Outcome and Safety after 103 Radioembolizations with Yttrium-90 Resin Microspheres in 73 Patients with Unresectable Intrahepatic Cholangiocarcinoma—An Evaluation of Predictors
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Dosimetry for Radioembolization
2.3. Radioembolization Procedure
2.4. Follow-Up: Response, Toxicity, Survival
2.5. Statistical Analyses
3. Results
3.1. Outcome—Treatment Response (RECIST)
3.2. Outcome—Survival
3.3. Safety
4. Discussion
4.1. Outcomes, Previous Results, and Predictors
4.2. Safety of TARE in ICC
4.3. Treatment Alternatives
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jemal, A.; Siegel, R.; Ward, E.; Hao, Y.; Xu, J.; Thun, M.J. Cancer statistics, 2009. CA A Cancer J. Clin. 2009, 59, 225–249. [Google Scholar] [CrossRef]
- Patel, T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology 2001, 33, 1353–1357. [Google Scholar] [CrossRef] [PubMed]
- Blechacz, B.; Gores, G.J. Cholangiocarcinoma: Advances in pathogenesis, diagnosis, and treatment. Hepatology 2008, 48, 308–321. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, J.; Kosuge, T.; Takayama, T.; Shimada, K.; Makuuchi, M.; Yoshida, J.; Sakamoto, M.; Hirohashi, S.; Yamasaki, S.; Hasegawa, H. Surgical treatment of intrahepatic cholangiocarcinoma: Four patients surviving more than five years. Surgery 1992, 111, 617–622. [Google Scholar] [PubMed]
- Lieser, M.J.; Barry, M.K.; Rowland, C.; Ilstrup, D.M.; Nagorney, D.M. Surgical management of intrahepatic cholangiocarcinoma: A 31-year experience. J. Hepato-Biliary-Pancreat. Surg. 1998, 5, 41–47. [Google Scholar] [CrossRef]
- Khan, S.A.; Thomas, H.C.; Davidson, B.R.; Taylor-Robinson, S.D. Cholangiocarcinoma. Lancet 2005, 366, 1303–1314. [Google Scholar] [CrossRef]
- Cunningham, S.C.; Choti, M.A.; Bellavance, E.C.; Pawlik, T.M. Palliation of hepatic tumors. Surg. Oncol. 2007, 16, 277–291. [Google Scholar] [CrossRef] [PubMed]
- Lamarca, A.; Ross, P.; Wasan, H.S.; Hubner, R.A.; McNamara, M.G.; Lopes, A.; Manoharan, P.; Palmer, D.; Bridgewater, J.; Valle, J.W. Advanced intrahepatic cholangiocarcinoma: Post hoc analysis of the ABC-01,-02, and-03 clinical trials. J. Natl. Cancer Inst. 2020, 112, 200–210. [Google Scholar] [CrossRef]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef] [Green Version]
- Tepel, J.; Hinz, S.; Klomp, H.J.; Kapischke, M.; Kremer, B. Intraoperative radiofrequency ablation (RFA) for irresectable liver malignancies. Eur. J. Surg. Oncol. (EJSO) 2004, 30, 551–555. [Google Scholar] [CrossRef]
- Bruix, J.; Sherman, M. Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology 2005, 42, 1208–1236. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, O.; Elsayed, Z. External beam radiotherapy for unresectable hepatocellular carcinoma. Cochrane Database Syst. Rev. 2017, 3, CD011314. [Google Scholar] [CrossRef] [PubMed]
