Latest Advances in the Use of Therapeutic Focused Ultrasound in the Treatment of Pancreatic Cancer
Abstract
Simple Summary
Abstract
1. Introduction
1.1. Pancreatic Cancer
1.2. Immunotherapy
1.3. Therapeutic Focused Ultrasound
2. Immune Effects of Therapeutic Focused Ultrasound
2.1. Thermal Ablation
2.2. Pulsed Focused Ultrasound and Histotripsy
2.3. Low Intensity Focused Ultrasound
3. Treatment of Pancreatic Cancer with Therapeutic Focused Ultrasound
3.1. Treatment of Pancreatic Cancer Patients with “Thermal” HIFU
3.2. HIFU in Combination with Traditional Oncologic Interventions
3.3. Clinical Case Studies
3.4. Complications of HIFU Treatments in Pancreatic Cancer Patients
3.5. Clinical Use of “Mechanical” Therapeutic Focused Ultrasound for the Treatment of Pancreatic Cancer Patients
3.6. Drug Delivery and Sonodynamic Therapy Studies in Preclinical Pancreatic Cancer Models
4. The Immunological Effects of Therapeutic Focused Ultrasound in Pancreatic Cancer
5. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Mizrahi, J.D.; Surana, R.; Valle, J.W.; Shroff, R.T. Pancreatic cancer. Lancet 2020, 395, 2008–2020. [Google Scholar] [CrossRef]
- Buscail, L.; Bournet, B.; Cordelier, P. Role of oncogenic KRAS in the diagnosis, prognosis and treatment of pancreatic cancer. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 153–168. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Pruski, M.; Bland, R.; Younes, M.; Guha, S.; Thosani, N.; Maitra, A.; Cash, B.D.; McAllister, F.; Logsdon, C.D.; et al. Kras mutation rate precisely orchestrates ductal derived pancreatic intraepithelial neoplasia and pancreatic cancer. Lab. Invest. 2021, 101, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Christenson, E.S.; Jaffee, E.; Azad, N.S. Current and emerging therapies for patients with advanced pancreatic ductal adenocarcinoma: A bright future. Lancet Oncol. 2020, 21, e135–e145. [Google Scholar] [CrossRef]
- Connor, A.A.; Denroche, R.E.; Jang, G.H.; Lemire, M.; Zhang, A.; Chan-Seng-Yue, M.; Wilson, G.; Grant, R.C.; Merico, D.; Lungu, I.; et al. Integration of genomic and transcriptional features in pancreatic cancer reveals increased cell cycle progression in metastases. Cancer Cell 2019, 35, 267–282.e7. [Google Scholar] [CrossRef]
- Waddell, N.; Pajic, M.; Patch, A.M.; Chang, D.K.; Kassahn, K.S.; Bailey, P.; Johns, A.L.; Miller, D.; Nones, K.; Quek, K.; et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 2015, 518, 495–501. [Google Scholar] [CrossRef]
- Holter, S.; Borgida, A.; Dodd, A.; Grant, R.; Semotiuk, K.; Hedley, D.; Dhani, N.; Narod, S.; Akbari, M.; Moore, M.; et al. Germline BRCA mutations in a large clinic-based cohort of patients with pancreatic adenocarcinoma. J. Clin. Oncol. 2015, 33, 3124–3129. [Google Scholar] [CrossRef]
- Murphy, K.M.; Brune, K.A.; Griffin, C.; Sollenberger, J.E.; Petersen, G.M.; Bansal, R.; Hruban, R.H.; Kern, S.E. Evaluation of candidate genes MAP2K4, MADH4, ACVR1B, and BRCA2 in familial pancreatic cancer: Deleterious BRCA2 mutations in 17%. Cancer Res. 2002, 62, 3789–3793. [Google Scholar]
- Hung, Y.H.; Hsu, M.C.; Chen, L.T.; Hung, W.C.; Pan, M.R. Alteration of epigenetic modifiers in pancreatic cancer and its clinical implication. J. Clin. Med. 2019, 8, 903. [Google Scholar] [CrossRef]
- Bailey, P.; Chang, D.K.; Nones, K.; Johns, A.L.; Patch, A.M.; Gingras, M.C.; Miller, D.K.; Christ, A.N.; Bruxner, T.J.; Quinn, M.C.; et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 2016, 531, 47–52. [Google Scholar] [CrossRef]
- Hegde, S.; Krisnawan, V.E.; Herzog, B.H.; Zuo, C.; Breden, M.A.; Knolhoff, B.L.; Hogg, G.D.; Tang, J.P.; Baer, J.M.; Mpoy, C.; et al. Dendritic cell paucity leads to dysfunctional immune surveillance in pancreatic cancer. Cancer Cell 2020, 37, 289–307.e9. [Google Scholar] [CrossRef] [PubMed]
- Grünwald, B.; Harant, V.; Schaten, S.; Frühschütz, M.; Spallek, R.; Höchst, B.; Stutzer, K.; Berchtold, S.; Erkan, M.; Prokopchuk, O.; et al. Pancreatic premalignant lesions secrete tissue inhibitor of metalloproteinases-1, which activates hepatic stellate cells via CD63 signalling to create a premetastatic niche in the liver. Gastroenterology 2016, 151, 1011–1024.e7. [Google Scholar] [CrossRef] [PubMed]
- Springfeld, C.; Jäger, D.; Büchler, M.W.; Strobel, O.; Hackert, T.; Palmer, D.H.; Neoptolemos, J.P. Chemotherapy for pancreatic cancer. Presse Med. 2019, 48 Pt 2, e159–e174. [Google Scholar] [CrossRef]
- Burris, H.A., 3rd; Moore, M.J.; Andersen, J.; Green, M.R.; Rothenberg, M.L.; Modiano, M.R.; Cripps, M.C.; Portenoy, R.K.; Storniolo, A.M.; Tarassoff, P.; et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J. Clin. Oncol. 1997, 15, 2403–2413. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouche, O.; Guimbaud, R.; Becouarn, Y.; Adenis, A.; Raoul, J.L.; Gourgou-Bourgade, S.; de la Fouchardière, C.; et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.; El-Maraghi, R.H.; Hammel, P.; Heinemann, V.; Kunzmann, V.; Sastre, J.; Scheithauer, W.; Siena, S.; Tabernero, J.; Teixeira, L.; et al. Nab-paclitaxel plus gemcitabine for metastatic pancreatic cancer: Long-term survival from a phase III trial. J. Natl. Cancer Inst. 2015, 413, 107. [Google Scholar] [CrossRef]
- Alexandrov, L.B.; Nik-Zainal, S.; Wedge, D.C.; Aparicio, S.A.J.R.; Behjati, S.; Biankin, A.V.; Bignell, G.R.; Bolli, N.; Borg, A.; Borresen-Dale, A.-L.; et al. Signatures of mutational processes in human cancer. Nature 2013, 500, 415–421. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, Y.; Yang, F.; Zhu, L.; Zhu, X.Q.; Wang, Z.F.; Wu, X.L.; Zhou, C.H.; Yan, J.Y.; Hu, B.Y.; et al. The molecular biology of pancreatic adenocarcinoma: Translational challenges and clinical perspectives. Signal Transduct. Target. Ther. 2021, 6, 249. [Google Scholar] [CrossRef]
- Provenzano, P.P.; Cuevas, C.; Chang, A.E.; Goel, V.K.; Von Hoff, D.D.; Hingorani, S.R. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 2012, 21, 418–429. [Google Scholar] [CrossRef]
- Olive, K.P.; Jacobetz, M.A.; Davidson, C.J.; Gopinathan, A.; McIntyre, D.; Honess, D.; Madhu, B.; Goldgraben, M.A.; Caldwell, M.E.; Allard, D.; et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 2009, 324, 1457–1461. [Google Scholar] [CrossRef]
- Jacobetz, M.A.; Chan, D.S.; Neesse, A.; Bapiro, T.E.; Cook, N.; Frese, K.K.; Feig, C.; Nakagawa, T.; Caldwell, M.E.; Zecchini, H.I.; et al. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut 2013, 62, 112–120. [Google Scholar] [CrossRef]
- Singha, N.C.; Nekoroski, T.; Zhao, C.; Symons, R.; Jiang, P.; Frost, G.I.; Huang, Z.; Shepard, H.M. Tumor-associated hyaluronan limits efficacy of monoclonal antibody therapy. Mol. Cancer Ther. 2015, 14, 523–532. [Google Scholar] [CrossRef]
- Prieto, J.G.; Pulido, M.M.; Zapico, J.; Molina, A.J.; Gimeno, M.; Coronel, P.; Alvarez, A.I. Comparative study of hyaluronic derivatives: Rheological behaviour, mechanical and chemical degradation. Int. J. Biol. Macromol. 2005, 35, 63–69. [Google Scholar] [CrossRef]
- Ansari, D.; Carvajo, M.; Bauden, M.; Andersson, R. Pancreatic cancer stroma: Controversies and current insights. Scand. J. Gastroenterol. 2017, 52, 641–646. [Google Scholar] [CrossRef]
- Rhim, A.D.; Oberstein, P.E.; Thomas, D.H.; Mirek, E.T.; Palermo, C.F.; Sastra, S.A.; Dekleva, E.N.; Saunders, T.; Becerra, C.P.; Tattersall, I.W.; et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell 2014, 25, 735–747. [Google Scholar] [CrossRef]
- Vonderheide, R.H.; Bayne, L.J. Inflammatory networks and immune surveillance of pancreatic carcinoma. Curr. Opin. Immunol. 2013, 25, 200–205. [Google Scholar] [CrossRef]
- Sherman, M.H. Stellate cells in tissue repair, inflammation, and cancer. Annu. Rev. Cell Dev. Biol. 2018, 34, 333–355. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, S.; Zeng, S.; Shen, H. The critical roles of activated stellate cells-mediated paracrine signalling, metabolism and onco-immunology in pancreatic ductal adenocarcinoma. Mol. Cancer 2018, 17, 62. [Google Scholar] [CrossRef]
