Simple Summary
In oncology, treatment outcomes can be competing, which means that one treatment could benefit one outcome, like survival, and negatively influence another, like independence. The choice of treatment therefore depends on the patient’s preference for outcomes, which needs to be assessed explicitly. Especially in older patients, patient preferences are important. Our systematic review summarizes all studies that assessed patient preferences for various treatment outcome categories. A total of 28 studies with 4374 patients were included, of which only six studies included mostly older patients. Although quality of life was only included in half of the studies, overall quality of life (79%) was most frequently prioritized as highest or second highest, followed by overall survival (67%), progression- and disease-free survival (56%), absence of severe or persistent treatment side effects (54%), treatment response (50%), and absence of transient short-term side effects (16%). In shared decision-making, these results can be used by healthcare professionals to better tailor the information provision and treatment recommendations to the individual patient.
Abstract
For physicians, it is important to know which treatment outcomes are prioritized overall by older patients with cancer, since this will help them to tailor the amount of information and treatment recommendations. Older patients might prioritize other outcomes than younger patients. Our objective is to summarize which outcomes matter most to older patients with cancer. A systematic review was conducted, in which we searched Embase and Medline on 22 December 2020. Studies were eligible if they reported some form of prioritization of outcome categories relative to each other in patients with all types of cancer and if they included at least three outcome categories. Subsequently, for each study, the highest or second-highest outcome category was identified and presented in relation to the number of studies that included that outcome category. An adapted Newcastle–Ottawa Scale was used to assess the risk of bias. In total, 4374 patients were asked for their priorities in 28 studies that were included. Only six of these studies had a population with a median age above 70. Of all the studies, 79% identified quality of life as the highest or second-highest priority, followed by overall survival (67%), progression- and disease-free survival (56%), absence of severe or persistent treatment side effects (54%), and treatment response (50%). Absence of transient short-term side effects was prioritized in 16%. The studies were heterogeneous considering age, cancer type, and treatment settings. Overall, quality of life, overall survival, progression- and disease-free survival, and severe and persistent side effects of treatment are the outcomes that receive the highest priority on a group level when patients with cancer need to make trade-offs in oncologic treatment decisions.
1. Introduction
Being diagnosed with cancer is a major life event and the start of a complex decision-making process on cancer treatment. Often, several treatment options are available. Most cancer treatments are intensive and burdensome, and the outcome cannot be guaranteed [1,2]. Furthermore, outcomes can be competing. For example, adjuvant chemotherapy in stage III colon cancer may decrease the likelihood of recurrence and increase (cancer-specific) survival, but toxicity may impact quality of life in the short term while serious treatment-related complications could also impact long-term functioning. Trade-offs are therefore needed.
The gold standard for complex decisions in oncology is shared decision-making, of which an important step is explicitly discussing which outcomes matter most to the patient [3,4,5,6]. This is particularly relevant in older patients, who may have a less favorable balance of benefits and risks of treatment than younger patients [1,7,8,9]. They are often excluded from clinical trials, and as a consequence, their recommendations are less evidence based [10]. Furthermore, oncological treatments have a narrow therapeutic index between the possible benefit of cancer control, including cancer symptom reduction, and the price that is still considered acceptable in terms of side effects. This increases the uncertainty in decision-making and makes it even more important to know which treatment outcomes are most frequently prioritized by older patients with cancer.
Knowledge of the most frequently mentioned patient priorities allows for a tailoring of information provision and prevents information overload caused by summing up all the treatment and outcome possibilities during the shared decision-making [3,4,5,6]. Prior research has demonstrated that adequate information provision about treatment impact and adverse events reduces the likelihood of decision regret [11] and improves patient satisfaction [12].
In patient preference elicitation many methods exist. Some methods are more general and ask patients to explicitly indicate what they would prefer, like rating scales [13] or the Outcome Prioritization Tool (OPT), which explicitly asks patients to rate each outcome relative to other outcomes without having two values on the same level [14]. This uses a trade-off principle: By prioritizing one outcome, patients are willing to accept the deterioration of other outcomes. The outcomes that are assigned priorities in the OPT conversation include extending life, maintaining independence, reducing pain, and reducing other symptoms [15]. Other methods are more specific and implicit, like discrete choice experiment (DCE), conjoint analysis (CA), and probability trade-off (Trade-off). These methods present patients with hypothetical scenarios with information on the possible benefits and side effects that are associated with various treatments and the probability of those happening. By measuring the willingness of patients to choose a treatment option while providing different scenarios of the included variables, the relative importance of that variable can be calculated [13]. Furthermore, the analytic hierarchy process (AHP) gives patients pair-wise comparisons and asks them to rate them against each other. This is also leads to a calculated relative importance of all included variables [16]. All methods have their benefits, and the best method of preference assessment depends on the question it needs to answer and the (number of) trade-offs that are at stake [13].
Two types of treatment outcomes are described in the literature [17,18]: disease-centered outcomes, which measure the objective effect of the treatment on the tumor and the adverse events, such as treatment response, toxicity, and disease-free survival, and patient-centered outcomes, which focus on the patient’s perception of health, quality of life, and functional outcomes like maintaining independence. To get a complete overview of the patient priorities in older patients with cancer, we set out to gather all available evidence from trade-off studies regarding treatment outcomes (both disease-centered outcomes and patient-centered outcomes).
2. Materials and Methods
We performed a systematic review to collect all available quantitative evidence comparing the relative importance patients allocate to various patient- and disease-centered outcomes after a cancer diagnosis. During the process the PRISMA guidelines were used [19]. We registered the systematic review in the OSF registry from the Center for Open Science [20].
2.1. Search Strategy
On 22 December 2020, we performed a search in Embase and Medline with the following terms and their synonyms: “health or treatment outcomes,” “priorities,” “trade-offs,” and “cancer.” The full electronic search strategy is shown in Appendix A. The search was limited to studies on humans written in English and published in the past 15 years. After an initial search in older patients, which resulted in few specific data, the search was expanded to all ages.
The titles and abstracts of all studies retrieved by the searches were assessed by one reviewer (N.S.) to determine which ones warranted further examination. All potentially relevant titles were subsequently screened independently as full text by two reviewers (N.S., A.W.). If no full text was found, the reviewers tried to find the final report of the study by using names of the different authors in combination with key words from the title. If none were found, the studies were excluded.