- Paprottka, K.J.; Schoeppe, F.; Ingrisch, M.; Rubenthaler, J.; Sommer, N.N.; De Toni, E.; Ilhan, H.; Zacherl, M.; Todica, A.; Paprottka, P.M. Pre-therapeutic factors for predicting survival after radioembolization: A single-center experience in 389 patients. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1185–1193. [Google Scholar] [CrossRef]
- Fendler, W.P.; Lechner, H.; Todica, A.; Paprottka, K.J.; Paprottka, P.M.; Jakobs, T.F.; Michl, M.; Bartenstein, P.; Lehner, S.; Haug, A.R. Safety, Efficacy, and Prognostic Factors After Radioembolization of Hepatic Metastases from Breast Cancer: A Large Single-Center Experience in 81 Patients. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2016, 57, 517–523. [Google Scholar] [CrossRef] [Green Version]
- Buettner, S.; Braat, A.J.; Margonis, G.A.; Brown, D.B.; Taylor, K.B.; Borgmann, A.J.; Kappadath, S.C.; Mahvash, A.; IJzermans, J.N.; Weiss, M.J. Yttrium-90 radioembolization in intrahepatic cholangiocarcinoma: A multicenter retrospective analysis. J. Vasc. Interv. Radiol. 2020, 31, 1035–1043. [Google Scholar] [CrossRef]
- Köhler, M.; Harders, F.; Lohöfer, F.; Paprottka, P.M.; Schaarschmidt, B.M.; Theysohn, J.; Herrmann, K.; Heindel, W.; Schmidt, H.H.; Pascher, A. Prognostic factors for overall survival in advanced intrahepatic cholangiocarcinoma treated with yttrium-90 radioembolization. J. Clin. Med. 2020, 9, 56. [Google Scholar] [CrossRef] [Green Version]
- Edeline, J.; Touchefeu, Y.; Guiu, B.; Farge, O.; Tougeron, D.; Baumgaertner, I.; Ayav, A.; Campillo-Gimenez, B.; Beuzit, L.; Pracht, M. Radioembolization plus chemotherapy for first-line treatment of locally advanced intrahepatic cholangiocarcinoma: A phase 2 clinical trial. JAMA Oncol. 2020, 6, 51–59. [Google Scholar] [CrossRef]
- Levillain, H.; Derijckere, I.D.; Ameye, L.; Guiot, T.; Braat, A.; Meyer, C.; Vanderlinden, B.; Reynaert, N.; Hendlisz, A.; Lam, M. Personalised radioembolization improves outcomes in refractory intra-hepatic cholangiocarcinoma: A multicenter study. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2270–2279. [Google Scholar] [CrossRef]
- Ahmadzadehfar, H.; Biersack, H.J.; Ezziddin, S. Radioembolization of liver tumors with yttrium-90 microspheres. Semin. Nucl. Med. 2010, 40, 105–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakobs, T.F.; Hoffmann, R.T.; Tatsch, K.; Trumm, C.; Reiser, M.F.; Helmberger, T.K. Developments and perspectives in radioablative techniques. Der Radiol. 2007, 47, 1083–1088. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Health U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0; The National Cancer Institute: Washington, DC, USA, 2017.