- Farajzadeh Valilou, S.; Keshavarz-Fathi, M.; Silvestris, N.; Argentiero, A.; Rezaei, N. The role of inflammatory cytokines and tumor associated macrophages (TAMs) in microenvironment of pancreatic cancer. Cytokine Growth Factor Rev. 2018, 39, 46–61. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Mitsunaga, S.; Kinoshita, T.; Konishi, M.; Takahashi, S.; Gotohda, N.; Kato, Y.; Aizawa, M.; Ochiai, A. Impact of tumor-associated macrophages on invasive ductal carcinoma of the pancreas head. Cancer Sci. 2012, 103, 2012–2020. [Google Scholar] [CrossRef]
- Thyagarajan, A.; Alshehri, M.S.A.; Miller, K.L.R.; Sherwin, C.M.; Travers, J.B.; Sahu, R.P. Myeloid-derived suppressor cells and pancreatic cancer: Implications in novel therapeutic approaches. Cancers 2019, 11, 1627. [Google Scholar] [CrossRef]
- Pergamo, M.; Miller, G. Myeloid-derived suppressor cells and their role in pancreatic cancer. Cancer Gene Ther. 2017, 24, 100–105. [Google Scholar] [CrossRef]
- Von Ahrens, D.; Bhagat, T.D.; Nagrath, D.; Maitra, A.; Verma, A. The role of stromal cancer-associated fibroblasts in pancreatic cancer. J. Hematol. Oncol. 2017, 10, 76. [Google Scholar] [CrossRef]
- Manoukian, P.; Bijlsma, M.; van Laarhoven, H. The Cellular Origins of Cancer-Associated Fibroblasts and Their Opposing Contributions to Pancreatic Cancer Growth. Front Cell Dev Biol. 2021, 9, 743907. [Google Scholar] [CrossRef]
- Bulle, A.; Lim, K.H. Beyond just a tight fortress: Contribution of stroma to epithelial-mesenchymal transition in pancreatic cancer. Signal Transduct. Target. Ther. 2020, 5, 249. [Google Scholar] [CrossRef]
- Bachem, M.G.; Schünemann, M.; Ramadani, M.; Siech, M.; Beger, H.; Buck, A.; Zhou, S.; Schmid-Kotsas, A.; Adler, G. Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells. Gastroenterology 2005, 128, 907–921. [Google Scholar] [CrossRef]
- Straussman, R.; Morikawa, T.; Shee, K.; Barzily-Rokni, M.; Qian, Z.R.; Du, J.; Davis, A.; Mongare, M.M.; Gould, J.; Frederick, D.T.; et al. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature 2012, 487, 500–504. [Google Scholar] [CrossRef]
- Meyer, M.A.; Baer, J.M.; Knolhoff, B.L.; Nywening, T.M.; Panni, R.Z.; Su, X.; Weilbaecher, K.N.; Hawkins, W.G.; Ma, C.; Fields, R.C.; et al. Breast and pancreatic cancer interrupt IRF8-dependent dendritic cell development to overcome immune surveillance. Nat. Commun. 2018, 9, 1250. [Google Scholar] [CrossRef]
- Ye, H.; Zhou, Q.; Zheng, S.; Li, G.; Lin, Q.; Wei, L.; Fu, Z.; Zhang, B.; Liu, Y.; Li, Z.; et al. Tumor-associated macrophages promote progression and the Warburg effect via CCL18/NF-kB/VCAM-1 pathway in pancreatic ductal adenocarcinoma. Cell Death Dis. 2018, 9, 453. [Google Scholar] [CrossRef]
- Hutcheson, J.; Balaji, U.; Porembka, M.R.; Wachsmann, M.B.; McCue, P.A.; Knudsen, E.S.; Witkiewicz, A.K. Immunologic and metabolic features of pancreatic ductal adenocarcinoma define prognostic subtypes of disease. Clin. Cancer Res. 2016, 22, 3606–3617. [Google Scholar] [CrossRef]
- Principe, D.R.; DeCant, B.; Mascariñas, E.; Wayne, E.A.; Diaz, A.M.; Akagi, N.; Hwang, R. TGFβ signaling in the pancreatic tumor microenvironment promotes fibrosis and immune evasion to facilitate tumorigenesis. Cancer Res. 2016, 76, 2525–2539. [Google Scholar] [CrossRef]
- Yin, Z.; Ma, T.; Huang, B.; Lin, L.; Zhou, Y.; Yan, J.; Zou, Y.; Chen, S. Macrophage-derived exosomal microRNA-501-3p promotes progression of pancreatic ductal adenocarcinoma through the TGFBR3-mediated TGF-β signaling pathway. J. Exp. Clin. Cancer Res. 2019, 38, 310. [Google Scholar] [CrossRef]
- Catenacci, D.V.; Junttila, M.R.; Karrison, T.; Bahary, N.; Horiba, M.N.; Nattam, S.R.; Marsh, R.; Wallace, J.; Kozloff, M.; Rajdev, L.; et al. Randomized phase ib/ii study of gemcitabine plus placebo or vismodegib, a hedgehog pathway inhibitor, in patients with metastatic pancreatic cancer. J. Clin. Oncol. 2015, 33, 4284–4292. [Google Scholar] [CrossRef]
- Kim, E.J.; Sahai, V.; Abel, E.V.; Griffith, K.A.; Greenson, J.K.; Takebe, N.; Khan, G.N.; Blau, J.L.; Craig, R.; Balis, U.G.; et al. Pilot clinical trial of hedgehog pathway inhibitor GDC0449 (vismodegib) in combination with gemcitabine in patients with metastatic pancreatic adenocarcinoma. Clin. Cancer Res. 2014, 20, 5937–5945. [Google Scholar] [CrossRef]
- Özdemir, B.C.; Pentcheva-Hoang, T.; Carstens, J.L.; Zheng, X.; Wu, C.C.; Simpson, T.R.; Laklai, H.; Sugimoto, H.; Kahlert, C.; Novitskiy, S.V.; et al. Depletion of carcinoma associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 2014, 25, 719–734. [Google Scholar] [CrossRef]
- Hingorani, S.R.; Zheng, L.; Bullock, A.J.; Seery, T.E.; Harris, W.P.; Sigal, D.S.; Braiteh, F.; Ritch, P.S.; Zalupski, M.M.; Bahary, N.; et al. HALO 202: Randomized phase II study of PEGPH20 plus nab-paclitaxel/gemcitabine versus nab-paclitaxel/gemcitabine in patients with untreated, metastatic pancreatic ductal adenocarcinoma. J. Clin. Oncol. 2018, 36, 359–366. [Google Scholar] [CrossRef]
- Beatty, G.L.; Torigian, D.A.; Chiorean, E.G.; Saboury, B.; Brothers, A.; Alavi, A.; Troxel, A.B.; Sun, W.; Teitelbaum, U.R.; Vonderheide, R.H.; et al. A phase I study of an agonist CD40 monoclonal antibody (CP-870893) in combination with gemcitabine in patients with advanced pancreatic ductal adenocarcinoma. Clin. Cancer Res. 2013, 19, 6286–6295. [Google Scholar] [CrossRef]
- Mahalingam, D.; Wilkinson, G.A.; Eng, K.H.; Fields, P.; Raber, P.; Moseley, J.L.; Cheetham, K.K.; Coffey, M.; Nuovo, G.; Kalinski, P.; et al. Pembrolizumab in combination with the oncolytic virus pelareorep and chemotherapy in patients with advanced pancreatic adenocarcinoma: A phase Ib study. Clin. Cancer Res. 2020, 26, 71–81. [Google Scholar] [CrossRef]
- Le, D.T.; Wang-Gillam, A.; Picozzi, V.; Greten, T.F.; Crocenzi, T.; Springett, G.; Morse, M.; Zeh, H.; Cohen, D.; Fine, R.L.; et al. Safety and survival with GVAX pancreas prime and Listeria monocytogenes expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J. Clin. Oncol. 2015, 33, 1325–1333. [Google Scholar] [CrossRef]
- Beatty, G.L.; O’Hara, M.H.; Lacey, S.F.; Torigian, D.A.; Nazimuddin, F.; Chen, F.; Kulikovskaya, I.M.; Soulen, M.C.; McGarvey, M.; Nelson, A.M.; et al. Activity of mesothelin-specific chimeric antigen receptor T cells against pancreatic carcinoma metastases in a phase 1 trial. Gastroenterology 2018, 155, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Siddiqui, B.A.; Anandhan, S.; Yadav, S.S.; Subudhi, S.K.; Gao, J.; Goswami, S.; Allison, J.P. The Next Decade of Immune Checkpoint Therapy. Cancer Discov. 2021, 11, 838–857. [Google Scholar] [CrossRef] [PubMed]
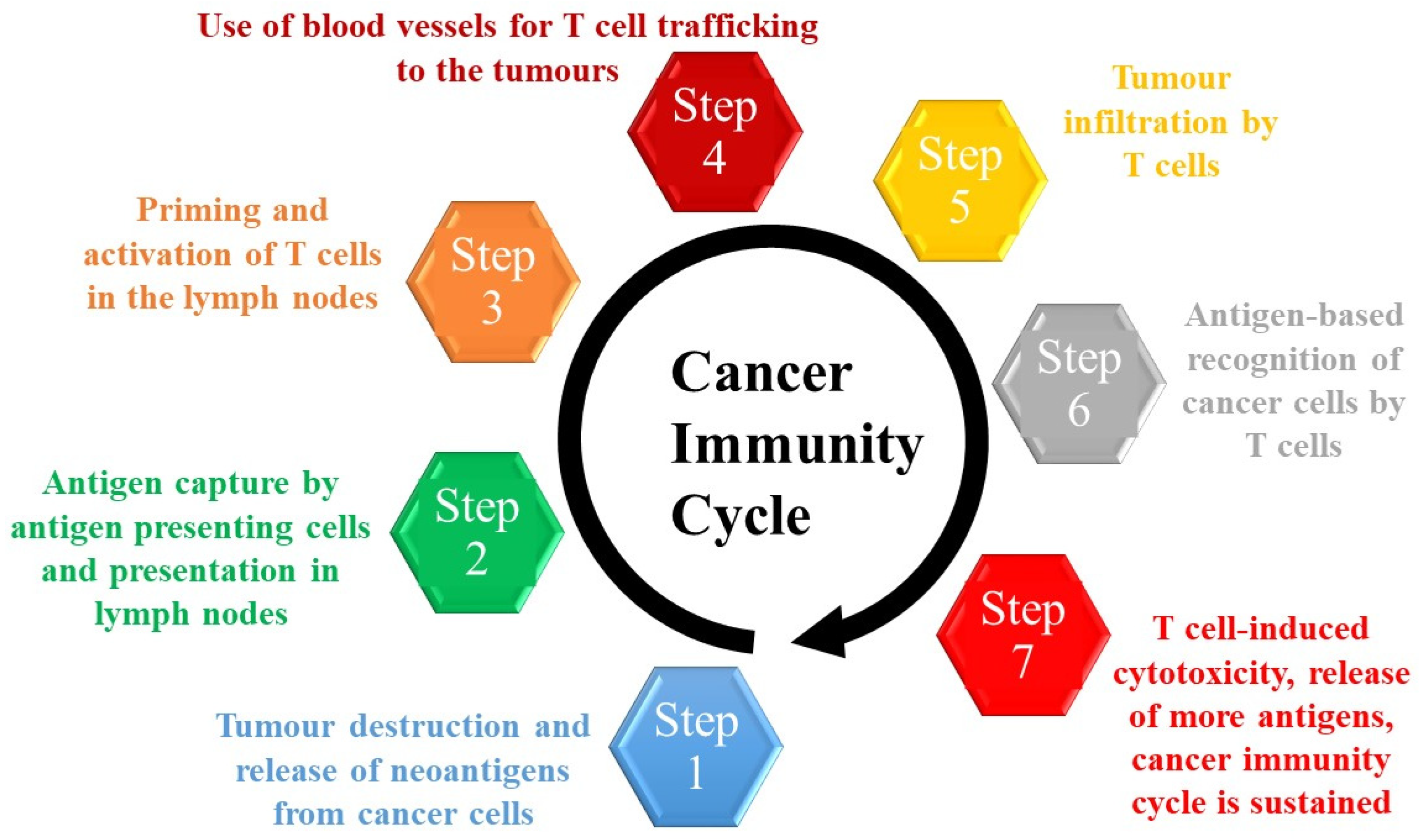
- Chen, D.S.; Mellman, I. Oncology meets immunology: The cancer-immunity cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Thomas, L.; Bondarenko, I.; O’Day, S.; Weber, J.; Garbe, C.; Lebbe, C.; Baurain, J.F.; Testori, A.; Grob, J.J.; et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 2011, 364, 2517–2526. [Google Scholar] [CrossRef]
- Schadendorf, D.; Hodi, F.S.; Robert, C.; Weber, J.S.; Margolin, K.; Hamid, O.; Patt, D.; Chen, T.T.; Berman, D.M.; Wolchok, J.D. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J. Clin. Oncol. 2015, 33, 1889–1894. [Google Scholar] [CrossRef]
- Robert, C.; Schachter, J.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus ipilimumab in advanced melanoma. N. Engl. J. Med. 2015, 372, 2521–2532. [Google Scholar] [CrossRef]
- Weber, J.S.; D’Angelo, S.P.; Minor, D.; Hodi, F.S.; Gutzmer, R.; Neyns, B.; Hoeller, C.; Khushalani, N.I.; Miller, W.H., Jr.; Lao, C.D.; et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): A randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015, 16, 375–384. [Google Scholar] [CrossRef]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef]
