Simple Summary
L-asparaginase (L-ASNase) therapy is key for achieving the very high cure rate of pediatric acute lymphoblastic leukemia (ALL), yet its use is mostly confined to this indication. One main reason preventing the expansion of today’s FDA-approved L-ASNases to solid cancers is their high toxicity and side effects, which become especially challenging in adult patients. The design of optimized L-ASNase molecules provides opportunities to overcome these unwanted toxicities. An additional challenge to broader application of L-ASNases is how cells can counter the pharmacological effect of this drug and the identification of L-ASNases resistance mechanisms. In this review, we discuss recent insights into L-ASNase adverse effects, resistance mechanisms, and how novel L-ASNase variants and drug combinations can expand its clinical applicability, with a focus on both hematological and solid tumors.
Abstract
L-Asparaginase (L-ASNase) is an enzyme that hydrolyses the amino acid asparagine into aspartic acid and ammonia. Systemic administration of bacterial L-ASNase is successfully used to lower the bioavailability of this non-essential amino acid and to eradicate rapidly proliferating cancer cells with a high demand for exogenous asparagine. Currently, it is a cornerstone drug in the treatment of the most common pediatric cancer, acute lymphoblastic leukemia (ALL). Since these lymphoblasts lack the expression of asparagine synthetase (ASNS), these cells depend on the uptake of extracellular asparagine for survival. Interestingly, recent reports have illustrated that L-ASNase may also have clinical potential for the treatment of other aggressive subtypes of hematological or solid cancers. However, immunogenic and other severe adverse side effects limit optimal clinical use and often lead to treatment discontinuation. The design of optimized and novel L-ASNase formulations provides opportunities to overcome these limitations. In addition, identification of multiple L-ASNase resistance mechanisms, including ASNS promoter reactivation and desensitization, has fueled research into promising novel drug combinations to overcome chemoresistance. In this review, we discuss recent insights into L-ASNase adverse effects, resistance both in hematological and solid tumors, and how novel L-ASNase variants and drug combinations can expand its clinical applicability.
1. Introduction
Asparagine (Asn) is a non-essential amino acid (AA), taken up via the diet or, alternatively, synthesized from central metabolic pathways in which Asn is produced from aspartic acid (Asp) and glutamine (Gln) by the enzyme asparagine synthetase (ASNS) [1,2,3,4]. L-asparaginase (L-ASNase) was first described in 1904 as a non-human enzyme that hydrolyses Asn into Asp and ammonia in different bovine tissues [5]. In 1953, Kidd et al. reported that the serum from guinea pigs could inhibit the growth of transplanted lymphomas in mice, but it took almost another 10 years before Broome et al. pinpointed that this inhibition was due to its L-ASNase enzyme activity [6,7]. In 1966, the first use of purified guinea pig serum to treat a boy with acute lymphoblastic leukemia (ALL) was reported [8]. As guinea pig serum is an expensive and limited resource for L-ASNase, the pharmaceutical industry rapidly moved towards alternative L-ASNases from bacterial sources for large-scale production of sufficient amounts of L-ASNase for cancer treatment purposes [9,10].
Currently, L-ASNase is mainly used as a cornerstone drug in the therapeutic regimen for childhood ALL. In contrast to healthy cells, ALL lymphoblasts largely lack ASNS expression, and thus depend on an extracellular source of Asn for their survival [11,12,13,14,15]. As large amounts of Asn are indeed necessary for ALL cell growth, depleting circulating Asn by L-ASNase administration results in starvation and selective apoptosis of the leukemic blasts (Figure 1A). More recently, accumulating evidence suggests that L-ASNase could also have clinical potential for the treatment of other subtypes of leukemia or certain aggressive solid tumors [16,17,18,19,20]. Nevertheless, use of this drug in adult cancer populations has largely been hampered by its toxicity profile, which includes severe immunological side effects as well as non-immune related toxicities such as pancreatitis, liver toxicities, coagulopathy, and neurotoxicity [21,22].
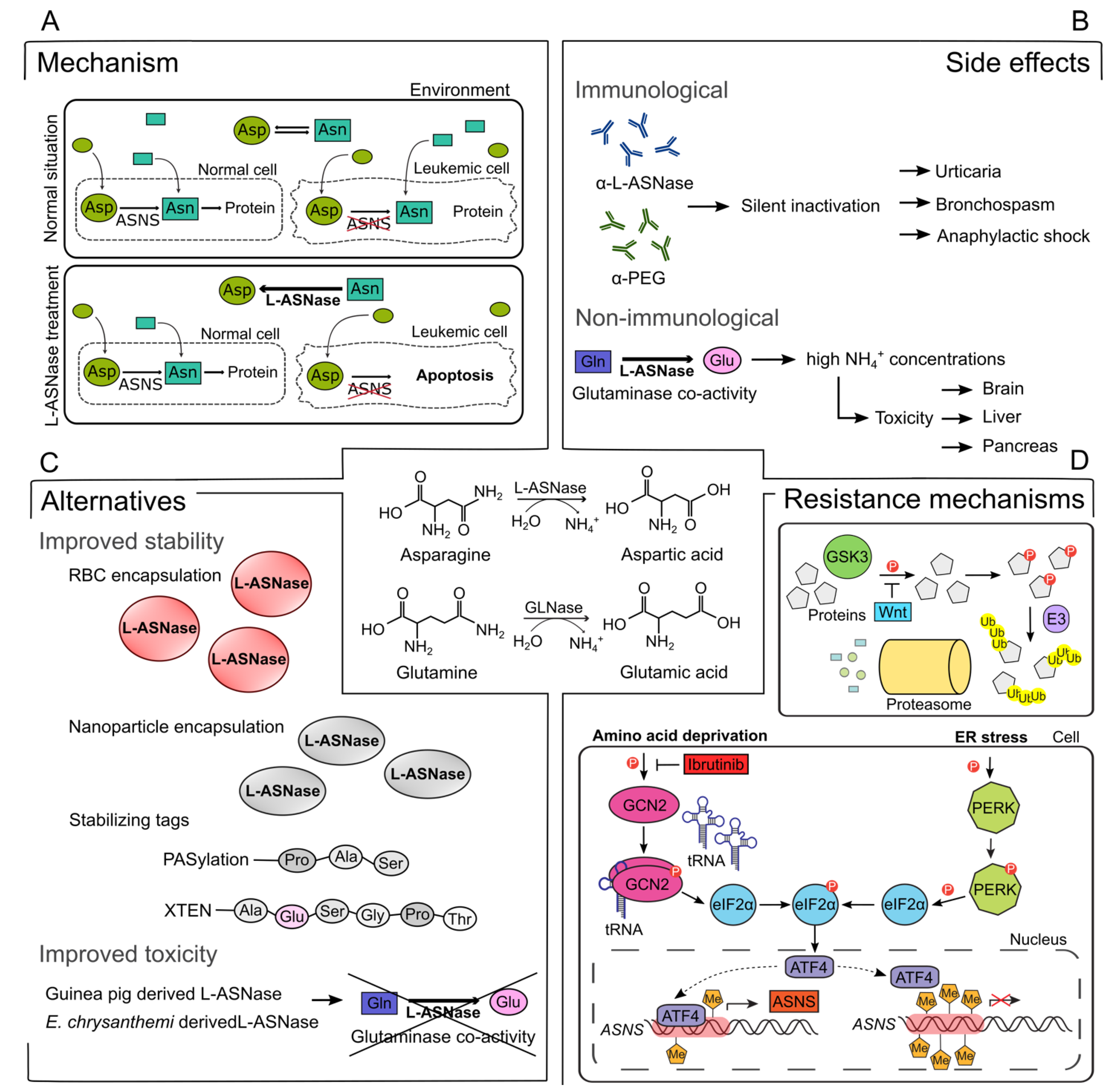
Figure 1.
Overview of L-asparaginase (L-ASNase) mechanism, side effects, alternatives, and combination therapies. (A) Mechanism of L-ASNase treatment in leukemic cells. Administration of L-ASNase depletes Asn from the environment, resulting in selective apoptosis of leukemic cells due to the lack of ASNS. Asn, asparagine; Asp, aspartic acid; ASNS, asparagine synthetase; L-ASNase, L-asparaginase (B) Immunological and non-immunological side effects related to ASNase treatment. Production of α-L-ASNase or α-PEG antibodies causes silent inactivation, which results in multiple side effects. The glutaminase co-activity of L-ASNase gives rise to a high concentration of NH4+, which are correlated with brain, liver, and pancreas toxicities. Gln, glutamine; Glu, glutamic acid, PEG, monomethoxypolyethylene glycol; GLNase, glutaminase (C) Multiple stabilizing strategies for ASNase based on encapsulation or tag addition that are under investigation or already approved in the clinic. RBC, red blood cell; Pro, proline; Ala, alanine; Ser, serine; Gly, glycine, Thr, Threonine (D) GSK3a-dependent protein ubiquitination and degradation and the GCN2-ATF4-ASNS axis are affected by ASNase treatment and have the ability to cause resistance. ER, endoplasmic reticulum; GSK3, glycogen synthase kinase-3; Wnt, Wingless/Integrated; E3, E3 ubiquitin ligase; Ub, ubiquitin; GCN2, general control nonderepressible 2; tRNA, transfer RNA; elF2α, eukaryotic translation initiation factor 2α; PERK, protein kinase RNA-like ER kinase; ATF4, activating transcription factor 4; Me, methyl.
2. Bacterial L-ASNase Used in the Clinic for ALL Therapy
Five different L-ASNase preparations are currently used in the clinic. Two variants, Erwinase® and Rylaze®, are both of the same Erwinia chrysanthemi ansB gene product but differ in their bio-manufacturing process [23,24]. The three others are derived from the Escherichia coli Type 2 L-ASNase. One is a native formulation (brand names Elspar®; Kidrolase®; Spectrila®; and others), while the two others are stabilized formulations, resulting from the covalent conjugation of monomethoxypolyethylene glycol (PEG) to lysine resides on the enzyme via a succinimidyl succinate linker (Oncaspar®) [25,26] or a succinimidyl carbonate linker (Asparlas®) [27,28]. The addition of the PEG-tag delays elimination from the body and increases the half-life of PEG-L-ASNase in humans towards 5.5 days (Oncaspar®) [29,30,31] and 16.1 days (Asparlas®) [28]. Given its enhanced stability and earlier development, Oncaspar® is the main enzymatic variant that is currently used in the clinic as front-line therapy for the treatment of childhood ALL [32,33,34]. An overview of all the above discussed L-ASNase variants is provided in Table 1.

Table 1.
Overview of L-ASNase variants currently used in the clinic. This table shows a comparison of different L-ASNase variants, their production hosts, and potential tags. In addition, remarks concerning the approval of the product and findings as observed in literature are shown.
In addition to their L-ASNase activity, all clinically approved bacterial L-ASNases also hydrolyze Gln into glutamic acid (Glu) and ammonia. The clinical relevance of this glutaminase co-activity in the context of ALL remains largely unclear, with conflicting reports in the literature about its putative antileukemic effect [38,39,40,41]. First, it was shown in vitro that this glutaminase co-activity was not required for its anticancer effect against ASNS negative leukemic cells [38]. In line with this notion, Nguyen et al. indeed showed that glutaminase co-activity was not essential for inducing an in vivo L-ASNase effect in a xenograft model of ASNS-negative SUP-B15 leukemia cells [40]. However, more recently, it was suggested that a durable and long-lasting anticancer effect in the same SUP-B15 xenograft model could only be obtained using an L-ASNase variant that retained its glutaminase co-activity [41]. Therefore, in the future, additional studies will be required to unambiguously show if glutaminase co-activity is truly required for a long-term antileukemic response in ASNS-negative tumor cells or not.
2.1. Immunological Side Effects
Due to its bacterial nature, immune responses to L-ASNase during treatment can occur in 30–70% of patients and have been associated with the generation of potentially neutralizing antibodies [30]. Clinical hypersensitivity reactions range from light local symptoms (urticaria) to systemic reactions (bronchospasm and anaphylactic shock) [30,42] (Figure 1B). The L-ASNase preparation, intensity, and consistency of dosing, as well as the administration route can all influence the likelihood of developing an immune reaction during L-ASNase therapy [34,43,44]. For example, it was shown that native E. coli L-ASNase is more immunogenic than the PEG-L-ASNase [45,46,47,48]. The antibodies generated during these hypersensitivity reactions can result in increased L-ASNase clearance and, therefore, a reduction or neutralization of the catalytic activity of L-ASNase [49,50]. In addition, circulating antibodies can also develop in the absence of any obvious clinical signs of allergy, a phenomenon known as silent inactivation, which can often go undetected [34,42,51]. However, recent work described that ALL patients treated with PEG-L-ASNase can also develop antibodies against the PEG-tag itself, thereby limiting tolerance and efficacy [52,53]. For this reason, additional research on more immunotolerant tags is currently ongoing, including XTEN, a non-immunogenic and biodegradable unstructured polymer [54] as well as PASylation [31,55,56] and red blood cell (RBC) encapsulation [57,58] (Figure 1C). Although the importance of switching from E. coli PEG-L-ASNase to E. chrysanthemi L-ASNase during ALL therapy after silent inactivation has been clearly established by several reports [49,59,60], hypersensitivity towards the Erwinia derived enzyme can also still occur in 3–33% of patients [23,42,61,62]. Albeit premedication with steroids or antihistamines is known to reduce clinical hypersensitivity symptoms, it may not prevent the development of antibodies [48]. Therefore, immunological responses against clinically used bacterial L-ASNase formulations can lead to early termination of treatment and decreased prognosis [50,63,64,65]. The active search towards and development of alternative L-ASNases aims to eliminate these problems by creating additional treatment options.
