Additive Intralesional Interleukin-2 Improves Progression-Free Survival in a Distinct Subgroup of Melanoma Patients with Prior Progression under Immunotherapy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Interleukin-2 Treatment
2.3. Histology and Immunohistochemistry
2.4. Statistical Analysis
3. Results
3.1. Clinical Characterization of Patients
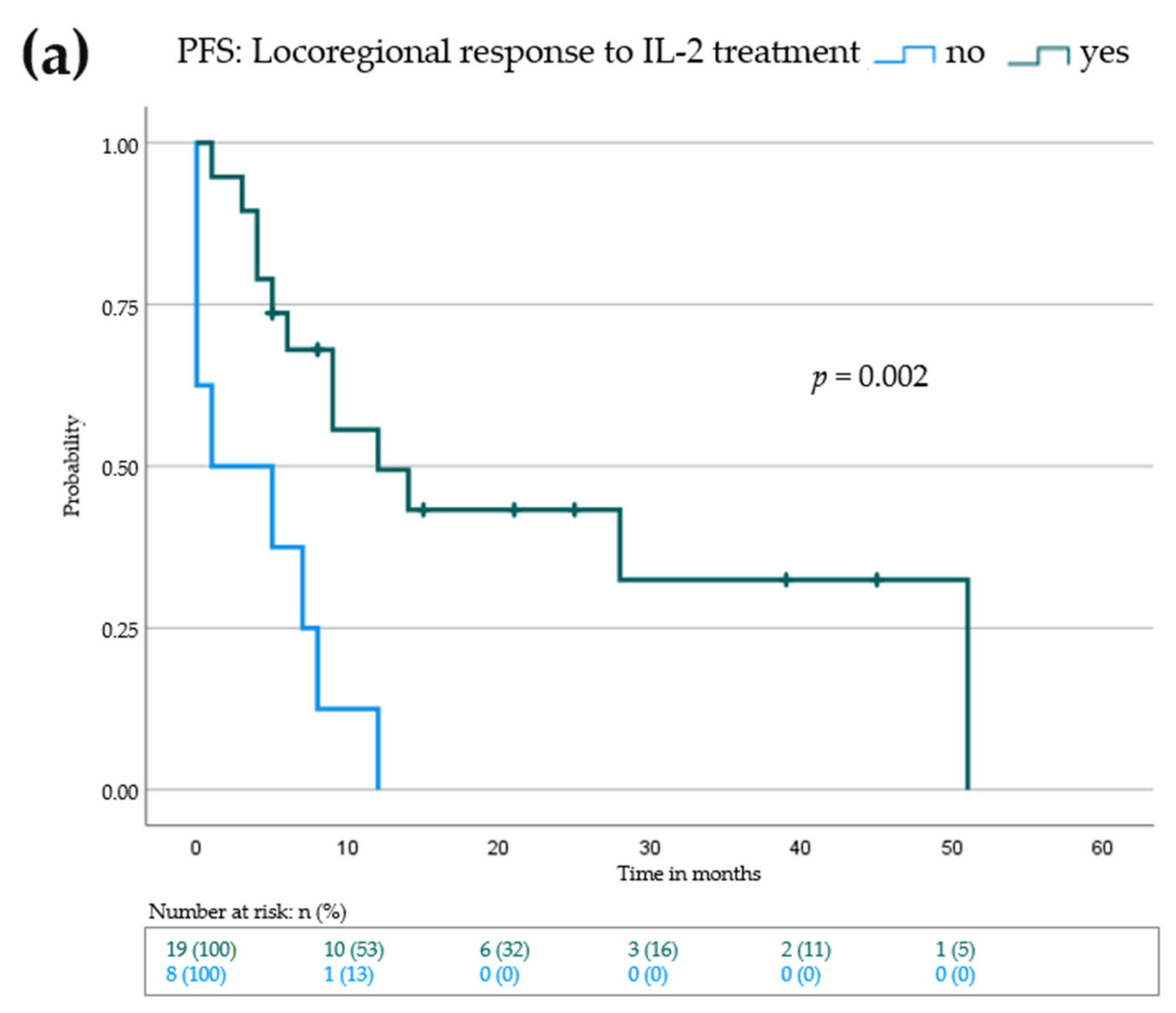
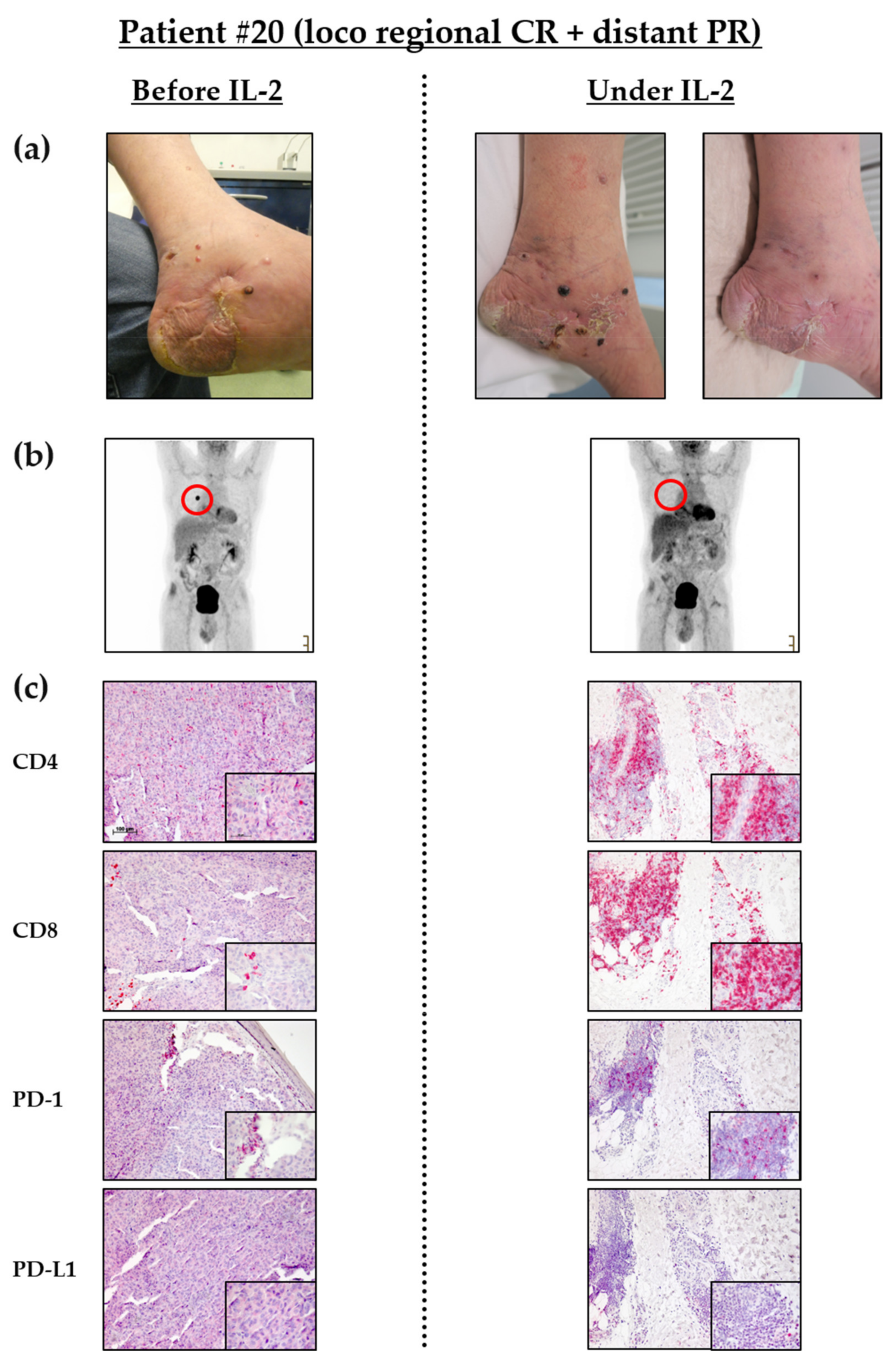
3.2. Intralesional IL-2 Treatment Leads to Locoregional Response in Most Patients and Can Induce Abscopal Effects in Distant Metastases
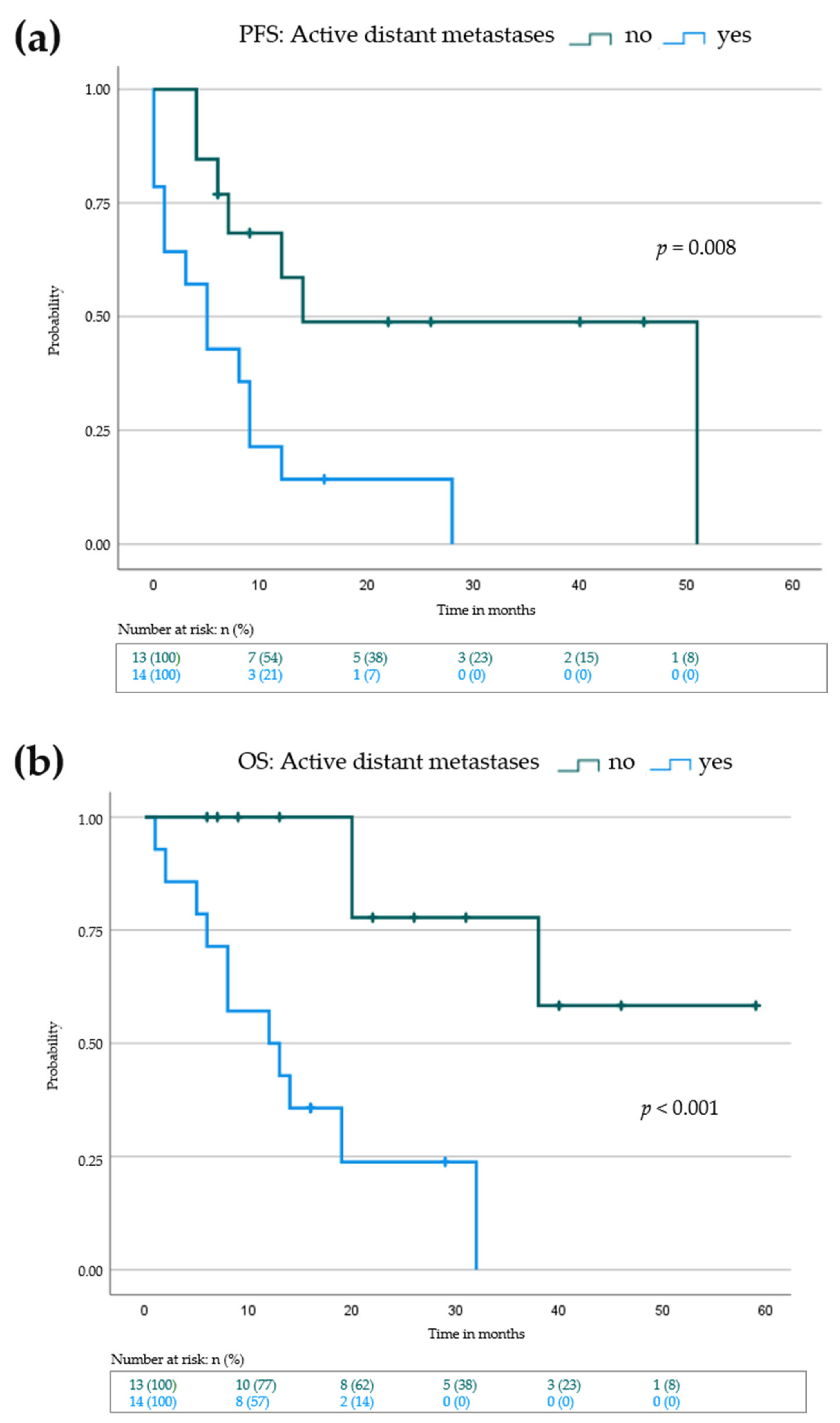
3.3. Lack of Active Distant Metastases and Locoregional Response to IL-2 Is Associated with Prolonged Progression-Free and Overall Survival
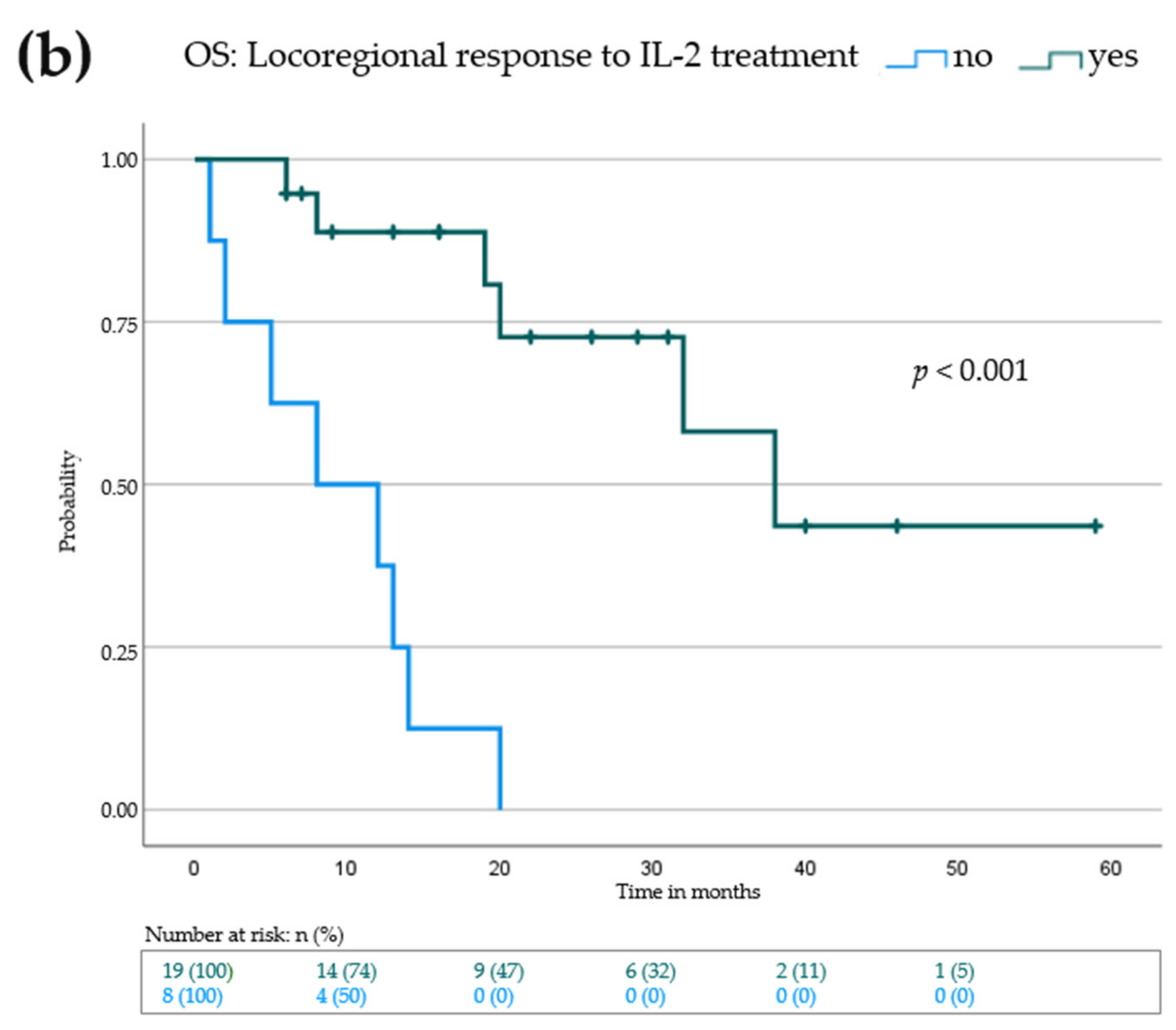
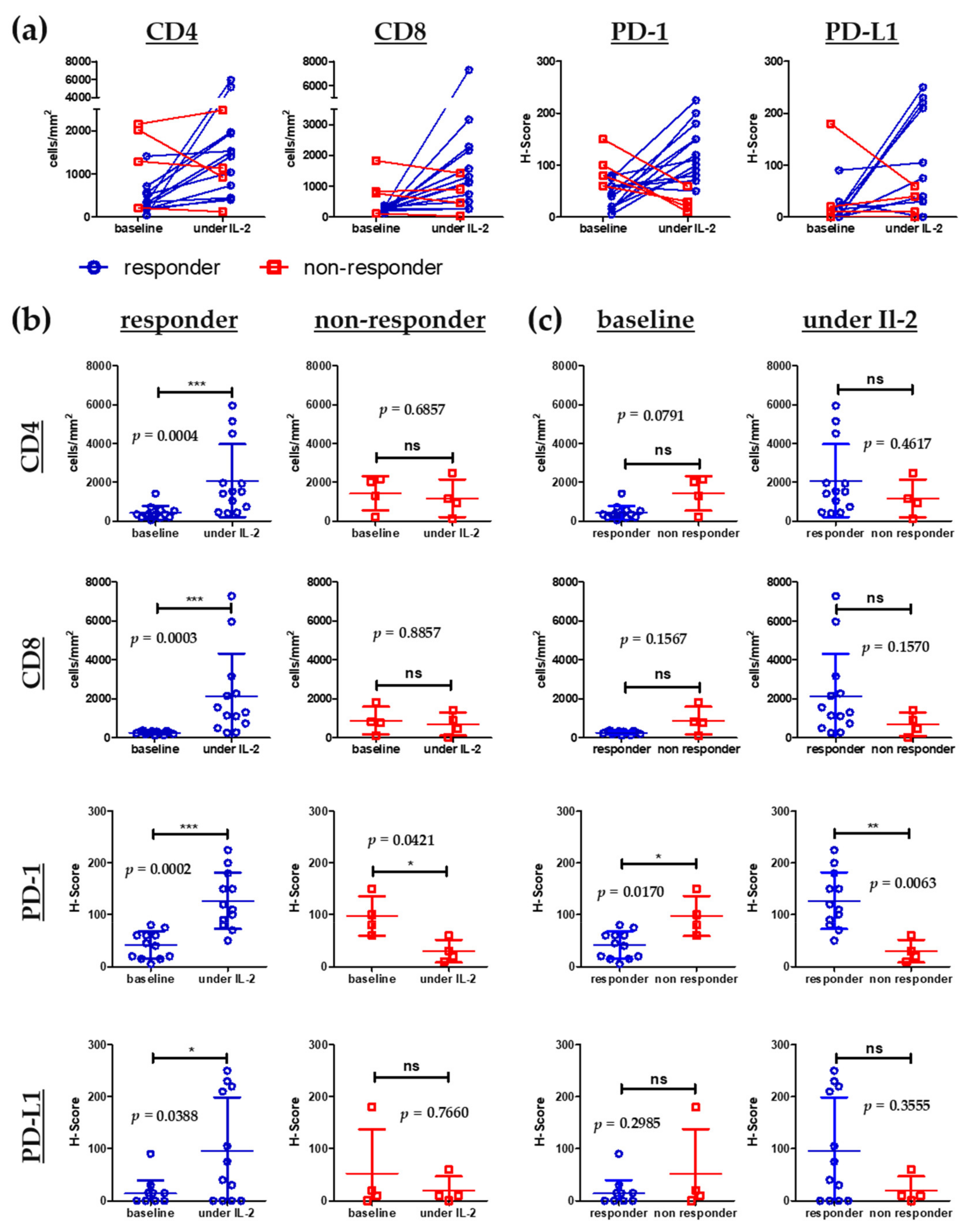
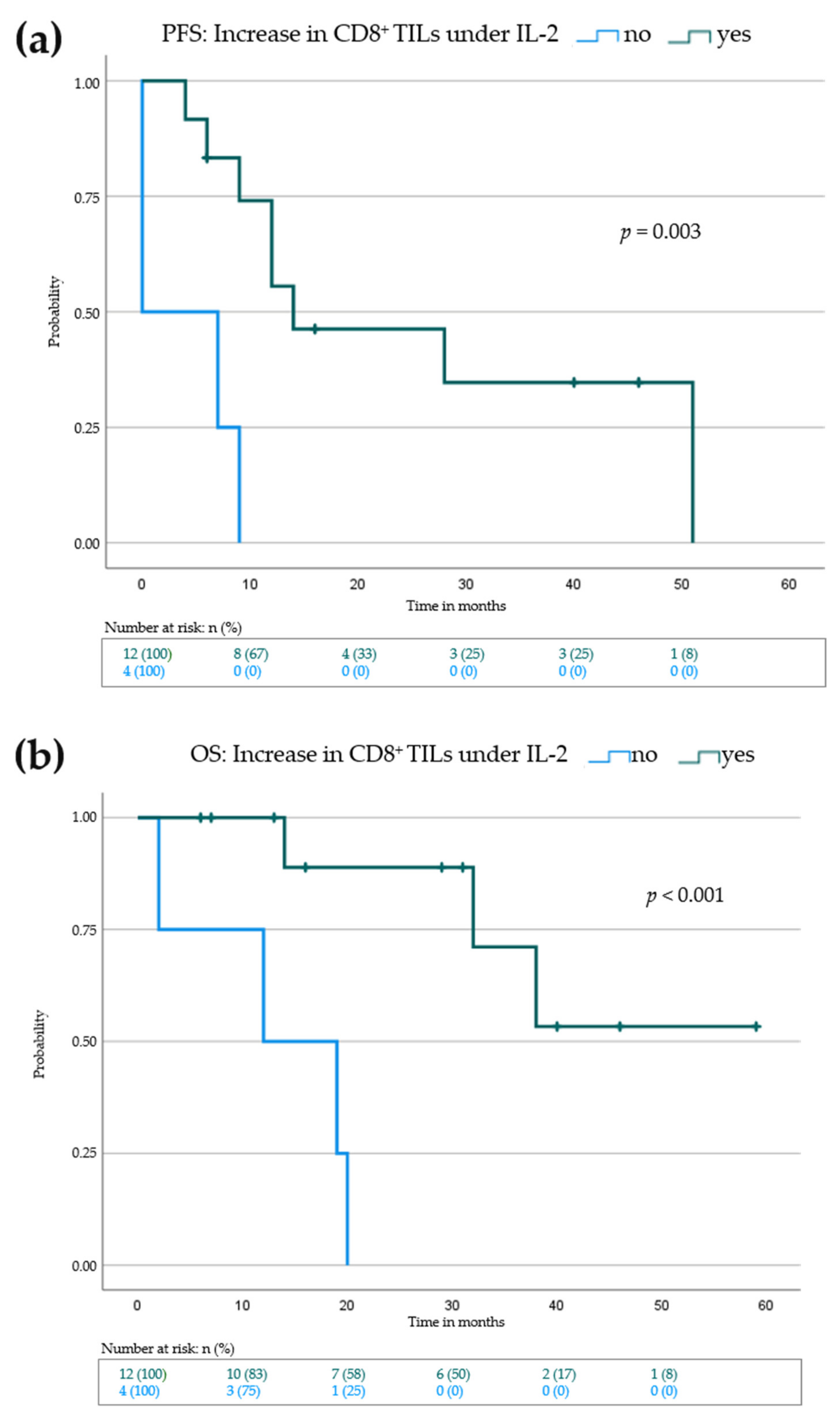
3.4. Elevation of Eosinophils in the Peripheral Blood and Increase of CD8+ Tumor Infiltrating Lymphocytes Is Associated with Improved Progression-Free and Overall Survival
3.5. Influx of CD8+ T Cells into Melanoma Metastases Correlates with Clinical Response
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marshall, H.T.; Djamgoz, M.B.A. Immuno-Oncology: Emerging Targets and Combination Therapies. Front. Oncol. 2018, 8, 315. [Google Scholar] [CrossRef] [PubMed]
- Pires da Silva, I.; Ahmed, T.; Reijers, I.L.M.; Weppler, A.M.; Betof Warner, A.; Patrinely, J.R.; Serra-Bellver, P.; Allayous, C.; Mangana, J.; Nguyen, K.; et al. Ipilimumab Alone or Ipilimumab plus Anti-PD-1 Therapy in Patients with Metastatic Melanoma Resistant to Anti-PD-(L)1 Monotherapy: A Multicentre, Retrospective, Cohort Study. Lancet Oncol. 2021, 22, 836–847. [Google Scholar] [CrossRef]
