Fibrosis in Mesothelioma: Potential Role of Lysyl Oxidases
Abstract
Simple Summary
Abstract
1. Introduction
2. Altered Expression of Lysyl Oxidases in Non-Cancerous Settings
2.1. Idiopathic Pulmonary Fibrosis (IPF)
2.2. Other Non-Cancerous Disease States
3. Altered Expression of Lysyl Oxidases in Cancerous Settings
3.1. Breast Cancer
3.2. Renal Cell Cancer
3.3. Pancreatic Cancer
3.4. Liver Cancer
3.5. Lung Cancer
4. Altered Expression of Lysyl Oxidases in Malignant Pleural Mesothelioma
5. Inhibitors of Lysyl Oxidases
5.1. First Generation of Lysyl Oxidase Inhibitors
5.1.1. β-Aminopropionitrile (BAPN)
5.1.2. Polyphenols Epigallocatechin Gallate (EGCG) and Ellagic Acid (EA)
5.1.3. Copper Chelators (Tetrathimolybdate and D-Penicillamine)
| Inhibitor | Company | Molecule Type | Target | Clinical Details | Clinical Trial Identifier and Name | Disease | Summary Results | Reference |
|---|---|---|---|---|---|---|---|---|
| Beta-aminopro pionitrile (BAPN) | National heart Institute | Small molecule inhibitor | Pan-LOXs, SSAO and DAO | Phase 1–3 g/day for 22–67 days n = 4 | Scleroderma | ↑urine HYD ↑α:β collagen chains implying ↓crosslinks Bone formation changes | [171] | |
| Beta-aminopro pionitrile (BAPN) | University of Arizona | Small molecule inhibitor | 250 mg 4 times daily for 3 weeks n = 5 | Fibrous based urethral strictures | No adverse effects. Unclear therapeutic benefit. Demonstrated efficacy. ↑ in acid-soluble collagen. ↓ dermal scar strength. | [162] | ||
| Simtuzumab (GS-6624) | Gilead | Humanised Antibody | LOXL2 | Simtuzumab (200 or 700 mg) with Ruxolitinib 6 cycles of 28 days (~6 months) n = 54 | Phase 2 NCT01369498 | Thrombocythaemia myelofibrosis | No clinical benefit in bone marrow fibrosis | [172] |
| Simtuzumab (GS-6624) | Gilead | Humanised Antibody | LOXL2 | 75 mg or 125 mg subcutaneous for 96 weeks n = 234 | Phase 2 NCT01672853 | Liver fibrosis in adults with primary sclerosing cholangitis | No significant clinical benefit to patients | [173] |
| Simtuzumab (GS-6624) | Gilead | Humanised Antibody | LOXL2 | Subcutaneous 125 mg/mL single dose once a week. Up to 254 wks | Phase 2 NCT01769196, NCT01759511 | Idiopathic pulmonary fibrosis | No ↑ progression free survival. Not recommended to progress in IPF | [174] |
| Simtuzumab (GS-6624) | Gilead | Humanised Antibody | LOXL2 | Intravenous 700 mg every 2 wks for 22 wks (~6 months) | Phase 2 NCT01707472 | Chronic Liver fibrosis in HIV and HCV–infected adults | Well tolerated and modulation of TGFB3 | [175] |
| Simtuzumab (GS-6624) | Gilead | Humanised Antibody | LOXL2 | Combined with Gemcitabine (1000 mg/m2) Simtuzumab either 200 or 700 mg (~3 months of treatment) n = 240 | Phase 2 NCT01472198 | Metastatic pancreatic adenocarcinoma | No ↑ OS (overall survival) | [176] |
| Simtuzumab (GS-6624) | Gilead | Humanised Antibody | LOXL2 | Combination with FOLFIRI, Simtuzumab 200 or 700 mg n = 249 (second line) (~6 months of treatment) | Phase 2 NCT01479465 | Metastatic KRAS mutant colorectal adenocarcinoma | Simtuzumab did not improve clinical outcomes | [177] |
| Simtuzumab (GS-6624) | Gilead | Humanised Antibody | LOXL2 | Subcutaneous weekly injections of 75 or 125 mg of Simtuzumab over 240 wks n = 219 | Phase 2 NCT01672866; NCT01672879; | Liver Fibrosis (nonalcoholic steatohepatitis, NASH) | Simtuzumab did not improve clinical outcomes | [178] |
| Simtuzumab (GS-6624) | Gilead | Humanised Antibody | LOXL2 | 200 mg, 700 mg or placebo by intravenous infusion every 2 weeks n = 258 | Phase 2 (NCT01672879) | Nonalcoholic Steatohepatitis | Regression of fibrosis associated reduced liver-related complications | [179] |
| Epigallocatechin Gallate (EGCG) | Northumbria University | Polyphenol | Aldehydes | Oral 135 and 270 mg single dose | Phase 1 NCT00981292 | Healthy subjects | No adverse effects | No Results Posted |
| Epigallocatechin Gallate (EGCG) | The University of Texas Health Science Center at San Antonio | Polyphenol | Aldehydes | Oral 450 mg twice a day for 1 year n = 50 | Phase 1 NCT02891538 | Primary colon or rectal adenocarcinoma | Study completion in 2023 | No Results Posted |
| Epigallocatechin Gallate (EGCG) | National Cancer Institute (NIH) | Polyphenol | Aldehydes | Oral 800 or 1200 mg for 14–28 days prior to resection | Phase 2 | Bladder cancer | PK: EGG levels increased. No significant changes in biomarkers | [180] |
| Epigallocatechin Gallate (EGCG) | University of California, San Francisco | Polyphenol | Aldehydes | Patients: 600 mg EGCG capsules once daily by mouth for two weeks | Phase1 NCT03928847 | Idiopathic pulmonary fibrosis | Reduction in serum biomarkers collagen oligomeric matrix protein (COMP) and periostin and in tissue Col1, snail, pSMAD3, fibronectin; n = 4 | [181] |
| Tetrathiomolybdate (TM) | University of Michigan | Copper chelator | Copper | n = 23 | Phase1/2 NCT00189176 | Idiopathic pulmonary fibrosis | Completed in 2006 | No Results Posted |
| Tetrathiomolybdate (TM) | New York University School of Medicine & University of Michigan | Copper chelator | Copper | n = 30 | Phase 2 | Malignant Pleural Mesothelioma | Following cytoreduction surgery, antiangiogenic effects observed with minimal toxicity. | [182] |
| Tetrathiomolybdate (TM) | University of Michigan | Copper chelator | Copper | Oral 180 mg/day n = 15 | Phase 2 | Advanced kidney cancer | Well-tolerated and reduces copper in serum. Potential as an antiangiogenic therapy | [183] |
| Tetrathiomolybdate (TM) | University of Michigan | Copper chelator | Copper | n = 18 90, 105, 120 mg/day 90 days | Phase 1 | Metastatic solid tumors including breast, colon, lung, and prostate cancers | Toxicity: mild anemia | [184] |
| Tetrathiomolybdate (TM) | Weill Cornell Medicine Iris Cantor Breast Center | Copper Chelator | Pan-LOXs | Oral 8–17 mg/dL for 2 years n = 75 | Phase 2 NCT00195091 | Breast Cancer stage II triple-negative breast cancer (TNBC), stage III and stage IV without any evidence of disease (NED) | No significant ↑ OS | [185] |
| ATN-224 | Cancer research UK | Copper chelator | Copper | Once daily | Phase 2 NCT00674557 | Breast cancer | Terminated 2009 No results posted | No Results Posted |
| D-penicillamine | National institute of respiratory diseases in Mexico City | Copper Chelator | Non specific Pan-LOXs | Daily 600 mg n = 56 Combined with colchicine 1 mg daily and prednisone 15 mg/d 5 year study | Phase 2 | Idiopathic pulmonary fibrosis | No improvement in disease progression | [186] |
| D-penicillamine | university of California | Copper Chelator | Non specific Pan-LOXs | Oral 750–1000 mg/day or 125 mg n = 134 24 months study | Phase 2 | Diffuse cutaneous systemic sclerosis | High dose had 80% adverse event related withdrawal A reduction in cardiomegaly | [187] |
| D-penicillamine | New approaches to brain tumour therapy CNS consortium (NCI) | Copper Chelator | Non specific Pan-LOXs | 250 mg/day n = 40 | Phase 2 | Glioblastoma —post resection | Adverse effects: hypocupremia No change in survival | [188] |
| PXS-5505 | Pharmaxis | Small molecule inhibitor | Pan-LOXs | Orally as 2 × 100 mg twice a day | Phase 1/2a NCT04676529 | Myelofibrosis | NA | NA |
| PXS-5505 | university of Rochester | Small molecule inhibitor | Pan-LOXs | Orally 100–200 mg BID in combination with Atezolizumab (Anti-PD-L1) 1200 mg every 3 weeks and Bevacizumab (Anti-VEGF) 15 mg/kg every 3 weeks | Phase 1b/2 NCT05109052 | Unresectable hepatocellular Carcinoma | NA | NA |
| PXS-6302 | Pharmaxis | Small molecule inhibitor | Pan-LOXs | Escalating dose 0.6–8 mg for 7 days topical | Phase 1/1c SOLARIA I ACTRN12621000322831 | Healthy subjects Acute and established scar | NA | NA |
| PXS-5382 | Pharmaxis | Small molecule inhibitor | LOXL2/3 | Single dose | Phase 1 NCT04183517 | Healthy subjects | No adverse effects | No Results Posted |
| PXS-5338 | Pharmaxis | Small molecule inhibitor | LOXL2/3 | Single dose | Phase 1 ACTRN12617001444370 | Healthy subjects | No adverse effects | No Results Posted [160] |
| PAT-1251 (GB2064) | PharmAkea (Now with Galecto) | Small molecule inhibitor | LOXL2 | Oral 150–4000 mg single dose | Phase 1 NCT02852551 | NA | No adverse effects | NCT02852551 |
| PAT-1251 (GB2064) | PharmAkea (Now with Galecto) | Small molecule inhibitor | LOXL2 | Oral 150–4000 mg single dose orally as 4 × 250 mg tablets twice a day | Phase 2a MYLOX-1 NCT04679870 NCT04054245—withdrawn MD Anderson | Myelofibrosis. | NA | NCT04679870 |
5.2. 2nd Generation Inhibitors
Lysyl Oxidase like 2 Antibodies (Simtuzumab)
5.3. Lysyl Oxidase Small Molecule Inhibitors: The Way of the Future
6. Discussion
- Is fibrosis limiting lymphatic drainage to cause pleural fluid buildup? Can lysyl oxidases inhibitors alleviate pleural fluid buildup?
- Will lysyl oxidase inhibitors be synergistic with chemotherapies and immune therapies in patients?
- How do lysyl oxidase inhibitors affect macrophages and their interaction with T-cells?
- Will tumors adapt to lysyl oxidase inhibition?
- Is there redundancy in the lysyl oxidase family members, particularly when treated with lysyl oxidase inhibitors?
- Are LOX/LOXL inhibitors synergistic with immune therapies and/or chemotherapies in preclinical mesothelioma and patients?
