Administering Docetaxel for Metastatic Hormone-Sensitive Prostate Cancer 1–6 Days Compared to More Than 14 Days after the Start of LHRH Agonist Is Associated with Better Clinical Outcomes Due to Androgen Flare
Abstract
Simple Summary
Abstract
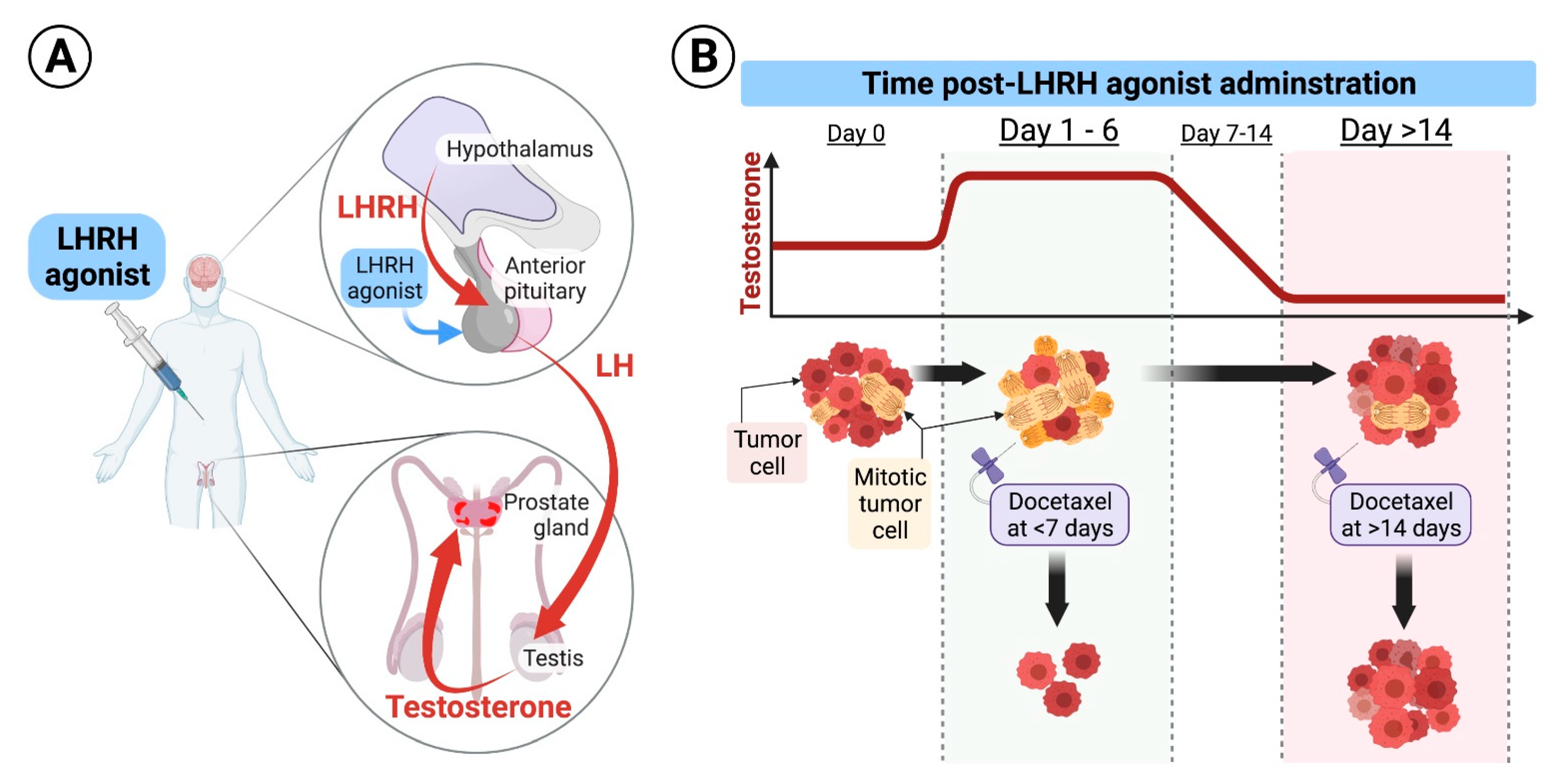
1. Introduction
2. Materials and Methods
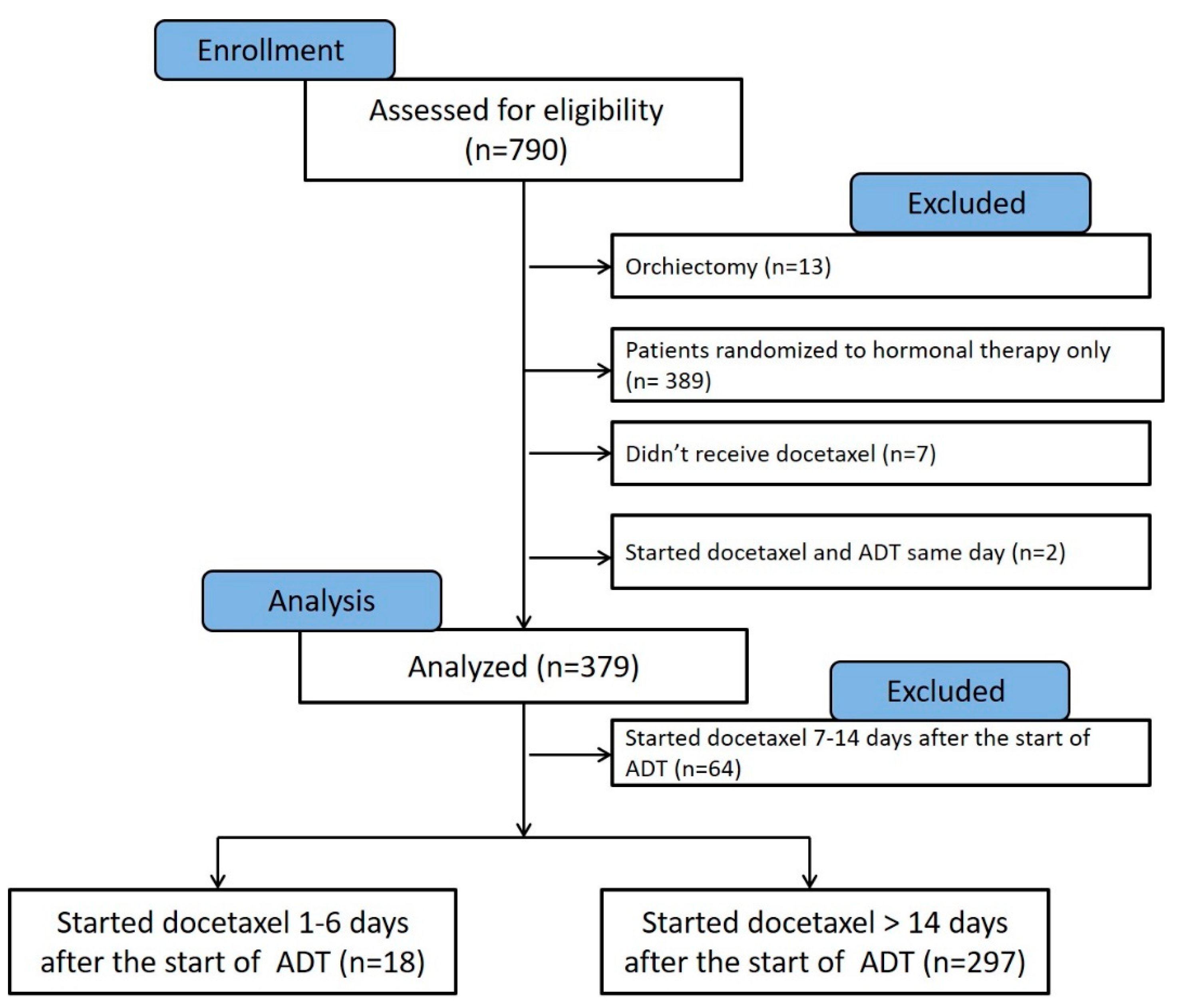
2.1. Patients
2.2. Statistical Analysis
3. Results
3.1. Comparison between Patients Who Initiated Docetaxel Therapy 1–6 Days versus >14 Days from the Start of LHRH Agonist
