Use of Omics Technologies for the Detection of Colorectal Cancer Biomarkers
Abstract
:Simple Summary
Abstract
1. Introduction
2. Omics Techniques
2.1. Genomics
2.2. Transcriptomics
2.3. Proteomics
2.4. Metabolomics
2.5. Glycomics
2.6. Volatolomics
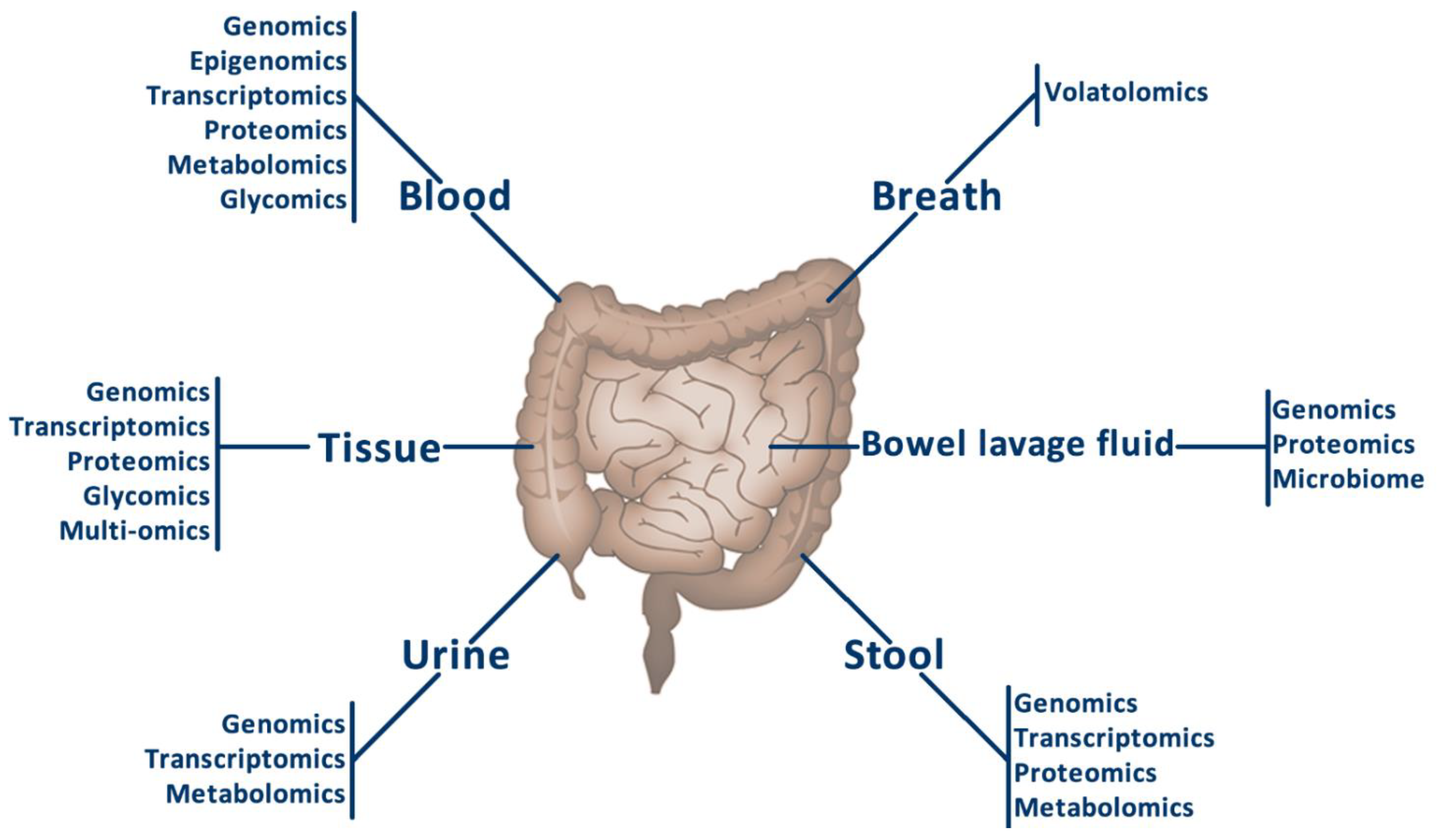
3. Sample Types for the Omics Analyses in Colorectal Cancer
3.1. Breath Samples
Volatolomics
3.2. Urine Samples
3.2.1. Genomics
3.2.2. Proteomics
3.2.3. Metabolomics
| Omics | Biomarker | Change | Reference |
|---|---|---|---|
| Metabolomics | 3-hydroxybutyric acid, L-dopa, L-histidinol, and N1, N12-diacetylspermine | Upregulated | [46] |
| Metabolomics | pyruvic acid, hydroquinone, tartaric acid, hippuric acid, butyraldehyde, ether, and 1,1,6-trimethyl-1,2-dihydronaphthalene | Downregulated | [46] |
| Metabolomics | Hydroxyproline dipeptide, tyrosine, glucuronic acid, tryptophan, pseudouridine, glucose, glycine, histidine, 5-oxoproline, isocitric acid, threonic acid | Upregulated | [49] |
| Metabolomics | Citric acid, octadecanoic acid, hexadecanoic acid, propanoic acid-2-methyl-1-(1,1-dimethylethyl)-2-methyl-1,3-propanediyl ester | Downregulated | [49] |
| Metabolomics | 3-(4-hydroxyphenyl)propionate, betaine, pipecolate, S-methylcysteine, choline, eicosapentaenoate (20:5n3), benzoate, S-adenosylhomocysteine, N-delta-acetylornithine, cysteine, 3-(4-hydroxyphenyl)lactate, gentisate, hippurate, 4-hydroxyhippurate, and salicylate. | Up- and downregulated | [52] |
3.3. Stool Samples
3.3.1. Genomics
3.3.2. Transcriptomics
3.3.3. Proteomics
3.3.4. Metabolomics
| Omics | Biomarker | Change | Reference |
|---|---|---|---|
| Genomics (metagenomics) | butyryl-CoA dehydrogenase from F. nucleatum | Upregulated | [19] |
| Genomics and Transcriptomics | baiF | Upregulated | [56] |
| Genomics (metagenomics) | Coprobacillus | Upregulated | [57] |
| Genomics (metagenomics) | m3 from Lachnoclostridium | Upregulated | [58] |
| Genomics (methylation) | COL4A1, COL4A2, TLX2, ITGA4 | Upregulated | [59] |
| Genomics (methylation) | GRIA4, VIPR2 | Upregulated | [60] |
| Genomics (methylation) | SDC2, NDRG4 | Upregulated | [61] |
| Genomics (methylation) | SDC2 | Upregulated | [62] |
| Genomics (methylation) | SDC2, ADHFE1, PPP2R5C | Upregulated | [64] |
| Genomics (methylation) | SOX21 | Upregulated | [65] |
| Transcriptomics (miRNAs) | miR-21, miR-106a, miR-96, miR-203, miR-20a, miR-326, miR-92 | Upregulated | [67] |
| Transcriptomics (miRNAs) | miR-320, miR-126, miR-484-5p, miR143, miR-145, miR-16, miR-125b | Downregulated | [67] |
| Transcriptomics (miRNAs) | miR-7, miR-17, miR-20a, miR-21, miR-92a, miR-96, miR-106a, miR-134, miR-183, miR-196a, miR-199a-3p, miR-214 | Upregulated | [68] |
| Transcriptomics (miRNAs) | miR-9, miR-29b, miR-127-5p, miR-138, miR-143, miR-146a, miR-222, miR-938 | Downregulated | [68] |
| Transcriptomics (lncRNAs) | CCAT1, CCAT2, H19, HOTAIR, HULC, MALAT1, PCAT1, MEG3, PTENP1, TUSC7 | Upregulated | [69] |
| Proteomics | Hp, LAMP1, SYNE2, LRG1, RBP4, FN1, ANXA6 | Upregulated | [71] |
| Metabolomics | Polyamines (cadaverine and putrescine) | Upregulated | [72] |
| Metabolomics | Cholesteryl esters, Sphingomyelins | Upregulated | [74] |
| Metabolomics | Oleic acid | Upregulated | [73] |
| Metabolomics | Butyrate, Alanine, Lactate, Glutamate, Succinate | Upregulated (except Butyrate downregulated) | [75] |
3.4. Blood Samples
3.4.1. Genomics
3.4.2. Transcriptomics
3.4.3. Proteomics
3.4.4. Metabolomics
3.4.5. Glycomics
| Omics | Biomarker | Change | Reference |
|---|---|---|---|
| Genomics | cfDNA | Increase | [80,81] |
| Genomics | KRAS, APC, TP53 | Mutation | [78,82] |
| Genomics | cfDNA Microsatellite instability | Increase | [84,101] |
| Transcriptomics | CK19, CK20, CEA, MDM2, DUSP6, CPEB4, MMD, EIF2S3, ANXA3, CLEC4D, LMNB1, PRRG4, TNFAIP6, VNN1, and IL2RB | Upregulated | [76,78,85] |
| Transcriptomics | miR-145, miR-143, miR-135, miR-17-92, miR-92a, miR-29a, miR-125b, miR-19a-3p, miR-223–3p, miR-92a-3p and miR-422a, miR-21 | Upregulated | [78,86,87] |
