Synthesis of Biotinylated PAMAM G3 Dendrimers Substituted with R-Glycidol and Celecoxib/Simvastatin as Repurposed Drugs and Evaluation of Their Increased Additive Cytotoxicity for Cancer Cell Lines
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Biochemical Reagents, Cell Lines and Materials
2.3. Syntheses
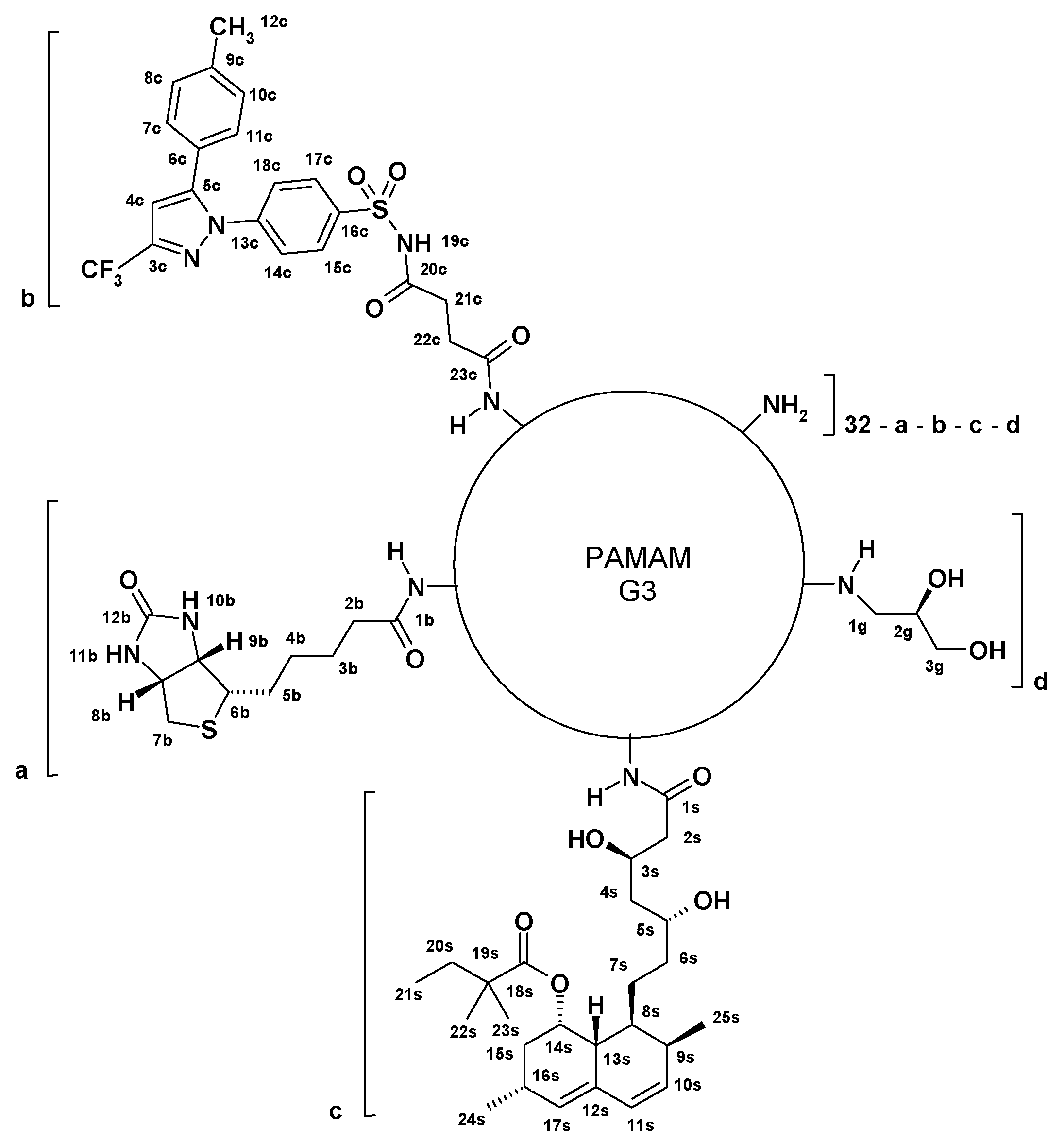
2.3.1. Preparation of G32B6C8gl Conjugate with Succinyl-Celecoxib
2.3.2. Conjugation of Simvastatin to G3 PAMAM
2.3.3. Preparation of G32B4S14gl Conjugate with Simvastatin
2.3.4. Preparation of G32B2C4S12gl Conjugate with Celecoxib and Simvastatin
2.3.5. Synthesis of G32B14gl Conjugate
2.4. Methods
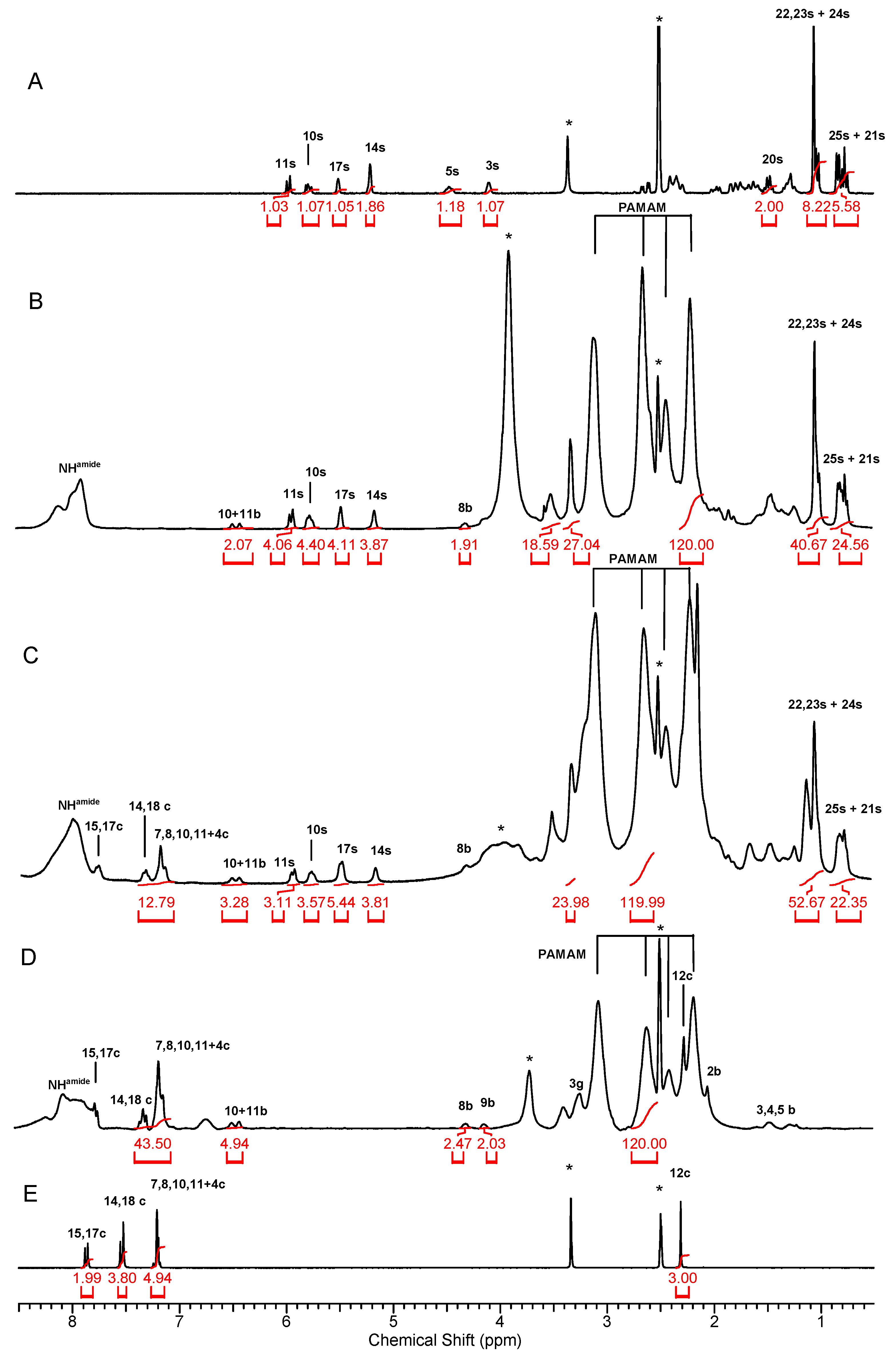
2.4.1. NMR Spectroscopy
2.4.2. Conjugate Size and ζ Potential Measurements
2.4.3. Cell Culture
2.4.4. Cytotoxicity Assays
2.4.5. Statistical Analysis
3. Results and Discussion
3.1. Syntheses and Characterization of Conjugates
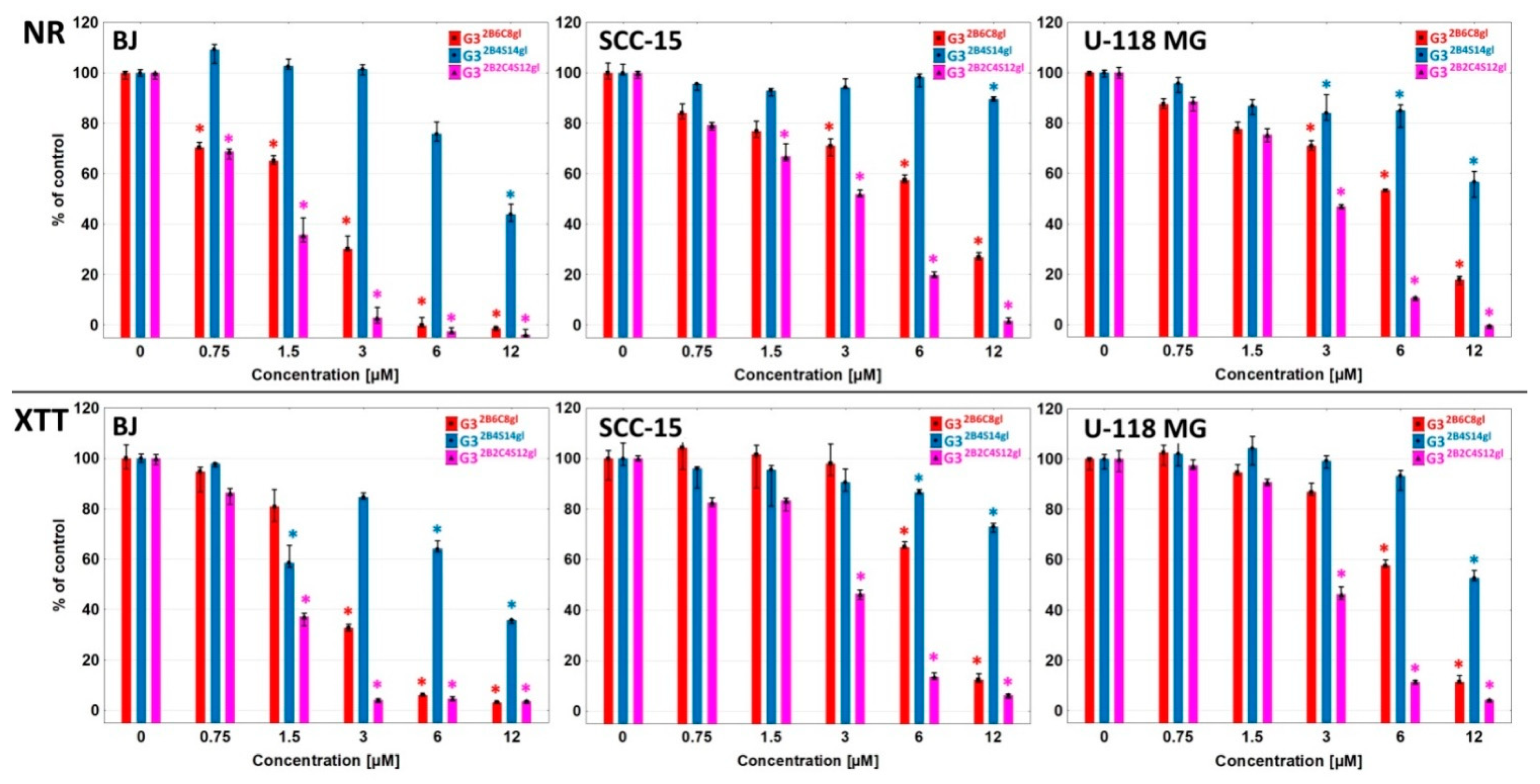
3.2. Conjugates Cytotoxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abedi-Gaballu, F.; Dehghan, G.; Ghaffari, M.; Yekta, R.; Abbaspour-Ravasjani, S.; Baradaran, B.; Ezzati Nazhad Dolatabadi, J.; Hamblin, M.R. PAMAM Dendrimers as Efficient Drug and Gene Delivery Nanosystems for Cancer Therapy. Appl. Mater. Today 2018, 12, 177–190. [Google Scholar] [CrossRef]
- Tołoczko-Iwaniuk, N.; Dziemiańczyk-Pakieła, D.; Nowaszewska, B.K.; Celińska-Janowicz, K.; Miltyk, W. Celecoxib in Cancer Therapy and Prevention-Review. Curr. Drug Targets 2019, 20, 302–315. [Google Scholar] [CrossRef]
- Uram, Ł.; Markowicz, J.; Misiorek, M.; Filipowicz-Rachwał, A.; Wołowiec, S.; Wałajtys-Rode, E. Celecoxib Substituted Biotinylated Poly(Amidoamine) G3 Dendrimer as Potential Treatment for Temozolomide Resistant Glioma Therapy and Anti-Nematode Agent. Eur. J. Pharm. Sci. 2020, 152, 105439. [Google Scholar] [CrossRef]