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carbone, M.; Adusumilli, P.S.; Alexander, H.R., Jr.; Baas, P.; Bardelli, F.; Bononi, A.; Bueno, R.; Felley-Bosco, E.; Galateau-Salle, F.; Jablons, D.; et al. Mesothelioma: Scientific clues for prevention, diagnosis, and therapy. CA Cancer J. Clin. 2019, 69, 402–429. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.; Baris, Y.I.; Bertino, P.; Brass, B.; Comertpay, S.; Dogan, A.U.; Gaudino, G.; Jube, S.; Kanodia, S.; Partridge, C.R.; et al. Erionite exposure in North Dakota and Turkish villages with mesothelioma. Proc. Natl. Acad. Sci. USA 2011, 108, 13618–13623. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.; Ly, B.H.; Dodson, R.F.; Pagano, I.; Morris, P.T.; Dogan, U.A.; Gazdar, A.F.; Pass, H.I.; Yang, H. Malignant mesothelioma: Facts, myths, and hypotheses. J. Cell Physiol. 2012, 227, 44–58. [Google Scholar] [CrossRef] [PubMed]
- Maher, B. Epidemiology: Fear in the dust. Nature 2010, 468, 884–885. [Google Scholar] [CrossRef]
- Arnold, D.T.; Maskell, N.A. Biomarkers in mesothelioma. Ann. Clin. Biochem. 2018, 55, 49–58. [Google Scholar] [CrossRef]
- Gunatilake, S.; Lodge, D.; Neville, D.; Jones, T.; Fogg, C.; Bassett, P.; Begum, S.; Kerley, S.; Marshall, L.; Glaysher, S.; et al. Predicting survival in malignant pleural mesothelioma using routine clinical and laboratory characteristics. BMJ Open Respir. Res. 2021, 8, e000506. [Google Scholar] [CrossRef]
- Shavelle, R.; Vavra-Musser, K.; Lee, J.; Brooks, J. Life Expectancy in Pleural and Peritoneal Mesothelioma. Lung. Cancer Int. 2017, 2017, 2782590. [Google Scholar] [CrossRef]
- Kindler, H.L.; Ismaila, N.; Armato, S.G., 3rd; Bueno, R.; Hesdorffer, M.; Jahan, T.; Jones, C.M.; Miettinen, M.; Pass, H.; Rimner, A.; et al. Treatment of Malignant Pleural Mesothelioma: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2018, 36, 1343–1373. [Google Scholar] [CrossRef]
- Vogelzang, N.J.; Rusthoven, J.J.; Symanowski, J.; Denham, C.; Kaukel, E.; Ruffie, P.; Gatzemeier, U.; Boyer, M.; Emri, S.; Manegold, C.; et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J. Clin. Oncol. 2003, 21, 2636–2644. [Google Scholar] [CrossRef]
- Zalcman, G.; Mazieres, J.; Margery, J.; Greillier, L.; Audigier-Valette, C.; Moro-Sibilot, D.; Molinier, O.; Corre, R.; Monnet, I.; Gounant, V.; et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): A randomised, controlled, open-label, phase 3 trial. Lancet 2016, 387, 1405–1414. [Google Scholar] [CrossRef]
- Nakajima, E.C.; Vellanki, P.J.; Larkins, E.; Chatterjee, S.; Mishra-Kalyani, P.S.; Bi, Y.; Qosa, H.; Liu, J.; Zhao, H.; Biable, M.; et al. FDA Approval Summary: Nivolumab in Combination with Ipilimumab for the Treatment of Unresectable Malignant Pleural Mesothelioma. Clin. Cancer Res. 2021, 28, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Baas, P.; Scherpereel, A.; Nowak, A.K.; Fujimoto, N.; Peters, S.; Tsao, A.S.; Mansfield, A.S.; Popat, S.; Jahan, T.; Antonia, S.; et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): A multicentre, randomised, open-label, phase 3 trial. Lancet 2021, 397, 375–386. [Google Scholar] [CrossRef]
- Fennell, D.A.; Ewings, S.; Ottensmeier, C.; Califano, R.; Hanna, G.G.; Hill, K.; Danson, S.; Steele, N.; Nye, M.; Johnson, L.; et al. Nivolumab versus placebo in patients with relapsed malignant mesothelioma (CONFIRM): A multicentre, double-blind, randomised, phase 3 trial. Lancet Oncol. 2021, 22, 1530–1540. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Kozuki, T.; Aoe, K.; Wada, S.; Harada, D.; Yoshida, M.; Sakurai, J.; Hotta, K.; Fujimoto, N. JME-001 phase II trial of first-line combination chemotherapy with cisplatin, pemetrexed, and nivolumab for unresectable malignant pleural mesothelioma. J. Immunother. Cancer 2021, 9, e003288. [Google Scholar] [CrossRef]
- Forde, P.M.; Anagnostou, V.; Sun, Z.; Dahlberg, S.E.; Kindler, H.L.; Niknafs, N.; Purcell, T.; Santana-Davila, R.; Dudek, A.Z.; Borghaei, H.; et al. Durvalumab with platinum-pemetrexed for unresectable pleural mesothelioma: Survival, genomic and immunologic analyses from the phase 2 PrE0505 trial. Nat. Med. 2021, 27, 1910–1920. [Google Scholar] [CrossRef]
- Forde, P.M.; Scherpereel, A.; Tsao, A.S. Use of Immune Checkpoint Inhibitors in Mesothelioma. Curr. Treat. Options Oncol. 2019, 20, 18. [Google Scholar] [CrossRef]
- Nowak, A.K.; McDonnell, A.; Cook, A. Immune checkpoint inhibition for the treatment of mesothelioma. Expert. Opin. Biol. Ther. 2019, 19, 697–706. [Google Scholar] [CrossRef]
- Baird, A.M.; Easty, D.; Jarzabek, M.; Shiels, L.; Soltermann, A.; Klebe, S.; Raeppel, S.; MacDonagh, L.; Wu, C.; Griggs, K.; et al. When RON MET TAM in Mesothelioma: All Druggable for One, and One Drug for All? Front. Endocrinol. 2019, 10, 89. [Google Scholar] [CrossRef]
- Busacca, S.; Sheaff, M.; Arthur, K.; Gray, S.G.; O’Byrne, K.J.; Richard, D.J.; Soltermann, A.; Opitz, I.; Pass, H.; Harkin, D.P.; et al. BRCA1 is an essential mediator of vinorelbine-induced apoptosis in mesothelioma. J. Pathol. 2012, 227, 200–208. [Google Scholar] [CrossRef]
- Fennell, D.A.; King, A.; Mohammed, S.; Branson, A.; Brookes, C.; Darlison, L.; Dawson, A.G.; Gaba, A.; Hutka, M.; Morgan, B.; et al. Rucaparib in patients with BAP1-deficient or BRCA1-deficient mesothelioma (MiST1): An open-label, single-arm, phase 2a clinical trial. Lancet Respir. Med. 2021, 9, 593–600. [Google Scholar] [CrossRef]
- Szlosarek, P.W.; Steele, J.P.; Nolan, L.; Gilligan, D.; Taylor, P.; Spicer, J.; Lind, M.; Mitra, S.; Shamash, J.; Phillips, M.M.; et al. Arginine Deprivation With Pegylated Arginine Deiminase in Patients With Argininosuccinate Synthetase 1-Deficient Malignant Pleural Mesothelioma: A Randomized Clinical Trial. JAMA Oncol. 2017, 3, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Szlosarek, P.W.; Phillips, M.M.; Pavlyk, I.; Steele, J.; Shamash, J.; Spicer, J.; Kumar, S.; Pacey, S.; Feng, X.; Johnston, A.; et al. Expansion Phase 1 Study of Pegargiminase Plus Pemetrexed and Cisplatin in Patients With Argininosuccinate Synthetase 1-Deficient Mesothelioma: Safety, Efficacy, and Resistance Mechanisms. JTO Clin. Res. Rep. 2020, 1, 100093. [Google Scholar] [CrossRef] [PubMed]
- Setargew, Y.F.I.; Wyllie, K.; Grant, R.D.; Chitty, J.L.; Cox, T.R. Targeting Lysyl Oxidase Family Meditated Matrix Cross-Linking as an Anti-Stromal Therapy in Solid Tumours. Cancers 2021, 13, 491. [Google Scholar] [CrossRef] [PubMed]
- Johnston, K.A.; Lopez, K.M. Lysyl oxidase in cancer inhibition and metastasis. Cancer Lett. 2018, 417, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Amendola, P.G.; Reuten, R.; Erler, J.T. Interplay Between LOX Enzymes and Integrins in the Tumor Microenvironment. Cancers 2019, 11, 729. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, S.; Li, W. LOX/LOXL in pulmonary fibrosis: Potential therapeutic targets. J. Drug Target. 2018, 27, 790–796. [Google Scholar] [CrossRef]
- Moon, H.J.; Finney, J.; Xu, L.; Moore, D.; Welch, D.R.; Mure, M. MCF-7 cells expressing nuclear associated lysyl oxidase-like 2 (LOXL2) exhibit an epithelial-to-mesenchymal transition (EMT) phenotype and are highly invasive in vitro. J. Biol. Chem. 2013, 288, 30000–30008. [Google Scholar] [CrossRef]
- Iturbide, A.; Garcia de Herreros, A.; Peiro, S. A new role for LOX and LOXL2 proteins in transcription regulation. FEBS J. 2015, 282, 1768–1773. [Google Scholar] [CrossRef]
- Cox, T.R.; Erler, J.T. Molecular pathways: Connecting fibrosis and solid tumor metastasis. Clin. Cancer Res. 2014, 20, 3637–3643. [Google Scholar] [CrossRef]
- Mitsuhashi, A.; Goto, H.; Saijo, A.; Trung, V.T.; Aono, Y.; Ogino, H.; Kuramoto, T.; Tabata, S.; Uehara, H.; Izumi, K.; et al. Fibrocyte-like cells mediate acquired resistance to anti-angiogenic therapy with bevacizumab. Nat. Commun. 2015, 6, 8792. [Google Scholar] [CrossRef]
- Nguyen, E.V.; Pereira, B.A.; Lawrence, M.G.; Ma, X.; Rebello, R.J.; Chan, H.; Niranjan, B.; Wu, Y.; Ellem, S.; Guan, X.; et al. Proteomic Profiling of Human Prostate Cancer-associated Fibroblasts (CAF) Reveals LOXL2-dependent Regulation of the Tumor Microenvironment. Mol. Cell Proteomics 2019, 18, 1410–1427. [Google Scholar] [CrossRef] [PubMed]
- Torres, S.; Garcia-Palmero, I.; Herrera, M.; Bartolome, R.A.; Pena, C.; Fernandez-Acenero, M.J.; Padilla, G.; Pelaez-Garcia, A.; Lopez-Lucendo, M.; Rodriguez-Merlo, R.; et al. LOXL2 Is Highly Expressed in Cancer-Associated Fibroblasts and Associates to Poor Colon Cancer Survival. Clin. Cancer Res. 2015, 21, 4892–4902. [Google Scholar] [CrossRef] [PubMed]
- Zeltz, C.; Pasko, E.; Cox, T.R.; Navab, R.; Tsao, M.S. LOXL1 Is Regulated by Integrin α11 and Promotes Non-Small Cell Lung Cancer Tumorigenicity. Cancers 2019, 11, 705. [Google Scholar] [CrossRef]
- Levene, C.I.; Bye, I.; Saffiotti, U. The effect of beta-aminopropionitrile on silicotic pulmonary fibrosis in the rat. Br. J. Exp. Pathol. 1968, 49, 152–159. [Google Scholar] [PubMed]
