Advancing Treatment of Bone Metastases through Novel Translational Approaches Targeting the Bone Microenvironment
Abstract
Simple Summary
Abstract
1. Clinical Burden of Bone Metastases
| Frequency of BM in Advanced Disease | Predominant Type | Frequency of SRE | |
|---|---|---|---|
| Prostate [17,18,23,27,28] | 80–90% | Blastic | 22% (pathologic fracture) 31–48% |
| Breast [29,30,31,32] | 65–80% | Lytic | 39% (pathologic fracture) 40–47% |
| NSCLC [20,33,34,35] | 30–60% | Lytic | 38–63% 20% (pathologic fracture) 10% cord compression |
| SCLC [36,37] | 50–66% | Lytic | 8–34% |
| Renal cell [38,39,40] | 20–68% | Lytic | 70–85% 28% (spinal cord/nerve root compression) |
| Urothelial ** [41] | 32–47% | Lytic | 7% |
| Melanoma [42,43,44] | 17–52% | Lytic | 47–58% |
| Thyroid [45,46,47,48,49] | 4 *–50% | Lytic | 32–78% |
| HCC [50,51,52,53] | 4.5–38% | Lytic | 56% |
| Biliary tract [54,55,56,57] | 2–35% | Lytic | 41% 16% (pathologic fracture) 8% (cord compression) |
| Gastric ** [58,59,60] | 4–45% | Lytic | 31–75% |
| Esophageal ** [61,62,63] | 15–24% | Lytic | 91% |
| Colorectal [64,65,66] | 3–24% | Lytic | 62–68% 8–10% (pathologic fracture) 6–9% (cord compression) |
| Pancreatic [67,68,69] | 2–12% | Blastic | 32–57% 6% (pathologic fracture) 3% (cord compression) |
| Squamous cell carcinoma of the head and neck [70,71,72,73] | 1–16% | Lytic | 9–31% 2–12% (pathologic fracture) 6–7% (cord compression) |
| Endometrial ** [74,75,76] | 1 *–25% | Lytic | NR |
| Ovarian ** [77,78,79] | 1 *–15% | NR | NR |
| Soft Tissue Sarcomas [80,81] | 9–11% | Lytic | 40% 22–31% (pathologic fracture) 13% (cord compression) |
| Multiple Myeloma [82,83,84,85,86] | 80–90% | Lytic | 22–60% 14–34% (pathologic fracture) 4.7–7.8% (cord compression) |
2. Complex Multicellular Composition of Bone and Bone Marrow Metastatic Niches
3. Models of Bone Metastasis
3.1. In Vivo Models
3.2. Bioengineered Microfluidic Models
4. Signaling Pathways Mediating Bone Metastasis and the Status of Clinical Trials of Biologic Agents Targeting Bone Metastases
| Drug | Drug Class | Molecular Target | Phase | Trial Number | Disease Type | Results | |
|---|---|---|---|---|---|---|---|
| Chemokine ligand or receptor | Balixafortide + eribulin | Targeted therapy + non-taxane mitotic inhibitor | Balixafortide—selective CXCR4 antagonist | I | NCT01837095 | HER2-negative BC | ORR 30%, MTD not reached [195] |
| Carlumab | Monoclonal antibody | CCL2 | I/II | NCT00992186 | mCRPC, advanced solid tumors | 9–34% of patients had SD [3] 3 mo, 39% improved pain scores [196,197] | |
| Reparixin + paclitaxel | Targeted therapy + taxane mitotic inhibitor | Reparixin—CXCR1/2 | IB | NCT02370238 | HER2-negative BC | 30% ORR, no DLTs [198] | |
| Propagermanium | Targeted therapy | Glycosylphosphatidylinositol-anchored proteins (CCL2 pathways) | I | UMIN000022494 | Perioperative BC | No DLTs [199] | |
| LY2510924 + carboplatin and etoposide | LY2510924—targeted therapy, carboplatin—platinating agent, etoposide—topoisomerase inhibitor | LY2510924—CXCR4 antagonist | II | NCT01439568 | SCLC | No difference in PFS, OS, or ORR, no additional toxicity [200,201] | |
| BMS-986253 | Monoclonal antibody | CXCL8 (IL-8) | I | NCT02536469 | Advanced solid tumors | No DLTs, 73% of patients had SD with median treatment duration 24 wks (range 4–54). No objective tumor response [202] | |
| TGF-beta | M7824 | First-in-class bifunctional checkpoint inhibitor | PD-L1 and TGF-beta | I | NCT02517398 | Advanced solid tumors | MTD not reached, 1 CR, 2 PR, 3 SD [203] |
| Fresolimumab + focal radiotherapy | Monoclonal antibody | TGF-beta | II | NCT01401062 | mBC with at least three distinct metastatic sites | 10 mg/kg dose resulted in increased median OS with HR of 2.73 (95%CI 1.02–7.3, p = 0.039) compared to 1 mg/kg dose [204] | |
| Galunisertib vs. placebo, in combination with gemcitabine | Galunisertib—targeted therapy, gemcitabine—antimetabolite | Galunisertib—TGB-beta kinase I inhibitor | Ib/II | NCT01373164 | Advanced pancreatic cancer, first-line therapy | Median OS 8.9 and 7.1 mo for G v P, HR = 0.79 (95%CI 0.59–1.09) with posterior probability HR < 1 = 0.93 [205] | |
| Matrix remodeling | BMS-275291 | MMP inhibitor | Broad range of MMPs | I | NCT00006229 (CAN-NCIC-BR18); NCT00039104; NCT00040755; NCT00036621 | Advanced solid tumors; NSCLC, PC, BC | 27% of patients had SD [206,207] |
| Marimastat vs. placebo | MMP inhibitor | Broad range of MMPs | III | NCT00003010 | mBC | No effect on PFS [208] | |
| Marimastat + carboplatin and paclitaxel | See above | See above | I | NCT00003011 | NSCLC; SCLC | 57% PR, 19% SD, tolerable combination therapy in NSCLC [209]. No effect on survival in SCLC [210] | |
| Other | KX2-391 | Dual function targeted therapy | Src and tubulin polymerization inhibitor | II | NCT01074138; NCT00658970 | Bone mCRPC, chemotherapy-naïve; advanced malignancies | 10% PSA response, median PFS 18.6 wks [211] |
| Dasatinib | Targeted therapy | Multikinase inhibitor | II | NCT00385580; NCT00918385 | mCRPC, chemotherapy-naïve | 43% SD rate at 12 wks [212] Significant changes in (18)F-fluoride incorporation in response to Tx [213] | |
| Dasatinib + ZA | See above | See above | I/II | NCT00566618 | HER2-negative bone metastatic BC | 23% PR, 36% CBR [214] | |
| Atrasentan | Targeted therapy | Selective ETA receptor antagonist | II/III | NCT00134056; NCT00181558; NCT00036543; NCT00039429 | CRPC, RCC | No effect on in PFS/OS [215] | |
| BHQ880 | Monoclonal antibody | DKK-1 | Ib | NCT00741377 | Relapsed MM | Increased bone density, tolerable with concurrent MM therapy including ZA [216] |
4.1. TGF-Beta Family
4.2. BMP Signaling
4.3. Wnt Signaling
4.4. Chemokines
4.5. Other Bone Invasive Pathways
4.6. Barriers to Discovery of Novel Signaling Pathways in Human Bone Metastatic Cancers
5. Future Directions
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hussain, A.; Lee, R.J.; Graff, J.N.; Halabi, S. The evolution and understanding of skeletal complication endpoints in clinical trials of tumors with metastasis to the bone. Crit. Rev. Oncol. Hematol. 2019, 139, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.E. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin. Cancer Res. 2006, 12, 6243s–6249s. [Google Scholar] [CrossRef]
- Mendoza, D.P.; Lin, J.J.; Rooney, M.M.; Chen, T.; Sequist, L.V.; Shaw, A.T.; Digumarthy, S.R. Imaging Features and Metastatic Patterns of Advanced ALK-Rearranged Non-Small Cell Lung Cancer. AJR Am. J. Roentgenol. 2020, 214, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Parkes, A.; Clifton, K.; Al-Awadhi, A.; Oke, O.; Warneke, C.L.; Litton, J.K.; Hortobagyi, G.N. Characterization of bone only metastasis patients with respect to tumor subtypes. NPJ Breast Cancer 2018, 4, 2. [Google Scholar] [CrossRef]
- Angsubhakorn, N.; Suvannasankha, A. Acute lymphoblastic leukaemia with osteolytic bone lesions: Diagnostic dilemma. BMJ Case Rep. 2018, 2018, bcr-2018. [Google Scholar] [CrossRef]
- Kim, B.; Yoon, Y.A.; Choi, Y.J. Adult B-lymphoblastic leukemia initially presenting as multiple osteolytic lesions: Caution in diagnostic approaches. Blood Res. 2021, 56, 119–121. [Google Scholar] [CrossRef]
- Lima, C.S.; Pinto Neto, J.V.; da Cunha, M.L.; Vassallo, J.; Cardinalli, I.A.; De Souza, C.A. Osteolytic lesions as a presenting sign of acute myeloid leukemia. Haematologia 2000, 30, 325–331. [Google Scholar] [CrossRef]
- Navarro, S.M.; Matcuk, G.R.; Patel, D.B.; Skalski, M.; White, E.A.; Tomasian, A.; Schein, A.J. Musculoskeletal Imaging Findings of Hematologic Malignancies. Radiographics 2017, 37, 881–900. [Google Scholar] [CrossRef]
- Hogler, W.; Wehl, G.; van Staa, T.; Meister, B.; Klein-Franke, A.; Kropshofer, G. Incidence of skeletal complications during treatment of childhood acute lymphoblastic leukemia: Comparison of fracture risk with the General Practice Research Database. Pediatr. Blood Cancer 2007, 48, 21–27. [Google Scholar] [CrossRef]
- Kwaan, H.C. Double hazard of thrombophilia and bleeding in leukemia. Hematol. Am. Soc. Hematol. Educ. Program 2007, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Morrison, V.A. Infections in patients with leukemia and lymphoma. Cancer Treat. Res. 2014, 161, 319–349. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Ferrer, S.; Bonnet, D.; Steensma, D.P.; Hasserjian, R.P.; Ghobrial, I.M.; Gribben, J.G.; Andreeff, M.; Krause, D.S. Bone marrow niches in haematological malignancies. Nat. Rev. Cancer 2020, 20, 285–298. [Google Scholar] [CrossRef]
- Mancuso, S.; Scaturro, D.; Santoro, M.; Di Gaetano, G.; Vitagliani, F.; Falco, V.; Siragusa, S.; Gonnelli, S.; Mauro, G.L. Bone damage after chemotherapy for lymphoma: A real-world experience. BMC Musculoskelet Disord. 2021, 22, 1024. [Google Scholar] [CrossRef] [PubMed]
- Mateos, M.V.; Fink, L.; Koneswaran, N.; Intorcia, M.; Giannopoulou, C.; Niepel, D.; Cavo, M. Bone complications in patients with multiple myeloma in five European countries: A retrospective patient chart review. BMC Cancer 2020, 20, 170. [Google Scholar] [CrossRef]
- Cho, Y.J.; Cho, Y.M.; Kim, S.H.; Shin, K.H.; Jung, S.T.; Kim, H.S. Clinical analysis of patients with skeletal metastasis of lung cancer. BMC Cancer 2019, 19, 303. [Google Scholar] [CrossRef]
- DePuy, V.; Anstrom, K.J.; Castel, L.D.; Schulman, K.A.; Weinfurt, K.P.; Saad, F. Effects of skeletal morbidities on longitudinal patient-reported outcomes and survival in patients with metastatic prostate cancer. Support Care Cancer 2007, 15, 869–876. [Google Scholar] [CrossRef]
- Howard, L.E.; De Hoedt, A.M.; Aronson, W.J.; Kane, C.J.; Amling, C.L.; Cooperberg, M.R.; Terris, M.K.; Divers, C.H.; Valderrama, A.; Freedland, S.J. Do skeletal-related events predict overall survival in men with metastatic castration-resistant prostate cancer? Prostate Cancer Prostatic Dis. 2016, 19, 380–384. [Google Scholar] [CrossRef]
- Kawai, A.T.; Martinez, D.; Saltus, C.W.; Vassilev, Z.P.; Soriano-Gabarro, M.; Kaye, J.A. Incidence of Skeletal-Related Events in Patients with Castration-Resistant Prostate Cancer: An Observational Retrospective Cohort Study in the US. Prostate Cancer 2019, 2019, 5971615. [Google Scholar] [CrossRef]
- Kong, P.; Yan, J.; Liu, D.; Ji, Y.; Wang, Y.; Zhuang, J.; Wang, J.; Hu, X.; Yue, X. Skeletal-related events and overall survival of patients with bone metastasis from nonsmall cell lung cancer—A retrospective analysis. Medicine 2017, 96, e9327. [Google Scholar] [CrossRef]
- Shinagare, A.B.; Ramaiya, N.H.; Jagannathan, J.P.; Fennessy, F.M.; Taplin, M.E.; Van den Abbeele, A.D. Metastatic pattern of bladder cancer: Correlation with the characteristics of the primary tumor. AJR Am. J. Roentgenol. 2011, 196, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, Y.; Tamiya, A.; Nakahama, K.; Naoki, Y.; Kanazu, M.; Omachi, N.; Okishio, K.; Kasai, T.; Atagi, S. Impact of metastatic status on the prognosis of EGFR mutation-positive non-small cell lung cancer patients treated with first-generation EGFR-tyrosine kinase inhibitors. Oncol. Lett. 2017, 14, 7589–7596. [Google Scholar] [CrossRef] [PubMed]