- Uram, Ł.; Filipowicz, A.; Misiorek, M.; Pieńkowska, N.; Markowicz, J.; Wałajtys-Rode, E.; Wołowiec, S. Biotinylated PAMAM G3 Dendrimer Conjugated with Celecoxib and/or Fmoc-l-Leucine and Its Cytotoxicity for Normal and Cancer Human Cell Lines. Eur. J. Pharm. Sci. 2018, 124, 1–9. [Google Scholar] [CrossRef]
- Hemmer, R.; Hall, A.; Spaulding, R.; Rossow, B.; Hester, M.; Caroway, M.; Haskamp, A.; Wall, S.; Bullen, H.A.; Morris, C.; et al. Analysis of Biotinylated Generation 4 Poly(Amidoamine) (PAMAM) Dendrimer Distribution in the Rat Brain and Toxicity in a Cellular Model of the Blood-Brain Barrier. Molecules 2013, 18, 11537–11552. [Google Scholar] [CrossRef]
- Fana, M.; Gallien, J.; Srinageshwar, B.; Dunbar, G.L.; Rossignol, J. PAMAM Dendrimer Nanomolecules Utilized as Drug Delivery Systems for Potential Treatment of Glioblastoma: A Systematic Review. Int. J. Nanomed. 2020, 15, 2789–2808. [Google Scholar] [CrossRef]
- Hanurry, E.Y.; Mekonnen, T.W.; Andrgie, A.T.; Darge, H.F.; Birhan, Y.S.; Hsu, W.-H.; Chou, H.-Y.; Cheng, C.-C.; Lai, J.-Y.; Tsai, H.-C. Biotin-Decorated PAMAM G4.5 Dendrimer Nanoparticles to Enhance the Delivery, Anti-Proliferative, and Apoptotic Effects of Chemotherapeutic Drug in Cancer Cells. Pharmaceutics 2020, 12, 443. [Google Scholar] [CrossRef]
- Tilija Pun, N.; Jeong, C.-H. Statin as a Potential Chemotherapeutic Agent: Current Updates as a Monotherapy, Combination Therapy, and Treatment for Anti-Cancer Drug Resistance. Pharmaceuticals 2021, 14, 470. [Google Scholar] [CrossRef]
- Sirtori, C.R. The Pharmacology of Statins. Pharm. Res. 2014, 88, 3–11. [Google Scholar] [CrossRef]
- Guerra, B.; Recio, C.; Aranda-Tavío, H.; Guerra-Rodríguez, M.; García-Castellano, J.M.; Fernández-Pérez, L. The Mevalonate Pathway, a Metabolic Target in Cancer Therapy. Front. Oncol. 2021, 11, 626971. [Google Scholar] [CrossRef]