2.2. Eligibility Criteria
We included original publications on the comparison of outcome priorities after a diagnosis of cancer; this included both studies in actual cancer patients and studies performed on other subjects asked to state their priority in the hypothetical situation of a cancer diagnosis. Studies were only included if they addressed at least three of the possible six outcome categories that were defined, which were transient short-term side effects, severe and persistent side effects, quality of life (including functioning), treatment response, progression- and disease-free survival, and overall survival (see Appendix B).
All methods of preference elicitation were allowed, as long as the studies provided a form of prioritization of the individual outcome categories. Therefore, studies were excluded if the relative importance of outcome categories could not be elucidated due to the way the results were elicited or reported.
2.3. Data Extraction
Both reviewers (N.S., A.W.) independently extracted the following characteristics: title, author, year of publication, country, cancer type, curative or palliative treatment setting, me(di)an age of the sample, sample size, and method of assessing preferences. In addition, any patient- or disease-centered outcomes that were included in the trade-offs in the study were extracted, together with the ranking or score regarding the priority for each of these outcomes. Outcomes relating to process attributes such as mode of administration, frequency of administration, or out of pockets costs were not included.
2.4. Quality Assessment
Quality assessment was carried out by two independent reviewers using a quality assessment based on the Newcastle–Ottawa Scale ([21]; N.S. and A.W.), adjusted for this purpose based on a validated checklist for conjoint analysis (Appendix C) [22,23,24]. Disagreements were discussed in a consensus meeting and in case of continuing disagreement, a third reviewer (M.H.) was consulted.
2.5. Data Synthesis and Analysis
Based on the outcomes used by the included studies, two reviewers (N.S., A.W.) defined six outcome categories: quality of life (including functioning), overall survival, progression- and disease-free survival, severe and persistent side effects of treatment, treatment response, and transient short-term side effects. Detailed definitions can be found in Appendix B.
Using this classification, each assessed outcome was allocated to one of the defined outcome categories by two independent reviewers (N.S. and A.W.). The scores that the study reported were used to prioritize outcome categories to identify the highest and second-highest priority. The results were reported using descriptive data, describing the proportion of studies that prioritized each outcome category as highest or second highest in relation to the number of studies addressing that outcome.
If multiple outcomes in the study were allocated to the same outcome category (e.g., diarrhea and nausea were both transient short-term side effects), an average score of these outcomes was used to decide on the prioritization order of the outcome categories. In case of discrepancies, items were discussed until consensus was achieved; if needed, a third reviewer (M.H.) was consulted. When a study assessed preferred outcomes with multiple methods, resulting in different prioritizations, only the discrete choice elicitation was used to limit the heterogeneity. To determine the robustness of the results, subgroup analyses were conducted for curative and palliative settings and for older patients (study populations with a median age of 70 years or higher).
3. Results
3.1. Search and Study Selection
The search resulted in 7321 hits (2042 from Medline and 5279 from Embase). After removing 2072 duplicates and 5222 studies for other reasons (Figure 1), a total of 27 publications were included. Cross-referencing yielded one more publication, resulting in a total of 28 studies in this systematic review.
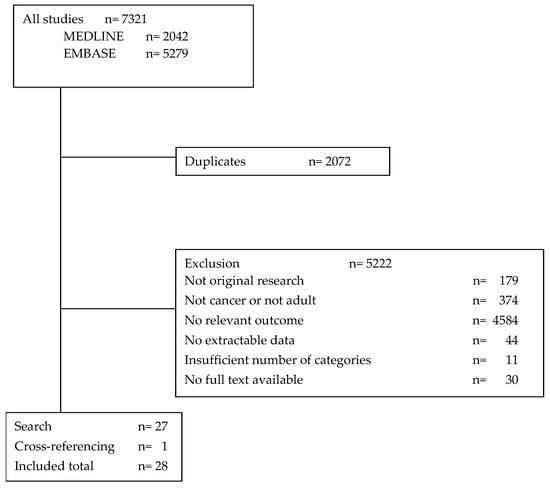
Figure 1.
Study selection.
3.2. Study Characteristics
The characteristics of 28 selected studies are summarized in Table 1. Most were published in the past five years. The total study population consisted of 4374 patients; the median sample size was 133 patients (range 36–419). The me(di)an age of participants varied between 35 and 78 years and six studies had a population with a median age over 70 years. The most frequently studied cancer type was gastrointestinal cancer (n = 8), followed by six studies that assessed various cancer types (see Table 1). Eight studies examined curative treatment, 11 studied palliative treatment, and nine studied both. The studies that assessed both often had a mix of all stages of cancer together. Various methods of preference elicitation were used. The majority of the studies (n = 15) used discrete choice elicitation, followed by conjoint analysis (n = 5), the Outcome Prioritization Tool (n = 3), and various types of rating scales. Both probability trade-off and the analytic hierarchy process were used in one study (Table 2).

Table 1.
Included studies and their characteristics.

Table 2.
Ranking of outcome categories per study.
3.3. Quality Assessment
Figure 2 provides an overview of the quality assessment. Details of each study can be found in Appendix D. In general, the representativeness of patients was good, although a few studies asked patients to provide answers for a hypothetical situation—for example, what they would choose if they had a different type or stage of cancer [29,40]. Some studies did not clearly report how specific outcomes were selected [28,29], or did not describe selection procedures at all [41,47,48,50]. Additionally, sometimes it was unclear how quality of life or other attributes were defined or were described to patients [27,39]. The analysis and outcome reporting were heterogeneous, but overall well described.
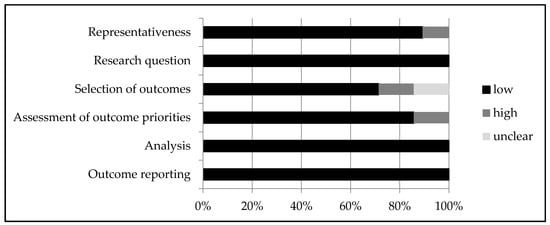
Figure 2.
Quality assessment—risk of bias.