- Norgaard, M.; Jensen, A.O.; Jacobsen, J.B.; Cetin, K.; Fryzek, J.P.; Sorensen, H.T. Skeletal related events, bone metastasis and survival of prostate cancer: A population based cohort study in Denmark (1999 to 2007). J. Urol. 2010, 184, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Landi, L.; D’Inca, F.; Gelibter, A.; Chiari, R.; Grossi, F.; Delmonte, A.; Passaro, A.; Signorelli, D.; Gelsomino, F.; Galetta, D.; et al. Bone metastases and immunotherapy in patients with advanced non-small-cell lung cancer. J. Immunother. Cancer 2019, 7, 316. [Google Scholar] [CrossRef] [PubMed]
- Parkes, A.; Warneke, C.L.; Clifton, K.; Al-Awadhi, A.; Oke, O.; Pestana, R.C.; Alhalabi, O.; Litton, J.K.; Hortobagyi, G.N. Prognostic Factors in Patients with Metastatic Breast Cancer with Bone-Only Metastases. Oncologist 2018, 23, 1282–1288. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.G.; Lee, H.M.; Cho, S.H.; Lee, S.A.; Hwang, S.H.; Jeong, J.; Lee, H.D. Prognostic factors for patients with bone-only metastasis in breast cancer. Yonsei Med. J. 2013, 54, 1168–1177. [Google Scholar] [CrossRef]
- Aly, A.; Onukwugha, E.; Woods, C.; Mullins, C.D.; Kwok, Y.; Qian, Y.; Arellano, J.; Balakumaran, A.; Hussain, A. Measurement of skeletal related events in SEER-Medicare: A comparison of claims-based methods. BMC Med. Res. Methodol. 2015, 15, 65. [Google Scholar] [CrossRef]
- Hussain, A.; Aly, A.; Daniel Mullins, C.; Qian, Y.; Arellano, J.; Onukwugha, E. Risk of skeletal related events among elderly prostate cancer patients by site of metastasis at diagnosis. Cancer Med. 2016, 5, 3300–3309. [Google Scholar] [CrossRef]
- Body, J.J.; Quinn, G.; Talbot, S.; Booth, E.; Demonty, G.; Taylor, A.; Amelio, J. Systematic review and meta-analysis on the proportion of patients with breast cancer who develop bone metastases. Crit. Rev. Oncol. Hematol. 2017, 115, 67–80. [Google Scholar] [CrossRef]
- Jensen, A.O.; Jacobsen, J.B.; Norgaard, M.; Yong, M.; Fryzek, J.P.; Sorensen, H.T. Incidence of bone metastases and skeletal-related events in breast cancer patients: A population-based cohort study in Denmark. BMC Cancer 2011, 11, 29. [Google Scholar] [CrossRef]
- Yanae, M.; Fujimoto, S.; Tane, K.; Tanioka, M.; Fujiwara, K.; Tsubaki, M.; Yamazoe, Y.; Morishima, Y.; Chiba, Y.; Takao, S.; et al. Increased risk of SSEs in bone-only metastatic breast cancer patients treated with zoledronic acid. J. Bone Oncol. 2017, 8, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Yong, M.; Jensen, A.O.; Jacobsen, J.B.; Norgaard, M.; Fryzek, J.P.; Sorensen, H.T. Survival in breast cancer patients with bone metastases and skeletal-related events: A population-based cohort study in Denmark (1999–2007). Breast Cancer Res. Treat. 2011, 129, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Santini, D.; Barni, S.; Intagliata, S.; Falcone, A.; Ferrau, F.; Galetta, D.; Moscetti, L.; La Verde, N.; Ibrahim, T.; Petrelli, F.; et al. Natural History of Non-Small-Cell Lung Cancer with Bone Metastases. Sci. Rep. 2015, 5, 18670. [Google Scholar] [CrossRef] [PubMed]
- Lagana, M.; Gurizzan, C.; Roca, E.; Cortinovis, D.; Signorelli, D.; Pagani, F.; Bettini, A.; Bonomi, L.; Rinaldi, S.; Berardi, R.; et al. High Prevalence and Early Occurrence of Skeletal Complications in EGFR Mutated NSCLC Patients with Bone Metastases. Front. Oncol. 2020, 10, 588862. [Google Scholar] [CrossRef] [PubMed]
- Hirsh, V.; Major, P.P.; Lipton, A.; Cook, R.J.; Langer, C.J.; Smith, M.R.; Brown, J.E.; Coleman, R.E. Zoledronic acid and survival in patients with metastatic bone disease from lung cancer and elevated markers of osteoclast activity. J. Thorac. Oncol. 2008, 3, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Xu, L.; Yuan, Z.; Wang, Z.; Zhao, L.; Wang, P. Clinical outcome for small cell lung cancer patients with bone metastases at the time of diagnosis. J. Bone Oncol. 2019, 19, 100265. [Google Scholar] [CrossRef] [PubMed]
- Megyesfalvi, Z.; Tallosy, B.; Pipek, O.; Fillinger, J.; Lang, C.; Klikovits, T.; Schwendenwein, A.; Hoda, M.A.; Renyi-Vamos, F.; Laszlo, V.; et al. The landscape of small cell lung cancer metastases: Organ specificity and timing. Thorac. Cancer 2021, 12, 914–923. [Google Scholar] [CrossRef]
- Grunwald, V.; Eberhardt, B.; Bex, A.; Florcken, A.; Gauler, T.; Derlin, T.; Panzica, M.; Durr, H.R.; Grotz, K.A.; Giles, R.H.; et al. An interdisciplinary consensus on the management of bone metastases from renal cell carcinoma. Nat. Rev. Urol. 2018, 15, 511–521. [Google Scholar] [CrossRef]
- Santoni, M.; Conti, A.; Procopio, G.; Porta, C.; Ibrahim, T.; Barni, S.; Guida, F.M.; Fontana, A.; Berruti, A.; Berardi, R.; et al. Bone metastases in patients with metastatic renal cell carcinoma: Are they always associated with poor prognosis? J. Exp. Clin. Cancer Res. 2015, 34, 10. [Google Scholar] [CrossRef]
- Woodward, E.; Jagdev, S.; McParland, L.; Clark, K.; Gregory, W.; Newsham, A.; Rogerson, S.; Hayward, K.; Selby, P.; Brown, J. Skeletal complications and survival in renal cancer patients with bone metastases. Bone 2011, 48, 160–166. [Google Scholar] [CrossRef]
- Wallmeroth, A.; Wagner, U.; Moch, H.; Gasser, T.C.; Sauter, G.; Mihatsch, M.J. Patterns of metastasis in muscle-invasive bladder cancer (pT2-4): An autopsy study on 367 patients. Urol. Int. 1999, 62, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Fon, G.T.; Wong, W.S.; Gold, R.H.; Kaiser, L.R. Skeletal metastases of melanoma: Radiographic, scintigraphic, and clinical review. AJR Am. J. Roentgenol. 1981, 137, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Mannavola, F.; Mandala, M.; Todisco, A.; Sileni, V.C.; Palla, M.; Minisini, A.M.; Pala, L.; Morgese, F.; Di Guardo, L.; Stucci, L.S.; et al. An Italian Retrospective Survey on Bone Metastasis in Melanoma: Impact of Immunotherapy and Radiotherapy on Survival. Front. Oncol. 2020, 10, 1652. [Google Scholar] [CrossRef]
- Zekri, J.; Marples, M.; Taylor, D.; Kandukurti, K.; McParland, L.; Brown, J.E. Complications of bone metastases from malignant melanoma. J. Bone Oncol. 2017, 8, 13–17. [Google Scholar] [CrossRef]
- Andrade, F.; Probstner, D.; Decnop, M.; Bulzico, D.; Momesso, D.; Corbo, R.; Vaisman, M.; Vaisman, F. The Impact of Zoledronic Acid and Radioactive Iodine Therapy on Morbi-Mortality of Patients with Bone Metastases of Thyroid Cancer Derived from Follicular Cells. Eur. Thyroid J. 2019, 8, 46–55. [Google Scholar] [CrossRef]
- Farooki, A.; Leung, V.; Tala, H.; Tuttle, R.M. Skeletal-related events due to bone metastases from differentiated thyroid cancer. J. Clin. Endocrinol. Metab. 2012, 97, 2433–2439. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Demura, S.; Shinmura, K.; Yokogawa, N.; Shimizu, T.; Tsuchiya, H. Current Management of Bone Metastases from Differentiated Thyroid Cancer. Cancers 2021, 13, 4429. [Google Scholar] [CrossRef]
- Nervo, A.; Ragni, A.; Retta, F.; Gallo, M.; Piovesan, A.; Liberini, V.; Gatti, M.; Ricardi, U.; Deandreis, D.; Arvat, E. Bone metastases from differentiated thyroid carcinoma: Current knowledge and open issues. J. Endocrinol. Investig. 2021, 44, 403–419. [Google Scholar] [CrossRef] [PubMed]
- Choksi, P.; Papaleontiou, M.; Guo, C.; Worden, F.; Banerjee, M.; Haymart, M. Skeletal Complications and Mortality in Thyroid Cancer: A Population-Based Study. J. Clin. Endocrinol. Metab. 2017, 102, 1254–1260. [Google Scholar] [CrossRef]
- Fukutomi, M.; Yokota, M.; Chuman, H.; Harada, H.; Zaitsu, Y.; Funakoshi, A.; Wakasugi, H.; Iguchi, H. Increased incidence of bone metastases in hepatocellular carcinoma. Eur. J. Gastroenterol. Hepatol. 2001, 13, 1083–1088. [Google Scholar] [CrossRef]
- Longo, V.; Brunetti, O.; D’Oronzo, S.; Ostuni, C.; Gatti, P.; Silvestris, F. Bone metastases in hepatocellular carcinoma: An emerging issue. Cancer Metastasis Rev. 2014, 33, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Natsuizaka, M.; Omura, T.; Akaike, T.; Kuwata, Y.; Yamazaki, K.; Sato, T.; Karino, Y.; Toyota, J.; Suga, T.; Asaka, M. Clinical features of hepatocellular carcinoma with extrahepatic metastases. J. Gastroenterol. Hepatol. 2005, 20, 1781–1787. [Google Scholar] [CrossRef] [PubMed]
- Uchino, K.; Tateishi, R.; Shiina, S.; Kanda, M.; Masuzaki, R.; Kondo, Y.; Goto, T.; Omata, M.; Yoshida, H.; Koike, K. Hepatocellular carcinoma with extrahepatic metastasis: Clinical features and prognostic factors. Cancer 2011, 117, 4475–4483. [Google Scholar] [CrossRef] [PubMed]
- Santini, D.; Brandi, G.; Aprile, G.; Russano, M.; Cereda, S.; Leone, F.; Lonardi, S.; Fornaro, L.; Scartozzi, M.; Silvestris, N.; et al. Bone metastases in biliary cancers: A multicenter retrospective survey. J. Bone Oncol. 2018, 12, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Thammaroj, P.; Chimcherd, A.; Chowchuen, P.; Panitchote, A.; Sumananont, C.; Wongsurawat, N. Imaging features of bone metastases from cholangiocarcinoma. Eur. J. Radiol. 2020, 129, 109118. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, G.Y.; Chen, M.; Wei, R.; Chen, J.; Wang, Z. Pattern of distant metastases in primary extrahepatic bile-duct cancer: A SEER-based study. Cancer Med. 2018, 7, 5006–5014. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Tu, Z.; Ye, C.; Cai, H.; Yang, S.; Chen, X.; Tu, J. Site-specific metastases of gallbladder adenocarcinoma and their prognostic value for survival: A SEER-based study. BMC Surg. 2021, 21, 59. [Google Scholar] [CrossRef]
- Mikami, J.; Kimura, Y.; Makari, Y.; Fujita, J.; Kishimoto, T.; Sawada, G.; Nakahira, S.; Nakata, K.; Tsujie, M.; Ohzato, H. Clinical outcomes and prognostic factors for gastric cancer patients with bone metastasis. World J. Surg. Oncol. 2017, 15, 8. [Google Scholar] [CrossRef]
- Petrillo, A.; Giunta, E.F.; Pappalardo, A.; Bosso, D.; Attademo, L.; Cardalesi, C.; Diana, A.; Fabbrocini, A.; Fabozzi, T.; Giordano, P.; et al. Bone Metastases from Gastric Cancer: What We Know and How to Deal with Them. J. Clin. Med. 2021, 10, 1777. [Google Scholar] [CrossRef]
- Turkoz, F.P.; Solak, M.; Kilickap, S.; Ulas, A.; Esbah, O.; Oksuzoglu, B.; Yalcin, S. Bone metastasis from gastric cancer: The incidence, clinicopathological features, and influence on survival. J. Gastric Cancer 2014, 14, 164–172. [Google Scholar] [CrossRef]
- Ai, D.; Zhu, H.; Ren, W.; Chen, Y.; Liu, Q.; Deng, J.; Ye, J.; Fan, J.; Zhao, K. Patterns of distant organ metastases in esophageal cancer: A population-based study. J. Thorac. Dis. 2017, 9, 3023–3030. [Google Scholar] [CrossRef] [PubMed]
- Imura, Y.; Yamamoto, S.; Wakamatsu, T.; Tanaka, T.; Tamiya, H.; Sugimura, K.; Miyata, H.; Ishihara, R.; Yano, M.; Naka, N. Clinical features and prognostic factors in patients with esophageal cancer with bone metastasis. Oncol. Lett. 2020, 19, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-G.; Zhang, W.-W.; Sun, J.-Y.; Li, F.-Y.; Lin, Q.; He, Z.-Y. Patterns of Distant Metastasis Between Histological Types in Esophageal Cancer. Front. Oncol. 2018, 8, 302. [Google Scholar] [CrossRef] [PubMed]
- Christensen, T.D.; Jensen, S.G.; Larsen, F.O.; Nielsen, D.L. Systematic review: Incidence, risk factors, survival and treatment of bone metastases from colorectal cancer. J. Bone Oncol. 2018, 13, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Chun, Y.J.; Kim, H.S.; Kim, J.H.; Lee, C.K.; Beom, S.H.; Shin, S.J.; Ahn, J.B. Clinical features and KRAS mutation in colorectal cancer with bone metastasis. Sci. Rep. 2020, 10, 21180. [Google Scholar] [CrossRef] [PubMed]