- Duarte, J.A.; de Barros, A.L.B.; Leite, E.A. The Potential Use of Simvastatin for Cancer Treatment: A Review. Biomed. Pharmacother. 2021, 141, 111858. [Google Scholar] [CrossRef]
- Göbel, A.; Zinna, V.M.; Dell’Endice, S.; Jaschke, N.; Kuhlmann, J.D.; Wimberger, P.; Rachner, T.D. Anti-Tumor Effects of Mevalonate Pathway Inhibition in Ovarian Cancer. BMC Cancer 2020, 20, 703. [Google Scholar] [CrossRef]
- Nath, S.D.; Linh, N.T.B.; Sadiasa, A.; Lee, B.T. Encapsulation of Simvastatin in PLGA Microspheres Loaded into Hydrogel Loaded BCP Porous Spongy Scaffold as a Controlled Drug Delivery System for Bone Tissue Regeneration. J. Biomater. Appl. 2014, 28, 1151–1163. [Google Scholar] [CrossRef]
- Kulhari, H.; Pooja, D.; Prajapati, S.K.; Chauhan, A.S. Performance Evaluation of PAMAM Dendrimer Based Simvastatin Formulations. Int. J. Pharm. 2011, 405, 203–209. [Google Scholar] [CrossRef]
- Kulhari, H.; Kulhari, D.P.; Prajapati, S.K.; Chauhan, A.S. Pharmacokinetic and Pharmacodynamic Studies of Poly(Amidoamine) Dendrimer Based Simvastatin Oral Formulations for the Treatment of Hypercholesterolemia. Mol. Pharm. 2013, 10, 2528–2533. [Google Scholar] [CrossRef]
- Sharma, R.; Liaw, K.; Sharma, A.; Jimenez, A.; Chang, M.; Salazar, S.; Amlani, I.; Kannan, S.; Kannan, R.M. Glycosylation of PAMAM Dendrimers Significantly Improves Tumor Macrophage Targeting and Specificity in Glioblastoma. J. Control. Release 2021, 337, 179–192. [Google Scholar] [CrossRef]
- Yan, S.; Ren, J.; Jian, Y.; Wang, W.; Yun, W.; Yin, J. Injectable Maltodextrin-Based Micelle/Hydrogel Composites for Simvastatin-Controlled Release. Biomacromolecules 2018, 19, 4554–4564. [Google Scholar] [CrossRef]
- Shojaei, S.; Koleini, N.; Samiei, E.; Aghaei, M.; Cole, L.K.; Alizadeh, J.; Islam, M.I.; Vosoughi, A.-R.; Albokashy, M.; Butterfield, Y.; et al. Simvastatin Increases Temozolomide-Induced Cell Death by Targeting the Fusion of Autophagosomes and Lysosomes. FEBS J. 2020, 287, 1005–1034. [Google Scholar] [CrossRef]
