The Central Role of the Ubiquitin–Proteasome System in EBV-Mediated Oncogenesis
Abstract
Simple Summary
Abstract
1. Introduction
2. EBV Latent Antigens Manipulate the Ubiquitin–Proteasome System for Targeted Protein Degradation
3. EBV Lytic Antigens Modulate the Activities of the Ubiquitin–Proteasome System for Protein Degradation
4. EBV-Encoded Proteins Can Be Modified by the Ubiquitin–Proteasome System
5. Targeting EBV-Associated Oncogenesis with Proteasome Inhibitors
6. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Glickman, M.H.; Ciechanover, A. The ubiquitin–proteasome proteolytic pathway: Destruction for the sake of construction. Physiol. Rev. 2002, 82, 373–428. [Google Scholar] [CrossRef]
- Komander, D.; Rape, M. The ubiquitin code. Annu. Rev. Biochem. 2012, 81, 203–229. [Google Scholar] [CrossRef]
- Shi, D.; Grossman, S.R. Ubiquitin becomes ubiquitous in cancer: Emerging roles of ubiquitin ligases and deubiquitinases in tumorigenesis and as therapeutic targets. Cancer. Biol. Ther. 2010, 10, 737–747. [Google Scholar] [CrossRef]
- Weissman, A.M. Themes and variations on ubiquitylation. Nat. Rev. Mol. Cell Biol. 2001, 2, 169–178. [Google Scholar] [CrossRef]
- Kutok, J.L.; Wang, F. Spectrum of Epstein–Barr virus-associated diseases. Annu. Rev. Pathol. 2006, 1, 375–404. [Google Scholar] [CrossRef]
- Pei, Y.; Wong, J.H.; Robertson, E.S. Herpesvirus Epigenetic Reprogramming and Oncogenesis. Annu. Rev. Virol. 2020, 7, 309–331. [Google Scholar] [CrossRef]
- Luo, H. Interplay between the virus and the ubiquitin–proteasome system: Molecular mechanism of viral pathogenesis. Curr. Opin. Virol. 2016, 17, 1–10. [Google Scholar] [CrossRef]
- Hui, K.F.; Tam, K.P.; Chiang, A.K.S. Therapeutic Strategies against Epstein–Barr Virus-Associated Cancers Using Proteasome Inhibitors. Viruses 2017, 9, 352. [Google Scholar] [CrossRef]
- Ovaa, H.; Kessler, B.M.; Rolen, U.; Galardy, P.J.; Ploegh, H.L.; Masucci, M.G. Activity-based ubiquitin-specific protease (USP) profiling of virus-infected and malignant human cells. Proc. Natl. Acad. Sci. USA 2004, 101, 2253–2258. [Google Scholar] [CrossRef]
- Holowaty, M.N.; Zeghouf, M.; Wu, H.; Tellam, J.; Athanasopoulos, V.; Greenblatt, J.; Frappier, L. Protein profiling with Epstein–Barr nuclear antigen-1 reveals an interaction with the herpesvirus-associated ubiquitin-specific protease HAUSP/USP7. J. Biol. Chem. 2003, 278, 29987–29994. [Google Scholar] [CrossRef]
- Sivachandran, N.; Cao, J.Y.; Frappier, L. Epstein–Barr virus nuclear antigen 1 Hijacks the host kinase CK2 to disrupt PML nuclear bodies. J. Virol. 2010, 84, 11113–11123. [Google Scholar] [CrossRef] [PubMed]
- Saridakis, V.; Sheng, Y.; Sarkari, F.; Holowaty, M.N.; Shire, K.; Nguyen, T.; Zhang, R.G.; Liao, J.; Lee, W.; Edwards, A.M.; et al. Structure of the p53 binding domain of HAUSP/USP7 bound to Epstein–Barr nuclear antigen 1 implications for EBV-mediated immortalization. Mol. Cell. 2005, 18, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Holowaty, M.N.; Frappier, L. HAUSP/USP7 as an Epstein–Barr virus target. Biochem. Soc. Trans. 2004, 32, 731–732. [Google Scholar] [CrossRef]
- Tomkinson, B.; Robertson, E.; Kieff, E. Epstein–Barr virus nuclear proteins EBNA-3A and EBNA-3C are essential for B-lymphocyte growth transformation. J. Virol. 1993, 67, 2014–2025. [Google Scholar] [CrossRef]
- Migliazza, A.; Martinotti, S.; Chen, W.; Fusco, C.; Ye, B.H.; Knowles, D.M.; Offit, K.; Chaganti, R.S.; Dalla-Favera, R. Frequent somatic hypermutation of the 5’ noncoding region of the BCL6 gene in B-cell lymphoma. Proc. Natl. Acad. Sci. USA 1995, 92, 12520–12524. [Google Scholar] [CrossRef]
- Gaidano, G.; Carbone, A.; Pastore, C.; Capello, D.; Migliazza, A.; Gloghini, A.; Roncella, S.; Ferrarini, M.; Saglio, G.; Dalla-Favera, R. Frequent mutation of the 5’ noncoding region of the BCL-6 gene in acquired immunodeficiency syndrome-related non-Hodgkin’s lymphomas. Blood 1997, 89, 3755–3762. [Google Scholar]
