Association of Preoperative Physical Activity with Short- and Long-Term Outcomes in Patients Undergoing Palliative Resection for Metastatic Colorectal Cancer: An Inverse Probability of Treatment Weighting Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. MET Values
2.3. Inverse Probability of Treatment Weighting
2.4. Outcomes and Covariables
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Short-Term Outcomes
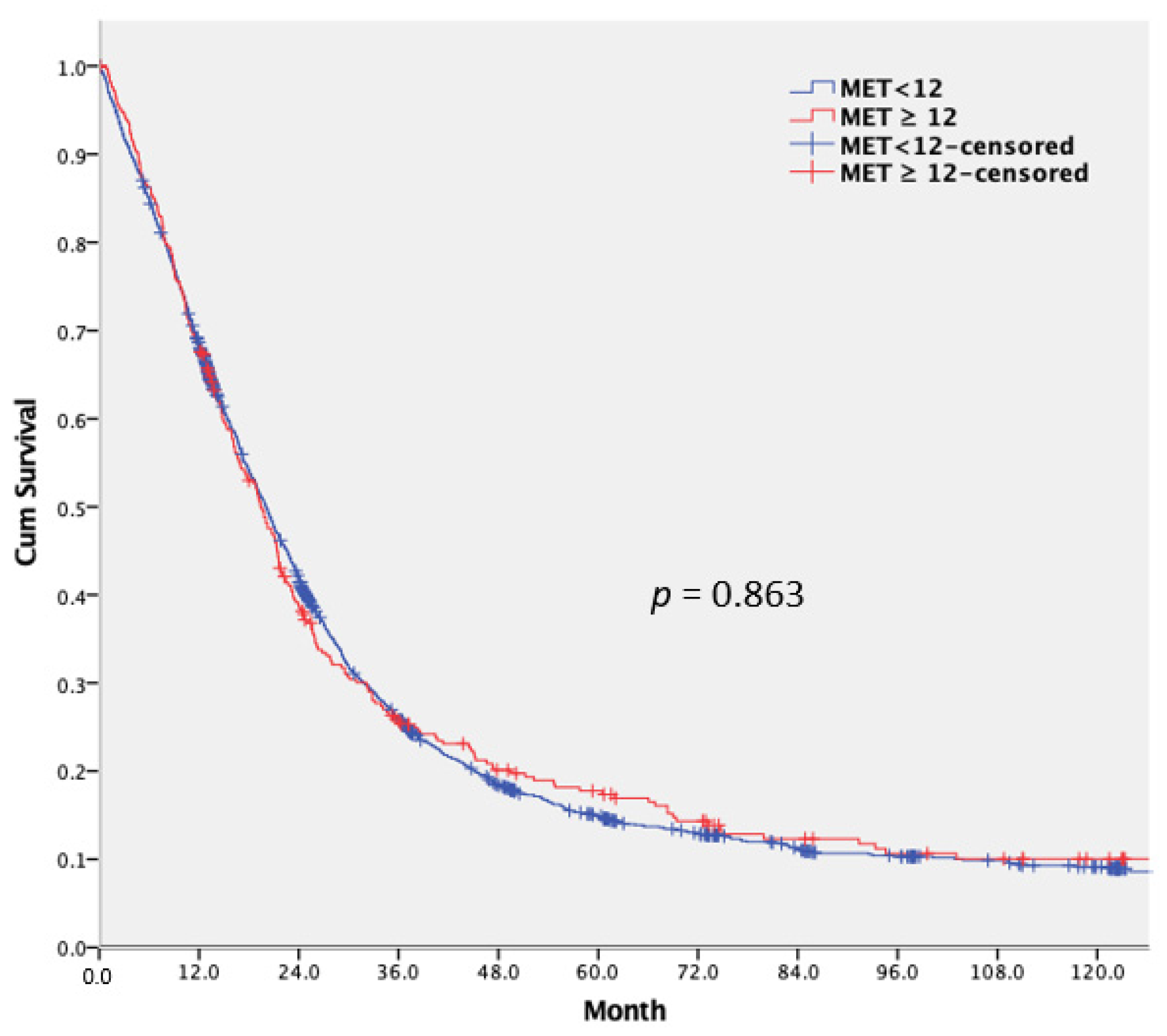
3.3. Long-Term Outcomes and Prognostic Factor Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Syngal, S.; Brand, R.E.; Church, J.M.; Giardiello, F.M.; Hampel, H.L.; Burt, R.W. ACG clinical guideline: Genetic testing and management of hereditary gastrointestinal cancer syndromes. Am. J. Gastroenterol. 2015, 110, 223–262. [Google Scholar] [CrossRef] [Green Version]
- Henrikson, N.B.; Webber, E.M.; Goddard, K.A.; Scrol, A.; Piper, M.; Williams, M.S.; Zallen, D.T.; Calonge, N.; Ganiats, T.G.; Janssens, A.C.; et al. Family history and the natural history of colorectal cancer: Systematic review. Genet. Med. 2015, 17, 702–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krämer, H.U.; Schottker, B.; Raum, E.; Brenner, H. Type 2 diabetes mellitus and colorectal cancer: Meta-analysis on sex-specific differences. Eur. J. Cancer 2012, 48, 1269–1282. [Google Scholar] [CrossRef]
- Moore, S.C.; Lee, I.M.; Weiderpass, E.; Campbell, P.T.; Sampson, J.N.; Kitahara, C.M.; Keadle, S.K.; Arem, H.; Berrington de Gonzalez, A.; Hartge, P.; et al. Association of leisure-time physical activity with risk of 26 types of cancer in 1.44 million adults. JAMA Intern Med. 2016, 176, 816–825. [Google Scholar] [CrossRef]
- Botteri, E.; Iodice, S.; Bagnardi, V.; Raimondi, S.; Lowenfels, A.B.; Maisonneuve, P. Smoking and colorectal cancer: A meta-analysis. JAMA 2008, 300, 2765–2778. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Li, Y.; Ding, Y.; Chen, K.; Jin, M. Alcohol drinking and the risk of colorectal cancer death: A meta-analysis. Eur. J. Cancer Prev. 2014, 23, 532–539. [Google Scholar] [CrossRef]