- Zaremba, A.; Philip, M.; Hassel, J.C.; Glutsch, V.; Fiocco, Z.; Loquai, C.; Rafei-Shamsabadi, D.; Gutzmer, R.; Utikal, J.; Haferkamp, S.; et al. Clinical Characteristics and Therapy Response in Unresectable Melanoma Patients Stage IIIB-IIID with in-Transit and Satellite Metastases. Eur. J. Cancer 2021, 152, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Weide, B.; Derhovanessian, E.; Pflugfelder, A.; Eigentler, T.K.; Radny, P.; Zelba, H.; Pföhler, C.; Pawelec, G.; Garbe, C. High Response Rate after Intratumoral Treatment with Interleukin-2: Results from a Phase 2 Study in 51 Patients with Metastasized Melanoma. Cancer 2010, 116, 4139–4146. [Google Scholar] [CrossRef]
- Danielli, R.; Patuzzo, R.; Di Giacomo, A.M.; Gallino, G.; Di Florio, A.; Cutaia, O.; Lazzeri, A.; Fazio, C.; Giovannoni, L.; Ruffini, P.A.; et al. A Phase II Study of Intratumoral Application of L19IL2/L19TNF in Melanoma Patients in Clinical Stage III or Stage IV M1a with Presence of Injectable Cutaneous and/or Subcutaneous Lesions. J. Clin. Oncol. 2014, 32, 5. [Google Scholar] [CrossRef]
- Danielli, R.; Patuzzo, R.; Ruffini, P.A.; Maurichi, A.; Giovannoni, L.; Elia, G.; Neri, D.; Santinami, M. Armed Antibodies for Cancer Treatment: A Promising Tool in a Changing Era. Cancer Immunol. Immunother. 2015, 64, 113–121. [Google Scholar] [CrossRef]
