Hematotoxicity and Nephrotoxicity in Prostate Cancer Patients Undergoing Radioligand Therapy with [177Lu]Lu-PSMA I&T
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Cohort
2.2. Pre-Therapy Work-Up
2.3. Treatment Protocol
2.4. Toxicity Assessment
- “Decline in GFR category (≥90 (G1), 60–89 (G2), 45–59 (G3a), 30–44 (G3b), 15–29 (G4), <15 (G5) mL/min/1.73 m2). A certain drop in eGFR is defined as a drop in GFR category accompanied by a 25% or greater drop in eGFR from baseline.
- Rapid progression is defined as a sustained decline in eGFR of more than 5 mL/min/1.73 m2/year” [17].
2.5. Statistical Analysis
3. Results
3.1. Patients
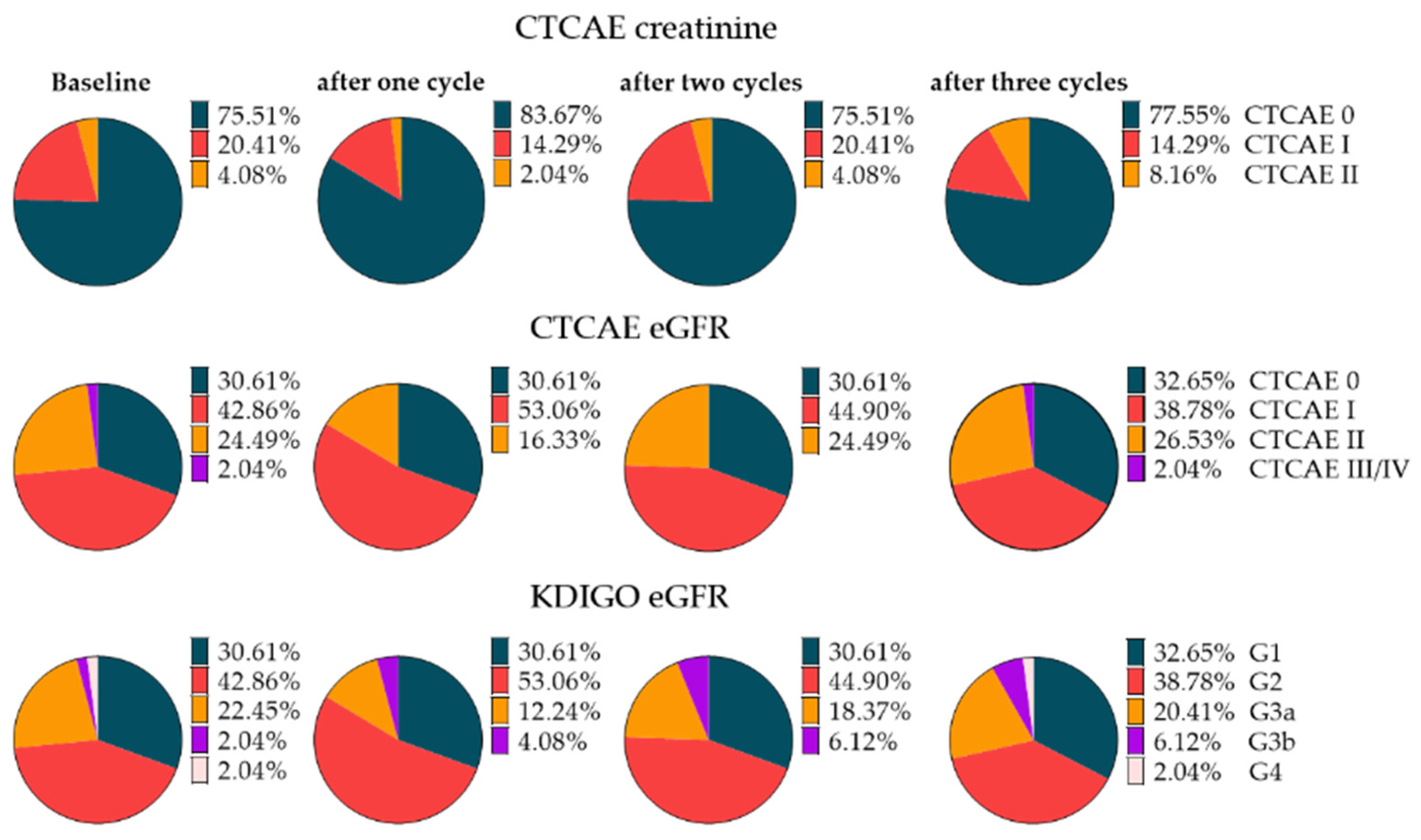
3.2. Baseline Categorization of Renal Toxicity
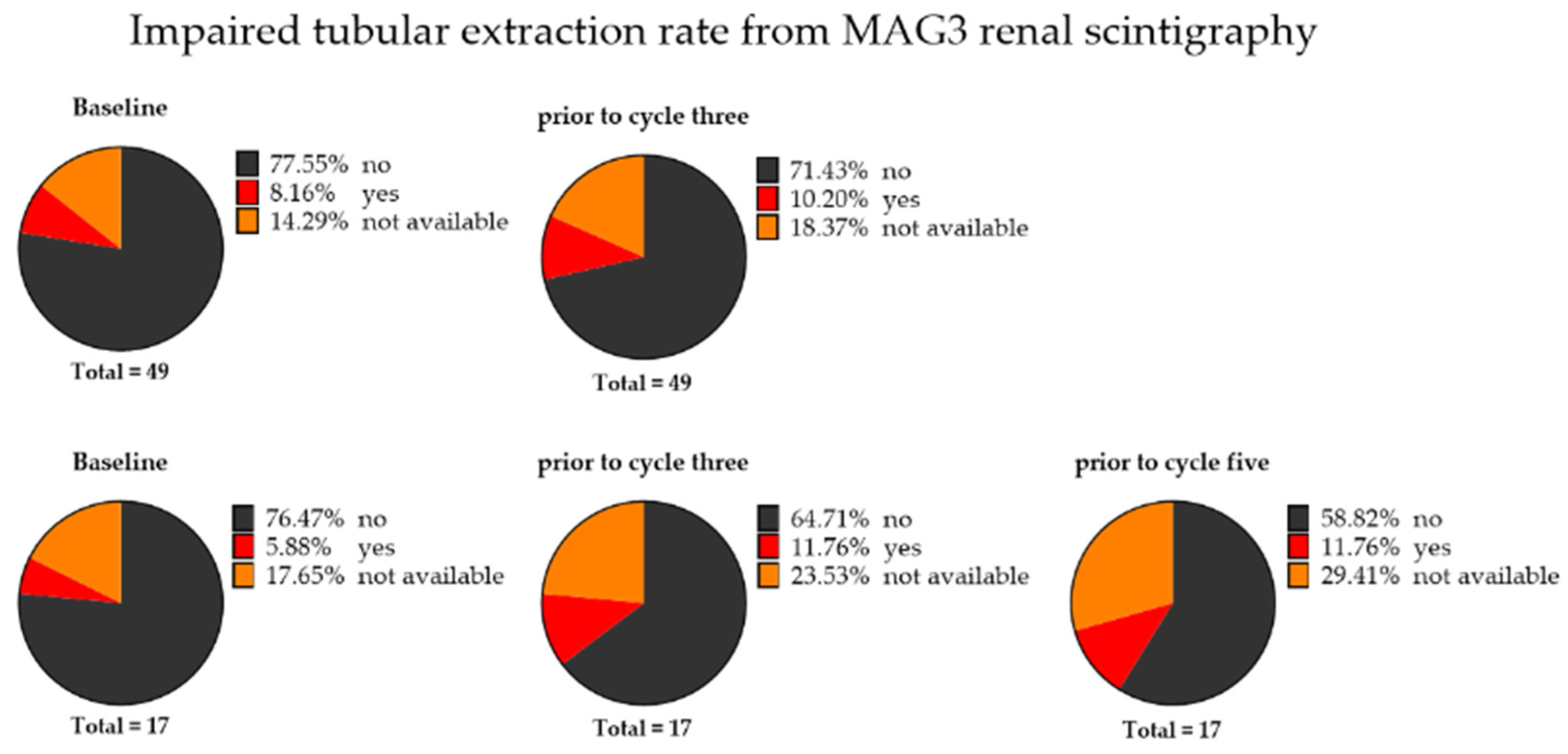
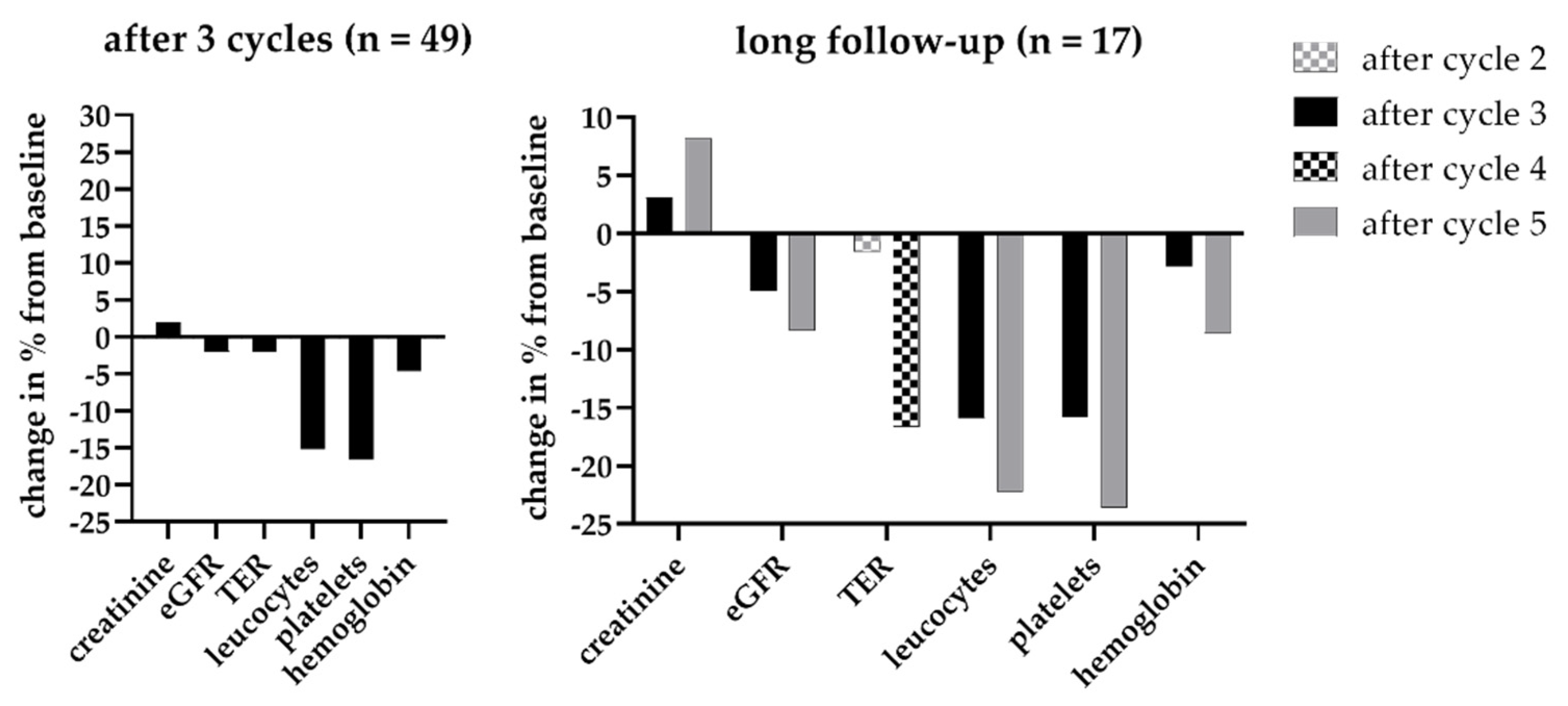
3.3. Renal Toxicity with RLT
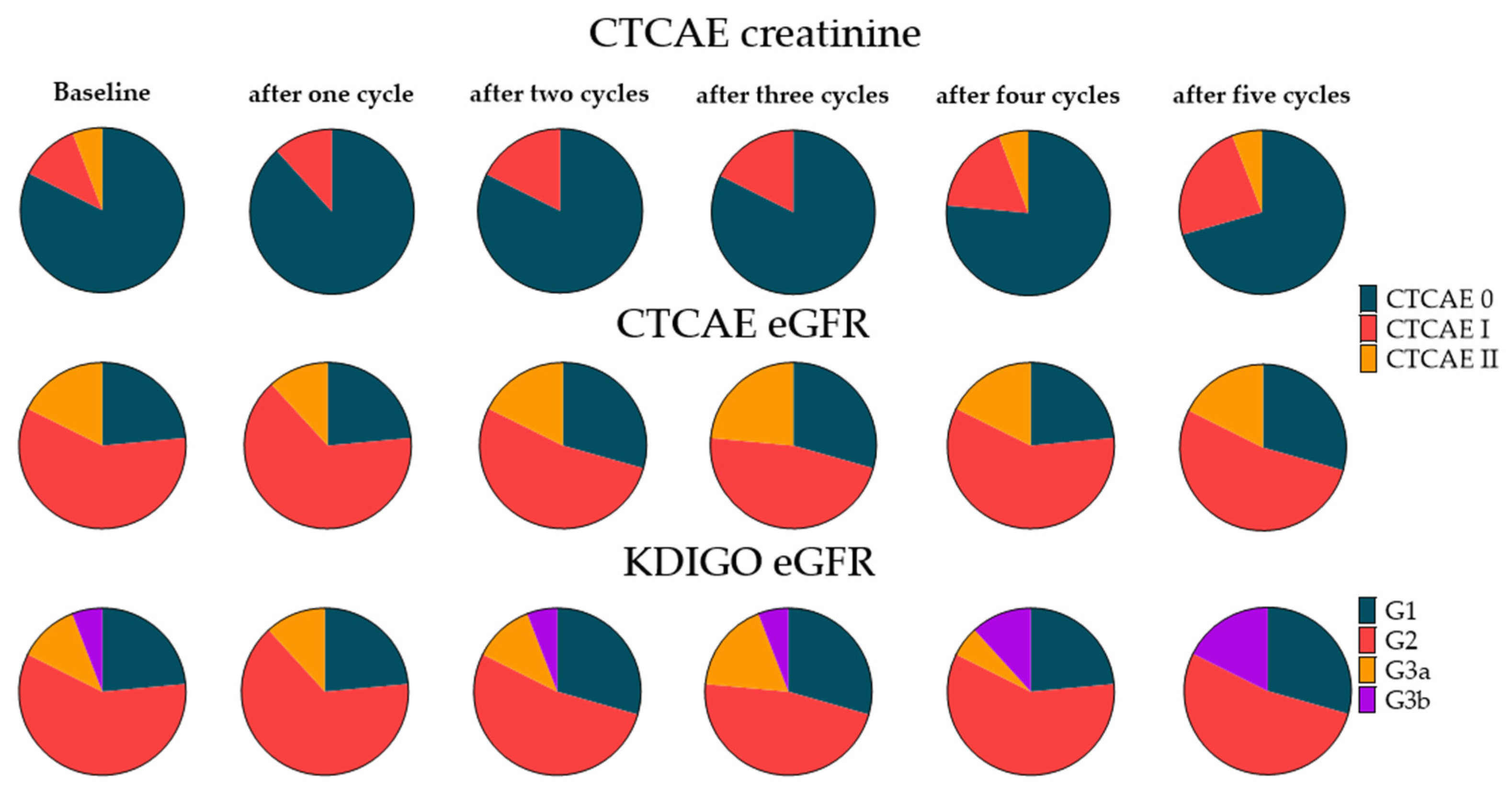
3.4. Extended Follow-Up Cohort
3.5. Risk Factor Assessment for Renal Toxicity
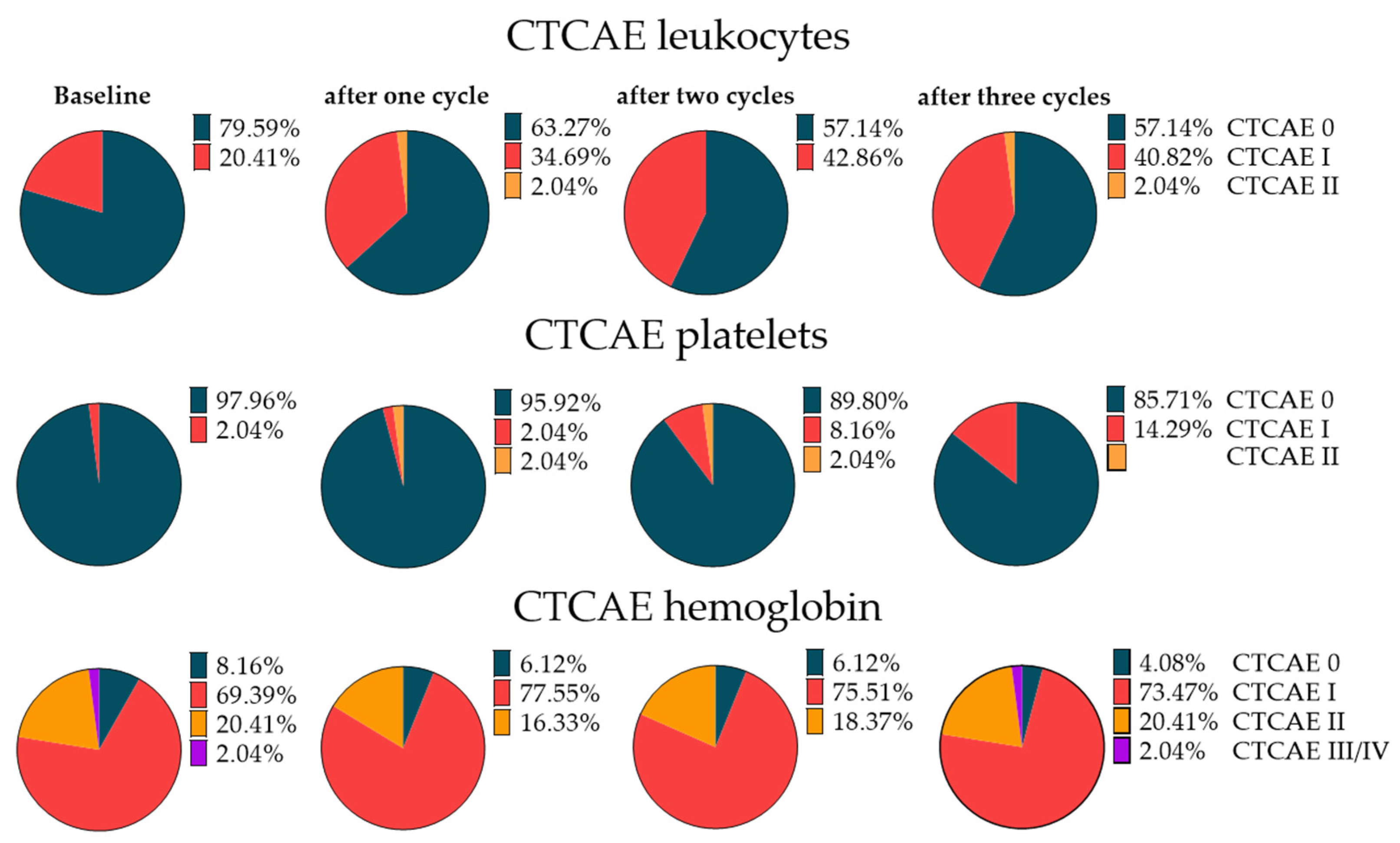
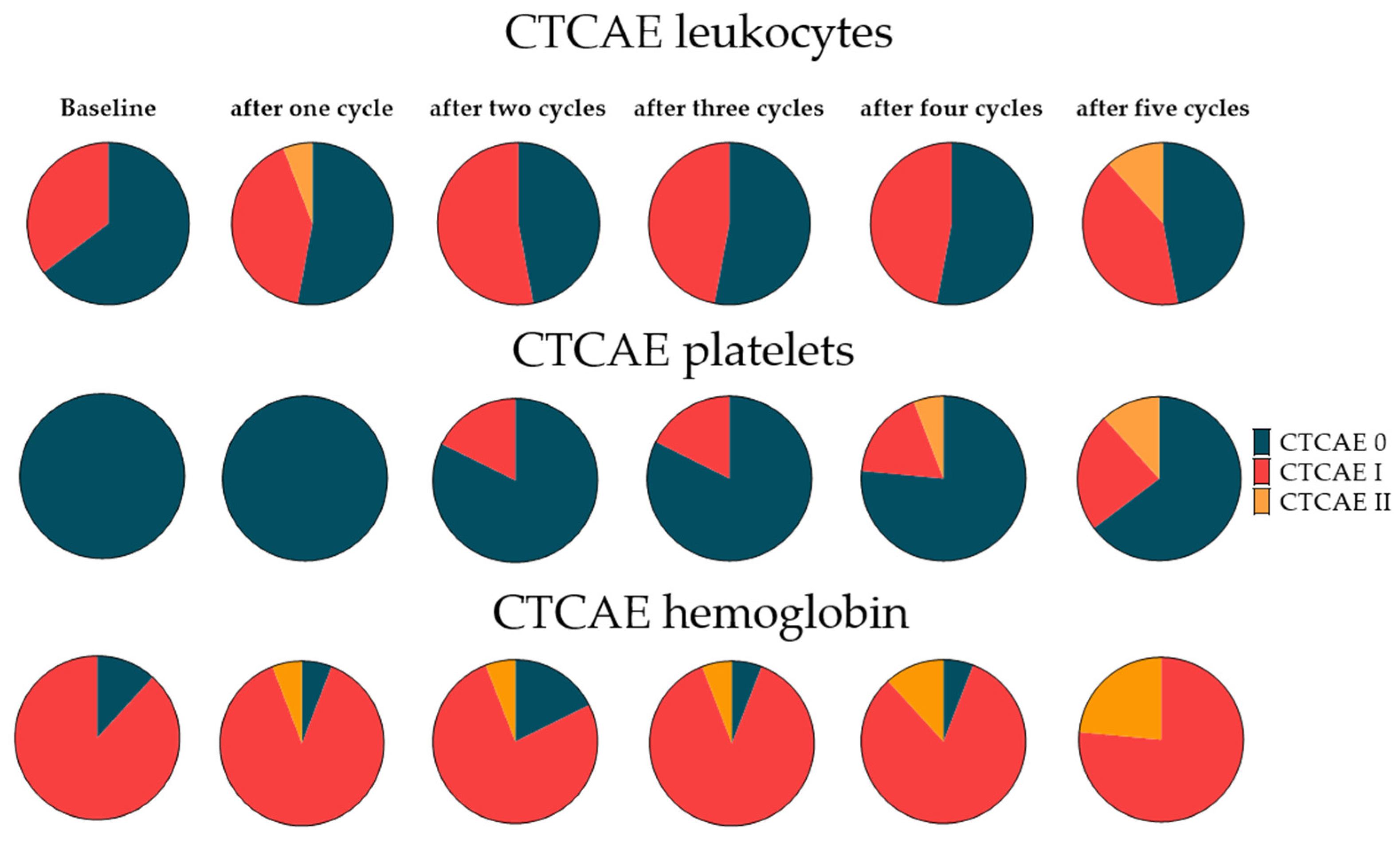
3.6. Hematotoxicity with RLT
3.7. Extended Follow-Up Cohort Hematotoxicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ryan, C.J.; Smith, M.R.; de Bono, J.S.; Molina, A.; Logothetis, C.J.; de Souza, P.; Fizazi, K.; Mainwaring, P.; Piulats, J.M.; Ng, S.; et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N. Engl. J. Med. 