| Epigenomics | SEPT9 | Methylation | [76] |
| Proteomics | CEA, CA19-9 and SAA | Increase | [76,88] |
| Proteomics | MST1/STK4 and S100A9 | Increase | [83] |
| Proteomics | Cyr61 | Increase | [89] |
| Proteomics | Antibodies against EDIL3, GTF2B, HCK, p53, PIM1 and STK4 | Increase | [91] |
| Metabolomics | Glucose and long-chain hydroxy fatty acids | Decrease | [93,94] |
| Metabolomics | Pyruvic acid, lysine, glycolic acid, ornithine, fumaric acid | Increase | [96] |
| Metabolomics | Palmitoleic acid, tryptophan, lysine, 3-hydroxyisovaleric acid | Decrease | [96] |
| Glycomics | Galactosylation and sialylation of fucosylated IgG glycan structures | Decrease | [97] |
| Glycomics | Bisecting GlcNAc in IgG glycan structures | Increase | [97] |
| Glycomics | Glycans with no galactose residues, tri- and tetra-galactosylated glycans, tri- and tetra-sialyted structures, highly branched glycans | Increase | [98] |
| Glycomics | Mono- and di-galactosylated structures, mono-sialyted glycans, galactosylated and sialylated bi-antennary GlcNAc glycans, neutral core fucosylated glycans with one or two galactose residues | Decrease | [98] |
| Glycomics | Mannose-rich HexNAc2Hex7, fucosylated bi-antennary glycan HexNAc4Hex5Fuc1NeuAc2, tetra-antennary HexNAc6Hex7NeuAc3 | Upregulated | [100] |
3.5. Bowel Lavage Fluid Samples
3.5.1. Genomics
3.5.2. Proteomics
3.5.3. Microbiome Study
| Omics | Biomarker | Change | Reference |
|---|---|---|---|
| Genomics | KRAS, P53 | Mutation | [103,104] |
| Genomics | TGFβ RII, APC | Mutation | [104] |
| Genomics | miR-124-3, LOC386758, SFRP1 | Methylation | [107] |
| Genomics | SDC2 | Methylation | [108] |
| Genomics (metagenomics) | Proteobacteria, Fusobacteria | Increase | [105] |
| Genomics (metagenomics) | Firmicutes | Decrease | [105] |
| Microbiome study | Bacteroides fragilis | Presence | [110] |
3.6. Tumour Tissue Samples
3.6.1. Genomics
3.6.2. Transcriptomics
3.6.3. Proteomics
3.6.4. Glycomics
| Omics | Biomarker | Change | Reference |
|---|---|---|---|
| Transcriptomics | CYP1B1 | Upregulated | [120] |
| Transcriptomics | FAS, GSR | Downregulated | [120] |
| Transcriptomics | AC125603.2, LINC00909, AC0168676.1, MIR210HG, AC009237, LINC01063 | Prognosis biomarkers | [122] |
| Proteomics | Transgelin | Decrease | [124] |
| Proteomics | CD8 T cell infiltration | Decrease | [125] |
| Proteomics | Glycolysis in MSI-H tumours | Increase | [125] |
| Glycomics | Glypican-3, syndecan-1 | Downregulated | [25] |
| Glycomics | Glycosylceramide, lactosylceramide, monosialic acid ganglioside, globoside 4 | Upregulated | [25] |
| Glycomics | Heparan sulphate | Decrease | [130] |
| Glycomics | Chondroitin sulphate, dermatan sulphate | Increase | [130] |
| Glycomics | Complex N-glycans, α2,3-sialylation | Decrease | [126] |
| Glycomics | High mannose, hybrid and paucimannosidic type N-glycans | Increase | [126] |
| Glycomics | Bisecting GlNAcylation, Lewis-Type fucosylation | Decrease | [127] |
| Glycomics | α2,6-sialylation, total sialylation, mannose type N-glycan structures | Increase | [127] |
| Glycomics | M/Z 9732+, 10552+, 10602+, 10752+, 11622+, 11772+, 12642+, 12792+, 13522+ | Decrease | [128] |
| Glycomics | M/Z 10132+, 11162+, 12282+ | Increase | [128] |
| Glycomics | Fucosylation levels, highly branched N-glycans | Decrease | [129] |
| Glycomics | Sialylation, high-mannose glycans | Increase | [129] |
| Glycomics | Glycan-Tn/STn-MUC1 | Increase | [131] |
| Glycomics | Oligomannosidic, bi-antennary hypogalactosylated, branched compositions | Increase | [100] |
3.6.5. Multi-Omics
4. Use of Extracellular Vesicles as Colorectal Cancer Biomarkers
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Sociedad Española de Oncología Médica Las Cifras del Cáncer en España 2020. 2020. Available online: https://seom.org/prensa/el-cancer-en-cifras (accessed on 24 December 2021).