- Hahn, F.; Müller, L.; Jungmann, F.; Mähringer-Kunz, A.; Tanyildizi, Y.; Düber, C.; Galle, P.R.; Weinmann, A.; Kloeckner, R. Survival prediction for patients with non-resectable intrahepatic cholangiocarcinoma undergoing chemotherapy: A retrospective analysis comparing the tumor marker CA 19-9 with cross-sectional imaging. J. Cancer Res. Clin. Oncol. 2020, 146, 1883–1890. [Google Scholar] [CrossRef] [Green Version]
- Mouli, S.; Memon, K.; Baker, T.; Benson, A.B., 3rd; Mulcahy, M.F.; Gupta, R.; Ryu, R.K.; Salem, R.; Lewandowski, R.J. Yttrium-90 radioembolization for intrahepatic cholangiocarcinoma: Safety, response, and survival analysis. J. Vasc. Interv. Radiol. 2013, 24, 1227–1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salem, R.; Lewandowski, R.J.; Mulcahy, M.F.; Riaz, A.; Ryu, R.K.; Ibrahim, S.; Atassi, B.; Baker, T.; Gates, V.; Miller, F.H.; et al. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: A comprehensive report of long-term outcomes. Gastroenterology 2010, 138, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Livraghi, T.; Meloni, F.; Di Stasi, M.; Rolle, E.; Solbiati, L.; Tinelli, C.; Rossi, S. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology 2008, 47, 82–89. [Google Scholar] [CrossRef]
- Brandi, G.; Rizzo, A.; Dall’Olio, F.G.; Felicani, C.; Ercolani, G.; Cescon, M.; Frega, G.; Tavolari, S.; Palloni, A.; De Lorenzo, S. Percutaneous radiofrequency ablation in intrahepatic cholangiocarcinoma: A retrospective single-center experience. Int. J. Hyperth. 2020, 37, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, M.V.; Albert, M.; McNally, M.; Robertson, M.; Sun, W.; Fraker, D.; Olthoff, K.; Christians, K.; Pappas, S.; Rilling, W.; et al. Chemoembolization of intrahepatic cholangiocarcinoma with cisplatinum, doxorubicin, mitomycin C, ethiodol, and polyvinyl alcohol: A 2-center study. Cancer 2011, 117, 1498–1505. [Google Scholar] [CrossRef] [PubMed]
