Validation of IL-7R as an Immunological Biomarker for Human Pancreatic Ductal Adenocarcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients
2.3. Sample Collection
2.4. Isolation of Total RNA from PBMCs
2.5. Quantitative Real Time PCR of IL-7R Gene Expression
2.6. Statistical Analysis
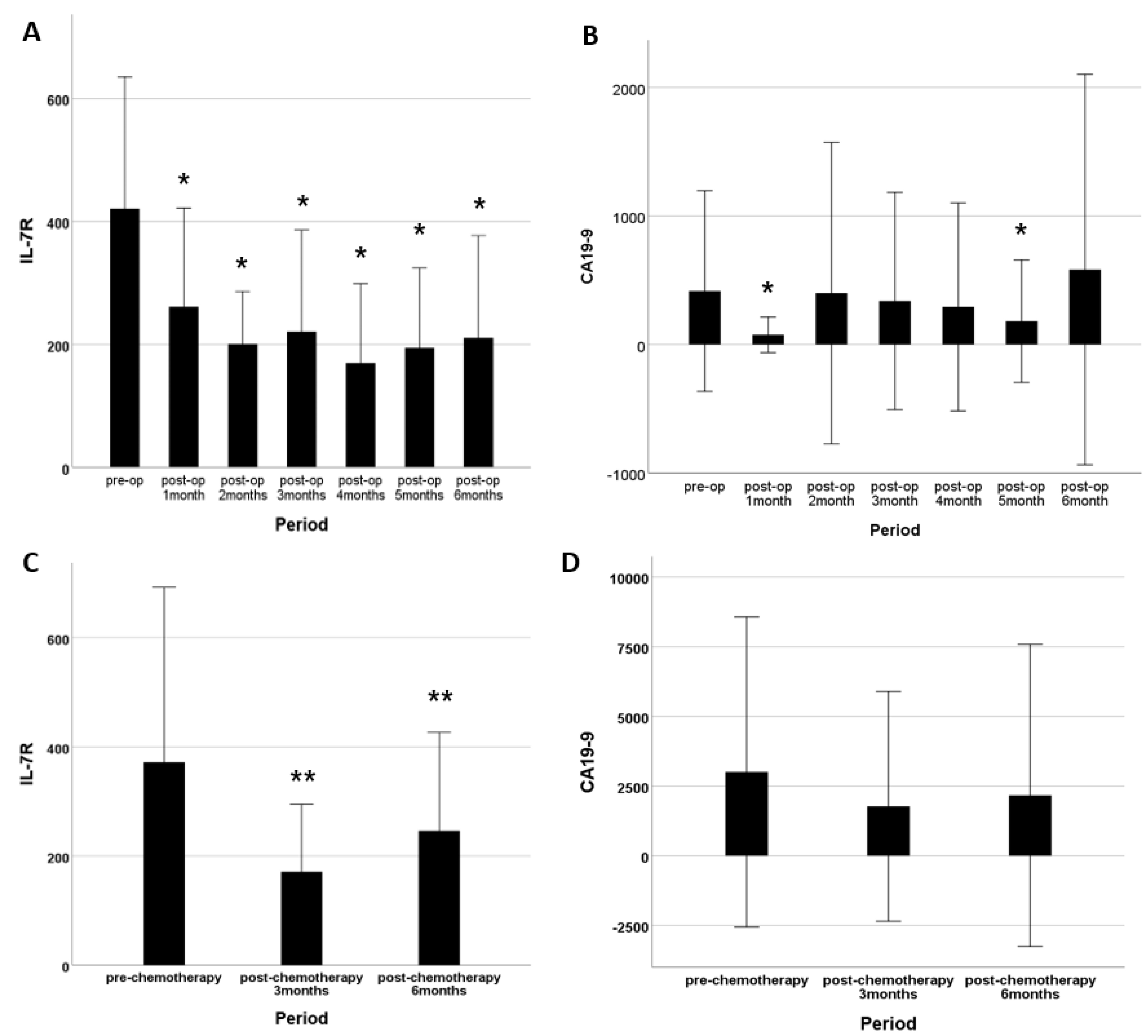
3. Results
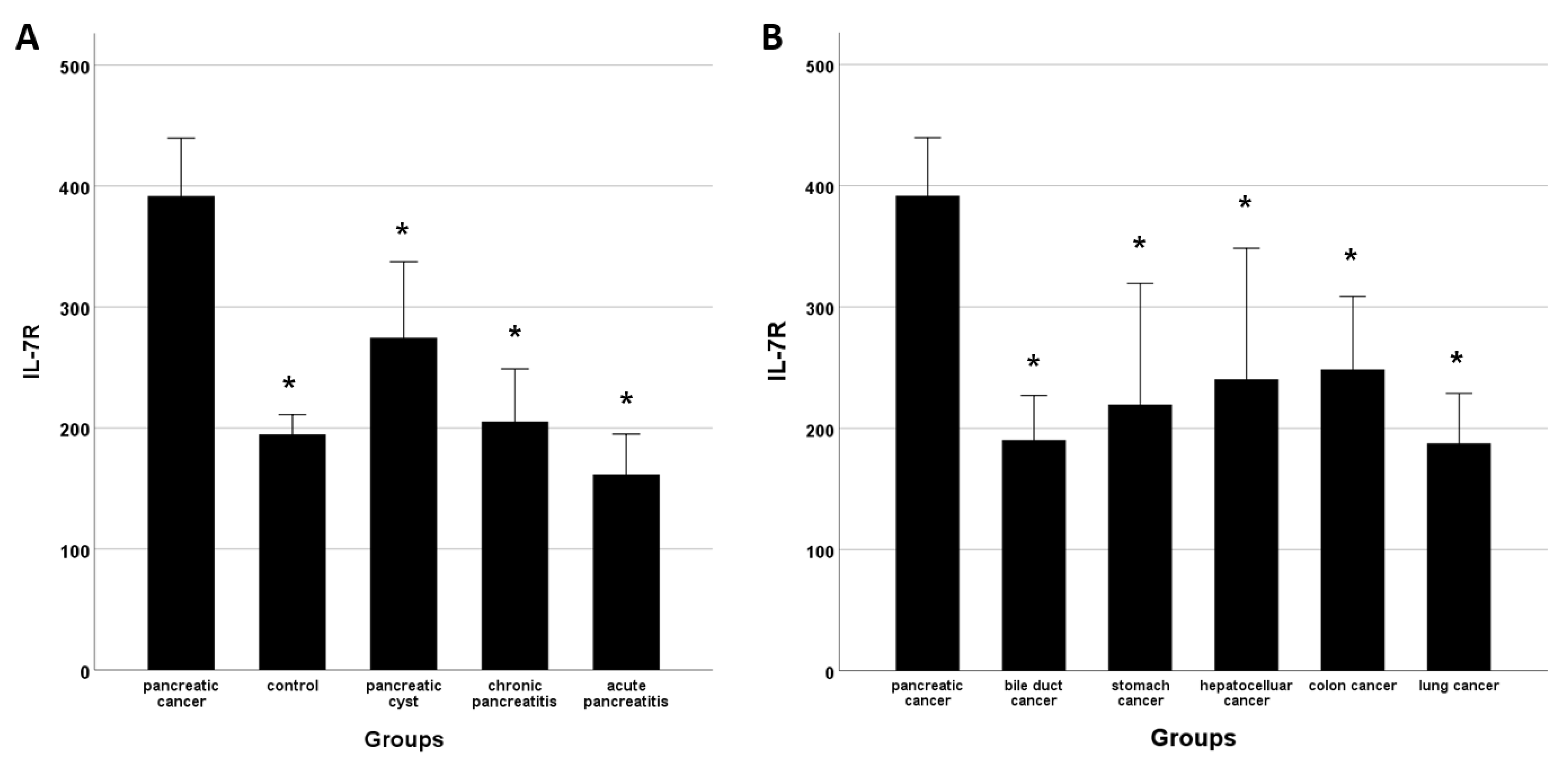
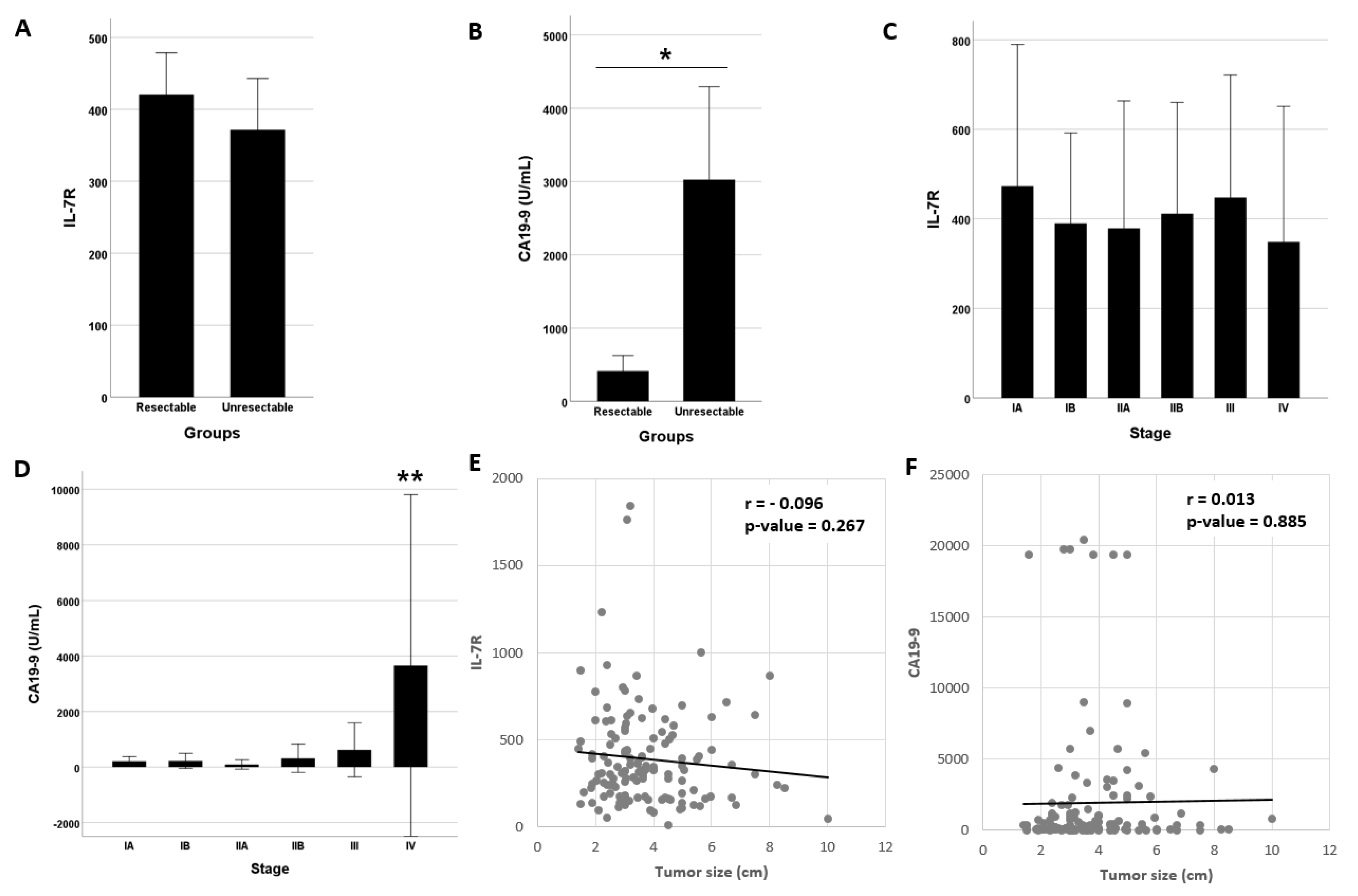
3.1. IL-7R Levels and Subject Characteristics
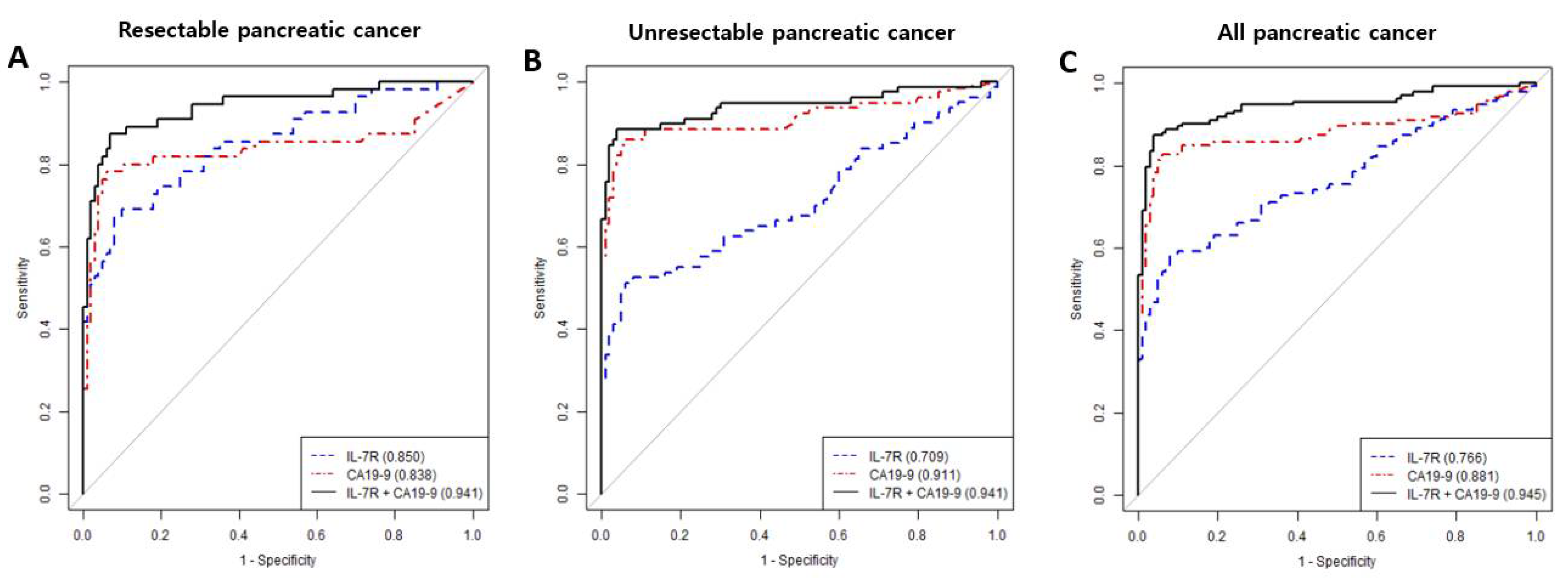
3.2. Diagnostic Performance of IL7-R and/or CA19-9
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paulson, A.S.; Tran Cao, H.S.; Tempero, M.A.; Lowy, A.M. Therapeutic Advances in Pancreatic Cancer. Gastroenterology 2013, 144, 1316–1326. [Google Scholar] [CrossRef] [PubMed]
- Takaori, K.; Bassi, C.; Biankin, A.; Brunner, T.B.; Cataldo, I.; Campbell, F.; Cunningham, D.; Falconi, M.; Frampton, A.E.; Furuse, J.; et al. International association of pancreatology (iap)/european pancreatic club (epc) consensus review of guidelines for the treatment of pancreatic cancer. Pancreatology 2016, 16, 14–27. [Google Scholar] [CrossRef]
- Bellone, G.; Novarino, A.; Vizio, B.; Brondino, G.; Addeo, A.; Prati, A.; Giacobino, A.; Campra, D.; Fronda, G.R.; Ciuffreda, L. Impact of surgery and chemotherapy on cellular immunity in pancreatic carcinoma patients in view of an integration of standard cancer treatment with immunotherapy. Int. J. Oncol. 2009, 34, 1701–1715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neesse, A.; Michl, P.; Frese, K.K.; Feig, C.; Cook, N.; Jacobetz, M.A.; Lolkema, M.P.; Buchholz, M.; Olive, K.P.; Gress, T.M.; et al. Stromal biology and therapy in pancreatic cancer. Gut 2011, 60, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Tsen, A.; Barbara, M.; Rosenkranz, L. Dilemma of elevated CA 19-9 in biliary pathology. Pancreatology 2018, 18, 862–867. [Google Scholar] [CrossRef]
