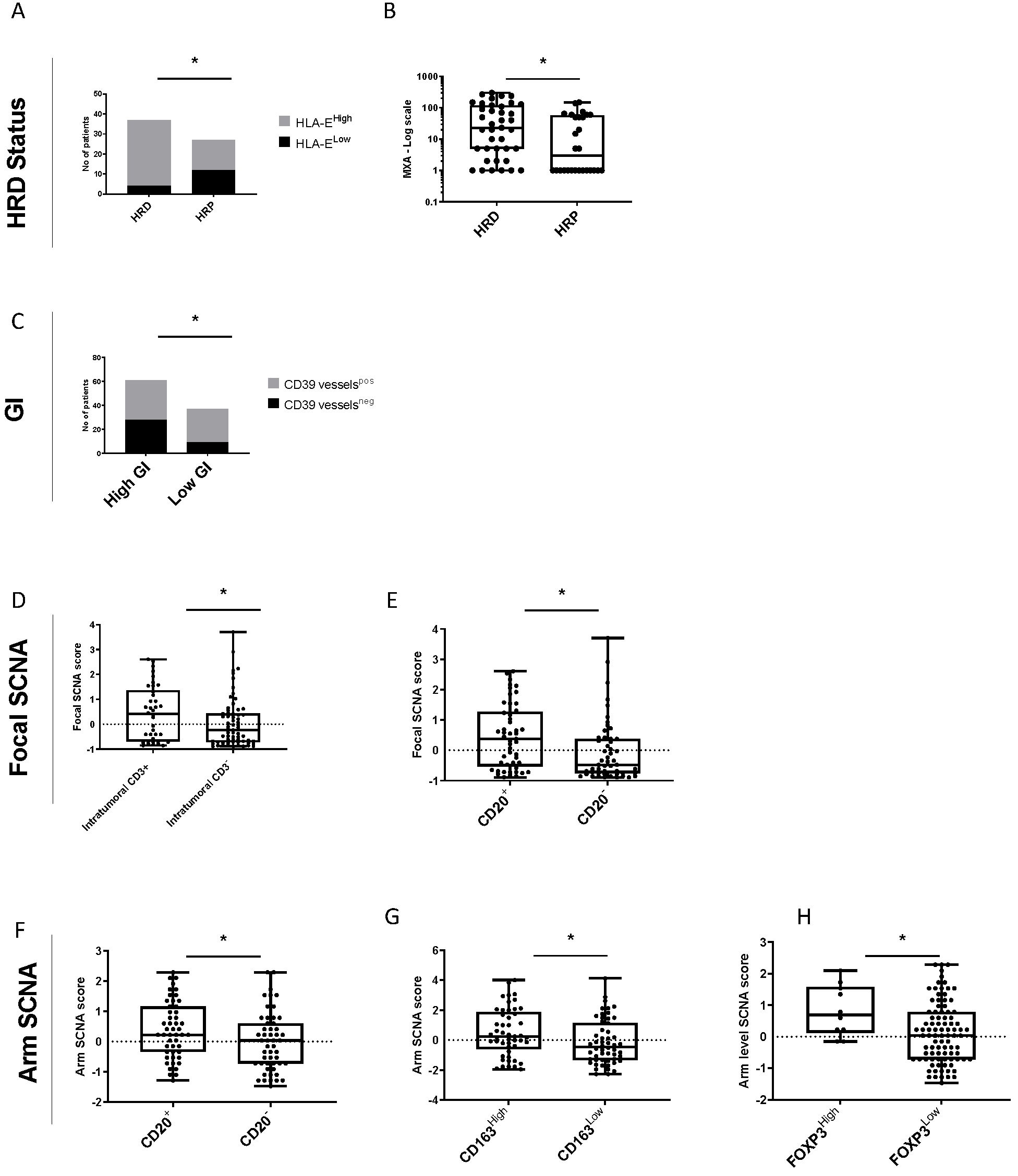
3.5. Relationship between Genomic Instability and Tumor Immune Microenvironment for HGSOC
3.6. Multivariate Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CGH | comparative genomic hybridization |
| GI | genomic index |
| HGSOC | high grade serous ovarian carcinoma |
| HRD | homologous recombination deficiency |
| ICI | immune checkpoint inhibitors |
| IHC | immunohistochemistry |
| ITT | intention to treat |
| LOH | loss of heterozygosity |
| LST | large-scale state transitions |
| OS | overall survival |
| PFS | progression free survival |
| SCNA | somatic copy number alterations |
| TAI | telomeric allelic imbalance |
| TMA | tissue microarray |
| TME | tumor immune microenvironment |
References
- Moore, K.; Colombo, N.; Scambia, G.; Kim, B.-G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N. Engl. J. Med. 2018, 379, 2495–2505. [Google Scholar] [CrossRef] [PubMed]
- González-Martín, A.; Pothuri, B.; Vergote, I.; DePont Christensen, R.; Graybill, W.; Mirza, M.R.; McCormick, C.; Lorusso, D.; Hoskins, P.; Freyer, G.; et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N. Engl. J. Med. 2019, 381, 2391–2402. [Google Scholar] [CrossRef] [PubMed]
- Burger, R.A.; Fleming, G.F.; Mannel, R.S.; Greer, B.E.; Liang, S.X. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N. Engl. J. Med. 2011, 365, 2473–2483. [Google Scholar] [CrossRef] [PubMed]
- Perren, T.J.; Swart, A.M.; Pfisterer, J.; Ledermann, J.A.; Pujade-Lauraine, E.; Kristensen, G.; Carey, M.S.; Beale, P.; Cervantes, A.; Kurzeder, C.; et al. A Phase 3 trial of bevacizumab in ovarian cancer. N. Engl. J. Med. 2011, 365, 2484–2496. [Google Scholar] [CrossRef] [PubMed]
- Ray-Coquard, I.; Pautier, P.; Pignata, S.; Pérol, D.; González-Martín, A.; Berger, R.; Fujiwara, K.; Vergote, I.; Colombo, N.; Mäenpää, J.; et al. Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N. Engl. J. Med. 2019, 381, 2416–2428. [Google Scholar] [CrossRef]
- Popova, T.; Manié, E.; Rieunier, G.; Caux-Moncoutier, V.; Tirapo, C.; Dubois, T.; Delattre, O.; Sigal-Zafrani, B.; Bollet, M.; Longy, M.; et al. Ploidy and large-scale genomic instability consistently identify basal-like breast carcinomas with BRCA1/2 inactivation. Cancer Res. 2012, 72, 5454–5462. [Google Scholar] [CrossRef]