- Zamorano-Leon, J.J.; López-De-Andres, A.; Álvarez-González, A.; Maestre-Miquel, C.; Astasio-Arbiza, P.; López-Farré, A.; De-Miguel-Diez, J.; Jiménez-García, R.; Albaladejo-Vicente, R. Trends and Predictors for the Uptake of Colon Cancer Screening Using the Fecal Occult Blood Test in Spain from 2011 to 2017. Int. J. Environ. Res. Public Health 2020, 17, 6222. [Google Scholar] [CrossRef] [PubMed]
- Roselló, S.; Simón, S.; Cervantes, A. Programmed colorectal cancer screening decreases incidence and mortality. Transl. Gastroenterol. Hepatol. 2019, 4, 84. [Google Scholar] [CrossRef] [PubMed]
- Cheshomi, H.; Matin, M.M. Exosomes and their importance in metastasis, diagnosis, and therapy of colorectal cancer. J. Cell. Biochem. 2019, 120, 2671–2686. [Google Scholar] [CrossRef]
- Mammes, A.; Pasquier, J.; Mammes, O.; Conti, M.; Douard, R.; Loric, S. Extracellular vesicles: General features and usefulness in diagnosis and therapeutic management of colorectal cancer. World J. Gastrointest. Oncol. 2021, 13, 1561–1598. [Google Scholar] [CrossRef]
- Brenner, H.; Stock, C.; Hoffmeister, M. Effect of screening sigmoidoscopy and screening colonoscopy on colorectal cancer incidence and mortality: Systematic review and meta-analysis of randomised controlled trials and observational studies. BMJ 2014, 348, g2467. [Google Scholar] [CrossRef] [Green Version]
- Elmunzer, B.J.; Hayward, R.A.; Schoenfeld, P.S.; Saini, S.D.; Deshpande, A.; Waljee, A.K. Effect of Flexible Sigmoidoscopy-Based Screening on Incidence and Mortality of Colorectal Cancer: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. PLoS Med. 2012, 9, e1001352. [Google Scholar] [CrossRef]
- Gini, A.; Jansen, E.E.L.; Zielonke, N.; Meester, R.G.S.; Senore, C.; Anttila, A.; Segnan, N.; Mlakar, D.N.; de Koning, H.J.; Lansdorp-Vogelaar, I.; et al. Impact of colorectal cancer screening on cancer-specific mortality in Europe: A systematic review. Eur. J. Cancer 2020, 127, 224–235. [Google Scholar] [CrossRef] [Green Version]
- Deandrea, S.; Molina-Barceló, A.; Uluturk, A.; Moreno, J.; Neamtiu, L.; Peiró-Pérez, R.; Saz-Parkinson, Z.; Lopez-Alcalde, J.; Lerda, D.; Salas, D. Presence, characteristics and equity of access to breast cancer screening programmes in 27 European countries in 2010 and 2014. Results from an international survey. Prev. Med. 2016, 91, 250–263. [Google Scholar] [CrossRef]
- Borràs, J.M.; Colomer, C.; Soria, P.; López, R. Priorities for cancer control in Spain. Ann. Oncol. 2010, 21, iii111–iii114. [Google Scholar] [CrossRef]
- Cobo-Cuenca, A.I.; Laredo-Aguilera, J.A.; Rodríguez-Borrego, M.-A.; Santacruz-Salas, E.; Carmona-Torres, J.M. Temporal Trends in Fecal Occult Blood Test: Associated Factors (2009–2017). Int. J. Environ. Res. Public Heal. 2019, 16, 2120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.S.; Piper, M.A.; Perdue, L.A.; Rutter, C.M.; Webber, E.M.; O’Connor, E.; Smith, N.; Whitlock, E.P. Screening for colorectal cancer: Updated evidence report and systematic review for the US preventive services task force. JAMA J. Am. Med. Assoc. 2016, 315, 2576–2594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niedermaier, T.; Balavarca, Y.; Brenner, H. Stage-Specific Sensitivity of Fecal Immunochemical Tests for Detecting Colorectal Cancer: Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2020, 115, 56–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Definition of genomics—NCI Dictionary of Cancer Terms—National Cancer Institute. Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/genomics (accessed on 27 December 2021).
- Grady, W.M.; Yu, M.; Markowitz, S.D. Epigenetic Alterations in the Gastrointestinal Tract: Current and Emerging Use for Biomarkers of Cancer. Gastroenterology 2021, 160, 690–709. [Google Scholar] [CrossRef] [PubMed]
- Clough, E.; Barrett, T. The Gene Expression Omnibus Database. Methods Mol. Biol. 2016, 1418, 93–110. [Google Scholar] [PubMed] [Green Version]
- Wang, Z.; Jensen, M.A.; Zenklusen, J.C. A Practical Guide to The Cancer Genome Atlas (TCGA). Methods Mol. Biol. 2016, 1418, 111–141. [Google Scholar] [PubMed]
- Yu, J.; Feng, Q.; Wong, S.H.; Zhang, D.; Liang, Q.Y.; Qin, Y.; Tang, L.; Zhao, H.; Stenvang, J.; Li, Y.; et al. Metagenomic analysis of faecal microbiome as a tool towards targeted non-invasive biomarkers for colorectal cancer. Gut 2017, 66, 70–78. [Google Scholar] [CrossRef]
- Definition of transcriptomics—NCI Dictionary of Cancer Terms—National Cancer Institute. Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/transcriptomics (accessed on 27 December 2021).
- Ahluwalia, P.; Kolhe, R.; Gahlay, G.K. The clinical relevance of gene expression based prognostic signatures in colorectal cancer. Biochim. Biophys. Acta —Rev. Cancer 2021, 1875, 188513. [Google Scholar] [CrossRef]
- Definition of proteomics—NCI Dictionary of Cancer Terms—National Cancer Institute. Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/proteomics (accessed on 27 December 2021).
- Boja, E.S.; Rodriguez, H. Proteogenomic convergence for understanding cancer pathways and networks. Clin. Proteom. 2014, 11, 22. [Google Scholar] [CrossRef] [Green Version]
- Nannini, G.; Meoni, G.; Amedei, A.; Tenori, L. Metabolomics profile in gastrointestinal cancers: Update and future perspectives. World J. Gastroenterol. 2020, 26, 2514–2532. [Google Scholar] [CrossRef]
- Joo, E.J.; Weyers, A.; Li, G.; Gasimli, L.; Li, L.; Choi, W.J.; Lee, K.B.; Linhardt, R.J. Carbohydrate-Containing Molecules as Potential Biomarkers in Colon Cancer. OMICS: A J. Integr. Biol. 2014, 18, 231–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drake, R.R. Glycosylation and Cancer: Moving Glycomics to the Forefront, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2015; Volume 126. [Google Scholar]
- Holst, S.; Wuhrer, M.; Rombouts, Y. Glycosylation Characteristics of Colorectal Cancer, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2015; Volume 126. [Google Scholar]
- Hakim, M.; Broza, Y.Y.; Barash, O.; Peled, N.; Phillips, M.; Amann, A.; Haick, H. Volatile organic compounds of lung cancer and possible biochmical pathways. Chem. Rev. 2012, 112, 5949–5966. [Google Scholar] [CrossRef] [PubMed]
- Bosch, S.; Berkhout, D.J.; Larbi, B.I.; De Meij, T.G.; De Boer, N.K. Fecal volatile organic compounds for early detection of colorectal cancer: Where are we now? J. Cancer Res. Clin. Oncol. 2019, 145, 223–234. [Google Scholar] [CrossRef] [PubMed]