- Burger, I.; Hong, K.; Schulick, R.; Georgiades, C.; Thuluvath, P.; Choti, M.; Kamel, I.; Geschwind, J.F. Transcatheter arterial chemoembolization in unresectable cholangiocarcinoma: Initial experience in a single institution. J. Vasc. Interv. Radiol. 2005, 16, 353–361. [Google Scholar] [CrossRef]
- Schicho, A.; Pereira, P.L.; Putzler, M.; Michalik, K.; Albrecht, T.; Nolte-Ernsting, C.; Stroszczynski, C.; Wiggermann, P. Degradable Starch Microspheres Transcatheter Arterial Chemoembolization (DSM-TACE) in Intrahepatic Cholangiocellular Carcinoma (ICC): Results from a National Multi-Center Study on Safety and Efficacy. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2017, 23, 796–800. [Google Scholar] [CrossRef] [Green Version]
- Edeline, J.; Lamarca, A.; McNamara, M.G.; Jacobs, T.; Hubner, R.A.; Palmer, D.; Koerkamp, B.G.; Johnson, P.; Guiu, B.; Valle, J.W. Locoregional therapies in patients with intrahepatic cholangiocarcinoma: A systematic review and pooled analysis. Cancer Treat. Rev. 2021, 99, 102258. [Google Scholar] [CrossRef]
| Variable | No. of Patients | % |
|---|---|---|
| Total cohort | 73 | 100 |
| Age | ||
| <65 years | 31 | 42 |
| ≥65 years | 42 | 58 |
| Male | 40 | 55 |
| Previous therapies | ||
| Surgery | 23 | 32 |
| Radiofrequency ablation | 1 | 1 |
| Prior trans-arterial chemoembolization | 4 | 5 |
| Prior chemotherapy | 52 | 71 |
| Tumor characteristics | ||
| Extra-hepatic disease | 37 | 51 |
| Tumor burden | ||
| ≤25% | 44 | 60 |
| 26–50% | 24 | 33 |
| >50% | 5 | 7 |
| Laboratory data | ||
| Bilirubin (absolute) | ||
| ≤0.6 mg/dL | 44 | 60 |
| 0.7–1.2 mg/dL | 29 | 40 |
| Cholinesterase (absolute) | ||
| Within normal limits (≥4.62 kU/L) | 59 | 81 |
| Below normal limits (<4.62 kU/L) | 14 | 19 |