- Marabelle, A.; Fakih, M.; Lopez, J.; Shah, M.; Shapira-Frommer, R.; Nakagawa, K.; Chung, H.C.; Kindler, H.L.; Lopez-Martin, J.A.; Miller, W.H., Jr.; et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: Prospective biomarker analysis of the multicohort, open label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020, 21, 1353–1365. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef] [PubMed]
- Yau, T.; Kang, Y.K.; Kim, T.Y.; El-Khoueiry, A.B.; Santoro, A.; Sangro, B.; Melero, I.; Kudo, M.; Hou, M.; Matilla, A.; et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: The CheckMate 040 randomized clinical trial. JAMA Oncol. 2020, 6, e204564. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Arén Frontera, O.; Melichar, B.; Choueiri, T.K.; Plimack, E.R.; Barthélémy, P.; Porta, C.; George, S.; et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290. [Google Scholar] [CrossRef]
- Motzer, R.J.; Escudier, B.; McDermott, D.F.; Arén Frontera, O.; Melichar, B.; Powles, T.; Donskov, F.; Plimack, E.R.; Barthélémy, P.; Hammers, H.J.; et al. Survival outcomes and independent response assessment with nivolumab plus ipilimumab versus sunitinib in patients with advanced renal cell carcinoma: 42-month follow-up of a randomized phase 3 clinical trial. J. Immunother. Cancer 2020, 8, e000891. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Paz-Ares, L.; Bernabe Caro, R.; Zurawski, B.; Kim, S.W.; Carcereny Costa, E.; Park, K.; Alexandru, A.; Lupinacci, L.; de la Mora Jimenez, E.; et al. Nivolumab plus ipilimumab in advanced non–small-cell lung cancer. N. Engl. J. Med. 2019, 381, 2020–2031. [Google Scholar] [CrossRef]
- Scherpereel, A.; Mazieres, J.; Greillier, L.; Lantuejoul, S.; Dô, P.; Bylicki, O.; Monnet, I.; Corre, R.; Audigier-Valette, C.; Locatelli-Sanchez, M.; et al. Nivolumab or nivolumab plus ipilimumab in patients with relapsed malignant pleural mesothelioma (IFCT-1501 MAPS2): A multicentre, open-label, randomised, non-comparative, phase 2 trial. Lancet Oncol. 2019, 20, 239–253. [Google Scholar] [CrossRef]
- Overman, M.J.; Lonardi, S.; Wong, K.Y.M.; Lenz, H.J.; Gelsomino, F.; Aglietta, M.; Morse, M.A.; Van Cutsem, E.; McDermott, R.; Hill, A.; et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J. Clin. Oncol. 2018, 36, 773–779. [Google Scholar] [CrossRef]
- Wei, S.C.; Levine, J.H.; Cogdill, A.P.; Zhao, Y.; Anang, N.A.S.; Andrews, M.C.; Sharma, P.; Wang, J.; Wargo, J.A.; Pe’er, D.; et al. Distinct Cellular Mechanisms Underlie Anti-CTLA-4 and Anti-PD-1 Checkpoint Blockade. Cell 2017, 170, 1120–1133.e17. [Google Scholar] [CrossRef]
- Wei, S.C.; Duffy, C.R.; Allison, J.P. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018, 8, 1069–1086. [Google Scholar] [CrossRef]
- Fares, C.M.; Van Allen, E.M.; Drake, C.G.; Allison, J.P.; Hu-Lieskovan, S. Mechanisms of Resistance to Immune Checkpoint Blockade: Why Does Checkpoint Inhibitor Immuno-therapy Not Work for All Patients? Am. Soc. Clin. Oncol. Educ. Book 2019, 39, 147–164. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.R.; Bamber, J.C.; ter Haar, G.R. Physical principles of medical ultrasonics. J. Acoust. Soc. Am. 2004, 116, 2707. [Google Scholar] [CrossRef]
- Ter Haar, G.; Aubry, J.F.; Grüll, H.; Lafon, C. Ultrasound Therapy. In EFSUMB Course Book, 2nd ed.; EFSUMB: London, UK, 2021. [Google Scholar]
- Sapareto, S.A.; Dewey, W.C. Thermal dose determination in cancer therapy. Int. J. Radiat. Oncol. Biol. Phys. 1984, 10, 787–800. [Google Scholar] [CrossRef]
- Van den Tempel, N.; Horsman, M.R.; Kanaar, R. Improving efficacy of hyperthermia in oncology by exploiting biological mechanisms. Int. J. Hyperth. 2016, 32, 446–454. [Google Scholar] [CrossRef]
- Henle, K.J.; Dethlefsen, L.A. Heat fractionation and thermotolerance: A review. Cancer Res. 1978, 38, 1843–1851. [Google Scholar] [PubMed]
- Mouratidis, P.X.E.; Rivens, I.; Civale, J.; Symonds-Tayler, R.; Ter Haar, G. Relationship between thermal dose and cell death for “rapid” ablative and “slow” hyperthermic heating. Int. J. Hyperth. 2019, 36, 229–243. [Google Scholar] [CrossRef]
- Mouratidis, P.X.; Rivens, I.; Ter Haar, G. A study of thermal dose-induced autophagy, apoptosis and necroptosis in colon cancer cells. Int. J. Hyperth. 2015, 31, 476–488. [Google Scholar] [CrossRef]
- Mouratidis, P.X.; ter Haar, G. HSP90 inhibition acts synergistically with heat to induce a pro-immunogenic form of cell death in colon cancer cells. Int. J. Hyperth. 2021, 38, 1443–1456. [Google Scholar] [CrossRef]
- Dewey, W.; Hopwood, L.; Sapareto, S.; Gerweck, L. Cellular responses to combinations of hyperthermia and radiation. Radiology 1977, 123, 463–474. [Google Scholar] [CrossRef]
- Sapareto, S.A.; Raaphorst, G.P.; Dewey, W.C. Cell killing and the sequencing of hyperthermia and radiation. Int. J. Radiat. Oncol. Biol. Phys. 1979, 5, 343–347. [Google Scholar] [CrossRef]
- Streffer, C.; Van Beuningen, D. The biological basis for tumour therapy by hyperthermia and radiation. In Hyperthermia and the Therapy of Malignant Tumors; Springer: Berlin/Heidelberg, Germany, 1987; pp. 24–70. [Google Scholar]
- Horsman, M.R.; Overgaard, J. Hyperthermia: A potent enhancer of radiotherapy. Clin. Oncol. (R. Coll. Radiol.) 2007, 19, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Guthkelch, A.; Carter, L.; Cassady, J.; Hynynen, K.; Iacono, R.; Johnson, P.; Obbens, E.A.; Roemer, R.B.; Seeger, J.F.; Shimm, D.S. Treatment of malignant brain tumors with focused ultrasound hyperthermia and radiation: Results of a phase I trial. J. Neuro-Oncol. 1991, 10, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Gray, M.D.; Lyon, P.C.; Mannaris, C.; Folkes, L.K.; Stratford, M.; Campo, L.; Chung, D.Y.F.; Scott, S.; Anderson, M.; Goldin, R.; et al. Focused Ultrasound Hyperthermia for Targeted Drug Release from Thermosensitive Liposomes: Results from a Phase I Trial. Radiology 2019, 291, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Lyon, P.C.; Griffiths, L.F.; Lee, J.; Chung, D.; Carlisle, R.; Wu, F.; Middleton, M.R.; Gleeson, F.V.; Coussios, C.C. Clinical trial protocol for TARDOX: A phase I study to investigate the feasibility of targeted release of lysothermosensitive liposomal doxorubicin (ThermoDox®) using focused ultrasound in patients with liver tumours. J. Ther. Ultrasound 2017, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Warwick, R.; Pond, J. Trackless lesions in nervous tissues produced by high intensity focused ultrasound (high-frequency mechanical waves). J. Anat. 1968, 102 Pt 3, 387. [Google Scholar]
- Barnard, J.; Fry, W.; Fry, F.; Brennan, J. Small localized ultrasonic lesions in the white and gray matter of the cat brain. AMA Arch. Neurol. Psychiatry 1956, 75, 15–35. [Google Scholar] [CrossRef]
- Roberts, W.W.; Hall, T.L.; Ives, K.; Wolf, J.S., Jr.; Fowlkes, J.B.; Cain, C.A. Pulsed cavitational ultrasound: A noninvasive technology for controlled tissue ablation (histotripsy) in the rabbit kidney. J. Urol. 2006, 175, 734–738. [Google Scholar] [CrossRef]
- Xu, Z.; Ludomirsky, A.; Eun, L.Y.; Hall, T.L.; Tran, B.C.; Fowlkes, J.B.; Cain, C.A. Controlled ultrasound tissue erosion. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2004, 51, 726–736. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, X. Effect of pulse duration and pulse repetition frequency of cavitation histotripsy on erosion at the surface of soft material. Ultrasonics 2018, 84, 296–309. [Google Scholar] [CrossRef]
- Khokhlova, V.A.; Bailey, M.R.; Reed, J.A.; Cunitz, B.W.; Kaczkowski, P.J.; Crum, L.A. Effects of nonlinear propagation, cavitation, and boiling in lesion formation by high intensity focused ultrasound in a gel phantom. J. Acoust. Soc. Am. 2006, 119, 1834–1848. [Google Scholar] [CrossRef] [PubMed]