- Kuol, N.; Stojanovska, L.; Apostolopoulos, V.; Nurgali, K. Crosstalk between cancer and the neuro-immune system. J. Neuroimmunol. 2018, 315, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Yako, Y.Y.; Brand, M.; Smith, M.; Kruger, D. Inflammatory cytokines and angiogenic factors as potential biomarkers in South African pancreatic ductal adenocarcinoma patients: A preliminary report. Pancreatology 2017, 17, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Yako, Y.Y.; Kruger, D.; Smith, M.; Brand, M. Cytokines as biomarkers of pancreatic ductal adenocarcinoma: A systematic review. PLoS ONE 2016, 11, e0154016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heo, S.-H.; Jang, S.I.; Kim, S.Y.; Choi, B.; Lee, D.K.; Lee, H.K.; Chang, E.-J. Characterization of Circulating IL-7R Positive Cell Populations for Early Detection of Pancreatic Ductal Adenocarcinoma. J. Clin. Med. 2021, 10, 4157. [Google Scholar] [CrossRef]
- Chun, Y.S.; Pawlik, T.M.; Vauthey, J.N. 8th Edition of the AJCC Cancer Staging Manual: Pancreas and Hepatobiliary Cancers. Ann. Surg. Oncol. 2018, 25, 845–847. [Google Scholar] [CrossRef] [PubMed]
- Lennon, A.M.; Buchanan, A.H.; Kinde, I.; Warren, A.; Honushefsky, A.; Cohain, A.T.; Ledbetter, D.H.; Sanfilippo, F.; Sheridan, K.; Rosica, D.; et al. Feasibility of blood testing combined with PET-CT to screen for cancer and guide intervention. Science 2020, 369, eabb9601. [Google Scholar] [CrossRef] [PubMed]