The efficiency of Asn depletion can be determined by serum Asn concentration, the presence of PEG-L-ASNase antibodies, and L-ASNase activity. However, due to the lack of tests for anti-L-ASNase antibodies that distinguish between inactivating or non-inactivating antibodies and the fast ex vivo metabolism of Asn in the presence of L-ASNase, the evaluation of L-ASNase activity remains the only option to monitor silent inactivation in patients during therapy [34,42,66]. Indeed, real-time monitoring of L-ASNase activity during treatment regimens is critical to avoid unfavorable outcomes in pediatric ALL due to suboptimal L-ASNase therapy, as recently shown in the context of ALL patients treated according to the Nordic Society of Pediatric Hematology and Oncology (NOPHO) ALL2008 protocol [67].
Of note, in recent years the drug supply of E. chrysanthemi L-ASNase was often limited due to manufacturing issues, thereby potentially harming optimal treatment outcome for hypersensitive ALL patients. To address this, recombinant DNA technology has recently been explored to increase L-ASNase production yield and overcome problems associated with drug shortage. Indeed, a new recombinant version of E. chrysanthemi L-ASNase, Rylaze®, also known as JZP-458, has been developed and taken forward into a clinical trial (NCT04145531). Recently, it has been approved by the FDA as part of a treatment regimen for children and adults with ALL or lymphoblastic lymphoma [24,68,69]. Rylaze® will help alleviate the shortage of additional L-ASNase for the treatment of ALL patients that develop hypersensitivity against PEG-L-ASNase [24].
2.2. Non-Immunological Side Effects
Finally, non-immune related toxicities that have been associated with L-ASNase administration occur with lower frequency but can develop with higher severity to affect treatment outcomes and include pancreatitis, liver toxicity, coagulopathy, and neurotoxicity [21,30,70,71] (Figure 1B). These toxicities are, at least partially, caused by residual glutaminase co-activity that is present in all clinically used L-ASNases [72,73,74,75,76]. This co-activity causes hydrolyzation of Gln into Glu and ammonia. The extensive ammonia production might be an important factor that contributes to L-ASNase-induced liver dysfunction and neurotoxicity [74,77,78,79]. It remains to be established whether this glutaminase co-activity is truly required for a long-term antileukemic response in ASNS-negative tumor cells [38,39,40,41]. Therefore, reducing the glutaminase co-activity of available L-ASNase may be advantageous to lessen toxic side effects, enabling patients to complete their treatments and, improve their outcomes as well as be used more frequently in the treatment of adults with ALL.
Of note, besides L-glutamine, also asparaginyl-transfer RNA has been reported to be a substrate for L-ASNase [80]. On top, some L-ASNases may have alternative activities that putatively play a partial role in their therapeutic activity and associated adverse side-effect. E. coli L-ASNase has been demonstrated to bind and inhibit the concanavalin A receptor [81,82]. More recently, an asparagine-independent cytotoxicity mechanism was reported specifically for a Rodosprillum rubrum mutant L-ASNase [83]. In contrast to other bacterial L-ASNases, this RrA variant can penetrate cancer cells via a clathrin receptor-dependent manner resulting in suppression of telomerase activity and asparagine-independent cytotoxicity [83,84].
3. ASNS Promoter Methylation as Biomarker for L-ASNase Sensitivity in ALL
Human ASNS activity is highly regulated at the transcriptional level via two main signaling pathways which are switched on after cellular stress to ensure survival [4]. The amino acid response (AAR) pathway is activated in response to intracellular AA depletion and an imbalance in AA homeostasis [85,86]. AAs are required for the synthesis of many proteins, lipids or nucleic acids and serve as essential nutrients for rapidly proliferating cancer cells [87,88]. GCN2 is a serine/threonine-protein kinase that senses AA deficiency, through binding with uncharged transfer RNA (tRNA), thereby functioning as a key regulator of the AAR [89,90,91,92,93]. Likewise, endoplasmic reticulum stress can also increase ASNS transcription via the activation of PERK kinases in the unfolded protein response (UPR) pathway [94]. The activation of both GCN2 and PERK kinases leads to the phosphorylation of the eukaryotic initiator factor eIF2α, which increases the levels of the transcription factor ATF4 [95,96,97]. If the promoter region of ASNS is hypomethylated, ATF4 can bind and cause stress-induced ASNS upregulation. In contrast, when the ASNS promoter is hypermethylated, no ATF4-induced ASNS expression will be observed [98] (Figure 1D).
In line with this notion, Jiang et al. recently showed that DNA hypermethylation at the ASNS promoter prevents its transcriptional expression following AA depletion in ALL cells, potentially explaining why most ALL cells are so exquisitely sensitive to this AA depletion therapy [99]. Furthermore, Touzart et al. also recently showed that the ASNS promoter methylation status strongly correlates with L-ASNase sensitivity in T-ALL cell lines and patient derived xenografts [100]. Therefore, although it was initially thought that low or absent ASNS expression would determine L-ASNase sensitivity in ALL [101,102], these more recent studies now suggest that ASNS promoter methylation in combination with low ASNS expression at diagnosis most likely serves as a better biomarker to predict L-ASNase sensitivity in this disease [99,100,103,104,105].
4. Expanding the Use of L-ASNase Therapy beyond ALL in Children
The observation that more cancer subtypes have lower ASNS expression compared to their normal healthy counterparts also prompted efforts to expand the use of L-ASNase outside the field of pediatric ALL (Table 2).

Table 2.
Overview of solid tumors with potential to benefit from L-ASNase treatment. This table shows a comparison of different cancer types and their correlating L-ASNase sensitivity mechanism. In addition, possible combination therapies and findings as observed in literature are shown.
4.1. Acute Monocytic Leukemia
For example, in the context of leukemia, variable ASNS expression has also been observed in AML. In particular acute monocytic leukemia (AML-M5) patients and AML with monosomy of chromosome 7 have been reported to have lower ASNS expression, which was correlated with sensitivity to L-ASNase administration ex vivo [118,119]. Subsequently, in vivo efficacy for L-ASNase in both adult and pediatric AML was seen when combined with methotrexate or a high dose cytarabine [107,109]. However, as the correlation between L-ASNase-sensitivity and ASNS expression in AML is not strong, and bioavailability of Gln has been demonstrated to be important for AML growth, debate is ongoing whether the cytotoxic effects of bacterial L-ASNase on AML cells is also due to their glutaminase co-activity [106,108]. In addition, a recent study also investigated the effects of L-ASNase treatment on the leukemic bone marrow microenvironment and its relationship with L-ASNase sensitivity in AML. More specifically, while L-ASNase had a clear cytotoxic effect on AML blasts, including the leukemic stem cell subpopulation, the effect of the drug was shown to be counteracted by the mesenchymal stromal cells and the monocytes/macrophages within this niche. This protective effect could be either attributed to the release of Asn or due to drug clearance mediated by lysosomal cysteine proteases such as cathepsine B [120,121]. More research will be necessary to further evaluate the role of the tumor microenvironment on L-ASNase efficacy.
4.2. Solid Cancers
In relation to non-hematological tumors, tissue microarrays have been used to identify ASNS-free cancer cells within a variety of solid cancer subtypes. It was suggested that ASNS protein levels could be used as a predictive biomarker to estimate susceptibility to L-ASNase treatment [122]. Lorenzi et al. indeed confirmed the potential use of this biomarker in ovarian cancer and demonstrated a strong negative correlation between ASNS expression levels and L-ASNase sensitivity [18]. Furthermore, 15% of tested ovarian cancers in a tissue microarray had low ASNS levels, especially ovarian clear cell carcinomas (OCCC). Based on this, it was hypothesized that patients with advanced OCCC, who are known to have a worse prognosis, could potentially benefit from L-ASNase therapy. As a result, a clinical trial for the use of PEG-L-ASNase in advanced ovarian cancer was initiated. However, the study was prematurely terminated in Phase II due to excessive toxicities observed, including persistent nausea, pancreatitis, and allergic reactions. Similar to AML, the glutaminase co-activity might play an important role in the cytotoxic activity of the bacterial L-ASNases in ovarian cancer. Purwaha et al. reported that cytotoxic effects induced by PEG-L-ASNase were primarily associated with Gln concentrations instead of Asn concentrations in culture conditions of OVCAR-8 cells [110].
The genetic background of tumor cells might also be an important variable that contributes to the susceptibility of tumor types to L-ASNase treatment or other antimetabolic treatments. For example, pancreatic cancer is generally very sensitive to AA depletion. In the majority of pancreatic ductal adenocarcinoma (PDAC) cases, KRAS has been found to be mutationally activated [123]. Continuous KRAS activation alters and regulates Gln metabolism to optimize cancer cell growth [124,125]. Gln is metabolized through upregulation of glutamate-oxaloacetate transaminase 2 (GOT2) into aspartate [126]. As a result, Gln and Asn dependencies are often seen in PDAC, creating a key vulnerability that can be exploited using L-ASNase treatment [111]. Dufour et al. screened 99 human PDAC for ASNS expression levels and showed that 52% of the pancreatic tumors could be classified as ASNS-low tumors as compared to healthy pancreatic tissue, which displayed high ASNS levels [17]. Furthermore, KRAS mutations are also frequently identified in colorectal cancer, suggesting that L-ASNase therapy might also be relevant in a subset of colorectal cancer patients [127]. Indeed, in combination with rapamycin, PEG-L-ASNase significantly suppressed the growth of KRAS-mutant colorectal cancer [112]. In this context, SOX12 was identified as an important regulator of GOT2 and ASNS, and knockdown of either one of these enzymes suppressed SOX12-mediated proliferation and metastasis in colorectal cancer [128].
Using metastatic breast carcinoma cell lines, Knott et al. recently demonstrated that Asn bioavailability governs their metastatic potential [20]. In vitro, Asn supplementation in AA deficient culture conditions significantly promoted the invasive properties of these cells. In vivo, ASNS silencing or Asn deprivation from food did not affect primary tumor growth but significantly reduced the number and size of metastatic nodules in the lungs [20]. This decrease in metastatic potential was correlated with the absence of Twist, a regulator of epithelial to mesenchymal transitions [20,129]. It was proposed that invasive breast cancer cells, which obtained mesenchymal traits, are more in need of Asn levels compared to their less invasive epithelial counterparts [20,130]. Alternatively, their altered expression profile, e.g., by activation of the WNT/STOP pathway, may force them to become dependent on alternative sources of free Asn [3,131]. On the other hand, supplementation of Asn restored cell proliferation and survival of multiple human breast cancer cell lines grown in Gln-depleted media [132]. Gln is known to be important for primary tumor growth in several breast cancer subtypes [111]. For example, many MYC-expressing Triple Negative Breast Cancer (TNBC) tumors show Gln addiction [133]. It was shown that L-ASNase together with a glutamine synthetase inhibitor may exert a complete inhibition of the Gln metabolism and enhances the anticancer effect in solid tumor types that are highly dependent on Gln including hepatocellular carcinoma and TNBC [134,135,136]. So, L-ASNase variants with glutaminase co-activity may be the most effective for breast cancer patients as they would both suppress growth of Gln-dependent primary tumors as well as Asn-dependent metastasis. However, a five-drug combination was tested for treatment of metastatic breast cancer in a clinical trial in 1980: 5-fluorouracil, adriamycin, cyclophosphamide, methotrexate, and L-ASNase. Addition of L-ASNase and methotrexate led to increased symptoms of stomatitis and increased diarrhea, and the trial had to be terminated due to overall increased toxicity [137]. Therefore, also for breast cancer, L-ASNases with low glutaminase co-activity may allow decreased toxicity and prolonged treatment.
4.3. Glioblastoma
ASNS was revealed to be both a tumor growth suppressor and a critical component for metastasis in hepatocellular carcinoma. Low ASNS levels were correlated with a worse prognosis and increased metastatic potential; however, it was correlated with higher sensitivity to L-ASNase both in vitro and in vivo [114]. In addition, Li et al. noticed that a subset of hepatic cancers showed a hypermethylated ASNS promoter alongside low ASNS expression [115]. As mentioned above, ASNS promoter methylation inhibits the ability to restore ASNS expression and acquire L-ASNase resistance. As such, those hepatocellular carcinoma patients with low ASNS expression and hypermethylation of the ASNS promoter may benefit the most from long-term L-ASNase treatment.