- Khokhlova, V.A.; Fowlkes, J.B.; Roberts, W.W.; Schade, G.R.; Xu, Z.; Khokhlova, T.D.; Hall, T.L.; Maxwell, A.D.; Wang, Y.-N.; Cain, C.A. Histotripsy methods in mechanical disintegration of tissue: Towards clinical applications. Int. J. Hyperth. 2015, 31, 145–162. [Google Scholar] [CrossRef] [PubMed]
- Bader, K.B.; Vlaisavljevich, E.; Maxwell, A.D. For Whom the Bubble Grows: Physical Principles of Bubble Nucleation and Dynamics in Histotripsy Ultrasound Therapy. Ultrasound Med. Biol. 2019, 45, 1056–1080. [Google Scholar] [CrossRef] [PubMed]
- Ter Haar, G.R.; Daniels, S. Evidence for ultrasonically induced cavitation in vivo. Phys. Med. Biol. 1981, 26, 1145–1149. [Google Scholar] [CrossRef] [PubMed]
- Ter Haar, G. Therapeutic applications of ultrasound. Prog. Biophys. Mol. Biol. 2007, 93, 111–129. [Google Scholar] [CrossRef]
- Rutten, S.; van den Bekerom, M.P.; Sierevelt, I.N.; Nolte, P.A. Enhancement of bone-healing by low-intensity pulsed ultrasound: A systematic review. JBJS Rev. 2016, 4, e6. [Google Scholar] [CrossRef]
- Warden, S.J.; McMeeken, J.M. Ultrasound usage and dosage in sports physiotherapy. Ultrasound Med. Biol. 2002, 28, 1075–1080. [Google Scholar] [CrossRef]
- Padilla, F.; Puts, R.; Vico, L.; Raum, K. Stimulation of bone repair with ultrasound: A review of the possible mechanic effects. Ultrasonics 2014, 54, 1125–1145. [Google Scholar] [CrossRef]
- Tsai, C.L.; Chang, W.H.; Liu, T.K. Preliminary studies of duration and intensity of ultrasonic treatments on fracture repair. Chin. J. Physiol. 1992, 35, 21–26. [Google Scholar]
- Corry, P.M.; Barlogie, B.; Tilchen, E.J.; Armour, E.P. Ultrasound-induced hyperthermia for the treatment of human superficial tumors. Int. J. Radiat. Oncol. Biol. Phys. 1982, 8, 1225–1229. [Google Scholar] [CrossRef]
- Simon, J.; Sapozhnikov, O.; Khokhlova, V.; Wang, Y.; Crum, L.; Bailey, M. Tissue atomization by high intensity focused ultrasound. IEEE Int. Ultrason. Symp. 2012, 2012, 1003–1006. [Google Scholar] [PubMed]
- Scarponi, C.; Nasorri, F.; Pavani, F.; Madonna, S.; Sestito, R.; Simonacci, M.; De Pità, O.; Cavani, A.; Albanesi, C. Low-Frequency Low-Intensity Ultrasounds Do Not Influence the Survival and Immune Functions of Cultured Keratinocytes and Dendritic Cells. J. Biomed. Biotechnol. 2009, 2009, 193260. [Google Scholar] [CrossRef]
- Wu, F.; Wang, Z.B.; Chen, W.Z.; Zou, J.Z.; Bai, J.; Zhu, H.; Li, K.Q.; Xie, F.L.; Jin Su, H.B.; Gao, G.W. Extracorporeal focused ultrasound surgery for treatment of human solid carcinomas: Early Chinese clinical experience. Ultrasound Med. Biol. 2004, 30, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Cohen, G.; Chandran, P.; Lorsung, R.M.; Aydin, O.; Tomlinson, L.E.; Rosenblatt, R.B.; Burks, S.R.; Frank, J.A. Pulsed-Focused Ultrasound Slows B16 Melanoma and 4T1 Breast Tumor Growth through Differential Tumor Microenvironmental Changes. Cancers 2021, 13, 1546. [Google Scholar] [CrossRef]
- FUSF State of the Field Report. Available online: https://www.fusfoundation.org/the-foundation/news-media/july-15-2021-focus-feature-state-of-the-field-report-2021 (accessed on 25 September 2021).
- Qu, S.; Worlikar, T.; Felsted, A.E.; Ganguly, A.; Beems, M.V.; Hubbard, R.; Pepple, A.L.; Kevelin, A.A.; Garavaglia, H.; Dib, J.; et al. Non-thermal histotripsy tumor ablation promotes abscopal immune responses that enhance cancer immunotherapy. J. Immunother. Cancer 2020, 8, e000200. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Zhu, X.Q.; Xu, Z.L.; Zhou, Q.; Zhang, J.; Wu, F. Increased infiltration of activated tumor-infiltrating lymphocytes after high intensity focused ultrasound ablation of human breast cancer. Surgery 2009, 145, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.L.; Zhu, X.Q.; Lu, P.; Zhou, Q.; Zhang, J.; Wu, F. Activation of tumor-infiltrating antigen presenting cells by high intensity focused ultrasound ablation of human breast cancer. Ultrasound Med. Biol. 2009, 35, 50–57. [Google Scholar] [CrossRef]
- Deng, J.; Zhang, Y.; Feng, J.; Wu, F. Dendritic cells loaded with ultrasound-ablated tumour induce in vivo specific antitumour immune responses. Ultrasound Med. Biol. 2010, 36, 441–448. [Google Scholar] [CrossRef]
- Mouratidis, P.X.E.; Costa, M.; Rivens, I.; Repasky, E.E.; Ter Haar, G. Pulsed focused ultrasound can improve the anti-cancer effects of immune checkpoint inhibitors in murine pancreatic cancer. J. R. Soc. Interface 2021, 18, 20210266. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Quinn, T.J.; Scandiuzzi, L.; Basu, I.; Partanen, A.; Tomé, W.A.; Macian, F.; Guha, C. Low-Intensity Focused Ultrasound Induces Reversal of Tumor-Induced T Cell Tolerance and Prevents Immune Escape. J. Immunol. 2016, 196, 1964–1976. [Google Scholar] [CrossRef]
- Adkins, I.; Sadilkova, L.; Hradilova, N.; Tomala, J.; Kovar, M.; Spisek, R. Severe, but not mild heat-shock treatment induces immunogenic cell death in cancer cells. Oncoimmunology 2017, 6, e1311433. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.Z.; Xie, F.L.; Ran, L.F.; Xie, X.P.; Fan, Y.M.; Wu, F. High-intensity focused ultrasound tumor ablation activates autologous tumor-specific cytotoxic T lymphocytes. Ultrasound Med. Biol. 2012, 38, 1363–1371. [Google Scholar] [CrossRef] [PubMed]
- Chavez, M.; Silvestrini, M.T.; Ingham, E.S.; Fite, B.Z.; Mahakian, L.M.; Tam, S.M.; Ilovitsh, A.; Monjazeb, A.M.; Murphy, W.J.; Hubbard, N.E.; et al. Distinct immune signatures in directly treated and distant tumors result from TLR adjuvants and focal ablation. Theranostics 2018, 8, 3611–3628. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Yang, X.Y.; Liu, Y.; Sankin, G.N.; Pua, E.C.; Morse, M.A.; Lyerly, H.K.; Clay, T.M.; Zhong, P. Investigation of HIFU-induced anti-tumor immunity in a murine tumor model. J. Transl. Med. 2007, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yuan, F.; Liang, M.; Lo, H.W.; Shinohara, M.L.; Robertson, C.; Zhong, P. M-HIFU inhibits tumor growth, suppresses STAT3 activity and enhances tumor specific immunity in a transplant tumor model of prostate cancer. PLoS ONE 2012, 7, e41632. [Google Scholar]
- Aydin, O.; Chandran, P.; Lorsung, R.R.; Cohen, G.; Burks, S.R.; Frank, J.A. The proteomic effects of Pulsed Focused Ultrasound on Tumor Microenvironments of Murine Melanoma and Breast Cancer Models. Ultrasound Med. Biol. 2019, 45, 3232–3245. [Google Scholar] [CrossRef]
- Rosenblatt, R.B.; Frank, J.A.; Burks, S.R. Cytosolic Ca2+ transients during pulsed focused ultrasound generate reactive oxygen species and cause DNA damage in tumor cells. Theranostics 2021, 11, 602–613. [Google Scholar] [CrossRef]
- Cohen, G.; Chandran, P.; Lorsung, R.M.; Tomlinson, L.E.; Sundby, M.; Burks, S.R.; Frank, J.A. The Impact of Focused Ultrasound in Two Tumor Models: Temporal Alterations in the Natural History on Tumor Microenvironment and Immune Cell Response. Cancers 2020, 12, 350. [Google Scholar] [CrossRef]
- Pahk, K.J.; Shin, C.H.; Bae, I.Y.; Yang, Y.; Kim, S.H.; Pahk, K.; Kim, H.; Oh, S.J. Boiling histotripsy-induced partial mechanical ablation modulates tumour microenvironment by promoting immunogenic cell death of cancers. Sci. Rep. 2019, 9, 9050. [Google Scholar] [CrossRef]
- Eranki, A.; Srinivasan, P.; Ries, M.; Kim, A.; Lazarski, C.A.; Rossi, C.T.; Khokhlova, T.D.; Wilson, E.; Knoblach, S.M.; Sharma, K.V.; et al. High-Intensity Focused Ultrasound (HIFU) Triggers Immune Sensitization of Refractory Murine Neuroblastoma to Checkpoint Inhibitor Therapy. Clin. Cancer Res. 2020, 26, 1152–1161. [Google Scholar] [CrossRef]
- Schade, G.R.; Wang, Y.N.; D’Andrea, S.; Hwang, J.H.; Liles, W.C.; Khokhlova, T.D. Boiling Histotripsy Ablation of Renal Cell Carcinoma in the Eker Rat Promotes a Systemic Inflammatory Response. Ultrasound Med. Biol. 2019, 45, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.L.; Hsieh, H.Y.; Lu, L.A.; Kang, C.W.; Wu, M.F.; Lin, C.Y. Low-pressure pulsed focused ultrasound with microbubbles promotes an anticancer immunological response. J. Transl. Med. 2012, 10, 221. [Google Scholar] [CrossRef]