3.4. Categories
The 28 publications reported 30 prioritizations: Two studies had a separate prioritization for patients with curative and palliative stages of disease. The median amount of outcome categories per study was four (range 3–6). The most frequently assessed outcome category was severe and persistent side effects (24 studies, 83%), followed by overall survival and transient short-term side effects (both, n = 19, 66%). Quality of life and progression- and disease-free survival were assessed in 14 (48%) and 15 studies (52%), respectively. Only one study included all six outcome categories (28), and there was no outcome category that was assessed in all studies.
3.5. Most Important Outcome Categories
For each study, the highest and second-highest outcome categories are identified and shown in Figure 3 and Table 2 relative to the number of studies that assessed that outcome category. For example, quality of life was assessed in total in 14 studies and was in 11 studies the highest or second-highest priority (n = 11/14, 79%). Overall survival (67%), progression- and disease-free survival (56%), and severe and persistent side effects (54%) were also commonly prioritized. When focusing only on studies addressing a palliative setting, quality of life and overall survival were most important (both in 75%); in contrast, progression- and disease-free survival (67%) and treatment response (67%) were given the highest priority in a curative treatment setting (Table 2, Figure 4).
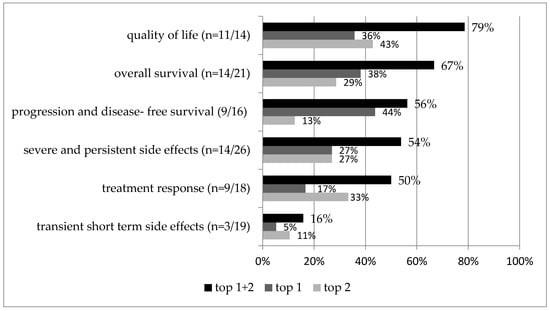
Figure 3.
Outcome category prioritization of all studies.
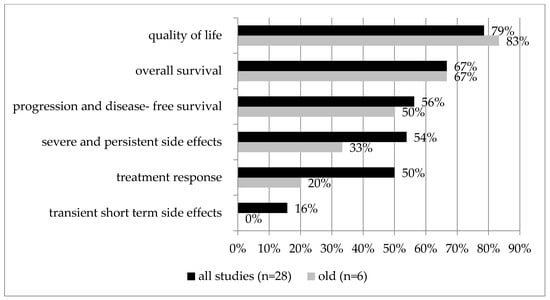
Figure 4.
Older patient subgroup analysis. Outcome category prioritization of all studies (n = 28) compared to the subgroup of older patients (n = 6). A higher percentage means more frequently prioritized as the first or second priority. Percentages are relative to the number of studies that included that outcome category.
The higher the percentage, the more frequently that outcome category was prioritized. Top 1 and top 2 priorities are shown. Percentages are given relative to the number of studies that assessed that outcome category. In total, 30 rankings from 28 studies are included. n represents the number of rankings that assessed the category.
In a subgroup analysis of six studies focusing specifically on older patients [28,36,37,41,47,50] (median age of the study population of 70 years or higher or separate data of this subgroup), quality of life and overall survival were included in five of the six studies. They were also the highest or second-highest priority in most of them (n = 5/6, 83%, and n = 4/6, 67%, respectively), followed by progression- and disease-free survival (n = ½; 50%), severe and persistent side effects (n = 1/3; 33%), treatment response (n = 1/5; 20%), and transient short-term side effects (n = 0/2; 0%; see Table 2, Figure 4).
4. Discussion
In this systematic review, we examined which outcomes of treatment matter most to patients with cancer. While we were particularly interested in the priorities of older patients, the search was expanded because very few studies exist on patient preferences in older adults with cancer. In the 28 included studies, quality of life received high priority most frequently, followed by overall survival, progression- and disease-free survival, and severe and persistent side effects. In the palliative setting and in the subgroup analysis of older patients, quality of life and overall survival were most often prioritized, but in the curative setting, progression- and disease-free survival and treatment response were more important. This suggests that even though quality of life is most important overall on a group level, priorities might change depending on the intent of the treatment, contextual factors, and the age of the patient.
In this systematic review, in which the majority of the studies included younger patients, quality of life was often considered important. Although severe and persistent side effects were assessed in the majority of studies, quality of life was underappreciated and included only in half of the studies. These findings are in line with previous research [52,53,54,55,56,57,58]. In our subgroup analysis of older patients, quality of life was assessed in the majority (75%), and together with overall survival, most frequently prioritized (83%). The outcome category of quality of life included functional and other patient-centered outcomes, but often was described in an unspecific way. However, especially in older patients, specific components of quality of life like cognition and functional abilities are considered important and few patients are willing to trade cognition for survival [59,60]. Thus, due to the inclusion of all ages and the unclear descriptions of quality of life, our review might underestimate the importance of certain aspects of quality of life in older patients.
A recent systematic review on information needs in older patients with cancer showed that after patients received a cancer diagnosis, their focus was on short-term issues like understanding the situation, treatment options, and other practicalities, whereas information on functioning, quality of life, and dealing with late effects were given lower priority [6]. However, decision regret is more often linked to negative long-term outcomes [61], something that was discussed in half of the patients [62]. Since our study also shows that both quality of life (79%) and severe and persistent side effects (54%) were more frequently prioritized outcomes than transient short-term side effects (16%), patients should be aided in assessing and explicitly expressing which long-term outcomes matter most to them.
Although both patients and physicians consider efficacy and physical side effects important in treatment choice, patients also incorporate other factors in their decision-making, like the impact on their daily life, family responsibilities, and the ability to attend important life events [63]. In the translation of general treatment outcomes to their personal situation, patients might interpret the outcomes differently than the physician. In addition, they might not realize that one treatment might have multiple competing effects. If the patient’s interpretation is left unrevealed, this may lead to a treatment choice that may not provide the patient with the benefit that they desire, or may also lead to a negative effect that lessens the benefit. For example, a patient with metastatic cancer may state that extending survival is most important, with an unexpressed underlying desire to care for an ailing partner for as long as possible. If the physician then tailors the treatment to value extending life, intensive palliative chemotherapy may be started. Although this may increase survival, it may in fact also hamper the patient’s caregiving abilities due to the side effects of treatment.