- Abkevich, V.; Timms, K.M.; Hennessy, B.T.; Potter, J.; Carey, M.S.; Meyer, L.A.; Smith-McCune, K.; Broaddus, R.; Lu, K.H.; Chen, J.; et al. Patterns of genomic loss of heterozygosity predict homologous recombination repair defects in epithelial ovarian cancer. Br. J. Cancer 2012, 107, 1776–1782. [Google Scholar] [CrossRef]
- Birkbak, N.J.; Wang, Z.C.; Kim, J.-Y.; Eklund, A.C.; Li, Q.; Tian, R.; Bowman-Colin, C.; Li, Y.; Greene-Colozzi, A.; Iglehart, J.D.; et al. Telomeric allelic imbalance indicates defective dna repair and sensitivity to dna-damaging agents. Cancer Discov. 2012, 2, 366–375. [Google Scholar] [CrossRef]
- Timms, K.M.; Abkevich, V.; Hughes, E.; Neff, C.; Reid, J.; Morris, B.; Kalva, S.; Potter, J.; Tran, T.V.; Chen, J.; et al. Association of BRCA1/2defects with genomic scores predictive of DNA damage repair deficiency among breast cancer subtypes. Breast Cancer Res. 2014, 16, 475. [Google Scholar] [CrossRef]
- Polak, P.; Kim, J.; Braunstein, L.Z.; Karlic, R.; Haradhavala, N.J.; Tiao, G.; Rosebrock, D.; Livitz, D.; Kübler, K.; Mouw, K.W.; et al. A mutational signature reveals alterations underlying deficient homologous recombination repair in breast cancer. Nat. Genet. 2017, 49, 1476–1486. [Google Scholar] [CrossRef]
- Davies, H.; Glodzik, D.; Morganella, S.; Yates, L.R.; Staaf, J.; Zou, X.; Ramakrishna, M.; Martin, S.; Boyault, S.; Sieuwerts, A.M.; et al. HRDetect is a predictor of BRCA1 and BRCA2 deficiency based on mutational signatures. Nat. Med. 2017, 23, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.L.; Fleming, G.F.; Brady, M.F.; Swisher, E.M.; Steffensen, K.D.; Friedlander, M.; Okamoto, A.; Moore, K.N.; Efrat Ben-Baruch, N.; Werner, T.L.; et al. Veliparib with first-line chemotherapy and as maintenance therapy in ovarian cancer. N. Engl. J. Med. 2019, 381, 2403–2415. [Google Scholar] [CrossRef]
- Le Saux, O.; Dubois, B.; Stern, M.-H.; Terme, M.; Tartour, E.; Classe, J.-M.; Chopin, N.; Trédan, O.; Caux, C.; Ray-Coquard, I. Les avancées actuelles de l’immunothérapie dans le cancer de l’ovaire. Bull. Cancer 2020, 107, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Fumet, J.-D.; Richard, C.; Ledys, F.; Klopfenstein, Q.; Joubert, P.; Routy, B.; Truntzer, C.; Gagné, A.; Hamel, M.-A.; Guimaraes, C.F.; et al. Prognostic and predictive role of CD8 and PD-L1 determination in lung tumor tissue of patients under anti-PD-1 therapy. Br. J. Cancer 2018, 119, 950–960. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.N.; Bookman, M.; Sehouli, J.; Miller, A.; Anderson, C.; Scambia, G.; Myers, T.; Taskiran, C.; Robison, K.; Mäenpää, J.; et al. Atezolizumab, bevacizumab, and chemotherapy for newly diagnosed stage III or IV ovarian cancer: Placebo-controlled randomized phase III trial (IMagyn050/GOG 3015/ENGOT-OV39). J. Clin. Oncol. 2021, 39, 1842–1855. [Google Scholar] [CrossRef]
- Pujade-Lauraine, E.; Fujiwara, K.; Ledermann, J.A.; Oza, A.M.; Kristeleit, R.; Ray-Coquard, I.-L.; Richardson, G.E.; Sessa, C.; Yonemori, K.; Banerjee, S.; et al. Avelumab alone or in combination with chemotherapy versus chemotherapy alone in platinum-resistant or platinum-refractory ovarian cancer (JAVELIN Ovarian 200): An open-label, three-arm, randomised, phase 3 study. Lancet Oncol. 2021, 22, 1034–1046. [Google Scholar] [CrossRef]
- Monk, B.J.; Colombo, N.; Oza, A.M.; Fujiwara, K.; Birrer, M.J.; Randall, L.; Poddubskaya, E.V.; Scambia, G.; Shparyk, Y.V.; Lim, M.C.; et al. Chemotherapy with or without avelumab followed by avelumab maintenance versus chemotherapy alone in patients with previously untreated epithelial ovarian cancer (JAVELIN Ovarian 100): An open-label, randomised, phase 3 trial. Lancet Oncol. 2021, 22, 1275–1289. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Chen, R.; Bai, Y.; Lu, X. The prognostic value of tumor-infiltrating T lymphocytes in ovarian cancer. Oncotarget 2017, 8, 15621–15631. [Google Scholar] [CrossRef]
- Zhang, L.; Conejo-Garcia, J.R.; Katsaros, D.; Gimotty, P.A.; Massobrio, M.; Regnani, G.; Makrigiannakis, A.; Gray, H.; Schlienger, K.; Liebman, M.N.; et al. Intratumoral T Cells, Recurrence, and Survival in Epithelial Ovarian Cancer. N. Engl. J. Med. 2003, 348, 203–213. [Google Scholar] [CrossRef]
- Henriksen, J.R.; Donskov, F.; Waldstrøm, M.; Jakobsen, A.; Hjortkjaer, M.; Petersen, C.B.; Steffensen, K.D. Favorable prognostic impact of Natural Killer cells and T cells in high-grade serous ovarian carcinoma. Acta Oncol. 2020, 59, 652–659. [Google Scholar] [CrossRef]
- Liu, R.; Hu, R.; Zeng, Y.; Zhang, W.; Zhou, H.-H. Tumour immune cell infiltration and survival after platinum-based chemotherapy in high-grade serous ovarian cancer subtypes: A gene expression-based computational study. EBioMedicine 2020, 51, 102602. [Google Scholar] [CrossRef] [PubMed]