- Kovacs, Z.I.; Burks, S.R.; Frank, J.A. Focused ultrasound with microbubbles induces sterile inflammatory response proportional to the blood brain barrier opening: Attention to experimental conditions. Theranostics 2018, 8, 2245–2248. [Google Scholar] [CrossRef] [PubMed]
- McMahon, D.; Hynynen, K. Acute Inflammatory Response Following Increased Blood-Brain Barrier Permeability Induced by Focused Ultrasound is Dependent on Microbubble Dose. Theranostics 2017, 7, 3989–4000. [Google Scholar] [CrossRef] [PubMed]
- Fite, B.Z.; Wang, J.; Kare, A.J.; Ilovitsh, A.; Chavez, M.; Ilovitsh, T.; Zhang, N.; Chen, W.; Robinson, E.; Zhang, H.; et al. Immune modulation resulting from MR-guided high intensity focused ultrasound in a model of murine breast cancer. Sci. Rep. 2021, 11, 927. [Google Scholar] [CrossRef]
- Schuster, T.G.; Wei, J.T.; Hendlin, K.; Jahnke, R.; Roberts, W.W. Histotripsy treatment of benign prostatic enlargement using the Vortx Rx system: Initial human safety and efficacy outcomes. Urology 2018, 114, 184–187. [Google Scholar] [CrossRef]
- Wang, X.; Sun, J. High-intensity focused ultrasound in patients with late-stage pancreatic carcinoma. Chin. Med. J. 2002, 115, 1332–1335. [Google Scholar]
- Wu, F.; Wang, Z.B.; Zhu, H.; Chen, W.Z.; Zou, J.Z.; Bai, J.; Li, K.Q.; Jin, C.B.; Xie, F.L.; Su, H.B. Feasibility of US-guided high-intensity focused ultrasound treatment in patients with advanced pancreatic cancer: Initial experience. Radiology 2005, 236, 1034–1040. [Google Scholar] [CrossRef]
- Xiong, L.L.; Hwang, J.H.; Huang, X.B.; Yao, S.S.; He, C.J.; Ge, X.H.; Ge, H.Y.; Wang, X.F. Early clinical experience using high intensity focused ultrasound for palliation of inoperable pancreatic cancer. JOP 2009, 10, 123–129. [Google Scholar]
- Wang, K.; Chen, Z.; Meng, Z.; Lin, J.; Zhou, Z.; Wang, P.; Chen, L.; Liu, L. Analgesic effect of high intensity focused ultrasound therapy for unresectable pancreatic cancer. Int. J. Hyperth. 2011, 27, 101–107. [Google Scholar] [CrossRef]
- Sung, H.Y.; Jung, S.E.; Cho, S.H.; Zhou, K.; Han, J.Y.; Han, S.T.; Kim, J.I.; Kim, J.K.; Choi, J.Y.; Yoon, S.K.; et al. Long-term outcome of high-intensity focused ultrasound in advanced pancreatic cancer. Pancreas 2011, 40, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.F.; Wang, K.; Meng, Z.Q.; Chen, Z.; Lin, J.H.; Zhou, Z.H.; Wang, P.; Shi, W.D.; Sheng, Y.H. High intensity focused ultrasound treatment for patients with local advanced pancreatic cancer. Hepatogastroenterology 2013, 60, 1906–1910. [Google Scholar]
- Ning, Z.Y.; Cheng, C.S.; Xie, J.; Chen, Q.W.; Xu, L.T.; Zhuang, L.P.; Zhang, C.Y.; Song, L.B.; Shi, W.D.; Zhu, X.Y.; et al. A retrospective analysis of survival factors of high intensity focused ultrasound (HIFU) treatment for unresectable pancreatic cancer. Discov. Med. 2016, 21, 435–445. [Google Scholar] [PubMed]
- Wang, G.; Zhou, D. Preoperative ultrasound ablation for borderline resectable pancreatic cancer: A report of 30 cases. Ultrason. Sonochem. 2015, 27, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Orsi, F.; Zhang, L.; Arnone, P.; Orgera, G.; Bonomo, G.; Della Vigna, P.; Monfardini, L.; Zhou, K.; Chen, W.; Wang, Z.; et al. High-intensity focused ultrasound ablation: Effective and safe therapy for solid tumors in difficult locations. AJR Am. J. Roentgenol. 2010, 195, W245–W252. [Google Scholar] [CrossRef] [PubMed]
- Anzidei, M.; Marincola, B.C.; Bezzi, M.; Brachetti, G.; Nudo, F.; Cortesi, E.; Berloco, P.; Catalano, C.; Napoli, A. Magnetic resonance-guided high-intensity focused ultrasound treatment of locally advanced pancreatic adenocarcinoma: Preliminary experience for pain palliation and local tumor control. Invest. Radiol. 2014, 49, 759–765. [Google Scholar] [CrossRef]
- Marinova, M.; Huxold, H.C.; Henseler, J.; Mücke, M.; Conrad, R.; Rolke, R.; Ahmadzadehfar, H.; Rauch, M.; Fimmers, R.; Luechters, G.; et al. Clinical Effectiveness and Potential Survival Benefit of US-Guided High-Intensity Focused Ultrasound Therapy in Patients with Advanced-Stage Pancreatic Cancer. Ultraschall Med. 2019, 40, 625–637. [Google Scholar] [CrossRef]
- Marinova, M.; Feradova, H.; Gonzalez-Carmona, M.A.; Conrad, R.; Tonguc, T.; Thudium, M.; Becher, M.U.; Kun, Z.; Gorchev, G.; Tomov, S.; et al. Improving quality of life in pancreatic cancer patients following high-intensity focused ultrasound (HIFU) in two European centers. Eur. Radiol. 2021, 31, 5818–5829. [Google Scholar] [CrossRef]
- Ning, Z.; Xie, J.; Chen, Q.; Zhang, C.; Xu, L.; Song, L.; Meng, Z. HIFU is safe, effective, and feasible in pancreatic cancer patients: A monocentric retrospective study among 523 patients. Onco Targets Ther. 2019, 12, 1021–1029. [Google Scholar] [CrossRef]
- Zhao, H.; Yang, G.; Wang, D.; Yu, X.; Zhang, Y.; Zhu, J.; Ji, Y.; Zhong, B.; Zhao, W.; Yang, Z.; et al. Concurrent gemcitabine and high-intensity focused ultrasound therapy in patients with locally advanced pancreatic cancer. Anticancer Drugs 2010, 21, 447–452. [Google Scholar] [CrossRef]
- Xie, D.R.; Chen, D.; Teng, H. A multicenter non-randomized clinical study of high intensity focused ultrasound in treating patients with local advanced pancreatic carcinoma. Chin. J. Clin. Oncol. 2003, 30, 630–634. [Google Scholar]
- Tao, S.F.; Gu, W.H.; Gu, J.C.; Zhu, M.L.; Wang, Q.; Zheng, L.Z. A Retrospective Case Series of High-Intensity Focused Ultrasound (HIFU) in Combination with Gemcitabine and Oxaliplatin (Gemox) on Treating Elderly Middle and Advanced Pancreatic Cancer. Onco Targets Ther. 2019, 12, 9735–9745. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Yan, T.; Wang, G.; Zhao, W.; Zhang, T.; Zhou, D. High-intensity focused ultrasound therapy in combination with gemcitabine for unresectable pancreatic carcinoma. Ther. Clin. Risk Manag. 2016, 12, 687–691. [Google Scholar] [PubMed]
- Li, Y.J.; Huang, G.L.; Sun, X.L.; Zhao, X.C.; Li, Z.G. The combination therapy of high-intensity focused ultrasound with radiotherapy in locally advanced pancreatic carcinoma. World J. Surg. Oncol. 2016, 14, 60. [Google Scholar] [CrossRef]
- Sofuni, A.; Moriyasu, F.; Sano, T.; Itokawa, F.; Tsuchiya, T.; Kurihara, T.; Ishii, K.; Tsuji, S.; Ikeuchi, N.; Tanaka, R.; et al. Safety trial of high-intensity focused ultrasound therapy for pancreatic cancer. World J. Gastroenterol. 2014, 20, 9570–9577. [Google Scholar] [CrossRef]
- Vidal-Jove, J.; Perich, E.; Del Castillo, M.A. Ultrasound Guided High Intensity Focused Ultrasound for malignant tumors: The Spanish experience of survival advantage in stage III and IV pancreatic cancer. Ultrason. Sonochem. 2015, 27, 703–706. [Google Scholar] [CrossRef]
- Marinova, M.; Rauch, M.; Mücke, M.; Rolke, R.; Gonzalez-Carmona, M.A.; Henseler, J.; Cuhls, H.; Radbruch, L.; Strassburg, C.P.; Zhang, L.; et al. High-intensity focused ultrasound (HIFU) for pancreatic carcinoma: Evaluation of feasibility, reduction of tumour volume and pain intensity. Eur. Radiol. 2016, 26, 4047–4056. [Google Scholar] [CrossRef] [PubMed]
- Strunk, H.M.; Henseler, J.; Rauch, M.; Mücke, M.; Kukuk, G.; Cuhls, H.; Radbruch, L.; Zhang, L.; Schild, H.H.; Marinova, M. Clinical Use of High-Intensity Focused Ultrasound (HIFU) for Tumor and Pain Reduction in Advanced Pancreatic Cancer. Rofo 2016, 188, 662–670. [Google Scholar] [CrossRef]
- Lau, P.C.P.; Zheng, S.F.; Ng, W.T.; Yu, S.C.H. Inoperable pancreatic adenocarcinoma rendered complete remission by high-intensity focused ultrasound concurrent with gemcitabine-capecitabine chemotherapy: Case report and topic review. J. Dig. Dis. 2012, 13, 60–64. [Google Scholar] [CrossRef]
- Dimitrov, D.; Stanislavova, N.; Yotsov, T.; Zhou, K. Recurrent pancreatic cancer patient treated by chemotherapy and focused ultrasound surgery. A case report. Med. Ultrason. 2020, 22, 247–249. [Google Scholar] [CrossRef]