Over the last decade, the use of L-ASNase for the treatment of glioblastoma has also been evaluated. Glioblastoma is associated with very poor survival rates and a lot of research is currently being performed to establish new therapeutic options for these patients [138]. One of the major problems is the presence of the blood-brain barrier (BBB), which makes it difficult for therapeutic agents with molecular weight over 400 Da and low lipid solubility to reach the tumors [139]. However, in the case of L-ASNase treatment, Asn is depleted systemically in the blood and therefore potentially overcomes the challenge of crossing the BBB. Multiple studies in children with ALL have already hypothesized that the loss of Asn in the peripheral blood is followed by a decline of Asn in the cerebrospinal fluid (CSF), but unfortunately the underlying mechanism of this pharmacodynamic effect is not fully understood [140,141,142]. While literature shows that different L-ASNase formulations can deplete Asn levels in the CSF in ALL [143,144,145], the impact on clinical outcomes has not been clear [142,146,147]. It has been reported that insufficient Asn depletion in the CSF may be associated with a higher likelihood of having a central nervous system (CNS) relapse in ALL [73]. Many studies fail to show any measurable L-ASNase enzyme activity in the CSF, although Riccardi et al. hypothesized the presence of activity in the CSF of rhesus monkeys after native L-ASNase administration [148]. More recently, Ballerini et al. showed a complete Asn depletion in serum and CSF in healthy rats with both the native L-ASNase formulations derived from E. chrysanthemi and E. coli, and to a lesser extent for PEG-L-ASNase. In addition, they demonstrated that L-ASNase enzymatic activity could be measured in the CSF and was more evident for non-PEGylated formulations, which may be explained by the difference in molecular weight between the different L-ASNase preparations [149]. These results indicate that L-ASNase may be beneficial for the treatment of brain tumors, including glioblastoma.
Panosyan et al. demonstrated that L-ASNase treatment in vitro resulted in a significant growth reduction in multiple glioblastoma cell lines. The authors also showed the possibility of combining L-ASNase with DNA-damaging drugs, such as temozolomide. Experiments on xenograft tumors in vivo revealed potential synergistic effects between L-ASNase and temozolomide treatment [116]. The combination of both drugs attenuated tumor growth significantly more than either the control or the temozolomide monotherapy groups. However, similar to what has been observed for other solid cancers, it remains unclear whether growth inhibition of glioblastoma is a consequence of Asn or Gln deprivation. Ohba et al. demonstrated that the combination of L-ASNase and 6-Diazo-5-oxo-l-norleucine, a Gln analog that binds to the active sites of Gln-utilizing enzymes and inhibits glutaminase and ASNS, induced a synergistic anti-proliferative effect in glioma cells [117]. In this way, L-ASNase resistance via the upregulation of ASNS can be overcome in glioblastoma.
In summary, many hematological and solid cancer types have potential for sensitivity to Asn deprivation. However, further studies are required to better understand this vulnerability at the molecular level. To expand the use of L-ASNase treatment to other neoplasms, a combination of predictive biomarkers will be necessary to identify the subgroups of patients susceptible to this metabolic therapy. More specifically, we believe that low ASNS expression in combination with ASNS promoter hypermethylation would serve as a good predictor for L-ASNase sensitivity.
5. The Development of Novel L-ASNase Variants
L-ASNase has clinical potential for the treatment of other cancer subtypes in addition to childhood ALL. However, the clinical utilization of this drug for adult malignancies has not been widely possible due to its high toxicity profile. There is a clear unmet clinical need for alternative L-ASNase formulations that possess fewer non-immune related toxicities while also being less immunogenic [143]. These novel agents would enable long term L-ASNase treatment in adult cancer patients, improving patient outcomes, and should be prioritized for clinical testing in specific adult cancer populations.
5.1. L-ASNase Red Blood Cell Encapsulation
Erytech Pharma created an alternative L-ASNase formulation by encapsulating E. coli L-ASNase into RBCs (GRASPA®), resulting in a significant increase in half-life as RBCs have a lifespan of about 120 days. In addition, this encapsulation also protects the enzyme, resulting in decreased immunogenicity [150,151]. The formulation of GRASPA®, initially called “Eryaspase”, has evolved during its development. Two sources of L-ASNase have been used as raw material and are encapsulated in the RBCs: native (Kidrolase®) or recombinant L-ASNase (Spectrila®). Several completed studies were conducted with Kidrolase® and showed promising results: three studies in ALL-GRASPALL 2005-01, 2009-06, SA2-2008- and one study in pancreatic carcinoma GRASPANC 2008-02 [152,153]. Later, additional trials were performed with Spectrila® in ALL (GRASPALL 2012-09) and pancreatic cancer (GRASPANC 2013-03). However, in 2018, GRASPA® was not approved for the treatment of ALL by the European Medicines Agency (EMA) due to insufficient evidence and data on safety and efficacy. At the time of withdrawal, the EMA concluded that the benefits of GRASPA® did not outweigh its risks [154]. Notably, for pancreatic cancer, this variant successfully underwent Phase 2b clinical trials [155,156] and is currently in Phase 3 trials [157]. It is also in a Phase 2 clinical trial for TNBC (TRYbeCA-2 trial). Very recently, Eryaspase® was granted Fast Track Designation by the FDA in patients who developed hypersensitivity to E. coli L-ASNase [158].
5.2. L-ASNase Variants with Reduced or Absent Co-Activity
Most non-immune related toxic side effects of L-ASNase therapy have been attributed to its glutaminase co-activity. Therefore, a reduction of glutaminase co-activity might effectively improve the toxicity profile of L-ASNase variants for the treatment of tumors with low or absent ASNS expression combined with ASNS promoter methylation [31,36]. As previously discussed in this review, a mutant form of E. chrysanthemi L-ASNase has been reported with ultra-low glutaminase co-activity [40]. Due to low toxicity, this variant might be suitable for clinical testing in the adult ALL setting. In addition, it would also be interesting to evaluate the cytotoxic effect of this L-ASNase variant in a variety of non-ALL cancer cell lines.
Wolinella succinogenes derived L-ASNase (WoA) was initially reported as a glutaminase-free L-ASNase variant. Reinert et al. compared this variant with E. coli derived L-ASNase and showed that WoA did not suppress the immune response in mice and lacked hepatotoxicity [159,160,161,162]. In addition, it was demonstrated that PEG-WoA caused less toxic side effects compared to PEGylated E. coli L-ASNase, suggesting that WoA would potentially be a safer drug due to its glutaminase-free properties [163]. Unfortunately, in the early 2000s, WoA was evaluated clinically through a US National Cancer Institute Rapid Access to Intervention Development (NCI RAID) grant, and was found to be very toxic and unexpectedly had significant L-glutaminase activity [164]. Meanwhile, whole genome sequencing of W. succinogenes revealed a serine at position 121 [165], in conflict with a proline at this position as previously reported by Lubkowski et al. [166]. In 2017, it was confirmed by Nguyen et al. that the WoA variant with a proline at position 121 (WoA-P121) had a higher L-glutaminase activity in contrast to the WoA variant with serine at position 121 (WoA-S121), highlighting the critical role of this residue regarding substrate specificity [164].
In addition, Reinert et al. also show that Gln is reduced in liver and spleen after a single administration of E. coli L-ASNase, which was not observed for WoA derived L-ASNase [167]. Remarkably, a significant increase in Glu levels was observed for the WoA treated group. The authors suggested that although Gln is not enzymatically cleaved by WoA, its turnover in mice is increased in response to Asn depletion, as Gln provides the NH3-group required to make more Asn, leading to a greater production of Glu [167]. Interestingly, this impact on Gln levels during L-ASNase treatment is not always clear in the clinic. This is demonstrated by Tong et al. who showed no depletion of Gln during L-ASNase therapy in ALL pediatric patients [49]. Nevertheless, the decrease of Gln levels after L-ASNase therapy was also observed by Emadi et al. who showed that crisantaspase, produced by E. chrysanthemi, caused nearly complete Gln depletion in R/R acute myeloid leukemia (AML) patients with no dose-limiting toxicity, while retaining its antileukemic activity [108]. More recently, they also demonstrated that the long-acting PEG-crisantaspase completely depletes Gln serum levels in AML mice [168]. Nikonorova et al. looked at liver function and toxicity after E. coli L-ASNase administration in juvenile and adult mice, and found significantly decreased levels of Gln, together with increased Glu levels [169]. Finally, Nguyen et al. clearly show that E. chrysanthemi derived L-ASNase significantly reduced the Gln levels in mice 24 h after L-ASNase administration [40].
Another interesting class of L-ASNase variants are derived from guinea pigs [170,171,172,173]. They are completely glutaminase-free and represent the only mammalian L-ASNase possessing clinically relevant kinetic properties [171]. Interestingly, from an immunogenic perspective, this enzyme already shares ~70% of its sequence homology with the largely inactive human enzyme. Unfortunately, the wild-type versions of known human L-asparaginases are not suitable replacements for the clinically used bacterial enzymes since they possess a very high KM (Michaelis constant) value for Asn [9,174,175], which indicates a low affinity for its substrate. Given the physiological concentration of Asn in blood (~50 μM) [4], the enzyme must have an Asn KM in the low micromolar range to be clinically relevant [172]. Recent work showed that further humanization of this guinea pig L-ASNase towards 85% homology has been possible through DNA shuffling without losing its in vitro antileukemic activity [170,171,172]. Therefore, this humanized guinea pig L-ASNase might serve as an optimal candidate for further clinical development as it might combine fewer non-immune related toxicities with less immunogenicity.
5.3. Enzyme Engineering and Bioprospecting
Many attempts have been made to improve the characteristics of existing L-ASNase variants through enzyme engineering. Several methods have been employed, examples being site-directed mutagenesis and directed evolution. The main goal is four-fold: the improvement of in vitro and in vivo stability and enzymatic activity and the reduction of immunogenicity and toxicity. A longer half-life can be obtained by modifying protease cleavage sites in the enzyme. Recently, it was shown that an N24S mutation in E. coli L-ASNase generates increased protease-resistance and thermal stability [176]. Secondly, as mentioned before, toxicity of L-ASNases can be at least partly contributed to glutaminase co-activity. It is therefore desirable to limit this activity. Nguyen et al. created L-ASNase variants with ultra-low glutaminase co-activity by adapting specific amino acids in the active site to increase specificity towards Asn. Era-TM2, an Erwinia chrysanthemi A31I/E63Q/S254Q triple mutant, proved to be strongly efficacious both in vitro and in vivo against T-ALL and B-ALL, with reduced side effects [40,177]. Site-directed mutagenesis was also employed by Offman et al., who created a N24A/Y250L E. coli L-ASNase mutant with decreased glutaminase activity [178]. Immunogenicity on the other hand can be reduced by identifying and modifying antigenic residues in the enzyme. Jianhua et al. reduced antigenicity in E. coli L-ASNase through R195A/K1961/H197A mutations [179].
An alternative strategy to enzyme engineering is bioprospecting other microbial sources for L-ASNases with improved characteristics. In particular, extremophilic bacteria are an interesting novel L-ASNase source due to their superior pharmacokinetic properties. Therapeutics derived from halophilic bacteria often showcase increased biological activity and tolerance to the osmolarity of blood. Ghasemi et al. isolated L-ASNase from Halomonas elongata, which showed cytotoxicity in vitro, ameliorated half-life, and optimal activity at a temperature of 37 °C [180]. In the food industry, where L-ASNases are used for the reduction of carcinogenic acrylamide formation when frying food, thermophilic bacteria are used as a L-ASNase source, as these have increased thermostability [9]. This increased stability can also greatly benefit pharmaceutical applications. However, at physiological temperatures their activity is often greatly reduced. Research therefore is focused on generating specific mutations that preserve stability but ameliorate activity at these lower temperatures. Recently, a K274E mutant of Pyrococcus furiosus L-ASNases was created that preserved thermostability and greatly increased efficacy in vitro against human cell lines [181].
6. Combination Therapies
Identification of L-ASNase resistance mechanisms has fueled research into promising novel drug combinations. For example, the GCN2-ATF4 axis can be responsible for reinduction of ASNS upon AA starvation by L-ASNase in specific tumor cell lines [122,182,183] (Figure 1D). Of note, this L-ASNase resistance mechanism can be counteracted by using recently developed GCN2 inhibitors. Indeed, this combination therapy was shown to be highly efficient in the context of ALL, AML, pancreatic cancer, and melanoma [184,185,186]. Similarly, a recent study showed that the BTK inhibitor Ibrutinib strongly synergized with L-ASNase in ALL through a comparable mechanism that involved suppression of GCN2 activity [187]. In addition, it was shown that SLC1A3, an aspartate/glutamate transporter, contributes to L-ASNase resistance in PC3 prostate cancer cells and plays an important role in tumor initiation and progression in a mouse model for breast cancer metastasis [188]. These findings suggest that restrictive aspartate and glutamate uptake might improve L-ASNase efficacy in solid tumors.
Finally, recent studies revealed that upstream activation of the Wnt pathway can induce L-ASNase sensitivity in drug resistant ALL cells. Indeed, Wnt activation inhibits GSK3a-dependent protein ubiquitination and degradation, resulting in decreased Asn recycling and higher susceptibility towards L-ASNase treatment (Figure 1D). Notably, pharmacological inhibition of GSK3a was sufficient to mimic this effect in vivo using leukemia mouse models [189]. Interestingly, the same concept was also shown to be applicable for APC or β-catenin-mutant CRC [113], suggesting that GSK3a inhibition might act as a more general mechanism to sensitize a variety of tumor types towards L-ASNase therapy.