- Santini, D.; Tampellini, M.; Vincenzi, B.; Ibrahim, T.; Ortega, C.; Virzi, V.; Silvestris, N.; Berardi, R.; Masini, C.; Calipari, N.; et al. Natural history of bone metastasis in colorectal cancer: Final results of a large Italian bone metastases study. Ann. Oncol. 2012, 23, 2072–2077. [Google Scholar] [CrossRef] [PubMed]
- Borad, M.J.; Saadati, H.; Lakshmipathy, A.; Campbell, E.; Hopper, P.; Jameson, G.; Von Hoff, D.D.; Saif, M.W. Skeletal metastases in pancreatic cancer: A retrospective study and review of the literature. Yale J. Biol. Med. 2009, 82, 1–6. [Google Scholar] [PubMed]
- Outani, H.; Akita, H.; Nakai, T.; Takada, R.; Imura, Y.; Tanaka, T.; Tamiya, H.; Oshima, K.; Takahashi, H.; Ohkawa, K.; et al. Clinical Features and Prognosis of Patients with the Bone Metastasis of Pancreatic Cancer: A Single-Institutional Cohort Study. Pancreas 2018, 47, e43–e46. [Google Scholar] [CrossRef]
- Puri, A.; Chang, J.; Dragovich, T.; Lucente, P.; Kundranda, M.N. Skeletal metastases in advanced pancreatic ductal adenocarcinoma (PDAC): A retrospective analysis. J. Clin. Oncol. 2018, 36, 245. [Google Scholar] [CrossRef]
- Basu, D.; Siegel, B.A.; McDonald, D.J.; Nussenbaum, B. Detection of occult bone metastases from head and neck squamous cell carcinoma: Impact of positron emission tomography computed tomography with fluorodeoxyglucose F 18. Arch. Otolaryngol. Head Neck Surg. 2007, 133, 801–805. [Google Scholar] [CrossRef][Green Version]
- Grisanti, S.; Bianchi, S.; Locati, L.D.; Triggiani, L.; Vecchio, S.; Bonetta, A.; Bergamini, C.; Conte, P.; Airoldi, M.; Merlano, M.; et al. Bone metastases from head and neck malignancies: Prognostic factors and skeletal-related events. PLoS ONE 2019, 14, e0213934. [Google Scholar] [CrossRef] [PubMed]
- Pietropaoli, M.P.; Damron, T.A.; Vermont, A.I. Bone metastases from squamous cell carcinoma of the head and neck. J. Surg. Oncol. 2000, 75, 136–141. [Google Scholar] [CrossRef]
- Sakisuka, T.; Kashiwagi, N.; Doi, H.; Takahashi, H.; Arisawa, A.; Matsuo, C.; Masuda, Y.; Inohara, H.; Sato, K.; Outani, H.; et al. Prognostic factors for bone metastases from head and neck squamous cell carcinoma: A case series of 97 patients. Mol. Clin. Oncol. 2021, 15, 246. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Cristiano, L.; Peters, E.; Ioffe, Y. Detection of bone metastases in uterine cancer: How common are they and should PET/CT be the standard for diagnosis? Gynecol. Oncol. Rep. 2021, 35, 100698. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chi, S.; Zhou, X.; Zhao, R.; Xiao, C.; Wang, H. Prognostic value of distant metastatic sites in stage IV endometrial cancer: A SEER database study of 2948 women. Int. J. Gynaecol. Obstet 2020, 149, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Uccella, S.; Morris, J.M.; Bakkum-Gamez, J.N.; Keeney, G.L.; Podratz, K.C.; Mariani, A. Bone metastases in endometrial cancer: Report on 19 patients and review of the medical literature. Gynecol. Oncol. 2013, 130, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Deng, K.; Yang, C.; Tan, Q.; Song, W.; Lu, M.; Zhao, W.; Lou, G.; Li, Z.; Li, K.; Hou, Y. Sites of distant metastases and overall survival in ovarian cancer: A study of 1481 patients. Gynecol. Oncol. 2018, 150, 460–465. [Google Scholar] [CrossRef]
- Thomakos, N.; Diakosavvas, M.; Machairiotis, N.; Fasoulakis, Z.; Zarogoulidis, P.; Rodolakis, A. Rare Distant Metastatic Disease of Ovarian and Peritoneal Carcinomatosis: A Review of the Literature. Cancers 2019, 11, 1044. [Google Scholar] [CrossRef]
- Zhang, C.; Guo, X.; Peltzer, K.; Ma, W.; Qi, L.; Zhang, Y.; Han, X.; Baklaushev, V.P.; Yao, Y.; Wang, G.; et al. The prevalence, associated factors for bone metastases development and prognosis in newly diagnosed ovarian cancer: A large population based real-world study. J. Cancer 2019, 10, 3133–3139. [Google Scholar] [CrossRef]
- Vincenzi, B.; Frezza, A.M.; Schiavon, G.; Santini, D.; Dileo, P.; Silletta, M.; Delisi, D.; Bertoldo, F.; Badalamenti, G.; Baldi, G.G.; et al. Bone metastases in soft tissue sarcoma: A survey of natural history, prognostic value and treatment options. Clin. Sarcoma Res. 2013, 3, 6. [Google Scholar] [CrossRef]
- Yoshikawa, H.; Myoui, A.; Ochi, T.; Araki, N.; Ueda, T.; Kudawara, I.; Nakanishi, K.; Tanaka, H.; Nakamura, H. Bone Metastases from Soft Tissue Sarcomas. Semin. Musculoskelet. Radiol. 1999, 3, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Berenson, J.R.; Lichtenstein, A.; Porter, L.; Dimopoulos, M.A.; Bordoni, R.; George, S.; Lipton, A.; Keller, A.; Ballester, O.; Kovacs, M.J.; et al. Efficacy of pamidronate in reducing skeletal events in patients with advanced multiple myeloma. Myeloma Aredia Study Group. N. Engl. J. Med. 1996, 334, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Bhatta, S.; Cyprien, L.; Fonseca, R.; Hernandez, R.K. Incidence of skeletal-related events among multiple myeloma patients in the United States at oncology clinics: Observations from real-world data. J. Bone Oncol. 2019, 14, 100215. [Google Scholar] [CrossRef] [PubMed]
- Parrondo, R.D.; Sher, T. Prevention Of Skeletal Related Events In Multiple Myeloma: Focus On The RANK-L Pathway In The Treatment Of Multiple Myeloma. Onco. Targets Ther. 2019, 12, 8467–8478. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Sweiss, K.; Han, J.; Ko, N.Y.; Patel, P.R.; Chiu, B.C.; Calip, G.S. Evaluation of Frequency of Administration of Intravenous Bisphosphonate and Recurrent Skeletal-Related Events in Patients With Multiple Myeloma. JAMA Netw. Open 2021, 4, e2118410. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Cai, L.; Zhou, F. Management of acute spinal cord compression in multiple myeloma. Crit. Rev. Oncol. Hematol. 2021, 160, 103205. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.; Subudhi, S.K.; Aparicio, A.; Ge, Z.; Guan, B.; Miura, Y.; Sharma, P. Differences in Tumor Microenvironment Dictate T Helper Lineage Polarization and Response to Immune Checkpoint Therapy. Cell 2019, 179, 1177–1190.e1113. [Google Scholar] [CrossRef] [PubMed]
- Beer, T.M.; Kwon, E.D.; Drake, C.G.; Fizazi, K.; Logothetis, C.; Gravis, G.; Ganju, V.; Polikoff, J.; Saad, F.; Humanski, P.; et al. Randomized, Double-Blind, Phase III Trial of Ipilimumab Versus Placebo in Asymptomatic or Minimally Symptomatic Patients with Metastatic Chemotherapy-Naive Castration-Resistant Prostate Cancer. J. Clin. Oncol. 2017, 35, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Inomata, M.; Shukuya, T.; Takahashi, T.; Ono, A.; Nakamura, Y.; Tsuya, A.; Kenmotsu, H.; Naito, T.; Murakami, H.; Harada, H.; et al. Continuous administration of EGFR-TKIs following radiotherapy after disease progression in bone lesions for non-small cell lung cancer. Anticancer Res. 2011, 31, 4519–4523. [Google Scholar]
- Lee, Y.; Masub, N.; Wechter, T.; Zhong, J.; Moran, U.; Darvishian, F.; Polsky, D.; Berman, R.S.; Shapiro, R.L.; Weber, J.S.; et al. Bone metastasis to predict treatment response rate and overall survival of patients with metastatic melanoma. J. Clin. Oncol. 2018, 36, e21585. [Google Scholar] [CrossRef]
- Meads, M.B.; Hazlehurst, L.A.; Dalton, W.S. The bone marrow microenvironment as a tumor sanctuary and contributor to drug resistance. Clin. Cancer Res. 2008, 14, 2519–2526. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Aoki, T.; Hanagiri, T.; Yoshii, C.; Mukae, H.; Uramoto, H.; Korogi, Y. Osteosclerotic lesions in patients treated with gefitinib for lung adenocarcinomas: A sign of favorable therapeutic response. Skeletal. Radiol. 2012, 41, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Nakata, E.; Sugihara, S.; Sugawara, Y.; Kozuki, T.; Harada, D.; Nogami, N.; Nakahara, R.; Furumatsu, T.; Tetsunaga, T.; Kunisada, T.; et al. Early response of bone metastases can predict tumor response in patients with non-small-cell lung cancer with bone metastases in the treatment with nivolumab. Oncol. Lett. 2020, 20, 2977–2986. [Google Scholar] [CrossRef] [PubMed]
- Rong, D.; Mao, Y.; Yang, Q.; Xu, S.; Zhao, Q.; Zhang, R. Early osteosclerotic changes predict chemotherapy response in non-small-cell lung cancer patients with bone metastases. Eur. Radiol. 2018, 28, 4362–4369. [Google Scholar] [CrossRef]
- Subudhi, S.K.; Siddiqui, B.A.; Aparicio, A.M.; Yadav, S.S.; Basu, S.; Chen, H.; Jindal, S.; Tidwell, R.S.S.; Varma, A.; Logothetis, C.J.; et al. Combined CTLA-4 and PD-L1 blockade in patients with chemotherapy-naive metastatic castration-resistant prostate cancer is associated with increased myeloid and neutrophil immune subsets in the bone microenvironment. J. Immunother. Cancer 2021, 9, e002919. [Google Scholar] [CrossRef]
- Zhao, E.; Wang, L.; Dai, J.; Kryczek, I.; Wei, S.; Vatan, L.; Altuwaijri, S.; Sparwasser, T.; Wang, G.; Keller, E.T.; et al. Regulatory T cells in the bone marrow microenvironment in patients with prostate cancer. Oncoimmunology 2012, 1, 152–161. [Google Scholar] [CrossRef]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fossa, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef]
- Fizazi, K.; Carducci, M.; Smith, M.; Damiao, R.; Brown, J.; Karsh, L.; Milecki, P.; Shore, N.; Rader, M.; Wang, H.; et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: A randomised, double-blind study. Lancet 2011, 377, 813–822. [Google Scholar] [CrossRef]
- Rosen, L.S.; Gordon, D.; Kaminski, M.; Howell, A.; Belch, A.; Mackey, J.; Apffelstaedt, J.; Hussein, M.A.; Coleman, R.E.; Reitsma, D.J.; et al. Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma: A randomized, double-blind, multicenter, comparative trial. Cancer 2003, 98, 1735–1744. [Google Scholar] [CrossRef]
- Rosen, L.S.; Gordon, D.; Tchekmedyian, S.; Yanagihara, R.; Hirsh, V.; Krzakowski, M.; Pawlicki, M.; de Souza, P.; Zheng, M.; Urbanowitz, G.; et al. Zoledronic acid versus placebo in the treatment of skeletal metastases in patients with lung cancer and other solid tumors: A phase III, double-blind, randomized trial--the Zoledronic Acid Lung Cancer and Other Solid Tumors Study Group. J. Clin. Oncol. 2003, 21, 3150–3157. [Google Scholar] [CrossRef]
- Saad, F.; Gleason, D.M.; Murray, R.; Tchekmedyian, S.; Venner, P.; Lacombe, L.; Chin, J.L.; Vinholes, J.J.; Goas, J.A.; Chen, B.; et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J. Natl. Cancer Inst. 2002, 94, 1458–1468. [Google Scholar] [CrossRef] [PubMed]
- Saravana-Bawan, S.; David, E.; Sahgal, A.; Chow, E. Palliation of bone metastases-exploring options beyond radiotherapy. Ann. Palliat. Med. 2019, 8, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Kim, J.K.; Shiozawa, Y.; Wang, J.; Mishra, A.; Joseph, J.; Berry, J.E.; McGee, S.; Lee, E.; Sun, H.; et al. Recruitment of mesenchymal stem cells into prostate tumours promotes metastasis. Nat. Commun. 2013, 4, 1795. [Google Scholar] [CrossRef] [PubMed]
- Lescarbeau, R.M.; Seib, F.P.; Prewitz, M.; Werner, C.; Kaplan, D.L. In vitro model of metastasis to bone marrow mediates prostate cancer castration resistant growth through paracrine and extracellular matrix factors. PLoS ONE 2012, 7, e40372. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Gajdosik, M.S.; Josic, D.; Clifton, J.G.; Logothetis, C.; Yu-Lee, L.Y.; Gallick, G.E.; Maity, S.N.; Lin, S.H. Secretome analysis of an osteogenic prostate tumor identifies complex signaling networks mediating cross-talk of cancer and stromal cells within the tumor microenvironment. Mol. Cell Proteom. 2015, 14, 471–483. [Google Scholar] [CrossRef]