- Gehrke, T.; Scherzad, A.; Hackenberg, S.; Ickrath, P.; Schendzielorz, P.; Hagen, R.; Kleinsasser, N. Additive Antitumor Effects of Celecoxib and Simvastatin on Head and Neck Squamous Cell Carcinoma in Vitro. Int. J. Oncol. 2017, 51, 931–938. [Google Scholar] [CrossRef]
- Uram, Ł.; Szuster, M.; Filipowicz, A.; Zaręba, M.; Wałajtys-Rode, E.; Wołowiec, S. Cellular Uptake of Glucoheptoamidated Poly(Amidoamine) PAMAM G3 Dendrimer with Amide-Conjugated Biotin, a Potential Carrier of Anticancer Drugs. Bioorg. Med. Chem. 2017, 25, 706–713. [Google Scholar] [CrossRef]
- Malinga-Drozd, M.; Uram, Ł.; Wróbel, K.; Wołowiec, S. Chiral Recognition of Homochiral Poly (Amidoamine) Dendrimers Substituted with R- and S-Glycidol by Keratinocyte (HaCaT) and Squamous Carcinoma (SCC-15) Cells In Vitro. Polymer 2021, 13, 1049. [Google Scholar] [CrossRef] [PubMed]
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A New Class of Polymers: Starburst-Dendritic Macromolecules. Polym. J. 1985, 17, 117–132. [Google Scholar] [CrossRef]
- Uram, Ł.; Szuster, M.; Gargasz, K.; Filipowicz, A.; Wałajtys-Rode, E.; Wołowiec, S. In Vitro Cytotoxicity of the Ternary PAMAM G3-Pyridoxal-Biotin Bioconjugate. Int. J. Nanomed. 2013, 8, 4707–4720. [Google Scholar] [CrossRef][Green Version]
- Kaczorowska, A.; Malinga-Drozd, M.; Kałas, W.; Kopaczyńska, M.; Wołowiec, S.; Borowska, K. Biotin-Containing Third Generation Glucoheptoamidated Polyamidoamine Dendrimer for 5-Aminolevulinic Acid Delivery System. Int. J. Mol. Sci. 2021, 22, 1982. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, J.; Kim, H.; Kang, S.; Yoon, J.-H.; Kim, D.-D.; Kim, Y.M.; Jung, Y. N-Succinylaspart-1-Yl Celecoxib Is a Potential Colon-Specific Prodrug of Celecoxib with Improved Therapeutic Properties. J. Pharm. Sci. 2012, 101, 1831–1842. [Google Scholar] [CrossRef]