7. Conclusions
In summary, L-ASNase therapy has been proven to be a successful anticancer therapy, but most of the L-ASNase preparations currently used elicit undesirable and potentially treatment-limiting side effects. The development of improved microbial L-ASNases is a challenge due to their immunogenicity resulting in subsequent failure to achieve sufficient long-term L-ASNase activity.
In addition, studies show that L-ASNase may also have clinical potential for the treatment of solid cancers with low or no ASNS expression. However, because of the tolerability and toxicity issues, which are especially pronounced in adults, the clinical potential of L-ASNase for the treatment of these solid tumors has not fully been pursued. Therefore, one could speculate that the development of safer, less toxic, and non-immunogenic L-ASNase variants could expand its use to many other aggressive types of cancer with poor outcomes, including glioblastoma, breast, pancreas, and hepatocellular carcinoma. However, the role of glutaminase co-activity for the anticancer activity of L-ASNase is still a matter of debate, as literature shows conflicting reports. Some cancers may be dependent on Gln for their growth or become Gln dependent after Asn depletion and eventually will not benefit from alternative low glutaminase L-ASNase variants. These questions will have to be addressed to assess the therapeutic potential of alternative L-ASNases in clinical development. Further, characterization of L-ASNase resistance mechanisms provide a piece of the puzzle for further development of more personalized combination therapies, not only for ALL but also for solid cancer subtypes.
Author Contributions
Conceptualization, M.V.T., E.P. and Y.D.V.; writing—original draft preparation, M.V.T., E.P., Y.D.V., T.L., S.G. and P.V.V.; writing—review and editing, M.V.T., E.P., Y.D.V., A.M.S., V.M., B.D.M., A.L., T.L., S.G. and P.V.V.; supervision, T.L, S.G. and P.V.V. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been supported by the Belgian Foundation Against Cancer (project grants to P.V.V. and S.G.), the Belgian Stand Up to Cancer Foundation (project grants to P.V.V. and S.G.), and vzw Kinderkankerfonds (grant to T.L.). E.P. is a predoctoral fellow with the Research Foundation Flanders (FWO). The respective funding sources were not involved in the writing or preparation of this manuscript.
Conflicts of Interest
A.L. is a co-founder of Enzyme by Design Inc and an inventor on patent applications relating to asparaginases. A.S. is a co-founder of Enzyme by Design Inc. and an inventor on patent applications relating to asparaginases.
References
- Choi, B.H.; Coloff, J.L. The Diverse Functions of Non-Essential Amino Acids in Cancer. Cancers 2019, 11, 675. [Google Scholar] [CrossRef] [PubMed]
- Endicott, M.; Jones, M.; Hull, J. Amino acid metabolism as a therapeutic target in cancer: A review. Amino Acids 2021, 53, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Batra, S.; Zhang, J. Asparagine: A Metabolite to Be Targeted in Cancers. Metabolites 2021, 11, 402. [Google Scholar] [CrossRef] [PubMed]
- Lomelino, C.L.; Andring, J.T.; McKenna, R.; Kilberg, M.S. Asparagine synthetase: Function, structure, and role in disease. J. Biol. Chem. 2017, 292, 19952–19958. [Google Scholar] [CrossRef] [PubMed]
- Lang, S. Uber desamidierung im Tierkorper. Beitr. Chem. Physiol. Pathol. 1904, 5, 321–345. [Google Scholar]
- Broome, J.D. Evidence that the L-asparaginase of guinea pig serum is responsible for its antilymphoma effects. I. Properties of the L-asparaginase of guinea pig serum in relation to those of the antilymphoma substance. J. Exp. Med. 1963, 118, 99–120. [Google Scholar] [CrossRef]
- Kidd, J.G. Regression of transplanted lymphomas induced in vivo by means of normal guinea pig serum. I. Course of transplanted cancers of various kinds in mice and rats given guinea pig serum, horse serum, or rabbit serum. J. Exp. Med. 1953, 98, 565–582. [Google Scholar] [CrossRef]
- Dolowy, W.C.; Henson, D.; Cornet, J.; Sellin, H. Toxic and antineoplastic effects of L-asparaginase. Study of mice with lymphoma and normal monkeys and report on a child with leukemia. Cancer 1966, 19, 1813–1819. [Google Scholar] [CrossRef]
- Batool, T.; Makky, E.A.; Jalal, M.; Yusoff, M.M. A Comprehensive Review on L-Asparaginase and Its Applications. Appl. Biochem. Biotechnol. 2016, 178, 900–923. [Google Scholar] [CrossRef]
- Cachumba, J.J.; Antunes, F.A.; Peres, G.F.; Brumano, L.P.; Santos, J.C.; Da Silva, S.S. Current applications and different approaches for microbial l-asparaginase production. Braz. J. Microbiol. 2016, 47 (Suppl. S1), 77–85. [Google Scholar] [CrossRef]
- Kumar, K.; Kaur, J.; Walia, S.; Pathak, T.; Aggarwal, D. L-asparaginase: An effective agent in the treatment of acute lymphoblastic leukemia. Leuk. Lymphoma 2014, 55, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Richards, N.G.; Kilberg, M.S. Asparagine synthetase chemotherapy. Annu. Rev. Biochem. 2006, 75, 629–654. [Google Scholar] [CrossRef] [PubMed]
- Prager, M.D.; Bachynsky, N. Asparagine synthetase in normal and malignant tissues: Correlation with tumor sensitivity to asparaginase. Arch. Biochem. Biophys. 1968, 127, 645–654. [Google Scholar] [CrossRef]
- Haskell, C.M.; Canellos, G.P. l-asparaginase resistance in human leukemia--asparagine synthetase. Biochem. Pharm. 1969, 18, 2578–2580. [Google Scholar] [CrossRef]
- Chiu, M.; Taurino, G.; Bianchi, M.G.; Kilberg, M.S.; Bussolati, O. Asparagine Synthetase in Cancer: Beyond Acute Lymphoblastic Leukemia. Front. Oncol. 2019, 9, 1480. [Google Scholar] [CrossRef]
- Emadi, A.; Zokaee, H.; Sausville, E.A. Asparaginase in the treatment of non-ALL hematologic malignancies. Cancer Chemother. Pharm. 2014, 73, 875–883. [Google Scholar] [CrossRef]
- Dufour, E.; Gay, F.; Aguera, K.; Scoazec, J.Y.; Horand, F.; Lorenzi, P.L.; Godfrin, Y. Pancreatic tumor sensitivity to plasma L-asparagine starvation. Pancreas 2012, 41, 940–948. [Google Scholar] [CrossRef]
- Lorenzi, P.L.; Reinhold, W.C.; Rudelius, M.; Gunsior, M.; Shankavaram, U.; Bussey, K.J.; Scherf, U.; Eichler, G.S.; Martin, S.E.; Chin, K.; et al. Asparagine synthetase as a causal, predictive biomarker for L-asparaginase activity in ovarian cancer cells. Mol. Cancer 2006, 5, 2613–2623. [Google Scholar] [CrossRef]
- Yu, M.; Henning, R.; Walker, A.; Kim, G.; Perroy, A.; Alessandro, R.; Virador, V.; Kohn, E.C. L-asparaginase inhibits invasive and angiogenic activity and induces autophagy in ovarian cancer. J. Cell Mol. Med. 2012, 16, 2369–2378. [Google Scholar] [CrossRef]
- Knott, S.R.V.; Wagenblast, E.; Khan, S.; Kim, S.Y.; Soto, M.; Wagner, M.; Turgeon, M.O.; Fish, L.; Erard, N.; Gable, A.L.; et al. Asparagine bioavailability governs metastasis in a model of breast cancer. Nature 2018, 554, 378–381. [Google Scholar] [CrossRef]
- Hijiya, N.; van der Sluis, I.M. Asparaginase-associated toxicity in children with acute lymphoblastic leukemia. Leuk. Lymphoma 2016, 57, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Thu Huynh, V.; Bergeron, S. Asparaginase Toxicities: Identification and Management in Patients with Acute Lymphoblastic Leukemia. Clin. J. Oncol. Nurs. 2017, 21, E248–E259. [Google Scholar] [CrossRef] [PubMed]
- Pieters, R.; Hunger, S.P.; Boos, J.; Rizzari, C.; Silverman, L.; Baruchel, A.; Goekbuget, N.; Schrappe, M.; Pui, C.H. L-asparaginase treatment in acute lymphoblastic leukemia: A focus on Erwinia asparaginase. Cancer 2011, 117, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Maese, L.; Rizzari, C.; Coleman, R.; Power, A.; van der Sluis, I.; Rau, R.E. Can recombinant technology address asparaginase Erwinia chrysanthemi shortages? Pediatr. Blood Cancer 2021, 68, e29169. [Google Scholar] [CrossRef] [PubMed]
- Kawedia, J.D.; Rytting, M.E. Asparaginase in acute lymphoblastic leukemia. Clin. Lymphoma Myeloma Leuk. 2014, 14, S14–S17. [Google Scholar] [CrossRef] [PubMed]
- Heo, Y.A.; Syed, Y.Y.; Keam, S.J. Pegaspargase: A Review in Acute Lymphoblastic Leukaemia. Drugs 2019, 79, 767–777. [Google Scholar] [CrossRef]
- Lew, G. Space for Calaspargase? A New Asparaginase for Acute Lymphoblastic Leukemia. Clin. Cancer Res. 2020, 26, 325–327. [Google Scholar] [CrossRef]
- Angiolillo, A.L.; Schore, R.J.; Devidas, M.; Borowitz, M.J.; Carroll, A.J.; Gastier-Foster, J.M.; Heerema, N.A.; Keilani, T.; Lane, A.R.; Loh, M.L.; et al. Pharmacokinetic and pharmacodynamic properties of calaspargase pegol Escherichia coli L-asparaginase in the treatment of patients with acute lymphoblastic leukemia: Results from Children’s Oncology Group Study AALL07P4. J. Clin. Oncol. 2014, 32, 3874–3882. [Google Scholar] [CrossRef]
- Soares, A.L.; Guimarães, G.M.; Polakiewicz, B.; de Moraes Pitombo, R.N.; Abrahão-Neto, J. Effects of polyethylene glycol attachment on physicochemical and biological stability of E. coli L-asparaginase. Int. J. Pharm. 2002, 237, 163–170. [Google Scholar] [CrossRef]
- Cecconello, D.K.; Magalhães, M.R.; Werlang, I.C.R.; Lee, M.L.M.; Michalowski, M.B.; Daudt, L.E. Asparaginase: An old drug with new questions. Hematol. Transfus. Cell 2020, 42, 275–282. [Google Scholar] [CrossRef]
- Brumano, L.P.; da Silva, F.V.S.; Costa-Silva, T.A.; Apolinário, A.C.; Santos, J.H.P.M.; Kleingesinds, E.K.; Monteiro, G.; Rangel-Yagui, C.d.O.; Benyahia, B.; Junior, A.P. Development of L-Asparaginase Biobetters: Current Research Status and Review of the Desirable Quality Profiles. Front. Bioeng. Biotechnol. 2019, 6, 212. [Google Scholar] [CrossRef] [PubMed]
- Bender, C.; Maese, L.; Carter-Febres, M.; Verma, A. Clinical Utility of Pegaspargase in Children, Adolescents and Young Adult Patients with Acute Lymphoblastic Leukemia: A Review. Blood Lymphat. Cancer 2021, 11, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Müller, H.J.; Boos, J. Use of l-asparaginase in childhood ALL. Crit. Rev. Oncol./Hematol. 1998, 28, 97–113. [Google Scholar] [CrossRef]
- Mondelaers, V.; Ferster, A.; Uyttebroeck, A.; Brichard, B.; van der Werff Ten Bosch, J.; Norga, K.; Francotte, N.; Piette, C.; Vandemeulebroecke, K.; Verbeke, C.; et al. Prospective, real-time monitoring of pegylated Escherichia coli and Erwinia asparaginase therapy in childhood acute lymphoblastic leukaemia and non-Hodgkin lymphoma in Belgium. Br. J. Haematol. 2020, 190, 105–114. [Google Scholar] [CrossRef]
- Fernandez, C.; Stewart, E.; Panetta, J.; Wilkinson, M.; Morrison, A.; Finkelman, F.; Sandlund, J.; Pui, C.; Jeha, S.; Relling, M. Successful challenges using native E. coli asparaginase after hypersensitivity reactions to PEGylated E. coli asparaginase. Cancer Chemother. Pharmacol. 2014, 73, 1307–1313. [Google Scholar] [CrossRef]