- Sofuni, A.; Fujita, M.; Asai, Y.; Tsuchiya, T.; Ishii, K.; Tanaka, R.; Tonozuka, R.; Honjo, M.; Mukai, S.; Nagai, K.; et al. A case of unresectable pancreatic cancer with long-term survival in High-Intensity Focused Ultrasound (HIFU) therapy. Ultrasound Int. Open 2019, 5, E89–E92. [Google Scholar] [CrossRef]
- Jung, S.E.; Cho, S.H.; Jang, J.H.; Han, J.Y. High-intensity focused ultrasound ablation in hepatic and pancreatic cancer: Complications. Abdom. Imaging 2011, 36, 185–195. [Google Scholar] [CrossRef]
- Ji, Y.; Zhang, Y.; Zhu, J.; Zhu, L.; Zhu, Y.; Hu, K.; Zhao, H. Response of patients with locally advanced pancreatic adenocarcinoma to high-intensity focused ultrasound treatment: A single-center, prospective, case series in China. Cancer Manag. Res. 2018, 10, 4439–4446. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Li, J.; Diao, L.; Ma, K.; Fan, Y.; Yang, W. High-intensity focused ultrasound ablation for advanced pancreatic cancer. J Cancer Res. Ther. 2019, 15, 831–835. [Google Scholar]
- Wang, K.; Zhu, H.; Meng, Z.; Chen, Z.; Lin, J.; Shen, Y.; Gao, H. Safety evaluation of high-intensity focused ultrasound in patients with pancreatic cancer. Onkologie 2013, 36, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Thudium, M.; Bette, B.; Tonguc, T.; Ghaei, S.; Conrad, R.; Becher, M.U.; Mücke, M.; Luechters, G.; Strunk, H.; Marinova, M. Multidisciplinary management and outcome in pancreatic cancer patients treated with high-intensity focused ultrasound. Int. J. Hyperth. 2020, 37, 456–462. [Google Scholar] [CrossRef]
- Shi, Y.; Ying, X.; Hu, X.; Zhao, J.; Fang, X.; Wu, M.; Chen, T.Z.; Shen, H. Influence of high intensity focused ultrasound (HIFU) treatment to the pancreatic function in pancreatic cancer patients. Pak. J. Pharm. Sci. 2015, 28, 1097–1100. [Google Scholar] [PubMed]
- Guo, X.; Zhu, H.; Zhou, K.; Jin, C.; Yang, Y.; Zhang, J.; Yang, W.; Ran, L.; Dimitrov, D. Effects of high-intensity focused ultrasound treatment on peripancreatic arterial and venous blood vessels in pancreatic cancer. Oncol. Lett. 2020, 19, 3839–3850. [Google Scholar] [CrossRef]
- Strunk, H.M.; Lützow, C.; Henseler, J.; Mücke, M.; Rauch, M.; Marx, C.; Schild, H.H.; Marinova, M. Mesenteric Vessel Patency Following HIFU Therapy in Patients with Locally Invasive Pancreatic Cancer. Ultraschall Med. 2018, 39, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhao, F.; Shi, Y.; Deng, Y.; Hu, X.; Shen, H. The efficacy of a new high intensity focused ultrasound therapy for locally advanced pancreatic cancer. J. Cancer Res. Clin. Oncol. 2017, 143, 2105–2111. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Shen, H.; Hu, X.; Wang, Y.; Yuan, Y. The efficacy of a new high-intensity focused ultrasound therapy for metastatic pancreatic cancer. Int. J. Hyperth. 2021, 38, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Yao, Y.; Zhu, X.; Gao, Y.; Chen, Y.; Wang, R.; Xu, P.; Wei, X.; Jiang, L. In Vivo Flow Cytometric Evaluation of Circulating Metastatic Pancreatic Tumor Cells after High-Intensity Focused Ultrasound Therapy. Cytom. A 2020, 97, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, C.J.; Bartels, L.W.; van Stralen, M.; de Senneville, B.D.; Moonen, C.T.W.; Bos, C. Fluid filling of the digestive tract for improved proton resonance frequency shift-based MR thermometry in the pancreas. J. Magn. Reson. Imaging 2018, 47, 692–701. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, C.J.; Bos, C.; de Senneville, B.D.; Borman, P.; Stemkens, B.; Tijssen, R.; Moonen, C.; Bartels, L. A planning strategy for combined motion-assisted/gated MR guided focused ultrasound treatment of the pancreas. Int. J. Hyperth. 2019, 36, 702–711. [Google Scholar]
- Diodato, A.; Cafarelli, A.; Schiappacasse, A.; Tognarelli, S.; Ciuti, G.; Menciassi, A. Motion compensation with skin contact control for high intensity focused ultrasound surgery in moving organs. Phys. Med. Biol. 2018, 63, 035017. [Google Scholar] [CrossRef]
- Celicanin, Z.; Auboiroux, V.; Bieri, O.; Petrusca, L.; Santini, F.; Viallon, M.; Scheffler, K.; Salomir, R. Real-time method for motion-compensated MR thermometry and MRgHIFU treatment in abdominal organs. Magn. Reson. Med. 2014, 72, 1087–1095. [Google Scholar] [CrossRef]
- Celicanin, Z.; Manasseh, G.; Petrusca, L.; Scheffler, K.; Auboiroux, V.; Crowe, L.A.; Hyacinthe, J.N.; Natsuaki, Y.; Santini, F.; Becker, C.D.; et al. Hybrid ultrasound-MR guided HIFU treatment method with 3D motion compensation. Magn. Reson. Med. 2018, 79, 2511–2523. [Google Scholar] [CrossRef]
- Lee, J.Y.; Choi, B.I.; Ryu, J.K.; Kim, Y.T.; Hwang, J.H.; Kim, S.H.; Han, J.K. Concurrent chemotherapy and pulsed high-intensity focused ultrasound therapy for the treatment of unresectable pancreatic cancer: Initial experiences. Korean J. Radiol. 2011, 12, 176–186. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, H.; Kim, Y.J.; Lee, J.Y.; Han, J.K.; Choi, B.I. Dynamic contrast-enhanced ultrasonographic (DCE-US) assessment of the early response after combined gemcitabine and HIFU with low-power treatment for the mouse xenograft model of human pancreatic cancer. Eur. Radiol. 2014, 24, 2059–2068. [Google Scholar] [CrossRef]
- Li, T.; Wang, Y.N.; Khokhlova, T.D.; D’Andrea, S.; Starr, F.; Chen, H.; McCune, J.S.; Risler, L.J.; Mashadi-Hossein, A.; Hingorani, S.R.; et al. Pulsed High-Intensity Focused Ultrasound Enhances Delivery of Doxorubicin in a Preclinical Model of Pancreatic Cancer. Cancer Res. 2015, 75, 3738–3746. [Google Scholar] [CrossRef]
- Farr, N.; Wang, Y.N.; D’ Andrea, S.; Starr, F.; Partanen, A.; Gravelle, K.M.; McCune, J.S.; Risler, L.J.; Whang, S.G.; Chang, A.; et al. Hyperthermia-enhanced targeted drug delivery using magnetic resonance-guided focussed ultrasound: A pre-clinical study in a genetic model of pancreatic cancer. Int. J. Hyperth. 2018, 34, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Kotopoulis, S.; Delalande, A.; Popa, M.; Mamaeva, V.; Dimcevski, G.; Gilja, O.H.; Postema, M.; Gjertsen, B.T.; McCormack, E. Sonoporation-enhanced chemotherapy significantly reduces primary tumour burden in an orthotopic pancreatic cancer xenograft. Mol. Imaging Biol. 2014, 16, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Huang, P.; Jiang, C.L.; Jia, D.X.; Du, X.X.; Zhou, J.H.; Han, Y.; Sui, H.; Wei, X.L.; Liu, L.; et al. Sonodynamically induced anti-tumor effect of 5-aminolevulinic acid on pancreatic cancer cells. Ultrasound Med. Biol. 2014, 40, 2671–2679. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Xu, H.; Liu, Q.; Liu, C.; Hu, J.; Liu, P.; Fang, T.; Bai, Y.; Zhu, J.; Xie, R. 5-Aminolevulinic acid hydrochloride loaded microbubbles-mediated sonodynamic therapy in pancreatic cancer cells. Artif. Cells Nanomed. Biotechnol. 2020, 48, 1178–1188. [Google Scholar] [CrossRef]
- McEwan, C.; Kamila, S.; Owen, J.; Nesbitt, H.; Callan, B.; Borden, M.; Nomikou, N.; Hamoudi, R.A.; Taylor, M.A.; Stride, E.; et al. Combined sonodynamic and antimetabolite therapy for the improved treatment of pancreatic cancer using oxygen loaded microbubbles as a delivery vehicle. Biomaterials 2016, 80, 20–32. [Google Scholar] [CrossRef]
- Sheng, Y.; Beguin, E.; Nesbitt, H.; Kamila, S.; Owen, J.; Barnsley, L.C.; Callan, B.; O’Kane, C.; Nomikou, N.; Hamoudi, R.; et al. Magnetically responsive microbubbles as delivery vehicles for targeted sonodynamic and antimetabolite therapy of pancreatic cancer. J. Control. Release 2017, 262, 192–200. [Google Scholar] [CrossRef]
- Prescott, M.; Mitchell, J.; Totti, S.; Lee, J.; Velliou, E.; Bussemaker, M. Sonodynamic therapy combined with novel anti-cancer agents, sanguinarine and ginger root extract: Synergistic increase in toxicity in the presence of PANC-1 cells in vitro. Ultrason Sonochem. 2018, 40 Pt B, 72–80. [Google Scholar] [CrossRef]
- Nesbitt, H.; Sheng, Y.; Kamila, S.; Logan, K.; Thomas, K.; Callan, B.; Taylor, M.A.; Love, M.; O’Rourke, D.; Kelly, P.; et al. Gemcitabine loaded microbubbles for targeted chemo-sonodynamic therapy of pancreatic cancer. J. Control. Release 2018, 279, 8–16. [Google Scholar] [CrossRef]