These unique patient values that underlie the preference are not easily incorporated into disease- and treatment-specific decision aids. Moreover, physicians are not good at estimating their patients’ preferences [50]. Thus it can be helpful to have an additional preference assessment conversation as part of decision-making. This will help to clarify what a priority of a specific outcome means to the patient and why it is important to them, and will prevent treatment selection based on wrong interpretations from the patient or misunderstandings by the physician.
In our review, some studies [41,50] used a non-disease and non-treatment-specific generic communication tool developed for patients with multimorbidity by Terri Fried: the Outcome Prioritization Tool (OPT) [15]. During this conversation, the healthcare professional verifies whether he or she understands the trade-offs correctly and invites the patient to explain why the outcomes are important and how they were interpreted [14]. Although this tool has been used in oncology patients before [41,50,64], it might be worthwhile to adapt this tool specifically for cancer patients and the treatment decisions they have to make.
This study has some limitations. Firstly, the studies were heterogeneous; various types of cancer and various tumor stages were included. Furthermore, the studies assessed the priorities with their own defined benefits and risks. Depending on what was most appropriate given the characteristics of cancer-specific treatment regimens, they each asked the outcome categories differently and used different levels of the various attributes. Although the studies had sufficient common denominators to allow for the categorization and combination of results, this does make comparison between the palliative and curative treatment settings more difficult. For example, in a curative setting, overall survival might not be prioritized when described as a small increase in an already high survival rate. However, in the situation of a metastatic disease with a poor prognosis, overall survival might be prioritized, because living a few more months might be important for a patient who is awaiting their first grandchild.
Moreover, multiple methods of assessing these outcome preferences were used, all with their own benefits and risks of bias [13,65]. To be able to compare the various outcome categories relative to each other and to minimize the effect of chance on ending up as a high priority, only studies that assessed at least three categories were included. Studies comparing only two categories and studies where it was not possible to elucidate the relevance of the various outcome categories were excluded. This might have changed the results, but made comparisons possible and allowed an actual trade-off between the benefits and negative effects of treatments to be registered.
Finally, due to the heterogeneity of methods and reporting, we had to simplify the results of the studies to a ranking. This does not give details on whether the outcome priorities are close together or far apart within a study or on how much benefit gain or risk avoidance leads to a change in priority; thus, some information may have been lost. It did, however, allow us to identify the most important outcome categories on a group level. In clinical practice, these could be presented to individual patients who need to make and define their own trade-offs anyway. This pre-selection may prevent them from being overwhelmed by too many choices.
5. Conclusions
In conclusion, understanding how patients prioritize potential outcomes of oncologic treatment and the trade-offs they are willing to make is an important component of shared decision-making. Our systematic review shows that quality of life, overall survival, progression- and disease-free survival, and avoiding severe and persistent side effects of treatment are the outcomes that receive the highest priority in patients with cancer.
Author Contributions
Conceptualization, M.E.H.; methodology M.E.H., P.A.L.S. and A.W.; software, P.A.L.S.; validation, P.A.L.S., A.W. and M.E.H.; formal analysis, P.A.L.S. and A.W.; investigation, P.A.L.S. and M.E.H.; resources M.E.H.; data curation, P.A.L.S. and A.W.; writing—original draft preparation, P.A.L.S.; writing—review and editing, P.A.L.S., M.E.H., J.E.A.P., S.F., M.E.S., P.S., S.R. and S.O.; visualization, P.A.L.S. and M.E.H.; supervision, M.E.H. and J.E.A.P.; project administration, P.A.L.S.; funding acquisition, M.E.H. and P.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by GERONTE. The GERONTE project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 945218.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Appendix A. Search
The following search was performed on 22 December 2020, in both Embase and Medline (((“treatment*”[tiab] OR “health”[tiab]) AND “outcome*”[tiab]) OR “scenario*”[tiab] OR “vignet*”[tiab] OR (“research”[tiab] AND “agenda”[tiab])) AND ((“priorit*”[tiab] OR “preference*”[tiab] OR “trade”[tiab] OR “trade off*”[tiab]) AND (“patient”[tiab] OR “patients”[tiab]) AND (“cancer”[tiab] OR “oncolog*”[tiab] OR “malignan*”[tiab]). Limitations: humans, English or Dutch, 2006, and onward study selection.
Appendix B

Table A1.
Descriptions of the Outcome Categories.
Table A1.
Descriptions of the Outcome Categories.
| Outcome Categories | Descriptions |
|---|---|
| Quality of life | Long-term maintenance of quality of life and functional status. Also includes other patient reported/centered measurements such as keeping one’s independence and social/role functioning. E.g., quality of life, maintaining independence of (instrumental) activities of daily living (iADL), worries, anxiety |
| Transient short-term side effects | Short-term and transient treatment-related toxicity/adverse events that terminate after cessation of treatment or that only require minimal medication. E.g., diarrhea, hair loss, nausea/vomiting, rash and skin change |
| Severe and persistent side effects | More severe adverse treatment events that require intensive treatment, hospitalization or discontinuation of treatment. Often patients will recover from these events, but it can take a long time (>6 months) and involves intensive treatment. Persistent side effects/sequelae inherent or caused by the treatment are also included here. E.g., severe bleeding, gastrointestinal perforation, heart attack, colostomy, neuropathy, fatigue, scars, permanent fecal incontinence, urinary incontinence, infertility |
| Treatment response | All treatment benefits, except for time-dependant measurements. Treatment benefits other than survival, like symptom reduction, response seen on scans, or risk reductions. E.g., symptom control, local control, complete response, partial response, recurrence risk |
| Progression- and disease-free survival | Progression-free survival or disease-free survival, but not overall survival. A time-dependent measurement that indicates how long the disease is under control or cured. Also a measurement of efficacy, but time-dependent. E.g., disease-free survival, progression-free survival, time to treatment failure |
| Overall survival | Overall survival and mortality independent of the cancer (treatment) at a certain time point during follow-up. E.g., overall survival, mortality at a certain time point, extending life |
Appendix C

Table A2.
Quality assessment based on the Newcastle–Ottawa Scale.
Table A2.