- Counts, D.F.; Evans, J.N.; Dipetrillo, T.A.; Sterling, K.M., Jr.; Kelley, J. Collagen lysyl oxidase activity in the lung increases during bleomycin-induced lung fibrosis. J. Pharmacol. Exp. Ther. 1981, 219, 675–678. [Google Scholar]
- Ledwozyw, A. The effect of beta-aminopropionitrile on bleomycin-induced lung injury in rats. Acta Physiol. Hung. 1995, 83, 91–99. [Google Scholar]
- Yao, Y.; Findlay, A.; Stolp, J.; Rayner, B.; Ask, K.; Jarolimek, W. Pan-lysyl oxidase inhibitor PXS-5505 ameliorates multiple-organ fibrosis by inhibiting collagen crosslinks in rodent models of systemic sclerosis. Authorea Prepr. 2021, 10, 19877269. [Google Scholar]
- Chien, J.W.; Richards, T.J.; Gibson, K.F.; Zhang, Y.; Lindell, K.O.; Shao, L.; Lyman, S.K.; Adamkewicz, J.I.; Smith, V.; Kaminski, N.; et al. Serum lysyl oxidase-like 2 levels and idiopathic pulmonary fibrosis disease progression. Eur. Respir. J. 2014, 43, 1430–1438. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, Y.; Tan, J.; Meng, X.; Xie, H.; Wang, R. Lysyl oxidase promotes epithelial-to-mesenchymal transition during paraquat-induced pulmonary fibrosis. Mol. BioSystems 2016, 12, 499–507. [Google Scholar] [CrossRef]
- Lu, J.; Qian, Y.; Jin, W.; Tian, R.; Zhu, Y.; Wang, J.; Meng, X.; Wang, R. Hypoxia-inducible factor-1α regulates epithelial-to-mesenchymal transition in paraquat-induced pulmonary fibrosis by activating lysyl oxidase. Exp. Ther. Med. 2018, 15, 2287–2294. [Google Scholar] [CrossRef]
- Aumiller, V.; Strobel, B.; Romeike, M.; Schuler, M.; Stierstorfer, B.E.; Kreuz, S. Comparative analysis of lysyl oxidase (like) family members in pulmonary fibrosis. Sci. Rep. 2017, 7, 149. [Google Scholar] [CrossRef]
- Fu, Q.; Bai, Y.; Liu, Y.; Zhou, J.; Zheng, Y. The serum level and significance of lysyl oxidase-like 2 in patients with rheumatoid arthritis-associated interstitial lung disease. Clin. Rheumatol. 2018, 37, 193–198. [Google Scholar] [CrossRef]
- Tjin, G.; White, E.S.; Faiz, A.; Sicard, D.; Tschumperlin, D.J.; Mahar, A.; Kable, E.P.W.; Burgess, J.K. Lysyl oxidases regulate fibrillar collagen remodelling in idiopathic pulmonary fibrosis. Dis. Model. Mech. 2017, 10, 1301–1312. [Google Scholar] [CrossRef]
- Bellaye, P.S.; Shimbori, C.; Upagupta, C.; Sato, S.; Shi, W.; Gauldie, J.; Ask, K.; Kolb, M. Lysyl Oxidase-Like 1 Protein Deficiency Protects Mice from Adenoviral Transforming Growth Factor-β1-induced Pulmonary Fibrosis. Am. J. Respir. Cell Mol. Biol. 2018, 58, 461–470. [Google Scholar] [CrossRef]
- Matsuo, A.; Tanida, R.; Yanagi, S.; Tsubouchi, H.; Miura, A.; Shigekusa, T.; Matsumoto, N.; Nakazato, M. Significance of nuclear LOXL2 inhibition in fibroblasts and myofibroblasts in the fibrotic process of acute respiratory distress syndrome. Eur. J. Pharmacol. 2021, 892, 173754. [Google Scholar] [CrossRef]
- Wei, Y.; Dong, W.; Jackson, J.; Ho, T.C.; Le Saux, C.J.; Brumwell, A.; Li, X.; Klesney-Tait, J.; Cohen, M.L.; Wolters, P.J.; et al. Blocking LOXL2 and TGFβ1 signalling induces collagen I turnover in precision-cut lung slices derived from patients with idiopathic pulmonary fibrosis. Thorax 2021, 76, 729–732. [Google Scholar] [CrossRef]
- Velázquez-Enríquez, J.M.; Santos-Álvarez, J.C.; Ramírez-Hernández, A.A.; Reyes-Jiménez, E.; López-Martínez, A.; Pina-Canseco, S.; Aguilar-Ruiz, S.R.; Romero-Tlalolini, M.; Castro-Sánchez, L.; Arellanes-Robledo, J.; et al. Proteomic Analysis Reveals Key Proteins in Extracellular Vesicles Cargo Associated with Idiopathic Pulmonary Fibrosis In Vitro. Biomedicines 2021, 9, 1058. [Google Scholar] [CrossRef]
- Choi, S.E.; Jeon, N.; Choi, H.Y.; Shin, J.I.; Jeong, H.J.; Lim, B.J. Lysyl oxidase-like 2 is expressed in kidney tissue and is associated with the progression of tubulointerstitial fibrosis. Mol. Med. Rep. 2017, 16, 2477–2482. [Google Scholar] [CrossRef]
- Cosgrove, D.; Dufek, B.; Meehan, D.T.; Delimont, D.; Hartnett, M.; Samuelson, G.; Gratton, M.A.; Phillips, G.; MacKenna, D.A.; Bain, G. Lysyl oxidase like-2 contributes to renal fibrosis in Col4α3/Alport mice. Kidney Int. 2018, 94, 303–314. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Li, X.; Zhou, W.Q.; Liu, X.; Huang, J.L.; Zhang, Y.Y.; Lindholm, B.; Yu, C. Serum Lysyl Oxidase Is a Potential Diagnostic Biomarker for Kidney Fibrosis. Am. J. Nephrol. 2020, 51, 907–918. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Saad, S.; Shi, Y.; Wang, R.; Chou, A.S.Y.; Gill, A.; Yao, Y.; Jarolimek, W.; Pollock, C.A. Lysyl oxidase inhibitors attenuate cyclosporin A-induced nephropathy in mouse. Sci. Rep. 2021, 11, 12437. [Google Scholar] [CrossRef]
- Siegel, R.C.; Chen, K.H.; Greenspan, J.S.; Aguiar, J.M. Biochemical and immunochemical study of lysyl oxidase in experimental hepatic fibrosis in the rat. Proc. Natl. Acad. Sci. USA 1978, 75, 2945–2949. [Google Scholar] [CrossRef] [PubMed]
- McPhie, J.L. The activity of lysyl oxidase in experimental hepatic fibrosis. Hepatogastroenterology 1981, 28, 240–241. [Google Scholar]
- Carter, E.A.; McCarron, M.J.; Alpert, E.; Isselbacher, K.J. Lysyl oxidase and collagenase in experimental acute and chronic liver injury. Gastroenterology 1982, 82, 526–534. [Google Scholar] [CrossRef]
- Wakasaki, H.; Ooshima, A. Synthesis of lysyl oxidase in experimental hepatic fibrosis. Biochem. Biophys Res. Commun. 1990, 166, 1201–1204. [Google Scholar] [CrossRef]
- Guo, C.J.; Xiao, X.; Sheng, L.; Chen, L.; Zhong, W.; Li, H.; Hua, J.; Ma, X. RNA Sequencing and Bioinformatics Analysis Implicate the Regulatory Role of a Long Noncoding RNA-mRNA Network in Hepatic Stellate Cell Activation. Cell Physiol. Biochem. 2017, 42, 2030–2042. [Google Scholar] [CrossRef]
- Mannaerts, I.; Schroyen, B.; Verhulst, S.; Van Lommel, L.; Schuit, F.; Nyssen, M.; van Grunsven, L.A. Gene expression profiling of early hepatic stellate cell activation reveals a role for Igfbp3 in cell migration. PLoS ONE 2013, 8, e84071. [Google Scholar] [CrossRef]
- Perepelyuk, M.; Terajima, M.; Wang, A.Y.; Georges, P.C.; Janmey, P.A.; Yamauchi, M.; Wells, R.G. Hepatic stellate cells and portal fibroblasts are the major cellular sources of collagens and lysyl oxidases in normal liver and early after injury. Am. J. Physiol. Gastrointest Liver Physiol. 2013, 304, G605–G614. [Google Scholar] [CrossRef]
- Liu, S.B.; Ikenaga, N.; Peng, Z.W.; Sverdlov, D.Y.; Greenstein, A.; Smith, V.; Schuppan, D.; Popov, Y. Lysyl oxidase activity contributes to collagen stabilization during liver fibrosis progression and limits spontaneous fibrosis reversal in mice. FASEB J. 2016, 30, 1599–1609. [Google Scholar] [CrossRef]
- Murawaki, Y.; Kusakabe, Y.; Hirayama, C. Serum lysyl oxidase activity in chronic liver disease in comparison with serum levels of prolyl hydroxylase and laminin. Hepatology 1991, 14, 1167–1173. [Google Scholar] [CrossRef]
- Kim, Y.; Peyrol, S.; So, C.K.; Boyd, C.D.; Csiszar, K. Coexpression of the lysyl oxidase-like gene (LOXL) and the gene encoding type III procollagen in induced liver fibrosis. J. Cell Biochem. 1999, 72, 181–188. [Google Scholar] [CrossRef]
- Vadasz, Z.; Kessler, O.; Akiri, G.; Gengrinovitch, S.; Kagan, H.M.; Baruch, Y.; Izhak, O.B.; Neufeld, G. Abnormal deposition of collagen around hepatocytes in Wilson’s disease is associated with hepatocyte specific expression of lysyl oxidase and lysyl oxidase like protein-2. J. Hepatol. 2005, 43, 499–507. [Google Scholar] [CrossRef]
- Zhang, Y.; Ghazwani, M.; Li, J.; Sun, M.; Stolz, D.B.; He, F.; Fan, J.; Xie, W.; Li, S. MiR-29b inhibits collagen maturation in hepatic stellate cells through down-regulating the expression of HSP47 and lysyl oxidase. Biochem. Biophys. Res. Commun. 2014, 446, 940–944. [Google Scholar] [CrossRef]
- Mesarwi, O.A.; Shin, M.K.; Drager, L.F.; Bevans-Fonti, S.; Jun, J.C.; Putcha, N.; Torbenson, M.S.; Pedrosa, R.P.; Lorenzi-Filho, G.; Steele, K.E.; et al. Lysyl Oxidase as a Serum Biomarker of Liver Fibrosis in Patients with Severe Obesity and Obstructive Sleep Apnea. Sleep 2015, 38, 1583–1591. [Google Scholar] [CrossRef]
- Ikenaga, N.; Peng, Z.W.; Vaid, K.A.; Liu, S.B.; Yoshida, S.; Sverdlov, D.Y.; Mikels-Vigdal, A.; Smith, V.; Schuppan, D.; Popov, Y.V. Selective targeting of lysyl oxidase-like 2 (LOXL2) suppresses hepatic fibrosis progression and accelerates its reversal. Gut 2017, 66, 1697–1708. [Google Scholar] [CrossRef]
- Dongiovanni, P.; Meroni, M.; Baselli, G.A.; Bassani, G.A.; Rametta, R.; Pietrelli, A.; Maggioni, M.; Facciotti, F.; Trunzo, V.; Badiali, S.; et al. Insulin resistance promotes Lysyl …… O…Oxidase Like 2 induction and fibrosis accumulation in non-alcoholic fatty liver disease. Clin. Sci. 2017, 131, 1301–1315. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, A.; Chen, W.; Wang, P.; Liu, T.; Cong, M.; Xu, A.; Yan, X.; Jia, J.; You, H. Inhibition of lysyl oxidase-like 1 (LOXL1) expression arrests liver fibrosis progression in cirrhosis by reducing elastin crosslinking. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 1129–1137. [Google Scholar] [CrossRef]