- McAlpine, J.N.; Porter, H.; Köbel, M.; Nelson, B.; Prentice, L.M.; Kalloger, S.E.; Senz, J.; Milne, K.; Ding, J.; Shah, S.P.; et al. BRCA1 and BRCA2 mutations correlate with TP53 abnormalities and presence of immune cell infiltrates in ovarian high-grade serous carcinoma. Mod. Pathol. 2012, 25, 740–750. [Google Scholar] [CrossRef] [PubMed]
- Strickland, K.C.; Howitt, B.E.; Shukla, S.A.; Rodig, S.; Ritterhouse, L.L.; Liu, J.F.; Garber, J.E.; Chowdhury, D.; Wu, C.J.; D’Andrea, A.D.; et al. Association and prognostic significance of BRCA1/2-mutation status with neoantigen load, number of tumor-infiltrating lymphocytes and expression of PD-1/PD-L1 in high grade serous ovarian cancer. Oncotarget 2016, 7, 13587–13598. [Google Scholar] [CrossRef] [PubMed]
- Dunphy, G.; Flannery, S.M.; Almine, J.F.; Connolly, D.J.; Paulus, C.; Jønsson, K.L.; Jakobsen, M.R.; Nevels, M.M.; Bowie, A.G.; Unterholzner, L. Non-canonical Activation of the DNA Sensing Adaptor STING by ATM and IFI16 Mediates NF-κB Signaling after Nuclear DNA Damage. Mol. Cell 2018, 71, 745–760. [Google Scholar] [CrossRef]
- Davoli, T.; Uno, H.; Wooten, E.C.; Elledge, S.J. Tumor aneuploidy correlates with markers of immune evasion and with reduced response to immunotherapy. Science 2017, 355, eaaf8399. [Google Scholar] [CrossRef]
- Morse, C.B.; Toukatly, M.N.; Kilgore, M.R.; Agnew, K.J.; Bernards, S.S.; Norquist, B.M.; Pennington, K.P.; Garcia, R.L.; Liao, J.B.; Swisher, E.M. Tumor infiltrating lymphocytes and homologous recombination deficiency are independently associated with improved survival in ovarian carcinoma. Gynecol. Oncol. 2019, 153, 217–222. [Google Scholar] [CrossRef]
- du Bois, A.; Kristensen, G.; Ray-Coquard, I.; Reuss, A.; Pignata, S.; Colombo, N.; Denison, U.; Vergote, I.; del Campo, J.M.; Ottevanger, P.; et al. Standard first-line chemotherapy with or without nintedanib for advanced ovarian cancer (AGO-OVAR 12): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 2016, 17, 78–89. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Halper-Stromberg, E.; Scharpf, R.B. Wave Correction for Arrays. Available online: https://rdrr.io/bioc/ArrayTV/f/inst/doc/ArrayTV.pdf (accessed on 20 December 2021).
- Chang, W.; Cheng, J.; Allaire, J.J.; Sievert, C.; Schloerke, B.; Xie, Y.; Allen, J.; McPherson, J.; Dipert, A.; Borges, B.; et al. Shiny: Web Application Framework for R. 2021. Available online: https://shiny.rstudio.com/reference/shiny/1.4.0/shiny-package.html (accessed on 20 December 2021).
