Interstitial Control-Released Polymer Carrying a Targeting Small-Molecule Drug Reduces PD-L1 and MGMT Expression in Recurrent High-Grade Gliomas with TMZ Resistance
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
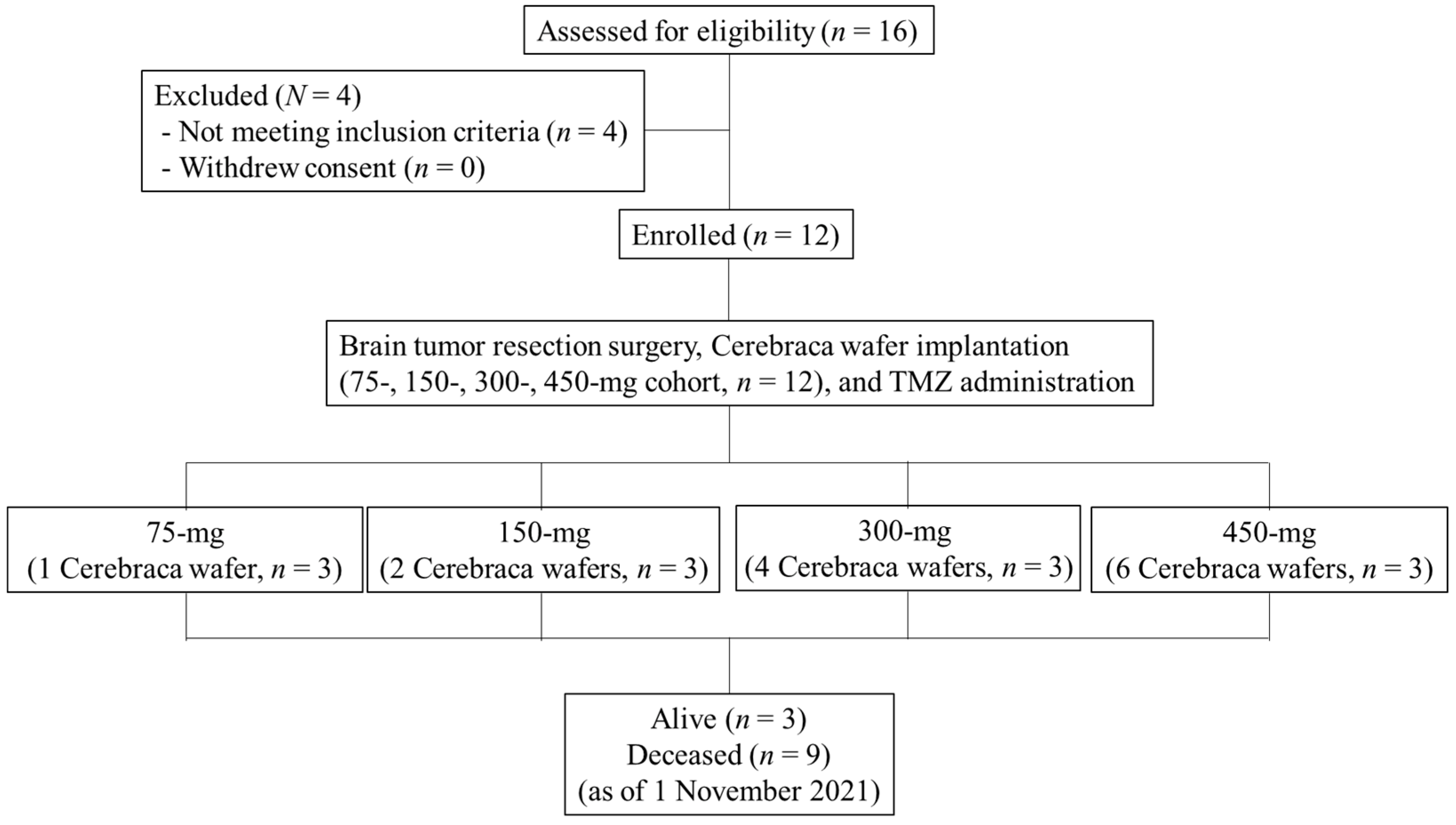
2.1. Study Design
- Female or male, age ≥20 years old.
- Patient has diagnosed recurrent high grade glioma, including anaplastic astrocytoma and glioblastoma multiforme.
- Patient has unilateral tumor in cerebrum that can be excised in one operation.
- Patient has recurrence of glioma.