- Cao, J.; Sun, Y.; Zhang, C.; Wang, X.; Zeng, Y.; Zhang, T.; Huang, P. Tablet-like TiO 2/C nanocomposites for repeated type I sonodynamic therapy of pancreatic cancer. Acta Biomater. 2021, 129, 269–279. [Google Scholar] [CrossRef]
- Zhang, K.; Xu, H.; Jia, X.; Chen, Y.; Ma, M.; Sun, L.; Chen, H. Ultrasound-Triggered Nitric Oxide Release Platform Based on Energy Transformation for Targeted Inhibition of Pancreatic Tumor. ACS Nano 2016, 10, 10816–10828. [Google Scholar] [CrossRef]
- Sun, Y.; Cao, J.; Wang, X.; Zhang, C.; Luo, J.; Zeng, Y.; Zhang, C.; Li, Q.; Zhang, Y.; Xu, W.; et al. Hypoxia-Adapted Sono-chemodynamic Treatment of Orthotopic Pancreatic Carcinoma Using Copper Metal-Organic Frameworks Loaded with an Ultrasound-Induced Free Radical Initiator. ACS Appl. Mater. Interfaces 2021, 13, 38114–38126. [Google Scholar] [CrossRef]
- Browning, R.J.; Able, S.; Ruan, J.L.; Bau, L.; Allen, P.D.; Kersemans, V.; Wallington, S.; Kinchesh, P.; Smart, S.; Karsonaki, C.; et al. Combining sonodynamic therapy with chemoradiation for the treatment of pancreatic cancer. J. Control. Release 2021, 337, 371–377. [Google Scholar] [CrossRef]
- Maeda, M.; Muragaki, Y.; Okamoto, J.; Yoshizawa, S.; Abe, N.; Nakamoto, H.; Ishii, H.; Kawabata, K.; Kawabata, K.; Umemura, S.; et al. Sonodynamic Therapy Based on Combined Use of Low Dose Administration of Epirubicin-Incorporating Drug Delivery System and Focused Ultrasound. Ultrasound Med. Biol. 2017, 43, 2295–2301. [Google Scholar] [CrossRef]
- Royal, R.; Levy, C.; Turner, K.; Mathur, A.; Hughes, M.; Kammula, U.; Sherry, R.; Topalian, S.; Yang, J.; Lowy, I.; et al. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J. Immunother. 2010, 33, 828–833. [Google Scholar] [CrossRef]
- Aglietta, M.; Barone, C.; Sawyer, M.B.; Moore, M.J.; Miller, W.H., Jr.; Bagala, C.; Colombi, F.; Cagnazzo, C.; Gioeni, L.; Wang, E.; et al. A phase I dose escalation trial of tremelimumab (CP-675,206) in combination with gemcitabine in chemotherapy-naive patients with metastatic pancreatic cancer. Ann. Oncol. 2014, 25, 1750–1755. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Tykodi, S.S.; Chow, L.Q.M.; Hwu, W.J.; Topalian, S.L.; Hwu, P.; Drake, C.G.; Camacho, L.H.; Kauh, J.; Odunsi, K.; et al. Safety and activity of anti–PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012, 366, 2455–2465. [Google Scholar] [CrossRef]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.-P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.; et al. Efficacy of Pembrolizumab in Patients with Non-colorectal High Microsatellite Instability/Mismatch Repair–Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef]
- O’Reilly, E.M.; Oh, D.Y.; Dhani, N.; Renouf, D.J.; Lee, M.A.; Sun, W.; Fisher, G.; Hezel, A.; Chang, S.-C.; Vlahovic, G.; et al. Durvalumab with or Without Tremelimumab for Patients With Metastatic Pancreatic Ductal Adenocarcinoma. JAMA Oncol. 2019, 5, 1431–1438. [Google Scholar] [CrossRef]
- Weiss, G.J.; Waypa, J.; Blaydorn, L.; Coats, J.; McGahey, K.; Sangal, A.; Niu, J.; Lynch, C.A.; Farley, J.H.; Khemka, V. A phase Ib study of pembrolizumab plus chemotherapy in patients with advanced cancer (PembroPlus). Br. J. Cancer 2017, 117, 33–40. [Google Scholar] [CrossRef]
- Weiss, G.J.; Blaydorn, L.; Beck, J.; Bornemann-Kolatzki, K.; Urnovitz, H.; Schütz, E.; Khemka, V. Phase Ib/II study of gemcitabine, nab-paclitaxel, and pembrolizumab in metastatic pancreatic adenocarcinoma. Investig. New Drugs 2017, 36, 96–102. [Google Scholar] [CrossRef]
- Bockorny, B.; Semenisty, V.; Macarulla, T.; Borazanci, E.; Wolpin, B.M.; Stemmer, S.M.; Golan, T.; Geva, R.; Borad, M.J.; Pedersen, K.S.; et al. BL-8040, a CXCR4 antagonist, in combination with pembrolizumab and chemotherapy for pancreatic cancer: The COMBAT trial. Nat. Med. 2020, 26, 878–885. [Google Scholar] [CrossRef]
- Doi, T.; Muro, K.; Ishii, H.; Kato, T.; Tsushima, T.; Takenoyama, M.; Oizumi, S.; Gemmoto, K.; Suna, H.; Enokitani, K.; et al. A Phase I Study of the Anti-CC Chemokine Receptor 4 Antibody, Mogamulizumab, in Combination with Nivolumab in Patients with Advanced or Metastatic Solid Tumors. Clin. Cancer Res. 2019, 25, 6614–6622. [Google Scholar] [CrossRef]
- Hong, D.; Rasco, D.; Veeder, M.; Luke, J.J.; Chandler, J.; Balmanoukian, A.; George, T.; Munster, P.; Berlin, J.D.; Gutierrez, M.; et al. A Phase 1b/2 Study of the Bruton Tyrosine Kinase Inhibitor Ibrutinib and the PD-L1 Inhibitor Durvalumab in Patients with Pretreated Solid Tumors. Oncology 2019, 97, 102–111. [Google Scholar] [CrossRef]
- Le, D.T.; Lutz, E.; Uram, J.N.; Sugar, E.A.; Onners, B.; Solt, S.; Zheng, L.; Diaz, L.; Donehower, R.C.; Jaffee, E.; et al. Evaluation of Ipilimumab in Combination with Allogeneic Pancreatic Tumor Cells Transfected with a GM-CSF Gene in Previously Treated Pancreatic Cancer. J. Immunother. 2013, 36, 382–389. [Google Scholar] [CrossRef]
- Mohindra, N.A.; Kircher, S.M.; Nimeiri, H.S.; Benson, A.B.; Rademaker, A.; Alonso, E.; Blatner, N.; Khazaie, K.; Mulcahy, M.F. Results of the phase Ib study of ipilimumab and gemcitabine for advanced pancreas cancer. J. Clin. Oncol. 2015, 33, e15281. [Google Scholar] [CrossRef]
- Kalyan, A.; Kircher, S.M.; Mohindra, N.A.; Nimeiri, H.S.; Maurer, V.; Rademaker, A.; Benson, A.B.; Mulcahy, M.F. Ipilimumab and gemcitabine for advanced pancreas cancer: A phase Ib study. J. Clin. Oncol. 2016, 34, e15747. [Google Scholar] [CrossRef]
- Kamath, S.D.; Kalyan, A.; Kircher, S.; Nimeiri, H.; Fought, A.J.; Benson, A.; Mulcahy, M. Ipilimumab and Gemcitabine for Advanced Pancreatic Cancer: A Phase Ib Study. Oncology 2019, 25, e808–e815. [Google Scholar] [CrossRef]
- Dimitrov, D.; Andreev, T.; Feradova, H.; Ignatov, B.; Zhou, K.; Johnson, C.; Delijski, T.; Gortchev, G.; Tomov, S. Multimodality treatment by FOLFOX plus HIFU in a case of advanced pancreatic carcinoma. A case report. JOP 2015, 16, 66–69. [Google Scholar]
- Ungaro, A.; Orsi, F.; Casadio, C.; Galdy, S.; Spada, F.; Cella, C.A.; Tonno, C.D.; Bonomo, G.; Vigna, P.D.; Murgioni, S.; et al. Successful palliative approach with high-intensity focused ultrasound in a patient with metastatic anaplastic pancreatic carcinoma: A case report. Ecancermedicalscience 2016, 10, 635. [Google Scholar] [CrossRef][Green Version]
- Tonguc, T.; Strunk, H.; Gonzalez-Carmona, M.A.; Recker, F.; Lütjohann, D.; Thudium, M.; Conrad, R.; Becher, M.U.; Savchenko, O.; Davidova, D.; et al. US-guided high-intensity focused ultrasound (HIFU) of abdominal tumors: Outcome, early ablation-related laboratory changes and inflammatory reaction. A single-center experience from Germany. Int. J. Hyperth. 2021, 38, 65–74. [Google Scholar] [CrossRef]
- Hendricks-Wenger, A.; Sereno, J.; Gannon, J.; Zeher, A.; Brock, R.M.; Beitel-White, N.; Simon, A.; Davalos, R.V.; Coutermarsh-Ott, S.; Vlaisavljevich, E.; et al. Histotripsy Ablation Alters the Tumor Microenvironment and Promotes Immune System Activation in a Subcutaneous Model of Pancreatic Cancer. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2021, 68, 2987–3000. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, D.; Nesbitt, H.; Farrell, S.; Logan, K.; McMullin, E.; Gillan, T.; Kelly, P.; O’Rourke, D.; Porter, S.; Thomas, K.; et al. Exploiting a Rose Bengal-bearing, oxygen-producing nanoparticle for SDT and associated immune-mediated therapeutic effects in the treatment of pancreatic cancer. Eur. J. Pharm. Biopharm. 2021, 163, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Nesbitt, H.; Logan, K.; Thomas, K.; Callan, B.; Gao, J.; McKaig, T.; Taylor, M.; Love, M.; Stride, E.; McHale, A.P.; et al. Sonodynamic therapy complements PD-L1 immune checkpoint inhibition in a murine model of pancreatic cancer. Cancer Lett. 2021, 517, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Humphris, J.L.; Patch, A.M.; Nones, K.; Bailey, P.J.; Johns, A.L.; McKay, S.; Chang, D.K.; Miller, D.K.; Pajic, M.; Kassahn, K.S.; et al. Hypermutation in pancreatic cancer. Gastroenterology 2017, 152, 68–74.e2. [Google Scholar] [CrossRef] [PubMed]