- Kyrgiou, M.; Kalliala, I.; Markozannes, G.; Gunter, M.J.; Paraskevaidis, E.; Gabra, H.; Martin-Hirsch, P.; Tsilidis, K.K. Adiposity and cancer at major anatomical sites: Umbrella review of the literature. BMJ 2017, 356, j477. [Google Scholar] [CrossRef] [Green Version]
- Chan, D.S.; Lau, R.; Aune, D.; Vieira, R.; Greenwood, D.C.; Kampman, E.; Norat, T. Red and processed meat and colorectal cancer incidence: Meta-analysis of prospective studies. PLoS ONE 2011, 6, e20456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matthews, C.E.; Moore, S.C.; Arem, H.; Cook, M.B.; Trabert, B.; Håkansson, N.; Larsson, S.C.; Wolk, A.; Gapstur, S.M.; Lynch, B.M.; et al. Amount and intensity of leisure-time physical activity and lower cancer risk. J. Clin. Oncol. 2020, 38, 686–697. [Google Scholar] [CrossRef]
- Lee, C.H.A.; Kong, J.C.; Ismail, H.; Riedel, B.; Heriot, A. Systematic review and meta-analysis of objective assessment of physical fitness in patients undergoing colorectal cancer surgery. Dis. Colon Rectum 2018, 61, 400–409. [Google Scholar] [CrossRef]
- You, J.F.; Hsu, Y.J.; Chern, Y.J.; Hung, H.Y.; Hsieh, P.S.; Yeh, C.Y.; Chiang, J.M.; Tsai, W.S. Association of a Preoperative Leisure-Time Physical Activity With Short-and Long-term Outcomes of Patients Undergoing Curative Resection for Stage I to III Colorectal Cancer: A Propensity Score Matching Analysis. Dis. Colon Rectum 2020, 63, 796–806. [Google Scholar] [CrossRef]
- Ainsworth, B.E.; Haskell, W.L.; Herrmann, S.D.; Meckes, N.; Bassett, D.R., Jr.; Tudor-Locke, C.; Greer, J.L.; Vezina, J.; Whitt-Glover, M.C.; Leon, A.S. 2011 Compendium of physical activities: A second update of codes and MET values. Med. Sci. Sports Exerc. 2011, 43, 1575–1581. [Google Scholar] [CrossRef] [Green Version]
- Ainsworth, B.E.; Haskell, W.L.; Leon, A.S.; Jacobs, D.R., Jr.; Montoye, H.J.; Sallis, J.F.; Paffenbarger, R.S., Jr. Compendium of physical activities: Classification of energy costs of human physical activities. Med. Sci. Sports Exerc. 1993, 25, 71–80. [Google Scholar] [CrossRef]