- André, T.; de Gramont, A.; Vernerey, D.; Chibaudel, B.; Bonnetain, F.; Tijeras-Raballand, A.; Scriva, A.; Hickish, T.; Tabernero, J.; Van Laethem, J.L.; et al. Adjuvant Fluorouracil, Leucovorin, and Oxaliplatin in Stage II to III Colon Cancer: Updated 10-Year Survival and Outcomes According to BRAF Mutation and Mismatch Repair Status of the MOSAIC Study. J. Clin. Oncol. 2015, 33, 4176–4187. [Google Scholar] [CrossRef] [PubMed]
- Nassiri, F.; Chakravarthy, A.; Feng, S.; Shen, S.Y.; Nejad, R.; Zuccato, J.A.; Voisin, M.R.; Patil, V.; Horbinski, C.; Aldape, K.; et al. Detection and discrimination of intracranial tumors using plasma cell-free DNA methylomes. Nat. Med. 2020, 26, 1044–1047. [Google Scholar] [CrossRef] [PubMed]
- Nuzzo, P.V.; Berchuck, J.E.; Korthauer, K.; Spisak, S.; Nassar, A.H.; Alaiwi, S.A.; Chakravarthy, A.; Shen, S.Y.; Bakouny, Z.; Boccardo, F.; et al. Detection of renal cell carcinoma using plasma and urine cell-free DNA methylomes. Nat. Med. 2020, 26, 1041–1043. [Google Scholar] [CrossRef] [PubMed]
- Feig, C.; Gopinathan, A.; Neesse, A.; Chan, D.S.; Cook, N.; Tuveson, D.A. The Pancreas Cancer Microenvironment. Clin. Cancer Res. 2012, 18, 4266–4276. [Google Scholar] [CrossRef] [Green Version]
- Evans, A.; Costello, E. The role of inflammatory cells in fostering pancreatic cancer cell growth and invasion. Front. Physiol. 2012, 3, 270. [Google Scholar]
- Roshani, R.; McCarthy, F.; Hagemann, T. Inflammatory cytokines in human pancreatic cancer. Cancer Lett. 2014, 345, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Akashi, K.; Kondo, M.; Weissman, I.L. Role of interleukin-7 in T-cell development from hematopoietic stem cells. Immunol. Rev. 1998, 165, 13–28. [Google Scholar] [CrossRef] [PubMed]