- Basso, K.; Saito, M.; Sumazin, P.; Margolin, A.A.; Wang, K.; Lim, W.K.; Kitagawa, Y.; Schneider, C.; Alvarez, M.J.; Califano, A.; et al. Integrated biochemical and computational approach identifies BCL6 direct target genes controlling multiple pathways in normal germinal center B cells. Blood 2010, 115, 975–984. [Google Scholar] [CrossRef]
- Basso, K.; Dalla-Favera, R. BCL6: Master regulator of the germinal center reaction and key oncogene in B cell lymphomagenesis. Adv. Immunol. 2010, 105, 193–210. [Google Scholar] [CrossRef]
- Cerchietti, L.C.; Ghetu, A.F.; Zhu, X.; Da Silva, G.F.; Zhong, S.; Matthews, M.; Bunting, K.L.; Polo, J.M.; Fares, C.; Arrowsmith, C.H.; et al. A small-molecule inhibitor of BCL6 kills DLBCL cells in vitro and in vivo. Cancer Cell 2010, 17, 400–411. [Google Scholar] [CrossRef]
- Cardenas, M.G.; Yu, W.; Beguelin, W.; Teater, M.R.; Geng, H.; Goldstein, R.L.; Oswald, E.; Hatzi, K.; Yang, S.N.; Cohen, J.; et al. Rationally designed BCL6 inhibitors target activated B cell diffuse large B cell lymphoma. J. Clin. Investig. 2016, 126, 3351–3362. [Google Scholar] [CrossRef]
- Pei, Y.; Banerjee, S.; Jha, H.C.; Sun, Z.; Robertson, E.S. An essential EBV latent antigen 3C binds Bcl6 for targeted degradation and cell proliferation. PLoS Pathog. 2017, 13, e1006500. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Halder, S.; Upadhyay, S.K.; Lu, J.; Kumar, P.; Murakami, M.; Cai, Q.; Robertson, E.S. Epstein–Barr virus nuclear antigen 3C facilitates G1-S transition by stabilizing and enhancing the function of cyclin D1. PLoS Pathog. 2011, 7, e1001275. [Google Scholar] [CrossRef]
- Pei, Y.; Singh, R.K.; Shukla, S.K.; Lang, F.; Zhang, S.; Robertson, E.S. Epstein–Barr Virus Nuclear Antigen 3C Facilitates Cell Proliferation by Regulating Cyclin D2. J. Virol. 2018, 92, e00663-18. [Google Scholar] [CrossRef]
- Xiong, Y.; Hannon, G.J.; Zhang, H.; Casso, D.; Kobayashi, R.; Beach, D. p21 is a universal inhibitor of cyclin kinases. Nature 1993, 366, 701–704. [Google Scholar] [CrossRef]
- Toyoshima, H.; Hunter, T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell 1994, 78, 67–74. [Google Scholar] [CrossRef]
- Abukhdeir, A.M.; Park, B.H. P21 and p27: Roles in carcinogenesis and drug resistance. Expert Rev. Mol. Med. 2008, 10, e19. [Google Scholar] [CrossRef]
- Knight, J.S.; Sharma, N.; Robertson, E.S. SCFSkp2 complex targeted by Epstein–Barr virus essential nuclear antigen. Mol. Cell. Biol. 2005, 25, 1749–1763. [Google Scholar] [CrossRef]
- Banerjee, S.; Lu, J.; Cai, Q.; Sun, Z.; Jha, H.C.; Robertson, E.S. EBNA3C augments Pim-1 mediated phosphorylation and degradation of p21 to promote B-cell proliferation. PLoS Pathog. 2014, 10, e1004304. [Google Scholar] [CrossRef]
- Hu, X.F.; Li, J.; Vandervalk, S.; Wang, Z.; Magnuson, N.S.; Xing, P.X. PIM-1-specific mAb suppresses human and mouse tumor growth by decreasing PIM-1 levels, reducing Akt phosphorylation, and activating apoptosis. J. Clin. Investig. 2009, 119, 362–375. [Google Scholar] [CrossRef]
- McFarland, E.D.C.; Izumi, K.M.; Mosialos, G. Epstein–barr virus transformation: Involvement of latent membrane protein 1-mediated activation of NF-kappaB. Oncogene 1999, 18, 6959–6964. [Google Scholar] [CrossRef]
- Cahir-McFarland, E.D.; Davidson, D.M.; Schauer, S.L.; Duong, J.; Kieff, E. NF-kappa B inhibition causes spontaneous apoptosis in Epstein–Barr virus-transformed lymphoblastoid cells. Proc. Natl. Acad. Sci. USA 2000, 97, 6055–6060. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.S.; Ghosh, S. Signaling to NF-kappaB. Genes Dev. 2004, 18, 2195–2224. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Wang, C.; Spencer, E.; Yang, L.; Braun, A.; You, J.; Slaughter, C.; Pickart, C.; Chen, Z.J. Activation of the IkappaB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell 2000, 103, 351–361. [Google Scholar] [CrossRef]
- Wang, C.; Deng, L.; Hong, M.; Akkaraju, G.R.; Inoue, J.; Chen, Z.J. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature 2001, 412, 346–351. [Google Scholar] [CrossRef]