- Ma, L.; Zeng, Y.; Wei, J.; Yang, D.; Ding, G.; Liu, J.; Shang, J.; Kang, Y.; Ji, X. Knockdown of LOXL1 inhibits TGF-β1-induced proliferation and fibrogenesis of hepatic stellate cells by inhibition of Smad2/3 phosphorylation. Biomed. Pharmacother. 2018, 107, 1728–1735. [Google Scholar] [CrossRef]
- Schilter, H.; Findlay, A.D.; Perryman, L.; Yow, T.T.; Moses, J.; Zahoor, A.; Turner, C.I.; Deodhar, M.; Foot, J.S.; Zhou, W.; et al. The lysyl oxidase like 2/3 enzymatic inhibitor, PXS-5153A, reduces crosslinks and ameliorates fibrosis. J. Cell Mol. Med. 2019, 23, 1759–1770. [Google Scholar] [CrossRef]
- Puente, A.; Fortea, J.I.; Posadas, M.; Garcia Blanco, A.; Rasines, L.; Cabezas, J.; Arias Loste, M.T.; Llerena, S.; Iruzubieta, P.; Fábrega, E.; et al. Changes in Circulating Lysyl Oxidase-Like-2 (LOXL2) Levels, HOMA, and Fibrosis after Sustained Virological Response by Direct Antiviral Therapy. J. Clin. Med. 2019, 8, 1242. [Google Scholar] [CrossRef]
- Klepfish, M.; Gross, T.; Vugman, M.; Afratis, N.A.; Havusha-Laufer, S.; Brazowski, E.; Solomonov, I.; Varol, C.; Sagi, I. LOXL2 Inhibition Paves the Way for Macrophage-Mediated Collagen Degradation in Liver Fibrosis. Front. Immunol. 2020, 11, 480. [Google Scholar] [CrossRef]
- Guo, C.J.; Pan, Q.; Li, D.G.; Sun, H.; Liu, B.W. miR-15b and miR-16 are implicated in activation of the rat hepatic stellate cell: An essential role for apoptosis. J. Hepatol. 2009, 50, 766–778. [Google Scholar] [CrossRef]
- Ma, L.; Liu, J.; Xiao, E.; Ning, H.; Li, K.; Shang, J.; Kang, Y. MiR-15b and miR-16 suppress TGF-β1-induced proliferation and fibrogenesis by regulating LOXL1 in hepatic stellate cells. Life Sci. 2021, 270, 119144. [Google Scholar] [CrossRef]
- Yang, A.; Yan, X.; Fan, X.; Shi, Y.; Huang, T.; Li, W.; Chen, W.; Jia, J.; You, H. Hepatic stellate cells-specific LOXL1 deficiency abrogates hepatic inflammation, fibrosis, and corrects lipid metabolic abnormalities in non-obese NASH mice. Hepatol. Int. 2021, 15, 1122–1135. [Google Scholar] [CrossRef]
- Malaspina, A.; Kaushik, N.; de Belleroche, J. Differential expression of 14 genes in amyotrophic lateral sclerosis spinal cord detected using gridded cDNA arrays. J. Neurochem. 2001, 77, 132–145. [Google Scholar] [CrossRef]
- Li, P.A.; He, Q.; Cao, T.; Yong, G.; Szauter, K.M.; Fong, K.S.; Karlsson, J.; Keep, M.F.; Csiszar, K. Up-regulation and altered distribution of lysyl oxidase in the central nervous system of mutant SOD1 transgenic mouse model of amyotrophic lateral sclerosis. Brain Res. Mol. Brain Res. 2004, 120, 115–122. [Google Scholar] [CrossRef]
- Meyringer, R.; Neumann, E.; Judex, M.; Landthaler, M.; Kullmann, F.; Scholmerich, J.; Gay, S.; Tarner, I.H.; Distler, O.; Müller-Ladner, U. Analysis of gene expression patterns in systemic sclerosis fibroblasts using RNA arbitrarily primed-polymerase chain reaction for differential display. J. Rheumatol. 2007, 34, 747–753. [Google Scholar]
- Nguyen, X.X.; Nishimoto, T.; Takihara, T.; Mlakar, L.; Bradshaw, A.D.; Feghali-Bostwick, C. Lysyl oxidase directly contributes to extracellular matrix production and fibrosis in systemic sclerosis. Am. J. Physiol. Lung Cell Mol. Physiol. 2021, 320, L29–L40. [Google Scholar] [CrossRef]
- Rimar, D.; Rosner, I.; Nov, Y.; Slobodin, G.; Rozenbaum, M.; Halasz, K.; Haj, T.; Jiries, N.; Kaly, L.; Boulman, N.; et al. Brief report: Lysyl oxidase is a potential biomarker of fibr.rosis in systemic sclerosis. Arthritis Rheumatol. 2014, 66, 726–730. [Google Scholar] [CrossRef]
- Vadasz, Z.; Balbir Gurman, A.; Meroni, P.; Farge, D.; Levi, Y.; Ingegnoli, F.; Braun-Moscovici, Y.; Rosner, I.; Slobodin, G.; Rozenbaum, M.; et al. Lysyl oxidase-a possible role in systemic sclerosis-associated pulmonary hypertension: A multicentre study. Rheumatology 2019, 58, 1547–1555. [Google Scholar] [CrossRef]
- Huang, M.; Cai, G.; Baugh, L.M.; Liu, Z.; Smith, A.; Watson, M.; Popovich, D.; Zhang, T.; Stawski, L.S.; Trojanowska, M.; et al. Systemic Sclerosis Dermal Fibroblasts Induce Cutaneous Fibrosis Through Lysyl Oxidase-like 4: New Evidence From Three-Dimensional Skin-like Tissues. Arthritis Rheumatol. 2020, 72, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Richeldi, L.; Collard, H.R.; Jones, M.G. Idiopathic pulmonary fibrosis. Lancet 2017, 389, 1941–1952. [Google Scholar] [CrossRef]
- van Geffen, C.; Deißler, A.; Quante, M.; Renz, H.; Hartl, D.; Kolahian, S. Regulatory Immune Cells in Idiopathic Pulmonary Fibrosis: Friends or Foes? Front. Immunol. 2021, 12, 663203. [Google Scholar] [CrossRef]
- Planté-Bordeneuve, T.; Pilette, C.; Froidure, A. The Epithelial-Immune Crosstalk in Pulmonary Fibrosis. Front. Immunol. 2021, 12, 631235. [Google Scholar] [CrossRef]
- Kishore, A.; Petrek, M. Roles of Macrophage Polarization and Macrophage-Derived miRNAs in Pulmonary Fibrosis. Front. Immunol. 2021, 12, 678457. [Google Scholar] [CrossRef]
- Chen, L.J.; Li, W.D.; Li, S.F.; Su, X.W.; Lin, G.Y.; Huang, Y.J.; Yan, G.M. Bleomycin induces upregulation of lysyl oxidase in cultured human fetal lung fibroblasts. Acta Pharmacol. Sin. 2010, 31, 554–559. [Google Scholar] [CrossRef]
- Yang, J.; Savvatis, K.; Kang, J.S.; Fan, P.; Zhong, H.; Schwartz, K.; Barry, V.; Mikels-Vigdal, A.; Karpinski, S.; Kornyeyev, D.; et al. Targeting LOXL2 for cardiac interstitial fibrosis and heart failure treatment. Nat. Commun. 2016, 7, 13710. [Google Scholar] [CrossRef]
- Rodríguez, C.; Martínez-González, J. The Role of Lysyl Oxidase Enzymes in Cardiac Function and Remodeling. Cells 2019, 8, 1483. [Google Scholar] [CrossRef]
- Nagaraju, C.K.; Robinson, E.L.; Abdesselem, M.; Trenson, S.; Dries, E.; Gilbert, G.; Janssens, S.; Van Cleemput, J.; Rega, F.; Meyns, B.; et al. Myofibroblast Phenotype and Reversibility of Fibrosis in Patients With End-Stage Heart Failure. J. Am. Coll. Cardiol. 2019, 73, 2267–2282. [Google Scholar] [CrossRef]
- Wu, Y.; Can, J.; Hao, S.; Qiang, X.; Ning, Z. LOXL2 Inhibitor Attenuates Angiotensin II-Induced Atrial Fibrosis and Vulnerability to Atrial Fibrillation through Inhibition of Transforming Growth Factor Beta-1 Smad2/3 Pathway. Cerebrovasc. Dis. 2021, 1–11. [Google Scholar] [CrossRef]
- Subramanian, M.L.; Stein, T.D.; Siegel, N.; Ness, S.; Fiorello, M.G.; Kim, D.; Roy, S. Upregulation of Lysyl Oxidase Expression in Vitreous of Diabetic Subjects: Implications for Diabetic Retinopathy. Cells 2019, 8, 1122. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Mecham, R.P.; Nguyen, N.H.; Roy, S. Decreased lysyl oxidase level protects against development of retinal vascular lesions in diabetic retinopathy. Exp. Eye Res. 2019, 184, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Lee, D.; Trackman, P.C.; Roy, S. Effects of High Glucose-Induced Lysyl Oxidase Propeptide on Retinal Endothelial Cell Survival: Implications for Diabetic Retinopathy. Am. J. Pathol. 2019, 189, 1945–1952. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Kim, D.; Nguyen, N.H.; Roy, S. Inhibition of Diabetes-Induced Lysyl Oxidase Overexpression Prevents Retinal Vascular Lesions Associated With Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5965–5972. [Google Scholar] [CrossRef]
- Xiao, W.; He, J.; Fu, W.; Xu, Y.; Zhang, Z. LOX gene polymorphisms are associated with osteoporotic vertebral compression fracture in postmenopausal Chinese women. Gene 2020, 741, 144543. [Google Scholar] [CrossRef]
- Ida, T.; Kaku, M.; Kitami, M.; Terajima, M.; Rosales Rocabado, J.M.; Akiba, Y.; Nagasawa, M.; Yamauchi, M.; Uoshima, K. Extracellular matrix with defective collagen cross-linking affects the differentiation of bone cells. PLoS ONE 2018, 13, e0204306. [Google Scholar] [CrossRef]
- Cox, T.R. The matrix in cancer. Nat. Rev. Cancer 2021, 21, 217–238. [Google Scholar] [CrossRef]
- Piersma, B.; Hayward, M.K.; Weaver, V.M. Fibrosis and cancer: A strained relationship. Biochim. Biophys. Acta Rev. Cancer 2020, 1873, 188356. [Google Scholar] [CrossRef]
- Saatci, O.; Kaymak, A.; Raza, U.; Ersan, P.G.; Akbulut, O.; Banister, C.E.; Sikirzhytski, V.; Tokat, U.M.; Aykut, G.; Ansari, S.A.; et al. Targeting lysyl oxidase (LOX) overcomes chemotherapy resistance in triple negative breast cancer. Nat. Commun. 2020, 11, 2416. [Google Scholar] [CrossRef]
- Maller, O.; Drain, A.P.; Barrett, A.S.; Borgquist, S.; Ruffell, B.; Zakharevich, I.; Pham, T.T.; Gruosso, T.; Kuasne, H.; Lakins, J.N.; et al. Tumour-associated macrophages drive stromal cell-dependent collagen crosslinking and stiffening to promote breast cancer aggression. Nat. Mater. 2021, 20, 548–559. [Google Scholar] [CrossRef]