- McLane, L.M.; Abdel-Hakeem, M.S.; Wherry, E.J. CD8 T Cell Exhaustion During Chronic Viral Infection and Cancer. Annu. Rev. Immunol. 2019, 37, 457–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belarif, L.; Danger, R.; Kermarrec, L.; Nerrière-Daguin, V.; Pengam, S.; Durand, T.; Mary, C.; Kerdreux, E.; Gauttier, V.; Kucik, A.; et al. IL-7 receptor influences anti-TNF responsiveness and T cell gut homing in inflammatory bowel disease. J. Clin. Investig. 2019, 129, 1910–1925. [Google Scholar] [CrossRef] [Green Version]
- Barata, J.T.; Durum, S.K.; Seddon, B. Flip the coin: IL-7 and IL-7R in health and disease. Nat. Immunol. 2019, 20, 1584–1593. [Google Scholar] [CrossRef]
- Mazzucchelli, R.; Durum, S.K. Interleukin-7 receptor expression: Intelligent design. Nat. Rev. Immunol. 2007, 7, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Krzystek-Korpacka, M.; Zawadzki, M.; Neubauer, K.; Bednarz-Misa, I.; Górska, S.; Wiśniewski, J.; Witkiewicz, W.; Gamian, A. Elevated systemic interleukin-7 in patients with colorectal cancer and individuals at high risk of cancer: Association with lymph node involvement and tumor location in the right colon. Cancer Immunol. Immunother. 2017, 66, 171–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Churchman, S.M.; El-Jawhari, J.J.; Burska, A.N.; Parmar, R.; Goëb, V.; Conaghan, P.G.; Emery, P.; Ponchel, F. Modulation of peripheral T-cell function by interleukin-7 in rheumatoid arthritis. Arthritis Res. Ther. 2014, 16, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, L.