Equal Efficacy and Safety Profile in Elderly Patients with Hepatocellular Carcinoma Receiving Palliative Treatment
Abstract
Simple Summary
Abstract
1. Introduction
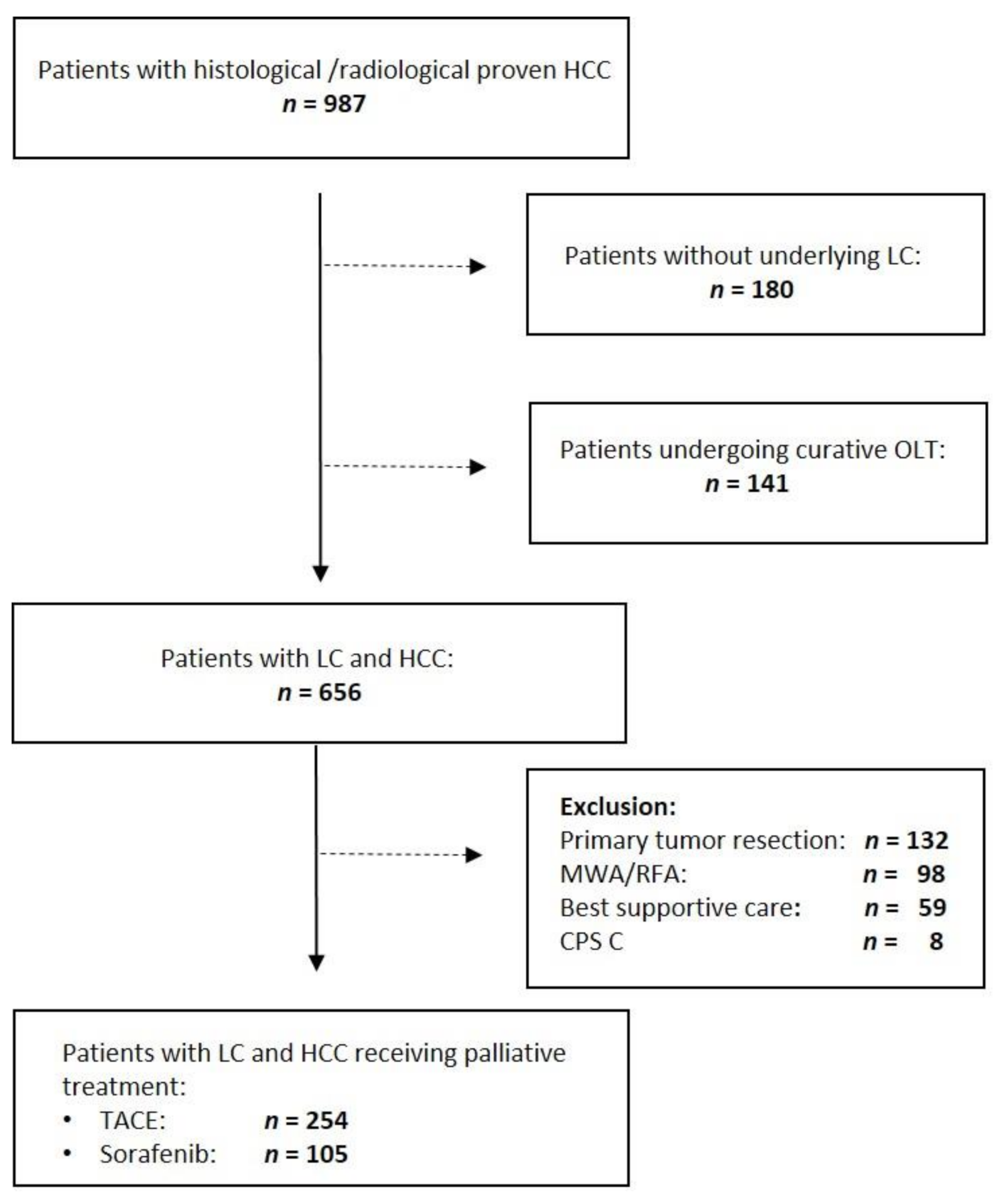
2. Materials and Methods
2.1. Study Population
2.2. Transarterial Chemoembolization
2.3. Systemic Therapy
2.4. Statistical Analysis
2.5. Ethical Approval
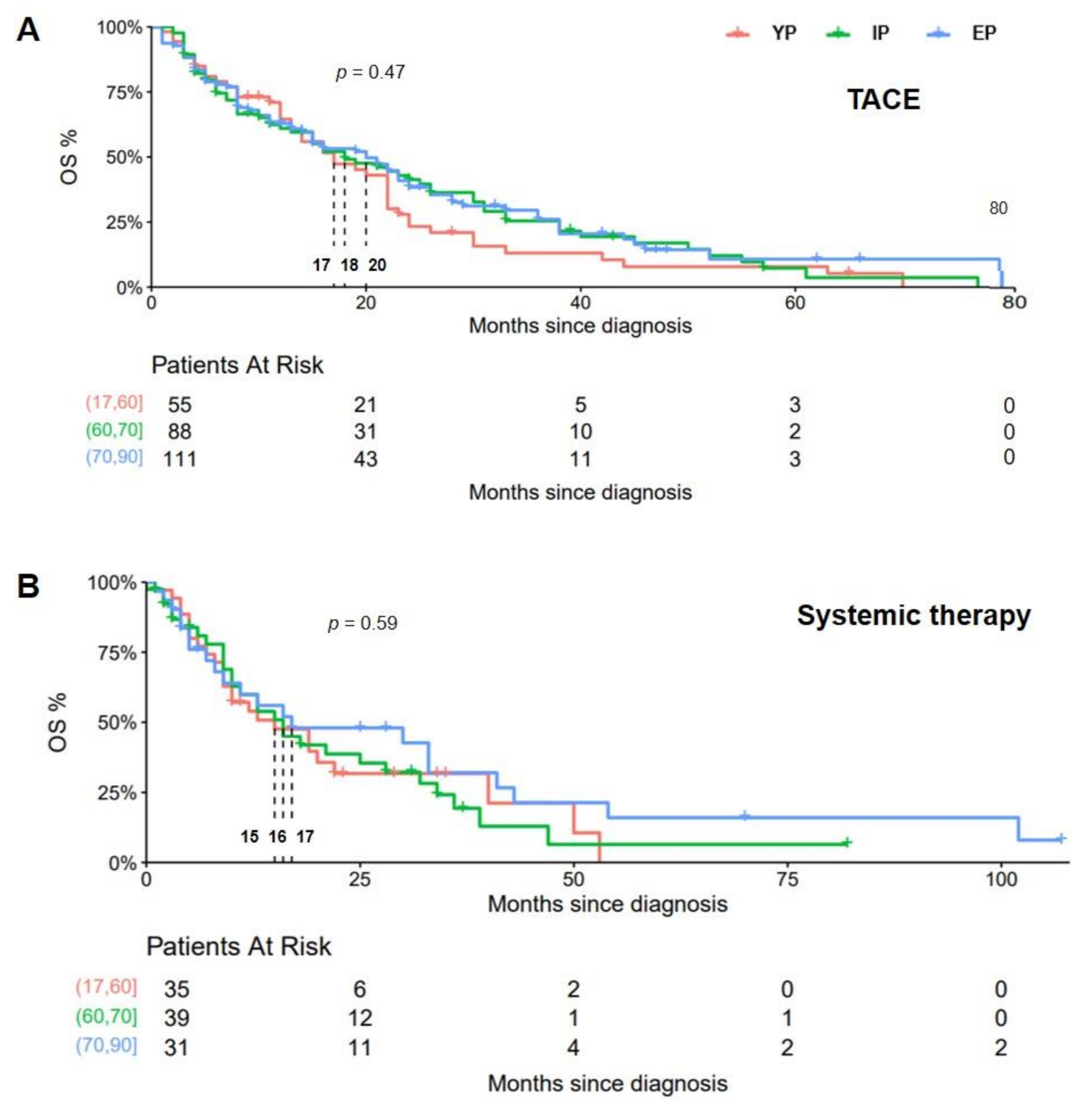
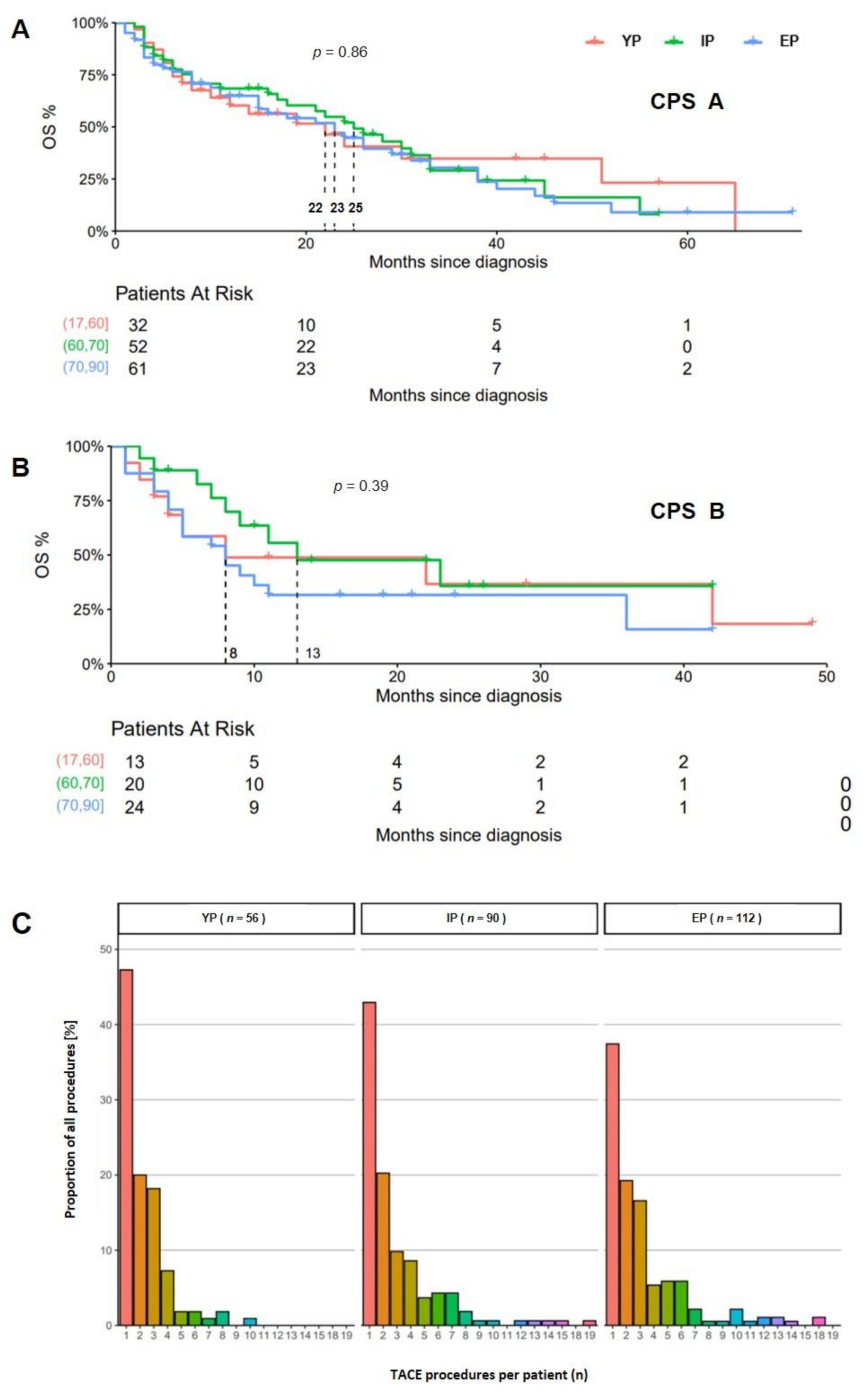
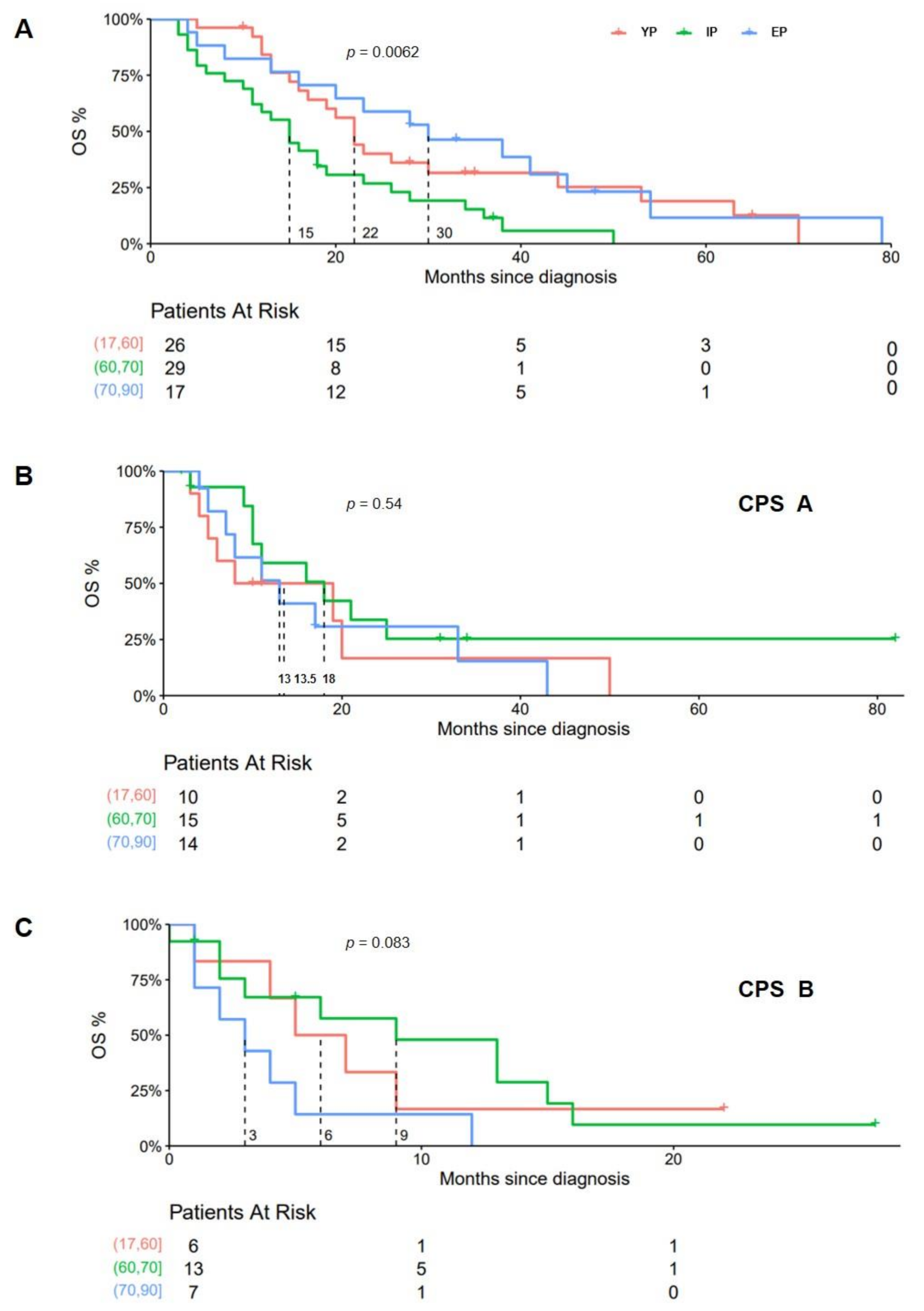
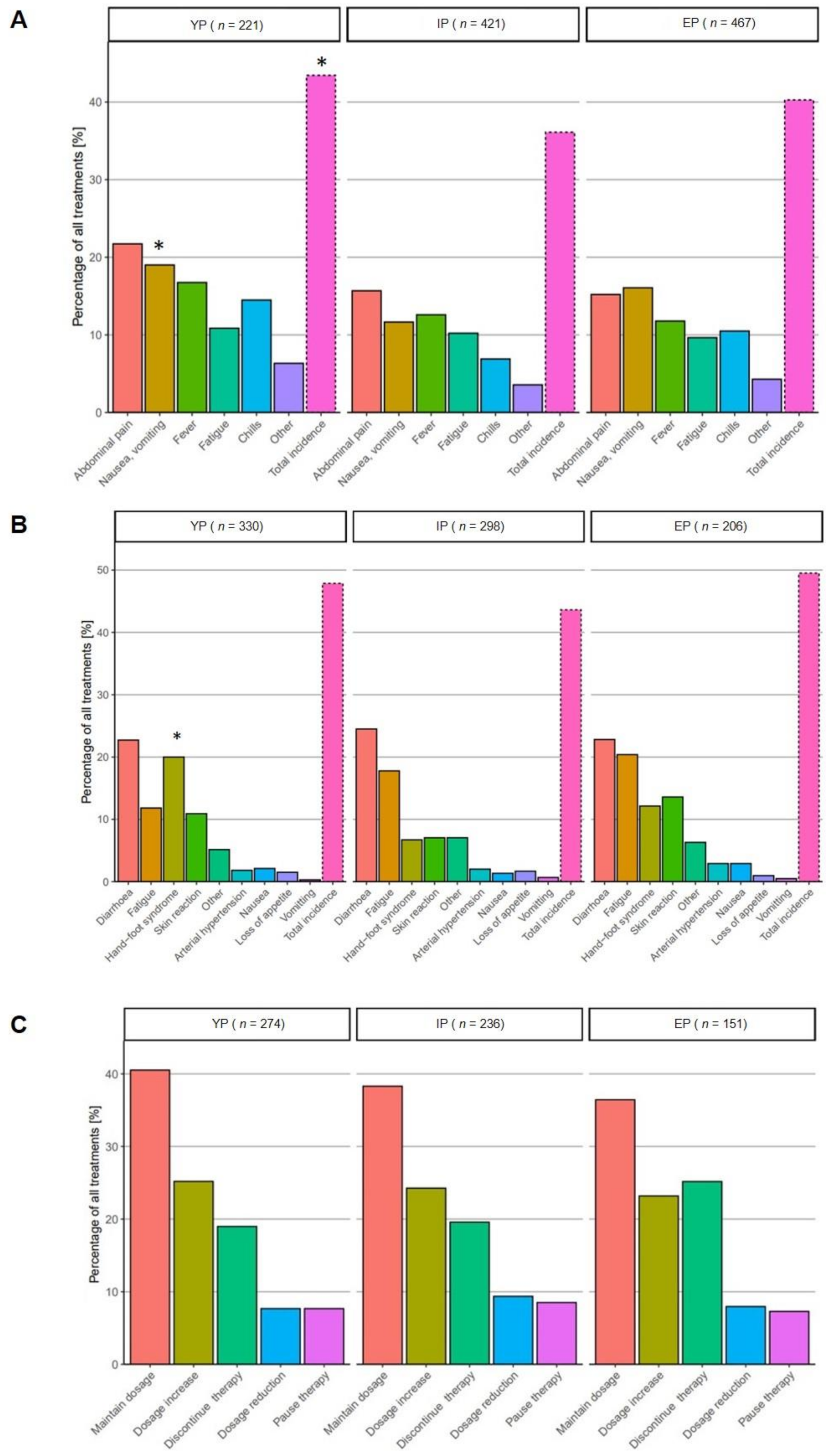
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bertuccio, P.; Turati, F.; Carioli, G.; Rodriguez, T.; La Vecchia, C.; Malvezzi, M.; Negri, E. Global Trends and Predictions in Hepatocellular Carcinoma Mortality. J. Hepatol. 2017, 67, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Huang, D.Q.; El-Serag, H.B.; Loomba, R. Global Epidemiology of NAFLD-Related HCC: Trends, Predictions, Risk Factors and Prevention. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 223–238. [Google Scholar] [CrossRef] [PubMed]