- Dinca, S.C.; Greiner, D.; Weidenfeld, K.; Bond, L.; Barkan, D.; Jorcyk, C.L. Novel mechanism for OSM-promoted extracellular matrix remodeling in breast cancer: LOXL2 upregulation and subsequent ECM alignment. Breast Cancer Res. 2021, 23, 56. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Wang, Y.; Wu, Y.; Zhang, X.; Liu, J.; Wang, T.; Fan, J.; Sun, J.; Yang, A.; Zhang, R. EZH2-mediated Epigenetic Silencing of miR-29/miR-30 targets LOXL4 and contributes to Tumorigenesis, Metastasis, and Immune Microenvironment Remodeling in Breast Cancer. Theranostics 2020, 10, 8494–8512. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.K.; Kim, H.S.; Jin, T.; Moon, W.K. LOXL4 knockdown enhances tumor growth and lung metastasis through collagen-dependent extracellular matrix changes in triple-negative breast cancer. Oncotarget 2017, 8, 11977–11989. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, C.; Xu, S.; Tian, Y.; Ju, A.; Hou, Q.; Liu, J.; Fu, Y.; Luo, Y. Lysyl Oxidase-Like Protein 2 Promotes Tumor Lymphangiogenesis and Lymph Node Metastasis in Breast Cancer. Neoplasia 2019, 21, 413–427. [Google Scholar] [CrossRef]
- Leo, C.; Cotic, C.; Pomp, V.; Fink, D.; Varga, Z. Overexpression of Lox in triple-negative breast cancer. Ann. Diagn. Pathol. 2018, 34, 98–102. [Google Scholar] [CrossRef]
- Salvador, F.; Martin, A.; López-Menéndez, C.; Moreno-Bueno, G.; Santos, V.; Vázquez-Naharro, A.; Santamaria, P.G.; Morales, S.; Dubus, P.R.; Muinelo-Romay, L.; et al. Lysyl Oxidase-like Protein LOXL2 Promotes Lung Metastasis of Breast Cancer. Cancer Res. 2017, 77, 5846–5859. [Google Scholar] [CrossRef]
- Janyasupab, M.; Lee, Y.H.; Zhang, Y.; Liu, C.W.; Cai, J.; Popa, A.; Samia, A.C.; Wang, K.W.; Xu, J.; Hu, C.C.; et al. Detection of Lysyl Oxidase-Like 2 (LOXL2), a Biomarker of Metastasis from Breast Cancers Using Human Blood Samples. Recent Pat. Biomark. 2015, 5, 93–100. [Google Scholar] [CrossRef]
- Peyrol, S.; Raccurt, M.; Gerard, F.; Gleyzal, C.; Grimaud, J.A.; Sommer, P. Lysyl oxidase gene expression in the stromal reaction to in situ and invasive ductal breast carcinoma. Am. J. Pathol. 1997, 150, 497–507. [Google Scholar]
- Decitre, M.; Gleyzal, C.; Raccurt, M.; Peyrol, S.; Aubert-Foucher, E.; Csiszar, K.; Sommer, P. Lysyl oxidase-like protein localizes to sites of de novo fibrinogenesis in fibrosis and in the early stromal reaction of ductal breast carcinomas. Lab. Investig. 1998, 78, 143–151. [Google Scholar]
- Lin, S.; Zheng, L.; Lu, Y.; Xia, Q.; Zhou, P.; Liu, Z. Comprehensive analysis on the expression levels and prognostic values of LOX family genes in kidney renal clear cell carcinoma. Cancer Med. 2020, 9, 8624–8638. [Google Scholar] [CrossRef]
- Hong, X.; Yu, J.J. Silencing of lysyl oxidase-like 2 inhibits the migration, invasion and epithelial-to-mesenchymal transition of renal cell carcinoma cells through the Src/FAK signaling pathway. Int. J. Oncol. 2019, 54, 1676–1690. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, V.; Torsello, B.; Bianchi, C.; Cifola, I.; Mangano, E.; Bovo, G.; Cassina, V.; De Marco, S.; Corti, R.; Meregalli, C.; et al. Major Action of Endogenous Lysyl Oxidase in Clear Cell Renal Cell Carcinoma Progression and Collagen Stiffness Revealed by Primary Cell Cultures. Am. J. Pathol. 2016, 186, 2473–2485. [Google Scholar] [CrossRef] [PubMed]
- Stassar, M.J.; Devitt, G.; Brosius, M.; Rinnab, L.; Prang, J.; Schradin, T.; Simon, J.; Petersen, S.; Kopp-Schneider, A.; Zöller, M. Identification of human renal cell carcinoma associated genes by suppression subtractive hybridization. Br. J. Cancer 2001, 85, 1372–1382. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mongkolrob, R.; Tharabenjasin, P.; Bualuang, A.; Jarjanazi, H.; Pabalan, N. Influence of Lysyl oxidase Polymorphisms in Cancer Risk: An Updated Meta-analysis. Genet. Test. Mol. Biomarkers 2021, 25, 411–418. [Google Scholar] [CrossRef]
- Wang, G.; Shen, Y.; Cheng, G.; Bo, H.; Lin, J.; Zheng, M.; Li, J.; Zhao, Y.; Li, W. Lysyl Oxidase Gene G473A Polymorphism and Cigarette Smoking in Association with a High Risk of Lung and Colorectal Cancers in a North Chinese Population. Int. J. Environ. Res. Public Health 2016, 13, 635. [Google Scholar] [CrossRef]
- Shi, W.; Yang, B.; Li, X.; Sun, S.; Wang, L.; Jiao, S. The effect of lysyl oxidase polymorphism on susceptibility and prognosis of nonsmall cell lung cancer. Tumour. Biol. 2012, 33, 2379–2383. [Google Scholar] [CrossRef]
- Hou, X.; Du, H.; Quan, X.; Shi, L.; Zhang, Q.; Wu, Y.; Liu, Y.; Xiao, J.; Li, Y.; Lu, L.; et al. Silibinin Inhibits NSCLC Metastasis by Targeting the EGFR/LOX Pathway. Front. Pharmacol. 2018, 9, 21. [Google Scholar] [CrossRef]
- Zhan, P.; Lv, X.J.; Ji, Y.N.; Xie, H.; Yu, L.K. Increased lysyl oxidase-like 2 associates with a poor prognosis in non-small cell lung cancer. Clin. Respir. J. 2018, 12, 712–720. [Google Scholar] [CrossRef]
- Liu, J.; Ping, W.; Zu, Y.; Sun, W. Correlations of lysyl oxidase with MMP2/MMP9 expression and its prognostic value in non-small cell lung cancer. Int. J. Clin. Exp. Pathol. 2014, 7, 6040–6047. [Google Scholar]
- Wilgus, M.L.; Borczuk, A.C.; Stoopler, M.; Ginsburg, M.; Gorenstein, L.; Sonett, J.R.; Powell, C.A. Lysyl oxidase: A lung adenocarcinoma biomarker of invasion and survival. Cancer 2011, 117, 2186–2191. [Google Scholar] [CrossRef]
- Zhan, P.; Shen, X.K.; Qian, Q.; Zhu, J.P.; Zhang, Y.; Xie, H.Y.; Xu, C.H.; Hao, K.K.; Hu, W.; Xia, N.; et al. Down-regulation of lysyl oxidase-like 2 (LOXL2) is associated with disease progression in lung adenocarcinomas. Med. Oncol. 2012, 29, 648–655. [Google Scholar] [CrossRef]
- Peng, D.H.; Ungewiss, C.; Tong, P.; Byers, L.A.; Wang, J.; Canales, J.R.; Villalobos, P.A.; Uraoka, N.; Mino, B.; Behrens, C.; et al. ZEB1 induces LOXL2-mediated collagen stabilization and deposition in the extracellular matrix to drive lung cancer invasion and metastasis. Oncogene 2017, 36, 1925–1938. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Song, X.R.; Sun, J.J.; Wang, X.W.; Xie, L.; Lv, L.Y. Lysyl oxidase may play a critical role in hypoxia-induced NSCLC cells invasion and migration. Cancer Biother Radiopharm. 2012, 27, 672–677. [Google Scholar] [CrossRef] [PubMed]
- Kamikawaji, K.; Seki, N.; Watanabe, M.; Mataki, H.; Kumamoto, T.; Takagi, K.; Mizuno, K.; Inoue, H. Regulation of LOXL2 and SERPINH1 by antitumor microRNA-29a in lung cancer with idiopathic pulmonary fibrosis. J. Hum. Genet. 2016, 61, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Nicolas-Boluda, A.; Vaquero, J.; Vimeux, L.; Guilbert, T.; Barrin, S.; Kantari-Mimoun, C.; Ponzo, M.; Renault, G.; Deptula, P.; Pogoda, K.; et al. Tumor stiffening reversion through collagen crosslinking inhibition improves T cell migration and anti-PD-1 treatment. Elife 2021, 10, e58688. [Google Scholar] [CrossRef] [PubMed]
- Grønborg, M.; Kristiansen, T.Z.; Iwahori, A.; Chang, R.; Reddy, R.; Sato, N.; Molina, H.; Jensen, O.N.; Hruban, R.H.; Goggins, M.G.; et al. Biomarker discovery from pancreatic cancer secretome using a differential proteomic approach. Mol. Cell Proteomics. 2006, 5, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Yamada, S.; Sonohara, F.; Suenaga, M.; Hayashi, M.; Takami, H.; Niwa, Y.; Hattori, N.; Iwata, N.; Kanda, M.; et al. Clinical Implications of Lysyl Oxidase-Like Protein 2 Expression in Pancreatic Cancer. Sci. Rep. 2018, 8, 9846. [Google Scholar] [CrossRef]
- Le Calvé, B.; Griveau, A.; Vindrieux, D.; Maréchal, R.; Wiel, C.; Svrcek, M.; Gout, J.; Azzi, L.; Payen, L.; Cros, J.; et al. Lysyl oxidase family activity promotes resistance of pancreatic ductal adenocarcinoma to chemotherapy by limiting the intratumoral anticancer drug distribution. Oncotarget 2016, 7, 32100–32112. [Google Scholar] [CrossRef]
- Miller, B.W.; Morton, J.P.; Pinese, M.; Saturno, G.; Jamieson, N.B.; McGhee, E.; Timpson, P.; Leach, J.; McGarry, L.; Shanks, E.; et al. Targeting the LOX/hypoxia axis reverses many of the features that make pancreatic cancer deadly: Inhibition of LOX abrogates metastasis and enhances drug efficacy. EMBO Mol. Med. 2015, 7, 1063–1076. [Google Scholar] [CrossRef]
- Jiang, H.; Torphy, R.J.; Steiger, K.; Hongo, H.; Ritchie, A.J.; Kriegsmann, M.; Horst, D.; Umetsu, S.E.; Joseph, N.M.; McGregor, K.; et al. Pancreatic ductal adenocarcinoma progression is restrained by stromal matrix. J. Clin. Investig. 2020, 130, 4704–4709. [Google Scholar] [CrossRef]
- Ma, W.; Li, T.; Wu, S.; Li, J.; Wang, X.; Li, H. LOX and ACSL5 as potential relapse markers for pancreatic cancer patients. Cancer Biol. Ther. 2019, 20, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.L.; Tsai, M.C.; Chang, Y.H.; Wang, C.C.; Chu, P.Y.; Lin, H.Y.; Huang, Y.H. MIR29A Impedes Metastatic Behaviors in Hepatocellular Carcinoma via Targeting LOX, LOXL2, and VEGFA. Int. J. Mol. Sci. 2021, 22, 6001. [Google Scholar] [CrossRef]
- Lin, W.; Zhang, T.; Ding, G.; Hao, L.; Zhang, B.; Yu, J.; Pang, Y.; Geng, F.; Zhan, L.; Zhou, M.; et al. Circular RNA circ-CCT3 promotes hepatocellular carcinoma progression by regulating the miR-1287-5p/TEAD1/PTCH1/LOX axis. Mol. Med. Rep. 2021, 23, 375. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhou, X.; Tang, F.; Wang, X.; Zhu, X. Identification of LOXL3-associating immune infiltration landscape and prognostic value in hepatocellular carcinoma. Virchows Arch. 2021, 479, 1153–1165. [Google Scholar] [CrossRef] [PubMed]