- Ramya, L.N.; Doble, M.; Rekha, V.P.; Pulicherla, K.K. L-Asparaginase as potent anti-leukemic agent and its significance of having reduced glutaminase side activity for better treatment of acute lymphoblastic leukaemia. Appl. Biochem. Biotechnol. 2012, 167, 2144–2159. [Google Scholar] [CrossRef]
- Völler, S.; Pichlmeier, U.; Zens, A.; Hempel, G. Pharmacokinetics of recombinant asparaginase in children with acute lymphoblastic leukemia. Cancer Chemother. Pharmacol. 2018, 81, 305–314. [Google Scholar] [CrossRef]
- Chan, W.K.; Lorenzi, P.L.; Anishkin, A.; Purwaha, P.; Rogers, D.M.; Sukharev, S.; Rempe, S.B.; Weinstein, J.N. The glutaminase activity of L-asparaginase is not required for anticancer activity against ASNS-negative cells. Blood 2014, 123, 3596–3606. [Google Scholar] [CrossRef]
- Parmentier, J.H.; Maggi, M.; Tarasco, E.; Scotti, C.; Avramis, V.I.; Mittelman, S.D. Glutaminase activity determines cytotoxicity of L-asparaginases on most leukemia cell lines. Leuk. Res. 2015, 39, 757–762. [Google Scholar] [CrossRef]
- Nguyen, H.A.; Su, Y.; Zhang, J.Y.; Antanasijevic, A.; Caffrey, M.; Schalk, A.M.; Liu, L.; Rondelli, D.; Oh, A.; Mahmud, D.L.; et al. A Novel l-Asparaginase with low l-Glutaminase Coactivity Is Highly Efficacious against Both T- and B-cell Acute Lymphoblastic Leukemias In Vivo. Cancer Res. 2018, 78, 1549–1560. [Google Scholar] [CrossRef]
- Chan, W.K.; Horvath, T.D.; Tan, L.; Link, T.; Harutyunyan, K.G.; Pontikos, M.A.; Anishkin, A.; Du, D.; Martin, L.A.; Yin, E.; et al. Glutaminase Activity of L-Asparaginase Contributes to Durable Preclinical Activity against Acute Lymphoblastic Leukemia. Mol. Cancer 2019, 18, 1587–1592. [Google Scholar] [CrossRef] [PubMed]
- Van der Sluis, I.M.; Vrooman, L.M.; Pieters, R.; Baruchel, A.; Escherich, G.; Goulden, N.; Mondelaers, V.; Sanchez de Toledo, J.; Rizzari, C.; Silverman, L.B.; et al. Consensus expert recommendations for identification and management of asparaginase hypersensitivity and silent inactivation. Haematologica 2016, 101, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Shinnick, S.E.; Browning, M.L.; Koontz, S.E. Managing hypersensitivity to asparaginase in pediatrics, adolescents, and young adults. J. Pediatr. Oncol. Nurs. 2013, 30, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Pui, C.H.; Liu, Y.; Relling, M.V. How to solve the problem of hypersensitivity to asparaginase? Pediatr. Blood Cancer 2018, 65. [Google Scholar] [CrossRef] [PubMed]
- Højfeldt, S.G.; Wolthers, B.O.; Tulstrup, M.; Abrahamsson, J.; Gupta, R.; Harila-Saari, A.; Heyman, M.; Henriksen, L.T.; Jónsson, Ò.G.; Lähteenmäki, P.M.; et al. Genetic predisposition to PEG-asparaginase hypersensitivity in children treated according to NOPHO ALL2008. Br. J. Haematol. 2019, 184, 405–417. [Google Scholar] [CrossRef]
- Henriksen, L.T.; Harila-Saari, A.; Ruud, E.; Abrahamsson, J.; Pruunsild, K.; Vaitkeviciene, G.; Jónsson, Ó.G.; Schmiegelow, K.; Heyman, M.; Schrøder, H.; et al. PEG-asparaginase allergy in children with acute lymphoblastic leukemia in the NOPHO ALL2008 protocol. Pediatr. Blood Cancer 2015, 62, 427–433. [Google Scholar] [CrossRef]
- Schore, R.J.; Devidas, M.; Bleyer, A.; Reaman, G.H.; Winick, N.; Loh, M.L.; Raetz, E.A.; Carroll, W.L.; Hunger, S.P.; Angiolillo, A.L. Plasma asparaginase activity and asparagine depletion in acute lymphoblastic leukemia patients treated with pegaspargase on Children’s Oncology Group AALL07P4. Leuk. Lymphoma 2019, 60, 1740–1748. [Google Scholar] [CrossRef]
- Salzer, W.; Bostrom, B.; Messinger, Y.; Perissinotti, A.J.; Marini, B. Asparaginase activity levels and monitoring in patients with acute lymphoblastic leukemia. Leuk. Lymphoma 2018, 59, 1797–1806. [Google Scholar] [CrossRef]
- Tong, W.H.; Pieters, R.; Kaspers, G.J.; te Loo, D.M.; Bierings, M.B.; van den Bos, C.; Kollen, W.J.; Hop, W.C.; Lanvers-Kaminsky, C.; Relling, M.V.; et al. A prospective study on drug monitoring of PEGasparaginase and Erwinia asparaginase and asparaginase antibodies in pediatric acute lymphoblastic leukemia. Blood 2014, 123, 2026–2033. [Google Scholar] [CrossRef]
- Yen, H.J.; Chang, W.H.; Liu, H.C.; Yeh, T.C.; Hung, G.Y.; Wu, K.H.; Peng, C.T.; Chang, Y.H.; Chang, T.K.; Hsiao, C.C.; et al. Outcomes Following Discontinuation of E. coli l-Asparaginase Upon Severe Allergic Reactions in Children With Acute Lymphoblastic Leukemia. Pediatr. Blood Cancer 2016, 63, 665–670. [Google Scholar] [CrossRef]
- Avramis, V.I.; Panosyan, E.H. Pharmacokinetic/pharmacodynamic relationships of asparaginase formulations: The past, the present and recommendations for the future. Clin. Pharm. 2005, 44, 367–393. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, J.K.; Hempel, G.; Koling, S.; Chan, L.S.; Fisher, T.; Meiselman, H.J.; Garratty, G. Antibody against poly(ethylene glycol) adversely affects PEG-asparaginase therapy in acute lymphoblastic leukemia patients. Cancer 2007, 110, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Kloos, R.; van der Sluis, I.M.; Mastrobattista, E.; Hennink, W.; Pieters, R.; Verhoef, J.J. Acute lymphoblastic leukaemia patients treated with PEGasparaginase develop antibodies to PEG and the succinate linker. Br. J. Haematol. 2020, 189, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Song, M.; Sim, B.C.; Gu, C.; Podust, V.N.; Wang, C.W.; McLaughlin, B.; Shah, T.P.; Lax, R.; Gast, R.; et al. Multivalent antiviral XTEN-peptide conjugates with long in vivo half-life and enhanced solubility. Bioconjug. Chem. 2014, 25, 1351–1359. [Google Scholar] [CrossRef]
- Schlapschy, M.; Binder, U.; Börger, C.; Theobald, I.; Wachinger, K.; Kisling, S.; Haller, D.; Skerra, A. PASylation: A biological alternative to PEGylation for extending the plasma half-life of pharmaceutically active proteins. Protein Eng. Des. Sel. 2013, 26, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, M.; Skerra, A. Prospects of PASylation® for the design of protein and peptide therapeutics with extended half-life and enhanced action. Bioorg. Med. Chem. 2018, 26, 2882–2887. [Google Scholar] [CrossRef]
- Ortac, I.; Ruff, L.; Yeh, Y.; Esener, S.; Messmer, B.T. Nanoparticle Encapsulated L-Asparaginase. Blood 2013, 122, 2669. [Google Scholar] [CrossRef]
- Teodor, E.; Litescu, S.-C.; Lazar, V.; Somoghi, R. Hydrogel-magnetic nanoparticles with immobilized l-asparaginase for biomedical applications. J. Mater. Sci. Mater. Med. 2009, 20, 1307–1314. [Google Scholar] [CrossRef]
- Woo, M.H.; Hak, L.J.; Storm, M.C.; Evans, W.E.; Sandlund, J.T.; Rivera, G.K.; Wang, B.; Pui, C.H.; Relling, M.V. Anti-asparaginase antibodies following E. coli asparaginase therapy in pediatric acute lymphoblastic leukemia. Leukemia 1998, 12, 1527–1533. [Google Scholar] [CrossRef]
- Zalewska-Szewczyk, B.; Andrzejewski, W.; Młynarski, W.; Jedrychowska-Dańska, K.; Witas, H.; Bodalski, J. The anti-asparagines antibodies correlate with L-asparagines activity and may affect clinical outcome of childhood acute lymphoblastic leukemia. Leuk. Lymphoma 2007, 48, 931–936. [Google Scholar] [CrossRef]
- Vrooman, L.M.; Kirov, I.I.; Dreyer, Z.E.; Kelly, M.; Hijiya, N.; Brown, P.; Drachtman, R.A.; Messinger, Y.H.; Ritchey, A.K.; Hale, G.A.; et al. Activity and Toxicity of Intravenous Erwinia Asparaginase Following Allergy to E. coli-Derived Asparaginase in Children and Adolescents With Acute Lymphoblastic Leukemia. Pediatr. Blood Cancer 2016, 63, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Salzer, W.L.; Asselin, B.; Supko, J.G.; Devidas, M.; Kaiser, N.A.; Plourde, P.; Winick, N.J.; Reaman, G.H.; Raetz, E.; Carroll, W.L.; et al. Erwinia asparaginase achieves therapeutic activity after pegaspargase allergy: A report from the Children’s Oncology Group. Blood 2013, 122, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Wang, C.; Raetz, E.A.; Schore, R.; Salzer, W.L.; Larsen, E.C.; Maloney, K.W.; Mattano, L.A., Jr.; Carroll, W.L.; Winick, N.J.; et al. Impact of Asparaginase Discontinuation on Outcome in Childhood Acute Lymphoblastic Leukemia: A Report From the Children’s Oncology Group. J. Clin. Oncol. 2020, 38, 1897–1905. [Google Scholar] [CrossRef] [PubMed]
- Panosyan, E.H.; Seibel, N.L.; Martin-Aragon, S.; Gaynon, P.S.; Avramis, I.A.; Sather, H.; Franklin, J.; Nachman, J.; Ettinger, L.J.; La, M.; et al. Asparaginase antibody and asparaginase activity in children with higher-risk acute lymphoblastic leukemia: Children’s Cancer Group Study CCG-1961. J. Pediatr. Hematol. Oncol. 2004, 26, 217–226. [Google Scholar] [CrossRef]
- Silverman, L.B.; Gelber, R.D.; Dalton, V.K.; Asselin, B.L.; Barr, R.D.; Clavell, L.A.; Hurwitz, C.A.; Moghrabi, A.; Samson, Y.; Schorin, M.A.; et al. Improved outcome for children with acute lymphoblastic leukemia: Results of Dana-Farber Consortium Protocol 91-01. Blood 2001, 97, 1211–1218. [Google Scholar] [CrossRef]
- Liu, C.; Kawedia, J.D.; Cheng, C.; Pei, D.; Fernandez, C.A.; Cai, X.; Crews, K.R.; Kaste, S.C.; Panetta, J.C.; Bowman, W.P.; et al. Clinical utility and implications of asparaginase antibodies in acute lymphoblastic leukemia. Leukemia 2012, 26, 2303–2309. [Google Scholar] [CrossRef]
- Gottschalk Højfeldt, S.; Grell, K.; Abrahamsson, J.; Lund, B.; Vettenranta, K.; Jónsson, Ó.G.; Frandsen, T.L.; Wolthers, B.O.; Marquart, H.V.; Vaitkeviciene, G.; et al. Relapse risk following truncation of pegylated asparaginase in childhood acute lymphoblastic leukemia. Blood 2021, 137, 2373–2382. [Google Scholar] [CrossRef]
- Lin, T.; Hernandez-Illas, M.; Rey, A.; Jenkins, J.; Chandula, R.; Silverman, J.A.; Choi, M.R. A Randomized Phase I Study to Evaluate the Safety, Tolerability, and Pharmacokinetics of Recombinant Erwinia Asparaginase (JZP-458) in Healthy Adult Volunteers. Clin. Transl. Sci. 2021, 14, 870–879. [Google Scholar] [CrossRef]
- Lin, T.; Dumas, T.; Kaullen, J.; Berry, N.S.; Choi, M.R.; Zomorodi, K.; Silverman, J.A. Population Pharmacokinetic Model Development and Simulation for Recombinant Erwinia Asparaginase Produced in Pseudomonas fluorescens (JZP-458). Clin. Pharm. Drug Dev. 2021, 10, 1503–1513. [Google Scholar] [CrossRef]
- Aldoss, I.; Douer, D.; Behrendt, C.E.; Chaudhary, P.; Mohrbacher, A.; Vrona, J.; Pullarkat, V. Toxicity profile of repeated doses of PEG-asparaginase incorporated into a pediatric-type regimen for adult acute lymphoblastic leukemia. Eur. J. Haematol. 2016, 96, 375–380. [Google Scholar] [CrossRef]
- Tong, W.H.; Pieters, R.; de Groot-Kruseman, H.A.; Hop, W.C.; Boos, J.; Tissing, W.J.; van der Sluis, I.M. The toxicity of very prolonged courses of PEGasparaginase or Erwinia asparaginase in relation to asparaginase activity, with a special focus on dyslipidemia. Haematologica 2014, 99, 1716–1721. [Google Scholar] [CrossRef] [PubMed]
- Grigoryan, R.S.; Panosyan, E.H.; Seibel, N.L.; Gaynon, P.S.; Avramis, I.A.; Avramis, V.I. Changes of amino acid serum levels in pediatric patients with higher-risk acute lymphoblastic leukemia (CCG-1961). In Vivo 2004, 18, 107–112. [Google Scholar] [PubMed]