- El-Serag, H.B.; Kanwal, F. Epidemiology of Hepatocellular Carcinoma in the United States: Where Are We? Where Do We Go? Hepatology 2014, 60, 1767–1775. [Google Scholar] [CrossRef]
- Nishikawa, H.; Kimura, T.; Kita, R.; Osaki, Y. Treatment for Hepatocellular Carcinoma in Elderly Patients: A Literature Review. J. Cancer 2013, 4, 635–643. [Google Scholar] [CrossRef] [PubMed]
- El-Serag, H.B.; Rudolph, K.L. Hepatocellular Carcinoma: Epidemiology and Molecular Carcinogenesis. Gastroenterology 2007, 132, 2557–2576. [Google Scholar] [CrossRef] [PubMed]
- Poon, R.T.; Fan, S.T.; Lo, C.M.; Liu, C.L.; Ngan, H.; Ng, I.O.; Wong, J. Hepatocellular Carcinoma in the Elderly: Results of Surgical and Nonsurgical Management. Am. J. Gastroenterol. 1999, 94, 2460–2466. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.K.; Barbas, A.S.; Turley, R.S.; Gamblin, T.C.; Geller, D.A.; Marsh, J.W.; Tsung, A.; Clary, B.M.; Lagoo-Deenadayalan, S. Major Liver Resection in Elderly Patients: A Multi-Institutional Analysis. J. Am. Coll. Surg. 2011, 212, 787–795. [Google Scholar] [CrossRef]