- Lee, Y.C.; Lin, S.C.; Yu, G.; Cheng, C.J.; Liu, B.; Liu, H.C.; Hawke, D.H.; Parikh, N.U.; Varkaris, A.; Corn, P.; et al. Identification of Bone-Derived Factors Conferring De Novo Therapeutic Resistance in Metastatic Prostate Cancer. Cancer Res. 2015, 75, 4949–4959. [Google Scholar] [CrossRef]
- Ridge, S.M.; Sullivan, F.J.; Glynn, S.A. Mesenchymal stem cells: Key players in cancer progression. Mol. Cancer 2017, 16, 31. [Google Scholar] [CrossRef]
- Wang, H.; Tian, L.; Liu, J.; Goldstein, A.; Bado, I.; Zhang, W.; Arenkiel, B.R.; Li, Z.; Yang, M.; Du, S.; et al. The Osteogenic Niche Is a Calcium Reservoir of Bone Micrometastases and Confers Unexpected Therapeutic Vulnerability. Cancer Cell 2018, 34, 823–839.e827. [Google Scholar] [CrossRef]
- Wang, H.; Yu, C.; Gao, X.; Welte, T.; Muscarella, A.M.; Tian, L.; Zhao, H.; Zhao, Z.; Du, S.; Tao, J.; et al. The osteogenic niche promotes early-stage bone colonization of disseminated breast cancer cells. Cancer Cell 2015, 27, 193–210. [Google Scholar] [CrossRef]
- Bonewald, L.F. The amazing osteocyte. J. Bone Miner. Res. 2011, 26, 229–238. [Google Scholar] [CrossRef]
- Toscani, D.; Bolzoni, M.; Ferretti, M.; Palumbo, C.; Giuliani, N. Role of Osteocytes in Myeloma Bone Disease: Anti-sclerostin Antibody as New Therapeutic Strategy. Front. Immunol. 2018, 9, 2467. [Google Scholar] [CrossRef] [PubMed]
- Riquelme, M.A.; Cardenas, E.R.; Jiang, J.X. Osteocytes and Bone Metastasis. Front. Endocrinol. 2020, 11, 567844. [Google Scholar] [CrossRef] [PubMed]
- Rapaport, E.; Fishman, R.F.; Gercel, C. Growth inhibition of human tumor cells in soft-agar cultures by treatment with low levels of adenosine 5’-triphosphate. Cancer Res. 1983, 43, 4402–4406. [Google Scholar] [PubMed]
- Delgado-Calle, J.; Anderson, J.; Cregor, M.D.; Hiasa, M.; Chirgwin, J.M.; Carlesso, N.; Yoneda, T.; Mohammad, K.S.; Plotkin, L.I.; Roodman, G.D.; et al. Bidirectional Notch Signaling and Osteocyte-Derived Factors in the Bone Marrow Microenvironment Promote Tumor Cell Proliferation and Bone Destruction in Multiple Myeloma. Cancer Res. 2016, 76, 1089–1100. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, N.; Ferretti, M.; Bolzoni, M.; Storti, P.; Lazzaretti, M.; Dalla Palma, B.; Bonomini, S.; Martella, E.; Agnelli, L.; Neri, A.; et al. Increased osteocyte death in multiple myeloma patients: Role in myeloma-induced osteoclast formation. Leukemia 2012, 26, 1391–1401. [Google Scholar] [CrossRef]
- Toscani, D.; Palumbo, C.; Dalla Palma, B.; Ferretti, M.; Bolzoni, M.; Marchica, V.; Sena, P.; Martella, E.; Mancini, C.; Ferri, V.; et al. The Proteasome Inhibitor Bortezomib Maintains Osteocyte Viability in Multiple Myeloma Patients by Reducing Both Apoptosis and Autophagy: A New Function for Proteasome Inhibitors. J. Bone Miner. Res. 2016, 31, 815–827. [Google Scholar] [CrossRef]
- Sottnik, J.L.; Dai, J.; Zhang, H.; Campbell, B.; Keller, E.T. Tumor-induced pressure in the bone microenvironment causes osteocytes to promote the growth of prostate cancer bone metastases. Cancer Res. 2015, 75, 2151–2158. [Google Scholar] [CrossRef]
- Wang, W.; Yang, X.; Dai, J.; Lu, Y.; Zhang, J.; Keller, E.T. Prostate cancer promotes a vicious cycle of bone metastasis progression through inducing osteocytes to secrete GDF15 that stimulates prostate cancer growth and invasion. Oncogene 2019, 38, 4540–4559. [Google Scholar] [CrossRef]
- Feuerer, M.; Rocha, M.; Bai, L.; Umansky, V.; Solomayer, E.F.; Bastert, G.; Diel, I.J.; Schirrmacher, V. Enrichment of memory T cells and other profound immunological changes in the bone marrow from untreated breast cancer patients. Int. J. Cancer 2001, 92, 96–105. [Google Scholar] [CrossRef]
- Schmitz-Winnenthal, F.H.; Volk, C.; Z’Graggen, K.; Galindo, L.; Nummer, D.; Ziouta, Y.; Bucur, M.; Weitz, J.; Schirrmacher, V.; Buchler, M.W.; et al. High frequencies of functional tumor-reactive T cells in bone marrow and blood of pancreatic cancer patients. Cancer Res. 2005, 65, 10079–10087. [Google Scholar] [CrossRef]
- Riley, R.S.; Williams, D.; Ross, M.; Zhao, S.; Chesney, A.; Clark, B.D.; Ben-Ezra, J.M. Bone marrow aspirate and biopsy: A pathologist’s perspective. II. interpretation of the bone marrow aspirate and biopsy. J. Clin. Lab. Anal. 2009, 23, 259–307. [Google Scholar] [CrossRef] [PubMed]
- van Lochem, E.G.; van der Velden, V.H.; Wind, H.K.; te Marvelde, J.G.; Westerdaal, N.A.; van Dongen, J.J. Immunophenotypic differentiation patterns of normal hematopoiesis in human bone marrow: Reference patterns for age-related changes and disease-induced shifts. Cytom. B Clin. Cytom. 2004, 60, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhao, E.; Xu, H.; Wang, L.; Kryczek, I.; Wu, K.; Hu, Y.; Wang, G.; Zou, W. Bone marrow and the control of immunity. Cell Mol. Immunol. 2012, 9, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Dieli, F.; Gebbia, N.; Poccia, F.; Caccamo, N.; Montesano, C.; Fulfaro, F.; Arcara, C.; Valerio, M.R.; Meraviglia, S.; Di Sano, C.; et al. Induction of gammadelta T-lymphocyte effector functions by bisphosphonate zoledronic acid in cancer patients in vivo. Blood 2003, 102, 2310–2311. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Han, X.; Qiao, X.; Chen, S. The Immune Landscape and Prognostic Immune Key Genes Potentially Involved in Modulating Synaptic Functions in Prostate Cancer. Front. Oncol. 2020, 10, 1330. [Google Scholar] [CrossRef] [PubMed]
- Heninger, E.; Sethakorn, N.; Kosoff, D.; Hematti, P.; Kuczler, M.D.; Pienta, K.J.; Lang, J.M. Immune profiling of the bone marrow microenvironment in patients with high-risk localized prostate cancer. Oncotarget 2020, 11, 4253–4265. [Google Scholar] [CrossRef]
- Kfoury, Y.; Baryawno, N.; Severe, N.; Mei, S.; Gustafsson, K.; Hirz, T.; Brouse, T.; Scadden, E.W.; Igolkina, A.A.; Kokkaliaris, K.; et al. Human prostate cancer bone metastases have an actionable immunosuppressive microenvironment. Cancer Cell 2021, 39, 1464–1478.e1468. [Google Scholar] [CrossRef]
- Hiraoka, K.; Zenmyo, M.; Watari, K.; Iguchi, H.; Fotovati, A.; Kimura, Y.N.; Hosoi, F.; Shoda, T.; Nagata, K.; Osada, H.; et al. Inhibition of bone and muscle metastases of lung cancer cells by a decrease in the number of monocytes/macrophages. Cancer Sci. 2008, 99, 1595–1602. [Google Scholar] [CrossRef]
- Soki, F.N.; Cho, S.W.; Kim, Y.W.; Jones, J.D.; Park, S.I.; Koh, A.J.; Entezami, P.; Daignault-Newton, S.; Pienta, K.J.; Roca, H.; et al. Bone marrow macrophages support prostate cancer growth in bone. Oncotarget 2015, 6, 35782–35796. [Google Scholar] [CrossRef]
- Lo, C.H.; Lynch, C.C. Multifaceted Roles for Macrophages in Prostate Cancer Skeletal Metastasis. Front. Endocrinol. 2018, 9, 247. [Google Scholar] [CrossRef]
- Biswas, S.K.; Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat. Immunol. 2010, 11, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Teitelbaum, S.L. Osteoclasts: New Insights. Bone Res. 2013, 1, 11–26. [Google Scholar] [CrossRef]
- Chang, M.K.; Raggatt, L.J.; Alexander, K.A.; Kuliwaba, J.S.; Fazzalari, N.L.; Schroder, K.; Maylin, E.R.; Ripoll, V.M.; Hume, D.A.; Pettit, A.R. Osteal tissue macrophages are intercalated throughout human and mouse bone lining tissues and regulate osteoblast function in vitro and in vivo. J. Immunol. 2008, 181, 1232–1244. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Movila, A.; Kataoka, S.; Wisitrasameewong, W.; Ruiz Torruella, M.; Murakoshi, M.; Murakami, S.; Kawai, T. Proinflammatory M1 Macrophages Inhibit RANKL-Induced Osteoclastogenesis. Infect. Immun. 2016, 84, 2802–2812. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Reinoso, V.; McCauley, L.K.; Fournier, P.G.J. Contribution of Macrophages and T Cells in Skeletal Metastasis. Cancers 2020, 12, 1014. [Google Scholar] [CrossRef] [PubMed]
- Zoni, E.; van der Pluijm, G. The role of microRNAs in bone metastasis. J. Bone Oncol. 2016, 5, 104–108. [Google Scholar] [CrossRef]
- Ke, K.; Sul, O.J.; Rajasekaran, M.; Choi, H.S. MicroRNA-183 increases osteoclastogenesis by repressing heme oxygenase-1. Bone 2015, 81, 237–246. [Google Scholar] [CrossRef]
- Soki, F.N.; Koh, A.J.; Jones, J.D.; Kim, Y.W.; Dai, J.; Keller, E.T.; Pienta, K.J.; Atabai, K.; Roca, H.; McCauley, L.K. Polarization of prostate cancer-associated macrophages is induced by milk fat globule-EGF factor 8 (MFG-E8)-mediated efferocytosis. J. Biol. Chem. 2014, 289, 24560–24572. [Google Scholar] [CrossRef]
- Ozdemir, B.C.; Hensel, J.; Secondini, C.; Wetterwald, A.; Schwaninger, R.; Fleischmann, A.; Raffelsberger, W.; Poch, O.; Delorenzi, M.; Temanni, R.; et al. The molecular signature of the stroma response in prostate cancer-induced osteoblastic bone metastasis highlights expansion of hematopoietic and prostate epithelial stem cell niches. PLoS ONE 2014, 9, e114530. [Google Scholar] [CrossRef]
- Wu, A.C.; He, Y.; Broomfield, A.; Paatan, N.J.; Harrington, B.S.; Tseng, H.W.; Beaven, E.A.; Kiernan, D.M.; Swindle, P.; Clubb, A.B.; et al. CD169(+) macrophages mediate pathological formation of woven bone in skeletal lesions of prostate cancer. J. Pathol. 2016, 239, 218–230. [Google Scholar] [CrossRef]
- Roca, H.; Jones, J.D.; Purica, M.C.; Weidner, S.; Koh, A.J.; Kuo, R.; Wilkinson, J.E.; Wang, Y.; Daignault-Newton, S.; Pienta, K.J.; et al. Apoptosis-induced CXCL5 accelerates inflammation and growth of prostate tumor metastases in bone. J. Clin. Investig. 2018, 128, 248–266. [Google Scholar] [CrossRef]
- Sulciner, M.L.; Serhan, C.N.; Gilligan, M.M.; Mudge, D.K.; Chang, J.; Gartung, A.; Lehner, K.A.; Bielenberg, D.R.; Schmidt, B.; Dalli, J.; et al. Resolvins suppress tumor growth and enhance cancer therapy. J. Exp. Med. 2018, 215, 115–140. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.; Koo, J.S. The Role of Adipokines and Bone Marrow Adipocytes in Breast Cancer Bone Metastasis. Int. J. Mol. Sci. 2020, 21, 4967. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, G.L.; Cao, S.; Zhao, M.C.; Liu, Y.Q.; Chen, X.X.; Qian, C. Adipogenic niches for melanoma cell colonization and growth in bone marrow. Lab. Investig. 2017, 97, 737–745. [Google Scholar] [CrossRef]
- Luo, G.; He, Y.; Yu, X. Bone Marrow Adipocyte: An Intimate Partner With Tumor Cells in Bone Metastasis. Front. Endocrinol. 2018, 9, 339. [Google Scholar] [CrossRef]
- Clements, V.K.; Long, T.; Long, R.; Figley, C.; Smith, D.M.C.; Ostrand-Rosenberg, S. Frontline Science: High fat diet and leptin promote tumor progression by inducing myeloid-derived suppressor cells. J. Leukoc. Biol. 2018, 103, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Svensson, R.U.; Parker, S.J.; Eichner, L.J.; Kolar, M.J.; Wallace, M.; Brun, S.N.; Lombardo, P.S.; Van Nostrand, J.L.; Hutchins, A.; Vera, L.; et al. Inhibition of acetyl-CoA carboxylase suppresses fatty acid synthesis and tumor growth of non-small-cell lung cancer in preclinical models. Nat. Med. 2016, 22, 1108–1119. [Google Scholar] [CrossRef]
- Goto, H.; Hozumi, A.; Osaki, M.; Fukushima, T.; Sakamoto, K.; Yonekura, A.; Tomita, M.; Furukawa, K.; Shindo, H.; Baba, H. Primary human bone marrow adipocytes support TNF-alpha-induced osteoclast differentiation and function through RANKL expression. Cytokine 2011, 56, 662–668. [Google Scholar] [CrossRef]