Quality assessment based on the Newcastle–Ottawa Scale.
| selection | 1. Representativeness of the cohort | + | Participants are patients who have cancer |
| − | Participants are asked to answer as if they have cancer or as if they have a different stage of cancer | ||
| ? | The sampling strategy leads to non-representativeness | ||
| study design | 2. Research question | + | Well-defined research question and hypothesis |
| − | Vaguely described research question | ||
| ? | No research question present | ||
| 3. Selection of outcomes included | + | Clearly described why the different outcomes (= attributes) and levels are included in the study, e.g., based on previous research, pilot study, or qualitative research | |
| − | Described vaguely, but not clear why these items or levels are selected, e.g., in a discussion with researchers | ||
| ? | No description on why these outcomes are chosen | ||
| 4. Assessment of outcome priorities | + | Clear description of the definition of the outcomes and method of assessment and clear understanding of how the outcomes are described to the patient | |
| − | Unclear description of outcomes or unclear method of assessment, e.g., quality of life is mentioned without clear description | ||
| ? | No description of definitions and unclear method of assessment | ||
| outcome | 5. Analysis | + | Clear description of method of analysis |
| − | Unclear description of method of analysis | ||
| ? | No description | ||
| 6. Outcome reporting | + | Scores for all outcomes separately reported | |
| ± | Outcomes reported, but hard to make a ranking or early stage and advanced stage of disease not separately reported | ||
| − | Not all scores reported | ||
| ? | Unclear whether all outcomes are reported |
Appendix D

Table A3.
Quality Assessment Per Study.
Table A3.
Quality Assessment Per Study.
| Author, Year | Representativeness | Research Question | Selection of Outcomes | Assessment of Outcome Priorities | Analysis | Outcome Reporting |
|---|---|---|---|---|---|---|
| Johnson, 2006 | + | + | + | + | + | + |
| Pieterse, 2007 | + | + | + | + | + | + |
| Thrumurthy, 2011 | + | + | + | − | + | + |
| Mohamed, 2011 | + | + | + | + | + | + |
| Park, 2012 | − | + | + | + | + | ± |
| Jorgensen, 2013 | + | + | − | + | + | + |
| Havrilesky, 2014 | + | + | + | + | + | + |
| DaCosta, 2014 | + | + | + | + | + | + |
| Molinari, 2014 | − | + | − | + | + | + |
| Muhlbacher, 2015 | + | + | + | + | + | + |
| Uemura, 2016 | + | + | + | + | + | + |
| Thill,2016 | + | + | + | + | + | ± |
| Chau, 2016 | + | + | + | + | + | + |
| Gonzalez, 2017 | + | + | + | + | + | + |
| Schmidt, 2017 | + | + | + | + | + | + |
| Bröckelmann, 2019 | + | + | + | + | + | + |
| Sun, 2019 | + | + | + | + | + | + |
| Liu, 2019 | + | + | + | − | + | + |
| Festen, 2019 | + | + | ? | + | + | + |
| van der Valk, 2020 | + | + | + | + | + | + |
| Valenti, 2020 | + | + | ? | + | + | ± |
| Wong, 2020 | − | + | + | + | + | + |
| Stegmann, 2020 | + | + | ? | + | + | ± |
| Fifer, 2020 | + | + | + | − | + | + |
| Khan, 2020 | + | + | − | + | + | + |
| Festen, 2021 | + | + | ? | + | + | + |
| Werner, 2021 | + | + | + | + | + | + |
| Weilandt, 2021 | + | + | − | − | + | + |
References
- Soto-Perez-De-Celis, E.; Li, D.; Yuan, Y.; Lau, M.; Hurria, A. Geriatric Oncology 2, Functional versus Chronological Age: Geriatric Assessments to Guide Decision Making in Older Patients with Cancer. Lancet Oncol. 2018, 19, e305–e316. [Google Scholar] [CrossRef]
- DuMontier, C.; Loh, K.P.; Soto-Perez-de-Celis, E.; Dale, W. Decision Making in Older Adults with Cancer. J. Clin. Oncol. 2021, 39, 2164–2174. [Google Scholar] [CrossRef] [PubMed]
- Kane, H.L.; Halpern, M.T.; Squiers, L.B.; Treiman, K.A.; McCormack, L.A. Implementing and Evaluating Shared Decision Making in Oncology Practice. CA Cancer J. Clin. 2014, 64, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.P.; Miller-Sonet, E.; Nipp, R.D.; Kamal, A.H.; Love, S.; Rocque, G.B. Importance of Quality-of-Life Priorities and Preferences Surrounding Treatment Decision Making in Patients with Cancer and Oncology Clinicians. Cancer 2020, 126, 3534–3541. [Google Scholar] [CrossRef] [PubMed]
- Stiggelbout, A.M.; Pieterse, A.H.; De Haes, J.C.J.M. Shared Decision Making: Concepts, Evidence, and Practice. Patient Educ. Couns. 2015, 98, 1172–1179. [Google Scholar] [CrossRef]
- Hamaker, M.E.; van Walree, I.C.; Seghers, P.A.L.; van den Bos, F.; Soubeyran, P.; O’Hanlon, S.; Rostoft, S. Information Needs of Older Patients Newly Diagnosed with Cancer. J. Geriatr. Oncol. 2021, in press. [CrossRef]
- Extermann, M. Interaction between Comorbidity and Cancer. Cancer Control 2007, 14, 14–22. [Google Scholar] [CrossRef]
- Extermann, M.; Lee, H. Measurement and Impact of Comorbidity in Older Cancer Patients. Crit. Rev. Oncol. Hematol. 2000, 35, 181–200. [Google Scholar] [CrossRef]
- Sarfati, D.; Koczwara, B.; Jackson, C. The Impact of Comorbidity on Cancer and Its Treatment. CA Cancer J. Clin. 2016, 66, 337–350. [Google Scholar] [CrossRef]