-F.; Logronio, K.; Tu, G.H.; Zhai, W.; Ni, I.; Mei, L.; Dilley, J.; Yu, J.; Rajpal, A.; Brown, C.; et al. Anti-IL-7 receptor- reverses established type 1 diabetes in nonobese diabetic mice by modulating effector T-cell function. Proc. Natl. Acad. Sci. USA 2012, 109, 12674–12679. [Google Scholar] [CrossRef] [Green Version]
- Karawajew, L.; Ruppert, V.; Wuchter, C.; Kosser, A.; Schrappe, M.; Dorken, B.; Ludwig, W.D. Inhibition of in vitro spontaneous apoptosis by il-7 correlates with bcl-2 up-regulation, cortical/mature immunophenotype, and better early cytoreduction of childhood t-cell acute lymphoblastic leukemia. Blood 2000, 96, 29–306. [Google Scholar] [CrossRef]
- Cattaruzza, L.; Gloghini, A.; Olivo, K.; Di Francia, R.; Lorenzon, D.; De Filippi, R.; Carbone, A.; Colombatti, A.; Pinto, A.; Aldinucci, D. Functional coexpression of interleukin (il)-7 and its receptor (il-7r) on hodgkin and reed-sternberg cells: Involvement of il-7 in tumor cell growth and microenvironmental interactions of hodgkin’s lymphoma. Int. J. Cancer 2009, 125, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Shaw, V.E.; Lane, B.; Jenkinson, C.; Cox, T.; Greenhalf, W.; Halloran, C.M.; Tang, J.; Sutton, R.; Neoptolemos, J.P.; Costello, E. Serum cytokine biomarker panels for discriminating pancreatic cancer from benign pancreatic disease. Mol. Cancer 2014, 13, 114. [Google Scholar] [CrossRef] [Green Version]
- Kruger, D.; Yako, Y.Y.; Devar, J.; Lahoud, N.; Smith, M. Inflammatory cytokines and combined biomarker panels in pancreatic ductal adenocarcinoma: Enhancing diagnostic accuracy. PLoS ONE 2019, 14, e0221169. [Google Scholar] [CrossRef]