- Eeckhoutte, A.; Houy, A.; Manié, E.; Reverdy, M.; Bièche, I.; Marangoni, E.; Goundiam, O.; Vincent-Salomon, A.; Stoppa-Lyonnet, D.; Bidard, F.-C.; et al. ShallowHRD: Detection of homologous recombination deficiency from shallow whole genome sequencing. Bioinformatics 2020, 36, 3888–3889. [Google Scholar] [CrossRef]
- Lartigue, L.; Neuville, A.; Lagarde, P.; Brulard, C.; Rutkowski, P.; Tos, A.D.; Wardelmann, E.; Debiec-Rychter, M.; Italiano, A.; Coindre, J.-M.; et al. Genomic index predicts clinical outcome of intermediate-risk gastrointestinal stromal tumours, providing a new inclusion criterion for imatinib adjuvant therapy. Eur. J. Cancer 2015, 51, 75–83. [Google Scholar] [CrossRef]
- Sullivan, L.C.; Clements, C.S.; Rossjohn, J.; Brooks, A.G. The major histocompatibility complex class Ib molecule HLA-E at the interface between innate and adaptive immunity. Tissue Antigens 2008, 72, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Gooden, M.J.; van Hall, T. Infiltrating CTLs are bothered by HLA-E on tumors. OncoImmunology 2012, 1, 92–93. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lanier, L.L.; Corliss, B.; Wu, J.; Phillips, J.H. Association of DAP12 with Activating CD94/NKG2C NK cell receptors. Immunity 1998, 8, 693–701. [Google Scholar] [CrossRef]
- HLA Loss Facilitates Immune Escape. Cancer Discov. 2018, 8, 8. [CrossRef]
- Zheng, H.; Lu, R.; Xie, S.; Wen, X.; Wang, H.; Gao, X.; Guo, L. Human leukocyte antigen-E alleles and expression in patients with serous ovarian cancer. Cancer Sci. 2015, 106, 522–528. [Google Scholar] [CrossRef]
- Eugène, J.; Jouand, N.; Ducoin, K.; Dansette, D.; Oger, R.; Deleine, C.; Leveque, E.; Meurette, G.; Podevin, J.; Matysiak, T.; et al. The inhibitory receptor CD94/NKG2A on CD8+ tumor-infiltrating lymphocytes in colorectal cancer: A promising new druggable immune checkpoint in the context of HLAE/β2m overexpression. Mod. Pathol. 2020, 33, 468–482. [Google Scholar] [CrossRef]
- Nguyen, S.; Béziat, V.; Dhedin, N.; Kuentz, M.; Vernant, J.P.; Debre, P.; Vieillard, V. HLA-E upregulation on IFN-γ-activated AML blasts impairs CD94/NKG2A-dependent NK cytolysis after haplo-mismatched hematopoietic SCT. Bone Marrow Transplant. 2009, 43, 693–699. [Google Scholar] [CrossRef]
- Malmberg, K.-J.; Levitsky, V.; Norell, H.; De Matos, C.T.; Carlsten, M.; Schedvins, K.; Rabbani, H.; Moretta, A.; Söderström, K.; Levitskaya, J.; et al. IFN-γ protects short-term ovarian carcinoma cell lines from CTL lysis via a CD94/NKG2A-dependent mechanism. J. Clin. Investig. 2002, 110, 1515–1523. [Google Scholar] [CrossRef]
- Gasser, S.; Raulet, D. The DNA damage response, immunity and cancer. Semin. Cancer Biol. 2006, 16, 344–347. [Google Scholar] [CrossRef]