- Nizioł, J.; Uram, Ł.; Szuster, M.; Sekuła, J.; Ruman, T. Biological Activity of N(4)-Boronated Derivatives of 2′-Deoxycytidine, Potential Agents for Boron-Neutron Capture Therapy. Bioorganic Med. Chem. 2015, 23, 6297–6304. [Google Scholar] [CrossRef]
- Mukaiyama, T. New Synthetic Reactions Based on the Onium Salts of Aza-Arenes [New Synthetic Methods (29)]. Angew. Chem. Int. Ed. Engl. 1979, 18, 707–721. [Google Scholar] [CrossRef]
- Uram, Ł.; Misiorek, M.; Pichla, M.; Filipowicz-Rachwał, A.; Markowicz, J.; Wołowiec, S.; Wałajtys-Rode, E. The Effect of Biotinylated PAMAM G3 Dendrimers Conjugated with COX-2 Inhibitor (Celecoxib) and PPARγ Agonist (Fmoc-L-Leucine) on Human Normal Fibroblasts, Immortalized Keratinocytes and Glioma Cells in Vitro. Molecules 2019, 24, 3801. [Google Scholar] [CrossRef]
- Brus, J.; Jegorov, A. Through-Bonds and Through-Space Solid-State NMR Correlations at Natural Isotopic Abundance: Signal Assignment and Structural Study of Simvastatin. J. Phys. Chem. A 2004, 108, 3955–3964. [Google Scholar] [CrossRef]
- Mukherjee, S.P.; Davoren, M.; Byrne, H.J. In Vitro Mammalian Cytotoxicological Study of PAMAM Dendrimers-towards Quantitative Structure Activity Relationships. Toxicol. In Vitro 2010, 24, 169–177. [Google Scholar] [CrossRef]
- AAT Bioquest, Inc. Quest GraphTM ANOVA Calculator. Available online: Https://www.aatbio.com/tools/anova-analysis-of-variance-one-two-way-calculator (accessed on 3 December 2021).
- Menter, D.G.; Ramsauer, V.P.; Harirforoosh, S.; Chakraborty, K.; Yang, P.; Hsi, L.; Newman, R.A.; Krishnan, K. Differential Effects of Pravastatin and Simvastatin on the Growth of Tumor Cells from Different Organ Sites. PLoS ONE 2011, 6, e28813. [Google Scholar] [CrossRef]