- Hardaway, A.L.; Herroon, M.K.; Rajagurubandara, E.; Podgorski, I. Marrow adipocyte-derived CXCL1 and CXCL2 contribute to osteolysis in metastatic prostate cancer. Clin. Exp. Metastasis 2015, 32, 353–368. [Google Scholar] [CrossRef]
- Hozumi, A.; Osaki, M.; Goto, H.; Sakamoto, K.; Inokuchi, S.; Shindo, H. Bone marrow adipocytes support dexamethasone-induced osteoclast differentiation. Biochem. Biophys. Res. Commun. 2009, 382, 780–784. [Google Scholar] [CrossRef]
- Shiozawa, Y.; Eber, M.R.; Berry, J.E.; Taichman, R.S. Bone marrow as a metastatic niche for disseminated tumor cells from solid tumors. Bonekey Rep. 2015, 4, 689. [Google Scholar] [CrossRef] [PubMed]
- Peinado, H.; Aleckovic, M.; Lavotshkin, S.; Matei, I.; Costa-Silva, B.; Moreno-Bueno, G.; Hergueta-Redondo, M.; Williams, C.; Garcia-Santos, G.; Ghajar, C.; et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 2012, 18, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Shiozawa, Y.; Pedersen, E.A.; Havens, A.M.; Jung, Y.; Mishra, A.; Joseph, J.; Kim, J.K.; Patel, L.R.; Ying, C.; Ziegler, A.M.; et al. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J. Clin. Investig. 2011, 121, 1298–1312. [Google Scholar] [CrossRef] [PubMed]
- Price, T.T.; Burness, M.L.; Sivan, A.; Warner, M.J.; Cheng, R.; Lee, C.H.; Olivere, L.; Comatas, K.; Magnani, J.; Kim Lyerly, H.; et al. Dormant breast cancer micrometastases reside in specific bone marrow niches that regulate their transit to and from bone. Sci. Transl. Med. 2016, 8, 340ra373. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Eber, M.R.; Shiozawa, Y. Models of Prostate Cancer Bone Metastasis. Methods Mol. Biol. 2019, 1914, 295–308. [Google Scholar] [CrossRef]
- Parekh, C.; Crooks, G.M. Critical differences in hematopoiesis and lymphoid development between humans and mice. J. Clin. Immunol. 2013, 33, 711–715. [Google Scholar] [CrossRef]
- Brendel, C.; Rio, P.; Verhoeyen, E. Humanized mice are precious tools for evaluation of hematopoietic gene therapies and preclinical modeling to move towards a clinical trial. Biochem. Pharmacol. 2020, 174, 113711. [Google Scholar] [CrossRef]
- McGovern, J.A.; Shafiee, A.; Wagner, F.; Lahr, C.A.; Landgraf, M.; Meinert, C.; Williams, E.D.; Russell, P.J.; Clements, J.A.; Loessner, D.; et al. Humanization of the Prostate Microenvironment Reduces Homing of PC3 Prostate Cancer Cells to Human Tissue-Engineered Bone. Cancers 2018, 10, 438. [Google Scholar] [CrossRef]
- Landgraf, M.; Lahr, C.A.; Sanchez-Herrero, A.; Meinert, C.; Shokoohmand, A.; Pollock, P.M.; Hutmacher, D.W.; Shafiee, A.; McGovern, J.A. Humanized bone facilitates prostate cancer metastasis and recapitulates therapeutic effects of zoledronic acid in vivo. Bone Res. 2019, 7, 31. [Google Scholar] [CrossRef]
- Lipton, A.; Fizazi, K.; Stopeck, A.T.; Henry, D.H.; Brown, J.E.; Yardley, D.A.; Richardson, G.E.; Siena, S.; Maroto, P.; Clemens, M.; et al. Superiority of denosumab to zoledronic acid for prevention of skeletal-related events: A combined analysis of 3 pivotal, randomised, phase 3 trials. Eur. J. Cancer 2012, 48, 3082–3092. [Google Scholar] [CrossRef]
- Scagliotti, G.V.; Hirsh, V.; Siena, S.; Henry, D.H.; Woll, P.J.; Manegold, C.; Solal-Celigny, P.; Rodriguez, G.; Krzakowski, M.; Mehta, N.D.; et al. Overall survival improvement in patients with lung cancer and bone metastases treated with denosumab versus zoledronic acid: Subgroup analysis from a randomized phase 3 study. J. Thorac. Oncol. 2012, 7, 1823–1829. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jacamo, R.; Shi, Y.X.; Wang, R.Y.; Battula, V.L.; Konoplev, S.; Strunk, D.; Hofmann, N.A.; Reinisch, A.; Konopleva, M.; et al. Human extramedullary bone marrow in mice: A novel in vivo model of genetically controlled hematopoietic microenvironment. Blood 2012, 119, 4971–4980. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Heckl, D.; Parekkadan, B. Multiple genetically engineered humanized microenvironments in a single mouse. Biomater. Res. 2016, 20, 19. [Google Scholar] [CrossRef] [PubMed]
- Abarrategi, A.; Mian, S.A.; Passaro, D.; Rouault-Pierre, K.; Grey, W.; Bonnet, D. Modeling the human bone marrow niche in mice: From host bone marrow engraftment to bioengineering approaches. J. Exp. Med. 2018, 215, 729–743. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Diaz, E.C.; Sinha, S.; Avedian, R.S.; Yang, F. Tissue-engineered 3D models for elucidating primary and metastatic bone cancer progression. Acta Biomater. 2019, 99, 18–32. [Google Scholar] [CrossRef]
- Holmes, D.; Gawad, S. The Application of Microfluidics in Biology. In Microengineering in Biotechnology; Hughes, M.P., Hoettges, K.F., Eds.; Humana Press: Totowa, NJ, USA, 2010; pp. 55–80. [Google Scholar]
- Castiaux, A.D.; Spence, D.M.; Martin, R.S. Review of 3D Cell Culture with Analysis in Microfluidic Systems. Anal. Methods 2019, 11, 4220–4232. [Google Scholar] [CrossRef] [PubMed]
- Ayuso, J.M.; Vitek, R.; Swick, A.D.; Skala, M.C.; Wisinski, K.B.; Kimple, R.J.; Lambert, P.F.; Beebe, D.J. Effects of culture method on response to EGFR therapy in head and neck squamous cell carcinoma cells. Sci. Rep. 2019, 9, 12480. [Google Scholar] [CrossRef]
- Tehranirokh, M.; Kouzani, A.Z.; Francis, P.S.; Kanwar, J.R. Microfluidic devices for cell cultivation and proliferation. Biomicrofluidics 2013, 7, 51502. [Google Scholar] [CrossRef]
- De Palma, M.; Biziato, D.; Petrova, T.V. Microenvironmental regulation of tumour angiogenesis. Nat. Rev. Cancer 2017, 17, 457–474. [Google Scholar] [CrossRef]
- Sackmann, E.K.; Fulton, A.L.; Beebe, D.J. The present and future role of microfluidics in biomedical research. Nature 2014, 507, 181–189. [Google Scholar] [CrossRef]
- Buchanan, C.F.; Verbridge, S.S.; Vlachos, P.P.; Rylander, M.N. Flow shear stress regulates endothelial barrier function and expression of angiogenic factors in a 3D microfluidic tumor vascular model. Cell Adhes. Migr. 2014, 8, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Laranga, R.; Duchi, S.; Ibrahim, T.; Guerrieri, A.N.; Donati, D.M.; Lucarelli, E. Trends in Bone Metastasis Modeling. Cancers 2020, 12, 2315. [Google Scholar] [CrossRef] [PubMed]
- Marlow, R.; Honeth, G.; Lombardi, S.; Cariati, M.; Hessey, S.; Pipili, A.; Mariotti, V.; Buchupalli, B.; Foster, K.; Bonnet, D.; et al. A novel model of dormancy for bone metastatic breast cancer cells. Cancer Res. 2013, 73, 6886–6899. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Shuman, L.A.; Sosnoski, D.M.; Dhurjati, R.; Vogler, E.A.; Mastro, A.M. Dynamic interaction between breast cancer cells and osteoblastic tissue: Comparison of two- and three-dimensional cultures. J. Cell Physiol. 2011, 226, 2150–2158. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, S.; Ramasundaram, P.; Dziopa, E.; Mannion, C.; Kissin, Y.; Tricoli, L.; Albanese, C.; Lee, W.; Zilberberg, J. Human ex vivo 3D bone model recapitulates osteocyte response to metastatic prostate cancer. Sci. Rep. 2018, 8, 17975. [Google Scholar] [CrossRef] [PubMed]
- Salamanna, F.; Borsari, V.; Brogini, S.; Giavaresi, G.; Parrilli, A.; Cepollaro, S.; Cadossi, M.; Martini, L.; Mazzotti, A.; Fini, M. An in vitro 3D bone metastasis model by using a human bone tissue culture and human sex-related cancer cells. Oncotarget 2016, 7, 76966–76983. [Google Scholar] [CrossRef]
- Sieh, S.; Taubenberger, A.V.; Lehman, M.L.; Clements, J.A.; Nelson, C.C.; Hutmacher, D.W. Paracrine interactions between LNCaP prostate cancer cells and bioengineered bone in 3D in vitro culture reflect molecular changes during bone metastasis. Bone 2014, 63, 121–131. [Google Scholar] [CrossRef]
- Paindelli, C.; Navone, N.; Logothetis, C.J.; Friedl, P.; Dondossola, E. Engineered bone for probing organotypic growth and therapy response of prostate cancer tumoroids in vitro. Biomaterials 2019, 197, 296–304. [Google Scholar] [CrossRef]
- Shokoohmand, A.; Ren, J.; Baldwin, J.; Atack, A.; Shafiee, A.; Theodoropoulos, C.; Wille, M.L.; Tran, P.A.; Bray, L.J.; Smith, D.; et al. Microenvironment engineering of osteoblastic bone metastases reveals osteomimicry of patient-derived prostate cancer xenografts. Biomaterials 2019, 220, 119402. [Google Scholar] [CrossRef]
- Bersini, S.; Jeon, J.S.; Dubini, G.; Arrigoni, C.; Chung, S.; Charest, J.L.; Moretti, M.; Kamm, R.D. A microfluidic 3D in vitro model for specificity of breast cancer metastasis to bone. Biomaterials 2014, 35, 2454–2461. [Google Scholar] [CrossRef]
- Hao, S.; Ha, L.; Cheng, G.; Wan, Y.; Xia, Y.; Sosnoski, D.M.; Mastro, A.M.; Zheng, S.Y. A Spontaneous 3D Bone-On-a-Chip for Bone Metastasis Study of Breast Cancer Cells. Small 2018, 14, e1702787. [Google Scholar] [CrossRef] [PubMed]
- Chong, M.S.; Lim, J.; Goh, J.; Sia, M.W.; Chan, J.K.; Teoh, S.H. Cocultures of mesenchymal stem cells and endothelial cells as organotypic models of prostate cancer metastasis. Mol. Pharm. 2014, 11, 2126–2133. [Google Scholar] [CrossRef] [PubMed]
- Kerr, S.C.; Morgan, M.M.; Gillette, A.A.; Livingston, M.K.; Lugo-Cintron, K.M.; Favreau, P.F.; Florek, L.; Johnson, B.P.; Lang, J.M.; Skala, M.C.; et al. A bioengineered organotypic prostate model for the study of tumor microenvironment-induced immune cell activation. Integr. Biol. 2020, 12, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Ayuso, J.M.; Truttschel, R.; Gong, M.M.; Humayun, M.; Virumbrales-Munoz, M.; Vitek, R.; Felder, M.; Gillies, S.D.; Sondel, P.; Wisinski, K.B.; et al. Evaluating natural killer cell cytotoxicity against solid tumors using a microfluidic model. Oncoimmunology 2019, 8, 1553477. [Google Scholar] [CrossRef] [PubMed]
- Bischel, L.L.; Sung, K.E.; Jimenez-Torres, J.A.; Mader, B.; Keely, P.J.; Beebe, D.J. The importance of being a lumen. FASEB J. 2014, 28, 4583–4590. [Google Scholar] [CrossRef]
- Zervantonakis, I.K.; Hughes-Alford, S.K.; Charest, J.L.; Condeelis, J.S.; Gertler, F.B.; Kamm, R.D. Three-dimensional microfluidic model for tumor cell intravasation and endothelial barrier function. Proc. Natl. Acad. Sci. USA 2012, 109, 13515–13520. [Google Scholar] [CrossRef]
- Parikh, M.R.; Belch, A.R.; Pilarski, L.M.; Kirshner, J. A three-dimensional tissue culture model to study primary human bone marrow and its malignancies. J. Vis. Exp. 2014, 85, 50947. [Google Scholar] [CrossRef]
- Torisawa, Y.S.; Spina, C.S.; Mammoto, T.; Mammoto, A.; Weaver, J.C.; Tat, T.; Collins, J.J.; Ingber, D.E. Bone marrow-on-a-chip replicates hematopoietic niche physiology in vitro. Nat. Methods 2014, 11, 663–669. [Google Scholar] [CrossRef]
- Glaser, D.E.; Curtis, M.B.; Sariano, P.A.; Rollins, Z.A.; Shergill, B.S.; Anand, A.; Deely, A.M.; Shirure, V.S.; Anderson, L.; Lowen, J.M.; et al. Organ-on-a-chip model of vascularized human bone marrow niches. Biomaterials 2021, 121245. [Google Scholar] [CrossRef]
- Zhang, W.; Bado, I.; Wang, H.; Lo, H.C.; Zhang, X.H. Bone Metastasis: Find Your Niche and Fit in. Trends Cancer 2019, 5, 95–110. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists’ Collaborative, G. Adjuvant bisphosphonate treatment in early breast cancer: Meta-analyses of individual patient data from randomised trials. Lancet 2015, 386, 1353–1361. [Google Scholar] [CrossRef]