- Patient has undergone standard therapy for their prior glioma episode; for patients with anaplastic astrocytoma, the prior standard therapy should include surgical resection, radiation and adjuvant temozolomide (or PCV [procarzine, lomustine and vincristine]); for patients with glioblastoma multiforme, the prior standard therapy should include surgical resection, radiation and adjuvant temozolomide.
- Patient has a Karnofsky Performance Score (KPS) ≥50.
- Patient is recovered from toxicities from prior systemic therapies and has adequate hematopoietic function at screening and before using study medication.
- Patient with no or mild organ impairment.
- Patient agrees not to use food or dietary supplements that contain Angelica sinensis from Screening Visit to Day 21.
- All male patients and female patients with child-bearing potential (between puberty and two years after menopause) should use appropriate contraception method(s) for at least four weeks after Cerebraca wafer treatment and TMZ treatment (whichever is longer).
- Patient has participated in other investigational studies within four weeks prior to receiving Cerebraca wafer.
- Patient with known or suspected hypersensitivity to Cerebraca wafer, TMZ or the excipient.
- Patient has tumor that cannot be surgically removed without significantly affecting vital function.
- Patient has external-beam radiation therapy within four weeks before study entry.
- Patient has immuno-compromised condition, or has a known autoimmune condition, or is human immunodeficiency virus (HIV) seropositive.
- Patient has on-going moderate to severe organ impairment other than study indication that may confound the efficacy evaluation, safety evaluation or usage of TMZ.
2.2. Imaging
2.3. Safety Evaluation
2.4. Patient-Derived Primary Tumor Cultures
2.5. RNA Extraction and Reverse Transcription from Total RNA
2.6. Real-Time Polymerase Chain Reaction (Real-Time PCR) Using SYBR Green
2.7. Flow Cytometry
2.8. IFN-γ ELISA Assay
2.9. MGMT Promoter Methylation Determination
3. Results
3.1. Patient Characteristics
3.2. Treatment Program
3.3. Cerebraca Wafer Implantation Demonstrated a Good Safety Profile
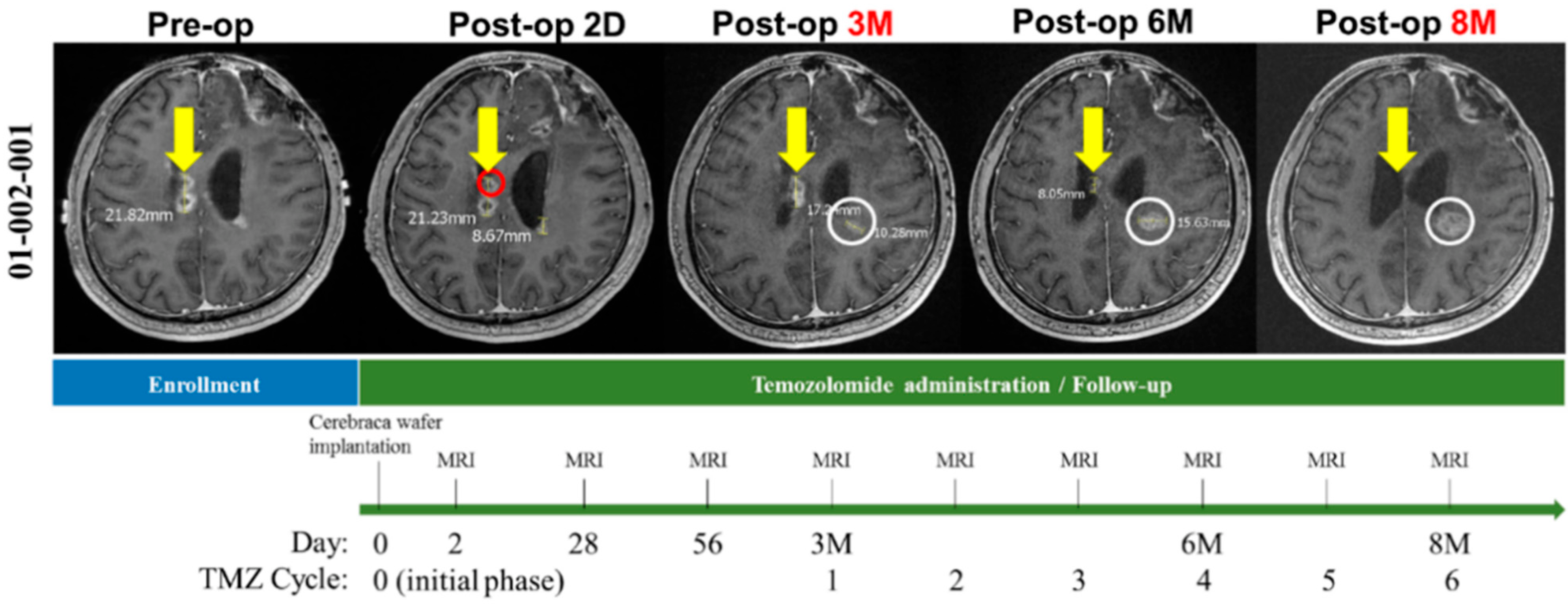
3.4. Cerebraca Wafer Implantation plus TMZ Administration Resulted in Residual Tumor Shrinkage after Surgical Resection
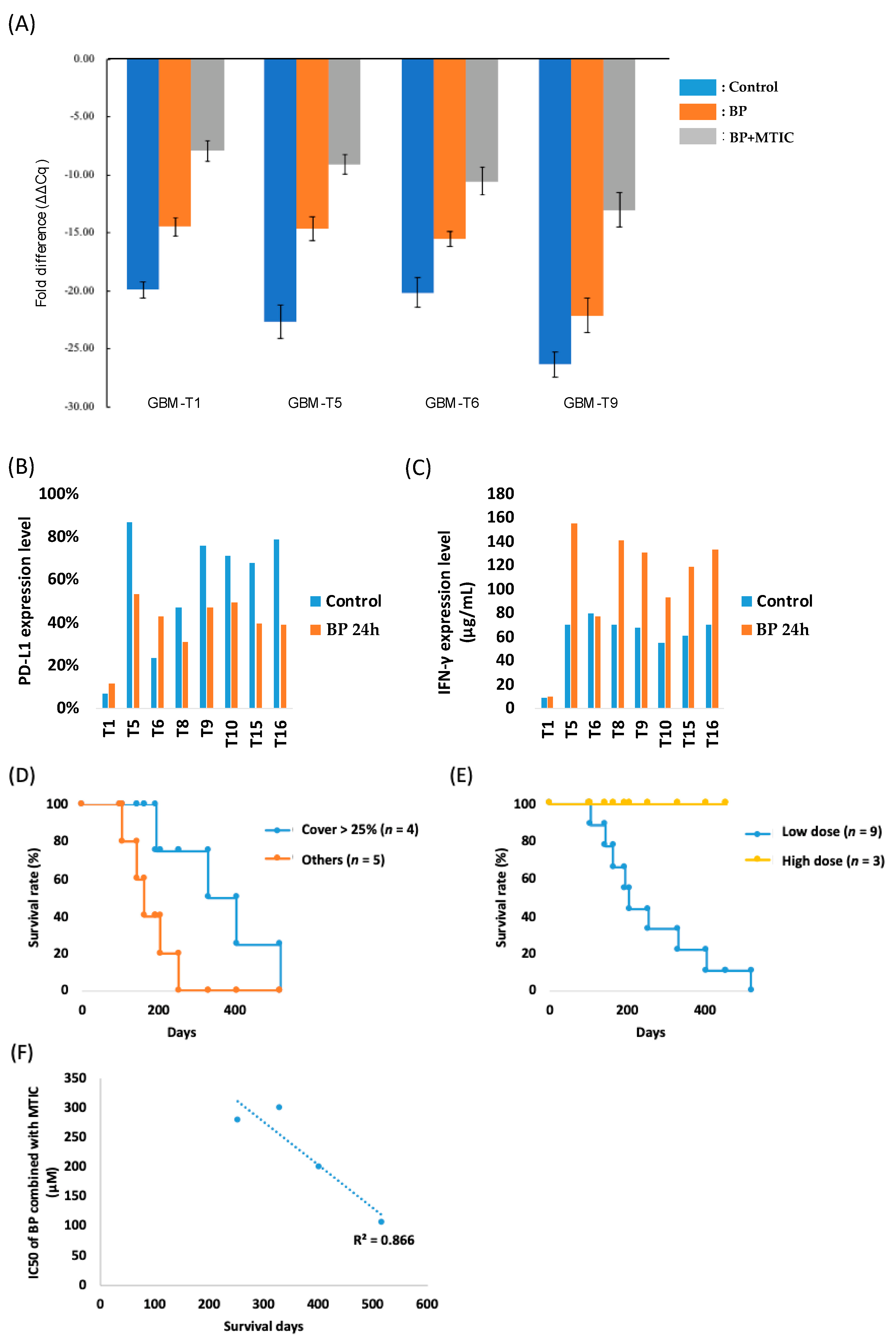
3.5. Molecular Analysis of Primary Glioma Cell Lines Obtained from Surgery Revealed the Beneficial Effects of Cerebraca Wafer
3.6. Cerebraca Wafer Reduced the Cancer Stem Cell Percentage in Recurrent GBM