- Danielli, R.; Patuzzo, R.; Di Giacomo, A.M.; Gallino, G.; Maurichi, A.; Di Florio, A.; Cutaia, O.; Lazzeri, A.; Fazio, C.; Miracco, C.; et al. Intralesional Administration of L19-IL2/L19-TNF in Stage III or Stage IVM1a Melanoma Patients: Results of a Phase II Study. Cancer Immunol. Immunother. 2015, 64, 999–1009. [Google Scholar] [CrossRef]
- Andtbacka, R.H.I.; Kaufman, H.L.; Collichio, F.; Amatruda, T.; Senzer, N.; Chesney, J.; Delman, K.A.; Spitler, L.E.; Puzanov, I.; Agarwala, S.S.; et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. JCO 2015, 33, 2780–2788. [Google Scholar] [CrossRef]
- Ribas, A.; Dummer, R.; Puzanov, I.; VanderWalde, A.; Andtbacka, R.H.I.; Michielin, O.; Olszanski, A.J.; Malvehy, J.; Cebon, J.; Fernandez, E.; et al. Oncolytic Virotherapy Promotes Intratumoral T Cell Infiltration and Improves Anti-PD-1 Immunotherapy. Cell 2017, 170, 1109–1119. [Google Scholar] [CrossRef] [Green Version]
- Milhem, M.; Gonzales, R.; Medina, T.; Kirkwood, J.M.; Buchbinder, E.; Mehmi, I.; Niu, J.; Shaheen, M.; Weight, R.; Margolin, K.; et al. Intratumoral Toll-like Receptor 9 (TLR9) Agonist, CMP-001, in Combination with Pembrolizumab Can Reverse Resistance to PD-1 Inhibition in a Phase Ib Trial in Subjects with Advanced Melanoma [Abstract]. Cancer Res. 2018, 78, CT144. [Google Scholar] [CrossRef]
- Rafei-Shamsabadi, D.; Lehr, S.; von Bubnoff, D.; Meiss, F. Successful Combination Therapy of Systemic Checkpoint Inhibitors and Intralesional Interleukin-2 in Patients with Metastatic Melanoma with Primary Therapeutic Resistance to Checkpoint Inhibitors Alone. Cancer Immunol. Immunother. 2019, 68, 1417–1428. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Hirsch, F.R.; Varella-Garcia, M.; Bunn, P.A.; Di Maria, M.V.; Veve, R.; Bremnes, R.M.; Barón, A.E.; Zeng, C.; Franklin, W.A. Epidermal Growth Factor Receptor in Non–Small-Cell Lung Carcinomas: Correlation Between Gene Copy Number and Protein Expression and Impact on Prognosis. JCO 2003, 21, 3798–3807. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open Source Software for Digital Pathology Image Analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Postow, M.A.; Callahan, M.K.; Barker, C.A.; Yamada, Y.; Yuan, J.; Kitano, S.; Mu, Z.; Rasalan, T.; Adamow, M.; Ritter, E.; et al. Immunologic Correlates of the Abscopal Effect in a Patient with Melanoma. N. Engl. J. Med. 2012, 366, 925–931. [Google Scholar] [CrossRef] [Green Version]
- Kodet, O.; Němejcova, K.; Strnadová, K.; Havlínová, A.; Dundr, P.; Krajsová, I.; Štork, J.; Smetana, K.; Lacina, L. The Abscopal Effect in the Era of Checkpoint Inhibitors. Int. J. Mol. Sci. 2021, 22, 7204. [Google Scholar] [CrossRef] [PubMed]
- Zawit, M.; Swami, U.; Awada, H.; Arnouk, J.; Milhem, M.; Zakharia, Y. Current Status of Intralesional Agents in Treatment of Malignant Melanoma. Ann. Transl. Med. 2021, 9, 1038. [Google Scholar] [CrossRef] [PubMed]
- Puglisi, R.; Bellenghi, M.; Pontecorvi, G.; Pallante, G.; Carè, A.; Mattia, G. Biomarkers for Diagnosis, Prognosis and Response to Immunotherapy in Melanoma. Cancers 2021, 13, 2875. [Google Scholar] [CrossRef] [PubMed]
- Weide, B.; Martens, A.; Hassel, J.C.; Berking, C.; Postow, M.A.; Bisschop, K.; Simeone, E.; Mangana, J.; Schilling, B.; Di Giacomo, A.M.; et al. Baseline Biomarkers for Outcome of Melanoma Patients Treated with Pembrolizumab. Clin. Cancer Res. 2016, 22, 5487–5496. [Google Scholar] [CrossRef] [Green Version]