- De Vietro, N.; Aresta, A.; Rotelli, M.T.; Zambonin, C.; Lippolis, C.; Picciariello, A.; Altomare, D.F. Relationship between cancer tissue derived and exhaled volatile organic compound from colorectal cancer patients. Preliminary results. J. Pharm. Biomed. Anal. 2020, 180, 113055. [Google Scholar] [CrossRef] [PubMed]
- Kabir, K.M.M.; Donald, W.A. Cancer breath testing: A patent review. Expert Opin. Ther. Patents 2018, 28, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Politi, L.; Monasta, L.; Rigressi, M.N.; Princivalle, A.; Gonfiotti, A.; Camiciottoli, G.; Perbellini, L. Discriminant Profiles of Volatile Compounds in the Alveolar Air of Patients with Squamous Cell Lung Cancer, Lung Adenocarcinoma or Colon Cancer. Molecules 2021, 26, 550. [Google Scholar] [CrossRef]
- Van De Goor, R.M.G.E.; Leunis, N.; Van Hooren, M.R.A.; Francisca, E.; Masclee, A.; Kremer, B.; Kross, K.W. Feasibility of electronic nose technology for discriminating between head and neck, bladder, and colon carcinomas. Eur. Arch. Oto-Rhino-Laryngology 2017, 274, 1053–1060. [Google Scholar] [CrossRef] [Green Version]
- Amann, A.; Mochalski, P.; Ruzsanyi, V.; Broza, Y.Y.; Haick, H. Assessment of the exhalation kinetics of volatile cancer biomarkers based on their physicochemical properties. J. Breath Res. 2014, 8, 016003. [Google Scholar] [CrossRef] [Green Version]
- Haick, H.; Broza, Y.Y.; Mochalski, P.; Ruzsanyi, V.; Amann, A. Assessment, origin, and implementation of breath volatile cancer markers. Chem. Soc. Rev. 2014, 43, 1423–1449. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Tao, J.; Li, J.; Tao, S. Volatile organic compounds analysis as a potential novel screening tool for colorectal cancer A systematic review and meta-analysis. Medicine 2020, 99, e20937. [Google Scholar] [CrossRef]
- Van Keulen, K.E.; Jansen, M.E.; Schrauwen, R.W.M.; Kolkman, J.J.; Siersema, P.D. Volatile organic compounds in breath can serve as a non-invasive diagnostic biomarker for the detection of advanced adenomas and colorectal cancer. Aliment. Pharmacol. Ther. 2020, 51, 334–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, J.; Xue, W.; Yin, H.; Zhang, N.; Zhou, J.; Long, Z.; Wu, C.; Liang, Z.; Xie, K.; Li, S.; et al. Differential Metabolic Alterations and Biomarkers Between Gastric Cancer and Colorectal Cancer: A Systematic Review and Meta-Analysis. OncoTargets Ther. 2020, 13, 6093–6108. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Hakim, M.; Broza, Y.Y.; Billan, S.; Abdah-Bortnyak, R.; Kuten, A.; Tisch, U.; Haick, H. Detection of lung, breast, colorectal, and prostate cancers from exhaled breath using a single array of nanosensors. Br. J. Cancer 2010, 103, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Haick, H.; Hakim, M. Volatile organic compounds as diagnostic markers for various types of cancer. U.S. Patent No. 9,551,712, 6 January 2011. [Google Scholar]
- Altomare, D.F.; Picciariello, A.; Rotelli, M.T.; De Fazio, M.; Aresta, A.; Zambonin, C.G.; Vincenti, L.; Trerotoli, P.; Vietro, N. De Chemical signature of colorectal cancer: Case–control study for profiling the breath print. BJS Open 2020, 4, 1189–1199. [Google Scholar] [CrossRef]
- Ohta, R.; Yamada, T.; Sonoda, H.; Matsuda, A.; Shinji, S.; Takahashi, G.; Iwai, T.; Takeda, K.; Ueda, K.; Kuriyama, S.; et al. Detection of KRAS mutations in circulating tumour DNA from plasma and urine of patients with colorectal cancer. Eur. J. Surg. Oncol. (EJSO) 2021, 47, 3151–3156. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Cao, Z.; Gao, Y. Changes in the Urinary Proteome in a Patient-Derived Xenograft (PDX) Nude Mouse Model of Colorectal Tumor. Sci. Rep. 2019, 9, 4975. [Google Scholar] [CrossRef] [Green Version]
- Lalmahomed, Z.S.; Bröker, M.E.; A Van Huizen, N.; Braak, R.R.J.C.V.D.; Dekker, L.J.; Rizopoulos, D.; Verhoef, C.; Steyerberg, E.W.; Luider, T.M.; Ijzermans, J.N. Hydroxylated collagen peptide in urine as biomarker for detecting colorectal liver metastases. Am. J. Cancer Res. 2016, 6, 321–330. [Google Scholar]
- Erozenci, L.A.; Böttger, F.; Bijnsdorp, I.V.; Jimenez, C.R. Urinary exosomal proteins as (pan-)cancer biomarkers: Insights from the proteome. FEBS Lett. 2019, 593, 1580–1597. [Google Scholar] [CrossRef] [Green Version]
- Mallafré-Muro, C.; Llambrich, M.; Cumeras, R.; Pardo, A.; Brezmes, J.; Marco, S.; Gumà, J. Comprehensive Volatilome and Metabolome Signatures of Colorectal Cancer in Urine: A Systematic Review and Meta-Analysis. Cancers 2021, 13, 2534. [Google Scholar] [CrossRef]
- Erben, V.; Poschet, G.; Schrotz-King, P.; Brenner, H. Comparing Metabolomics Profiles in Various Types of Liquid Biopsies among Screening Participants with and without Advanced Colorectal Neoplasms. Diagnostics 2021, 11, 561. [Google Scholar] [CrossRef]
- Udo, R.; Katsumata, K.; Kuwabara, H.; Enomoto, M.; Ishizaki, T.; Sunamura, M.; Nagakawa, Y.; Soya, R.; Sugimoto, M.; Tsuchida, A. Urinary charged metabolite profiling of colorectal cancer using capillary electrophoresis-mass spectrometry. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ning, W.; Qiao, N.; Zhang, X.; Pei, D.; Wang, W. Metabolic profiling analysis for clinical urine of colorectal cancer. Asia-Pacific J. Clin. Oncol. 2021, 17, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Barichello, S.; Deng, L.; Ismond, K.P.; Loomes, D.; Kirwin, E.M.; Wang, H.; Chang, D.; Svenson, L.W.; Thanh, N. Comparative effectiveness and cost-effectiveness analysis of a urine metabolomics test vs. alternative colorectal cancer screening strategies. Int. J. Color. Dis. 2019, 34, 1953–1962. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.R.; Kwon, H.N.; Nam, H.; Kim, J.J.; Park, S.; Kim, Y.-H. Urine-NMR metabolomics for screening of advanced colorectal adenoma and early stage colorectal cancer. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Zarei, I.; Baxter, B.A.; Oppel, R.C.; Borresen, E.C.; Brown, R.J.; Ryan, E.P. Plasma and Urine Metabolite Profiles Impacted by Increased Dietary Navy Bean Intake in Colorectal Cancer Survivors: A Randomized-Controlled Trial. Cancer Prev. Res. 2021, 14, 497–508. [Google Scholar] [CrossRef]
- Ang, C.-S.; Baker, M.S.; Nice, E.C. Mass Spectrometry-Based Analysis for the Discovery and Validation of Potential Colorectal Cancer Stool Biomarkers. Methods Enzymol. 2017, 586, 247–274. [Google Scholar] [CrossRef]