- Ainsworth, B.E.; Haskell, W.L.; Whitt, M.C.; Irwin, M.L.; Swartz, A.M.; Strath, S.J.; O’Brien, W.L.; Bassett, D.R., Jr.; Schmitz, K.H.; Emplaincourt, P.O.; et al. Compendium of physical activities: An update of activity codes and MET intensities. Med. Sci. Sports Exerc. 2000, 32 (Suppl. S9), S498–S504. [Google Scholar] [CrossRef] [Green Version]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef] [Green Version]
- Wolin, K.Y.; Yan, Y.; Colditz, G.A.; Lee, I.M. Physical activity and colon cancer prevention: A meta-analysis. Br. J. Cancer 2009, 100, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Friedenreich, C.; Norat, T.; Steindorf, K.; Boutron-Ruault, M.C.; Pischon, T.; Mazuir, M.; Clavel-Chapelon, F.; Linseisen, J.; Boeing, H.; Bergman, M.; et al. Physical activity and risk of colon and rectal cancers: The European prospective investigation into cancer and nutrition. Cancer Epidemiol. Prev. Biomark. 2006, 15, 2398–2407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onerup, A.; Bock, D.; Börjesson, M.; Fagevik Olsén, M.; Gellerstedt, M.; Haglind, E.; Nilsson, H.; Angenete, E. Is preoperative physical activity related to post-surgery recovery?-a cohort study of colorectal cancer patients. Int. J. Colorectal Dis. 2016, 31, 1131–1140. [Google Scholar] [CrossRef] [PubMed]
- West, M.A.; Lythgoe, D.; Barben, C.P.; Noble, L.; Kemp, G.J.; Jack, S.; Grocott, M.P. Cardiopulmonary exercise variables are associated with postoperative morbidity after major colonic surgery: A prospective blinded observational study. Br. J. Anaesth. 2014, 112, 665–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nutt, C.L.; Russell, J.C. Use of the pre-operative shuttle walk test to predict morbidity and mortality after elective major colorectal surgery. Anaesthesia 2012, 67, 839–849. [Google Scholar] [CrossRef]
- Lai, C.W.; Minto, G.; Challand, C.P.; Hosie, K.B.; Sneyd, J.R.; Creanor, S.; Struthers, R.A. Patients’ inability to perform a preoperative cardiopulmonary exercise test or demonstrate an anaerobic threshold is associated with inferior outcomes after major colorectal surgery. Br. J. Anaesth. 2013, 111, 607–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikolopoulos, I.; Ellwood, M.; George, M.; Carapeti, E.; Williams, A. Cardiopulmonary exercise testing versus spirometry as predictors of cardiopulmonary complications after colorectal surgery. Eur. Surg. 2015, 47, 324–330. [Google Scholar] [CrossRef]