- Wildiers, H.; Mauer, M.; Pallis, A.; Hurria, A.; Mohile, S.G.; Luciani, A.; Curigliano, G.; Extermann, M.; Lichtman, S.M.; Ballman, K.; et al. End Points and Trial Design in Geriatric Oncology Research: A Joint European Organisation for Research and Treatment of Cancer-Alliance for Clinical Trials in Oncology-International Society of Geriatric Oncology Position Article. J. Clin. Oncol. 2013, 31, 3711–3718. [Google Scholar] [CrossRef]
- Karolina, A.; Maguire, R. A Systematic Review of the Factors Associated with Regret Post-Cancer Treatment. J. Psychosoc. Oncol. 2020, 40, 1–23. [Google Scholar] [CrossRef]
- Lis, C.G.; Rodeghier, M.; Gupta, D. Patient Preference and Adherence Distribution and Determinants of Patient Satisfaction in Oncology: A Review of the Literature. Patient Prefer. Adherence 2009, 3, 287–304. [Google Scholar] [PubMed]
- Blinman, P.; King, M.; Norman, R.; Viney, R.; Stockler, M.R. Preferences for Cancer Treatments: An Overview of Methods and Applications in Oncology. Ann. Oncol. 2012, 23, 1104–1110. [Google Scholar] [CrossRef] [PubMed]
- Stegmann, M.E.; Festen, S.; Brandenbarg, D.; Schuling, J.; van Leeuwen, B.; de Graeff, P.; Berendsen, A.J. Using the Outcome Prioritization Tool (OPT) to Assess the Preferences of Older Patients in Clinical Decision-Making: A Review. Maturitas 2019, 128, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Fried, T.R.; Tinetti, M.; Agostini, J.; Iannone, L.; Towle, V. Health Outcome Prioritization to Elicit Preferences of Older Persons with Multiple Health Conditions. Patient Educ. Couns. 2011, 83, 278–282. [Google Scholar] [CrossRef]
- Thill, M.; Pisa, G.; Isbary, G. Targets for Neoadjuvant Thearpy-The Preferences of Patients with Early Breast Cancer. Geburtshilfe Frauenheilkd. 2016, 76, 551–556. [Google Scholar]
- Doolin, J.W.; Halpin, M.; Berry, J.L.; Hshieh, T.; Zerillo, J.A. Why Focus on Patient-Reported Outcome Measures in Older Colorectal Cancer Patients? Eur. J. Surg. Oncol. 2020, 46, 394–401. [Google Scholar] [CrossRef]
- LeBlanc, T.W.; Abernethy, A.P. Patient-Reported Outcomes in Cancer Care-Hearing the Patient Voice at Greater Volume. Nat. Rev. Clin. Oncol. 2017, 14, 763–772. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Center for Open Science. OSF-Open Registry. Available online: https://osf.io/ekq6u (accessed on 19 January 2022).
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analysis. 2012. Available online: https://web.archive.org/web/20210716121605id_/http://www3.med.unipmn.it/dispense_ebm/2009-2010/Corso%20Perfezionamento%20EBM_Faggiano/NOS_oxford.pdf (accessed on 22 October 2021).
- Bridges, J.F.P.; Hauber, A.B.; Marshall, D.; Lloyd, A.; Prosser, L.A.; Regier, D.A.; Johnson, F.R.; Mauskopf, J. Conjoint Analysis Applications in Health—A Checklist: A Report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value Health 2011, 14, 403–413. [Google Scholar] [CrossRef]
- Yepes-Nuñez, J.J.; Zhang, Y.; Xie, F.; Alonso-Coello, P.; Selva, A.; Schünemann, H.; Guyatt, G. Forty-Two Systematic Reviews Generated 23 Items for Assessing the Risk of Bias in Values and Preferences’ Studies. J. Clin. Epidemiol. 2017, 85, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration (FDA). Patient Preference Information-Guidance for Industry, Food and Drug Administration Staff, and Other Stakeholders; FDA: Silver Spring, MD, USA, 2016.
- Pieterse, A.H.; Berkers, F.; Baas-Thijssen, M.C.M.; Marijnen, C.A.M.; Stiggelbout, A.M. Adaptive Conjoint Analysis as Individual Preference Assessment Tool: Feasibility through the Internet and Reliability of Preferences. Patient Educ. Couns. 2010, 78, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.F.; Hauber, A.B.; Neary, M.P. Patient Benefit-Risk Preferences for Targeted Agents in the Treatment of Renal Cell Carcinoma. Pharmacoeconomics 2011, 29, 977–988. [Google Scholar] [CrossRef] [PubMed]
- Thrumurthy, S.G.; Morris, J.J.A.; Mughal, M.M.; Ward, J.B. Discrete-Choice Preference Comparison between Patients and Doctors for the Surgical Management of Oesophagogastric Cancer. Br. J. Surg. 2011, 98, 1124–1131. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, M.L.; Young, J.M.; Solomon, M.J. Adjuvant Chemotherapy for Colorectal Cancer: Age Differences in Factors Influencing Patients’ Treatment Decisions. Patient Prefer. Adherence 2013, 7, 827–834. [Google Scholar] [CrossRef]
- Molinari, M.; de Coutere, S.; Krahn, M.; Helton, S.; Urbach, D.R. Patients’ Preferences and Trade-Offs for the Treatment of Early Stage Hepatocellular Carcinoma. J. Surg. Res. 2014, 189, 57–67. [Google Scholar] [CrossRef]
- van der Valk, M.J.M.; van der Sande, M.E.; Toebes, R.E.; Breukink, S.O.; Bröker, M.E.E.; Doornebosch, P.G.; Maliko, N.; Neijenhuis, P.A.; Marinelli, A.W.K.S.; Peters, F.P.; et al. Importance of Patient Reported and Clinical Outcomes for Patients with Locally Advanced Rectal Cancer and Their Treating Physicians. Do Clinicians Know What Patients Want? Eur. J. Surg. Oncol. 2020, 46, 1634–1641. [Google Scholar] [CrossRef]