3.7. Cerebraca Wafer Provides a Synergistic Effect with the Alkylating Agents and Reduces Their Drug Resistance
3.8. Preliminary Efficacy Analyses Suggest That Cerebraca Wafer Improved OS
3.9. Overall Survival Is Correlated with the Combination Effect of BP and TMZ
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hartenstein, R. Medical oncology—Current treatment options. Krankenpflege 1985, 39, 400. [Google Scholar] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Feldheim, J.; Kessler, A.F.; Monoranu, C.M.; Ernestus, R.I.; Löhr, M.; Hagemann, C. Changes of O6-Methylguanine DNA Methyltransferase (MGMT) Promoter Methylation in Glioblastoma Relapse—A Meta-Analysis Type Literature Review. Cancers 2019, 11, 1837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, R.; Chen, X.; You, Y.; Wang, X.; Liu, Y.; Hu, Q.; Yan, W. Comprehensive portrait of recurrent glioblastoma multiforme in molecular and clinical characteristics. Oncotarget 2015, 6, 30968–30974. [Google Scholar] [CrossRef] [Green Version]
- Smrdel, U.; Popovic, M.; Zwitter, M.; Bostjancic, E.; Zupan, A.; Kovac, V.; Glavac, D.; Bokal, D.; Jerebic, J. Long-term survival in glioblastoma: Methyl guanine methyl transferase (MGMT) promoter methylation as independent favourable prognostic factor. Radiol. Oncol. 2016, 50, 394–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, R.Q.; Liu, F.; Qiu, X.Y.; Chen, X.Q. The Prognostic and Therapeutic Value of PD-L1 in Glioma. Front. Pharmacol. 2018, 9, 1503. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Guo, G.; Guan, H.; Yu, Y.; Lu, J.; Yu, J. Challenges and potential of PD-1/PD-L1 checkpoint blockade immunotherapy for glioblastoma. J. Exp. Clin. Cancer Res. 2019, 38, 87. [Google Scholar] [CrossRef] [Green Version]
- Tomaszewski, W.; Sanchez-Perez, L.; Gajewski, T.F.; Sampson, J.H. Brain Tumor Microenvironment and Host State: Implications for Immunotherapy. Clin. Cancer Res. 2019, 25, 4202–4210. [Google Scholar] [CrossRef] [Green Version]
- Nduom, E.K.; Wei, J.; Yaghi, N.K.; Huang, N.; Kong, L.Y.; Gabrusiewicz, K.; Ling, X.; Zhou, S.; Ivan, C.; Chen, J.Q.; et al. PD-L1 expression and prognostic impact in glioblastoma. Neuro-Oncology 2016, 18, 195–205. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Zhang, C.B.; Liu, X.; Wang, Z.L.; Sun, L.H.; Li, G.Z.; Liang, J.S.; Hu, H.M.; Liu, Y.W.; Zhang, W.; et al. Molecular and clinical characterization of PD-L1 expression at transcriptional level via 976 samples of brain glioma. OncoImmunology 2016, 5, e1196310. [Google Scholar] [CrossRef] [Green Version]