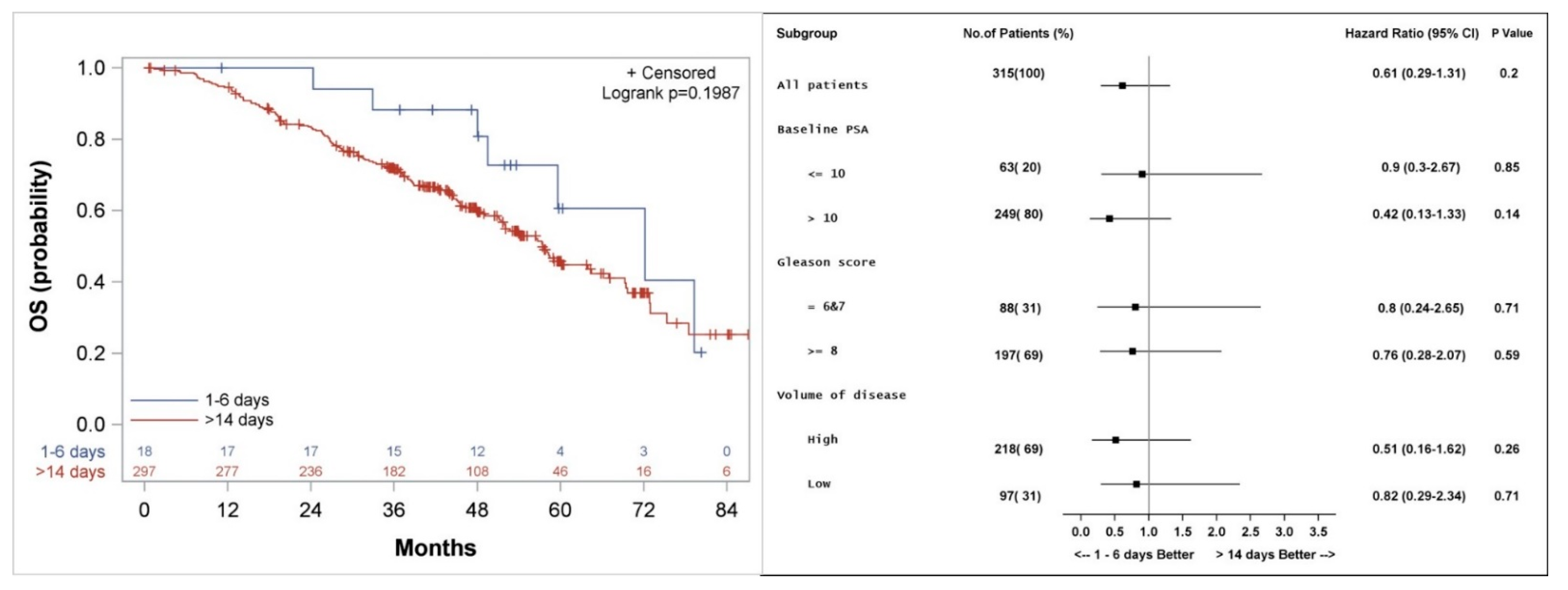
3.2. Overall Survival
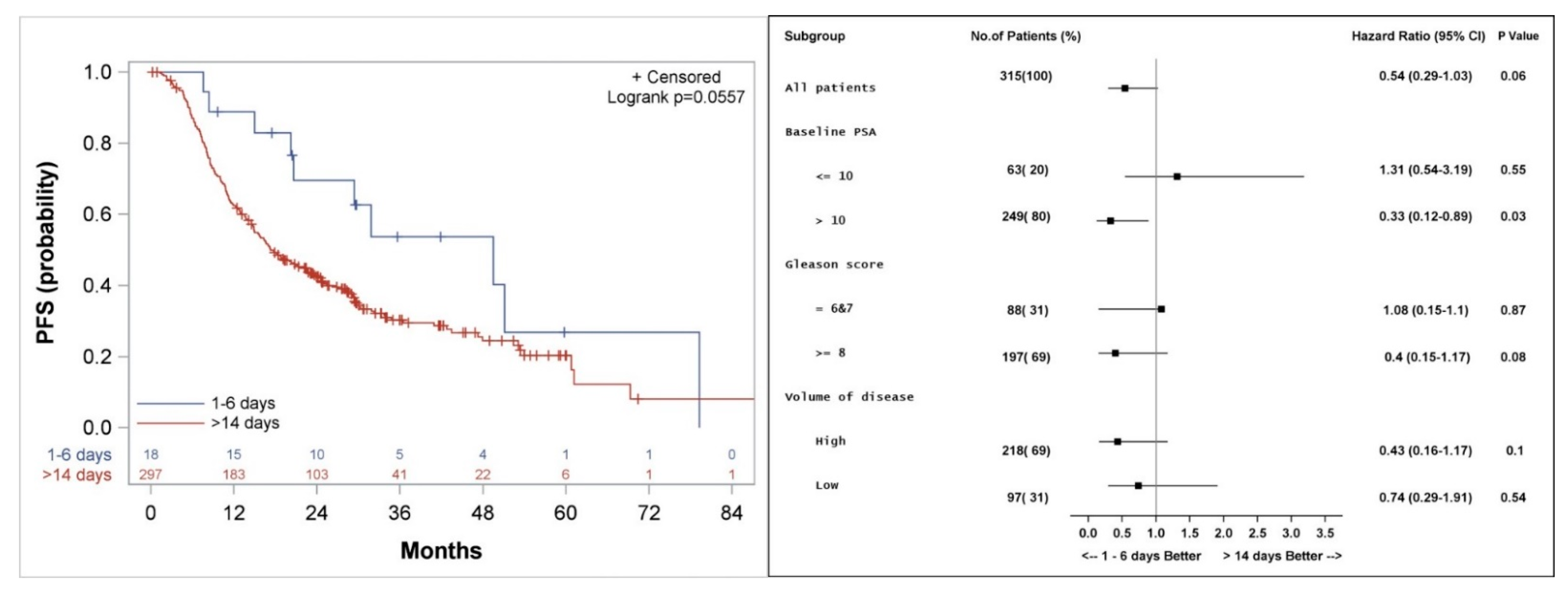
3.3. Progression Free Survival
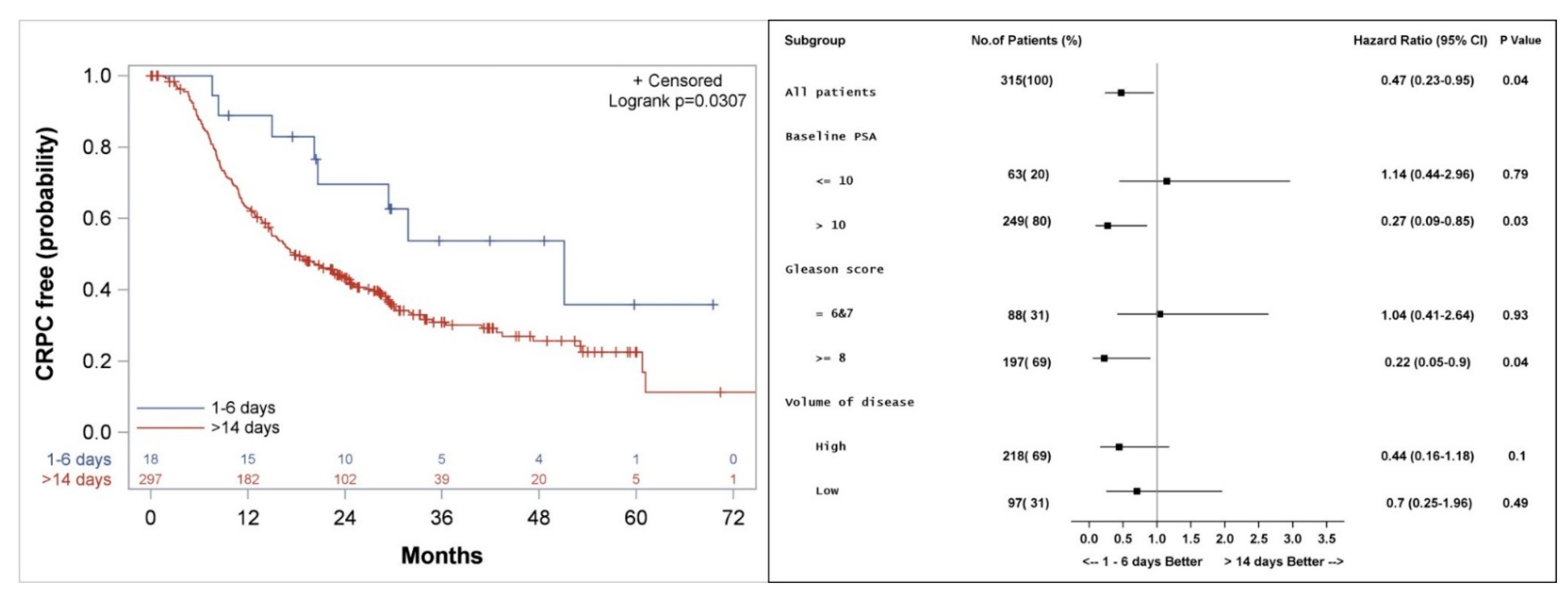
3.4. Freedom from Castrate Resistant Prostate Cancer
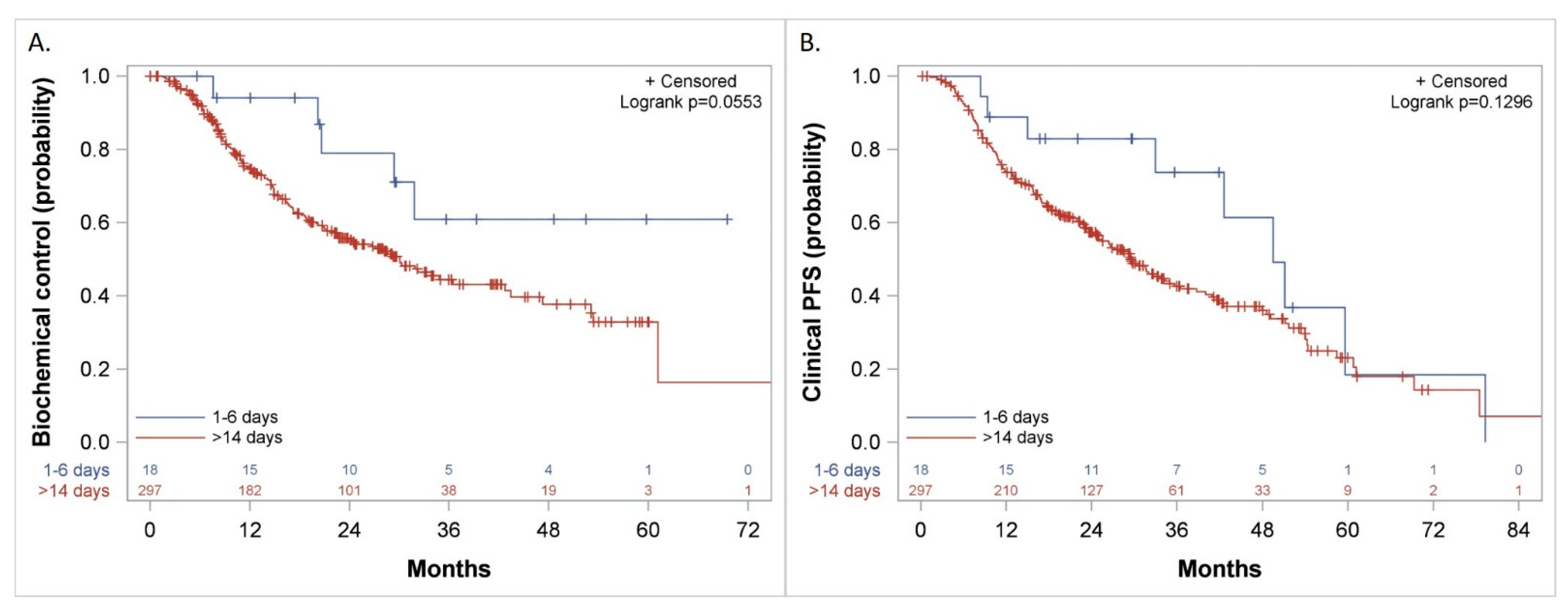
3.5. Biochemical Control, Freedom from CP, and Clinical PFS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA A Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Nasser, N.J.; Klein, J.; Agbarya, A. Markers of Toxicity and Response to Radiation Therapy in Patients with Prostate Cancer. Adv. Radiat. Oncol. 