- Huang, J.; Li, B.-K.; Chen, G.-H.; Li, J.-Q.; Zhang, Y.-Q.; Li, G.-H.; Yuan, Y.-F. Long-Term Outcomes and Prognostic Factors of Elderly Patients with Hepatocellular Carcinoma Undergoing Hepatectomy. J. Gastrointest. Surg. 2009, 13, 1627–1635. [Google Scholar] [CrossRef]
- Takahashi, H.; Mizuta, T.; Kawazoe, S.; Eguchi, Y.; Kawaguchi, Y.; Otuka, T.; Oeda, S.; Ario, K.; Iwane, S.; Akiyama, T.; et al. Efficacy and Safety of Radiofrequency Ablation for Elderly Hepatocellular Carcinoma Patients. Hepatol. Res. 2010, 40, 997–1005. [Google Scholar] [CrossRef]
- Song, T.; Lang, M.; Ren, S.; Gan, L.; Lu, W. The Past, Present and Future of Conversion Therapy for Liver Cancer. Am. J. Cancer Res. 2021, 11, 4711–4724. [Google Scholar] [PubMed]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD Guidelines for the Treatment of Hepatocellular Carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of Hepatocellular Carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Meyer, T.; Cheng, A.-L.; El-Khoueiry, A.B.; Rimassa, L.; Ryoo, B.-Y.; Cicin, I.; Merle, P.; Chen, Y.; Park, J.-W.; et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N. Engl. J. Med. 2018, 379, 54–63. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.-H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.-W.; Han, G.; Jassem, J.; et al. Lenvatinib versus Sorafenib in First-Line Treatment of Patients with Unresectable Hepatocellular Carcinoma: A Randomised Phase 3 Non-Inferiority Trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef]
- Zhu, A.X.; Park, J.O.; Ryoo, B.-Y.; Yen, C.-J.; Poon, R.; Pastorelli, D.; Blanc, J.-F.; Chung, H.C.; Baron, A.D.; Pfiffer, T.E.F.; et al. Ramucirumab versus Placebo as Second-Line Treatment in Patients with Advanced Hepatocellular Carcinoma Following First-Line Therapy with Sorafenib (REACH): A Randomised, Double-Blind, Multicentre, Phase 3 Trial. Lancet Oncol. 2015, 16, 859–870. [Google Scholar] [CrossRef]