2013, 368, 138–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beer, T.M.; Armstrong, A.J.; Rathkopf, D.E.; Loriot, Y.; Sternberg, C.N.; Higano, C.S.; Iversen, P.; Bhattacharya, S.; Carles, J.; Chowdhury, S.; et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N. Engl. J. Med. 2014, 371, 424–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tannock, I.F.; de Wit, R.; Berry, W.R.; Horti, J.; Pluzanska, A.; Chi, K.N.; Oudard, S.; Théodore, C.; James, N.D.; Turesson, I.; et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N. Engl. J. Med. 2004, 351, 1502–1512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berthold, D.R.; Pond, G.R.; Soban, F.; de Wit, R.; Eisenberger, M.; Tannock, I.F. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: Updated survival in the TAX 327 study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 242–245. [Google Scholar] [CrossRef]
- de Bono, J.S.; Oudard, S.; Ozguroglu, M.; Hansen, S.; Machiels, J.P.; Kocak, I.; Gravis, G.; Bodrogi, I.; Mackenzie, M.J.; Shen, L.; et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet 2010, 376, 1147–1154. [Google Scholar] [CrossRef]
- Calais, J.; Gafita, A.; Eiber, M.R.; Armstrong, W.R.; Gartmann, J.; Thin, P.; Nguyen, K.; Lok, V.; Gosa, L.; Grogan, T.; et al. Prospective phase 2 trial of PSMA-targeted molecular RadiothErapy with (177)Lu-PSMA-617 for metastatic Castration-reSISTant Prostate Cancer (RESIST-PC): Efficacy results of the UCLA cohort. J. Nucl. Med. 2021, 63. [Google Scholar] [CrossRef]
- Hofman, M.S.; Violet, J.; Hicks, R.J.; Ferdinandus, J.; Thang, S.P.; Akhurst, T.; Iravani, A.; Kong, G.; Ravi Kumar, A.; Murphy, D.G.; et al. [(177)Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): A single-centre, single-arm, phase 2 study. Lancet Oncol. 2018, 19, 825–833. [Google Scholar] [CrossRef]
- Violet, J.; Sandhu, S.; Iravani, A.; Ferdinandus, J.; Thang, S.P.; Kong, G.; Kumar, A.R.; Akhurst, T.; Pattison, D.A.; Beaulieu, A.; et al. Long-Term Follow-up and Outcomes of Retreatment in an Expanded 50-Patient Single-Center Phase II Prospective Trial of (177)Lu-PSMA-617 Theranostics in Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2020, 61, 857–865. [Google Scholar] [CrossRef]
- Hofman, M.S.; Emmett, L.; Sandhu, S.; Iravani, A.; Joshua, A.M.; Goh, J.C.; Pattison, D.A.; Tan, T.H.; Kirkwood, I.D.; Ng, S.; et al. [(177)Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): A randomised, open-label, phase 2 trial. Lancet 2021, 397, 797–804. [Google Scholar] [CrossRef]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef]