- Baine, M.J.; Menning, M.; Smith, L.M.; Mallya, K.; Kaur, S.; Rachagani, S.; Chakraborty, S.; Sasson, A.R.; Brand, R.E.; Batra, S.K. Differential gene expression analysis of peripheral blood mononuclear cells reveals novel test for early detection of pancreatic cancer. Cancer Biomark. 2011-2012, 11, 1–14. [Google Scholar] [CrossRef] [Green Version]
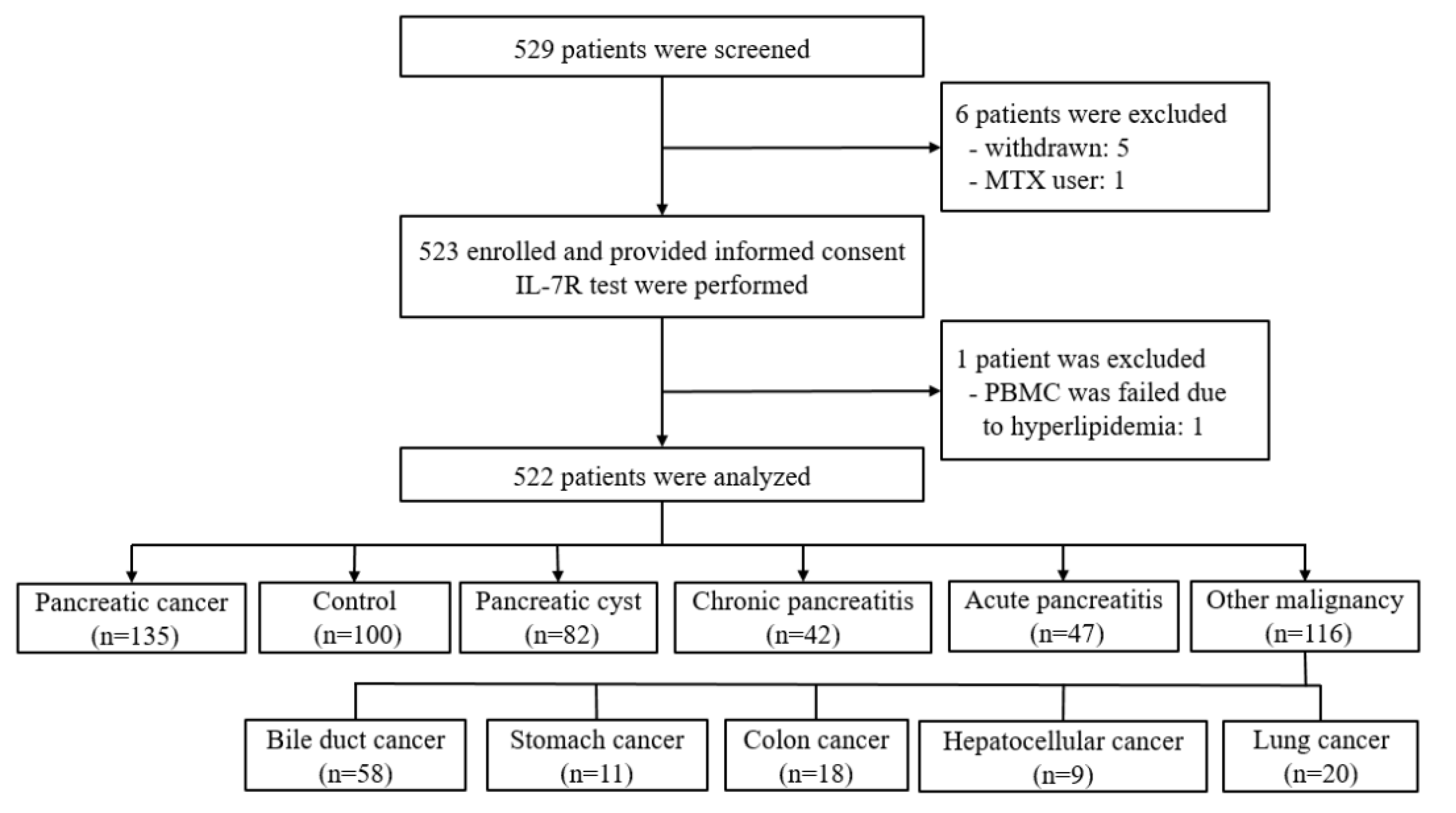
| Pancreatic Cancer (n = 135) | Control (n = 100) | Pancreatic Cyst (n = 82) | Chronic Pancreatitis (n = 42) | Acute Pancreatitis (n = 47) | |
|---|---|---|---|---|---|
| Age, years (mean ± SD) | 67.1 ± 11.5 | 51.9 ± 12.3 † | 64.5 ± 10.1 | 56.7 ± 13.2 † | 47.7 ± 15.2 † |
| male: female | 70:65 | 43:50 | 29:53 | 32:10 | 35:12 |
| BMI, kg/m2 (mean ± SD) | 23.0 ± 3.1 | 24.5 ± 4.1 † | 23.3 ± 3.1 | 23.2 ± 2.8 | 26.2 ± 4.8 † |
| DM, n (%) | 50 (37.0) | NA | 19 (23.2) | 22 (52.4) | 10 (21.3) |
| WBC, count/uL (mean ± SD) | 7358.4 ± 3704.7 | 6041.7 ± 1688.7 | 5997.4 ± 1837.3 | 9823.2 ± 14,658.8 | 8903.0 ± 4680.3 |
| neutrophil | 4873.1 ± 3290.6 | 3268.7 ± 1196.2 † | 3366.0 ± 1255.6 † | 4658.0 ± 2182.9 | 6417.0 ± 4696.0 † |
| lymphocyte | 1669.9 ± 640.1 | 2135.3 ± 633.0 † | 2259.1 ± 666.8 † | 2043.2 ± 796.8 † | 1590.2 ± 804.1 |
| monocyte | 605.3 ± 453.1 | 439.4 ± 166.3 † | 461.9 ± 165.0 † | 606.6 ± 230.6 | 683.6 ± 380.3 |
| eosinophil | 159.8 ± 138.2 | 154.1 ± 131.2 | 164.2 ± 194.5 | 218.0 ± 157.3 | 191.5 ± 176.6 |
| basophil | 39.2 ± 22.0 | 42.7 ± 33.0 | 39.0 ± 20.3 | 44.6 ± 22.9 | 35.7 ± 20.1 |
| platelet count 103/μL (mean ± SD) | 237.0 ± 82.7 | NA | 230.2 ± 55.7 | 248.3 ± 76.3 | 226.1 ± 78.1 |
| protein, g/dL (mean ± SD) | 6.8 ± 0.8 | 7.1 ± 0.4 † | 7.0 ± 0.5 † | 6.9 ± 0.6 | 6.2 ± 1.1 † |
| albumin, g/dL (mean ± SD) | 3.9 ± 0.4 | 4.3 ± 0.5 † | 4.3 ± 0.3 † | 4.1 ± 0.4 | 3.7 ± 0.6 |