- Fujiwara, D.; Tsubaki, M.; Takeda, T.; Tomonari, Y.; Koumoto, Y.; Sakaguchi, K.; Nishida, S. Statins Induce Apoptosis through Inhibition of Ras Signaling Pathways and Enhancement of Bim and P27 Expression in Human Hematopoietic Tumor Cells. Tumour Biol. 2017, 39, 1010428317734947. [Google Scholar] [CrossRef] [PubMed]
- Belhabib, I.; Zaghdoudi, S.; Lac, C.; Bousquet, C.; Jean, C. Extracellular Matrices and Cancer-Associated Fibroblasts: Targets for Cancer Diagnosis and Therapy? Cancers 2021, 13, 3466. [Google Scholar] [CrossRef]
- Jiang, W.; Hu, J.-W.; He, X.-R.; Jin, W.-L.; He, X.-Y. Statins: A Repurposed Drug to Fight Cancer. J. Exp. Clin. Cancer Res. 2021, 40, 241. [Google Scholar] [CrossRef]


| Compound | Size (nm) | Zeta Potential (mV) | ||||
|---|---|---|---|---|---|---|
| pH 7 | pH 5 | pH 7 | pH 5 | |||
| d(V) | d(N) | d(V) | d(N) | |||
| G32B14gl | 2.1 ± 0.49 | 1.8 ± 0.42 | 5.0 ± 0.96 | 3.3 ± 0.85 | 9.6 ± 0.89 | 7.8 ± 2.51 |
| G32B6C8gl | 7.8 ± 3.56 | 5.7 ± 2.35 | 5.8 ± 1.59 | 4.0 ± 1.46 | 18.6 ± 3.70 | 34.6 ± 2.52 |
| G32B4S14gl | 10.3 ± 1.67 | 8.6 ± 1.44 | 9.5 ± 1.97 | 6.5 ± 1.92 | 37.7 ± 3.82 | 36.5 ± 4.11 |
| G32B2Cel4S12gl | 155 ± 5.77 | 86.7 ± 6.83 | 8.5 ± 2.19 | 6.9 ± 2.14 | 30.3 ± 0.73 | 32.5 ± 3.14 |
| G312S | 85.8 ± 1.83 | 57.2 ± 2.96 | 44.2 ± 1.99 | 25.3 ± 7.07 | 42.4 ± 1.77 | 32.9 ± 2.84 |
| Assay | Cell Line | Conjugate/Carrier Alone/Drug Alone (IC50 µM) | ||||||
|---|---|---|---|---|---|---|---|---|
| G32B6C8gl | G32B4S14gl | G32B2C4S12gl | G32B14gl | C | S | C + S 0.5:1 Ratio | ||
| NR | BJ | 1.90 | 9.99 | 1.10 | 53.60 | 88.68 | 90.14 | 63.40 |
| 11.40 a | 39.96 b | 2.20 a | ||||||
| 4.40 b | ||||||||
| SCC-15 | 6.65 | >12.00 | 6.69 | >200.00 | 47.48 | 56.54 | 56.45 | |
| 39.9 a | >48.00 b | 13.38 a | ||||||
| 26.76 b | ||||||||
| U-118 MG | 5.86 | 16.90 | 2.73 | >200.00 | 65.57 | 26.19 | 37.65 | |
| 35.16 a | 67.60 b | 5.46 a | ||||||