- Sadaghiani, M.S.; Sheikhbahaei, S.; Werner, R.A.; Pienta, K.J.; Pomper, M.G.; Solnes, L.B.; Gorin, M.A.; Wang, N.Y.; Rowe, S.P. A Systematic Review and Meta-analysis of the Effectiveness and Toxicities of Lutetium-177-labeled Prostate-specific Membrane Antigen-targeted Radioligand Therapy in Metastatic Castration-Resistant Prostate Cancer. Eur. Urol. 2021, 80, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Herrmann, K.; Krause, B.J.; Rahbar, K.; Chi, K.N.; Morris, M.J.; Sartor, O.; Tagawa, S.T.; Kendi, A.T.; Vogelzang, N.J.; et al. 576MO Health-related quality of life (HRQoL), pain and safety outcomes in the phase III VISION study of 177Lu-PSMA-617 in patients with metastatic castration-resistant prostate cancer. Ann. Oncol. 2021, 32, S627–S628. [Google Scholar] [CrossRef]
- Heck, M.M.; Tauber, R.; Schwaiger, S.; Retz, M.; D’Alessandria, C.; Maurer, T.; Gafita, A.; Wester, H.J.; Gschwend, J.E.; Weber, W.A.; et al. Treatment Outcome, Toxicity, and Predictive Factors for Radioligand Therapy with (177)Lu-PSMA-I&T in Metastatic Castration-resistant Prostate Cancer. Eur. Urol. 2019, 75, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Fendler, W.P.; Eiber, M.; Baum, R.; Bozkurt, M.F.; Czernin, J.; Delgado Bolton, R.C.; Ezziddin, S.; Forrer, F.; Hicks, R.J.; et al. EANM procedure guidelines for radionuclide therapy with (177)Lu-labelled PSMA-ligands ((177)Lu-PSMA-RLT). Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2536–2544. [Google Scholar] [CrossRef] [PubMed]
- Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf (accessed on 6 May 2021).
- Yordanova, A.; Becker, A.; Eppard, E.; Kürpig, S.; Fisang, C.; Feldmann, G.; Essler, M.; Ahmadzadehfar, H. The impact of repeated cycles of radioligand therapy using [(177)Lu]Lu-PSMA-617 on renal function in patients with hormone refractory metastatic prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1473–1479. [Google Scholar] [CrossRef] [PubMed]
- Summary of Recommendation Statements. Kidney Int. Suppl. 2013, 3, 5–14. [CrossRef] [Green Version]
- Gallyamov, M.; Meyrick, D.; Barley, J.; Lenzo, N. Renal outcomes of radioligand therapy: Experience of (177)lutetium-prostate-specific membrane antigen ligand therapy in metastatic castrate-resistant prostate cancer. Clin. Kidney J. 2020, 13, 1049–1055. [Google Scholar] [CrossRef] [Green Version]
- Rosar, F.; Kochems, N.; Bartholomä, M.; Maus, S.; Stemler, T.; Linxweiler, J.; Khreish, F.; Ezziddin, S. Renal Safety of [(177)Lu]Lu-PSMA-617 Radioligand Therapy in Patients with Compromised Baseline Kidney Function. Cancers 2021, 13, 3095. [Google Scholar] [CrossRef]
- Blaufox, M.D.; De Palma, D.; Taylor, A.; Szabo, Z.; Prigent, A.; Samal, M.; Li, Y.; Santos, A.; Testanera, G.; Tulchinsky, M. The SNMMI and EANM practice guideline for renal scintigraphy in adults. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2218–2228. [Google Scholar] [CrossRef]