| Treatment Concept (Patients) | n (%) | Calculated Dose | Applied Dose | ||
|---|---|---|---|---|---|
| Median Dose | Interquartile Range | Median Dose | Interquartile Range | ||
| Overall | 73 | 1500 | 933–1940 | 1373 | 881–1848 |
| Location | |||||
| One lobe, single session | 25 (34%) | 1067 | 412–2500 | 1050 | 404–2157 |
| Whole liver, single session | 25 (34%) | 1960 | 750–2500 | 1887 | 740–2475 |
| Whole liver, two sessions | 17(23%) | 1000 | 400–1500 | 1009 | 390–1512 |
| Multiple treatments | 6 (8%) | 966.5 | 560–2000 | 1000.5 | 564–1874 |
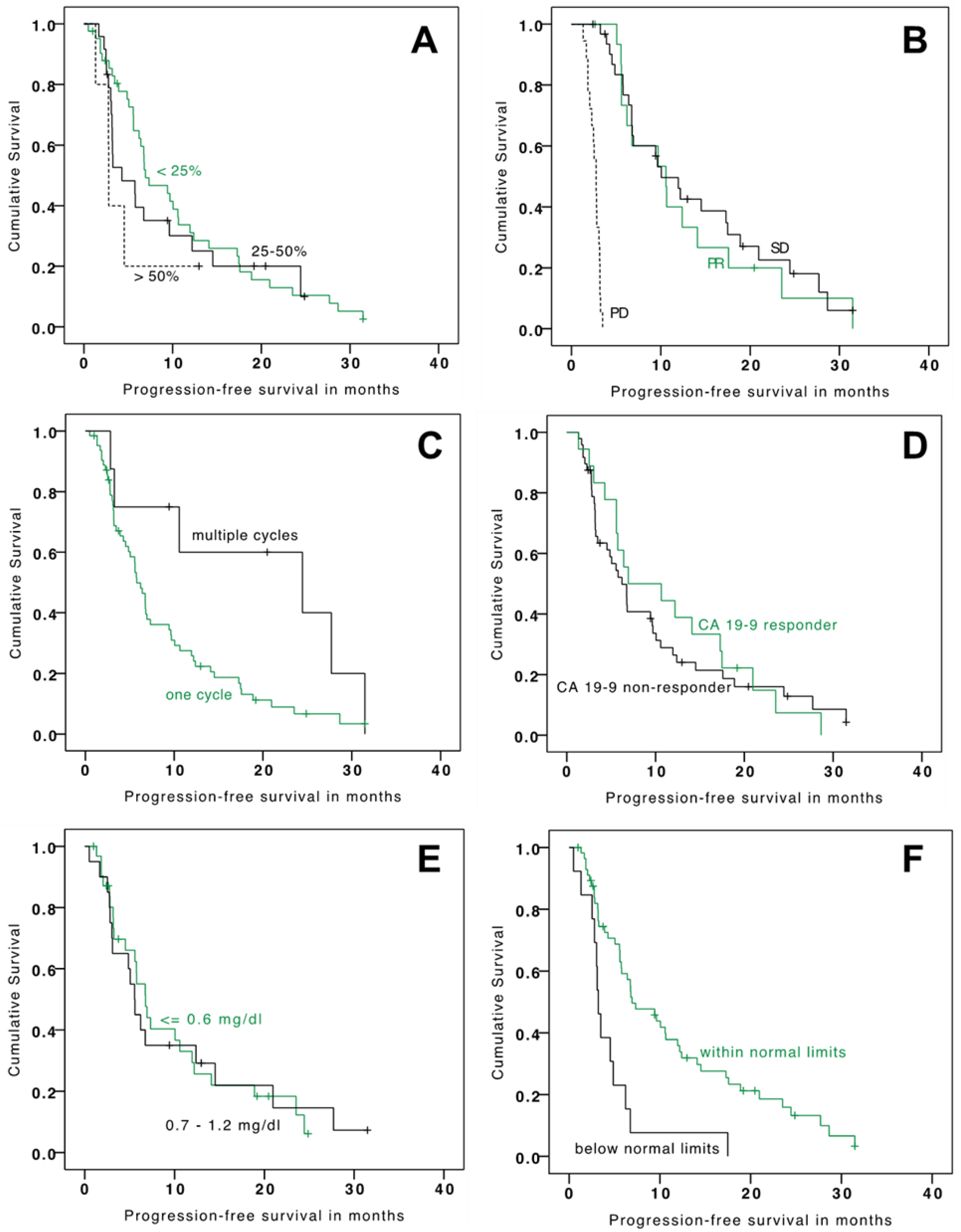
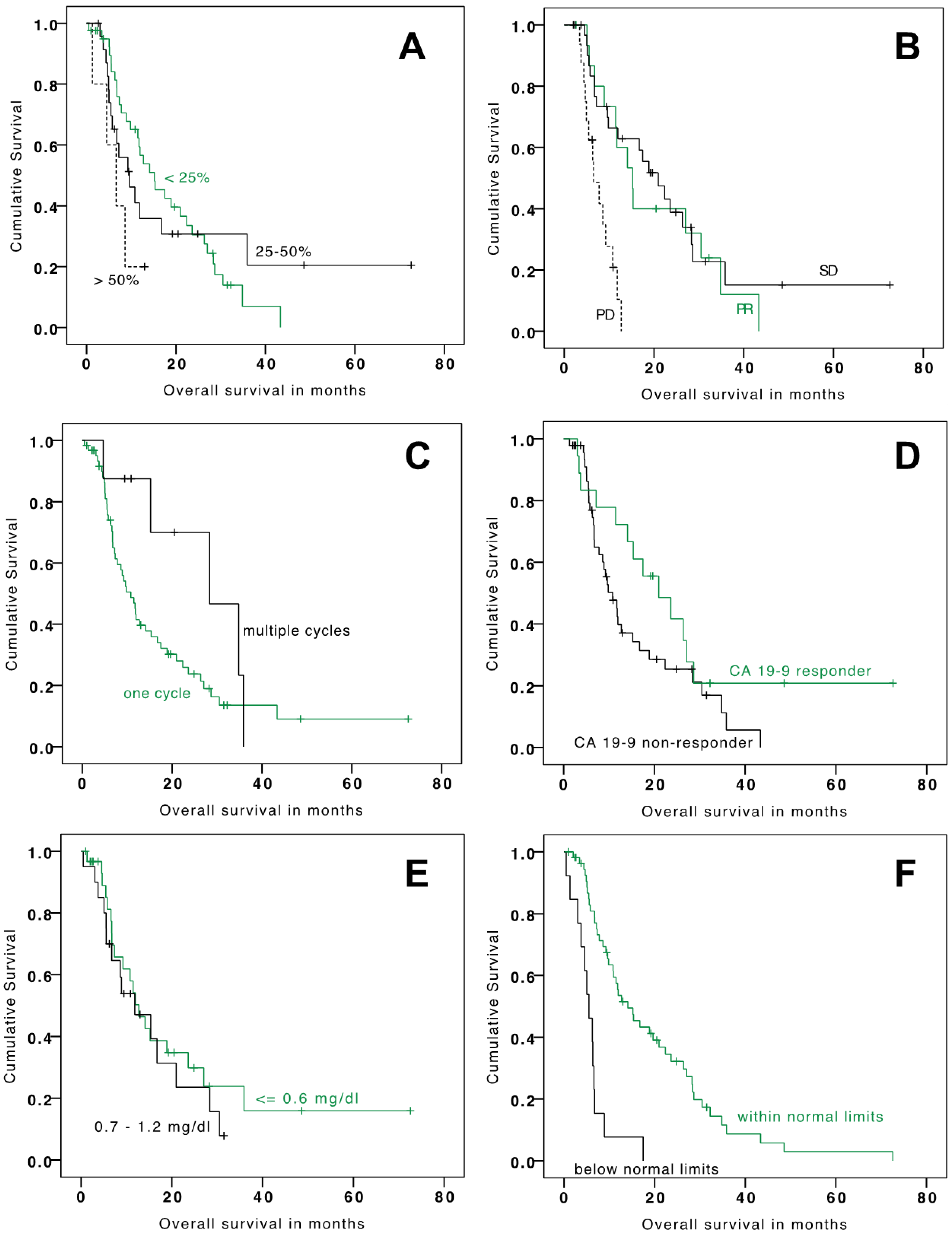
| Category | n (%) | Overall Survival (Months) | Progression Free Survival (Months) | ||||
|---|---|---|---|---|---|---|---|
| Median | 95% CI | p-value | Median | 95% CI | p-value | ||
| All patients | 11.82 | 7.32–16.32 | 6.41 | 5.20–7.61 | |||
| Tumor burden | 11.83 | 7.32–16.32 | 6.40 | 5.2–7.61 | |||
| ≤25% | 44 (60%) | 15.21 | 8.86–21.56 | (reference) | 6.93 | 3.67–10.19 | (reference) |
| 26–50% | 24 (33%) | 9.62 | 4.54–14.70 | 0.963 | 4.27 | 0.34–8.19 | 0.515 |
| >50% | 5 (7%) | 6.60 | 2.16–11.04 | 0.036 | 2.79 | 2.72–2.86 | 0.143 |
| CA-19-9 response | 11.95 | 7.53–16.38 | 6.73 | 5.58–7.88 | |||
| Yes | 20 (27%) | 20.96 | 10.44–31.48 | (reference) | 6.93 | 0.0–15.74 | (reference) |
| No | 53 (73%) | 10.81 | 7.74–13.88 | 0.098 | 6.21 | 4.38–8.04 | 0.654 |
| RECIST response | 11.82 | 7.32–16.32 | 6.40 | 5.20–7.60 | |||
| Partial response | 18 (25%) | 15.21 | 10.64–19.77 | (reference) | 10.59 | 5.68–15.47 | (reference) |
| Stable disease | 36 (49%) | 20.96 | 13.55–28.37 | 0.637 | 10.05 | 6.54–13.56 | 0.634 |
| Progressive disease | 19 (26%) | 6.60 | 3.99–9.21 | <0.001 | 2.76 | 2.28–3.23 | <0.001 |
| Bilirubin | 11.46 | 7.94–14.98 | 6.20 | 4.67–7.74 | |||