- Saito, S.; Murata, T.; Kanda, T.; Isomura, H.; Narita, Y.; Sugimoto, A.; Kawashima, D.; Tsurumi, T. Epstein–Barr virus deubiquitinase downregulates TRAF6-mediated NF-kappaB signaling during productive replication. J. Virol. 2013, 87, 4060–4070. [Google Scholar] [CrossRef]
- Schultheiss, U.; Puschner, S.; Kremmer, E.; Mak, T.W.; Engelmann, H.; Hammerschmidt, W.; Kieser, A. TRAF6 is a critical mediator of signal transduction by the viral oncogene latent membrane protein 1. EMBO J. 2001, 20, 5678–5691. [Google Scholar] [CrossRef]
- Arcipowski, K.M.; Stunz, L.L.; Graham, J.P.; Kraus, Z.J.; Vanden Bush, T.J.; Bishop, G.A. Molecular mechanisms of TNFR-associated factor 6 (TRAF6) utilization by the oncogenic viral mimic of CD40, latent membrane protein 1 (LMP1). J. Biol. Chem. 2011, 286, 9948–9955. [Google Scholar] [CrossRef]
- Li, L.; Li, W.; Xiao, L.; Xu, J.; Chen, X.; Tang, M.; Dong, Z.; Tao, Q.; Cao, Y. Viral oncoprotein LMP1 disrupts p53-induced cell cycle arrest and apoptosis through modulating K63-linked ubiquitination of p53. Cell Cycle 2012, 11, 2327–2336. [Google Scholar] [CrossRef]
- Thompson, H.G.; Harris, J.W.; Wold, B.J.; Lin, F.; Brody, J.P. p62 overexpression in breast tumors and regulation by prostate-derived Ets factor in breast cancer cells. Oncogene 2003, 22, 2322–2333. [Google Scholar] [CrossRef]
- Duran, A.; Linares, J.F.; Galvez, A.S.; Wikenheiser, K.; Flores, J.M.; Diaz-Meco, M.T.; Moscat, J. The signaling adaptor p62 is an important NF-kappaB mediator in tumorigenesis. Cancer Cell 2008, 13, 343–354. [Google Scholar] [CrossRef]
- Komatsu, M.; Waguri, S.; Koike, M.; Sou, Y.S.; Ueno, T.; Hara, T.; Mizushima, N.; Iwata, J.; Ezaki, J.; Murata, S.; et al. Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell 2007, 131, 1149–1163. [Google Scholar] [CrossRef] [PubMed]
- Shin, J. P62 and the sequestosome, a novel mechanism for protein metabolism. Arch. Pharm. Res. 1998, 21, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Wooten, M.W.; Geetha, T.; Seibenhener, M.L.; Babu, J.R.; Diaz-Meco, M.T.; Moscat, J. The p62 scaffold regulates nerve growth factor-induced NF-kappaB activation by influencing TRAF6 polyubiquitination. J. Biol. Chem. 2005, 280, 35625–35629. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Howell, M.E.A.; Sparks-Wallace, A.; Zhao, J.; Hensley, C.R.; Nicksic, C.A.; Horne, S.R.; Mohr, K.B.; Moorman, J.P.; Yao, Z.Q.; et al. The Ubiquitin Sensor and Adaptor Protein p62 Mediates Signal Transduction of a Viral Oncogenic Pathway. mBio 2021, 12, e01097-21. [Google Scholar] [CrossRef]
- Eliopoulos, A.G.; Caamano, J.H.; Flavell, J.; Reynolds, G.M.; Murray, P.G.; Poyet, J.L.; Young, L.S. Epstein–Barr virus-encoded latent infection membrane protein 1 regulates the processing of p100 NF-kappaB2 to p52 via an IKKgamma/NEMO-independent signalling pathway. Oncogene 2003, 22, 7557–7569. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Zhao, J.; Ren, J.; Hall, K.H.; Moorman, J.P.; Yao, Z.Q.; Ning, S. The Linear Ubiquitin Assembly Complex Modulates Latent Membrane Protein 1 Activation of NF-kappaB and Interferon Regulatory Factor 7. J. Virol. 2017, 91, e01138-16. [Google Scholar] [CrossRef]
- Hinz, M.; Scheidereit, C. The IkappaB kinase complex in NF-kappaB regulation and beyond. EMBO Rep. 2014, 15, 46–61. [Google Scholar] [CrossRef]
- Voigt, S.; Sterz, K.R.; Giehler, F.; Mohr, A.W.; Wilson, J.B.; Moosmann, A.; Kieser, A. A central role of IKK2 and TPL2 in JNK activation and viral B-cell transformation. Nat. Commun. 2020, 11, 685. [Google Scholar] [CrossRef]
- Ning, S.; Pagano, J.S. The A20 deubiquitinase activity negatively regulates LMP1 activation of IRF7. J. Virol. 2010, 84, 6130–6138. [Google Scholar] [CrossRef]
- Hsu, H.; Huang, J.; Shu, H.B.; Baichwal, V.; Goeddel, D.V. TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity 1996, 4, 387–396. [Google Scholar] [CrossRef]
- Kelliher, M.A.; Grimm, S.; Ishida, Y.; Kuo, F.; Stanger, B.Z.; Leder, P. The death domain kinase RIP mediates the TNF-induced NF-kappaB signal. Immunity 1998, 8, 297–303. [Google Scholar] [CrossRef]