- Andersson, E.; Poschke, I.; Villabona, L.; Carlson, J.; Lundqvist, A.; Kiessling, R.; Seliger, B.; Masucci, G.V. Non-classical HLA-class I expression in serous ovarian carcinoma: Correlation with the HLA-genotype, tumor infiltrating immune cells and prognosis. OncoImmunology 2016, 5, e1052213. [Google Scholar] [CrossRef]
- Gooden, M.; Lampen, M.; Jordanova, E.S.; Leffers, N.; Trimbos, J.B.; van der Burg, S.H.; Nijman, H.; van Hall, T. HLA-E expression by gynecological cancers restrains tumor-infiltrating CD8+ T lymphocytes. Proc. Natl. Acad. Sci. USA 2011, 108, 10656–10661. [Google Scholar] [CrossRef] [PubMed]
- André, P.; Denis, C.; Soulas, C.; Bourbon-Caillet, C.; Lopez, J.; Arnoux, T.; Bléry, M.; Bonnafous, C.; Gauthier, L.; Morel, A.; et al. Anti-NKG2A mAb Is a Checkpoint Inhibitor that Promotes Anti-tumor Immunity by Unleashing Both T and NK Cells. Cell 2018, 175, 1731–1743. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | All (n = 103) |
|---|---|
| median age | 58.8 (44; 72.9) |
| FIGO | |
| IIB | 6 (5.8%) |
| IIC | 4 (3.9%) |
| IIIB | 6 (5.8%) |
| IIIC | 57 (55.4%) |
| IV | 30 (29.1%) |
| Histology | |
| High grade serous | 103 (100%) |
| Optimal cytoreduction | |
| yes | 49 (52.4%) |
| no | 54 (47.6%) |
| Performance status | |
| 0 | 64 (62.1%) |
| 1 | 36 (35%) |
| 2 | 3 (2.9%) |
| Treatment | |
| nintedanib | 70 (68%) |
| placebo | 33 (32%) |
| Progression-Free Survival | Overall Survival | |||||
|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | Univariate Analysis | Multivariate Analysis | |||
| HR (95% CI) | p-Value | HR (95% CI) | HR (95% CI) | p-Value | HR (95% CI) | |
| Clinical | ||||||
| FIGO (IV vs. others) | 1.57 [0.96; 2.55] | 0.07 | 2.277 [1.19; 4.37] | 0.02 | ||
| Age (>60 vs. ≤60 y) | 1.15 [0.72; 1.83] | 0.55 | 1.41 [0.74; 2.70] | 0.3 | ||
| Complete cytoreduction CC-0 (Yes or no) | 0.45 [0.29; 0.73] | <0.001 | 0.69 [0.41; 1.00] | 0.31 [0.15; 0.64] | <0.001 | 0.46 [0.08; 1.00] |
| Performance status (0 vs. 1–2) | 1.48 [0.93; 2.35] | 0.1 | 1.38 [0.72; 2.64] | 0.3 | ||
| Treatment (Placebo vs. Nintedanib) | 0.6 [0.35; 1.03] | 0.07 | 0.9 [0.44; 1.82] | 0.78 | ||
| Immunological * | ||||||
| BDCA2 | 1.12 [0.7; 1.78] | 0.65 | 0.88 [0.45; 1.72] | 0.72 | ||
| CD163 | 1.05 [0.67; 1.66] | 0.82 | 0.86 [0.45; 1.66] | 0.66 | ||
| CD20 | 0.72 [0.45; 1.15] | 0.17 | 0.55 [0.28; 1.08] | 0.08 | ||
| CD3 stromal | 1 [0.63; 1.59] | 0.51 | 0.69 [0.36; 1.32] | 0.70 | ||
| CD3 tumor | 0.52 [0.32; 0.85] | 0.01 | 0.66 [0.39; 1.00] | 0.27 [0.11; 0.65] | 0.004 | |
| CD39 lymphocytes | 0.67 [0.37; 1.22] | 0.19 | 0.92 [0.46; 1.83] | 0.09 | ||
| CD39 vessels | 1 [0.62; 1.61] | 1.00 | 0.36 [0.11; 1.16] | 0.82 | ||
| CD73 stromal cells | 0.88 [0.55; 1.41] | 0.79 | 0.61 [0.3; 1.24] | 0.34 | ||
| CD73 vessels | 1.64 [0.95; 2.82] | 0.08 | 1.50 [1.00; 2.78] | 1.62 [0.75; 3.51] | 0.22 | |