- Bechis, S.K.; Carroll, P.R.; Cooperberg, M.R. Impact of Age at Diagnosis on Prostate Cancer Treatment and Survival. J. Clin. Oncol. 2011, 29, 235–241. [Google Scholar] [CrossRef]
- Adami, H.O.; Malker, B.; Holmberg, L.; Persson, I.; Stone, B. The Relation between Survival and Age at Diagnosis in Breast Cancer. N. Engl. J. Med. 1986, 315, 559–563. [Google Scholar] [CrossRef]
- Haymart, M.R. Understanding the Relationship Between Age and Thyroid Cancer. Oncologist 2009, 14, 216–221. [Google Scholar] [CrossRef]
- Leff, D.R.; Chen, A.; Roberts, D.; Grant, K.; Western, C.; Windsor, A.C.J.; Cohen, C.R.G. Colorectal Cancer in the Young Patient. Am. Surg. 2007, 73, 42–47. [Google Scholar] [CrossRef]
- Guo, H.; Wu, T.; Lu, Q.; Dong, J.; Ren, Y.-F.; Nan, K.-J.; Lv, Y.; Zhang, X.-F. Hepatocellular Carcinoma in Elderly: Clinical Characteristics, Treatments and Outcomes Compared with Younger Adults. PLoS ONE 2017, 12, e0184160. [Google Scholar] [CrossRef]
- Common Terminology Criteria for Adverse Events (CTCAE) v5.0. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_50 (accesses on 4 April 2021).