- Avramis, V.I.; Sencer, S.; Periclou, A.P.; Sather, H.; Bostrom, B.C.; Cohen, L.J.; Ettinger, A.G.; Ettinger, L.J.; Franklin, J.; Gaynon, P.S.; et al. A randomized comparison of native Escherichia coli asparaginase and polyethylene glycol conjugated asparaginase for treatment of children with newly diagnosed standard-risk acute lymphoblastic leukemia: A Children’s Cancer Group study. Blood 2002, 99, 1986–1994. [Google Scholar] [CrossRef]
- Heitink-Pollé, K.M.; Prinsen, B.H.; de Koning, T.J.; van Hasselt, P.M.; Bierings, M.B. High incidence of symptomatic hyperammonemia in children with acute lymphoblastic leukemia receiving pegylated asparaginase. JIMD Rep. 2013, 7, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Ollenschläger, G.; Roth, E.; Linkesch, W.; Jansen, S.; Simmel, A.; Mödder, B. Asparaginase-induced derangements of glutamine metabolism: The pathogenetic basis for some drug-related side-effects. Eur. J. Clin. Investig. 1988, 18, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Villa, P.; Corada, M.; Bartosek, I. L-asparaginase effects on inhibition of protein synthesis and lowering of the glutamine content in cultured rat hepatocytes. Toxicol. Lett. 1986, 32, 235–241. [Google Scholar] [CrossRef]
- Laterza, O.F.; Gerhardt, G.; Sokoll, L.J. Measurement of plasma ammonia is affected in patients receiving asparaginase therapy. Clin. Chem. 2003, 49, 1710–1711. [Google Scholar] [CrossRef]
- Jörck, C.; Kiess, W.; Weigel, J.F.; Mütze, U.; Bierbach, U.; Beblo, S. Transient hyperammonemia due to L-asparaginase therapy in children with acute lymphoblastic leukemia or non-Hodgkin lymphoma. Pediatr. Hematol. Oncol. 2011, 28, 3–9. [Google Scholar] [CrossRef]
- Nussbaum, V.; Lubcke, N.; Findlay, R. Hyperammonemia secondary to asparaginase: A case series. J. Oncol. Pharm. Pract. 2016, 22, 161–164. [Google Scholar] [CrossRef]
- Kessel, D. Asparaginyl-transfer RNA: A substrate for L-asparaginase. Biochim. Biophys. Acta (BBA)—Nucleic Acids Protein Synth. 1971, 240, 554–557. [Google Scholar] [CrossRef]
- Fidler, I.J.; Montgomery, P.C. Effects of L-asparaginase on lymphocyte surface and blastogenesis. Cancer Res. 1972, 32, 2400–2406. [Google Scholar] [PubMed]
- Ankel, E.G.; Zirneski, J.; Ring, B.J.; Holcenberg, J.S. Effect of asparaginase on cell membranes of sensitive and resistants mouse lymphoma cells. In Vitro 1984, 20, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Zhdanov, D.D.; Pokrovsky, V.S.; Pokrovskaya, M.V.; Alexandrova, S.S.; Eldarov, M.A.; Grishin, D.V.; Basharov, M.M.; Gladilina, Y.A.; Podobed, O.V.; Sokolov, N.N. Rhodospirillum rubrum L-asparaginase targets tumor growth by a dual mechanism involving telomerase inhibition. Biochem. Biophys. Res. Commun. 2017, 492, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Plyasova, A.A.; Pokrovskaya, M.V.; Lisitsyna, O.M.; Pokrovsky, V.S.; Alexandrova, S.S.; Hilal, A.; Sokolov, N.N.; Zhdanov, D.D. Penetration into cancer cells via clathrin-dependent mechanism allows l-asparaginase from rhodospirillum rubrum to inhibit telomerase. Pharmaceuticals 2020, 13, 286. [Google Scholar] [CrossRef]
- Balasubramanian, M.N.; Butterworth, E.A.; Kilberg, M.S. Asparagine synthetase: Regulation by cell stress and involvement in tumor biology. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E789–E799. [Google Scholar] [CrossRef]
- Kilberg, M.S.; Balasubramanian, M.; Fu, L.; Shan, J. The transcription factor network associated with the amino acid response in mammalian cells. Adv. Nutr. 2012, 3, 295–306. [Google Scholar] [CrossRef]
- Lieu, E.L.; Nguyen, T.; Rhyne, S.; Kim, J. Amino acids in cancer. Exp. Mol. Med. 2020, 52, 15–30. [Google Scholar] [CrossRef]
- Wei, Z.; Liu, X.; Cheng, C.; Yu, W.; Yi, P. Metabolism of Amino Acids in Cancer. Front. Cell Dev. Biol. 2020, 8, 603837. [Google Scholar] [CrossRef]
- Wek, S.A.; Zhu, S.; Wek, R.C. The histidyl-tRNA synthetase-related sequence in the eIF-2 alpha protein kinase GCN2 interacts with tRNA and is required for activation in response to starvation for different amino acids. Mol. Cell Biol. 1995, 15, 4497–4506. [Google Scholar] [CrossRef]
- Wek, R.C.; Ramirez, M.; Jackson, B.M.; Hinnebusch, A.G. Identification of positive-acting domains in GCN2 protein kinase required for translational activation of GCN4 expression. Mol. Cell Biol. 1990, 10, 2820–2831. [Google Scholar] [CrossRef]
- Shan, J.; Fu, L.; Balasubramanian, M.N.; Anthony, T.; Kilberg, M.S. ATF4-dependent regulation of the JMJD3 gene during amino acid deprivation can be rescued in Atf4-deficient cells by inhibition of deacetylation. J. Biol. Chem. 2012, 287, 36393–36403. [Google Scholar] [CrossRef] [PubMed]
- Siu, F.; Bain, P.J.; LeBlanc-Chaffin, R.; Chen, H.; Kilberg, M.S. ATF4 is a mediator of the nutrient-sensing response pathway that activates the human asparagine synthetase gene. J. Biol. Chem. 2002, 277, 24120–24127. [Google Scholar] [CrossRef] [PubMed]
- Su, N.; Thiaville, M.M.; Awad, K.; Gjymishka, A.; Brant, J.O.; Yang, T.P.; Kilberg, M.S. Protein or amino acid deprivation differentially regulates the hepatic forkhead box protein A (FOXA) genes through an activating transcription factor-4-independent pathway. Hepatology 2009, 50, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Kilberg, M.S.; Shan, J.; Su, N. ATF4-dependent transcription mediates signaling of amino acid limitation. Trends Endocrinol. Metab. 2009, 20, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Baird, T.D.; Wek, R.C. Eukaryotic initiation factor 2 phosphorylation and translational control in metabolism. Adv. Nutr. 2012, 3, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Harding, H.P.; Novoa, I.; Zhang, Y.; Zeng, H.; Wek, R.; Schapira, M.; Ron, D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell 2000, 6, 1099–1108. [Google Scholar] [CrossRef]
- Barbosa-Tessmann, I.P.; Chen, C.; Zhong, C.; Siu, F.; Schuster, S.M.; Nick, H.S.; Kilberg, M.S. Activation of the human asparagine synthetase gene by the amino acid response and the endoplasmic reticulum stress response pathways occurs by common genomic elements. J. Biol. Chem. 2000, 275, 26976–26985. [Google Scholar] [CrossRef]
- Ren, Y.; Roy, S.; Ding, Y.; Iqbal, J.; Broome, J.D. Methylation of the asparagine synthetase promoter in human leukemic cell lines is associated with a specific methyl binding protein. Oncogene 2004, 23, 3953–3961. [Google Scholar] [CrossRef]
- Jiang, J.; Srivastava, S.; Seim, G.; Pavlova, N.N.; King, B.; Zou, L.; Zhang, C.; Zhong, M.; Feng, H.; Kapur, R.; et al. Promoter demethylation of the asparagine synthetase gene is required for ATF4-dependent adaptation to asparagine depletion. J. Biol. Chem. 2019, 294, 18674–18684. [Google Scholar] [CrossRef]
- Touzart, A.; Lengliné, E.; Latiri, M.; Belhocine, M.; Smith, C.; Thomas, X.; Spicuglia, S.; Puthier, D.; Pflumio, F.; Leguay, T.; et al. Epigenetic Silencing Affects l-Asparaginase Sensitivity and Predicts Outcome in T-ALL. Clin. Cancer Res. 2019, 25, 2483–2493. [Google Scholar] [CrossRef]
- Horowitz, B.; Madras, B.K.; Meister, A.; Old, L.J.; Boyes, E.A.; Stockert, E. Asparagine synthetase activity of mouse leukemias. Science 1968, 160, 533–535. [Google Scholar] [CrossRef] [PubMed]
- Su, N.; Pan, Y.X.; Zhou, M.; Harvey, R.C.; Hunger, S.P.; Kilberg, M.S. Correlation between asparaginase sensitivity and asparagine synthetase protein content, but not mRNA, in acute lymphoblastic leukemia cell lines. Pediatr. Blood Cancer 2008, 50, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Akagi, T.; Yin, D.; Kawamata, N.; Bartram, C.R.; Hofmann, W.K.; Wolf, I.; Miller, C.W.; Koeffler, H.P. Methylation analysis of asparagine synthetase gene in acute lymphoblastic leukemia cells. Leukemia 2006, 20, 1303–1306. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; Miyake, K.; Nordlund, J.; Syvänen, A.C.; van der Weyden, L.; Honda, H.; Yamasaki, N.; Nagamachi, A.; Inaba, T.; Ikawa, T.; et al. Association of aberrant ASNS imprinting with asparaginase sensitivity and chromosomal abnormality in childhood BCP-ALL. Blood 2020, 136, 2319–2333. [Google Scholar] [CrossRef]
- Peng, H.; Shen, N.; Qian, L.; Sun, X.L.; Koduru, P.; Goodwin, L.O.; Issa, J.P.; Broome, J.D. Hypermethylation of CpG islands in the mouse asparagine synthetase gene: Relationship to asparaginase sensitivity in lymphoma cells. Partial methylation in normal cells. Br. J. Cancer 2001, 85, 930–935. [Google Scholar] [CrossRef][Green Version]
- Willems, L.; Jacque, N.; Jacquel, A.; Neveux, N.; Maciel, T.T.; Lambert, M.; Schmitt, A.; Poulain, L.; Green, A.S.; Uzunov, M.; et al. Inhibiting glutamine uptake represents an attractive new strategy for treating acute myeloid leukemia. Blood 2013, 122, 3521–3532. [Google Scholar] [CrossRef]
- Buaboonnam, J.; Cao, X.; Pauley, J.L.; Pui, C.H.; Ribeiro, R.C.; Rubnitz, J.E.; Inaba, H. Sequential administration of methotrexate and asparaginase in relapsed or refractory pediatric acute myeloid leukemia. Pediatr. Blood Cancer 2013, 60, 1161–1164. [Google Scholar] [CrossRef]
- Emadi, A.; Law, J.Y.; Strovel, E.T.; Lapidus, R.G.; Jeng, L.J.B.; Lee, M.; Blitzer, M.G.; Carter-Cooper, B.A.; Sewell, D.; Van Der Merwe, I.; et al. Asparaginase Erwinia chrysanthemi effectively depletes plasma glutamine in adult patients with relapsed/refractory acute myeloid leukemia. Cancer Chemother. Pharm. 2018, 81, 217–222. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, J.; Zeng, H.; Zhang, Y.; Zhang, Y.; Zhou, X.; Zhou, H. Antiproliferative effects of L-asparaginase in acute myeloid leukemia. Exp. Med. 2020, 20, 2070–2078. [Google Scholar] [CrossRef]
- Purwaha, P.; Lorenzi, P.L.; Silva, L.P.; Hawke, D.H.; Weinstein, J.N. Targeted metabolomic analysis of amino acid response to L-asparaginase in adherent cells. Metabolomics 2014, 10, 909–919. [Google Scholar] [CrossRef]
- Choi, Y.-K.; Park, K.-G. Targeting Glutamine Metabolism for Cancer Treatment. Biomol. Ther. 2018, 26, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Toda, K.; Kawada, K.; Iwamoto, M.; Inamoto, S.; Sasazuki, T.; Shirasawa, S.; Hasegawa, S.; Sakai, Y. Metabolic Alterations Caused by KRAS Mutations in Colorectal Cancer Contribute to Cell Adaptation to Glutamine Depletion by Upregulation of Asparagine Synthetase. Neoplasia 2016, 18, 654–665. [Google Scholar] [CrossRef] [PubMed]
- Hinze, L.; Labrosse, R.; Degar, J.; Han, T.; Schatoff, E.M.; Schreek, S.; Karim, S.; McGuckin, C.; Sacher, J.R.; Wagner, F.; et al. Exploiting the Therapeutic Interaction of WNT Pathway Activation and Asparaginase for Colorectal Cancer Therapy. Cancer Discov. 2020, 10, 1690–1705. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Dong, L.W.; Tan, Y.X.; Zhang, J.; Pan, Y.F.; Yang, C.; Li, M.H.; Ding, Z.W.; Liu, L.J.; Jiang, T.Y.; et al. Asparagine synthetase is an independent predictor of surgical survival and a potential therapeutic target in hepatocellular carcinoma. Br. J. Cancer 2013, 109, 14–23. [Google Scholar] [CrossRef]