- Van Blarigan, E.L.; Fuchs, C.S.; Niedzwiecki, D.; Zhang, S.; Saltz, L.B.; Mayer, R.J.; Mowat, R.B.; Whittom, R.; Hantel, A.; Benson, A.; et al. Association of survival with adherence to the American Cancer Society Nutrition and Physical Activity Guidelines for Cancer Survivors After Colon Cancer Diagnosis: The CALGB 89803/Alliance Trial. JAMA Oncol. 2018, 4, 783–790. [Google Scholar] [CrossRef]
- Phipps, A.I.; Shi, Q.; Zemla, T.J.; Dotan, E.; Gill, S.; Goldberg, R.M.; Hardikar, S.; Jahagirdar, B.; Limburg, P.J.; Newcomb, P.A.; et al. Physical activity and outcomes in patients with stage III colon cancer: A correlative analysis of Phase III Trial NCCTG N0147 (Alliance). Cancer Epidemiol. Prev. Biomark. 2018, 27, 696–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayasekara, H.; English, D.R.; Haydon, A.; Hodge, A.M.; Lynch, B.M.; Rosty, C.; Williamson, E.J.; Clendenning, M.; Southey, M.C.; Jenkins, M.A.; et al. Associations of alcohol intake, smoking, physical activity and obesity with survival following colorectal cancer diagnosis by stage, anatomic site and tumor molecular subtype. Int. J. Cancer 2018, 142, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.C.; Zemel, B.S.; Troxel, A.B.; Rickels, M.R.; Damjanov, N.; Ky, B.; Rhim, A.D.; Rustgi, A.K.; Courneya, K.S.; Schmitz, K.H. Dose-response effects of aerobic exercise on body composition among colon cancer survivors: A randomised controlled trial. Br. J. Cancer 2017, 117, 1614–1620. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci, E. An integrative approach for deciphering the causal associations of physical activity and cancer risk: The role of adiposity. J. Natl. Cancer Inst. 2018, 110, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, L.; Idorn, M.; Olofsson, G.H.; Lauenborg, B.; Nookaew, I.; Hansen, R.H.; Johannesen, H.H.; Becker, J.C.; Pedersen, K.S.; Dethlefsen, C.; et al. Voluntary running suppresses tumor growth through epinephrine- and IL-6-dependent NK cell mobilization and redistribution. Cell Metab. 2016, 23, 554–562. [Google Scholar] [CrossRef] [Green Version]