- Svensson, J.; Berg, G.; Wängberg, B.; Larsson, M.; Forssell-Aronsson, E.; Bernhardt, P. Renal function affects absorbed dose to the kidneys and haematological toxicity during ¹⁷⁷Lu-DOTATATE treatment. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 947–955. [Google Scholar] [CrossRef] [Green Version]
- Groener, D.; Nguyen, C.T.; Baumgarten, J.; Bockisch, B.; Davis, K.; Happel, C.; Mader, N.; Nguyen Ngoc, C.; Wichert, J.; Banek, S.; et al. Hematologic safety of (177)Lu-PSMA-617 radioligand therapy in patients with metastatic castration-resistant prostate cancer. EJNMMI Res. 2021, 11, 61. [Google Scholar] [CrossRef] [PubMed]
- Fendler, W.P.; Reinhardt, S.; Ilhan, H.; Delker, A.; Böning, G.; Gildehaus, F.J.; Stief, C.; Bartenstein, P.; Gratzke, C.; Lehner, S.; et al. Preliminary experience with dosimetry, response and patient reported outcome after 177Lu-PSMA-617 therapy for metastatic castration-resistant prostate cancer. Oncotarget 2017, 8, 3581–3590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Widjaja, L.; Werner, R.A.; Ross, T.L.; Bengel, F.M.; Derlin, T. Comparison of pretherapeutic osseous tumor volume and standard hematology for prediction of hematotoxicity after PSMA-targeted radioligand therapy. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4077–4088. [Google Scholar] [CrossRef] [PubMed]