- Smith, M.; Parker, C.; Saad, F.; Miller, K.; Tombal, B.; Ng, Q.S.; Boegemann, M.; Matveev, V.; Piulats, J.M.; Zucca, L.E.; et al. Addition of radium-223 to abiraterone acetate and prednisone or prednisolone in patients with castration-resistant prostate cancer and bone metastases (ERA 223): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 408–419. [Google Scholar] [CrossRef]
- McKay, R.R.; Bosse, D.; Gray, K.P.; Michaelson, M.D.; Krajewski, K.; Jacene, H.A.; Walsh, M.; Bellmunt, J.; Pomerantz, M.; Harshman, L.C.; et al. Radium-223 Dichloride in Combination with Vascular Endothelial Growth Factor-Targeting Therapy in Advanced Renal Cell Carcinoma with Bone Metastases. Clin. Cancer Res. 2018, 24, 4081–4088. [Google Scholar] [CrossRef] [PubMed]
- Pernas, S.; Martin, M.; Kaufman, P.A.; Gil-Martin, M.; Gomez Pardo, P.; Lopez-Tarruella, S.; Manso, L.; Ciruelos, E.; Perez-Fidalgo, J.A.; Hernando, C.; et al. Balixafortide plus eribulin in HER2-negative metastatic breast cancer: A phase 1, single-arm, dose-escalation trial. Lancet Oncol. 2018, 19, 812–824. [Google Scholar] [CrossRef]
- Sandhu, S.K.; Papadopoulos, K.; Fong, P.C.; Patnaik, A.; Messiou, C.; Olmos, D.; Wang, G.; Tromp, B.J.; Puchalski, T.A.; Balkwill, F.; et al. A first-in-human, first-in-class, phase I study of carlumab (CNTO 888), a human monoclonal antibody against CC-chemokine ligand 2 in patients with solid tumors. Cancer Chemother. Pharmacol. 2013, 71, 1041–1050. [Google Scholar] [CrossRef]
- Pienta, K.J.; Machiels, J.P.; Schrijvers, D.; Alekseev, B.; Shkolnik, M.; Crabb, S.J.; Li, S.; Seetharam, S.; Puchalski, T.A.; Takimoto, C.; et al. Phase 2 study of carlumab (CNTO 888), a human monoclonal antibody against CC-chemokine ligand 2 (CCL2), in metastatic castration-resistant prostate cancer. Investig. New Drugs 2013, 31, 760–768. [Google Scholar] [CrossRef]
- Goldstein, L.J.; Mansutti, M.; Levy, C.; Chang, J.C.; Henry, S.; Fernandez-Perez, I.; Prausova, J.; Staroslawska, E.; Viale, G.; Butler, B.; et al. A randomized, placebo-controlled phase 2 study of paclitaxel in combination with reparixin compared to paclitaxel alone as front-line therapy for metastatic triple-negative breast cancer (fRida). Breast Cancer Res. Treat. 2021, 190, 265–275. [Google Scholar] [CrossRef]
- Masuda, T.; Noda, M.; Kogawa, T.; Kitagawa, D.; Hayashi, N.; Jomori, T.; Nakanishi, Y.; Nakayama, K.I.; Ohno, S.; Mimori, K. Phase I dose-escalation trial to repurpose propagermanium, an oral CCL2 inhibitor, in patients with breast cancer. Cancer Sci. 2020, 111, 924–931. [Google Scholar] [CrossRef]
- Peng, S.B.; Zhang, X.; Paul, D.; Kays, L.M.; Gough, W.; Stewart, J.; Uhlik, M.T.; Chen, Q.; Hui, Y.H.; Zamek-Gliszczynski, M.J.; et al. Identification of LY2510924, a novel cyclic peptide CXCR4 antagonist that exhibits antitumor activities in solid tumor and breast cancer metastatic models. Mol. Cancer Ther. 2015, 14, 480–490. [Google Scholar] [CrossRef]
- Salgia, R.; Stille, J.R.; Weaver, R.W.; McCleod, M.; Hamid, O.; Polzer, J.; Roberson, S.; Flynt, A.; Spigel, D.R. A randomized phase II study of LY2510924 and carboplatin/etoposide versus carboplatin/etoposide in extensive-disease small cell lung cancer. Lung Cancer 2017, 105, 7–13. [Google Scholar] [CrossRef]
- Bilusic, M.; Heery, C.R.; Collins, J.M.; Donahue, R.N.; Palena, C.; Madan, R.A.; Karzai, F.; Marte, J.L.; Strauss, J.; Gatti-Mays, M.E.; et al. Phase I trial of HuMax-IL8 (BMS-986253), an anti-IL-8 monoclonal antibody, in patients with metastatic or unresectable solid tumors. J. Immunother. Cancer 2019, 7, 240. [Google Scholar] [CrossRef] [PubMed]
- Strauss, J.; Heery, C.R.; Schlom, J.; Madan, R.A.; Cao, L.; Kang, Z.; Lamping, E.; Marte, J.L.; Donahue, R.N.; Grenga, I.; et al. Phase I Trial of M7824 (MSB0011359C), a Bifunctional Fusion Protein Targeting PD-L1 and TGFbeta, in Advanced Solid Tumors. Clin. Cancer Res. 2018, 24, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Formenti, S.C.; Lee, P.; Adams, S.; Goldberg, J.D.; Li, X.; Xie, M.W.; Ratikan, J.A.; Felix, C.; Hwang, L.; Faull, K.F.; et al. Focal Irradiation and Systemic TGFbeta Blockade in Metastatic Breast Cancer. Clin. Cancer Res. 2018, 24, 2493–2504. [Google Scholar] [CrossRef] [PubMed]
- Melisi, D.; Garcia-Carbonero, R.; Macarulla, T.; Pezet, D.; Deplanque, G.; Fuchs, M.; Trojan, J.; Oettle, H.; Kozloff, M.; Cleverly, A.; et al. Galunisertib plus gemcitabine vs. gemcitabine for first-line treatment of patients with unresectable pancreatic cancer. Br. J. Cancer 2018, 119, 1208–1214. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, N.A.; Humphrey, J.S.; Ness, E.A.; Johnson, M.D.; Gupta, E.; Williams, K.; Daly, D.J.; Sonnichsen, D.; Conway, D.; Marshall, J.; et al. A phase I study of oral BMS-275291, a novel nonhydroxamate sheddase-sparing matrix metalloproteinase inhibitor, in patients with advanced or metastatic cancer. Clin. Cancer Res. 2004, 10, 1963–1970. [Google Scholar] [CrossRef]
- Leighl, N.B.; Paz-Ares, L.; Douillard, J.Y.; Peschel, C.; Arnold, A.; Depierre, A.; Santoro, A.; Betticher, D.C.; Gatzemeier, U.; Jassem, J.; et al. Randomized phase III study of matrix metalloproteinase inhibitor BMS-275291 in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: National Cancer Institute of Canada-Clinical Trials Group Study BR.18. J. Clin. Oncol. 2005, 23, 2831–2839. [Google Scholar] [CrossRef]
- Sparano, J.A.; Bernardo, P.; Stephenson, P.; Gradishar, W.J.; Ingle, J.N.; Zucker, S.; Davidson, N.E. Randomized phase III trial of marimastat versus placebo in patients with metastatic breast cancer who have responding or stable disease after first-line chemotherapy: Eastern Cooperative Oncology Group trial E2196. J. Clin. Oncol. 2004, 22, 4683–4690. [Google Scholar] [CrossRef]
- Goffin, J.R.; Anderson, I.C.; Supko, J.G.; Eder, J.P., Jr.; Shapiro, G.I.; Lynch, T.J.; Shipp, M.; Johnson, B.E.; Skarin, A.T. Phase I trial of the matrix metalloproteinase inhibitor marimastat combined with carboplatin and paclitaxel in patients with advanced non-small cell lung cancer. Clin. Cancer Res. 2005, 11, 3417–3424. [Google Scholar] [CrossRef]
- Shepherd, F.A.; Giaccone, G.; Seymour, L.; Debruyne, C.; Bezjak, A.; Hirsh, V.; Smylie, M.; Rubin, S.; Martins, H.; Lamont, A.; et al. Prospective, randomized, double-blind, placebo-controlled trial of marimastat after response to first-line chemotherapy in patients with small-cell lung cancer: A trial of the National Cancer Institute of Canada-Clinical Trials Group and the European Organization for Research and Treatment of Cancer. J. Clin. Oncol. 2002, 20, 4434–4439. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Heath, E.I.; Posadas, E.M.; Yu, E.Y.; Harrison, M.R.; Bruce, J.Y.; Cho, S.Y.; Wilding, G.E.; Fetterly, G.J.; Hangauer, D.G.; et al. A phase 2 study of KX2-391, an oral inhibitor of Src kinase and tubulin polymerization, in men with bone-metastatic castration-resistant prostate cancer. Cancer Chemother. Pharmacol. 2013, 71, 883–892. [Google Scholar] [CrossRef]
- Yu, E.Y.; Wilding, G.; Posadas, E.; Gross, M.; Culine, S.; Massard, C.; Morris, M.J.; Hudes, G.; Calabro, F.; Cheng, S.; et al. Phase II study of dasatinib in patients with metastatic castration-resistant prostate cancer. Clin. Cancer Res. 2009, 15, 7421–7428. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.Y.; Duan, F.; Muzi, M.; Deng, X.; Chin, B.B.; Alumkal, J.J.; Taplin, M.E.; Taub, J.M.; Herman, B.; Higano, C.S.; et al. Castration-resistant prostate cancer bone metastasis response measured by 18F-fluoride PET after treatment with dasatinib and correlation with progression-free survival: Results from American College of Radiology Imaging Network 6687. J. Nucl. Med. 2015, 56, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Mitri, Z.; Nanda, R.; Blackwell, K.; Costelloe, C.M.; Hood, I.; Wei, C.; Brewster, A.M.; Ibrahim, N.K.; Koenig, K.B.; Hortobagyi, G.N.; et al. TBCRC-010: Phase I/II Study of Dasatinib in Combination with Zoledronic Acid for the Treatment of Breast Cancer Bone Metastasis. Clin. Cancer Res. 2016, 22, 5706–5712. [Google Scholar] [CrossRef]
- Quinn, D.I.; Tangen, C.M.; Hussain, M.; Lara, P.N., Jr.; Goldkorn, A.; Moinpour, C.M.; Garzotto, M.G.; Mack, P.C.; Carducci, M.A.; Monk, J.P.; et al. Docetaxel and atrasentan versus docetaxel and placebo for men with advanced castration-resistant prostate cancer (SWOG S0421): A randomised phase 3 trial. Lancet Oncol. 2013, 14, 893–900. [Google Scholar] [CrossRef]
- Iyer, S.P.; Beck, J.T.; Stewart, A.K.; Shah, J.; Kelly, K.R.; Isaacs, R.; Bilic, S.; Sen, S.; Munshi, N.C. A Phase IB multicentre dose-determination study of BHQ880 in combination with anti-myeloma therapy and zoledronic acid in patients with relapsed or refractory multiple myeloma and prior skeletal-related events. Br. J. Haematol. 2014, 167, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Juarez, P.; Guise, T.A. TGF-beta in cancer and bone: Implications for treatment of bone metastases. Bone 2011, 48, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Jilka, R.L.; Weinstein, R.S.; Bellido, T.; Parfitt, A.M.; Manolagas, S.C. Osteoblast programmed cell death (apoptosis): Modulation by growth factors and cytokines. J. Bone Miner. Res. 1998, 13, 793–802. [Google Scholar] [CrossRef]
- Kassem, M.; Kveiborg, M.; Eriksen, E.F. Production and action of transforming growth factor-beta in human osteoblast cultures: Dependence on cell differentiation and modulation by calcitriol. Eur. J. Clin. Investig. 2000, 30, 429–437. [Google Scholar] [CrossRef]
- Rodriguez-Carballo, E.; Gamez, B.; Ventura, F. p38 MAPK Signaling in Osteoblast Differentiation. Front. Cell Dev. Biol. 2016, 4, 40. [Google Scholar] [CrossRef]
- Alliston, T.; Choy, L.; Ducy, P.; Karsenty, G.; Derynck, R. TGF-beta-induced repression of CBFA1 by Smad3 decreases cbfa1 and osteocalcin expression and inhibits osteoblast differentiation. EMBO J. 2001, 20, 2254–2272. [Google Scholar] [CrossRef]
- Wu, M.; Chen, G.; Li, Y.P. TGF-beta and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016, 4, 16009. [Google Scholar] [CrossRef]
- Karst, M.; Gorny, G.; Galvin, R.J.; Oursler, M.J. Roles of stromal cell RANKL, OPG, and M-CSF expression in biphasic TGF-beta regulation of osteoclast differentiation. J. Cell Physiol. 2004, 200, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, K.S.; Chen, C.G.; Balooch, G.; Stebbins, E.; McKenna, C.R.; Davis, H.; Niewolna, M.; Peng, X.H.; Nguyen, D.H.; Ionova-Martin, S.S.; et al. Pharmacologic inhibition of the TGF-beta type I receptor kinase has anabolic and anti-catabolic effects on bone. PLoS ONE 2009, 4, e5275. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; He, W.; Tulley, S.; Gupta, G.P.; Serganova, I.; Chen, C.R.; Manova-Todorova, K.; Blasberg, R.; Gerald, W.L.; Massague, J. Breast cancer bone metastasis mediated by the Smad tumor suppressor pathway. Proc. Natl. Acad. Sci. USA 2005, 102, 13909–13914. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.G.; Arnold, S.A.; Jiang, M.; Palmer, T.D.; Ketova, T.; Merkel, A.; Pickup, M.; Samaras, S.; Shyr, Y.; Moses, H.L.; et al. ALCAM/CD166 is a TGF-beta-responsive marker and functional regulator of prostate cancer metastasis to bone. Cancer Res. 2014, 74, 1404–1415. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Siegel, P.M.; Shu, W.; Drobnjak, M.; Kakonen, S.M.; Cordon-Cardo, C.; Guise, T.A.; Massague, J. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 2003, 3, 537–549. [Google Scholar] [CrossRef]