- McGlynn, K.A.; Petrick, J.L.; London, W.T. Global Epidemiology of Hepatocellular Carcinoma: An Emphasis on Demographic and Regional Variability. Clin. Liver Dis. 2015, 19, 223–238. [Google Scholar] [CrossRef]
- Rich, N.E.; Yopp, A.C.; Singal, A.G.; Murphy, C.C. Hepatocellular Carcinoma Incidence Is Decreasing Among Younger Adults in the United States. Clin. Gastroenterol. Hepatol. 2020, 18, 242–248.e5. [Google Scholar] [CrossRef] [PubMed]
- Oishi, K.; Itamoto, T.; Kobayashi, T.; Oshita, A.; Amano, H.; Ohdan, H.; Tashiro, H.; Asahara, T. Hepatectomy for Hepatocellular Carcinoma in Elderly Patients Aged 75 Years or More. J. Gastrointest. Surg. 2009, 13, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Dohmen, K.; Shigematsu, H.; Irie, K.; Ishibashi, H. Comparison of the Clinical Characteristics among Hepatocellular Carcinoma of Hepatitis B, Hepatitis C and Non-B Non-C Patients. Hepato-Gastroenterology 2003, 50, 2022–2027. [Google Scholar]
- Mirici-Cappa, F.; Gramenzi, A.; Santi, V.; Zambruni, A.; Micoli, A.D.; Frigerio, M.; Maraldi, F.; Nolfo, M.A.D.; Poggio, P.D.; Benvegnù, L.; et al. Treatments for Hepatocellular Carcinoma in Elderly Patients Are as Effective as in Younger Patients: A 20-Year Multicentre Experience. Gut 2010, 59, 387–396. [Google Scholar] [CrossRef]
- Liu, P.-H.; Hsu, C.-Y.; Lee, Y.-H.; Hsia, C.-Y.; Huang, Y.-H.; Su, C.-W.; Chiou, Y.-Y.; Lin, H.-C.; Huo, T.-I. Uncompromised Treatment Efficacy in Elderly Patients With Hepatocellular Carcinoma: A Propensity Score Analysis. Medicine 2014, 93, e264. [Google Scholar] [CrossRef]
- Hepatozelluläres Karzinom. Available online: https://www.dgvs.de/wissen-kompakt/leitlinien/leitlinien-der-dgvs/hepatozellulaeres-karzinom/ (accessed on 25 September 2021).
- Llovet, J.M.; Real, M.I.; Montaña, X.; Planas, R.; Coll, S.; Aponte, J.; Ayuso, C.; Sala, M.; Muchart, J.; Solà, R.; et al. Arterial Embolisation or Chemoembolisation versus Symptomatic Treatment in Patients with Unresectable Hepatocellular Carcinoma: A Randomised Controlled Trial. Lancet 2002, 359, 1734–1739. [Google Scholar] [CrossRef]
- Biselli, M.; Forti, P.; Mucci, F.; Foschi, F.G.; Marsigli, L.; Caputo, F.; Ravaglia, G.; Bernardi, M.; Stefanini, G.F. Chemoembolization versus Chemotherapy in Elderly Patients with Unresectable Hepatocellular Carcinoma and Contrast Uptake as Prognostic Factor. J. Gerontol. A Biol. Sci. Med. Sci. 1997, 52, M305–M309. [Google Scholar] [CrossRef][Green Version]
- Mosconi, C.; Gramenzi, A.; Biselli, M.; Cappelli, A.; Bruno, A.; De Benedittis, C.; Cucchetti, A.; Modestino, F.; Peta, G.; Bianchi, G.; et al. Survival and Tolerability of Transarterial Chemoembolization in Greater Versus Less than 70 Years of Age Patients with Unresectable Hepatocellular Carcinoma: A Propensity Score Analysis. Cardiovasc. Interv. Radiol. 2020, 43, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Yau, T.; Yao, T.J.; Chan, P.; Epstein, R.J.; Ng, K.K.; Chok, S.H.; Cheung, T.T.; Fan, S.T.; Poon, R.T.P. The Outcomes of Elderly Patients with Hepatocellular Carcinoma Treated with Transarterial Chemoembolization. Cancer 2009, 115, 5507–5515. [Google Scholar] [CrossRef] [PubMed]