| Variable | Mean ± SD |
|---|---|
| Age at first cycle of PSMA RLT (years) | 71.2 ± 8.4 |
| Treatment cycles per patient | 4.8 ± 1.8 |
| Administered cumulative activity (GBq) | 17.8 ± 1.5 |
| Gleason score | 8 ± 1 * |
| Previous Treatments | Patients (%) |
| Radical prostatectomy | 24 (49.0%) |
| Primary radiation therapy to the prostate | 9 (18.4%) |
| Adjuvant radiation therapy | 12 (24.5%) |
| Salvage radiation therapy | 6 (12.2%) |
| Antihormonal treatment | 49 (100%) |
| Enzalutamide | 38 (77.6%) |
| Abiraterone acetate | 35 (71.4%) |
| Previous chemotherapy | 28 (57.1%) |
| Clinical Risk Factors for Nephrotoxicity | Patients (%) |
| Pre-existing renal disease | 12 (24.5%) |
| Arterial hypertension | 32 (65.3%) |
| Diabetes mellitus | 8 (16.3%) |
| Nephrotoxic drugs | 34 (69.4%) |
| Tumor burden | |
| Extent of tumor | |
| 1 (2.0%) |
| 6 (12.2%) |
| 42 (85.7%) |
| 12 (24.5%) |
| Organ system | |
| 46 (93.9%) |
| 35 (71.4%) |
| 17 (34.7%) |
| 3 (6.1%) |
| 7 (14.3%) |
| Baseline Laboratory Values | Mean ± SD |
| PSA (μg/L) | 374.1 ± 623.1 |
| eGFR (mL/min/1.73 m2) | 76.5 ± 20.1 |