- Beyer, M. Interleukin-2 Treatment of Tumor Patients Can Expand Regulatory T Cells. Oncoimmunology 2012, 1, 1181–1182. [Google Scholar] [CrossRef] [Green Version]
- Mooradian, M.J.; Reuben, A.; Prieto, P.A.; Hazar-Rethinam, M.; Frederick, D.T.; Nadres, B.; Piris, A.; Juneja, V.; Cooper, Z.A.; Sharpe, A.H.; et al. A Phase II Study of Combined Therapy with a BRAF Inhibitor (Vemurafenib) and Interleukin-2 (Aldesleukin) in Patients with Metastatic Melanoma. OncoImmunology 2018, 7, e1423172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.; Guo, Y.; Liu, S.; Wang, H.; Zhu, J.; Ou, L.; Xu, X. Targeting Regulatory T Cells for Immunotherapy in Melanoma. Mol. Biomed. 2021, 2, 11. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Leonard, W.J. Biology of IL-2 and Its Therapeutic Modulation: Mechanisms and Strategies. J. Leukoc. Biol. 2018, 103, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Overwijk, W.W.; Tagliaferri, M.A.; Zalevsky, J. Engineering IL-2 to Give New Life to T Cell Immunotherapy. Annu. Rev. Med. 2021, 72, 281–311. [Google Scholar] [CrossRef]
- Charych, D.H.; Hoch, U.; Langowski, J.L.; Lee, S.R.; Addepalli, M.K.; Kirk, P.B.; Sheng, D.; Liu, X.; Sims, P.W.; VanderVeen, L.A.; et al. NKTR-214, an Engineered Cytokine with Biased IL2 Receptor Binding, Increased Tumor Exposure, and Marked Efficacy in Mouse Tumor Models. Clin. Cancer Res. 2016, 22, 680–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charych, D.; Khalili, S.; Dixit, V.; Kirk, P.; Chang, T.; Langowski, J.; Rubas, W.; Doberstein, S.K.; Eldon, M.; Hoch, U.; et