2021, 6, 100603. [Google Scholar] [CrossRef]
- Nasser, N.J.; Thoms, J.; Soosaipillai, A.; Pintilie, M.; Wang, R.; Diamandis, E.P.; Bristow, R.G. Human tissue Kallikreins: Blood levels and response to radiotherapy in intermediate risk prostate cancer. Radiother. Oncol. 2017, 124, 427–432. [Google Scholar] [CrossRef]
- Huggins, C.; Hodges, C.V. Studies on prostatic cancer. Cancer Res. 1941, 1, 293–297. [Google Scholar] [CrossRef]
- The Nobel Prize in Physiology or Medicine 1966. Available online: https://www.nobelprize.org/prizes/medicine/1966/huggins/facts/ (accessed on 21 January 2022).
- Guillemin, R. Peptides in the brain: The new endocrinology of the neuron. Science 1978, 202, 390–402. [Google Scholar] [CrossRef]
- Schally, A.V.; Block, N.L.; Rick, F.G. Discovery of LHRH and development of LHRH analogs for prostate cancer treatment. Prostate 2017, 77, 1036–1054. [Google Scholar] [CrossRef]
- Burgus, R.; Dunn, T.F.; Desiderio, D.; Ward, D.N.; Vale, W.; Guillemin, R. Characterization of ovine hypothalamic hypophysiotropic TSH-releasing factor. Nature 1970, 226, 321–325. [Google Scholar] [CrossRef]
- Schally, A.; Arimura, A.; Baba, Y.; Nair, R.; Matsuo, H.; Redding, T.; Debeljuk, L.; White, W. Isolation and properties of the FSH and LH-releasing hormone. Biochem. Biophys. Res. Commun. 1971, 43, 393–399. [Google Scholar] [CrossRef]
- The Nobel Prize in Physiology or Medicine 1977. Available online: https://www.nobelprize.org/prizes/medicine/1977/summary/ (accessed on 21 January 2022).