- Umezaki, N.; Nakagawa, S.; Yamashita, Y.I.; Kitano, Y.; Arima, K.; Miyata, T.; Hiyoshi, Y.; Okabe, H.; Nitta, H.; Hayashi, H.; et al. Lysyl oxidase induces epithelial-mesenchymal traansition and predicts intrahepatic metastasis of hepatocellular carcinoma. Cancer Sci. 2019, 110, 2033–2043. [Google Scholar] [PubMed]
- Zhu, J.; Huang, S.; Wu, G.; Huang, C.; Li, X.; Chen, Z.; Zhao, L.; Zhao, Y. Lysyl Oxidase Is Predictive of Unfavorable Outcomes and Essential for Regulation of Vascular Endothelial Growth Factor in Hepatocellular Carcinoma. Dig. Dis. Sci. 2015, 60, 3019–3031. [Google Scholar] [CrossRef] [PubMed]
- Shao, B.; Zhao, X.; Liu, T.; Zhang, Y.; Sun, R.; Dong, X.; Liu, F.; Zhao, N.; Zhang, D.; Wu, L.; et al. LOXL2 promotes vasculogenic mimicry and tumour aggressiveness in hepatocellular carcinoma. J. Cell Mol. Med. 2019, 23, 1363–1374. [Google Scholar] [CrossRef]
- Choi, J.; Chung, T.; Rhee, H.; Kim, Y.J.; Jeon, Y.; Yoo, J.E.; Noh, S.; Han, D.H.; Park, Y.N. Increased Expression of the Matrix-Modifying Enzyme Lysyl Oxidase-Like 2 in Aggressive Hepatocellular Carcinoma with Poor Prognosis. Gut Liver 2019, 13, 83–92. [Google Scholar] [CrossRef]
- Ninomiya, G.; Yamada, S.; Hayashi, M.; Takeda, S.; Suenaga, M.; Takami, H.; Kanda, M.; Iwata, N.; Niwa, Y.; Tanaka, C.; et al. Significance of Lysyl oxidase-like 2 gene expression on the epithelial-mesenchymal status of hepatocellular carcinoma. Oncol. Rep. 2018, 39, 2664–2672. [Google Scholar]
- Wong, C.C.; Tse, A.P.; Huang, Y.P.; Zhu, Y.T.; Chiu, D.K.; Lai, R.K.; Au, S.L.; Kai, A.K.; Lee, J.M.; Wei, L.L.; et al. Lysyl oxidase-like 2 is critical to tumor microenvironment and metastatic niche formation in hepatocellular carcinoma. Hepatology 2014, 60, 1645–1658. [Google Scholar] [CrossRef]
- Li, R.; Wang, Y.; Zhang, X.; Feng, M.; Ma, J.; Li, J.; Yang, X.; Fang, F.; Xia, Q.; Zhang, Z.; et al. Exosome-mediated secretion of LOXL4 promotes hepatocellular carcinoma cell invasion and metastasis. Mol. Cancer 2019, 18, 18. [Google Scholar] [CrossRef] [PubMed]
- Brett, E.A.; Sauter, M.A.; Machens, H.G.; Duscher, D. Tumor-associated collagen signatures: Pushing tumor boundaries. Cancer Metab. 2020, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Zhao, Y.; Wang, X.; Zhu, T. Intratumoral Fibrosis in Facilitating Renal Cancer Aggressiveness: Underlying Mechanisms and Promising Targets. Front. Cell Dev. Biol. 2021, 9, 651620. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Yin, H.; Liu, H.; Liu, F.; Du, Y.; Xu, T. The Significance of Fibrosis Quantification as a Marker in Assessing Pseudo-Capsule Status and Clear Cell Renal Cell Carcinoma Prognosis. Diagnostics 2020, 10, 895. [Google Scholar] [CrossRef]
- Ahmad, R.S.; Eubank, T.D.; Lukomski, S.; Boone, B.A. Immune Cell Modulation of the Extracellular Matrix Contributes to the Pathogenesis of Pancreatic Cancer. Biomolecules 2021, 11, 901. [Google Scholar] [CrossRef]
- Abayasiriwardana, K.S.; Wood, M.K.; Prêle, C.M.; Birnie, K.A.; Robinson, B.W.; Laurent, G.J.; McAnulty, R.J.; Mutsaers, S.E. Inhibition of collagen production delays malignant.t.t mesothelioma tumor growth in a murine model. Biochem. Biophys. Res. Commun. 2019, 510, 198–204. [Google Scholar] [CrossRef]
- Çakılkaya, P.; Sørensen, R.R.; Jürgensen, H.J.; Krigslund, O.; Gårdsvoll, H.; Nielsen, C.F.; Santoni-Rugiu, E.; Behrendt, N.; Engelholm, L.H. The Collagen Receptor uPARAP in Malignant Mesothelioma: A Potential Diagnostic Marker and Therapeutic Target. Int. J. Mol. Sci. 2021, 22, 11452. [Google Scholar] [CrossRef]
- Jagirdar, R.M.; Papazoglou, E.D.; Pitaraki, E.; Kouliou, O.A.; Rouka, E.; Giannakou, L.; Giannopoulos, S.; Sinis, S.I.; Hatzoglou, C.; Gourgoulianis, K.I.; et al. Cell and extracellular matrix interaction models in benign mesothelial and malignant pleural mesothelioma cells in 2D and 3D in-vitro. Clin. Exp. Pharmacol. Physiol. 2021, 48, 543–552. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, S.; Wang, X.; Liu, H.; Zhou, X.; Liu, H. COL1A1 Is a Potential Prognostic Biomarker and Correlated with Immune Infiltration in Mesothelioma. Biomed. Res. Int. 2021, 2021, 5320941. [Google Scholar] [CrossRef]
- Balancin, M.L.; Teodoro, W.R.; Baldavira, C.M.; Prieto, T.G.; Farhat, C.; Velosa, A.P.; da Costa Souza, P.; Yaegashi, L.B.; Ab’Saber, A.M.; Takagaki, T.Y.; et al. Different histological patterns of type-V collagen levels confer a matrices-privileged tissue microenvironment for invasion in malignant tumors with prognostic value. Pathol. Res. Pract. 2020, 216, 153277. [Google Scholar] [CrossRef]
- Balancin, M.L.; Teodoro, W.R.; Farhat, C.; de Miranda, T.J.; Assato, A.K.; de Souza Silva, N.A.; Velosa, A.P.; Falzoni, R.; Ab’Saber, A.M.; Roden, A.C.; et al. An integrative histopathologic clustering model based on immuno-matrix elements to predict the risk of death in malignant mesothelioma. Cancer Med. 2020, 9, 4836–4849. [Google Scholar] [CrossRef]
- Robledo, R.; Mossman, B. Cellular and molecular mechanisms of asbestos-induced fibrosis. J. Cell Physiol. 1999, 180, 158–166. [Google Scholar] [CrossRef]
- Senthebane, D.A.; Rowe, A.; Thomford, N.E.; Shipanga, H.; Munro, D.; Mazeedi, M.; Almazyadi, H.A.M.; Kallmeyer, K.; Dandara, C.; Pepper, M.S.; et al. The Role of Tumor Microenvironment in Chemoresistance: To Survive, Keep Your Enemies Closer. Int. J. Mol. Sci. 2017, 18, 1586. [Google Scholar] [CrossRef] [PubMed]
- Barker, H.E.; Cox, T.R.; Erler, J.T. The rationale for targeting the LOX family in cancer. Nat. Rev. Cancer 2012, 12, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Cox, T.R.; Gartland, A.; Erler, J.T. Lysyl Oxidase, a Targetable Secreted Molecule Involved in Cancer Metastasis. Cancer Res. 2016, 76, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Gordon, G.J.; Rockwell, G.N.; Jensen, R.V.; Rheinwald, J.G.; Glickman, J.N.; Aronson, J.P.; Pottorf, B.J.; Nitz, M.D.; Richards, W.G.; Sugarbaker, D.J.; et al. Identification of novel candidate oncogenes and tumor suppressors in malignant pleural mesothelioma using large-scale transcriptional profiling. Am. J. Pathol. 2005, 166, 1827–1840. [Google Scholar] [CrossRef]
- Rhodes, D.R.; Kalyana-Sundaram, S.; Mahavisno, V.; Varambally, R.; Yu, J.; Briggs, B.B.; Barrette, T.R.; Anstet, M.J.; Kincead-Beal, C.; Kulkarni, P.; et al. Oncomine 3.0: Genes, pathways, and networks in a collection of 18,000 cancer gene expression profiles. Neoplasia 2007, 9, 166–180. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Kim, H.W.; Jang, M.; Oh, S.S.; Yong, S.J.; Jeong, Y.; Jung, S.H.; Choi, J.W. LOX family and ZFPM2 as novel diagnostic biomarkers for malignant pleural mesothelioma. Biomark. Res. 2020, 8, 110. [Google Scholar] [CrossRef]
- Tang, Z.; Kang, B.; Li, C.; Chen, T.; Zhang, Z. GEPIA2: An enhanced web server for large-scale expression profiling and interactive analysis. Nucleic. Acids Res. 2019, 47, W556–W560. [Google Scholar] [CrossRef]
- Findlay, A.; Turner, C.; Schilter, H.; Deodhar, M.; Zhou, W.; Perryman, L.; Foot, J.; Zahoor, A.; Yao, Y.; Hamilton, R.; et al. An activity-based bioprobe differentiates a novel small molecule inhibitor from a LOXL2 antibody and provides renewed promise for anti-fibrotic therapeutic strategies. Clin. Transl. Med. 2021, 11, e572. [Google Scholar] [CrossRef]
- Dasler, W. Isolation of toxic crystals from sweet peas (Lathyrus odoratus). Science 1954, 120, 307–308. [Google Scholar] [CrossRef]
- Peacock, E.E.; Madden, J.W. Administration of beta-aminopropionitrile to human beings with urethral strictures: A prelimary report. Am. J. Surg. 1978, 136, 600–605. [Google Scholar] [CrossRef]
- Barrow, M.V.; Simpson, C.F.; Miller, E.J. Lathyrism: A review. Q. Rev. Biol. 1974, 49, 101–128. [Google Scholar] [CrossRef] [PubMed]
- Evtyugin, D.D.; Magina, S.; Evtuguin, D.V. Recent Advances in the Production and Applications of Ellagic Acid and Its Derivatives. A Review. Molecules 2020, 25, 2745. [Google Scholar] [CrossRef] [PubMed]
- Almatroodi, S.A.; Almatroudi, A.; Khan, A.A.; Alhumaydhi, F.A.; Alsahli, M.A.; Rahmani, A.H. Potential Therapeutic Targets of Epigallocatechin Gallate (EGCG), the Most Abundant Catechin in Green Tea, and Its Role in the Therapy of Various Types of Cancer. Molecules 2020, 25, 3146. [Google Scholar] [CrossRef] [PubMed]
- González-Sarrías, A.; García-Villalba, R.; Núñez-Sánchez, M.Á.; Tomé-Carneiro, J.; Zafrilla, P.; Mulero, J.; Tomás-Barberán, F.A.; Espín, J.C. Identifying the limits for ellagic acid bioavailability: A crossover pharmacokinetic study in healthy volunteers after consumption of pomegranate extracts. J. Funct. Foods 2015, 19, 225–235. [Google Scholar] [CrossRef]
- Nakagawa, K.; Miyazawa, T. Chemiluminescence-high-performance liquid chromatographic determination of tea catechin, (-)-epigallocatechin 3-gallate, at picomole levels in rat and human plasma. Anal. Biochem. 1997, 248, 41–49. [Google Scholar] [CrossRef]