- Takayasu, K.; Arii, S.; Kudo, M.; Ichida, T.; Matsui, O.; Izumi, N.; Matsuyama, Y.; Sakamoto, M.; Nakashima, O.; Ku, Y.; et al. Superselective Transarterial Chemoembolization for Hepatocellular Carcinoma. Validation of Treatment Algorithm Proposed by Japanese Guidelines. J. Hepatol. 2012, 56, 886–892. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.-F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.-L.; Forner, A.; et al. Sorafenib in Advanced Hepatocellular Carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.-L.; Kang, Y.-K.; Chen, Z.; Tsao, C.-J.; Qin, S.; Kim, J.S.; Luo, R.; Feng, J.; Ye, S.; Yang, T.-S.; et al. Efficacy and Safety of Sorafenib in Patients in the Asia-Pacific Region with Advanced Hepatocellular Carcinoma: A Phase III Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Oncol. 2009, 10, 25–34. [Google Scholar] [CrossRef]
- Raoul, J.-L.; Adhoute, X.; Penaranda, G.; Perrier, H.; Castellani, P.; Oules, V.; Bourlière, M. Sorafenib: Experience and Better Manage ment of Side Effects Improve Overall Survival in Hepatocellular Carcinoma Patients: A Real-Life Retrospective Analysis. Liver Cancer 2019, 8, 457–467. [Google Scholar] [CrossRef]
- Marrero, J.A.; Kudo, M.; Venook, A.P.; Ye, S.-L.; Bronowicki, J.-P.; Chen, X.-P.; Dagher, L.; Furuse, J.; Geschwind, J.-F.H.; de Guevara, L.L.; et al. Observational Registry of Sorafenib Use in Clinical Practice across Child-Pugh Subgroups: The GIDEON Study. J. Hepatol. 2016, 65, 1140–1147. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, S.; Chiba, T.; Ooka, Y.; Kanogawa, N.; Motoyama, T.; Suzuki, E.; Tawada, A.; Kanai, F.; Yoshikawa, M.; Yokosuka, O. Efficacy of Sorafenib in Intermediate-Stage Hepatocellular Carcinoma Patients Refractory to Transarterial Chemoembolization. Oncology 2014, 87, 330–341. [Google Scholar] [CrossRef] [PubMed]
- Arizumi, T.; Ueshima, K.; Minami, T.; Kono, M.; Chishina, H.; Takita, M.; Kitai, S.; Inoue, T.; Yada, N.; Hagiwara, S.; et al. Effectiveness of Sorafenib in Patients with Transcatheter Arterial Chemoembolization (TACE) Refractory and Intermediate-Stage Hepatocellular Carcinoma. Liver Cancer 2015, 4, 253–262. [Google Scholar] [CrossRef]
- Park, K.H.; Kim, J.H.; Choe, W.H.; Kwon, S.Y.; Yoo, B.C.; Hwang, J.H.; Park, S.W.; Kim, Y.J.; Park, H.S.; Yu, M.H.; et al. Risk Factors for Liver Function Deterioration after Transarterial Chemoembolization Refractoriness in Child-Pugh Class A Hepatocellular Carcinoma Patients. Korean J. Gastroenterol. 2020, 75, 147–156. [Google Scholar] [CrossRef]
- Di Costanzo, G.G.; Tortora, R.; Iodice, L.; Lanza, A.G.; Lampasi, F.; Tartaglione, M.T.; Picciotto, F.P.; Mattera, S.; De Luca, M. Safety and Effectiveness of Sorafenib in Patients with Hepatocellular Carcinoma in Clinical Practice. Dig. Liver Dis. 2012, 44, 788–792. [Google Scholar] [CrossRef]
- Reig, M.; Torres, F.; Rodriguez-Lope, C.; Forner, A.; LLarch, N.; Rimola, J.; Darnell, A.; Ríos, J.; Ayuso, C.; Bruix, J. Early Dermatologic Adverse Events Predict Better Outcome in HCC Patients Treated with Sorafenib. J. Hepatol. 2014, 61, 318–324. [Google Scholar] [CrossRef]
- Tak, K.Y.; Nam, H.C.; Choi, J.Y.; Yoon, S.K.; Kim, C.W.; Kim, H.Y.; Lee, S.W.; Lee, H.L.; Chang, U.I.; Song, D.S.; et al. Effectiveness of Sorafenib Dose Modifications on Treatment Outcome of Hepatocellular Carcinoma: Analysis in Real-Life Settings. Int. J. Cancer 2020, 147, 1970–1978. [Google Scholar] [CrossRef] [PubMed]
- Cochrane Library. Geriatric Factors Predict Chemotherapy Feasibility: Ancillary Results of FFCD 2001-02 Phase III Study in First-Line Chemotherapy for Metastatic Colorectal Cancer in Elderly Patients. Available online: https://www.cochranelibrary.com/central/doi/10.1002/central/CN-00877144/full (accessed on 7 December 2021).