- Sweeney, C.J.; Chen, Y.-H.; Carducci, M.; Liu, G.; Jarrard, D.F.; Eisenberger, M.; Wong, Y.-N.; Hahn, N.; Kohli, M.; Cooney, M.M. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N. Engl. J. Med. 2015, 373, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Gravis, G.; Fizazi, K.; Joly, F.; Oudard, S.; Priou, F.; Esterni, B.; Latorzeff, I.; Delva, R.; Krakowski, I.; Laguerre, B. Androgen-deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU 15): A randomised, open-label, phase 3 trial. Lancet Oncol. 2013, 14, 149–158. [Google Scholar] [CrossRef]
- Clarke, N.W.; Ali, A.; Ingleby, F.C.; Hoyle, A.; Amos, C.L.; Attard, G.; Brawley, C.D.; Calvert, J.; Chowdhury, S.; Cook, A.; et al. Addition of docetaxel to hormonal therapy in low- and high-burden metastatic hormone sensitive prostate cancer: Long-term survival results from the STAMPEDE trial. Ann. Oncol. 2019, 30, 1992–2003. [Google Scholar] [CrossRef] [PubMed]
- James, N.D.; Sydes, M.R.; Clarke, N.W.; Mason, M.D.; Dearnaley, D.P.; Spears, M.R.; Ritchie, A.W.; Parker, C.C.; Russell, J.M.; Attard, G. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): Survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 2016, 387, 1163–1177. [Google Scholar] [CrossRef]
- Kyriakopoulos, C.E.; Chen, Y.-H.; Carducci, M.A.; Liu, G.; Jarrard, D.F.; Hahn, N.M.; Shevrin, D.H.; Dreicer, R.; Hussain, M.; Eisenberger, M.; et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer: Long-Term Survival Analysis of the Randomized Phase III E3805 CHAARTED Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Carmel, A.; Joly, F.; Delva, R.; Gravis, G.; Rolland, F.; Priou, F.; Ferrero, J.-M.; Houede, N.; Mourey, L. Updated results of GETUG-12, a phase III trial of docetaxel-based chemotherapy in high-risk localized prostate cancer, with a 12-year follow-up. Ann. Oncol. 2018, 29, viii271. [Google Scholar] [CrossRef]
- Harris, W.P.; Mostaghel, E.A.; Nelson, P.S.; Montgomery, B. Androgen deprivation therapy: Progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat. Clin. Pract. Urol. 2009, 6, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, H.; Messing, E.M.; Chang, C. Androgen deprivation therapy for prostate cancer: Current status and future prospects. Prostate 2004, 61, 332–353. [Google Scholar] [CrossRef] [PubMed]
- Dearnaley, D.P.; Saltzstein, D.R.; Sylvester, J.E.; Karsh, L.; Mehlhaff, B.A.; Pieczonka, C.; Bailen, J.L.; Shi, H.; Ye, Z.; Faessel, H.M.; et al. The Oral Gonadotropin-releasing Hormone Receptor Antagonist Relugolix as Neoadjuvant/Adjuvant Androgen Deprivation Therapy to External Beam Radiotherapy in Patients with Localised Intermediate-risk Prostate Cancer: A Randomised, Open-label, Parallel-group Phase 2 Trial. Eur. Urol. 2020, 78, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Vargas, H.; Palacios, J.; Moreno-Bueno, G. Telling cells how to die: Docetaxel therapy in cancer cell lines. Cell Cycle 2007, 6, 780–783. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pienta, K.J. Preclinical mechanisms of action of docetaxel and docetaxel combinations in prostate cancer. Semin. Oncol. 2001, 28, 3–7. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Mirzaei, S.; Hashemi, F.; Zarrabi, A.; Zabolian, A.; Saleki, H.; Sharifzadeh, S.O.; Soleymani, L.; Daneshi, S.; Hushmandi, K.; et al. New insight towards development of paclitaxel and docetaxel resistance in cancer cells: EMT as a novel molecular mechanism and therapeutic possibilities. Biomed. Pharmacother. 2021, 141, 111824. [Google Scholar] [CrossRef]
- Mohammadian, J.; Sabzichi, M.; Molavi, O.; Shanehbandi, D.; Samadi, N. Combined treatment with stattic and docetaxel alters the Bax/Bcl-2 gene expression ratio in human prostate cancer cells. Asian Pac. J. Cancer Prev. APJCP 2016, 17, 5031. [Google Scholar] [PubMed]
- Kramer, G.; Schwarz, S.; Hägg, M.; Havelka, A.M.; Linder, S. Docetaxel induces apoptosis in hormone refractory prostate carcinomas during multiple treatment cycles. Br. J. Cancer 2006, 94, 1592–1598. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, F.; Amadori, D.; Carloni, S.; Brigliadori, G.; Tesei, A.; Ulivi, P.; Rosetti, M.; Vannini, I.; Arienti, C.; Zoli, W. Mitotic catastrophe and apoptosis induced by docetaxel in hormone-refractory prostate cancer cells. J. Cell. Physiol. 2008, 217, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Mang, J.; Merkle, K.; Heller, M.; Schüler, J.; Tolstov, Y.; Li, J.; Hohenfellner, M.; Duensing, S. Molecular complexity of taxane-induced cytotoxicity in prostate cancer cells. Urol. Oncol. Semin. Orig. Investig. 2017, 35, 32.e9–32.e16. [Google Scholar] [CrossRef]