- Middleton, G.; Greenhalf, W.; Costello, E.; Shaw, V.; Cox, T.; Ghaneh, P.; Palmer, D.H.; Neoptolemos, J.P. Immunobiological effects of gemcitabine and capecitabine combination chemotherapy in advanced pancreatic ductal adenocarcinoma. Br. J. Cancer 2016, 114, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Middleton, G.; Silcocks, P.; Cox, T.; Valle, J.; Wadsley, J.; Propper, D.; Coxon, F.; Ross, P.; Madhusudan, S.; Roques, T.; et al. Gemcitabine and capecitabine with or without telomerase peptide vaccine GV1001 in patients with locally advanced or metastatic pancreatic cancer (TeloVac): An open-label, randomised, phase 3 trial. Lancet Oncol. 2014, 15, 829–840. [Google Scholar] [CrossRef]
- Seifert, L.; Werba, G.; Tiwari, S.; Ly, N.N.G.; Nguy, S.; Alothman, S.; Alqunaibit, D.; Avanzi, A.; Daley, D.; Barilla, R.; et al. Radiation therapy induces macrophages to suppress T cell responses against pancreatic tumors in mice. Gastroenterology 2016, 150, 1659–1672.e5. [Google Scholar] [CrossRef]
- Pihlak, R.; Weaver, J.M.J.; Valle, J.W.; McNamara, M.G. Advances in Molecular Profiling and Categorisation of Pancreatic Adenocarcinoma and the Implications for Therapy. Cancers 2018, 10, 17. [Google Scholar] [CrossRef]
- Timmer, F.E.F.; Geboers, B.; Nieuwenhuizen, S.; Dijkstra, M.; Schouten, E.A.C.; Puijk, R.S.; de Vries, J.J.J.; van den Tol, M.P.; Bruynzeel, A.M.E.; Streppel, M.M.; et al. Pancreatic Cancer and Immunotherapy: A Clinical Overview. Cancers 2021, 13, 4138. [Google Scholar] [CrossRef]
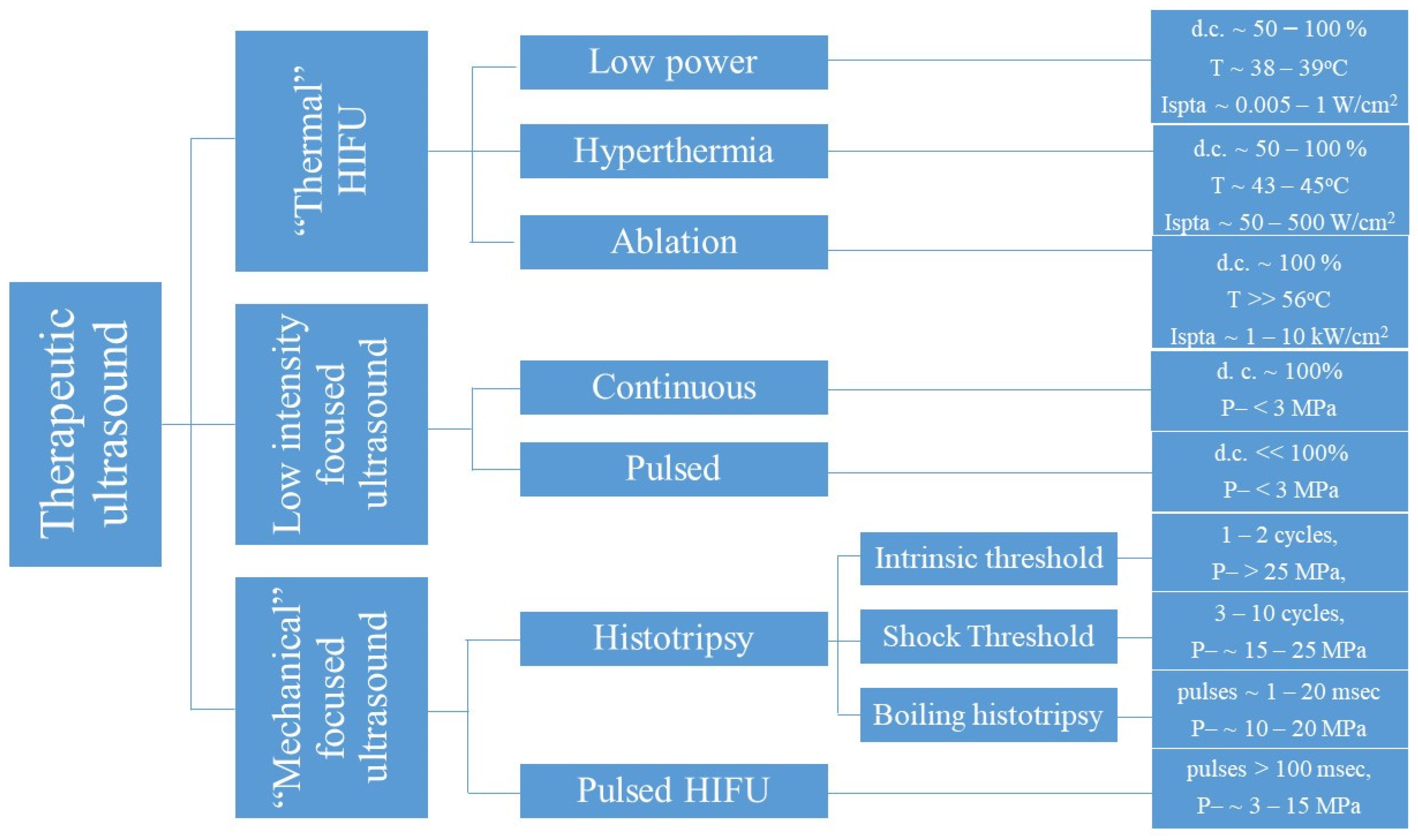
| Treatment Type | Frequency | Treatment Parameters (Intensity) | Therapeutic Intent | Translational Stage | Ref. |
|---|---|---|---|---|---|
| Low intensity focused ultrasound—continuous exposures | 1.5 MHz | 0.5 W/cm2 for 5, 10 and 25 min /day for 25 days | Fracture repair | In vivo preclinical | [101] |
| Thermal (Hyperthermia) | 1–3 MHz | 0.3–2 W/cm2 until temperature reached 45 °C | Tumour sensitisation in combination with radiotherapy/chemotherapy | Clinical | [102] |
| Thermal (ablation) | 0.8 MHz | 5 kW/ cm2 | Tumour ablation | Clinical | [105] |
| Mechanical (pHIFU) | 1.15 MHz | 2.7 kW/cm2 spatial average temporal peak | Tumour ablation | In vivo preclinical | [106] |
| Mechanical (Histotripsy) | 2 MHz | 14 kW/cm2 | Tissue atomisation | In situ preclinical | [103] |
| Low intensity focused ultrasound—pulsed exposures | 42 kHz | 0.15 W/cm2 | Cell proliferation | In vitro preclinical | [104] |
| Therapeutic Focused Ultrasound | Study and Tumour Type | Treatment Parameters | Immunologic Effect | Ref. | Year |
|---|---|---|---|---|---|
| Thermal hyperthermia | preclinical in vitro, colon cancer cells | TID: 60–120 CEM43 | DAMP release and induction of ICD | [80] | 2021 |
| Thermal ablation | preclinical in vitro, lung and ovarian | TID >> 240 CEM43 | DAMP release and induction of ICD | [114] | 2017 |
| Thermal ablation | preclinical in vivo, murine hepatocellular tumours | f: 9.5 MHz, acoustic power: 5 W, exposure time:220 s | Increased Tcytotoxic activity, IFNγ and TNFα, DC maturation | [111,115] | 2010, 2012 |
| Thermal ablation | preclinical, melanoma tumours | TID >> 240 CEM43 in combination with immunotherapy | Antigen cross-presentation, IFNγ release | [116] | 2018 |
| Thermal ablation | clinical, osteosarcoma, hepatocellular and renal cell carcinomas, breast cancer | acoustic focal peak intensities: 5–20 kW/cm2 | Increases in various immune cells in the blood and tumours including T cells and APC | [105,109,110] | 2004, 2009 |
| Mechanical pHIFU | preclinical, in vivo colon adenocarcinoma, prostate tumours | d.c.: 2%, P–: 10–12.5 MPa | DC maturation/accumulation of tumour-specific IFNγ-secreting cells | [117,118] | 2007, 2012 |
| Mechanical pHIFU | preclinical, in vivo melanoma, breast tumours | P–: ~6 MPa, d.c.: 10%, prf: 5 Hz, | Various pro-immune anti-tumour effects in the tumours, TDLN and spleen of subjects | [119,120,121] | 2019, 2021, 2020 |
| Mechanical histotripsy | preclinical in vivo, melanoma tumours expressing cancer antigen | f: 1 MHz, P–: 30 MPa, prf: 100 Hz | Stimulating cancer-specific lymphocyte responses | [108] | 2020 |
| Mechanical histotripsy | preclinical, in vitro breast cancer cells | 10 ms-long pulses, P–: 14 MPa, prf: 1 Hz, d.c.: 1%, | DAMP and cytokine release, induction of ICD | [122] | 2019 |
| Mechanical pHIFU | preclinical in vivo neuroblastoma tumours | f: 1.5 MHz, P–: 14 MPa, 13.33-ms long pulses, prf: 1 Hz, | Induction of systemic inflammation | [123] | 2020 |
| Mechanical histotripsy | preclinical in vivo renal cell carcinoma | P–: 17–20 MPa, 10 ms duration, prf = 1 Hz | Tumour infiltration of Tcytotoxic cells | [124] | 2019 |
| LOFU pulsed exposures | preclinical in vivo, colon tumours | P–: 1.4 MPa, 100 ms long pulses, prf: 1 Hz | Increases in Tcytotoxic cells Tcytotoxic/Tregulatory ratio | [125] | 2012 |
| LOFU continuous exposures | preclinical in vivo, melanoma tumours | P–: 3 MPa, d.c.: 100% | Reversion of T cell tolerance and anergy | [113] | 2016 |
| LOFU pulsed exposures | preclinical in vivo, murine brain | P–: 0.3 MPa, 10-ms bursts, 1% duty cycle | Sterile inflammation | [126,127] | 2018 2017 |
| Year | Tumour Type | Focused Ultrasound Parameters | Immunologic Effect | Ref. |
|---|---|---|---|---|
| 2002 | Clinical study—thermal ablation | Input power: 0.5–1.6 kW Beamed power: 1–1.4 MW | Increases NK cells, T cells, CD4+ cells | [130] |
| 2015 | Case study—thermal ablation | Power: 103 W, treatment time: 752 s | Abscopal effect | [201] |
| 2016 | Case study—thermal ablation | f: 0.8 MHz | Abscopal effect | [202] |
| 2021 | Clinical retrospective—thermal ablation | f: 0.8 MHz, power: 366 W, energy/volume: 12.8 kJ/mL | Increases in cytokines (IL-6) and leukocytes | [203] |
| 2021 | Preclinical histotripsy in murine subcutaneous Pan02 tumours | f: 1 MHz, prf: 250 Hz, pulses < 2 | Decreases in macrophages, regulatory T cells, increases in dendritic cells, release of DAMPS | [204] |
| 2021 | Preclinical sonodynamic therapy in syngeneic KPC bilateral tumours | f: 1 MHz, Isatp: 3 W/cm2 d.c.: 30% prf: 100 Hz treatment duration:3.5 min | Abscopal effect, increased Tcytotoxic and decreased Tregulatory cells | [205] |
| 2021 | Preclinical sonodynamic therapy—syngeneic KPC tumours | f: 1 MHz, d.c.: 30%, prf: 100 Hz, P–: 0.48 MPa, in combination with microbubbles/anti-PD1 | Increases in Tcytotoxic and CD4+ T cells in off-target tumours | [206] |
| 2021 | Preclinical pHIFU—murine syngeneic orthotopic KPC tumours | f: 1.5 MHz, d.c: 1%, prf: 1 Hz, P–: 17 MPa, 10 ms long pulses in combination with anti-CTLA-4/anti-PD-1 | Increased Tcytotoxic cells, IFNγ+ Tcytotoxic cells and the ratio of these cells to Tregulatory and MDSC in the tumours | [112] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mouratidis, P.X.E.; ter Haar, G. Latest Advances in the Use of Therapeutic Focused Ultrasound in the Treatment of Pancreatic Cancer. Cancers 2022, 14, 638. https://doi.org/10.3390/cancers14030638
Mouratidis PXE, ter Haar G. Latest Advances in the Use of Therapeutic Focused Ultrasound in the Treatment of Pancreatic Cancer. Cancers. 2022; 14(3):638. https://doi.org/10.3390/cancers14030638
Chicago/Turabian StyleMouratidis, Petros X. E., and Gail ter Haar. 2022. "Latest Advances in the Use of Therapeutic Focused Ultrasound in the Treatment of Pancreatic Cancer" Cancers 14, no. 3: 638. https://doi.org/10.3390/cancers14030638
APA StyleMouratidis, P. X. E., & ter Haar, G. (2022). Latest Advances in the Use of Therapeutic Focused Ultrasound in the Treatment of Pancreatic Cancer. Cancers, 14(3), 638. https://doi.org/10.3390/cancers14030638