- Gsur, A.; Baierl, A.; Brezina, S. Colorectal Cancer Study of Austria (CORSA): A Population-Based Multicenter Study. Biology 2021, 10, 722. [Google Scholar] [CrossRef]
- Tikk, K.; Weigl, K.; Hoffmeister, M.; Igel, S.; Schwab, M.; Hampe, J.; Klug, S.J.; Mansmann, U.; Kolligs, F.; Brenner, H. Study protocol of the RaPS study: Novel risk adapted prevention strategies for people with a family history of colorectal cancer. BMC Cancer 2018, 18, 720. [Google Scholar] [CrossRef] [Green Version]
- Wirbel, J.; Pyl, P.T.; Kartal, E.; Zych, K.; Kashani, A.; Milanese, A.; Fleck, J.S.; Voigt, A.Y.; Palleja, A.; Ponnudurai, R.; et al. Meta-analysis of fecal metagenomes reveals global microbial signatures that are specific for colorectal cancer. Nat. Med. 2019, 25, 679–689. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Li, D.; Yang, Z.; Dai, W.; Feng, X.; Liu, Y.; Jiang, Y.; Li, P.; Li, Y.; Tang, B.; et al. Establishing high-accuracy biomarkers for colorectal cancer by comparing fecal microbiomes in patients with healthy families. Gut Microbes 2020, 11, 918–929. [Google Scholar] [CrossRef]
- Liang, J.Q.; Li, T.; Nakatsu, G.; Chen, Y.-X.; Yau, T.O.; Chu, E.; Wong, S.; Szeto, C.H.; Ng, S.C.; Chan, F.K.L.; et al. A novel faecal Lachnoclostridium marker for the non-invasive diagnosis of colorectal adenoma and cancer. Gut 2020, 69, 1248–1257. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Wen, J.; Li, C.; Wang, H.; Wang, J.; Zou, H. High-Yield Methylation Markers for Stool-Based Detection of Colorectal Cancer. Am. J. Dig. Dis. 2019, 65, 1710–1719. [Google Scholar] [CrossRef] [PubMed]
- Vega-Benedetti, A.F.; Loi, E.; Moi, L.; Orrù, S.; Ziranu, P.; Pretta, A.; Lai, E.; Puzzoni, M.; Ciccone, L.; Casadei-Gardini, A.; et al. Colorectal Cancer Early Detection in Stool Samples Tracing CpG Islands Methylation Alterations Affecting Gene Expression. Int. J. Mol. Sci. 2020, 21, 4494. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Ye, Q.; Hong, Y.; Dai, W.; Zhang, C.; Liu, W.; Guo, Y.; Zhu, D.; Zhang, Z.; Chen, S.; et al. A systematic evaluation of stool DNA preparation protocols for colorectal cancer screening via analysis of DNA methylation biomarkers. Clin. Chem. Lab. Med. (CCLM) 2020, 59, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.J.; Oh, H.I.; Seo, Y.Y.; Jeong, D.; Kim, C.; Kang, H.W.; Han, Y.D.; Chung, H.C.; Kim, N.K.; An, S. Feasibility of quantifying SDC2 methylation in stool DNA for early detection of colorectal cancer. Clin. Epigenet. 2017, 9, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Liu, S.; Wang, H.; Zheng, L.; Zhou, C.; Li, G.; Huang, R.; Wang, H.; Li, C.; Fan, X.; et al. Robust performance of a novel stool DNA test of methylated SDC2 for colorectal cancer detection: A multicenter clinical study. Clin. Epigenet. 2020, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-C.; Wu, P.-H.; Chen, Y.-J.; Yang, C.-H.; Huang, J.-L.; Chou, Y.-C.; Chang, P.-K.; Wen, C.-C.; Jao, S.-W.; Huang, H.-H.; et al. Using Comorbidity Pattern Analysis to Detect Reliable Methylated Genes in Colorectal Cancer Verified by Stool DNA Test. Genes 2021, 12, 1539. [Google Scholar] [CrossRef] [PubMed]
- Moradi, K.; Babaei, E.; Feizi, M.A.H.; Safaralizadeh, R.; Rezvani, N. Quantitative detection of SRY-Box 21 (SOX21) gene promoter methylation as a stool-based noninvasive biomarker for early diagnosis of colorectal cancer by MethyLight method. Indian J. Cancer 2021, 58, 217–224. [Google Scholar]
- Ahmed, F.E.; Vos, P.; Ijames, S.; Lysle, D.T.; Allison, R.R.; Flake, G.; Sinar, D.R.; Naziri, W.; Marcuard, S.P.; Pennington, R. Transcriptomic molecular markers for screening human colon cancer in stool and tissue. Cancer Genom. Proteom. 2007, 4, 1–20. [Google Scholar]
- Ahmed, F.E.; Jeffries, C.D.; Vos, P.W.; Flake, G.; Nuovo, G.J.; Sinar, D.R.; Naziri, W.; Marcuard, S.P. Diagnostic microRNA markers for screening sporadic human colon cancer and active ulcerative colitis in stool and tissue. Cancer Genom. Proteom. 2009, 6, 281–295. [Google Scholar]
- Ahmed, F.E.; Ahmed, N.C.; Gouda, M.M.; Vos, P.W.; Bonnerup, C. RT-qPCR for Fecal Mature MicroRNA Quantification and Validation. Methods Mol. Biol. 2018, 1765, 203–215. [Google Scholar] [PubMed]
- Gharib, E.; Nazemalhosseini-Mojarad, E.; Baghdar, K.; Nayeri, Z.; Sadeghi, H.; Rezasoltani, S.; Jamshidi-Fard, A.; Larki, P.; Sadeghi, A.; Hashemi, M.; et al. Identification of a stool long non-coding RNAs panel as a potential biomarker for early detection of colorectal cancer. J. Clin. Lab. Anal. 2021, 35, e23601. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, A.; Boisvert, F.-M. Clinical Proteomics in Colorectal Cancer, a Promising Tool for Improving Personalised Medicine. Proteomes 2018, 6, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komor, M.A.; Bosch, L.J.; Coupé, V.; Rausch, C.; Pham, T.V.; Piersma, S.R.; Mongera, S.; Mulder, C.J.; Dekker, E.; Kuipers, E.J.; et al. Proteins in stool as biomarkers for non-invasive detection of colorectal adenomas with high risk of progression. J. Pathol. 2020, 250, 288–298. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Misra, B.B.; Liang, L.; Bi, D.; Weng, W.; Wu, W.; Cai, S.; Qin, H.; Goel, A.; Li, X.; et al. Integrated microbiome and metabolome analysis reveals a novel interplay between commensal bacteria and metabolites in colorectal cancer. Theranostics 2019, 9, 4101–4114. [Google Scholar] [CrossRef]
- Song, E.M.; Byeon, J.-S.; Lee, S.M.; Yoo, H.J.; Kim, S.J.; Chang, K.; Hwang, S.W.; Yang, D.-H.; Jeong, J.-Y. Fecal Fatty Acid Profiling as a Potential New Screening Biomarker in Patients with Colorectal Cancer. Am. J. Dig. Dis. 2018, 63, 1229–1236. [Google Scholar] [CrossRef]
- Clos-Garcia, M.; Garcia, K.; Alonso, C.; Iruarrizaga-Lejarreta, M.; D’Amato, M.; Crespo, A.; Iglesias, A.; Cubiella, J.; Bujanda, L.; Falcón-Pérez, J.M. Integrative Analysis of Fecal Metagenomics and Metabolomics in Colorectal Cancer. Cancers 2020, 12, 1142. [Google Scholar] [CrossRef]
- Lin, Y.; Ma, C.; Bezabeh, T.; Wang, Z.; Liang, J.; Huang, Y.; Zhao, J.; Liu, X.; Ye, W.; Tang, W.; et al. 1H NMR-based metabolomics reveal overlapping discriminatory metabolites and metabolic pathway disturbances between colorectal tumor tissues and fecal samples. Int. J. Cancer 2019, 145, 1679–1689. [Google Scholar] [CrossRef]