| <0.6 mg/dL | 44 (60%) | 11.95 | 8.75–15.16 | (reference) | 6.76 | 4.83–8.69 | (reference) |
| 0.7–1.2 mg/dL | 29 (40%) | 8.903 | 4.19–13.61 | 0.419 | 5.55 | 4.40–6.70 | 0.967 |
| Cholinesterase | 10.87 | 7.88–13.86 | 6.40 | 5.08–7.73 | |||
| ≥4.62 kU/L | 59 (81%) | 14.0 | 8.59–19.59 | (reference) | 6.93 | 3.56–10.30 | (reference) |
| <4.62 kU/L | 14 (19%) | 5.52 | 3.51–7.52 | <0.001 | 3.22 | 2.68–3.76 | 0.001 |
| Treatment cycles | 11.83 | 7.33–16.30 | 6.41 | 5.20–7.61 | |||
| One | 67 (92%) | 10.81 | 7.78–13.84 | (reference) | 5.78 | 4.69–6.88 | (reference) |
| Multiple | 6 (8%) | 28.35 | 9.74–46.97 | 0.156 | 24.44 | 0.0–52.067 | 0.04 |
| Laboratory Value | Baseline | Follow-Up (Discharge) | Follow-Up (3 Months Post Radioembolization) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | Change from Baseline | Median | IQR | Change from Baseline | |||
| Abs. | Rel. in % | Abs. | Rel. in % | |||||||
| Bilirubin (mg/dL) | 0.60 | 0.5–0.8 | 0.7 | 0.55–1.0 | 0.1 | 11.81 | 0.7 | 0.6–1.2 | 0.1 | 25 |
| Serum cholinesterase (kU/L) | 6.62 | 5.27–7.58 | 5.79 | 4.55–6.85 | −0.85 | −12.8 | 5.90 | 4.54–6.89 | −0.87 | −11.03 |
| Albumin (g/dL) | 4.25 | 4.0–4.5 | 3.8 | 3.5–4.0 | −0.40 | −9.64 | 4.1 | 3.3–4.4 | −0.2 | −4.13 |
| Thrombocytes (Thousand/µL) | 197 | 150.5–253.0 | 160.0 | 114.0–197.5 | −39.0 | −22.06 | 147.5 | 116.25–191.25 | −36.5 | −20.04 |
| C-reactive protein (mg/dL) | 0.9 | 0.5–1.85 | 1.8 | 0.8–5.2 | 0.4 | 50 | 1.25 | 0.6–2.7 | 0.3 | 36.67 |
| Symptoms | No Events | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | |
|---|---|---|---|---|---|---|---|
| Acute | Nausea | 51 (70%) | 8 (11%) | 11 (15%) | 3 (4%) | 0 | 0 |
| Vomiting | 65 (89%) | 8 (11%) | 0 | 0 | 0 | 0 | |
| Pain | 52 (71%) | 11 (15%) | 8 (11%) | 2 (3%) | 0 | 0 | |
| Fever | 67 (92%) | 5 (7%) | 1 (1%) | 0 | 0 | 0 | |