| CD8 | 0.7 [0.44; 1.12] | 0.13 | 0.58 [0.29; 1.15] | 0.12 | ||
| CDK12 | 1.03 [0.54; 1.97] | 0.93 | 0.85 [0.35; 2.04] | 0.71 | ||
| DC LAMP | 1.81 [0.78; 4.22] | 0.17 | 2.75 [1.07; 7.08] | 0.04 | ||
| FOXP3 | 0.74 [0.45; 1.24] | 0.25 | 0.54 [0.25; 1.15] | 0.11 | ||
| HLA-E | 0.7 [0.42; 1.18] | 0.18 | 0.36 [0.18; 0.72] | 0.004 | 0.23 [0.02; 1.00] | |
| ICOS | 0.81 [0.49; 1.34] | 0.41 | 0.92 [0.46; 1.87] | 0.82 | ||
| IgA | 0.76 [0.46; 1.24] | 0.27 | 0.89 [0.45; 1.78] | 0.75 | ||
| IgG | 0.74 [0.45; 1.22] | 0.24 | 0.58 [0.28; 1.19] | 0.14 | ||
| MXA | 1.21 [0.71; 2.06] | 0.49 | 1.75 [0.92; 3.33] | 0.09 | ||
| NKp46 | 0.68 [0.43; 1.09] | 0.1 | 0.65 [0.29; 1.00] | 0.83 [0.43; 1.62] | 0.59 | |
| PD-L1 (immune cells) | 0.94 [0.53; 1.67] | 0.84 | 0.51 [0.2; 1.32] | 0.17 | ||
| PD-L1 (tumor cells) | 1.49 [0.71; 3.14] | 0.29 | 0.77 [0.24; 2.5] | 0.66 | ||
| Genomic | ||||||
| HRD status (HRD vs. HRP) | 0.89 [0.49; 1.62] | 0.7 | 0.36 [0.15; 0.84] | 0.02 | ||
| GI (Low vs. High) | 0.59 [0.36; 0.97] | 0.03 | 0.41 [0.35; 1.5] | 0.41 | ||
| Focal CNA score | 1.5 [0.93; 2.39] | 0.09 | 2.15 [0.9; 5.47] | 0.08 | ||
| Arm CNA level | 0.7 [0.35; 1.39] | 0.3 | 1.28 [0.79; 2.10] | 0.31 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fumet, J.-D.; Lardenois, E.; Ray-Coquard, I.; Harter, P.; Joly, F.; Canzler, U.; Truntzer, C.; Tredan, O.; Liebrich, C.; Lortholary, A.; et al. Genomic Instability Is Defined by Specific Tumor Microenvironment in Ovarian Cancer: A Subgroup Analysis of AGO OVAR 12 Trial. Cancers 2022, 14, 1189. https://doi.org/10.3390/cancers14051189
Fumet J-D, Lardenois E, Ray-Coquard I, Harter P, Joly F, Canzler U, Truntzer C, Tredan O, Liebrich C, Lortholary A, et al. Genomic Instability Is Defined by Specific Tumor Microenvironment in Ovarian Cancer: A Subgroup Analysis of AGO OVAR 12 Trial. Cancers. 2022; 14(5):1189. https://doi.org/10.3390/cancers14051189
Chicago/Turabian StyleFumet, Jean-David, Emilie Lardenois, Isabelle Ray-Coquard, Philipp Harter, Florence Joly, Ulrich Canzler, Caroline Truntzer, Olivier Tredan, Clemens Liebrich, Alain Lortholary, and et al. 2022. "Genomic Instability Is Defined by Specific Tumor Microenvironment in Ovarian Cancer: A Subgroup Analysis of AGO OVAR 12 Trial" Cancers 14, no. 5: 1189. https://doi.org/10.3390/cancers14051189
APA StyleFumet, J.-D., Lardenois, E., Ray-Coquard, I., Harter, P., Joly, F., Canzler, U., Truntzer, C., Tredan, O., Liebrich, C., Lortholary, A., Pissaloux, D., Leary, A., Pfisterer, J., Eeckhoutte, A., Hilpert, F., Fabbro, M., Caux, C., Alexandre, J., Houlier, A., ... du Bois, A. (2022). Genomic Instability Is Defined by Specific Tumor Microenvironment in Ovarian Cancer: A Subgroup Analysis of AGO OVAR 12 Trial. Cancers, 14(5), 1189. https://doi.org/10.3390/cancers14051189