al. Modeling the Receptor Pharmacology, Pharmacokinetics, and Pharmacodynamics of NKTR-214, a Kinetically-Controlled Interleukin-2 (IL2) Receptor Agonist for Cancer Immunotherapy. PLoS ONE 2017, 12, e0179431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miura, J.T.; Zager, J.S. Neo-DREAM Study Investigating Daromun for the Treatment of Clinical Stage IIIB/C Melanoma. Future Oncol. 2019, 15, 3665–3674. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Patients (n = 27) |
|---|---|
| Median age (years) | 73 (22–88) |
| Sex | |
| Male | 15 (56%) |
| Female | 12 (44%) |
| ECOG 1 | |
| 0 | 21 (78%) |
| 1 | 6 (22%) |
| AJCC 2 stage (2017) | |
| III | 11 (41%) |
| IIIB | 2 (7%) |
| IIIC | 9 (34%) |
| IV | 16 (59%) |
| M1a | 3 (11%) |
| M1b | 4 (15%) |
| M1c | 7 (26%) |
| M1d | 2 (7%) |
| Baseline LDH 3 | |
| Normal | 13 (48%) |
| Elevated | 8 (30%) |
| NA 4 | 6 (22%) |
| Baseline S100 | |
| Normal | 14 (52%) |
| Elevated | 5 (19%) |
| NA | 8 (29%) |
| Baseline NLR 5 | |
| ≤2.5 (n = 12) | 12 (44%) |
| >2.5 (n = 12) | 12 (44%) |
| NA | 3 (11%) |
| BRAF-V600-Mutation | |
| Wild type | 22 (81%) |
| Positive | 5 (19%) |
| Median number of locoregional metastases | 10 (3–100) |
| Locoregional Progression on | |
| Adjuvant therapy | 9 (33%) |
| Palliative therapy | 18 (67%) |
| Type/localization of primary melanoma | |
| Cutaneous | 13 (48%) |
| Acral lentiginous | 11 (40%) |
| Uveal | 1 (4%) |
| CUP 6 | 1 (4%) |
| Mucosal | 1 (4%) |
| Median follow up time, months | 16 (1–59) |
| Type of Response | Patients |