- Hauptman, N.; Glava, D. Colorectal Cancer Blood-Based Biomarkers. Gastroenterol. Res. Pract. 2017, 2017. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Casanova, A.; Costa-Fraga, N.; Bao-Caamano, A.; López-López, R.; Muinelo-Romay, L.; Diaz-Lagares, A. Epigenetic Landscape of Liquid Biopsy in Colorectal Cancer. Front. Cell Dev. Biol. 2021, 9. [Google Scholar] [CrossRef]
- Gallardo-Gómez, M.; De Chiara, L.; Álvarez-Chaver, P.; Cubiella, J. Colorectal cancer screening and diagnosis: Omics-based technologies for development of a non-invasive blood-based method. Expert Rev. Anticancer Ther. 2021, 21, 723–738. [Google Scholar] [CrossRef] [PubMed]
- Petit, J.; Carroll, G.; Gould, T.; Pockney, P.; Dun, M.; Scott, R.J. Cell-Free DNA as a Diagnostic Blood-Based Biomarker for Colorectal Cancer: A Systematic Review. J. Surg. Res. 2019, 236, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Qian, C.; Shi, W.; Wu, X.; Jing, R.; Zhang, L.; Wang, Z.; Ju, S. Alu-based cell-free DNA: A potential complementary biomarker for diagnosis of colorectal cancer. Clin. Biochem. 2013, 46, 64–69. [Google Scholar] [CrossRef]
- Hao, T.B.; Shi, W.; Shen, X.J.; Qi, J.; Wu, X.H.; Wu, Y.; Tang, Y.Y.; Ju, S.Q. Circulating cell-free DNA in serum as a biomarker for diagnosis and prognostic prediction of colorectal cancer. Br. J. Cancer 2014, 111, 1482–1489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.-Y.; Hsieh, J.-S.; Chang, M.-Y.; Huang, T.-J.; Chen, F.-M.; Cheng, T.-L.; Alexandersen, K.; Huang, Y.-S.; Tzou, W.-S.; Lin, S.-R. Molecular Detection of APC, K-ras, and p53 Mutations in the Serum of Colorectal Cancer Patients as Circulating Biomarkers. World J. Surg. 2004, 28, 721–726. [Google Scholar] [CrossRef]
- Chen, X.; Sun, J.; Wang, X.; Yuan, Y.; Cai, L.; Xie, Y.; Fan, Z.; Liu, K.; Jiao, X. A Meta-Analysis of Proteomic Blood Markers of Colorectal Cancer. Curr. Med. Chem. 2021, 28, 1176–1196. [Google Scholar] [CrossRef]
- Kim, G.P.; Colangelo, L.H.; Wieand, H.S.; Paik, S.; Kirsch, I.R.; Wolmark, N.; Allegra, C.J. Prognostic and Predictive Roles of High-Degree Microsatellite Instability in Colon Cancer: A National Cancer Institute–National Surgical Adjuvant Breast and Bowel Project Collaborative Study. J. Clin. Oncol. 2007, 25, 767–772. [Google Scholar] [CrossRef]
- Lech, G.; Słotwiński, R.; Słodkowski, M.; Krasnodębski, I.W. Colorectal cancer tumour markers and biomarkers: Recent therapeutic advances. World J. Gastroenterol. 2016, 22, 1745–1755. [Google Scholar] [CrossRef]
- Huang, Z.; Huang, D.; Ni, S.; Peng, Z.; Sheng, W.; Du, X. Plasma microRNAs are promising novel biomarkers for early detection of colorectal cancer. Int. J. Cancer 2010, 127, 118–126. [Google Scholar] [CrossRef]
- Durán-Vinet, B.; Araya-Castro, K.; Calderón, J.; Vergara, L.; Weber, H.; Retamales, J.; Araya-Castro, P.; Leal-Rojas, P. CRISPR/Cas13-Based Platforms for a Potential Next-Generation Diagnosis of Colorectal Cancer through Exosomes Micro-RNA Detection: A Review. Cancers 2021, 13, 4640. [Google Scholar] [CrossRef]
- Giessen, C.; Nagel, D.; Glas, M.; Spelsberg, F.; Lau-Werner, U.; Modest, D.P.; Michl, M.; Heinemann, V.; Stieber, P.; Schulz, C. Evaluation of preoperative serum markers for individual patient prognosis in stage I–III rectal cancer. Tumor Biol. 2014, 35, 10237–10248. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.F.; Xu, Z.B.; Zhu, X.J.; Liu, J.L.; Gao, F.L.; Wu, C.L.; Song, B.; Tao, X.; Lin, Q. Serum Cyr61 as a potential biomarker for diagnosis of colorectal cancer. Clin. Transl. Oncol. 2017, 19, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, M.; Weigl, K.; Tikk, K.; Benner, A.; Schrotz-King, P.; Brenner, H. Multiplex screening of 275 plasma protein biomarkers to identify a signature for early detection of colorectal cancer. Mol. Oncol. 2020, 14, 8–21. [Google Scholar] [CrossRef] [Green Version]
- Villar-Vázquez, R.; Padilla, G.; Fernández-Aceñero, M.J.; Suárez, A.; Fuente, E.; Pastor, C.; Calero, M.; Barderas, R.; Casal, J.I. Development of a novel multiplex beads-based assay for autoantibody detection for colorectal cancer diagnosis. Proteomics 2016, 16, 1280–1290. [Google Scholar] [CrossRef] [PubMed]
- Hashim, N.A.A.; Ab-Rahim, S.; Ngah, W.Z.W.; Nathan, S.; Ab Mutalib, N.S.; Sagap, I.; Jamal, A.R.A.; Mazlan, M. Global metabolomics profiling of colorectal cancer in Malaysian patients. BioImpacts 2021, 11, 33–43. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, Y.; Zhao, W.; Deng, K.; Wang, Z.; Yang, C.; Ma, L.; Openkova, M.S.; Hou, Y.; Li, K. Metabolomics for biomarker discovery in the diagnosis, prognosis, survival and recurrence of colorectal cancer: A systematic review. Oncotarget 2017, 8, 35460–35472. [Google Scholar] [CrossRef] [Green Version]
- Hata, T.; Takemasa, I.; Takahashi, H.; Haraguchi, N.; Nishimura, J.; Hata, T.; Mizushima, T.; Doki, Y.; Mori, M. Downregulation of serum metabolite GTA-446 as a novel potential marker for early detection of colorectal cancer. Br. J. Cancer 2017, 117, 227–232. [Google Scholar] [CrossRef]
- Gu, J.; Xiao, Y.; Shu, D.; Liang, X.; Hu, X.; Xie, Y.; Lin, D.; Li, H. Metabolomics Analysis in Serum from Patients with Colorectal Polyp and Colorectal Cancer by 1H-NMR Spectrometry. Dis. Markers 2019, 2019, 3491852. [Google Scholar] [CrossRef] [Green Version]
- Nishiumi, S.; Kobayashi, T.; Kawana, S.; Unno, Y.; Sakai, T.; Okamoto, K.; Yamada, Y.; Sudo, K.; Yamaji, T.; Saito, Y.; et al. Investigations in the possibility of early detection of colorectal cancer by gas chromatography/triple-quadrupole mass spectrometry. Oncotarget 2017, 8, 17115–17126. [Google Scholar] [CrossRef] [Green Version]
- Theodoratou, E.; Thaçi, K.; Agakov, F.; Timofeeva, M.N.; Štambuk, J.; Pučić-Baković, M.; Vučković, F.; Orchard, P.; Agakova, A.; Din, F.V.N.; et al. Glycosylation of plasma IgG in colorectal cancer prognosis. Sci. Rep. 2016, 6, 28098. [Google Scholar] [CrossRef]
- Doherty, M.; Theodoratou, E.; Walsh, I.; Adamczyk, B.; Stöckmann, H.; Agakov, F.; Timofeeva, M.; Trbojević-Akmačić, I.; Vučković, F.; Duffy, F.; et al. Plasma N-glycans in colorectal cancer risk. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhang, L.; Zhang, R.; Han, J.; Qin, W.; Gu, Y.; Sha, J.; Xu, X.; Feng, Y.; Ren, Z.; et al. Screening and diagnosis of colorectal cancer and advanced adenoma by Bionic Glycome method and machine learning. Am. J. Cancer Res. 2021, 11, 3002–3020. [Google Scholar] [PubMed]