- Li, W.; Dong, C. Polymorphism in asparagine synthetase is associated with overall survival of hepatocellular carcinoma patients. BMC Gastroenterol. 2017, 17, 79. [Google Scholar] [CrossRef]
- Panosyan, E.H.; Wang, Y.; Xia, P.; Lee, W.N.; Pak, Y.; Laks, D.R.; Lin, H.J.; Moore, T.B.; Cloughesy, T.F.; Kornblum, H.I.; et al. Asparagine depletion potentiates the cytotoxic effect of chemotherapy against brain tumors. Mol. Cancer Res. 2014, 12, 694–702. [Google Scholar] [CrossRef]
- Ohba, S.; Hirose, Y. L-asparaginase and 6-diazo-5-oxo-L-norleucine synergistically inhibit the growth of glioblastoma cells. J. Neurooncol. 2020, 146, 469–475. [Google Scholar] [CrossRef]
- Dübbers, A.; Würthwein, G.; Müller, H.J.; Schulze-Westhoff, P.; Winkelhorst, M.; Kurzknabe, E.; Lanvers, C.; Pieters, R.; Kaspers, G.J.; Creutzig, U.; et al. Asparagine synthetase activity in paediatric acute leukaemias: AML-M5 subtype shows lowest activity. Br. J. Haematol. 2000, 109, 427–429. [Google Scholar] [CrossRef]
- Okada, S.; Hongo, T.; Yamada, S.; Watanabe, C.; Fujii, Y.; Ohzeki, T.; Horikoshi, Y.; Ito, T.; Yazaki, M.; Komada, Y.; et al. In vitro efficacy of l-asparaginase in childhood acute myeloid leukaemia. Br. J. Haematol. 2003, 123, 802–809. [Google Scholar] [CrossRef]
- Michelozzi, I.M.; Granata, V.; De Ponti, G.; Alberti, G.; Tomasoni, C.; Antolini, L.; Gambacorti-Passerini, C.; Gentner, B.; Dazzi, F.; Biondi, A.; et al. Acute myeloid leukaemia niche regulates response to L-asparaginase. Br. J. Haematol. 2019, 186, 420–430. [Google Scholar] [CrossRef]
- Kaspers, G.J.L. Acute myeloid leukaemia niche regulates response to L-asparaginase. Br. J. Haematol. 2019, 186, 397–399. [Google Scholar] [CrossRef] [PubMed]
- Scherf, U.; Ross, D.T.; Waltham, M.; Smith, L.H.; Lee, J.K.; Tanabe, L.; Kohn, K.W.; Reinhold, W.C.; Myers, T.G.; Andrews, D.T.; et al. A gene expression database for the molecular pharmacology of cancer. Nat. Genet. 2000, 24, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Waters, A.M.; Der, C.J. KRAS: The Critical Driver and Therapeutic Target for Pancreatic Cancer. Cold Spring Harb. Perspect. Med. 2018, 8, a031435. [Google Scholar] [CrossRef] [PubMed]
- Gaglio, D.; Metallo, C.M.; Gameiro, P.A.; Hiller, K.; Danna, L.S.; Balestrieri, C.; Alberghina, L.; Stephanopoulos, G.; Chiaradonna, F. Oncogenic K-Ras decouples glucose and glutamine metabolism to support cancer cell growth. Mol. Syst. Biol. 2011, 7, 523. [Google Scholar] [CrossRef] [PubMed]
- Son, J.; Lyssiotis, C.A.; Ying, H.; Wang, X.; Hua, S.; Ligorio, M.; Perera, R.M.; Ferrone, C.R.; Mullarky, E.; Shyh-Chang, N.; et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature 2013, 496, 101–105. [Google Scholar] [CrossRef]
- Li, D.; Fu, Z.; Chen, R.; Zhao, X.; Zhou, Y.; Zeng, B.; Yu, M.; Zhou, Q.; Lin, Q.; Gao, W.; et al. Inhibition of glutamine metabolism counteracts pancreatic cancer stem cell features and sensitizes cells to radiotherapy. Oncotarget 2015, 6, 31151–31163. [Google Scholar] [CrossRef]
- Porru, M.; Pompili, L.; Caruso, C.; Biroccio, A.; Leonetti, C. Targeting KRAS in metastatic colorectal cancer: Current strategies and emerging opportunities. J. Exp. Clin. Cancer Res. 2018, 37, 57. [Google Scholar] [CrossRef]
- Du, F.; Chen, J.; Liu, H.; Cai, Y.; Cao, T.; Han, W.; Yi, X.; Qian, M.; Tian, D.; Nie, Y.; et al. SOX12 promotes colorectal cancer cell proliferation and metastasis by regulating asparagine synthesis. Cell Death Dis. 2019, 10, 239. [Google Scholar] [CrossRef]
- Cao, J.; Wang, X.; Dai, T.; Wu, Y.; Zhang, M.; Cao, R.; Zhang, R.; Wang, G.; Jiang, R.; Zhou, B.P.; et al. Twist promotes tumor metastasis in basal-like breast cancer by transcriptionally upregulating ROR1. Theranostics 2018, 8, 2739–2751. [Google Scholar] [CrossRef]
- Luo, M.; Shang, L.; Brooks, M.D.; Jiagge, E.; Zhu, Y.; Buschhaus, J.M.; Conley, S.; Fath, M.A.; Davis, A.; Gheordunescu, E.; et al. Targeting Breast Cancer Stem Cell State Equilibrium through Modulation of Redox Signaling. Cell Metab. 2018, 28, 69–86. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, M.; Xu, F.; Jiang, S. Wnt signaling in breast cancer: Biological mechanisms, challenges and opportunities. Mol. Cancer 2020, 19, 165. [Google Scholar] [CrossRef]
- Pavlova, N.N.; Hui, S.; Ghergurovich, J.M.; Fan, J.; Intlekofer, A.M.; White, R.M.; Rabinowitz, J.D.; Thompson, C.B.; Zhang, J. As Extracellular Glutamine Levels Decline, Asparagine Becomes an Essential Amino Acid. Cell Metab. 2018, 27, 428–438. [Google Scholar] [CrossRef]
- Wise, D.R.; Thompson, C.B. Glutamine addiction: A new therapeutic target in cancer. Trends Biochem. Sci. 2010, 35, 427–433. [Google Scholar] [CrossRef]
- Chiu, M.; Tardito, S.; Pillozzi, S.; Arcangeli, A.; Armento, A.; Uggeri, J.; Missale, G.; Bianchi, M.G.; Barilli, A.; Dall’Asta, V.; et al. Glutamine depletion by crisantaspase hinders the growth of human hepatocellular carcinoma xenografts. Br. J. Cancer 2014, 111, 1159–1167. [Google Scholar] [CrossRef]
- Gross, M.I.; Demo, S.D.; Dennison, J.B.; Chen, L.; Chernov-Rogan, T.; Goyal, B.; Janes, J.R.; Laidig, G.J.; Lewis, E.R.; Li, J.; et al. Antitumor activity of the glutaminase inhibitor CB-839 in triple-negative breast cancer. Mol. Cancer 2014, 13, 890–901. [Google Scholar] [CrossRef]
- Fung, M.K.L.; Chan, G.C.-F. Drug-induced amino acid deprivation as strategy for cancer therapy. J. Hematol. Oncol. 2017, 10, 144. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Yap, H.Y.; Wiseman, C.L.; Blumenschein, G.R.; Buzdar, A.U.; Legha, S.S.; Gutterman, J.U.; Hersh, E.M.; Bodey, G.P. Chemoimmunotherapy for metastatic breast cancer with 5-fluorouracil, adriamycin, cyclophosphamide, methotrexate, L-asparaginase, Corynebacterium parvum, and Pseudomonas vaccine. Cancer Treat. Rep. 1980, 64, 157–159. [Google Scholar]
- Tan, A.C.; Ashley, D.M.; López, G.Y.; Malinzak, M.; Friedman, H.S.; Khasraw, M. Management of glioblastoma: State of the art and future directions. CA Cancer J. Clin. 2020, 70, 299–312. [Google Scholar] [CrossRef]
- Bhowmik, A.; Khan, R.; Ghosh, M.K. Blood brain barrier: A challenge for effectual therapy of brain tumors. Biomed. Res. Int. 2015, 2015, 320941. [Google Scholar] [CrossRef]
- Hawkins, D.S.; Park, J.R.; Thomson, B.G.; Felgenhauer, J.L.; Holcenberg, J.S.; Panosyan, E.H.; Avramis, V.I. Asparaginase pharmacokinetics after intensive polyethylene glycol-conjugated L-asparaginase therapy for children with relapsed acute lymphoblastic leukemia. Clin. Cancer Res. 2004, 10, 5335–5341. [Google Scholar] [CrossRef]
- Panetta, J.C.; Gajjar, A.; Hijiya, N.; Hak, L.J.; Cheng, C.; Liu, W.; Pui, C.H.; Relling, M.V. Comparison of native E. coli and PEG asparaginase pharmacokinetics and pharmacodynamics in pediatric acute lymphoblastic leukemia. Clin. Pharm. 2009, 86, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Vieira Pinheiro, J.P.; Wenner, K.; Escherich, G.; Lanvers-Kaminsky, C.; Würthwein, G.; Janka-Schaub, G.; Boos, J. Serum asparaginase activities and asparagine concentrations in the cerebrospinal fluid after a single infusion of 2,500 IU/m(2) PEG asparaginase in children with ALL treated according to protocol COALL-06-97. Pediatr. Blood Cancer 2006, 46, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Lanvers-Kaminsky, C. Asparaginase pharmacology: Challenges still to be faced. Cancer Chemother. Pharm. 2017, 79, 439–450. [Google Scholar] [CrossRef]
- Rizzari, C.; Zucchetti, M.; Conter, V.; Diomede, L.; Bruno, A.; Gavazzi, L.; Paganini, M.; Sparano, P.; Lo Nigro, L.; Aricò, M.; et al. L-asparagine depletion and L-asparaginase activity in children with acute lymphoblastic leukemia receiving i.m. or i.v. Erwinia C. or E. coli L-asparaginase as first exposure. Ann. Oncol. 2000, 11, 189–193. [Google Scholar] [CrossRef]
- Rizzari, C.; Citterio, M.; Zucchetti, M.; Conter, V.; Chiesa, R.; Colombini, A.; Malguzzi, S.; Silvestri, D.; D’Incalci, M. A pharmacological study on pegylated asparaginase used in front-line treatment of children with acute lymphoblastic leukemia. Haematologica 2006, 91, 24–31. [Google Scholar] [PubMed]
- Asselin, B.; Rizzari, C. Asparaginase pharmacokinetics and implications of therapeutic drug monitoring. Leuk. Lymphoma 2015, 56, 2273–2280. [Google Scholar] [CrossRef]
- Henriksen, L.T.; Nersting, J.; Raja, R.A.; Frandsen, T.L.; Rosthøj, S.; Schrøder, H.; Albertsen, B.K. Cerebrospinal fluid asparagine depletion during pegylated asparaginase therapy in children with acute lymphoblastic leukaemia. Br. J. Haematol. 2014, 166, 213–220. [Google Scholar] [CrossRef]
- Riccardi, R.; Holcenberg, J.S.; Glaubiger, D.L.; Wood, J.H.; Poplack, D.G. L-asparaginase pharmacokinetics and asparagine levels in cerebrospinal fluid of rhesus monkeys and humans. Cancer Res. 1981, 41, 4554–4558. [Google Scholar]
- Ballerini, A.; Moro, F.; Nerini, I.F.; Marzo, C.M.; Di Clemente, A.; Ferrari, M.; D’Incalci, M.; Biondi, A.; Colombini, A.; Conter, V.; et al. Pharmacodynamic effects in the cerebrospinal fluid of rats after intravenous administration of different asparaginase formulations. Cancer Chemother. Pharm. 2017, 79, 1267–1271. [Google Scholar] [CrossRef]
- Koleva, L.; Bovt, E.; Ataullakhanov, F.; Sinauridze, E. Erythrocytes as Carriers: From Drug Delivery to Biosensors. Pharmaceutics 2020, 12, 276. [Google Scholar] [CrossRef]
- Rossi, L.; Pierigè, F.; Aliano, M.P.; Magnani, M. Ongoing Developments and Clinical Progress in Drug-Loaded Red Blood Cell Technologies. BioDrugs 2020, 34, 265–272. [Google Scholar] [CrossRef]
- Domenech, C.; Thomas, X.; Chabaud, S.; Baruchel, A.; Gueyffier, F.; Mazingue, F.; Auvrignon, A.; Corm, S.; Dombret, H.; Chevallier, P.; et al. l-asparaginase loaded red blood cells in refractory or relapsing acute lymphoblastic leukaemia in children and adults: Results of the GRASPALL 2005-01 randomized trial. Br. J. Haematol. 2011, 153, 58–65. [Google Scholar] [CrossRef]
- Hunault-Berger, M.; Leguay, T.; Huguet, F.; Leprêtre, S.; Deconinck, E.; Ojeda-Uribe, M.; Bonmati, C.; Escoffre-Barbe, M.; Bories, P.; Himberlin, C.; et al. A Phase 2 study of L-asparaginase encapsulated in erythrocytes in elderly patients with Philadelphia chromosome negative acute lymphoblastic leukemia: The GRASPALL/GRAALL-SA2-2008 study. Am. J. Hematol. 2015, 90, 811–818. [Google Scholar] [CrossRef]
- European Medicines Agency. Graspa: Withdrawal of the Marketing Authorisation Application. Available online: https://www.ema.europa.eu/en/medicines/human/withdrawn-applications/graspa-0 (accessed on 16 December 2021).