- Shibutani, M.; En, W.; Okazaki, Y.; Kashiwagi, S.; Fukuoka, T.; Iseki, Y.; Hirakawa, K.; Ohira, M. The Efficacy and Safety of Trifluridine/Tipiracil Treatment for Elderly Patients With Metastatic Colorectal Cancer in a Real-World Setting. Anticancer Res. 2021, 41, 6211–6216. [Google Scholar] [CrossRef] [PubMed]
- Kunzmann, V.; Siveke, J.T.; Algül, H.; Goekkurt, E.; Siegler, G.; Martens, U.; Waldschmidt, D.; Pelzer, U.; Fuchs, M.; Kullmann, F.; et al. Nab-Paclitaxel plus Gemcitabine versus Nab-Paclitaxel plus Gemcitabine Followed by FOLFIRINOX Induction Chemotherapy in Locally Advanced Pancreatic Cancer (NEOLAP-AIO-PAK-0113): A Multicentre, Randomised, Phase 2 Trial. Lancet Gastroenterol. Hepatol. 2021, 6, 128–138. [Google Scholar] [CrossRef]
- Nieß, H.; Kleespies, A.; Andrassy, J.; Pratschke, P.; Angele, M.K.; Guba, M.; Jauch, K.-W.; Bruns, C.J. Pankreaskarzinom im hohen Alter. Chirurg 2013, 84, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Feilhauer, K.; Hennig, R.; Lenz, S.; Köninger, J. Pancreatic resection in the elderly: Is the risk justified? Chir. Z. Alle Geb. Oper. Medizen 2015, 86, 670–675. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | YP | IP | EP | p |
|---|---|---|---|---|
| n | 194 | 241 | 221 | |
| Age (median; min/max) | 56 (23–60) | 66 (61–70) | 75 (71–87) | |
| Male (n; %) | 164 (84.5%) | 198 (82.2%) | 180 (81%) | 0.61 |
| ECOG (n; %) | ||||
| 0 | 69 (40.83) | 76 (36.54) | 61 (33.52) | 0.85 |
| 1 | 63 (37.28) | 85 (40.87) | 75 (41.21) | |
| 2 | 28 (16.57) | 29 (13.94) | 32 (17.58) | |
| 3 | 7 (4.14) | 14 (6.73) | 11 (6.04) | |
| BCLC (n; %) | ||||
| A | 55 (31.07) | 44 (20.18) | 38 (19.69) | 0.0054 |
| B | 54 (30.51) | 100 (45.87) | 98 (50.78) | |
| C | 46 (25.99) | 52 (23.85) | 42 (21.76) | |
| D | 22 (12.43) | 22 (10.09) | 15 (7.77) | |
| CPS (n; %) | ||||
| A | 88 (53.33) | 122 (61) | 111 (66.07) | 0.01 |
| B | 47 (28.48) | 53 (26.5) | 47 (27.98) | |
| C | 30 (18.18) | 25 (12.5) | 10 (5.95) | |
| Etiology of LC (n; %) | ||||
| ALD | 95 (38.78) | 136 (49.45) | 105 (41.67) | 0.0005 |
| HBV | 52 (21.22) | 32 (11.64) | 17 (6.75) | |
| HCV | 79 (32.24) | 51 (18.55) | 40 (15.87) | |
| NAFLD/NASH | 6 (2.45) | 17 (6.18) | 24 (9.52) | |
| Other | 13 (5.31) | 39 (14.18) | 66 (26.19) | |
| No. of intrahepatic tumor | ||||
| lesions (n; %) | ||||
| 1 | 65 (43.33) | 80 (42.33) | 77 (44.25) | 0.54 |
| 2 | 35 (23.33) | 42 (22.22) | 33 (18.97) | |
| 3 | 13 (8.67) | 23 (12.17) | 28 (16.09) | |
| >4 | 37 (24.67) | 44 (23.28) | 36 (20.69) | |
| AFP (ng/mL) | ||||
| rang | 1.21–2,018,171.1 | 0.968–457,231.896 | 1.331–249,118.43 | 0.71 |
| median | 106.2 | 49.4 | 57.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fründt, T.W.; Casar, C.; von Felden, J.; Schöler, U.; Priebe, M.; Kraczyk, J.; Ahrend, H.; Salamon, J.; Adam, G.; Huber, S.; et al. Equal Efficacy and Safety Profile in Elderly Patients with Hepatocellular Carcinoma Receiving Palliative Treatment. Cancers 2022, 14, 768. https://doi.org/10.3390/cancers14030768
Fründt TW, Casar C, von Felden J, Schöler U, Priebe M, Kraczyk J, Ahrend H, Salamon J, Adam G, Huber S, et al. Equal Efficacy and Safety Profile in Elderly Patients with Hepatocellular Carcinoma Receiving Palliative Treatment. Cancers. 2022; 14(3):768. https://doi.org/10.3390/cancers14030768
Chicago/Turabian StyleFründt, Thorben W., Christian Casar, Johann von Felden, Ulrike Schöler, Maximilian Priebe, Jenny Kraczyk, Hannes Ahrend, Johannes Salamon, Gerhard Adam, Samuel Huber, and et al. 2022. "Equal Efficacy and Safety Profile in Elderly Patients with Hepatocellular Carcinoma Receiving Palliative Treatment" Cancers 14, no. 3: 768. https://doi.org/10.3390/cancers14030768
APA StyleFründt, T. W., Casar, C., von Felden, J., Schöler, U., Priebe, M., Kraczyk, J., Ahrend, H., Salamon, J., Adam, G., Huber, S., Lohse, A. W., Wege, H., & Schulze, K. (2022). Equal Efficacy and Safety Profile in Elderly Patients with Hepatocellular Carcinoma Receiving Palliative Treatment. Cancers, 14(3), 768. https://doi.org/10.3390/cancers14030768