|---|---|
| Locoregional (Stage III and IV) 1 | (n = 27) |
| Complete response | 11 (41%) |
| Partial response | 9 (33%) |
| Progressive disease | 7 (26%) |
| Distant organ metastases (Stage IV only) 1 | (n = 16) |
| Complete response | 1 (6%) |
| Partial response | 5 (31%) |
| Stable disease | 1 (6%) |
| Progressive disease | 7 (44%) |
| NA | 2 (13%) |
| Condition | Median PFS | 95% CI 1 | p Values |
|---|---|---|---|
| Sex | |||
| Female (n = 12) | 9 | 3.0–17.9 | 0.468 |
| Male (n = 15) | 7 | 1.8–12.2 | |
| Age over 70 | |||
| No (n = 12) | 12 | 6.9–17.0 | 0.613 |
| Yes (n = 15) | 7 | 3.5–10.5 | |
| Locoregional progression on: | |||
| Adjuvant therapy (n = 9) | 14 | - * | 0.125 |
| Palliative therapy (n = 18) | 6 | 1.8–10.2 | |
| Active distant metastases at baseline | |||
| No (n = 13) | 14 | 0.0–33.5 | 0.008 |
| Yes (n = 14) | 5 | 1.4–8.6 | |
| Baseline LDH 2 | |||
| Normal (n = 13) | 9 | 0.0–19.6 | 0.716 |
| Elevated (n = 8) | 9 | 2.5–15.5 | |
| Baseline S100 | |||
| Normal (n = 14) | 12 | 2.8–21.2 | 0.321 |
| Elevated (n = 5) | 5 | 0.0–13.6 | |
| Baseline NLR 3 | |||
| ≤2.5 (n = 12) | 9 | 0.5–17.5 | 0.706 |
| >2.5 (n = 12) | 8 | 2.0–13.9 | |
| BRAF-V600-Mutation | |||
| Wild type (n = 22) | 8 | 4.7–11.3 | 0.717 |
| Positive (5) | 14 | 0.0–29.6 |
| Condition | Median PFS | 95% CI 1 | p Values |
|---|---|---|---|
| Increase of AEC 2 under IL-2 | |||
| No (n = 12) | 3 | 0.0–8.9 | <0.001 |
| Yes (n = 13) | 28 | 5.9–12.1 | |
| Locoregional response to IL-2 | |||
| No (n = 8) | 1 | 0.0–7.9 | 0.002 |
| Yes (n = 19) | 12 | 2.5–21.5 | |
| Increase in CD4+ TILs 3 | |||