- Gupte, A.; Mumper, R.J. Elevated copper and oxidative stress in cancer cells as a target for cancer treatment. Cancer Treat. Rev. 2009, 35, 32–46. [Google Scholar] [CrossRef]
- Mounajjed, T.; Oxentenko, A.S.; Qureshi, H.; Smyrk, T.C. Revisiting the topic of histochemically detectable copper in various liver diseases with special focus on venous outflow impairment. Am. J. Clin. Pathol. 2013, 139, 79–86. [Google Scholar] [CrossRef]
- Baldari, S.; Di Rocco, G.; Toietta, G. Current Biomedical Use of Copper Chelation Therapy. Int. J. Mol. Sci. 2020, 21, 1069. [Google Scholar] [CrossRef]
- Keiser, H.R.; Sjoerdsma, A. Studies on beta-aminopropionitrile in patients with scleroderma. Clin. Pharmacol. Ther. 1967, 8, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Verstovsek, S.; Savona, M.R.; Mesa, R.A.; Dong, H.; Maltzman, J.D.; Sharma, S.; Silverman, J.; Oh, S.T.; Gotlib, J. A phase 2 study of simtuzumab in patients with primary, post-polycythaemia vera or post-essential thrombocythaemia myelofibrosis. Br. J. Haematol. 2017, 176, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Muir, A.J.; Levy, C.; Janssen, H.L.A.; Montano-Loza, A.J.; Shiffman, M.L.; Caldwell, S.; Luketic, V.; Ding, D.; Jia, C.; McColgan, B.J.; et al. Simtuzumab for Primary Sclerosing Cholangitis: Phase 2 Study Results With Insights on the Natural History of the Disease. Hepatology 2019, 69, 684–698. [Google Scholar] [CrossRef]
- Raghu, G.; Brown, K.K.; Collard, H.R.; Cottin, V.; Gibson, K.F.; Kaner, R.J.; Lederer, D.J.; Martinez, F.J.; Noble, P.W.; Song, J.W.; et al. Efficacy of simtuzumab versus placebo in patients with idiopathic pulmonary fibrosis: A randomised, double-blind, controlled, phase 2 trial. Lancet Respir. Med. 2017, 5, 22–32. [Google Scholar] [CrossRef]
- Meissner, E.G.; McLaughlin, M.; Matthews, L.; Gharib, A.M.; Wood, B.J.; Levy, E.; Sinkus, R.; Virtaneva, K.; Sturdevant, D.; Martens, C.; et al. Simtuzumab treatment of advanced liver fibrosis in HIV and HCV-infected adults: Results of a 6-month open-label safety trial. Liver Int. 2016, 36, 1783–1792. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.B., 3rd; Wainberg, Z.A.; Hecht, J.R.; Vyushkov, D.; Dong, H.; Bendell, J.; Kudrik, F. A Phase II Randomized, Double-Blind, Placebo-Controlled Study of Simtuzumab or Placebo in Combination with Gemcitabine for the First-Line Treatment of Pancreatic Adenocarcinoma. Oncologist 2017, 22, 241.e215. [Google Scholar] [CrossRef] [PubMed]
- Hecht, J.R.; Benson, A.B., 3rd; Vyushkov, D.; Yang, Y.; Bendell, J.; Verma, U. A Phase II, Randomized, Double-Blind, Placebo-Controlled Study of Simtuzumab in Combination with FOLFIRI for the Second-Line Treatment of Metastatic KRAS Mutant Colorectal Adenocarcinoma. Oncologist 2017, 22, 243.e223. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Abdelmalek, M.F.; Caldwell, S.; Shiffman, M.L.; Diehl, A.M.; Ghalib, R.; Lawitz, E.J.; Rockey, D.C.; Schall, R.A.; Jia, C.; et al. Simtuzumab Is Ineffective for Patients With Bridging Fibrosis or Compensated Cirrhosis Caused by Nonalcoholic Steatohepatitis. Gastroenterology 2018, 155, 1140–1153. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Anstee, Q.M.; Trauner, M.; Lawitz, E.J.; Abdelmalek, M.F.; Ding, D.; Han, L.; Jia, C.; Huss, R.S.; Chung, C.; et al. Cirrhosis Regression is Associated with Improved Clinical Outcomes in Patients with Nonalcoholic Steatohepatitis. Hepatology 2021. [Google Scholar] [CrossRef]
- Gee, J.R.; Saltzstein, D.R.; Kim, K.; Kolesar, J.; Huang, W.; Havighurst, T.C.; Wollmer, B.W.; Stublaski, J.; Downs, T.; Mukhtar, H.; et al. A Phase II Randomized, Double-blind, Presurgical Trial of Polyphenon E in Bladder Cancer Patients to Evaluate Pharmacodynamics and Bladder Tissue Biomarkers. Cancer Prev. Res. 2017, 10, 298–307. [Google Scholar] [CrossRef]
- Chapman, H.A.; Wei, Y.; Montas, G.; Leong, D.; Golden, J.A.; Trinh, B.N.; Wolters, P.J.; Le Saux, C.J.; Jones, K.D.; Hills, N.K.; et al. Reversal of TGFβ1-Driven Profibrotic State in Patients with Pulmonary Fibrosis. N. Engl. J. Med. 2020, 382, 1068–1070. [Google Scholar] [CrossRef] [PubMed]
- Pass, H.I.; Brewer, G.J.; Dick, R.; Carbone, M.; Merajver, S. A phase II trial of tetrathiomolybdate after surgery for malignant mesothelioma: Final results. Ann. Thorac. Surg. 2008, 86, 383–389, discussion 390. [Google Scholar] [CrossRef] [PubMed]
- Redman, B.G.; Esper, P.; Pan, Q.; Dunn, R.L.; Hussain, H.K.; Chenevert, T.; Brewer, G.J.; Merajver, S.D. Phase II trial of tetrathiomolybdate in patients with advanced kidney cancer. Clin. Cancer Res. 2003, 9, 1666–1672. [Google Scholar]
- Brewer, G.J.; Dick, R.D.; Grover, D.K.; LeClaire, V.; Tseng, M.; Wicha, M.; Pienta, K.; Redman, B.G.; Jahan, T.; Sondak, V.K.; et al. Treatment of metastatic cancer with tetrathiomolybdate, an anticopper, antiangiogenic agent: Phase I study. Clin. Cancer Res. 2000, 6, 1–10. [Google Scholar] [PubMed]
- Chan, N.; Willis, A.; Kornhauser, N.; Ward, M.M.; Lee, S.B.; Nackos, E.; Seo, B.R.; Chuang, E.; Cigler, T.; Moore, A.; et al. Influencing the Tumor Microenvironment: A Phase II Study of Copper Depletion Using Tetrathiomolybdate in Patients with Breast Cancer at High Risk for Recurrence and in Preclinical Models of Lung Metastases. Clin. Cancer Res. 2017, 23, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Selman, M.; Carrillo, G.; Salas, J.; Padilla, R.P.; Pérez-Chavira, R.; Sansores, R.; Chapela, R. Colchicine, D-penicillamine, and prednisone in the treatment of idiopathic pulmonary fibrosis: A controlled clinical trial. Chest 1998, 114, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Clements, P.J.; Seibold, J.R.; Furst, D.E.; Mayes, M.; White, B.; Wigley, F.; Weisman, M.D.; Barr, W.; Moreland, L.; Medsger, T.A., Jr.; et al. High-dose versus low-dose D-penicillamine in early diffuse systemic sclerosis trial: Lessons learned. Semin. Arthritis Rheum. 2004, 33, 249–263. [Google Scholar] [CrossRef]
- Brem, S.; Grossman, S.A.; Carson, K.A.; New, P.; Phuphanich, S.; Alavi, J.B.; Mikkelsen, T.; Fisher, J.D. Phase 2 trial of copper depletion and penicillamine as antiangiogenesis therapy of glioblastoma. Neuro Oncol. 2005, 7, 246–253. [Google Scholar] [CrossRef]
- Barry-Hamilton, V.; Spangler, R.; Marshall, D.; McCauley, S.; Rodriguez, H.M.; Oyasu, M.; Mikels, A.; Vaysberg, M.; Ghermazien, H.; Wai, C.; et al. Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nat. Med. 2010, 16, 1009–1017. [Google Scholar] [CrossRef]
- Rossow, L.; Veitl, S.; Vorlová, S.; Wax, J.K.; Kuhn, A.E.; Maltzahn, V.; Upcin, B.; Karl, F.; Hoffmann, H.; Gätzner, S.; et al. LOX-catalyzed collagen stabilization is a proximal cause for intrinsic resistance to chemotherapy. Oncogene 2018, 37, 4921–4940. [Google Scholar] [CrossRef]
- Chen, P.; Zhao, D.; Li, J.; Liang, X.; Li, J.; Chang, A.; Henry, V.K.; Lan, Z.; Spring, D.J.; Rao, G.; et al. Symbiotic Macrophage-Glioma Cell Interactions Reveal Synthetic Lethality in PTEN-Null Glioma. Cancer Cell 2019, 35, 868–884.e866. [Google Scholar] [CrossRef] [PubMed]
- Haj-Shomaly, J.; Vorontsova, A.; Barenholz-Cohen, T.; Levi-Galibov, O.; Devarasetty, M.; Timaner, M.; Raviv, Z.; Cooper, T.J.; Soker, S.; Hasson, P.; et al. T cells promote metastasis by regulating extracellular matrix remodeling following chemotherapy. Cancer Res. 2022, 82, 278–291. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.Y.; Wang, N.; Zhang, C.; Chan, Y.T.; Yuen, M.F.; Feng, Y. Lysyl Oxidase-Like 4 Fosters an Immunosuppressive Microenvironment During Hepatocarcinogenesis. Hepatology 2021, 73, 2326–2341. [Google Scholar] [CrossRef] [PubMed]
- Collier, J.L.; Weiss, S.A.; Pauken, K.E.; Sen, D.R.; Sharpe, A.H. Not-so-opposite ends of the spectrum: CD8(+) T cell dysfunction across chronic infection, cancer and autoimmunity. Nat. Immunol. 2021, 22, 809–819. [Google Scholar] [CrossRef] [PubMed]
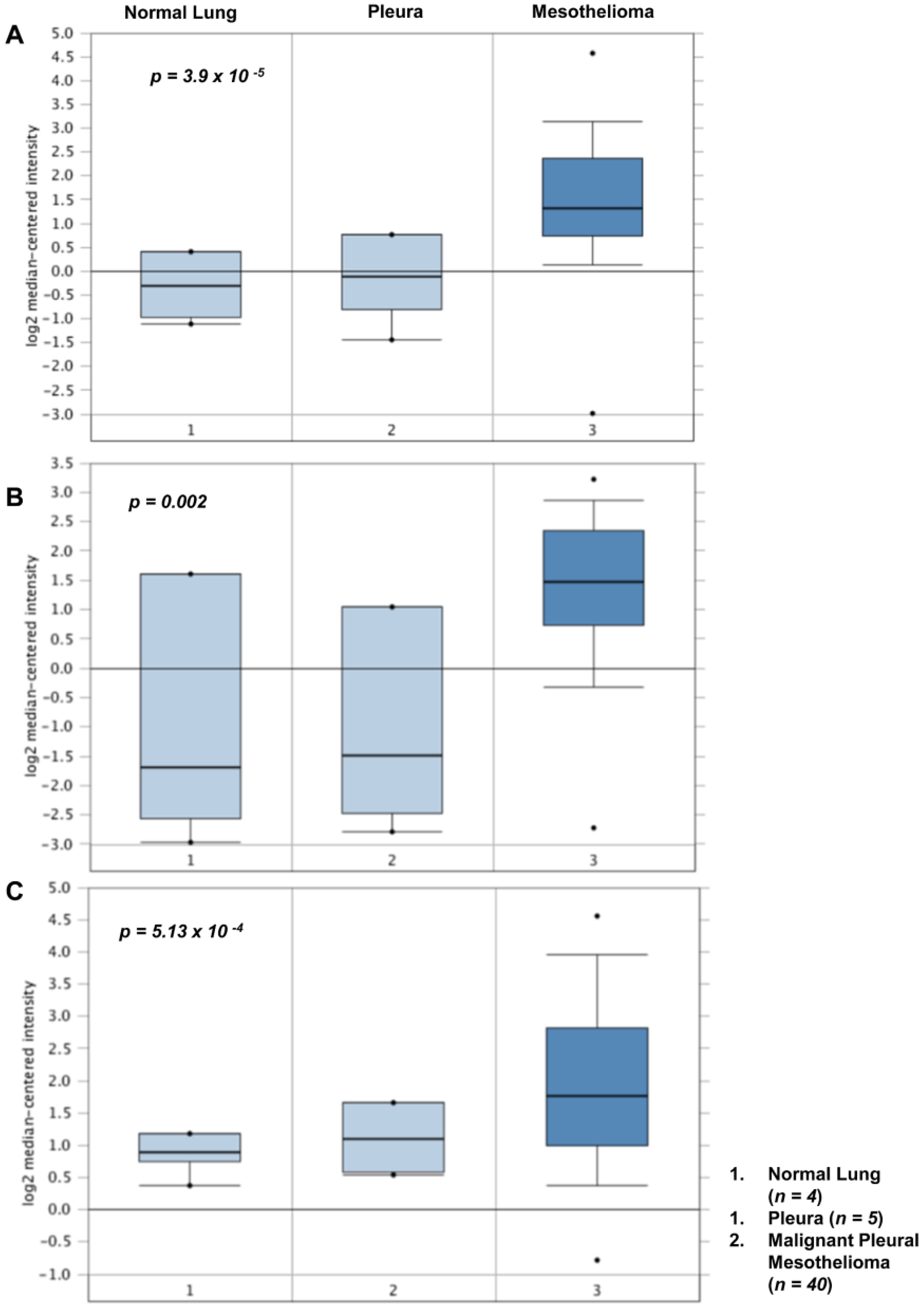
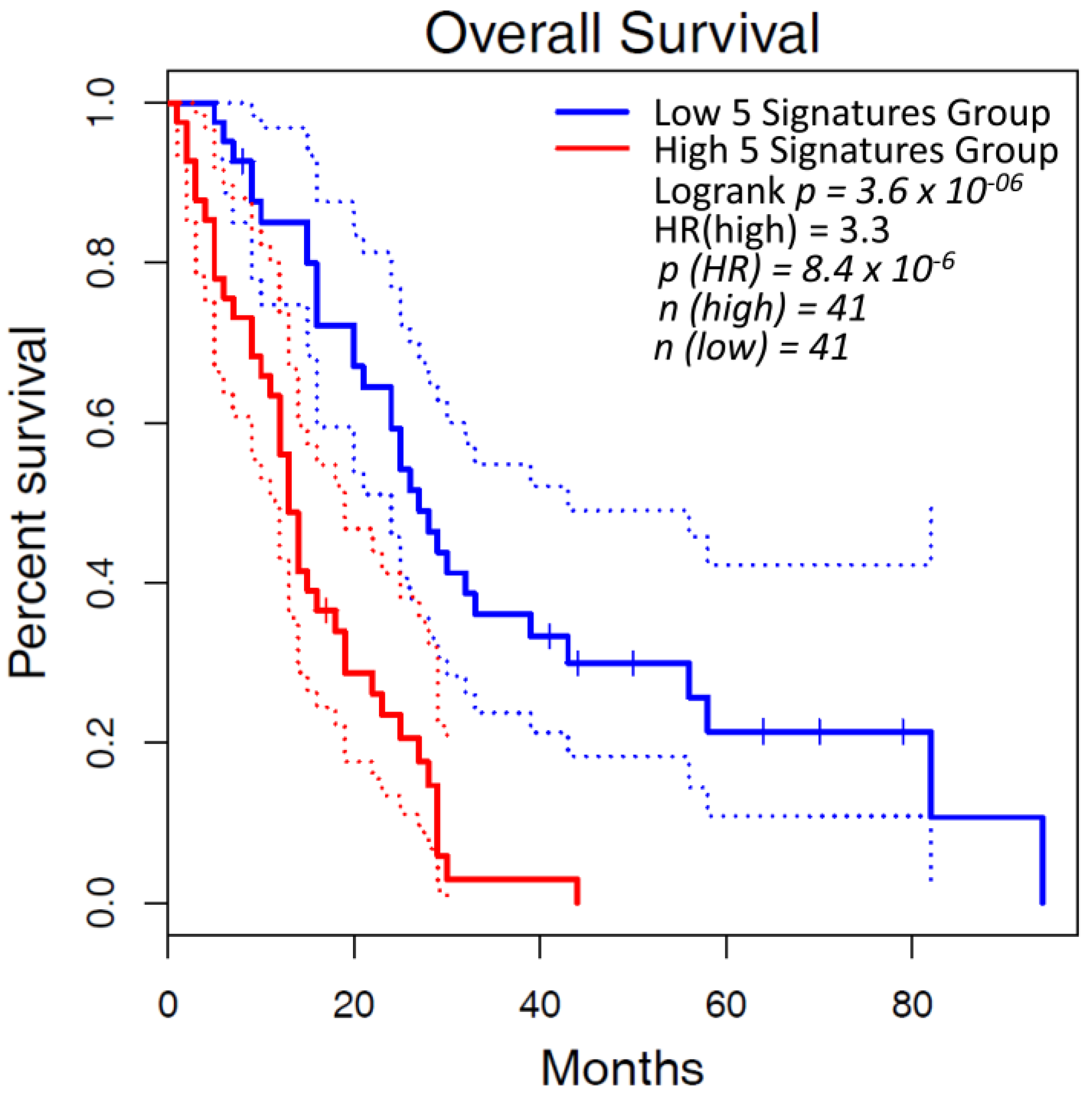
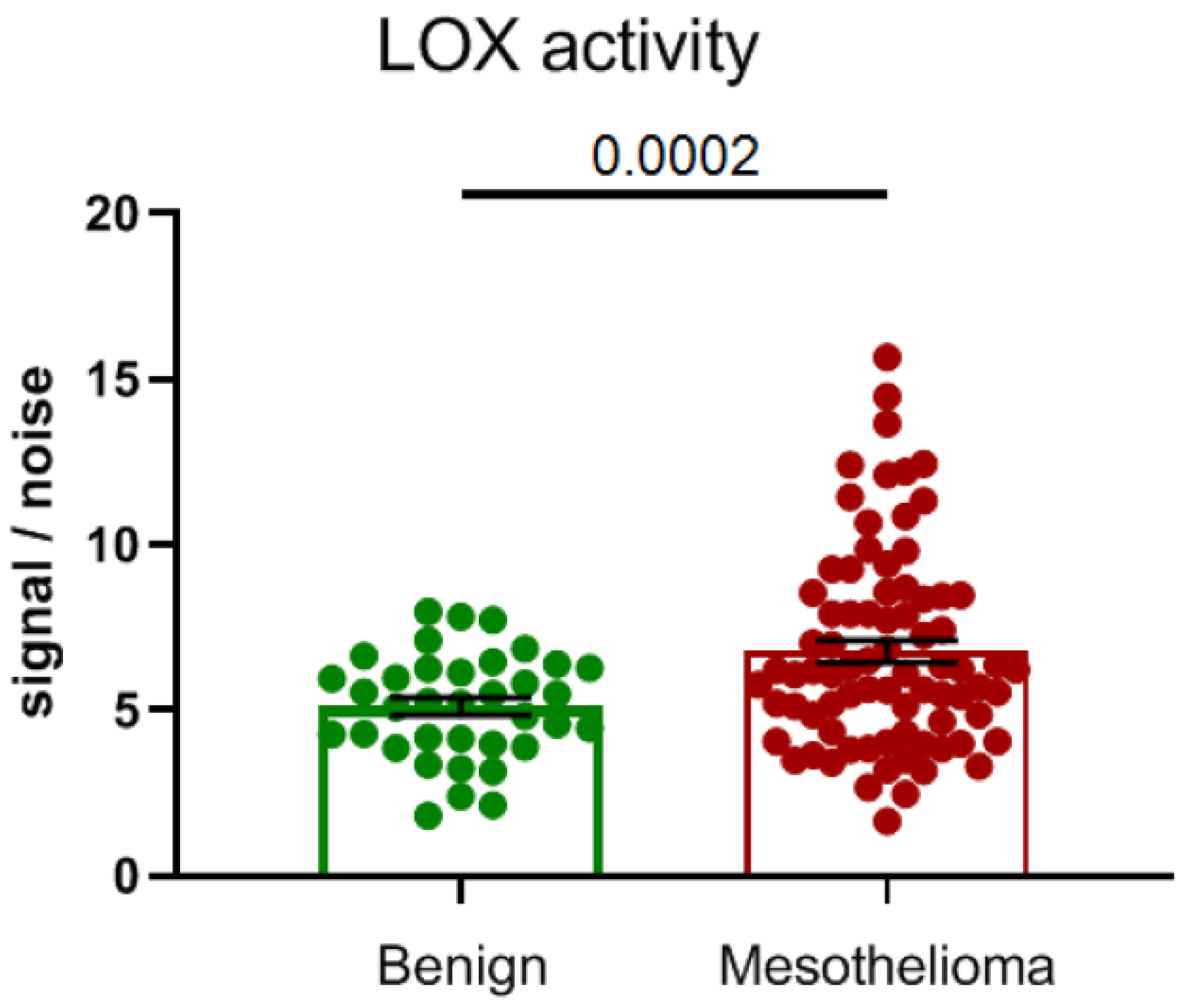
| Idiopathic Pulmonary Fibrosis (IPF) | |
| β-aminopropionitrile (BAPN) found to inhibit pulmonary fibrosis in a lung model of silicosis | [34] |
| LOX activity induced in bleomycin-induced lung fibrosis and alleviated by treatment with LOX/LOXL inhibitor | [35,36,37] |
| In IPF patients, elevated serum levels of LOXL2 are associated with an increased risk for disease progression. | [38] |
| LOX activity promotes the progress of EMT in a paraquat model of IPF. | [39,40] |
| A comparative analysis shows that LOX/LOXL2 are elevated in IPF fibroblasts, while LOXL2/3 activity is crucial for fibroblast-to-myofibroblast transition (FMT). | [41] |
| Elevated serum levels of LOXL2 are associated with rheumatoid arthritis -associated interstitial lung disease (RA-ILD). | [42] |
| Increased collagen fibril thickness in IPF versus non-IPF lung tissues is correlated with increased levels of LOXL1/LOXL2 protein, and a decrease in LOX protein expression. | [43] |