| bilirubin, mg/dL (mean ± SD) | 2.9 ± 4.6 | 0.7 ± 0.3 † | 0.7 ± 0.3 † | 0.7 ± 0.4 † | 1.2 ± 0.9 † |
| AST, IU/L (mean ± SD) | 91.8 ± 153.0 | 25.5 ± 10.4 † | 27.6 ± 13.3 † | 25.2 ± 10.5 † | 52.8 ± 68.9 |
| ALT, IU/L (mean ± SD) | 103.2 ± 173.6 | 23.2 ± 14.2 † | 24.1 ± 17.8 † | 21.8 ± 11.2 † | 56.7 ± 73.3 |
| CRP, mg/L (mean ± SD) | 20.4 ± 47.4 | NA | 2.8 ± 15.9 | 17.8 ± 44.6 | 71.6 ± 88.7 † |
| CEA, ng/L (mean ± SD) | 98.1 ± 699.9 | 1.7 ± 1.0 | 2.3 ± 1.7 | 3.8 ± 3.4 | 1.8 ± 1.4 |
| CA19-9, U/mL (mean ± SD) | 1946.2 ± 4513.5 | 13.6 ± 39.2 † | 25.3 ± 81.8 † | 48.2 ± 88.8 † | 13.0 ± 14.8 † |
| IL-7R, (mean ± SD) | 391.7 ± 281.9 | 194.7 ± 82.0 † | 274.6 ± 286.0 † | 205.3 ± 139.8 † | 161.8 ± 112.9 † |
| Marker | AUC | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) | |
|---|---|---|---|---|---|---|---|
| Resectable pancreatic cancer | IL-7R | 0.850 | 67.3 | 92.0 | 82.2 | 83.6 | 83.2 |
| CA19-9 | 0.838 | 65.5 | 96.0 | 90.0 | 83.5 | 85.2 | |
| IL-7R + CA19-9 | 0.941 | 87.3 | 93.0 | 87.3 | 93.0 | 91.0 | |
| IL-7R vs. CA19-9 | >0.999 | >0.999 | 0.735 | 0.823 | >0.999 | >0.999 | |
| IL-7R vs. combination † | 0.014 | 0.002 | >0.999 | 0.666 | 0.004 | 0.011 | |
| CA19-9 vs. combination | 0.027 | 0.003 | 0.761 | >0.999 | 0.008 | 0.173 | |
| Unresectablepancreatic cancer | IL-7R | 0.709 | 51.3 | 94.0 | 87.2 | 70.7 | 75.0 |
| CA19-9 | 0.911 | 78.8 | 96.0 | 94.0 | 85.0 | 88.3 | |
| IL-7R + CA19-9 | 0.941 | 86.3 | 96.0 | 94.5 | 89.7 | 91.7 | |
| IL-7R vs. CA19-9 | <0.001 | <0.001 | >0.999 | 0.657 | 0.001 | 0.002 | |
| IL-7R vs. combination | <0.001 | <0.001 | >0.999 | 0.299 | <0.001 | <0.001 | |
| CA19-9 vs. combination | 0.507 | 0.157 | >0.999 | >0.999 | 0.166 | 0.319 | |