- Huye, L.E.; Ning, S.; Kelliher, M.; Pagano, J.S. Interferon regulatory factor 7 is activated by a viral oncoprotein through RIP-dependent ubiquitination. Mol. Cell. Biol. 2007, 27, 2910–2918. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, Y.; Peng, S.; Yu, X.; Li, W.; Shi, F.; Luo, X.; Tang, M.; Tan, Z.; Bode, A.M.; et al. Epstein–Barr virus encoded latent membrane protein 1 suppresses necroptosis through targeting RIPK1/3 ubiquitination. Cell Death Dis. 2018, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Greenfeld, H.; Takasaki, K.; Walsh, M.J.; Ersing, I.; Bernhardt, K.; Ma, Y.; Fu, B.; Ashbaugh, C.W.; Cabo, J.; Mollo, S.B.; et al. TRAF1 Coordinates Polyubiquitin Signaling to Enhance Epstein–Barr Virus LMP1-Mediated Growth and Survival Pathway Activation. PLoS Pathog. 2015, 11, e1004890. [Google Scholar] [CrossRef]
- Xu, C.; Sun, L.; Liu, W.; Duan, Z. Latent Membrane Protein 1 of Epstein–Barr Virus Promotes RIG-I Degradation Mediated by Proteasome Pathway. Front. Immunol. 2018, 9, 1446. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Zhang, Q.; Li, X.; Zhao, D.; Liu, Y.; Shen, Q.; Yang, M.; Wang, C.; Li, N.; Cao, X. Cytoplasmic STAT4 Promotes Antiviral Type I IFN Production by Blocking CHIP-Mediated Degradation of RIG-I. J. Immunol. 2016, 196, 1209–1217. [Google Scholar] [CrossRef]
- Ikeda, M.; Ikeda, A.; Longan, L.C.; Longnecker, R. The Epstein–Barr virus latent membrane protein 2A PY motif recruits WW domain-containing ubiquitin-protein ligases. Virology 2000, 268, 178–191. [Google Scholar] [CrossRef]
- Fruehling, S.; Swart, R.; Dolwick, K.M.; Kremmer, E.; Longnecker, R. Tyrosine 112 of latent membrane protein 2A is essential for protein tyrosine kinase loading and regulation of Epstein–Barr virus latency. J. Virol. 1998, 72, 7796–7806. [Google Scholar] [CrossRef]
- Winberg, G.; Matskova, L.; Chen, F.; Plant, P.; Rotin, D.; Gish, G.; Ingham, R.; Ernberg, I.; Pawson, T. Latent membrane protein 2A of Epstein–Barr virus binds WW domain E3 protein-ubiquitin ligases that ubiquitinate B-cell tyrosine kinases. Mol. Cell. Biol. 2000, 20, 8526–8535. [Google Scholar] [CrossRef][Green Version]
- Ikeda, M.; Ikeda, A.; Longnecker, R. PY motifs of Epstein–Barr virus LMP2A regulate protein stability and phosphorylation of LMP2A-associated proteins. J. Virol. 2001, 75, 5711–5718. [Google Scholar] [CrossRef][Green Version]
- Ikeda, A.; Caldwell, R.G.; Longnecker, R.; Ikeda, M. Itchy, a Nedd4 ubiquitin ligase, downregulates latent membrane protein 2A activity in B-cell signaling. J. Virol. 2003, 77, 5529–5534. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ikeda, M.; Ikeda, A.; Longnecker, R. Lysine-independent ubiquitination of Epstein–Barr virus LMP2A. Virology 2002, 300, 153–159. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fish, K.; Sora, R.P.; Schaller, S.J.; Longnecker, R.; Ikeda, M. EBV latent membrane protein 2A orchestrates p27(kip1) degradation via Cks1 to accelerate MYC-driven lymphoma in mice. Blood 2017, 130, 2516–2526. [Google Scholar] [CrossRef] [PubMed]
- Sora, R.P.; Ikeda, M.; Longnecker, R. Two Pathways of p27(Kip1) Degradation Are Required for Murine Lymphoma Driven by Myc and EBV Latent Membrane Protein 2A. mBio 2019, 10, e00548-19. [Google Scholar] [CrossRef]
- Shackelford, J.; Maier, C.; Pagano, J.S. Epstein–Barr virus activates beta-catenin in type III latently infected B lymphocyte lines: Association with deubiquitinating enzymes. Proc. Natl. Acad. Sci. USA 2003, 100, 15572–15576. [Google Scholar] [CrossRef]
- Jang, K.L.; Shackelford, J.; Seo, S.Y.; Pagano, J.S. Up-regulation of beta-catenin by a viral oncogene correlates with inhibition of the seven in absentia homolog 1 in B lymphoma cells. Proc. Natl. Acad. Sci. USA 2005, 102, 18431–18436. [Google Scholar] [CrossRef]
- Morrison, J.A.; Klingelhutz, A.J.; Raab-Traub, N. Epstein–Barr virus latent membrane protein 2A activates beta-catenin signaling in epithelial cells. J. Virol. 2003, 77, 12276–12284. [Google Scholar] [CrossRef]
- Iwakiri, D.; Minamitani, T.; Samanta, M. Epstein–Barr virus latent membrane protein 2A contributes to anoikis resistance through ERK activation. J. Virol. 2013, 87, 8227–8234. [Google Scholar] [CrossRef]