- Shore, N.D.; Saad, F.; Cookson, M.S.; George, D.J.; Saltzstein, D.R.; Tutrone, R.; Akaza, H.; Bossi, A.; van Veenhuyzen, D.F.; Selby, B.; et al. Oral Relugolix for Androgen-Deprivation Therapy in Advanced Prostate Cancer. N. Engl. J. Med. 2020, 382, 2187–2196. [Google Scholar] [CrossRef]
- Mori, K.; Mostafaei, H.; Sari Motlagh, R.; Pradere, B.; Quhal, F.; Laukhtina, E.; Schuettfort, V.M.; Kramer, G.; Abufaraj, M.; Karakiewicz, P.I.; et al. Systemic therapies for metastatic hormone-sensitive prostate cancer: Network meta-analysis. BJU Int. 2021. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Li, D.; Ma, X.; Xu, K.; Ding, B.; Li, H.; Wang, Z.; Ouyang, W.; Long, G.; et al. Androgen Receptor Splice Variant 7 Predicts Shorter Response in Patients with Metastatic Hormone-sensitive Prostate Cancer Receiving Androgen Deprivation Therapy. Eur. Urol. 2021, 79, 879–886. [Google Scholar] [CrossRef]
- Hofmann, M.R.; Hussain, M.; Dehm, S.M.; Beltran, H.; Wyatt, A.W.; Halabi, S.; Sweeney, C.; Scher, H.I.; Ryan, C.J.; Feng, F.Y.; et al. Prostate Cancer Foundation Hormone-Sensitive Prostate Cancer Biomarker Working Group Meeting Summary. Urology 2021, 155, 165–171. [Google Scholar] [CrossRef]
- Cattrini, C.; España, R.; Mennitto, A.; Bersanelli, M.; Castro, E.; Olmos, D.; Lorente, D.; Gennari, A. Optimal Sequencing and Predictive Biomarkers in Patients with Advanced Prostate Cancer. Cancers 2021, 13, 4522. [Google Scholar] [CrossRef]
- Gourdin, T.S.; Lilly, M.B.; Hussain, A.; Savage, S.; Clarke, H.S.; Sion, A.M.; Grubb, R.; Sellman, D.; Dincman, T.; Mikoll, J. Preliminary results from a phase II trial of docetaxel before castration with degarelix in men with newly diagnosed metastatic prostate cancer. J. Clin. Oncol. 2021, 39, 116. [Google Scholar] [CrossRef]
- Thompson, I.M. Flare Associated with LHRH-Agonist Therapy. Rev. Urol. 2001, 3 (Suppl. 3), S10–S14. [Google Scholar]
- Verhelst, J.; Denis, L.; Van Vliet, P.; Van Poppel, H.; Braeckman, J.; Van Cangh, P.; Mattelaer, J.; D’Hulster, O.; Mahler, C. Endocrine profiles during administration of the new non-steroidal anti-androgen Casodex in prostate cancer. Clin. Endocrinol. 1994, 41, 525–530. [Google Scholar] [CrossRef]
- Migliari, R.; Muscas, G.; Melis, M.; Garau, M.; Sorgia, M.; Scarpa, R.M.; Usai, E. Monitoring of erection function in patients with prostatic carcinoma treated with Casodex. Arch. Ital Urol. Nefrol. 1991, 63, 155–161. [Google Scholar]
- McLEOD, D.G.; Iversen, P.; See, W.A.; Morris, T.; Armstrong, J.; Wirth, M.P.; Group, C.E.P.C.T. Bicalutamide 150 mg plus standard care vs standard care alone for early prostate cancer. BJU Int. 2006, 97, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Iversen, P. Antiandrogen monotherapy: Indications and results. Urology 2002, 60, 64–71. [Google Scholar] [CrossRef]
- Fizazi, K.; Galceran, J.C.; Foulon, S.; Roubaud, G.; McDermott, R.; Fléchon, A.; Tombal, B.; Supiot, S.; Berthold, D.; Ronchin, P. LBA5 A phase III trial with a 2 × 2 factorial design in men with de novo metastatic castration-sensitive prostate cancer: Overall survival with abiraterone acetate plus prednisone in PEACE-1. Ann. Oncol. 2021, 32, S1299. [Google Scholar] [CrossRef]
- Mout, L.; de Wit, R.; Stuurman, D.; Verhoef, E.; Mathijssen, R.; de Ridder, C.; Lolkema, M.; van Weerden, W. Testosterone Diminishes Cabazitaxel Efficacy and Intratumoral Accumulation in a Prostate Cancer Xenograft Model. EBioMedicine 2018, 27, 182–186. [Google Scholar] [CrossRef]
- Markham, A. Relugolix: First global approval. Drugs 2019, 79, 675–679. [Google Scholar] [CrossRef]
- Steinberg, M. Degarelix: A gonadotropin-releasing hormone antagonist for the management of prostate cancer. Clin. Ther. 2009, 31, 2312–2331. [Google Scholar] [CrossRef] [PubMed]