- Bachet, J.B.; Gay, F.; Maréchal, R.; Galais, M.P.; Adenis, A.; Ms, C.D.; Cros, J.; Demetter, P.; Svrcek, M.; Bardier-Dupas, A.; et al. Asparagine Synthetase Expression and Phase I Study With L-Asparaginase Encapsulated in Red Blood Cells in Patients With Pancreatic Adenocarcinoma. Pancreas 2015, 44, 1141–1147. [Google Scholar] [CrossRef]
- Hammel, P.; Bachet, J.B.; Portales, F.; Mineur, L.; Metges, J.P.; de la Fouchardiere, C.; Louvet, C.; El Hajbi, F.; Faroux, R.; Guimbaud, R.; et al. 621PD—A Phase 2b of eryaspase in combination with gemcitabine or FOLFOX as second-line therapy in patients with metastatic pancreatic adenocarcinoma (NCT02195180). Ann. Oncol. 2017, 28, v211. [Google Scholar] [CrossRef]
- Hammel, P.; Fabienne, P.; Mineur, L.; Metges, J.P.; Andre, T.; De La Fouchardiere, C.; Louvet, C.; El Hajbi, F.; Faroux, R.; Guimbaud, R.; et al. Erythrocyte-encapsulated asparaginase (eryaspase) combined with chemotherapy in second-line treatment of advanced pancreatic cancer: An open-label, randomized Phase IIb trial. Eur. J. Cancer 2020, 124, 91–101. [Google Scholar] [CrossRef]
- Erytech. ERYTECH Granted, U.S. FDA Fast Track Designation for Eryaspase in Hypersensitive ALL. Available online: https://investors.erytech.com/news-releases/news-release-details/erytech-granted-us-fda-fast-track-designation-eryaspase (accessed on 20 December 2021).
- Distasio, J.A.; Durden, D.L.; Paul, R.D.; Nadji, M. Alteration in spleen lymphoid populations associated with specific amino acid depletion during L-asparaginase treatment. Cancer Res. 1982, 42, 252–258. [Google Scholar]
- Distasio, J.A.; Niederman, R.A.; Kafkewitz, D. Antilymphoma activity of a glutaminase-free L-asparaginase of microbial origin. Proc. Soc. Exp. Biol. Med. 1977, 155, 528–531. [Google Scholar] [CrossRef]
- Durden, D.L.; Distasio, J.A. Comparison of the immunosuppressive effects of asparaginases from Escherichia coli and Vibrio succinogenes. Cancer Res. 1980, 40, 1125–1129. [Google Scholar]
- Distasio, J.A.; Niederman, R.A.; Kafkewitz, D.; Goodman, D. Purification and characterization of L-asparaginase with anti-lymphoma activity from Vibrio succinogenes. J. Biol. Chem. 1976, 251, 6929–6933. [Google Scholar] [CrossRef]
- Bendich, A.; Kafkewitz, D.; Abuchowski, A.; Davis, F.F. Immunological effects of native and polyethylene glycol-modified asparaginases from Vibrio succinogenes and Escherichia coli in normal and tumour-bearing mice. Clin. Exp. Immunol. 1982, 48, 273–278. [Google Scholar]
- Nguyen, H.A.; Durden, D.L.; Lavie, A. The differential ability of asparagine and glutamine in promoting the closed/active enzyme conformation rationalizes the Wolinella succinogenes L-asparaginase substrate specificity. Sci. Rep. 2017, 7, 41643. [Google Scholar] [CrossRef]
- Baar, C.; Eppinger, M.; Raddatz, G.; Simon, J.; Lanz, C.; Klimmek, O.; Nandakumar, R.; Gross, R.; Rosinus, A.; Keller, H.; et al. Complete genome sequence and analysis of Wolinella succinogenes. Proc. Natl. Acad. Sci. USA 2003, 100, 11690–11695. [Google Scholar] [CrossRef]
- Lubkowski, J.; Palm, G.J.; Gilliland, G.L.; Derst, C.; Röhm, K.H.; Wlodawer, A. Crystal structure and amino acid sequence of Wolinella succinogenes L-asparaginase. Eur. J. Biochem. 1996, 241, 201–207. [Google Scholar] [CrossRef]
- Reinert, R.B.; Oberle, L.M.; Wek, S.A.; Bunpo, P.; Wang, X.P.; Mileva, I.; Goodwin, L.O.; Aldrich, C.J.; Durden, D.L.; McNurlan, M.A.; et al. Role of glutamine depletion in directing tissue-specific nutrient stress responses to L-asparaginase. J. Biol. Chem. 2006, 281, 31222–31233. [Google Scholar] [CrossRef]
- Emadi, A.; Kapadia, B.; Bollino, D.; Bhandary, B.; Baer, M.R.; Niyongere, S.; Strovel, E.T.; Kaizer, H.; Chang, E.; Choi, E.Y.; et al. Venetoclax and pegcrisantaspase for complex karyotype acute myeloid leukemia. Leukemia 2021, 35, 1907–1924. [Google Scholar] [CrossRef]
- Nikonorova, I.A.; Zhu, Q.; Signore, C.C.; Mirek, E.T.; Jonsson, W.O.; Kong, B.; Guo, G.L.; Belden, W.J.; Anthony, T.G. Age modulates liver responses to asparaginase-induced amino acid stress in mice. J. Biol. Chem. 2019, 294, 13864–13875. [Google Scholar] [CrossRef]
- Schalk, A.M.; Lavie, A. Structural and kinetic characterization of guinea pig L-asparaginase type III. Biochemistry 2014, 53, 2318–2328. [Google Scholar] [CrossRef]
- Schalk, A.M.; Nguyen, H.A.; Rigouin, C.; Lavie, A. Identification and structural analysis of an L-asparaginase enzyme from guinea pig with putative tumor cell killing properties. J. Biol. Chem. 2014, 289, 33175–33186. [Google Scholar] [CrossRef]
- Rigouin, C.; Nguyen, H.A.; Schalk, A.M.; Lavie, A. Discovery of human-like L-asparaginases with potential clinical use by directed evolution. Sci. Rep. 2017, 7, 10224. [Google Scholar] [CrossRef]
- Tower, D.B.; Peters, E.L.; Curtis, W.C. Guinea pig serum L-asparaginase. Properties, purification, and application to determination of asparagine in biological samples. J. Biol. Chem. 1963, 238, 983–993. [Google Scholar] [CrossRef]
- Cantor, J.R.; Stone, E.M.; Chantranupong, L.; Georgiou, G. The human asparaginase-like protein 1 hASRGL1 is an Ntn hydrolase with beta-aspartyl peptidase activity. Biochemistry 2009, 48, 11026–11031. [Google Scholar] [CrossRef]
- Karamitros, C.S.; Konrad, M. Human 60-kDa lysophospholipase contains an N-terminal L-asparaginase domain that is allosterically regulated by L-asparagine. J. Biol. Chem. 2014, 289, 12962–12975. [Google Scholar] [CrossRef]
- Maggi, M.; Mittelman, S.D.; Parmentier, J.H.; Colombo, G.; Meli, M.; Whitmire, J.M.; Merrell, D.S.; Whitelegge, J.; Scotti, C. A protease-resistant Escherichia coli asparaginase with outstanding stability and enhanced anti-leukaemic activity in vitro. Sci. Rep. 2017, 7, 1–16. [Google Scholar] [CrossRef]
- Nguyen, H.A.; Su, Y.; Lavie, A. Design and characterization of erwinia chrysanthemi l-asparaginase variants with diminished l-glutaminase activity. J. Biol. Chem. 2016, 291, 17664–17676. [Google Scholar] [CrossRef]
- Offman, M.N.; Krol, M.; Patel, N.; Krishnan, S.; Liu, J.; Saha, V.; Bates, P.A. Rational engineering of L-asparaginase reveals importance of dual activity for cancer cell toxicity. Blood J. Am. Soc. Hematol. 2011, 117, 1614–1621. [Google Scholar] [CrossRef]
- Jianhua, C.; Yujun, W.; Ruibo, J.; Min, W.; Wutong, W. Probing the antigenicity of E. coli L-asparaginase by mutational analysis. Mol. Biotechnol. 2006, 33, 57–65. [Google Scholar]
- Ghasemi, A.; Asad, S.; Kabiri, M.; Dabirmanesh, B. Cloning and characterization of Halomonas elongata L-asparaginase, a promising chemotherapeutic agent. Appl. Microbiol. Biotechnol. 2017, 101, 7227–7238. [Google Scholar] [CrossRef]
- Bansal, S.; Srivastava, A.; Mukherjee, G.; Pandey, R.; Verma, A.K.; Mishra, P.; Kundu, B. Hyperthermophilic asparaginase mutants with enhanced substrate affinity and antineoplastic activity: Structural insights on their mechanism of action. FASEB J. 2012, 26, 1161–1171. [Google Scholar] [CrossRef]
- Ye, J.; Kumanova, M.; Hart, L.S.; Sloane, K.; Zhang, H.; De Panis, D.N.; Bobrovnikova-Marjon, E.; Diehl, J.A.; Ron, D.; Koumenis, C. The GCN2-ATF4 pathway is critical for tumour cell survival and proliferation in response to nutrient deprivation. EMBO J. 2010, 29, 2082–2096. [Google Scholar] [CrossRef]
- Bunpo, P.; Dudley, A.; Cundiff, J.K.; Cavener, D.R.; Wek, R.C.; Anthony, T.G. GCN2 protein kinase is required to activate amino acid deprivation responses in mice treated with the anti-cancer agent L-asparaginase. J. Biol. Chem. 2009, 284, 32742–32749. [Google Scholar] [CrossRef]
- Nakamura, A.; Nambu, T.; Ebara, S.; Hasegawa, Y.; Toyoshima, K.; Tsuchiya, Y.; Tomita, D.; Fujimoto, J.; Kurasawa, O.; Takahara, C.; et al. Inhibition of GCN2 sensitizes ASNS-low cancer cells to asparaginase by disrupting the amino acid response. Proc. Natl. Acad. Sci. USA 2018, 115, E7776–E7785. [Google Scholar] [CrossRef]
- Apfel, V.; Begue, D.; Cordo, V.; Holzer, L.; Martinuzzi, L.; Buhles, A.; Kerr, G.; Barbosa, I.; Naumann, U.; Piquet, M.; et al. Therapeutic Assessment of Targeting ASNS Combined with l-Asparaginase Treatment in Solid Tumors and Investigation of Resistance Mechanisms. ACS Pharm. Transl. Sci. 2021, 4, 327–337. [Google Scholar] [CrossRef]
- Fujimoto, J.; Kurasawa, O.; Takagi, T.; Liu, X.; Banno, H.; Kojima, T.; Asano, Y.; Nakamura, A.; Nambu, T.; Hata, A.; et al. Identification of Novel, Potent, and Orally Available GCN2 Inhibitors with Type I Half Binding Mode. ACS Med. Chem. Lett. 2019, 10, 1498–1503. [Google Scholar] [CrossRef]
- Butler, M.; van Ingen Schenau, D.S.; Yu, J.; Jenni, S.; Dobay, M.P.; Hagelaar, R.; Vervoort, B.M.T.; Tee, T.M.; Hoff, F.W.; Meijerink, J.P.; et al. BTK inhibition sensitizes Acute Lymphoblastic Leukemia to asparaginase by suppressing the Amino Acid Response pathway. Blood 2021, 138, 2383–2395. [Google Scholar] [CrossRef]
- Sun, J.; Nagel, R.; Zaal, E.A.; Ugalde, A.P.; Han, R.; Proost, N.; Song, J.Y.; Pataskar, A.; Burylo, A.; Fu, H.; et al. SLC1A3 contributes to L-asparaginase resistance in solid tumors. EMBO J. 2019, 38, e102147. [Google Scholar] [CrossRef]
- Hinze, L.; Pfirrmann, M.; Karim, S.; Degar, J.; McGuckin, C.; Vinjamur, D.; Sacher, J.; Stevenson, K.E.; Neuberg, D.S.; Orellana, E.; et al. Synthetic Lethality of Wnt Pathway Activation and Asparaginase in Drug-Resistant Acute Leukemias. Cancer Cell 2019, 35, 664–676. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).