| 10.92 b | ||||||||
| XTT | BJ | 2.40 | 8.75 | 1.28 | 23.45 | 100.13 | 100.86 | 66.00 |
| 14.40 a | 35.00 b | 2.56 a | ||||||
| 5.12 b | ||||||||
| SCC-15 | 6.98 | >12.00 | 2.98 | 64.58 | 97.13 | 84.38 | 60.14 | |
| 41.88 a | >48.00 b | 5.96 a | ||||||
| 11.92 b | ||||||||
| U-118 MG | 6.49 | 12.27 | 2.91 | 62.65 | 93.74 | 112.33 | 70.67 | |
| 38.94 a | 49.08 b | 5.82 a | ||||||
| 11.64 b | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wróbel, K.; Wołowiec, S.; Markowicz, J.; Wałajtys-Rode, E.; Uram, Ł. Synthesis of Biotinylated PAMAM G3 Dendrimers Substituted with R-Glycidol and Celecoxib/Simvastatin as Repurposed Drugs and Evaluation of Their Increased Additive Cytotoxicity for Cancer Cell Lines. Cancers 2022, 14, 714. https://doi.org/10.3390/cancers14030714
Wróbel K, Wołowiec S, Markowicz J, Wałajtys-Rode E, Uram Ł. Synthesis of Biotinylated PAMAM G3 Dendrimers Substituted with R-Glycidol and Celecoxib/Simvastatin as Repurposed Drugs and Evaluation of Their Increased Additive Cytotoxicity for Cancer Cell Lines. Cancers. 2022; 14(3):714. https://doi.org/10.3390/cancers14030714
Chicago/Turabian StyleWróbel, Konrad, Stanisław Wołowiec, Joanna Markowicz, Elżbieta Wałajtys-Rode, and Łukasz Uram. 2022. "Synthesis of Biotinylated PAMAM G3 Dendrimers Substituted with R-Glycidol and Celecoxib/Simvastatin as Repurposed Drugs and Evaluation of Their Increased Additive Cytotoxicity for Cancer Cell Lines" Cancers 14, no. 3: 714. https://doi.org/10.3390/cancers14030714
APA StyleWróbel, K., Wołowiec, S., Markowicz, J., Wałajtys-Rode, E., & Uram, Ł. (2022). Synthesis of Biotinylated PAMAM G3 Dendrimers Substituted with R-Glycidol and Celecoxib/Simvastatin as Repurposed Drugs and Evaluation of Their Increased Additive Cytotoxicity for Cancer Cell Lines. Cancers, 14(3), 714. https://doi.org/10.3390/cancers14030714