| 99mTc-MAG3-derived TER (mL/min/1.73 m2) | 213.3 ± 61.5 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hartrampf, P.E.; Weinzierl, F.-X.; Serfling, S.E.; Pomper, M.G.; Rowe, S.P.; Higuchi, T.; Seitz, A.K.; Kübler, H.; Buck, A.K.; Werner, R.A. Hematotoxicity and Nephrotoxicity in Prostate Cancer Patients Undergoing Radioligand Therapy with [177Lu]Lu-PSMA I&T. Cancers 2022, 14, 647. https://doi.org/10.3390/cancers14030647
Hartrampf PE, Weinzierl F-X, Serfling SE, Pomper MG, Rowe SP, Higuchi T, Seitz AK, Kübler H, Buck AK, Werner RA. Hematotoxicity and Nephrotoxicity in Prostate Cancer Patients Undergoing Radioligand Therapy with [177Lu]Lu-PSMA I&T. Cancers. 2022; 14(3):647. https://doi.org/10.3390/cancers14030647
Chicago/Turabian StyleHartrampf, Philipp E., Franz-Xaver Weinzierl, Sebastian E. Serfling, Martin G. Pomper, Steven P. Rowe, Takahiro Higuchi, Anna Katharina Seitz, Hubert Kübler, Andreas K. Buck, and Rudolf A. Werner. 2022. "Hematotoxicity and Nephrotoxicity in Prostate Cancer Patients Undergoing Radioligand Therapy with [177Lu]Lu-PSMA I&T" Cancers 14, no. 3: 647. https://doi.org/10.3390/cancers14030647
APA StyleHartrampf, P. E., Weinzierl, F.-X., Serfling, S. E., Pomper, M. G., Rowe, S. P., Higuchi, T., Seitz, A. K., Kübler, H., Buck, A. K., & Werner, R. A. (2022). Hematotoxicity and Nephrotoxicity in Prostate Cancer Patients Undergoing Radioligand Therapy with [177Lu]Lu-PSMA I&T. Cancers, 14(3), 647. https://doi.org/10.3390/cancers14030647