- Coura, M.D.M.A.; Barbosa, E.A.; Brand, G.D.; Bloch, C.; Sousa, J.B. De Identification of Differential N-Glycan Compositions in the Serum and Tissue of Colon Cancer Patients by Mass Spectrometry. Biology 2021, 10, 343. [Google Scholar] [CrossRef] [PubMed]
- Boland, C.R.; Thibodeau, S.N.; Hamilton, S.R.; Sidransky, D.; Eshleman, J.R.; Burt, R.W.; Meltzer, S.J.; Rodriguez-Bigas, M.A.; Fodde, R.; Ranzani, G.N.; et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: Development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998, 58, 5248–5257. [Google Scholar]
- Rocker, J.M.; DiPalma, J.A.; Pannell, L.K. Rectal Effluent as a Research Tool. Am. J. Dig. Dis. 2015, 60, 24–31. [Google Scholar] [CrossRef]
- Heinzlmann, M.; Neynaber, S.; Heldwein, W.; Folwaczny, C. K-ras and p53 mutations in colonic lavage fluid of patients with colorectal neoplasias. Digestion 2001, 63, 229–233. [Google Scholar] [CrossRef] [Green Version]
- Potter, M.A.; Morris, R.G.; Wyllie, A.H.; Ferguson, A. Detection of Mutations Associated With Colorectal Cancer in DNA From Whole-Gut Lavage Fluid. JNCI J. Natl. Cancer Inst. 1998, 90, 623–626. [Google Scholar] [CrossRef] [Green Version]
- Shen, W.; Sun, J.; Yao, F.; Lin, K.; Yuan, Y.; Chen, Y.; Han, H.; Li, Z.; Zou, J.; Jiao, X. Microbiome in Intestinal Lavage Fluid May Be A Better Indicator in Evaluating The Risk of Developing Colorectal Cancer Compared with Fecal Samples. Transl. Oncol. 2020, 13, 100772. [Google Scholar] [CrossRef]
- Yuan, Y.; Chen, Y.; Yao, F.; Zeng, M.; Xie, Q.; Shafiq, M.; Noman, S.M.; Jiao, X. Microbiomes and Resistomes in Biopsy Tissue and Intestinal Lavage Fluid of Colorectal Cancer. Front. Cell Dev. Biol. 2021, 9. [Google Scholar] [CrossRef]
- Harada, T.; Yamamoto, E.; Yamano, H.-O.; Nojima, M.; Maruyama, R.; Kumegawa, K.; Ashida, M.; Yoshikawa, K.; Kimura, T.; Harada, E.; et al. Analysis of DNA Methylation in Bowel Lavage Fluid for Detection of Colorectal Cancer. Cancer Prev. Res. 2014, 7, 1002–1010. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.S.; Kim, D.S.; Cho, S.W.; Park, J.W.; Jeon, S.J.; Moon, T.J.; Kim, S.H.; Son, B.K.; Oh, T.J.; An, S.; et al. Analysis of Syndecan-2 Methylation in Bowel Lavage Fluid for the Detection of Colorectal Neoplasm. Gut Liver 2018, 12, 508–515. [Google Scholar] [CrossRef] [Green Version]
- Brydon, W.G.; Ferguson, A. Haemoglobin in gut lavage fluid as a measure of gastrointestinal blood loss. Lancet 1992, 340, 1381–1382. [Google Scholar] [CrossRef]
- Namavar, F.; Theunissen, E.B.M.; Vught, A.M.J.J.V.-V.; Peerbooms, P.G.H.; Bal, M.; Hoitsma, H.F.W.; MacLaren, D.M. Epidemiology of the Bacteroides fragilis group in the colonic flora in 10 patients with colonic cancer. J. Med Microbiol. 1989, 29, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Arain, M.A.; Chen, Y.-J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Farkas, L.; et al. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 329–359. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Daza, J.; Itzel, T.; Betge, J.; Zhan, T.; Marmé, F.; Teufel, A. Prognostic Cancer Gene Expression Signatures: Current Status and Challenges. Cells 2021, 10, 648. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.-C.; Yeh, Y.-M.; Chan, R.-H.; Lin, B.-W.; Chen, P.-C.; Pan, C.-C.; Shen, M.-R. Sequential and co-occurring DNA damage response genetic mutations impact survival in stage III colorectal cancer patients receiving adjuvant oxaliplatin-based chemotherapy. BMC Cancer 2021, 21, 1–11. [Google Scholar] [CrossRef]
- Wills, C.; He, Y.; Summers, M.G.; Lin, Y.; Phipps, A.I.; Watts, K.; Law, P.J.; Al-Tassan, N.A.; Maughan, T.S.; Kaplan, R.; et al. A genome-wide search for determinants of survival in 1926 patients with advanced colorectal cancer with follow-up in over 22,000 patients. Eur. J. Cancer 2021, 159, 247–258. [Google Scholar] [CrossRef]
- Chiu, J.W.; Krzyzanowska, M.K.; Serra, S.; Knox, J.J.; Dhani, N.; Mackay, H.; Hedley, D.; Moore, M.; Liu, G.; Burkes, R.L.; et al. Molecular Profiling of Patients With Advanced Colorectal Cancer: Princess Margaret Cancer Centre Experience. Clin. Color. Cancer 2018, 17, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Guinney, J.; Dienstmann, R.; Wang, X.; De Reyniès, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef]
- Berg, I.V.D.; Smid, M.; Braak, R.R.J.C.V.D.; van de Wiel, M.A.; van Deurzen, C.H.; de Weerd, V.; Martens, J.W.M.; Ijzermans, J.N.M.; Wilting, S.M. A panel of DNA methylation markers for the classification of consensus molecular subtypes 2 and 3 in patients with colorectal cancer. Mol. Oncol. 2021, 15, 3348–3362. [Google Scholar] [CrossRef]
- Li, D.-H.; Du, X.-H.; Liu, M.; Zhang, R. A 10-gene-methylation-based signature for prognosis prediction of colorectal cancer. Cancer Genet. 2021, 252, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zang, Y.; Yang, Y.; Xiang, J.; Chen, Z. Candidate genes involved in metastasis of colon cancer identified by integrated analysis. Cancer Med. 2019, 8, 2338–2347. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Liu, Y.; Jiang, C.; Sun, L.; Zhou, H. Identification and clinical validation of metastasis-associated biomarkers based on large-scale samples in colon-adenocarcinoma. Pharmacol. Res. 2020, 160, 105087. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Xiao, H.; Shen, J.; Qiao, X.; Zhang, F.; Zhang, W.; Gao, Y.; Liu, Y.D. SELE gene as a characteristic prognostic biomarker of colorectal cancer. J. Int. Med. Res. 2021, 49. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Han, T.; Zhang, Z.; Yi, P.; Zhang, C.; Zhang, S.; Peng, W. Identification and Validation of Six Autophagy-related Long Non-coding RNAs as Prognostic Signature in Colorectal Cancer. Int. J. Med. Sci. 2020, 18, 88–98. [Google Scholar] [CrossRef]
- Xi, G.; Ziyu, X.; Yiting, L.; Zonghang, L.; Lifeng, Z. Construction of competing endogenous RNA network and identification of novel molecular biomarkers in colon cancer: A bioinformatic analysis. Medicine 2021, 100, e25369. [Google Scholar] [CrossRef]
- Buttacavoli, M.; Albanese, N.N.; Roz, E.; Pucci-Minafra, I.; Feo, S.; Cancemi, P. Proteomic Profiling of Colon Cancer Tissues: Discovery of New Candidate Biomarkers. Int. J. Mol. Sci. 2020, 21, 3096. [Google Scholar] [CrossRef]
- Vasaikar, S.; Huang, C.; Wang, X.; Petyuk, V.A.; Savage, S.R.; Wen, B.; Dou, Y.; Zhang, Y.; Shi, Z.; Arshad, O.A.; et al. Proteogenomic Analysis of Human Colon Cancer Reveals New Therapeutic Opportunities. Cell 2019, 177, 1035–1049. [Google Scholar] [CrossRef] [Green Version]