- DeKroon, R.M.; Gunawardena, H.P.; Edwards, R.; Raab-Traub, N. Global Proteomic Changes Induced by the Epstein–Barr Virus Oncoproteins Latent Membrane Protein 1 and 2A. mBio 2018, 9, e00959-18. [Google Scholar] [CrossRef]
- Li, Z.; Baccianti, F.; Delecluse, S.; Tsai, M.H.; Shumilov, A.; Cheng, X.; Ma, S.; Hoffmann, I.; Poirey, R.; Delecluse, H.J. The Epstein–Barr virus noncoding RNA EBER2 transactivates the UCHL1 deubiquitinase to accelerate cell growth. Proc. Natl. Acad. Sci. USA 2021, 118, e2115508118. [Google Scholar] [CrossRef]
- Ersing, I.; Nobre, L.; Wang, L.W.; Soday, L.; Ma, Y.; Paulo, J.A.; Narita, Y.; Ashbaugh, C.W.; Jiang, C.; Grayson, N.E.; et al. A Temporal Proteomic Map of Epstein–Barr Virus Lytic Replication in B Cells. Cell Rep. 2017, 19, 1479–1493. [Google Scholar] [CrossRef] [PubMed]
- Feederle, R.; Kost, M.; Baumann, M.; Janz, A.; Drouet, E.; Hammerschmidt, W.; Delecluse, H.J. The Epstein–Barr virus lytic program is controlled by the co-operative functions of two transactivators. EMBO J. 2000, 19, 3080–3089. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, J.; El-Guindy, A. Epstein–Barr Virus Lytic Cycle Reactivation. Curr. Top MicroBiol. Immunol. 2015, 391, 237–261. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.H.; Chen, C.S.; Wang, W.H.; Hsu, S.W.; Tsai, H.H.; Liu, S.T.; Chang, L.K. TRIM5alpha Promotes Ubiquitination of Rta from Epstein–Barr Virus to Attenuate Lytic Progression. Front. Microbiol. 2016, 7, 2129. [Google Scholar] [CrossRef] [PubMed]
- Quinn, L.L.; Williams, L.R.; White, C.; Forrest, C.; Zuo, J.; Rowe, M. The Missing Link in Epstein–Barr Virus Immune Evasion: The BDLF3 Gene Induces Ubiquitination and Downregulation of Major Histocompatibility Complex Class I (MHC-I) and MHC-II. J. Virol. 2016, 90, 356–367. [Google Scholar] [CrossRef]
- Yla-Anttila, P.; Gupta, S.; Masucci, M.G. The Epstein–Barr virus deubiquitinase BPLF1 targets SQSTM1/p62 to inhibit selective autophagy. Autophagy 2021, 17, 3461–3474. [Google Scholar] [CrossRef]
- Li, J.; Nagy, N.; Liu, J.; Gupta, S.; Frisan, T.; Hennig, T.; Cameron, D.P.; Baranello, L.; Masucci, M.G. The Epstein–Barr virus deubiquitinating enzyme BPLF1 regulates the activity of topoisomerase II during productive infection. PLoS Pathog. 2021, 17, e1009954. [Google Scholar] [CrossRef]
- Kumar, R.; Whitehurst, C.B.; Pagano, J.S. The Rad6/18 ubiquitin complex interacts with the Epstein–Barr virus deubiquitinating enzyme, BPLF1, and contributes to virus infectivity. J. Virol. 2014, 88, 6411–6422. [Google Scholar] [CrossRef]
- Gupta, S.; Yla-Anttila, P.; Callegari, S.; Tsai, M.H.; Delecluse, H.J.; Masucci, M.G. Herpesvirus deconjugases inhibit the IFN response by promoting TRIM25 autoubiquitination and functional inactivation of the RIG-I signalosome. PLoS Pathog. 2018, 14, e1006852. [Google Scholar] [CrossRef]
- Whitehurst, C.B.; Vaziri, C.; Shackelford, J.; Pagano, J.S. Epstein–Barr virus BPLF1 deubiquitinates PCNA and attenuates polymerase eta recruitment to DNA damage sites. J. Virol. 2012, 86, 8097–8106. [Google Scholar] [CrossRef]
- Whitehurst, C.B.; Ning, S.; Bentz, G.L.; Dufour, F.; Gershburg, E.; Shackelford, J.; Langelier, Y.; Pagano, J.S. The Epstein–Barr virus (EBV) deubiquitinating enzyme BPLF1 reduces EBV ribonucleotide reductase activity. J. Virol. 2009, 83, 4345–4353. [Google Scholar] [CrossRef] [PubMed]
- Jangra, S.; Bharti, A.; Lui, W.Y.; Chaudhary, V.; Botelho, M.G.; Yuen, K.S.; Jin, D.Y. Suppression of JAK-STAT Signaling by Epstein–Barr Virus Tegument Protein BGLF2 through Recruitment of SHP1 Phosphatase and Promotion of STAT2 Degradation. J. Virol. 2021, 95, e0102721. [Google Scholar] [CrossRef]
- Liu, X.; Sadaoka, T.; Krogmann, T.; Cohen, J.I. Epstein–Barr Virus (EBV) Tegument Protein BGLF2 Suppresses Type I Interferon Signaling To Promote EBV Reactivation. J. Virol. 2020, 94, e00258-20. [Google Scholar] [CrossRef] [PubMed]