- Werner, R.N.; Gaskins, M.; Dressler, C.; Nast, A.; Schaefer, C.; Aigner, F.; Siegel, R. Measuring Importance of Outcomes to Patients: A Cross-Sectional Survey for the German Anal Cancer Guideline. J. Clin. Epidemiol. 2021, 129, 40–50. [Google Scholar] [CrossRef]
- Park, M.H.; Jo, C.; Bae, E.Y.; Lee, E.K. A Comparison of Preferences of Targeted Therapy for Metastatic Renal Cell Carcinoma between the Patient Group and Health Care Professional Group in South Korea. Value Health 2012, 15, 933–939. [Google Scholar] [CrossRef]
- Havrilesky, L.J.; Secord, A.A.; Ehrisman, J.A.; Berchuck, A.; Valea, F.A.; Lee, P.S.; Gaillard, S.L.; Samsa, G.P.; Cella, D.; Weinfurt, K.P.; et al. Patient Preferences in Advanced or Recurrent Ovarian Cancer. Cancer 2014, 120, 3651–3659. [Google Scholar] [CrossRef]
- Dacosta Dibonaventura, M.; Copher, R.; Basurto, E.; Faria, C.; Lorenzo, R. Patient Preferences and Treatment Adherence among Women Diagnosed with Metastatic Breast Cancer. Am. Health Drug Benefits 2014, 7, 386–396. [Google Scholar] [PubMed]
- Mühlbacher, A.C.; Bethge, S. Patients’ Preferences: A Discrete-Choice Experiment for Treatment of Non-Small-Cell Lung Cancer. Eur. J. Health Econ. 2015, 16, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Uemura, H.; Matsubara, N.; Kimura, G.; Yamaguchi, A.; Ledesma, D.A.; DiBonaventura, M.; Mohamed, A.F.; Basurto, E.; McKinnon, I.; Wang, E.; et al. Patient Preferences for Treatment of Castration-Resistant Prostate Cancer in Japan: A Discrete-Choice Experiment. BMC Urol. 2016, 16, 63. [Google Scholar] [CrossRef] [PubMed]
- Chun-Leung Chau, D.; Wang, D.; Tedesco, A.; McGuffin, M.; di Prospero, L.; Fitch, M.; Cao, X.; Feldman-Stewart, D.; Ellis, J.; Szumachter, E. Prostate Cancer Patients’ Preferences for Intermittent vs. Continuous Androgen Deprivation—A Pilot Institutional Study. J. Med. Imaging Radiat. Sci. 2016, 47, 108–112. [Google Scholar] [CrossRef] [PubMed][Green Version]
- González, J.M.; Ogale, S.; Morlock, R.; Posner, J.; Hauber, B.; Sommer, N.; Grothey, A. Patient and Physician Preferences for Anticancer Drugs for the Treatment of Metastatic Colorectal Cancer: A Discrete-Choice Experiment. Cancer Manag. Res. 2017, 9, 149–158. [Google Scholar] [CrossRef]
- Liu, F.X.; Witt, E.A.; Ebbinghaus, S.; Dibonaventura Beyer, G.; Basurto, E.; Joseph, R.W. Patient and Oncology Nurse Preferences for the Treatment Options in Advanced Melanoma: A Discrete Choice Experiment. Cancer Nurs. 2019, 42, E52–E59. [Google Scholar] [CrossRef]
- Wong, X.Y.; Lim, A.Q.J.; Shen, Q.; Chia, J.W.K.; Chew, M.H.; Tan, W.S.; Wee, H.L. Patient Preferences and Predicted Relative Uptake for Targeted Therapies in Metastatic Colorectal Cancer: A Discrete Choice Experiment. Curr. Med. Res. Opin. 2020, 36, 1677–1686. [Google Scholar] [CrossRef] [PubMed]
- Stegmann, M.E.; Brandenbarg, D.; Berendsen, A.J.; Reyners, A.K.L.; van Geffen, W.H.; Hiltermann, T.J.N. Prioritisation of Treatment Goals among Older Patients with Non-Curable Cancer: The OPTion Randomised Controlled Trial in Dutch Primary Care. Br. J. Gen. Pract. 2020, 70, E450–E456. [Google Scholar] [CrossRef]
- Weilandt, J.; Diehl, K.; Schaarschmidt, M.L.; Kiecker, F.; Sasama, B.; Pronk, M.; Ohletz, J.; Könnecke, A.; Müller, V.; Utikal, J.; et al. Patient Preferences in Adjuvant and Palliative Treatment of Advanced Melanoma: A Discrete Choice Experiment. Acta Derm.-Venereol. 2020, 100, 1–9. [Google Scholar] [CrossRef]
- Johnson, F.R.; Hauber, A.B.; Osoba, D.; Hsu, M.A.; Coombs, J.; Copley-Merriman, C. Are Chemotherapy Patients’ HRQoL Importance Weights Consistent with Linear Scoring Rules? A Stated-Choice Approach. Qual. Life Res. 2006, 15, 285–298. [Google Scholar] [CrossRef]
- Schmidt, K.; Damm, K.; Vogel, A.; Golpon, H.; Manns, M.P.; Welte, T.; Graf von der Schulenburg, J.M. Therapy Preferences of Patients with Lung and Colon Cancer: A Discrete Choice Experiment. Patient Prefer. Adherence 2017, 11, 1647–1656. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wang, H.; Xu, N.; Li, J.; Shi, J.; Zhou, N.; Ni, M.; Hu, X.; Chen, Y. Patient Preferences for Chemotherapy in the Treatment of Non-Small Cell Lung Cancer: A Multicenter Discrete Choice Experiment (DCE) Study in China. Patient Prefer. Adherence 2019, 13, 1701–1709. [Google Scholar] [CrossRef] [PubMed]
- Bröckelmann, P.J.; McMullen, S.; Wilson, J.B.; Mueller, K.; Goring, S.; Stamatoullas, A.; Zagadailov, E.; Gautam, A.; Huebner, D.; Dalal, M.; et al. Patient and Physician Preferences for First-Line Treatment of Classical Hodgkin Lymphoma in Germany, France and the United Kingdom. Br. J. Haematol. 2019, 184, 202–214. [Google Scholar] [CrossRef]