- Berghoff, A.S.; Kiesel, B.; Widhalm, G.; Rajky, O.; Ricken, G.; Wöhrer, A.; Dieckmann, K.; Filipits, M.; Brandstetter, A.; Weller, M.; et al. Programmed death ligand 1 expression and tumor-infiltrating lymphocytes in glioblastoma. Neuro-Oncology 2015, 17, 1064–1075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, J.; Zhang, X.K.; Chen, H.D.; Zhong, Z.H.; Wu, Q.L.; Lin, S.X. Expression of programmed cell death-ligand 1 and its correlation with clinical outcomes in gliomas. Oncotarget 2016, 7, 8944–8955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perry, J.; Chambers, A.; Spithoff, K.; Laperriere, N. Gliadel wafers in the treatment of malignant glioma: A systematic review. Curr. Oncol. 2007, 14, 189–194. [Google Scholar] [CrossRef] [Green Version]
- Su, Q.; Zhao, A.; Peng, H.; Zhou, S. Preparation and characterization of biodegradable electrospun polyanhydride nano/microfibers. J. Nanosci. Nanotechnol. 2010, 10, 6369–6375. [Google Scholar] [CrossRef]
- Brem, H.; Tamargo, R.J.; Olivi, A.; Pinn, M.; Weingart, J.D.; Wharam, M.; Epstein, J.I. Biodegradable polymers for controlled delivery of chemotherapy with and without radiation therapy in the monkey brain. J. Neurosurg. 1994, 80, 283–290. [Google Scholar] [CrossRef]
- Tamargo, R.J.; Epstein, J.I.; Reinhard, C.S.; Chasin, M.; Brem, H. Brain biocompatibility of a biodegradable, controlled-release polymer in rats. J. Biomed. Mater. Res. 1989, 23, 253–266. [Google Scholar] [CrossRef]
- Westphal, M.; Hilt, D.C.; Bortey, E.; Delavault, P.; Olivares, R.; Warnke, P.C.; Whittle, I.R.; Jaaskelainen, J.; Ram, Z. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro-Oncology 2003, 5, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Yen, S.Y.; Harn, H.J.; Lin, S.Z.; Chen, S.R.; Lin, P.C.; Syu, F.J.; Hsieh, D.K.; Huang, M.H.; Chiou, T.W. (Z)-butylidenephthalide Restores Temozolomide Sensitivity to Temozolomide-resistant Malignant Glioma Cells by Downregulating Expression of the DNA Repair Enzyme MGMT. Eur. J. Cancer 2012, 48, S220–S221. [Google Scholar] [CrossRef]
- Harn, H.J.; Lin, S.Z.; Lin, P.C.; Liu, C.Y.; Liu, P.Y.; Chang, L.F.; Yen, S.Y.; Hsieh, D.K.; Liu, F.C.; Tai, D.F.; et al. Local interstitial delivery of z-butylidenephthalide by polymer wafers against malignant human gliomas. Neuro-Oncology 2011, 13, 635–648. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, C.; Jacobsson, H.; Hatschek, T.; Torkzad, M.R.; Boden, K.; Eriksson-Alm, Y.; Berg, E.; Fujii, H.; Kubo, A.; Blomqvist, L. Radiologic measurements of tumor response to treatment: Practical approaches and limitations. Radiographics 2008, 28, 329–344. [Google Scholar] [CrossRef] [Green Version]