- Fournier, P.G.; Juarez, P.; Jiang, G.; Clines, G.A.; Niewolna, M.; Kim, H.S.; Walton, H.W.; Peng, X.H.; Liu, Y.; Mohammad, K.S.; et al. The TGF-beta Signaling Regulator PMEPA1 Suppresses Prostate Cancer Metastases to Bone. Cancer Cell 2015, 27, 809–821. [Google Scholar] [CrossRef]
- Javelaud, D.; Mohammad, K.S.; McKenna, C.R.; Fournier, P.; Luciani, F.; Niewolna, M.; Andre, J.; Delmas, V.; Larue, L.; Guise, T.A.; et al. Stable overexpression of Smad7 in human melanoma cells impairs bone metastasis. Cancer Res. 2007, 67, 2317–2324. [Google Scholar] [CrossRef]
- Juarez, P.; Fournier, P.G.J.; Mohammad, K.S.; McKenna, R.C.; Davis, H.W.; Peng, X.H.; Niewolna, M.; Mauviel, A.; Chirgwin, J.M.; Guise, T.A. Halofuginone inhibits TGF-beta/BMP signaling and in combination with zoledronic acid enhances inhibition of breast cancer bone metastasis. Oncotarget 2017, 8, 86447–86462. [Google Scholar] [CrossRef]
- Mohammad, K.S.; Javelaud, D.; Fournier, P.G.; Niewolna, M.; McKenna, C.R.; Peng, X.H.; Duong, V.; Dunn, L.K.; Mauviel, A.; Guise, T.A. TGF-beta-RI kinase inhibitor SD-208 reduces the development and progression of melanoma bone metastases. Cancer Res. 2011, 71, 175–184. [Google Scholar] [CrossRef]
- Wan, X.; Li, Z.G.; Yingling, J.M.; Yang, J.; Starbuck, M.W.; Ravoori, M.K.; Kundra, V.; Vazquez, E.; Navone, N.M. Effect of transforming growth factor beta (TGF-beta) receptor I kinase inhibitor on prostate cancer bone growth. Bone 2012, 50, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.J.; Selander, K.; Chirgwin, J.M.; Dallas, M.; Grubbs, B.G.; Wieser, R.; Massague, J.; Mundy, G.R.; Guise, T.A. TGF-beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J. Clin. Investig. 1999, 103, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Fenaux, P.; Platzbecker, U.; Mufti, G.J.; Garcia-Manero, G.; Buckstein, R.; Santini, V.; Diez-Campelo, M.; Finelli, C.; Cazzola, M.; Ilhan, O.; et al. Luspatercept in Patients with Lower-Risk Myelodysplastic Syndromes. N. Engl. J. Med. 2020, 382, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Cappellini, M.D.; Viprakasit, V.; Taher, A.T.; Georgiev, P.; Kuo, K.H.M.; Coates, T.; Voskaridou, E.; Liew, H.K.; Pazgal-Kobrowski, I.; Forni, G.L.; et al. A Phase 3 Trial of Luspatercept in Patients with Transfusion-Dependent beta-Thalassemia. N. Engl. J. Med. 2020, 382, 1219–1231. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, L.U.; Hagberg Thulin, M.; Plas, P.; Olsson, A.; Damber, J.E.; Welen, K. Tasquinimod inhibits prostate cancer growth in bone through alterations in the bone microenvironment. Prostate 2016, 76, 383–393. [Google Scholar] [CrossRef]
- Alarmo, E.L.; Kallioniemi, A. Bone morphogenetic proteins in breast cancer: Dual role in tumourigenesis? Endocr. Relat. Cancer 2010, 17, R123–R139. [Google Scholar] [CrossRef]
- Rahman, M.S.; Akhtar, N.; Jamil, H.M.; Banik, R.S.; Asaduzzaman, S.M. TGF-beta/BMP signaling and other molecular events: Regulation of osteoblastogenesis and bone formation. Bone Res. 2015, 3, 15005. [Google Scholar] [CrossRef]
- Ketolainen, J.M.; Alarmo, E.L.; Tuominen, V.J.; Kallioniemi, A. Parallel inhibition of cell growth and induction of cell migration and invasion in breast cancer cells by bone morphogenetic protein 4. Breast Cancer Res. Treat. 2010, 124, 377–386. [Google Scholar] [CrossRef]
- Zabkiewicz, C.; Resaul, J.; Hargest, R.; Jiang, W.G.; Ye, L. Bone morphogenetic proteins, breast cancer, and bone metastases: Striking the right balance. Endocr. Relat. Cancer 2017, 24, R349–R366. [Google Scholar] [CrossRef]
- Katsuno, Y.; Hanyu, A.; Kanda, H.; Ishikawa, Y.; Akiyama, F.; Iwase, T.; Ogata, E.; Ehata, S.; Miyazono, K.; Imamura, T. Bone morphogenetic protein signaling enhances invasion and bone metastasis of breast cancer cells through Smad pathway. Oncogene 2008, 27, 6322–6333. [Google Scholar] [CrossRef]
- Lee, Y.C.; Cheng, C.J.; Bilen, M.A.; Lu, J.F.; Satcher, R.L.; Yu-Lee, L.Y.; Gallick, G.E.; Maity, S.N.; Lin, S.H. BMP4 promotes prostate tumor growth in bone through osteogenesis. Cancer Res. 2011, 71, 5194–5203. [Google Scholar] [CrossRef]
- Owens, P.; Pickup, M.W.; Novitskiy, S.V.; Giltnane, J.M.; Gorska, A.E.; Hopkins, C.R.; Hong, C.C.; Moses, H.L. Inhibition of BMP signaling suppresses metastasis in mammary cancer. Oncogene 2015, 34, 2437–2449. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, R.X.; Yuan, W.; Chen, H.Q.; Tian, D.D.; Li, H.; Jiang, X.; Deng, Z.L.; Wang, Y. Knockdown of Bone Morphogenetic Proteins Type 1a Receptor (BMPR1a) in Breast Cancer Cells Protects Bone from Breast Cancer-Induced Osteolysis by Suppressing RANKL Expression. Cell Physiol. Biochem. 2018, 45, 1759–1771. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Bryant, H.U.; Macdougald, O.A. Regulation of bone mass by Wnt signaling. J. Clin. Investig. 2006, 116, 1202–1209. [Google Scholar] [CrossRef] [PubMed]
- Woolf, D.K.; Padhani, A.R.; Makris, A. Assessing response to treatment of bone metastases from breast cancer: What should be the standard of care? Ann. Oncol. 2015, 26, 1048–1057. [Google Scholar] [CrossRef]
- Hall, C.L.; Bafico, A.; Dai, J.; Aaronson, S.A.; Keller, E.T. Prostate cancer cells promote osteoblastic bone metastases through Wnts. Cancer Res. 2005, 65, 7554–7560. [Google Scholar] [CrossRef]
- Xu, W.H.; Liu, Z.B.; Yang, C.; Qin, W.; Shao, Z.M. Expression of dickkopf-1 and beta-catenin related to the prognosis of breast cancer patients with triple negative phenotype. PLoS ONE 2012, 7, e37624. [Google Scholar] [CrossRef]
- Letamendia, A.; Labbe, E.; Attisano, L. Transcriptional regulation by Smads: Crosstalk between the TGF-beta and Wnt pathways. J. Bone Jt. Surg. Am. 2001, 83-A (Suppl. S1), S31–S39. [Google Scholar] [CrossRef]
- Johnson, R.W.; Merkel, A.R.; Page, J.M.; Ruppender, N.S.; Guelcher, S.A.; Sterling, J.A. Wnt signaling induces gene expression of factors associated with bone destruction in lung and breast cancer. Clin. Exp. Metastasis 2014, 31, 945–959. [Google Scholar] [CrossRef]
- Bu, G.; Lu, W.; Liu, C.C.; Selander, K.; Yoneda, T.; Hall, C.; Keller, E.T.; Li, Y. Breast cancer-derived Dickkopf1 inhibits osteoblast differentiation and osteoprotegerin expression: Implication for breast cancer osteolytic bone metastases. Int. J. Cancer 2008, 123, 1034–1042. [Google Scholar] [CrossRef]
- Pang, H.; Ma, N.; Shen, W.; Zhao, Q.; Wang, J.; Duan, L.; Chen, W.; Zhang, N.; Zhao, Z.; Liu, L.; et al. Effects of DKK1 overexpression on bone metastasis of SBC-3 cells. Oncol. Lett. 2018, 15, 6739–6744. [Google Scholar] [CrossRef] [PubMed]
- Thudi, N.K.; Martin, C.K.; Murahari, S.; Shu, S.T.; Lanigan, L.G.; Werbeck, J.L.; Keller, E.T.; McCauley, L.K.; Pinzone, J.J.; Rosol, T.J. Dickkopf-1 (DKK-1) stimulated prostate cancer growth and metastasis and inhibited bone formation in osteoblastic bone metastases. Prostate 2011, 71, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Clines, K.L.; Clines, G.A. DKK1 and Kremen Expression Predicts the Osteoblastic Response to Bone Metastasis. Transl. Oncol. 2018, 11, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Zhang, H.; Li, X.; Li, X.; Cong, M.; Peng, F.; Yu, J.; Zhang, X.; Yang, Q.; Hu, G. Differential effects on lung and bone metastasis of breast cancer by Wnt signalling inhibitor DKK1. Nat. Cell Biol. 2017, 19, 1274–1285. [Google Scholar] [CrossRef]
- Giri, J.; Das, R.; Nylen, E.; Chinnadurai, R.; Galipeau, J. CCL2 and CXCL12 Derived from Mesenchymal Stromal Cells Cooperatively Polarize IL-10+ Tissue Macrophages to Mitigate Gut Injury. Cell Rep. 2020, 30, 1923–1934.e1924. [Google Scholar] [CrossRef] [PubMed]
- Zimmerlin, L.; Park, T.S.; Zambidis, E.T.; Donnenberg, V.S.; Donnenberg, A.D. Mesenchymal stem cell secretome and regenerative therapy after cancer. Biochimie 2013, 95, 2235–2245. [Google Scholar] [CrossRef]
- Minn, A.J.; Gupta, G.P.; Siegel, P.M.; Bos, P.D.; Shu, W.; Giri, D.D.; Viale, A.; Olshen, A.B.; Gerald, W.L.; Massague, J. Genes that mediate breast cancer metastasis to lung. Nature 2005, 436, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Minn, A.J.; Kang, Y.; Serganova, I.; Gupta, G.P.; Giri, D.D.; Doubrovin, M.; Ponomarev, V.; Gerald, W.L.; Blasberg, R.; Massague, J. Distinct organ-specific metastatic potential of individual breast cancer cells and primary tumors. J. Clin. Investig. 2005, 115, 44–55. [Google Scholar] [CrossRef]
- Guerard, A.; Laurent, V.; Fromont, G.; Esteve, D.; Gilhodes, J.; Bonnelye, E.; Le Gonidec, S.; Valet, P.; Malavaud, B.; Reina, N.; et al. The Chemokine Receptor CCR3 Is Potentially Involved in the Homing of Prostate Cancer Cells to Bone: Implication of Bone-Marrow Adipocytes. Int. J. Mol. Sci. 2021, 22, 1994. [Google Scholar] [CrossRef]
- Bonapace, L.; Coissieux, M.M.; Wyckoff, J.; Mertz, K.D.; Varga, Z.; Junt, T.; Bentires-Alj, M. Cessation of CCL2 inhibition accelerates breast cancer metastasis by promoting angiogenesis. Nature 2014, 515, 130–133. [Google Scholar] [CrossRef]
- Esposito, M.; Mondal, N.; Greco, T.M.; Wei, Y.; Spadazzi, C.; Lin, S.C.; Zheng, H.; Cheung, C.; Magnani, J.L.; Lin, S.H.; et al. Bone vascular niche E-selectin induces mesenchymal-epithelial transition and Wnt activation in cancer cells to promote bone metastasis. Nat. Cell Biol. 2019, 21, 627–639. [Google Scholar] [CrossRef] [PubMed]
- Fedarko, N.S.; Jain, A.; Karadag, A.; Van Eman, M.R.; Fisher, L.W. Elevated serum bone sialoprotein and osteopontin in colon, breast, prostate, and lung cancer. Clin. Cancer Res. 2001, 7, 4060–4066. [Google Scholar]
- Zhao, H.; Chen, Q.; Alam, A.; Cui, J.; Suen, K.C.; Soo, A.P.; Eguchi, S.; Gu, J.; Ma, D. The role of osteopontin in the progression of solid organ tumour. Cell Death Dis. 2018, 9, 356. [Google Scholar] [CrossRef]
- Pecheur, I.; Peyruchaud, O.; Serre, C.M.; Guglielmi, J.; Voland, C.; Bourre, F.; Margue, C.; Cohen-Solal, M.; Buffet, A.; Kieffer, N.; et al. Integrin alpha(v)beta3 expression confers on tumor cells a greater propensity to metastasize to bone. FASEB J. 2002, 16, 1266–1268. [Google Scholar] [CrossRef]
- Kim, B.; Kim, H.; Jung, S.; Moon, A.; Noh, D.Y.; Lee, Z.H.; Kim, H.J.; Kim, H.H. A CTGF-RUNX2-RANKL Axis in Breast and Prostate Cancer Cells Promotes Tumor Progression in Bone. J. Bone Miner. Res. 2020, 35, 155–166. [Google Scholar] [CrossRef]
- Zhang, X.H.; Wang, Q.; Gerald, W.; Hudis, C.A.; Norton, L.; Smid, M.; Foekens, J.A.; Massague, J. Latent bone metastasis in breast cancer tied to Src-dependent survival signals. Cancer Cell 2009, 16, 67–78. [Google Scholar] [CrossRef]
- Zhang, X.H.; Jin, X.; Malladi, S.; Zou, Y.; Wen, Y.H.; Brogi, E.; Smid, M.; Foekens, J.A.; Massague, J. Selection of bone metastasis seeds by mesenchymal signals in the primary tumor stroma. Cell 2013, 154, 1060–1073. [Google Scholar] [CrossRef]
- Ganguly, S.S.; Hostetter, G.; Tang, L.; Frank, S.B.; Saboda, K.; Mehra, R.; Wang, L.; Li, X.; Keller, E.T.; Miranti, C.K. Notch3 promotes prostate cancer-induced bone lesion development via MMP-3. Oncogene 2020, 39, 204–218. [Google Scholar] [CrossRef] [PubMed]