- Festen, S.; Kok, M.; Hopstaken, J.S.; van der Wal-Huisman, H.; van der Leest, A.; Reyners, A.K.L.; de Bock, G.H.; de Graeff, P.; van Leeuwen, B.L. How to Incorporate Geriatric Assessment in Clinical Decision-Making for Older Patients with Cancer. An Implementation Study. J. Geriatr. Oncol. 2019, 10, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Valentí, V.; Ramos, J.; Pérez, C.; Capdevila, L.; Ruiz, I.; Tikhomirova, L.; Sánchez, M.; Juez, I.; Llobera, M.; Sopena, E.; et al. Increased Survival Time or Better Quality of Life? Trade-off between Benefits and Adverse Events in the Systemic Treatment of Cancer. Clin. Transl. Oncol. Off. Publ. Fed. Span. Oncol. Soc. Natl. Cancer Inst. Mex. 2020, 22, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Fifer, S.; Galinsky, J.; Richard, S. Myeloma Patient Value Mapping: A Discrete Choice Experiment on Myeloma Treatment Preferences in the UK. Patient Prefer. Adherence 2020, 14, 1283–1293. [Google Scholar] [CrossRef]
- Festen, S.; Stegmann, M.E.; Prins, A.; van Munster, B.C.; van Leeuwen, B.L.; Halmos, G.B.; de Graeff, P.; Brandenbarg, D. How Well Do Healthcare Professionals Know of the Priorities of Their Older Patients Regarding Treatment Outcomes? Patient Educ. Couns. 2021, 104, 2358–2363. [Google Scholar] [CrossRef]
- Khan, N.; Feliciano, J.; Müller, K.; He, M.; Tao, R.; Korol, E.; Dalal, M.; Rebeira, M.; Matasar, M. Patient Preferences for First-Line Treatment of Classical Hodgkin Lymphoma: A US Survey and Discrete Choice Experiment. Leuk. Lymphoma 2020, 2630–2637. [Google Scholar] [CrossRef]
- Shrestha, A.; Martin, C.; Burton, M.; Walters, S.; Collins, K.; Wyld, L. Quality of Life versus Length of Life Considerations in Cancer Patients: A Systematic Literature Review. Psycho-Oncology 2019, 28, 1367–1380. [Google Scholar] [CrossRef]
- Meropol, N.J.; Egleston, B.L.; Buzaglo, J.S.; Benson, A.B.; Cegala, D.J.; Diefenbach, M.A.; Fleisher, L.; Miller, S.M.; Sulmasy, D.P.; Weinfurt, K.P.; et al. Cancer Patient Preferences for Quality and Length of Life. Cancer 2008, 113, 3459–3466. [Google Scholar] [CrossRef]
- van der Plas-Krijgsman, W.G.; de Boer, A.Z.; de Jong, P.; Bastiaannet, E.; van den Bos, F.; Mooijaart, S.P.; Liefers, G.J.; Portielje, J.E.A.; de Glas, N.A. Predicting Disease-Related and Patient-Reported Outcomes in Older Patients with Breast Cancer—A Systematic Review. J. Geriatr. Oncol. 2021, 12, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Hamaker, M.E.; Schulkes, K.J.; ten Bokkel Huinink, D.; van Munster, B.C.; van Huis, L.H.; van den Bos, F. Evaluation and Reporting of Quality of Life Outcomes in Phase III Chemotherapy Trials for Poor Prognosis Malignancies. Qual. Life Res. 2017, 26, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Scheepers, E.R.M.; van Huis-Tanja, L.H.; Emmelot-Vonk, M.H.; Hamaker, M.E. Study Objectives in Clinical Trials in Older Patients with Solid Malignancies: Do We Measure What Matters? Qual. Life Res. 2021, 30, 1833–1839. [Google Scholar] [CrossRef] [PubMed]
- Bien, D.R.; Danner, M.; Vennedey, V.; Civello, D.; Evers, S.M.; Hiligsmann, M. Patients’ Preferences for Outcome, Process and Cost Attributes in Cancer Treatment: A Systematic Review of Discrete Choice Experiments. Patient 2017, 10, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Guerra, R.L.; Castaneda, L.; de Albuquerque, R.D.C.R.; Ferreira, C.B.T.; Corrêa, F.D.M.; Fernandes, R.R.A.; de Almeida, L.M. Patient Preferences for Breast Cancer Treatment Interventions: A Systematic Review of Discrete Choice Experiments. Patient 2019, 12, 559–569. [Google Scholar] [CrossRef]
- Fried, T.R.; Bradley, E.H. What Matters to Seriously Ill Older Persons Making End-of-Life Treatment Decisions? A Qualitative Study. J. Palliat. Med. 2003, 6, 237–244. [Google Scholar] [CrossRef]
- Dhakal, P.; Wichman, C.S.; Pozehl, B.; Weaver, M.; Fisher, A.L.; Bociek, R.G.; Bhatt, V.R.; Vose, J. Preferences of Adults with Cancer for Systemic Cancer Treatment: Do Preferences Differ Based on Age? Future Oncol. 2021, 18, 311–321. [Google Scholar] [CrossRef]
- Hoffman, R.M.; Lo, M.; Clark, J.A.; Albertsen, P.C.; Barry, M.J.; Goodman, M.; Penson, D.F.; Stanford, J.L.; Stroup, A.M.; Hamilton, A.S. Treatment Decision Regret Among Long-Term Survivors of Localized Prostate Cancer: Results From the Prostate Cancer Outcomes Study. J. Clin. Oncol. 2017, 35, 2306–2314. [Google Scholar] [CrossRef]
- Kuijpers, M.M.T.; Veenendaal, H.; Engelen, V.; Visserman, E.; Noteboom, E.A.; Stiggelbout, A.M.; May, A.M.; Wit, N.; Wall, E.; Helsper, C.W. Shared Decision Making in Cancer Treatment: A Dutch National Survey on Patients’ Preferences and Perceptions. Eur. J. Cancer Care 2021, 31, e13534. [Google Scholar] [CrossRef]
- Rocque, G.B.; Rasool, A.; Williams, B.R.; Wallace, A.S.; Niranjan, S.J.; Halilova, K.I.; Turkman, Y.E.; Ingram, S.A.; Williams, C.P.; Forero-Torres, A.; et al. What Is Important When Making Treatment Decisions in Metastatic Breast Cancer? A Qualitative Analysis of Decision-Making in Patients and Oncologists. Oncologist 2019, 24, 1313–1321. [Google Scholar] [CrossRef]
- Festen, S.; van Twisk, Y.Z.; van Munster, B.C.; de Graeff, P. ‘What Matters to You?’ Health Outcome Prioritisation in Treatment Decision-Making for Older Patients. Age Ageing 2021, 50, 2264–2269. [Google Scholar] [CrossRef] [PubMed]
- Hazlewood, G.S. Measuring Patient Preferences: An Overview of Methods with a Focus on Discrete Choice Experiments. Rheum. Dis. Clin. N. Am. 2018, 44, 337–347. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).