- Asakawa, J.; Iguchi, T.; Tamada, S.; Yasuda, S.; Ninomiya, N.; Kato, M.; Yamasaki, T.; Ohmachi, T.; Nakatani, T. A change from gonadotropin releasing hormone antagonist to gonadotropin releasing hormone agonist therapy does not affect the oncological outcomes in hormone sensitive prostate cancer. Basic Clin. Androl. 2018, 28, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, O.S.; Nyquist, M.D.; Schweizer, M.T.; Balk, S.P.; Corey, E.; Plymate, S.; Nelson, P.S.; Mostaghel, E.A. Supraphysiologic Testosterone Therapy in the Treatment of Prostate Cancer: Models, Mechanisms and Questions. Cancers 2017, 9, 166. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Mendonca, J.; Owoyemi, O.; Boyapati, K.; Thomas, N.; Kanacharoen, S.; Coffey, M.; Topiwala, D.; Gomes, C.; Ozbek, B. Supraphysiologic Testosterone Induces Ferroptosis and Activates Immune Pathways through Nucleophagy in Prostate Cancer. Cancer Res. 2021, 81, 5948–5962. [Google Scholar] [CrossRef] [PubMed]
- Nordeen, S.K.; Su, L.-J.; Osborne, G.A.; Hayman, P.M.; Orlicky, D.J.; Wessells, V.M.; van Bokhoven, A.; Flaig, T.W. Titration of Androgen Signaling: How Basic Studies Have Informed Clinical Trials Using High-Dose Testosterone Therapy in Castrate-Resistant Prostate Cancer. Life 2021, 11, 884. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.C.; Ro, J.; André, F.; Loi, S.; Verma, S.; Iwata, H.; Harbeck, N.; Loibl, S.; Huang Bartlett, C.; Zhang, K.; et al. Palbociclib in Hormone-Receptor–Positive Advanced Breast Cancer. N. Engl. J. Med. 2015, 373, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, R.L. The endocrinology of the menstrual cycle. In Human Fertility; Springer: Berlin/Heidelberg, Germany, 2014; pp. 145–169. [Google Scholar]
| Characteristic | Gap between LHRH and Docetaxel Intiation 1–6 Days (n = 18) | Gap between LHRH and Docetaxel Intiation >14 Days (n = 297) | p-Value |
|---|---|---|---|
| Age (yrs.) Average | 63.9 | 63.4 | 0.81 |
| Median | 64 | 64 | |
| Range | 47–73 | 42–83 | |
| Race—no. (%) | 0.81 | ||
| Black | 1 (6%) | 28 (9%) | |
| White | 16 (88%) | 258 (87%) | |
| Other | 0 (0%) | 3 (1%) | |
| Unknown | 1 (6%) | 8 (3%) | |
| Volume of metastases—no. (%) | 0.07 | ||
| Low | 9 (50%) | 85 (29%) | |
| High | 9 (50%) | 212 (71%) | |
| ECOG performance status—no. (%) | 0.21 | ||
| 0 | 14 (78%) | 204 (69%) | |
| 1 | 3 (17%) | 89 (30%) | |
| 2 | 1 (5%) | 4 (1%) | |
| Gleason score | 0.11 | ||
| 6 | 0 (0%) | 14 (5%) | |
| 7 | 8 (44%) | 66 (22%) | |
| 8 | 2 (11%) | 61 (21%) | |
| 9 | 5 (28%) | 101 (34%) | |
| 10 | 0 (0%) | 28 (9%) | |
| Unknown | 3 (17%) | 27 (9%) | |
| PSA level at start of ADT—ng/mL | 0.0004 | ||
| Median | 10.35 | 60.25 | |
| Range | 0.4–859 | 0.4–8540 | |
| Prior treatment for prostate cancer—no. (%) | 0.004 | ||
| No local therapy | 8 (44%) | 232 (78%) | |
| Primary radiation | 3 (17%) | 16 (5%) | |
| Prostatectomy | 7 (39%) | 49 (17%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasser, N.J.; Sun, K.; Scanlon, K.M.; Mishra, M.V.; Molitoris, J.K. Administering Docetaxel for Metastatic Hormone-Sensitive Prostate Cancer 1–6 Days Compared to More Than 14 Days after the Start of LHRH Agonist Is Associated with Better Clinical Outcomes Due to Androgen Flare. Cancers 2022, 14, 864. https://doi.org/10.3390/cancers14040864
Nasser NJ, Sun K, Scanlon KM, Mishra MV, Molitoris JK. Administering Docetaxel for Metastatic Hormone-Sensitive Prostate Cancer 1–6 Days Compared to More Than 14 Days after the Start of LHRH Agonist Is Associated with Better Clinical Outcomes Due to Androgen Flare. Cancers. 2022; 14(4):864. https://doi.org/10.3390/cancers14040864
Chicago/Turabian StyleNasser, Nicola J., Kai Sun, Karen M. Scanlon, Mark V. Mishra, and Jason K. Molitoris. 2022. "Administering Docetaxel for Metastatic Hormone-Sensitive Prostate Cancer 1–6 Days Compared to More Than 14 Days after the Start of LHRH Agonist Is Associated with Better Clinical Outcomes Due to Androgen Flare" Cancers 14, no. 4: 864. https://doi.org/10.3390/cancers14040864
APA StyleNasser, N. J., Sun, K., Scanlon, K. M., Mishra, M. V., & Molitoris, J. K. (2022). Administering Docetaxel for Metastatic Hormone-Sensitive Prostate Cancer 1–6 Days Compared to More Than 14 Days after the Start of LHRH Agonist Is Associated with Better Clinical Outcomes Due to Androgen Flare. Cancers, 14(4), 864. https://doi.org/10.3390/cancers14040864