| Loss of LOXL1 activity prevents the development of fibrosis in a transforming growth factor-β1-induced model of pulmonary fibrosis. | [44] |
| In bleomycin-induced lung fibrosis, nuclear expression of LOXL2 appears to be a major element in the progression of lung fibrosis. | [45] |
| LOXL2 inhibitor induces collagen turnover in ex vivo lung explants from patients with IPF. | [46] |
| In a study comparing extracellular vesicles (EVs) from IPF-derived pulmonary fibroblast cell lines versus normal pulmonary fibroblast cell lines for differentially expressed proteins, LOXL1 was found in IPF EVs. | [47] |
| Kidney Fibrosis | |
| LOXL2 is expressed in compartments of renal tissue, where it appears to contribute to the progression of tubulointerstitial fibrosis. | [48] |
| LOXL2 inhibition significantly reduced interstitial fibrosis in a mouse model of renal fibrosis. | [49] |
| Elevated serum LOX and LOXL2 levels may act as a potential biomarker for kidney fibrosis. | [50] |
| In a murine model of cyclosporine induced nephropathy, pan-LOX and LOXL2 specific inhibitors attenuated kidney damage. | [51] |
| Liver Fibrosis | |
| Increased LOX levels both in tissue and serum is associated with collagen in the extracellular space in animal models of hepatic fibrosis. | [52,53,54,55] |
| Hepatic stellate cell activation results in elevated LOX mRNA and protein in liver fibrosis. | [56,57,58,59] |
| Lysyl oxidase activity levels increase in patient serum from chronic persistent hepatitis to chronic active hepatitis to cirrhosis. | [60] |
| In a mouse model of liver fibrosis, increased steady state levels of LOXL mRNA occur early in fibrosis development. | [61] |
| Expression of LOX and LOXL2 in hepatocytes is linked to liver fibrosis. | [62] |
| Reduced levels of miR-29b are associated with elevated levels of LOX in models of liver fibrosis. | [63] |
| In patients with severe obesity or obstructive sleep apnoea, serum levels of lysyl oxidase can act as a potential biomarker of liver fibrosis. | [64] |
| Targeting of LOXL2 is associated with anti-fibrotic effects in a mouse model of hepatic fibrosis. | [65] |
| Links between LOXL2, insulin resistance and fibrosis accumulation in non-alcoholic fatty liver disease (NAFLD) are identified. | [66] |
| LOXL1 identified as a candidate therapeutic target for ameliorating liver fibrosis progression in cirrhosis, and inhibition of human hepatic stellate cell mediated fibrogenesis. | [67,68] |
| First demonstration that a small molecule dual inhibitor of LOXL2/3 (PXS-5153A) can ameliorate fibrosis in models of liver fibrosis and myocardial infarct | [69] |
| Patients with HCV who demonstrated sustained responses to antiviral therapy were shown to have regression in liver fibrosis associated with decreased LOXL2 expression. | [70] |
| anti-LOXL2 based therapy in a mouse model of liver fibrosis results in reduced fibrosis via accelerated collagenolytic activity by macrophages. | [71] |
| miR-15b/16 are downregulated in activated hepatic stellate cells (HSCs), and overexpression of these miRs is found to suppress LOXL1 expression in HSCs and induce a fibrogenic response. | [72,73] |
| Selective deletion of LOXL1 in HSCs in a murine NAFLD model ameliorates fibrosis, and serum levels of LOXL1 are positively correlated with histological fibrosis progression in NAFLD patients. | [74] |
| Amyotrophic Lateral Sclerosis (ALS) | |
| LOX transcripts are overexpressed in patient lumbar spinal cord samples. | [75] |
| LOX activity increased in animal model of ALS. | [76] |
| Systemic Sclerosis | |
| LOX mRNA transcripts overexpressed in fibroblasts from patients with systemic sclerosis | [77,78] |
| Elevated levels of LOX found in the serums of patients with systemic sclerosis | [78,79] |
| Elevated levels of LOX and LOXL2 in skin and lungs of systemic sclerosis patients | [37] |
| Elevated serum LOX levels and idiopathic pulmonary arterial hypertension (iPAH) found in patients with systemic sclerosis | [80] |
| LOXL4 activity as a cause of cutaneous fibrosis in fibroblasts from patients with system sclerosis identified | [81] |
| Breast Cancer | |
| Lysyl oxidases associated with chemotherapy resistance in triple negative breast cancer | [99] |
| Stromal expression of LOXL2 is associated with tumor aggression and disease-specific mortality. | [100] |
| Oncostatin-M-induced ECM remodelling via upregulated LOXL2 | [101] |
| Inhibition of LOXL4 decreased breast cancer cell proliferation, migration, and metastasis in vitro and in vivo. | [102,103] |
| LOXL2 promotes tumour lymph-angiogenesis and lymph node metastasis. | [104] |
| LOX expression is significantly higher in triple negative breast cancers versus other breast cancer subtypes. | [105] |
| LOXL2 involved with breast cancer metastasis to the lung | [106] |
| LOXL2 expression may serve as a biomarker for breast cancer and is detectable in serum and urine. | [107] |
| LOX and LOXL proteins are located in the stromal reaction of ductal carcinoma in situ (DCIS) breast cancer. | [108,109] |
| Renal Cell Cancer (RCC) | |
| LOX and LOXL2 significantly elevated in RCC and associated with poorer overall survival (OS) | [110] |
| LOXL2 associated with migration, invasion and EMT transition of RCC | [111] |
| Primary RCC cultuure endogenously express LOX, and plays major roles in progression via activities on cellular adhesion, migration, and collagen stiffness. | [112] |
| Early demonstration of overexpression of LOX mRNA in RCC | [113] |
| Non-Small Cell Lung Cancer (NSCLC) | |
| A novel LOX polymorphism G473A is associated with increased risk for lung cancer. | [114,115,116] |
| LOXL1 promotes lung cancer tumourigenicity via collagen matrix remodelling and collagen fibre alignment in vitro and in vivo. | [33] |
| High expression of LOX and LOXL2 mRNA and protein is associated with poor prognosis in NSCLC patients. | [117,118,119,120] |
| Low expression of the LOXL2 protein in adenocarcinomas is associated with a poorer N-stage, a higher pathological TNM stage and poorer differentiation. | [121] |
| The miR-200/ZEB1 axis drives lung cancer metastasis through LOXL2. | [122] |
| LOX activity is associated with increased invasion and migration of hypoxic NSCLC cells. | [123] |
| Reduced levels of microRNA-29a (miR-29a) in lung cancer is associated with overexpression of LOXL2 and concomitant fibrosis. | [124] |
| Pancreatic Cancer (PDAC) | |
| Tumor stiffening reversion through collagen crosslinking inhibition improves T-cell migration and anti-PD-1 treatment. | [125] |
| LOXL2 is highly up-regulated (≥20-fold) in the PDAC secretome. | [126] |
| High expression of LOXL2 protein is associated with worse DFS and OS in patients with PDAC. | [127] |
| Increased levels of LOX, LOXL1, and LOXL2 expression in PDAC are associated with poor responses to chemotherapy by limiting drug distribution, and inhibition of LOX enhances drug efficacy. | [128,129] |
| In an orthotopic PDX model of PDAC, targeting LOXL2 led to accelerated tumour growth, and poorer overall survival. | [130] |
| Levels of LOX are a potential prognostic markers for the prognosis of pancreatic cancer patients. | [131] |
| Liver Cancer | |
| Reduced levels of miR-29a in hepatocellular carcinoma (HCC) lead to elevated expression of its known targets LOX and LOXL2. | [132] |
| A circRNA network has been identified that activates LOX transcription in HCC. | [133] |
| High LOXL3 expression predicts poor outcomes for patients with HCC, and is correlated with immune infiltrates and T-cell activation. | [134] |
| High LOX expression is associated with an higher recurrence rate and poorer OS in patients with HCC. | [135,136] |
| LOXL2 is overexpressed in HCC and positively correlated with tumour grade, metastasis, and poor OS. | [137,138,139] |
| LOXL2 is significantly overexpressed in human HCC sera and may act as a good biomarker for HCC. | [140] |
| LOXL4 is upregulated in HCC tissues and associated with poor prognosis. Exosomal-mediated transfer of LOXL4 between HCC cells and human umbilical vein endothelial cells (HUVECs) promotes cell migration and angiogenesis, respectively | [141] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perryman, L.; Gray, S.G. Fibrosis in Mesothelioma: Potential Role of Lysyl Oxidases. Cancers 2022, 14, 981. https://doi.org/10.3390/cancers14040981
Perryman L, Gray SG. Fibrosis in Mesothelioma: Potential Role of Lysyl Oxidases. Cancers. 2022; 14(4):981. https://doi.org/10.3390/cancers14040981
Chicago/Turabian StylePerryman, Lara, and Steven G. Gray. 2022. "Fibrosis in Mesothelioma: Potential Role of Lysyl Oxidases" Cancers 14, no. 4: 981. https://doi.org/10.3390/cancers14040981
APA StylePerryman, L., & Gray, S. G. (2022). Fibrosis in Mesothelioma: Potential Role of Lysyl Oxidases. Cancers, 14(4), 981. https://doi.org/10.3390/cancers14040981