- Masud, H.; Yanagi, Y.; Watanabe, T.; Sato, Y.; Kimura, H.; Murata, T. Epstein–Barr Virus BBRF2 Is Required for Maximum Infectivity. Microorganisms 2019, 7, 705. [Google Scholar] [CrossRef] [PubMed]
- He, H.P.; Luo, M.; Cao, Y.L.; Lin, Y.X.; Zhang, H.; Zhang, X.; Ou, J.Y.; Yu, B.; Chen, X.; Xu, M.; et al. Structure of Epstein–Barr virus tegument protein complex BBRF2-BSRF1 reveals its potential role in viral envelopment. Nat. Commun. 2020, 11, 5405. [Google Scholar] [CrossRef] [PubMed]
- Levitskaya, J.; Sharipo, A.; Leonchiks, A.; Ciechanover, A.; Masucci, M.G. Inhibition of ubiquitin/proteasome-dependent protein degradation by the Gly-Ala repeat domain of the Epstein–Barr virus nuclear antigen 1. Proc. Natl. Acad. Sci. USA 1997, 94, 12616–12621. [Google Scholar] [CrossRef]
- Levitskaya, J.; Coram, M.; Levitsky, V.; Imreh, S.; Steigerwald-Mullen, P.M.; Klein, G.; Kurilla, M.G.; Masucci, M.G. Inhibition of antigen processing by the internal repeat region of the Epstein–Barr virus nuclear antigen-1. Nature 1995, 375, 685–688. [Google Scholar] [CrossRef]
- Sharipo, A.; Imreh, M.; Leonchiks, A.; Imreh, S.; Masucci, M.G. A minimal glycine-alanine repeat prevents the interaction of ubiquitinated I kappaB alpha with the proteasome: A new mechanism for selective inhibition of proteolysis. Nat. Med. 1998, 4, 939–944. [Google Scholar] [CrossRef]
- Dantuma, N.P.; Heessen, S.; Lindsten, K.; Jellne, M.; Masucci, M.G. Inhibition of proteasomal degradation by the gly-Ala repeat of Epstein–Barr virus is influenced by the length of the repeat and the strength of the degradation signal. Proc. Natl. Acad. Sci. USA 2000, 97, 8381–8385. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, Y.; Wang, C.; Liu, L.; Wang, H.; Zhang, Y.; Long, C.; Sun, X. Triptolide inhibits Epstein–Barr nuclear antigen 1 expression by increasing sensitivity of mitochondria apoptosis of nasopharyngeal carcinoma cells. J. Exp. Clin. Cancer Res. 2018, 37, 192. [Google Scholar] [CrossRef]
- Ohashi, M.; Holthaus, A.M.; Calderwood, M.A.; Lai, C.Y.; Krastins, B.; Sarracino, D.; Johannsen, E. The EBNA3 family of Epstein–Barr virus nuclear proteins associates with the USP46/USP12 deubiquitination complexes to regulate lymphoblastoid cell line growth. PLoS Pathog. 2015, 11, e1004822. [Google Scholar] [CrossRef] [PubMed]
- Aviel, S.; Winberg, G.; Massucci, M.; Ciechanover, A. Degradation of the Epstein–barr virus latent membrane protein 1 (LMP1) by the ubiquitin–proteasome pathway. Targeting via ubiquitination of the N-terminal residue. J. Biol. Chem. 2000, 275, 23491–23499. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.W.; Kim, S.M.; Jin, D.H.; Kim, Y.S.; Hur, D.Y. RPS27a enhances EBV-encoded LMP1-mediated proliferation and invasion by stabilizing of LMP1. BioChem. Biophys. Res. Commun. 2017, 491, 303–309. [Google Scholar] [CrossRef]
- Zhao, M.; Nanbo, A.; Becnel, D.; Qin, Z.; Morris, G.F.; Li, L.; Lin, Z. Ubiquitin Modification of the Epstein–Barr Virus Immediate Early Transactivator Zta. J. Virol. 2020, 94, e01298-20. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.C.; Yoshikai, Y.; Hsu, S.W.; Saitoh, H.; Chang, L.K. Role of RNF4 in the ubiquitination of Rta of Epstein–Barr virus. J. Biol. Chem. 2013, 288, 12866–12879. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Watanabe, T.; Suzuki, C.; Abe, Y.; Masud, H.; Inagaki, T.; Yoshida, M.; Suzuki, T.; Goshima, F.; Adachi, J.; et al. S-Like-Phase Cyclin-Dependent Kinases Stabilize the Epstein–Barr Virus BDLF4 Protein To Temporally Control Late Gene Transcription. J. Virol. 2019, 93, e01707-18. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Narita, Y.; Yoshida, M.; Sato, Y.; Goshima, F.; Kimura, H.; Murata, T. The Epstein–Barr Virus BDLF4 Gene Is Required for Efficient Expression of Viral Late Lytic Genes. J. Virol. 2015, 89, 10120–10124. [Google Scholar] [CrossRef]
- Lee, C.P.; Liu, G.T.; Kung, H.N.; Liu, P.T.; Liao, Y.T.; Chow, L.P.; Chang, L.S.; Chang, Y.H.; Chang, C.W.; Shu, W.C.; et al. The Ubiquitin Ligase Itch and Ubiquitination Regulate BFRF1-Mediated Nuclear Envelope Modification for Epstein–Barr Virus Maturation. J. Virol. 2016, 90, 8994–9007. [Google Scholar] [CrossRef]