| No (n = 5) | 7 | 0.0–22.0 | 0.140 |
| Yes (n = 11) | 14 | 9.0–18.9 | |
| Increase in CD8+ TILs | - * | ||
| No (n = 4) | 0 | 0.0–36.6 | 0.003 |
| Yes (n = 12) | 14 | ||
| Increase in PD-1 staining 4 | |||
| No (n = 6) | 7 | 0.0–17.8 | 0.365 |
| Yes (n = 10) | 12 | 7.2–16.7 | |
| Increase in PD-L1 staining 5 | |||
| No (n = 9) | 12 | 3.2–20.7 | 0.669 |
| Yes (n = 7) | 12 | 5.5–18.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rafei-Shamsabadi, D.; Lehr, S.; Behrens, M.; Meiss, F. Additive Intralesional Interleukin-2 Improves Progression-Free Survival in a Distinct Subgroup of Melanoma Patients with Prior Progression under Immunotherapy. Cancers 2022, 14, 540. https://doi.org/10.3390/cancers14030540
Rafei-Shamsabadi D, Lehr S, Behrens M, Meiss F. Additive Intralesional Interleukin-2 Improves Progression-Free Survival in a Distinct Subgroup of Melanoma Patients with Prior Progression under Immunotherapy. Cancers. 2022; 14(3):540. https://doi.org/10.3390/cancers14030540
Chicago/Turabian StyleRafei-Shamsabadi, David, Saskia Lehr, Max Behrens, and Frank Meiss. 2022. "Additive Intralesional Interleukin-2 Improves Progression-Free Survival in a Distinct Subgroup of Melanoma Patients with Prior Progression under Immunotherapy" Cancers 14, no. 3: 540. https://doi.org/10.3390/cancers14030540
APA StyleRafei-Shamsabadi, D., Lehr, S., Behrens, M., & Meiss, F. (2022). Additive Intralesional Interleukin-2 Improves Progression-Free Survival in a Distinct Subgroup of Melanoma Patients with Prior Progression under Immunotherapy. Cancers, 14(3), 540. https://doi.org/10.3390/cancers14030540