- Sethi, M.K.; Kim, H.; Park, C.K.; Baker, M.S.; Paik, Y.; Packer, N.H.; Hancock, W.S.; Fanayan, S.; Thaysen-andersen, M. In-depth N -glycome profiling of paired colorectal cancer and non-tumorigenic tissues reveals cancer-, stage- and EGFR-specific protein. Glycobiology 2015, 25, 1064–1078. [Google Scholar] [CrossRef] [Green Version]
- Sethi, M.K.; Hancock, W.S.; Fanayan, S. Identifying N-Glycan Biomarkers in Colorectal Cancer by Mass Spectrometry. Accounts Chem. Res. 2016, 49, 2099–2106. [Google Scholar] [CrossRef]
- Zhang, D.; Xie, Q.; Wang, Q.; Wang, Y.; Miao, J.; Li, L.; Zhang, T.; Cao, X.; Li, Y. Mass spectrometry analysis reveals aberrant N-glycans in colorectal cancer tissues. Glycobiology 2019, 29, 372–384. [Google Scholar] [CrossRef] [PubMed]
- Boyaval, F.; van Zeijl, R.; Dalebout, H.; Holst, S.; van Pelt, G.; Fariña-Sarasqueta, A.; Mesker, W.; Tollenaar, R.; Morreau, H.; Wuhrer, M.; et al. N -Glycomic Signature of Stage II Colorectal Cancer and Its Association with the Tumor Authors N -Glycomic Signature of Stage II Colorectal Cancer and Its Association with the Tumor Microenvironment. Mol. Cell Proteom. 2021, 20, 100057. [Google Scholar] [CrossRef] [PubMed]
- Marolla, A.P.C.; Waisberg, J.; Saba, G.T.; Waisberg, D.R.; Margeotto, F.B.; Pinhal, M.A.D.S. Glycomics expression analysis of sulfated glycosaminoglycans of human colorectal cancer tissues and non-neoplastic mucosa by electrospray ionization mass spectrometry. Einstein 2015, 13, 510–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishn, S.R.; Kaur, S.; Smith, L.M.; Johansson, S.L.; Jain, M.; Patel, A.; Gautam, S.K.; Hollingsworth, M.A.; Mandel, U.; Clausen, H.; et al. Mucins and associated glycan signatures in colon adenoma–carcinoma sequence: Prospective pathological implication(s) for early diagnosis of colon cancer. Cancer Lett. 2016, 374, 304–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, W.; Yang, Y.; Li, X.; Huang, M.; Xu, F.; Ge, W.; Zhang, S.; Zheng, S. Multi-omics Approach Reveals Distinct Differences in Left- and Right-Sided Colon Cancer. Mol. Cancer Res. 2018, 16, 476–485. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Jin, W.; Liu, H.; Wang, X.; Wu, J.; Gan, D.; Cui, C.; Han, Y.; Han, C.; Wang, Z. A novel prognostic model based on multi-omics features predicts the prognosis of colon cancer patients. Mol. Genet. Genom. Med. 2020, 8, e1255. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-López, L.; Blancas, I.; Garrido, J.M.; Mut-Salud, N.; Moya-Jódar, M.; Osuna, A.; Rodríguez-Serrano, F. The role of exosomes on colorectal cancer: A review. J. Gastroenterol. Hepatol. 2018, 33, 792–799. [Google Scholar] [CrossRef] [Green Version]
- Bracci, L.; Lozupone, F.; Parolini, I. The role of exosomes in colorectal cancer disease progression and respone to therapy. Cytokine Growth Factor Rev. 2020, 51, 84–91. [Google Scholar] [CrossRef]
- Zhou, J.; Li, X.-L.; Chen, Z.-R.; Chng, W.-J. Tumor-derived exosomes in colorectal cancer progression and their clinical applications. Oncotarget 2017, 8, 100781–100790. [Google Scholar] [CrossRef] [Green Version]
- Umwali, Y.; Yue, C.-B.; Gabriel, A.N.A.; Zhang, Y.; Zhang, X. Roles of exosomes in diagnosis and treatment of colorectal cancer. World J. Clin. Cases 2021, 9, 4467–4479. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhong, J.; Zhong, B.; Huang, J.; Jiang, L.; Jiang, Y.; Yuan, J.; Sun, J.; Dai, L.; Yang, C.; et al. Exosomes as potential sources of biomarkers in colorectal cancer. Cancer Lett. 2020, 476, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Siveen, K.S.; Raza, A.; Ahmed, E.I.; Khan, A.Q.; Prabhu, K.S.; Kuttikrishnan, S.; Mateo, J.M.; Zayed, H.; Rasul, K.; Azizi, F.; et al. The Role of Extracellular Vesicles as Modulators of the Tumor Microenvironment, Metastasis and Drug Resistance in Colorectal Cancer. Cancers 2019, 11, 746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, X.; Zhong, X.; Hu, Z.; Zhao, S.; Wei, P.; Li, D. An insight into small extracellular vesicles: Their roles in colorectal cancer progression and potential clinical applications An insight into small extracellular vesicles: Their roles in colorectal cancer progression and potential clinical applications. Clin. Transl. Med. 2020, 10, e249. [Google Scholar] [CrossRef] [PubMed]
| Omics | Biomarker | Change | Reference |
|---|---|---|---|
| Volatolomics (GC-MS) | Benzaldehyde, Benzene ethyl, Indole | Upregulated | [30] |
| Volatolomics (GC-IMR-MS) | 1,3-butadiene, N2O | Upregulated | [32] |
| Volatolomics (GC-IMR-MS) | Acetic acid, HNO2 | Downregulated | [32] |
| Volatolomics (GC-MS) | 1,3,5-cycloheptatriene | Upregulated | [40] |
| Volatolomics (GC-MS) | Tetradecane, Ethylbenzene, Methylbenzene, 5,9-Undecadien-2-one, 6,10-dimethyl, Benzaldehyde, Decane, Benzoic acid, 1,3-Bis(1-methylethenyl) benzene, Dodecane, Ethanone, 1[4-(1-methylethenyl)phenyl], acetic acid | Upregulated | [40,41] |
| Volatolomics (GC-MS) | Decanal, 2-Ethyl-1-hexanol | Downregulated | [40] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alorda-Clara, M.; Torrens-Mas, M.; Morla-Barcelo, P.M.; Martinez-Bernabe, T.; Sastre-Serra, J.; Roca, P.; Pons, D.G.; Oliver, J.; Reyes, J. Use of Omics Technologies for the Detection of Colorectal Cancer Biomarkers. Cancers 2022, 14, 817. https://doi.org/10.3390/cancers14030817
Alorda-Clara M, Torrens-Mas M, Morla-Barcelo PM, Martinez-Bernabe T, Sastre-Serra J, Roca P, Pons DG, Oliver J, Reyes J. Use of Omics Technologies for the Detection of Colorectal Cancer Biomarkers. Cancers. 2022; 14(3):817. https://doi.org/10.3390/cancers14030817
Chicago/Turabian StyleAlorda-Clara, Marina, Margalida Torrens-Mas, Pere Miquel Morla-Barcelo, Toni Martinez-Bernabe, Jorge Sastre-Serra, Pilar Roca, Daniel Gabriel Pons, Jordi Oliver, and Jose Reyes. 2022. "Use of Omics Technologies for the Detection of Colorectal Cancer Biomarkers" Cancers 14, no. 3: 817. https://doi.org/10.3390/cancers14030817
APA StyleAlorda-Clara, M., Torrens-Mas, M., Morla-Barcelo, P. M., Martinez-Bernabe, T., Sastre-Serra, J., Roca, P., Pons, D. G., Oliver, J., & Reyes, J. (2022). Use of Omics Technologies for the Detection of Colorectal Cancer Biomarkers. Cancers, 14(3), 817. https://doi.org/10.3390/cancers14030817