- Spear, P.G.; Longnecker, R. Herpesvirus entry: An update. J. Virol. 2003, 77, 10179–10185. [Google Scholar] [CrossRef]
- Zhang, H.J.; Tian, J.; Qi, X.K.; Xiang, T.; He, G.P.; Zhang, H.; Yu, X.; Zhang, X.; Zhao, B.; Feng, Q.S.; et al. Epstein–Barr virus activates F-box protein FBXO2 to limit viral infectivity by targeting glycoprotein B for degradation. PLoS Pathog. 2018, 14, e1007208. [Google Scholar] [CrossRef]
- Richardson, P.G.; Mitsiades, C.; Hideshima, T.; Anderson, K.C. Bortezomib: Proteasome inhibition as an effective anticancer therapy. Annu. Rev. Med. 2006, 57, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Kane, R.C.; Farrell, A.T.; Sridhara, R.; Pazdur, R. United States Food and Drug Administration approval summary: Bortezomib for the treatment of progressive multiple myeloma after one prior therapy. Clin. Cancer Res 2006, 12, 2955–2960. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.X.; Tanhehco, Y.C.; Chen, J.; Foss, C.A.; Fox, J.J.; Lemas, V.; Chong, J.M.; Ambinder, R.F.; Pomper, M.G. Virus-associated tumor imaging by induction of viral gene expression. Clin. Cancer Res. 2007, 13, 1453–1458. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fu, D.X.; Tanhehco, Y.; Chen, J.; Foss, C.A.; Fox, J.J.; Chong, J.M.; Hobbs, R.F.; Fukayama, M.; Sgouros, G.; Kowalski, J.; et al. Bortezomib-induced enzyme-targeted radiation therapy in herpesvirus-associated tumors. Nat. Med. 2008, 14, 1118–1122. [Google Scholar] [CrossRef] [PubMed]
- Shirley, C.M.; Chen, J.; Shamay, M.; Li, H.; Zahnow, C.A.; Hayward, S.D.; Ambinder, R.F. Bortezomib induction of C/EBPbeta mediates Epstein–Barr virus lytic activation in Burkitt lymphoma. Blood 2011, 117, 6297–6303. [Google Scholar] [CrossRef] [PubMed]
- Muz, B.; Ghazarian, R.N.; Ou, M.; Luderer, M.J.; Kusdono, H.D.; Azab, A.K. Spotlight on ixazomib: Potential in the treatment of multiple myeloma. Drug Des. Dev. Ther. 2016, 10, 217–226. [Google Scholar] [CrossRef]
- Shirley, M. Ixazomib: First Global Approval. Drugs 2016, 76, 405–411. [Google Scholar] [CrossRef]
- Ganguly, S.; Kuravi, S.; Alleboina, S.; Mudduluru, G.; Jensen, R.A.; McGuirk, J.P.; Balusu, R. Targeted Therapy for EBV-Associated B-cell Neoplasms. Mol. Cancer Res. 2019, 17, 839–844. [Google Scholar] [CrossRef]
- Kupperman, E.; Lee, E.C.; Cao, Y.; Bannerman, B.; Fitzgerald, M.; Berger, A.; Yu, J.; Yang, Y.; Hales, P.; Bruzzese, F.; et al. Evaluation of the proteasome inhibitor MLN9708 in preclinical models of human cancer. Cancer Res. 2010, 70, 1970–1980. [Google Scholar] [CrossRef]
- Meng, L.; Mohan, R.; Kwok, B.H.; Elofsson, M.; Sin, N.; Crews, C.M. Epoxomicin, a potent and selective proteasome inhibitor, exhibits in vivo antiinflammatory activity. Proc. Natl. Acad. Sci. USA 1999, 96, 10403–10408. [Google Scholar] [CrossRef]
- Hanada, M.; Sugawara, K.; Kaneta, K.; Toda, S.; Nishiyama, Y.; Tomita, K.; Yamamoto, H.; Konishi, M.; Oki, T. Epoxomicin, a new antitumor agent of microbial origin. J. Antibiot. 1992, 45, 1746–1752. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.B.; Crews, C.M. From epoxomicin to carfilzomib: Chemistry, biology, and medical outcomes. Nat. Prod. Rep. 2013, 30, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Myung, J.; Kim, K.B.; Crews, C.M. The ubiquitin–proteasome pathway and proteasome inhibitors. Med. Res. Rev. 2001, 21, 245–273. [Google Scholar] [CrossRef] [PubMed]
- Harshbarger, W.; Miller, C.; Diedrich, C.; Sacchettini, J. Crystal structure of the human 20S proteasome in complex with carfilzomib. Structure 2015, 23, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Jager, S.; Groll, M.; Huber, R.; Wolf, D.H.; Heinemeyer, W. Proteasome beta-type subunits: Unequal roles of propeptides in core particle maturation and a hierarchy of active site function. J. Mol. Biol. 1999, 291, 997–1013. [Google Scholar] [CrossRef] [PubMed]