- Delaney, C.; Garg, S.K.; Yung, R. Analysis of DNA Methylation by Pyrosequencing. Methods Mol. Biol. 2015, 1343, 249–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, E.T.; Gautam, S.; Malchow, C.; Lun, M.; Pan, E.; Brem, S. Bevacizumab for recurrent glioblastoma multiforme: A meta-analysis. J. Natl. Compr. Cancer Netw. 2011, 9, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.Y.; Sheu, J.J.; Lin, P.C.; Lin, C.T.; Liu, Y.J.; Ho, L.I.; Chang, L.F.; Wu, W.C.; Chen, S.R.; Chen, J.; et al. Expression of Nur77 induced by an N-butylidenephthalide derivative promotes apoptosis and inhibits cell growth in oral squamous cell carcinoma. Investig. New Drugs 2012, 30, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Kleinberg, L.R.; Weingart, J.; Burger, P.; Carson, K.; Grossman, S.A.; Li, K.; Olivi, A.; Wharam, M.D.; Brem, H. Clinical course and pathologic findings after Gliadel and radiotherapy for newly diagnosed malignant glioma: Implications for patient management. Cancer Investig. 2004, 22, 1–9. [Google Scholar] [CrossRef]
- Weber, E.L.; Goebel, E.A. Cerebral edema associated with Gliadel wafers: Two case studies. Neuro-Oncology 2005, 7, 84–89. [Google Scholar] [CrossRef]
- Kuramitsu, S.; Motomura, K.; Natsume, A.; Wakabayashi, T. Double-edged Sword in the Placement of Carmustine (BCNU) Wafers along the Eloquent Area: A Case Report. NMC Case Rep. J. 2015, 2, 40–45. [Google Scholar] [CrossRef] [Green Version]
- Auffinger, B.; Tobias, A.L.; Han, Y.; Lee, G.; Guo, D.; Dey, M.; Lesniak, M.S.; Ahmed, A.U. Conversion of differentiated cancer cells into cancer stem-like cells in a glioblastoma model after primary chemotherapy. Cell Death Differ. 2014, 21, 1119–1131. [Google Scholar] [CrossRef]
- Peng, L.; Fu, J.; Wang, W.; Hofman, F.M.; Chen, T.C.; Chen, L. Distribution of cancer stem cells in two human brain gliomas. Oncol. Lett. 2019, 17, 2123–2130. [Google Scholar] [CrossRef]
- Quinn, J.A.; Jiang, S.X.; Carter, J.; Reardon, D.A.; Desjardins, A.; Vredenburgh, J.J.; Rich, J.N.; Gururangan, S.; Friedman, A.H.; Bigner, D.D.; et al. Phase II trial of Gliadel plus O6-benzylguanine in adults with recurrent glioblastoma multiforme. Clin. Cancer Res. 2009, 15, 1064–1068. [Google Scholar] [CrossRef] [Green Version]
- Davis, M.E. Glioblastoma: Overview of Disease and Treatment. Clin. J. Oncol. Nurs. 2016, 20, S2–S8. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Zhen, Z.; Na, J.; Wang, Q.; Gao, L.; Yuan, Y. Associations of therapeutic hypothermia with clinical outcomes in patients receiving ECPR after cardiac arrest: Systematic review with meta-analysis. Scand. J. Trauma Resusc. Emerg. Med. 2020, 28, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owen, K.L.; Brockwell, N.K.; Parker, B.S. JAK-STAT Signaling: A Double-Edged Sword of Immune Regulation and Cancer Progression. Cancers 2019, 11, 2002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, P.C.; Chen, Y.L.; Chiu, S.C.; Yu, Y.L.; Chen, S.P.; Chien, M.H.; Chen, K.Y.; Chang, W.L.; Lin, S.Z.; Chiou, T.W.; et al. Orphan nuclear receptor, Nurr-77 was a possible target gene of butylidenephthalide chemotherapy on glioblastoma multiform brain tumor. J. Neurochem. 2008, 106, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.C.; Lin, S.Z.; Chen, Y.L.; Chang, J.S.; Ho, L.I.; Liu, P.Y.; Chang, L.F.; Harn, Y.C.; Chen, S.P.; Sun, L.Y.; et al. Butylidenephthalide suppresses human telomerase reverse transcriptase (TERT) in human glioblastomas. Ann. Surg. Oncol. 2011, 18, 3514–3527. [Google Scholar] [CrossRef]
- Huang, M.H.; Lin, S.Z.; Lin, P.C.; Chiou, T.W.; Harn, Y.W.; Ho, L.I.; Chan, T.M.; Chou, C.W.; Chuang, C.H.; Su, H.L.; et al. Brain tumor senescence might be mediated by downregulation of S-phase kinase-associated protein 2 via butylidenephthalide leading to decreased cell viability. Tumor Biol. 2014, 35, 4875–4884. [Google Scholar] [CrossRef] [PubMed]
- Yen, S.Y.; Chen, S.R.; Hsieh, J.; Li, Y.S.; Chuang, S.E.; Chuang, H.M.; Huang, M.H.; Lin, S.Z.; Harn, H.J.; Chiou, T.W. Biodegradable interstitial release polymer loading a novel small molecule targeting Axl receptor tyrosine kinase and reducing brain tumour migration and invasion. Oncogene 2016, 35, 2156–2165. [Google Scholar] [CrossRef] [Green Version]
- Yen, S.Y.; Chuang, H.M.; Huang, M.H.; Lin, S.Z.; Chiou, T.W.; Harn, H.J. n-Butylidenephthalide Regulated Tumor Stem Cell Genes EZH2/AXL and Reduced Its Migration and Invasion in Glioblastoma. Int. J. Mol. Sci. 2017, 18, 372. [Google Scholar] [CrossRef] [Green Version]
- Elashi, A.A.; Sasidharan Nair, V.; Taha, R.Z.; Shaath, H.; Elkord, E. DNA methylation of immune checkpoints in the peripheral blood of breast and colorectal cancer patients. Oncoimmunology 2019, 8, e1542918. [Google Scholar] [CrossRef] [Green Version]
- Lu, K.Y.; Primus Dass, K.T.; Lin, S.Z.; Tseng, Y.H.; Liu, S.P.; Harn, H.J. N-butylidenephthalide ameliorates high-fat diet-induced obesity in mice and promotes browning through adrenergic response/AMPK activation in mouse beige adipocytes. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 159033. [Google Scholar] [CrossRef]
- Huang, M.H.; Chou, Y.W.; Li, M.H.; Shih, T.E.; Lin, S.Z.; Chuang, H.M.; Chiou, T.W.; Su, H.L.; Harn, H.J. Epigenetic targeting DNMT1 of pancreatic ductal adenocarcinoma using interstitial control release biodegrading polymer reduced tumor growth through hedgehog pathway inhibition. Pharmacol. Res. 2019, 139, 50–61. [Google Scholar] [CrossRef]