- Sethi, N.; Dai, X.; Winter, C.G.; Kang, Y. Tumor-derived JAGGED1 promotes osteolytic bone metastasis of breast cancer by engaging notch signaling in bone cells. Cancer Cell 2011, 19, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Bae, Y.; Kasimir-Bauer, S.; Tang, R.; Chen, J.; Ren, G.; Yuan, M.; Esposito, M.; Li, W.; Wei, Y.; et al. Therapeutic Antibody Targeting Tumor- and Osteoblastic Niche-Derived Jagged1 Sensitizes Bone Metastasis to Chemotherapy. Cancer Cell 2017, 32, 731–747.e736. [Google Scholar] [CrossRef]
- Shahriari, K.; Shen, F.; Worrede-Mahdi, A.; Liu, Q.; Gong, Y.; Garcia, F.U.; Fatatis, A. Cooperation among heterogeneous prostate cancer cells in the bone metastatic niche. Oncogene 2017, 36, 2846–2856. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Huang, D.; Blick, T.; Connor, A.; Reiter, L.A.; Hardink, J.R.; Lynch, C.C.; Waltham, M.; Thompson, E.W. An MMP13-selective inhibitor delays primary tumor growth and the onset of tumor-associated osteolytic lesions in experimental models of breast cancer. PLoS ONE 2012, 7, e29615. [Google Scholar] [CrossRef] [PubMed]
- Cathcart, J.; Pulkoski-Gross, A.; Cao, J. Targeting Matrix Metalloproteinases in Cancer: Bringing New Life to Old Ideas. Genes Dis. 2015, 2, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Tauro, M.; Shay, G.; Sansil, S.S.; Laghezza, A.; Tortorella, P.; Neuger, A.M.; Soliman, H.; Lynch, C.C. Bone-Seeking Matrix Metalloproteinase-2 Inhibitors Prevent Bone Metastatic Breast Cancer Growth. Mol. Cancer Ther. 2017, 16, 494–505. [Google Scholar] [CrossRef] [PubMed]
- Gamez, B.; Rodriguez-Carballo, E.; Graupera, M.; Rosa, J.L.; Ventura, F. Class I PI-3-Kinase Signaling Is Critical for Bone Formation Through Regulation of SMAD1 Activity in Osteoblasts. J. Bone Miner. Res. 2016, 31, 1617–1630. [Google Scholar] [CrossRef]
- Moon, J.B.; Kim, J.H.; Kim, K.; Youn, B.U.; Ko, A.; Lee, S.Y.; Kim, N. Akt induces osteoclast differentiation through regulating the GSK3beta/NFATc1 signaling cascade. J. Immunol. 2012, 188, 163–169. [Google Scholar] [CrossRef]
- Valer, J.A.; Sanchez-de-Diego, C.; Gamez, B.; Mishina, Y.; Rosa, J.L.; Ventura, F. Inhibition of phosphatidylinositol 3-kinase alpha (PI3Kalpha) prevents heterotopic ossification. EMBO Mol. Med. 2019, 11, e10567. [Google Scholar] [CrossRef]
- Yuan, G.; Lian, Z.; Liu, Q.; Lin, X.; Xie, D.; Song, F.; Wang, X.; Shao, S.; Zhou, B.; Li, C.; et al. Phosphatidyl inositol 3-kinase (PI3K)-mTOR inhibitor PKI-402 inhibits breast cancer induced osteolysis. Cancer Lett. 2019, 443, 135–144. [Google Scholar] [CrossRef]
- Mancini, A.; Colapietro, A.; Pompili, S.; Del Fattore, A.; Delle Monache, S.; Biordi, L.A.; Angelucci, A.; Mattei, V.; Liang, C.; Gravina, G.L.; et al. Dual PI3 K/mTOR inhibition reduces prostate cancer bone engraftment altering tumor-induced bone remodeling. Tumour. Biol. 2018, 40, 1010428318771773. [Google Scholar] [CrossRef]
- Hinz, N.; Jucker, M. AKT in Bone Metastasis of Solid Tumors: A Comprehensive Review. Cancers 2021, 13, 2287. [Google Scholar] [CrossRef]
- Haber, T.; Jockel, E.; Roos, F.C.; Junker, K.; Prawitt, D.; Hampel, C.; Thuroff, J.W.; Brenner, W.; German Renal Cell Tumor, N. Bone Metastasis in Renal Cell Carcinoma is Preprogrammed in the Primary Tumor and Caused by AKT and Integrin alpha5 Signaling. J. Urol. 2015, 194, 539–546. [Google Scholar] [CrossRef]
- Buschhaus, J.M.; Humphries, B.A.; Eckley, S.S.; Robison, T.H.; Cutter, A.C.; Rajendran, S.; Haley, H.R.; Bevoor, A.S.; Luker, K.E.; Luker, G.D. Targeting disseminated estrogen-receptor-positive breast cancer cells in bone marrow. Oncogene 2020, 39, 5649–5662. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhou, W.; Ge, J.; Zhang, Z. Prostaglandin E2 receptor EP4 is involved in the cell growth and invasion of prostate cancer via the cAMPPKA/PI3KAkt signaling pathway. Mol. Med. Rep. 2018, 17, 4702–4712. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Hu, X.; Xu, J.; Cheng, Y.; Shao, Y.; Peng, Y. Effect of PI3K/Akt Signaling Pathway on the Process of Prostate Cancer Metastasis to Bone. Cell Biochem. Biophys. 2015, 72, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Andre, F.; Ciruelos, E.M.; Juric, D.; Loibl, S.; Campone, M.; Mayer, I.A.; Rubovszky, G.; Yamashita, T.; Kaufman, B.; Lu, Y.S.; et al. Alpelisib plus fulvestrant for PIK3CA-mutated, hormone receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: Final overall survival results from SOLAR-1. Ann. Oncol. 2021, 32, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Andre, F.; Ciruelos, E.; Rubovszky, G.; Campone, M.; Loibl, S.; Rugo, H.S.; Iwata, H.; Conte, P.; Mayer, I.A.; Kaufman, B.; et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer. N. Engl. J. Med. 2019, 380, 1929–1940. [Google Scholar] [CrossRef]
- Wortzel, I.; Dror, S.; Kenific, C.M.; Lyden, D. Exosome-Mediated Metastasis: Communication from a Distance. Dev. Cell 2019, 49, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Giannandrea, D.; Citro, V.; Lesma, E.; Bignotto, M.; Platonova, N.; Chiaramonte, R. Restoring Tissue Homeostasis at Metastatic Sites: A Focus on Extracellular Vesicles in Bone Metastasis. Front. Oncol. 2021, 11, 644109. [Google Scholar] [CrossRef]
- Dai, J.; Escara-Wilke, J.; Keller, J.M.; Jung, Y.; Taichman, R.S.; Pienta, K.J.; Keller, E.T. Primary prostate cancer educates bone stroma through exosomal pyruvate kinase M2 to promote bone metastasis. J. Exp. Med. 2019, 216, 2883–2899. [Google Scholar] [CrossRef]
- Raimondo, S.; Saieva, L.; Vicario, E.; Pucci, M.; Toscani, D.; Manno, M.; Raccosta, S.; Giuliani, N.; Alessandro, R. Multiple myeloma-derived exosomes are enriched of amphiregulin (AREG) and activate the epidermal growth factor pathway in the bone microenvironment leading to osteoclastogenesis. J. Hematol. Oncol. 2019, 12, 2. [Google Scholar] [CrossRef]
- Taverna, S.; Pucci, M.; Giallombardo, M.; Di Bella, M.A.; Santarpia, M.; Reclusa, P.; Gil-Bazo, I.; Rolfo, C.; Alessandro, R. Amphiregulin contained in NSCLC-exosomes induces osteoclast differentiation through the activation of EGFR pathway. Sci. Rep. 2017, 7, 3170. [Google Scholar] [CrossRef] [PubMed]
- Tiedemann, K.; Sadvakassova, G.; Mikolajewicz, N.; Juhas, M.; Sabirova, Z.; Tabaries, S.; Gettemans, J.; Siegel, P.M.; Komarova, S.V. Exosomal Release of L-Plastin by Breast Cancer Cells Facilitates Metastatic Bone Osteolysis. Transl. Oncol. 2019, 12, 462–474. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Qian, N.; Ling, S.; Li, Y.; Sun, W.; Li, J.; Du, R.; Zhong, G.; Liu, C.; Yu, G.; et al. Breast cancer exosomes contribute to pre-metastatic niche formation and promote bone metastasis of tumor cells. Theranostics 2021, 11, 1429–1445. [Google Scholar] [CrossRef] [PubMed]
- Probert, C.; Dottorini, T.; Speakman, A.; Hunt, S.; Nafee, T.; Fazeli, A.; Wood, S.; Brown, J.E.; James, V. Communication of prostate cancer cells with bone cells via extracellular vesicle RNA; a potential mechanism of metastasis. Oncogene 2019, 38, 1751–1763. [Google Scholar] [CrossRef]
- Cai, C.; Wang, H.; He, H.H.; Chen, S.; He, L.; Ma, F.; Mucci, L.; Wang, Q.; Fiore, C.; Sowalsky, A.G.; et al. ERG induces androgen receptor-mediated regulation of SOX9 in prostate cancer. J. Clin. Investig. 2013, 123, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Haider, M.; Zhang, X.; Coleman, I.; Ericson, N.; True, L.D.; Lam, H.M.; Brown, L.G.; Ketchanji, M.; Nghiem, B.; Lakely, B.; et al. Epithelial mesenchymal-like transition occurs in a subset of cells in castration resistant prostate cancer bone metastases. Clin. Exp. Metastasis 2016, 33, 239–248. [Google Scholar] [CrossRef]
- Hornberg, E.; Ylitalo, E.B.; Crnalic, S.; Antti, H.; Stattin, P.; Widmark, A.; Bergh, A.; Wikstrom, P. Expression of androgen receptor splice variants in prostate cancer bone metastases is associated with castration-resistance and short survival. PLoS ONE 2011, 6, e19059. [Google Scholar] [CrossRef]
- Ylitalo, E.B.; Thysell, E.; Jernberg, E.; Lundholm, M.; Crnalic, S.; Egevad, L.; Stattin, P.; Widmark, A.; Bergh, A.; Wikstrom, P. Subgroups of Castration-resistant Prostate Cancer Bone Metastases Defined Through an Inverse Relationship Between Androgen Receptor Activity and Immune Response. Eur. Urol. 2017, 71, 776–787. [Google Scholar] [CrossRef]
- Zheng, G.; Lin, M.T.; Lokhandwala, P.M.; Beierl, K.; Netto, G.J.; Gocke, C.D.; Eshleman, J.R.; McCarthy, E.; Illei, P.B. Clinical mutational profiling of bone metastases of lung and colon carcinoma and malignant melanoma using next-generation sequencing. Cancer Cytopathol. 2016, 124, 744–753. [Google Scholar] [CrossRef]
- Zehir, A.; Benayed, R.; Shah, R.H.; Syed, A.; Middha, S.; Kim, H.R.; Srinivasan, P.; Gao, J.; Chakravarty, D.; Devlin, S.M.; et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat. Med. 2017, 23, 703–713. [Google Scholar] [CrossRef]
- Abbasi, M.R.; Rifatbegovic, F.; Brunner, C.; Mann, G.; Ziegler, A.; Potschger, U.; Crazzolara, R.; Ussowicz, M.; Benesch, M.; Ebetsberger-Dachs, G.; et al. Impact of Disseminated Neuroblastoma Cells on the Identification of the Relapse-Seeding Clone. Clin. Cancer Res. 2017, 23, 4224–4232. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Ma, Y.; Zou, Y.; Yin, A.; Li, W.; Dong, D. HCMDB: The human cancer metastasis database. Nucleic Acids Res. 2018, 46, D950–D955. [Google Scholar] [CrossRef]
- Chalfin, H.J.; Glavaris, S.A.; Malihi, P.D.; Sperger, J.M.; Gorin, M.A.; Lu, C.; Goodwin, C.R.; Chen, Y.; Caruso, E.A.; Dumpit, R.; et al. Prostate Cancer Disseminated Tumor Cells are Rarely Detected in the Bone Marrow of Patients with Localized Disease Undergoing Radical Prostatectomy across Multiple Rare Cell Detection Platforms. J. Urol. 2018, 199, 1494–1501. [Google Scholar] [CrossRef] [PubMed]
- Chinnadurai, R.; Rajakumar, A.; Schneider, A.J.; Bushman, W.A.; Hematti, P.; Galipeau, J. Potency Analysis of Mesenchymal Stromal Cells Using a Phospho-STAT Matrix Loop Analytical Approach. Stem Cells 2019, 37, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
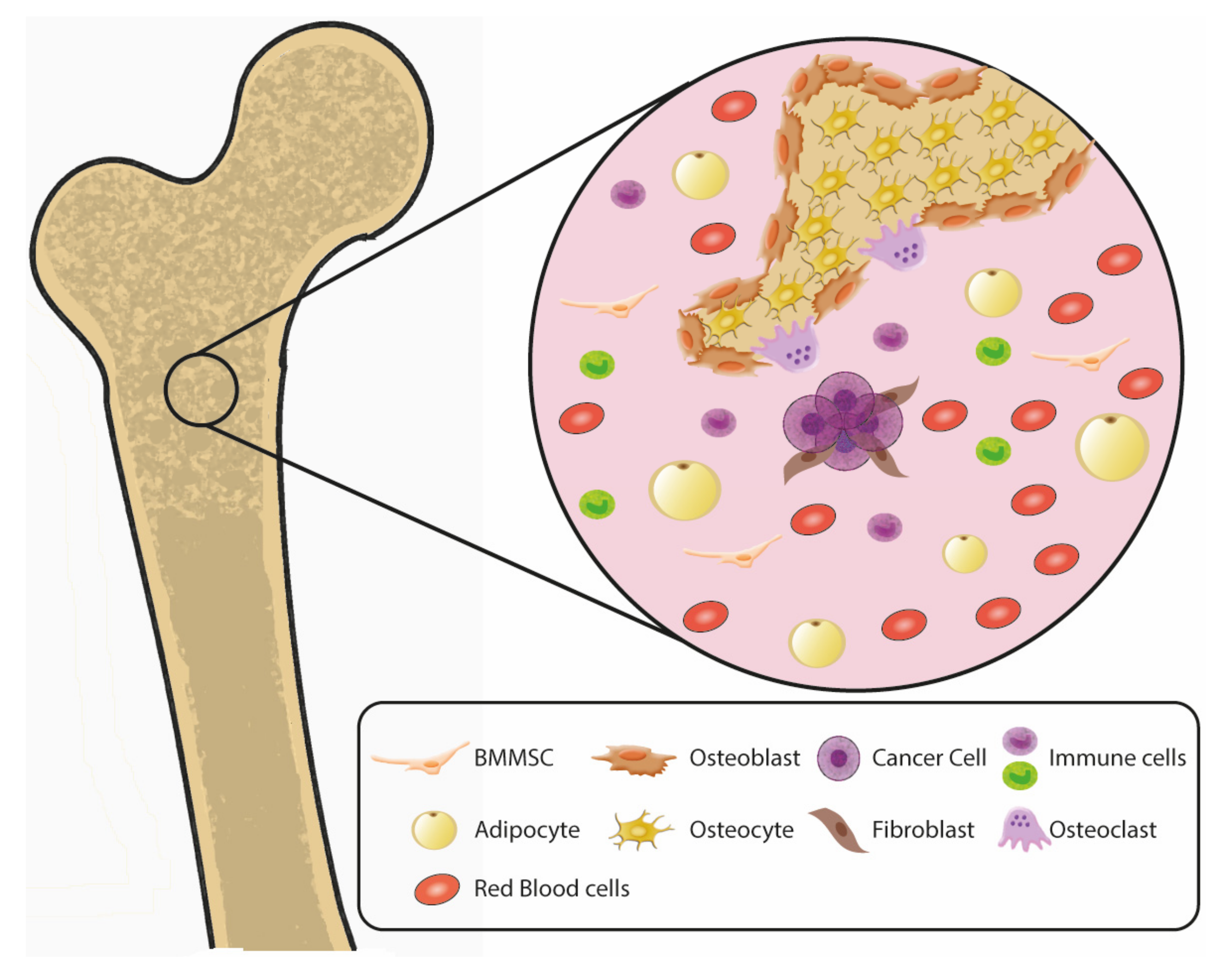
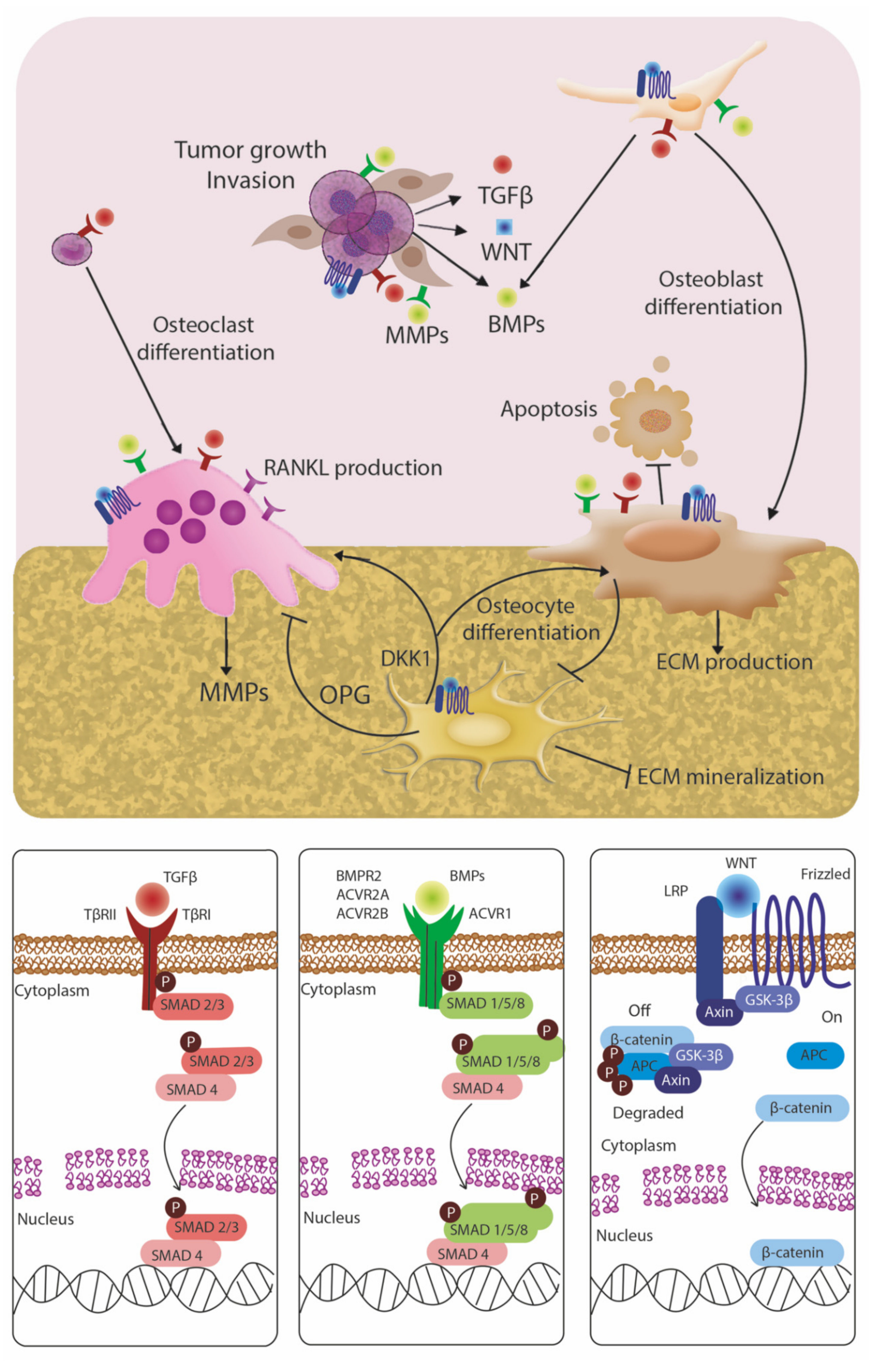
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sethakorn, N.; Heninger, E.; Sánchez-de-Diego, C.; Ding, A.B.; Yada, R.C.; Kerr, S.C.; Kosoff, D.; Beebe, D.J.; Lang, J.M. Advancing Treatment of Bone Metastases through Novel Translational Approaches Targeting the Bone Microenvironment. Cancers 2022, 14, 757. https://doi.org/10.3390/cancers14030757
Sethakorn N, Heninger E, Sánchez-de-Diego C, Ding AB, Yada RC, Kerr SC, Kosoff D, Beebe DJ, Lang JM. Advancing Treatment of Bone Metastases through Novel Translational Approaches Targeting the Bone Microenvironment. Cancers. 2022; 14(3):757. https://doi.org/10.3390/cancers14030757
Chicago/Turabian StyleSethakorn, Nan, Erika Heninger, Cristina Sánchez-de-Diego, Adeline B. Ding, Ravi Chandra Yada, Sheena C. Kerr, David Kosoff, David J. Beebe, and Joshua M. Lang. 2022. "Advancing Treatment of Bone Metastases through Novel Translational Approaches Targeting the Bone Microenvironment" Cancers 14, no. 3: 757. https://doi.org/10.3390/cancers14030757
APA StyleSethakorn, N., Heninger, E., Sánchez-de-Diego, C., Ding, A. B., Yada, R. C., Kerr, S. C., Kosoff, D., Beebe, D. J., & Lang, J. M. (2022). Advancing Treatment of Bone Metastases through Novel Translational Approaches Targeting the Bone Microenvironment. Cancers, 14(3), 757. https://doi.org/10.3390/cancers14030757