- Herndon, T.M.; Deisseroth, A.; Kaminskas, E.; Kane, R.C.; Koti, K.M.; Rothmann, M.D.; Habtemariam, B.; Bullock, J.; Bray, J.D.; Hawes, J.; et al. U.s. Food and Drug Administration approval: Carfilzomib for the treatment of multiple myeloma. Clin. Cancer Res. 2013, 19, 4559–4563. [Google Scholar] [CrossRef] [PubMed]
- Vij, R.; Siegel, D.S.; Jagannath, S.; Jakubowiak, A.J.; Stewart, A.K.; McDonagh, K.; Bahlis, N.; Belch, A.; Kunkel, L.A.; Wear, S.; et al. An open-label, single-arm, phase 2 study of single-agent carfilzomib in patients with relapsed and/or refractory multiple myeloma who have been previously treated with bortezomib. Br. J. Haematol. 2012, 158, 739–748. [Google Scholar] [CrossRef]
- Fricker, L.D. Proteasome Inhibitor Drugs. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 457–476. [Google Scholar] [CrossRef]
- Kobayashi, E.; Hwang, D.; Bheda-Malge, A.; Whitehurst, C.B.; Kabanov, A.V.; Kondo, S.; Aga, M.; Yoshizaki, T.; Pagano, J.S.; Sokolsky, M.; et al. Inhibition of UCH-L1 Deubiquitinating Activity with Two Forms of LDN-57444 Has Anti-Invasive Effects in Metastatic Carcinoma Cells. Int. J. Mol. Sci. 2019, 20, 3733. [Google Scholar] [CrossRef]
- Tikhmyanova, N.; Schultz, D.C.; Lee, T.; Salvino, J.M.; Lieberman, P.M. Identification of a new class of small molecules that efficiently reactivate latent Epstein–Barr Virus. ACS Chem. Biol. 2014, 9, 785–795. [Google Scholar] [CrossRef][Green Version]
- Tikhmyanova, N.; Tutton, S.; Martin, K.A.; Lu, F.; Kossenkov, A.V.; Paparoidamis, N.; Kenney, S.; Salvino, J.M.; Lieberman, P.M. Small molecule perturbation of the CAND1-Cullin1-ubiquitin cycle stabilizes p53 and triggers Epstein–Barr virus reactivation. PLoS Pathog. 2017, 13, e1006517. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhang, Y.; Shi, M.; Sun, Y.; Chen, C.; Niu, M.; Zhang, Q.; Zeng, L.; Yao, R.; Li, H.; et al. Blockade of deubiquitinase USP7 overcomes bortezomib resistance by suppressing NF-kappaB signaling pathway in multiple myeloma. J. Leukoc. Biol. 2018, 104, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Hui, K.F.; Leung, Y.Y.; Yeung, P.L.; Middeldorp, J.M.; Chiang, A.K. Combination of SAHA and bortezomib up-regulates CDKN2A and CDKN1A and induces apoptosis of Epstein–Barr virus-positive Wp-restricted Burkitt lymphoma and lymphoblastoid cell lines. Br. J. Haematol. 2014, 167, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, J.L.; Mina, R.; Jakubowiak, A.J.; Zimmerman, T.L.; Wolf, J.J.; Lewis, C.; Gleason, C.; Sharp, C.; Martin, T.; Heffner, L.T.; et al. Combining carfilzomib and panobinostat to treat relapsed/refractory multiple myeloma: Results of a Multiple Myeloma Research Consortium Phase I Study. Blood Cancer J. 2019, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- McConkey, D.J.; Zhu, K. Mechanisms of proteasome inhibitor action and resistance in cancer. Drug Resist. Updat. 2008, 11, 164–179. [Google Scholar] [CrossRef]
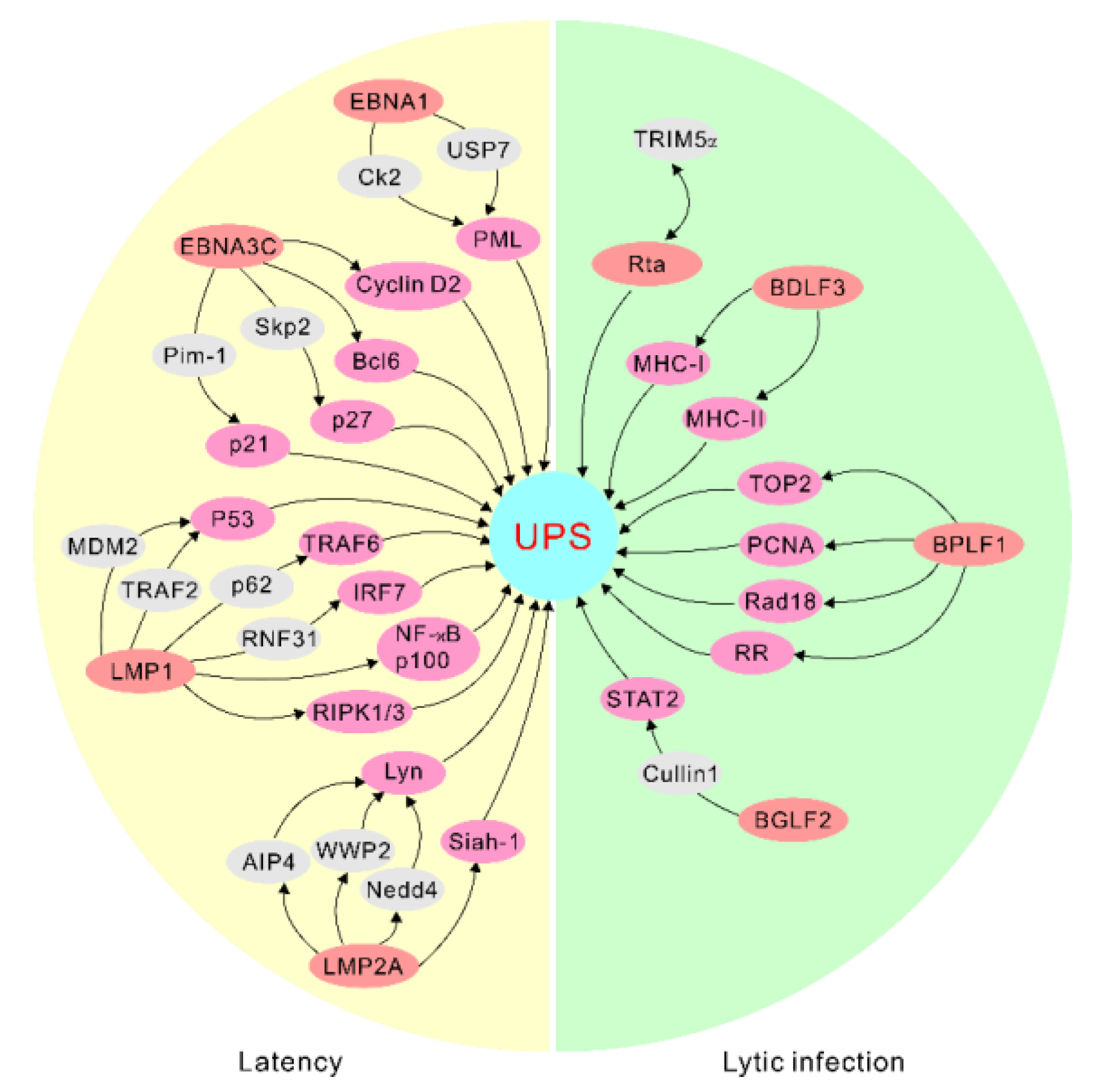

| EBV Life Cycle | EBV Antigens | Cellular Factors |
|---|---|---|
| Latent cycle | EBNA1 | USP7 CK2 PRMT5 |
| EBNA3C | Bcl6 Cyclin D1 Cyclin D2 Skp2 Pim-1 | |
| LMP1 | RIPK1 RIPK3 P53 TRAF1 TRAF6 NF-κB2 p100 RNF31 IRF7 CHIP TRAFD1 | |
| LMP2A | AIP4 WWP2 Nedd4 Siah-1 | |
| Lytic cycle | Rta | TRIM5α |
| BDLF3 | MHC-I MHC-II | |
| BPLF1 | P62 TOP2 TRIM25 PCNA Rad18 RR | |
| BGLF2 | Cullin 1 TYK2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pei, Y.; Robertson, E.S. The Central Role of the Ubiquitin–Proteasome System in EBV-Mediated Oncogenesis. Cancers 2022, 14, 611. https://doi.org/10.3390/cancers14030611
Pei Y, Robertson ES. The Central Role of the Ubiquitin–Proteasome System in EBV-Mediated Oncogenesis. Cancers. 2022; 14(3):611. https://doi.org/10.3390/cancers14030611
Chicago/Turabian StylePei, Yonggang, and Erle S. Robertson. 2022. "The Central Role of the Ubiquitin–Proteasome System in EBV-Mediated Oncogenesis" Cancers 14, no. 3: 611. https://doi.org/10.3390/cancers14030611
APA StylePei, Y., & Robertson, E. S. (2022). The Central Role of the Ubiquitin–Proteasome System in EBV-Mediated Oncogenesis. Cancers, 14(3), 611. https://doi.org/10.3390/cancers14030611