| Variables | Cerebraca Wafer (75 mg API/300 mg Excipient) Treatment Groups | ||
|---|---|---|---|
| 75–150 mg API n = 6 | 300 mg API n = 3 | 450 mg API n = 3 | |
| Age (years) | |||
| mean (min-max) | 51.7 (37.9–68.9) | 40.3 (27.0–51.1) | 55.7 (50.1–65.5) |
| Gender | |||
| male:female | 5:1 | 2:1 | 2:1 |
| Time from diagnosis (months) | |||
| Mean (min-max) | 17.0 (4.5–24.1) | 15.7 (9.1–25.7) | 26.8 (10.4–43.8) |
| Recurrence | |||
| First | 4 | 3 | 2 |
| Second | 2 | 0 | 0 |
| Third or more | 0 | 0 | 1 |
| Prior treatment lines (mean) | 3.5 | 3.3 | 3.7 |
| Prior bevacizumab (Yes:No) | 1:5 | 1:2 | 0:3 |
| Grade IV:Grade III | |||
| At screening | 4:2 | 3:0 | 2:1 |
| At study entry | 5:1 | 3:0 | 3:0 |
| Resection rate | |||
| >95% | 2/6 | 1/3 | 2/3 |
| >75% | 4/6 | 1/3 | 2/3 |
| ≤75% | 2/6 | 2/3 | 1/3 |
| Biopsy | 1/6 | 1/3 | 0/3 |
| wafer coverage rate | |||
| >25% (exclude 95% resection) | 3/4 | 2/2 | 1/1 |
| Molecular subtype | |||
| Classical (EGFR) | 3 | 0 | 2 |
| Mesenchymal (NF1) | 1 | 3 | 1 |
| Neural (NEFL) | 1 | 0 | 0 |
| Proneural (IDHR132H) | 1 | 0 | 0 |
| MGMT promoter methylation | |||
| Unmethylated:Methylated | 5:1 | 3:0 | 2:1 |
| KPS (mean, min-max) | |||
| At screening | 81.7 (73.1–90.3) | 90.0 (90.0–90.0) | 80.0 (71.3–88.7) |
| At Day 28 (±1 day) | 73.3 (62.0–84.6) | 86.7 (83.8–89.6) | 70.0 (60.0–80.0) |
| QLQ-C30 (mean, min-max) | |||
| Health status | |||
| At screening | 45.8 (36.4–55.2) | 75.0 (70.8–79.2) | 55.6 (34.6–76.6) |
| At Day 28 (±1 day) | 59.7 (47.2–72.2) | 55.6 (46.0–65.2) | 61.1 (41.9–80.3) |
| Functional scales | |||
| At screening | 67.8 (52.4–83.2) | 85.2 (80.1–90.3) | 57.0 (39.7–74.3) |
| At Day 28 (±1 day) | 61.5 (48.5–74.5) | 80.7 (74.6–86.8) | 46.7 (27.4–66.0) |
| Symptom scales | |||
| At screening | 14.5 (10.1–18.9) | 08.5 (07.0–10.0) | 29.9 (27.2–32.6) |
| At Day 28 (±1 day) | 22.6 (17.7–27.5) | 18.8 (12.2–25.4) | 20.5 (13.4–27.6) |
| Steroid use (Yes:No:Unknown) | |||
| At study entry | 1:5:0 | 0:3:0 | 0:3:0 |
| At Day 0–21 | 3:3:0 | 1:2:0 | 0:3:0 |
| Event term | n (ratio) | n (ratio) | n (ratio) |
| ≥Grade 3 AE | 2/6 | 1/3 | 1/3 |
| Liver function abnormal | 1/6 | ||
| Wound complication | 1/6 | ||
| Sepsis | 1/3 | ||
| Lung infection | 1/3 | ||
| ≥Grade 3 neurologic AE | 0/6 | 1/3 | 1/3 |
| CSF leakage | 1/3 | ||
| Hydrocephalus | 1/3 | ||
| Incidence of SAE | 1/6 | 2/3 | 3/3 |
| Wound complication | 1/6 | ||
| Spinal compression fracture | 1/3 | ||
| Sepsis | 1/3 | ||
| Lung infection | 1/3 | ||
| CSF leakage | 1/3 | ||
| Hydrocephalus | 1/3 | ||