| All pancreatic cancer | IL-7R | 0.766 | 58.5 | 92.0 | 90.8 | 62.2 | 72.8 |
| CA19-9 | 0.881 | 73.3 | 96.0 | 96.1 | 72.7 | 83.0 | |
| IL-7R + CA19-9 | 0.945 | 85.9 | 96.0 | 96.7 | 83.5 | 90.2 | |
| IL-7R vs. CA19-9 | 0.017 | 0.044 | 0.735 | 0.412 | 0.028 | 0.021 | |
| IL-7R vs. combination | <0.001 | <0.001 | 0.459 | 0.132 | <0.001 | <0.001 | |
| CA19-9 vs. combination | 0.008 | 0.001 | >0.999 | >0.999 | 0.001 | 0.004 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, S.-I.; Cho, J.-H.; Kim, S.-Y.; Hong, I.-Y.; Park, J.-S.; Lee, H.-S.; Park, G.; Kim, J.-K.; Lee, H.-K.; Lee, D.-K. Validation of IL-7R as an Immunological Biomarker for Human Pancreatic Ductal Adenocarcinoma. Cancers 2022, 14, 853. https://doi.org/10.3390/cancers14030853
Jang S-I, Cho J-H, Kim S-Y, Hong I-Y, Park J-S, Lee H-S, Park G, Kim J-K, Lee H-K, Lee D-K. Validation of IL-7R as an Immunological Biomarker for Human Pancreatic Ductal Adenocarcinoma. Cancers. 2022; 14(3):853. https://doi.org/10.3390/cancers14030853
Chicago/Turabian StyleJang, Sung-Ill, Jae-Hee Cho, So-Young Kim, In-Young Hong, Joon-Seong Park, Hye-Sun Lee, Goeun Park, Jong-Kyoung Kim, Hyung-Keun Lee, and Dong-Ki Lee. 2022. "Validation of IL-7R as an Immunological Biomarker for Human Pancreatic Ductal Adenocarcinoma" Cancers 14, no. 3: 853. https://doi.org/10.3390/cancers14030853
APA StyleJang, S.-I., Cho, J.-H., Kim, S.-Y., Hong, I.-Y., Park, J.-S., Lee, H.-S., Park, G., Kim, J.-K., Lee, H.-K., & Lee, D.-K. (2022). Validation of IL-7R as an Immunological Biomarker for Human Pancreatic Ductal Adenocarcinoma. Cancers, 14(3), 853. https://doi.org/10.3390/cancers14030853