| Late | Gastritis | 66 (90%) | 0 | 3 (4%) | 4 (5%) | 0 | 0 |
| Pancreatitis | 71 (97%) | 2 (3%) | 0 | 0 | 0 | 0 | |
| Cholecystits | 73 (100%) | 0 | 0 | 0 | 0 | 0 | |
| Up to 3 months after radioembolization | REILD | 73 (100%) | 0 | 0 | 0 | 0 | 0 |
| Skin necrosis | 73 (100%) | 0 | 0 | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paprottka, K.J.; Galiè, F.; Ingrisch, M.; Geith, T.; Ilhan, H.; Todica, A.; Michl, M.; Nadjiri, J.; Paprottka, P.M. Outcome and Safety after 103 Radioembolizations with Yttrium-90 Resin Microspheres in 73 Patients with Unresectable Intrahepatic Cholangiocarcinoma—An Evaluation of Predictors. Cancers 2021, 13, 5399. https://doi.org/10.3390/cancers13215399
Paprottka KJ, Galiè F, Ingrisch M, Geith T, Ilhan H, Todica A, Michl M, Nadjiri J, Paprottka PM. Outcome and Safety after 103 Radioembolizations with Yttrium-90 Resin Microspheres in 73 Patients with Unresectable Intrahepatic Cholangiocarcinoma—An Evaluation of Predictors. Cancers. 2021; 13(21):5399. https://doi.org/10.3390/cancers13215399
Chicago/Turabian StylePaprottka, Karolin J., Franziska Galiè, Michael Ingrisch, Tobias Geith, Harun Ilhan, Andrei Todica, Marlies Michl, Jonathan Nadjiri, and Philipp M. Paprottka. 2021. "Outcome and Safety after 103 Radioembolizations with Yttrium-90 Resin Microspheres in 73 Patients with Unresectable Intrahepatic Cholangiocarcinoma—An Evaluation of Predictors" Cancers 13, no. 21: 5399. https://doi.org/10.3390/cancers13215399
APA StylePaprottka, K. J., Galiè, F., Ingrisch, M., Geith, T., Ilhan, H., Todica, A., Michl, M., Nadjiri, J., & Paprottka, P. M. (2021). Outcome and Safety after 103 Radioembolizations with Yttrium-90 Resin Microspheres in 73 Patients with Unresectable Intrahepatic Cholangiocarcinoma—An Evaluation of Predictors. Cancers, 13(21), 5399. https://doi.org/10.3390/cancers13215399