| Survival status | n (ratio) | n (ratio) | n (ratio) |
| OS | |||
| At 6M | 5/6 | 1/3 | 3/3 |
| At 9M | 3/6 | 0/3 | 3/3 |
| At 12M | 2/6 | 0/3 | 3/3 |
| PFS | |||
| At 6M | 2/6 | 1/3 | 3/3 |
| At 9M | 0/6 | 0/3 | 1/3 |
| At 12M | 0/6 | 0/3 | 1/3 |
| Sample ID | T1 | T5 | T6 | T8 | T9 | T10 | T15 | T16 |
|---|---|---|---|---|---|---|---|---|
| Cohort | Cohort I | Cohort II | Cohort III | Cohort IV | ||||
| CD133 | 0.29% | 88.12% | 94.26% | 91.81% | 92.40% | 89.70% | 84.28% | 91.20% |
| SOX2 | 0.50% | 93.20% | 90.75% | 89.87% | 94.26% | 92.45% | 80.40% | 89.62% |
| BCNU IC50 | 1200 μM | 2000 μM | >2000 μM | 2000 μM | >2000 μM | >2000 μM | 1800 μM | 1600 μM |
| BP IC50 | 300 μM | 400 μM | 420 μM | 400 μM | 600μM | 410 μM | 390 μM | 405 μM |
| MGMT unmethylation | + | + | + | − | + | + | + | + |
| MTIC IC50 | 300 μM | 700 μM | 300 μM | 400 μM | >800 μM | 375 μM | 500 μM | 460 μM |
| BP + MTIC IC50 | 105 μM | 200 μM | 200 μM | 300 μM | 280 μM | 250 μM | 300 μM | 250 μM |
| BP 8 h + MTIC IC50 | 100 μM | 225 μM | 155 μM | 150 μM | 275 μM | 260 μM | 200 μM | 190 μM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.-A.; Liu, W.-H.; Ma, H.-I.; Chen, Y.-H.; Hueng, D.-Y.; Tsai, W.-C.; Lin, S.-Z.; Harn, H.-J.; Chiou, T.-W.; Liu, J.-W.; et al. Interstitial Control-Released Polymer Carrying a Targeting Small-Molecule Drug Reduces PD-L1 and MGMT Expression in Recurrent High-Grade Gliomas with TMZ Resistance. Cancers 2022, 14, 1051. https://doi.org/10.3390/cancers14041051
Liu C-A, Liu W-H, Ma H-I, Chen Y-H, Hueng D-Y, Tsai W-C, Lin S-Z, Harn H-J, Chiou T-W, Liu J-W, et al. Interstitial Control-Released Polymer Carrying a Targeting Small-Molecule Drug Reduces PD-L1 and MGMT Expression in Recurrent High-Grade Gliomas with TMZ Resistance. Cancers. 2022; 14(4):1051. https://doi.org/10.3390/cancers14041051
Chicago/Turabian StyleLiu, Ching-Ann, Wei-Hsiu Liu, Hsin-I Ma, Yuan-Hao Chen, Dueng-Yuan Hueng, Wen-Chiuan Tsai, Shinn-Zong Lin, Horng-Jyh Harn, Tzyy-Wen Chiou, Jen-Wei Liu, and et al. 2022. "Interstitial Control-Released Polymer Carrying a Targeting Small-Molecule Drug Reduces PD-L1 and MGMT Expression in Recurrent High-Grade Gliomas with TMZ Resistance" Cancers 14, no. 4: 1051. https://doi.org/10.3390/cancers14041051
APA StyleLiu, C.-A., Liu, W.-H., Ma, H.-I., Chen, Y.-H., Hueng, D.-Y., Tsai, W.-C., Lin, S.-Z., Harn, H.-J., Chiou, T.-W., Liu, J.-W., Lee, J.-H., & Chiu, T.-L. (2022). Interstitial Control-Released Polymer Carrying a Targeting Small-Molecule Drug Reduces PD-L1 and MGMT Expression in Recurrent High-Grade Gliomas with TMZ Resistance. Cancers, 14(4), 1051. https://doi.org/10.3390/cancers14041051