- Slattery, M.L.; Curtin, K.; Wolff, R.K.; Herrick, J.S.; Caan, B.J.; Samowitz, W. Diet, physical activity, and body size associations with rectal tumor mutations and epigenetic changes. Cancer Causes Control 2010, 21, 1237–1245. [Google Scholar] [CrossRef] [Green Version]
- Carlomagno, C.; De Stefano, A.; Rosanova, M.; De Falco, S.; Attademo, L.; Fiore, G.; De Placido, S. Multiple treatment lines and prognosis in metastatic colorectal cancer patients. Cancer Metastasis Rev. 2019, 38, 307–313. [Google Scholar] [CrossRef]
- Groot Koerkamp, B.; Rahbari, N.N.; Büchler, M.W.; Koch, M.; Weitz, J. Circulating tumor cells and prognosis of patients with resectable colorectal liver metastases or widespread metastatic colorectal cancer: A meta-analysis. Ann. Surg. Oncol. 2013, 20, 2156–2165. [Google Scholar] [CrossRef]
- Riedl, J.M.; Posch, F.; Moik, F.; Bezan, A.; Szkandera, J.; Smolle, M.A.; Kasparek, A.K.; Pichler, M.; Stöger, H.; Stotz, M.; et al. Inflammatory biomarkers in metastatic colorectal cancer: Prognostic and predictive role beyond the first line setting. Oncotarget 2017, 8, 96048–96061. [Google Scholar] [CrossRef] [Green Version]
- Nozawa, H.; Ishihara, S.; Kawai, K.; Hata, K.; Kiyomatsu, T.; Tanaka, T.; Nishikawa, T.; Otani, K.; Yasuda, K.; Sasaki, K.; et al. Conversion to Resection in Patients Receiving Systemic Chemotherapy for Unresectable and/or Metastatic Colorectal Cancer-Predictive Factors and Prognosis. Clin. Colorectal Cancer 2018, 17, e91–e97. [Google Scholar] [CrossRef] [Green Version]
- Reed, M.; Patrick, C.; Croft, B.; Walde, N.; Voutsadakis, I.A. The metabolic syndrome and its components as prognostic factors in metastatic colorectal cancer. Indian J. Gastroenterol. 2019, 38, 15–22. [Google Scholar] [CrossRef]
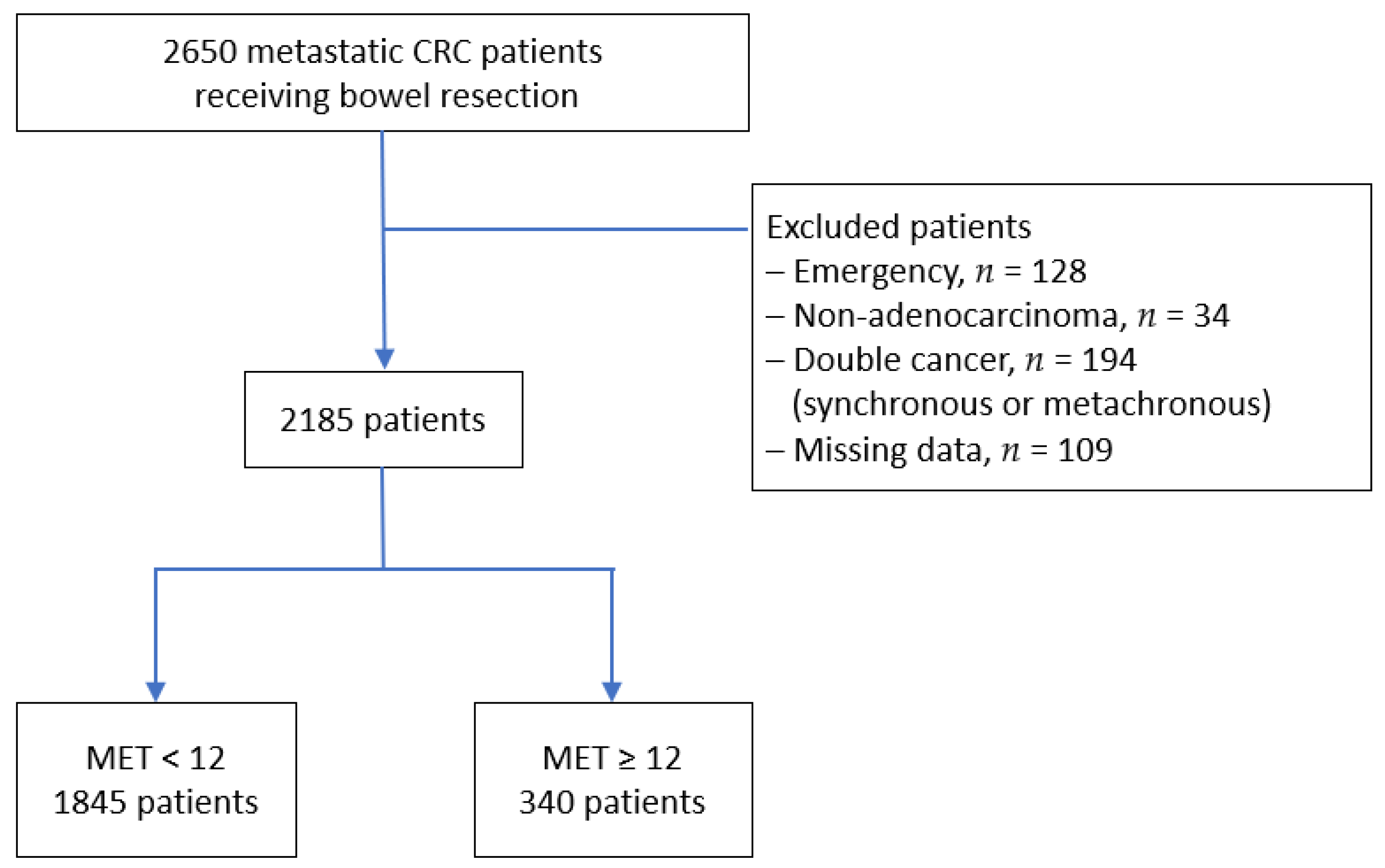
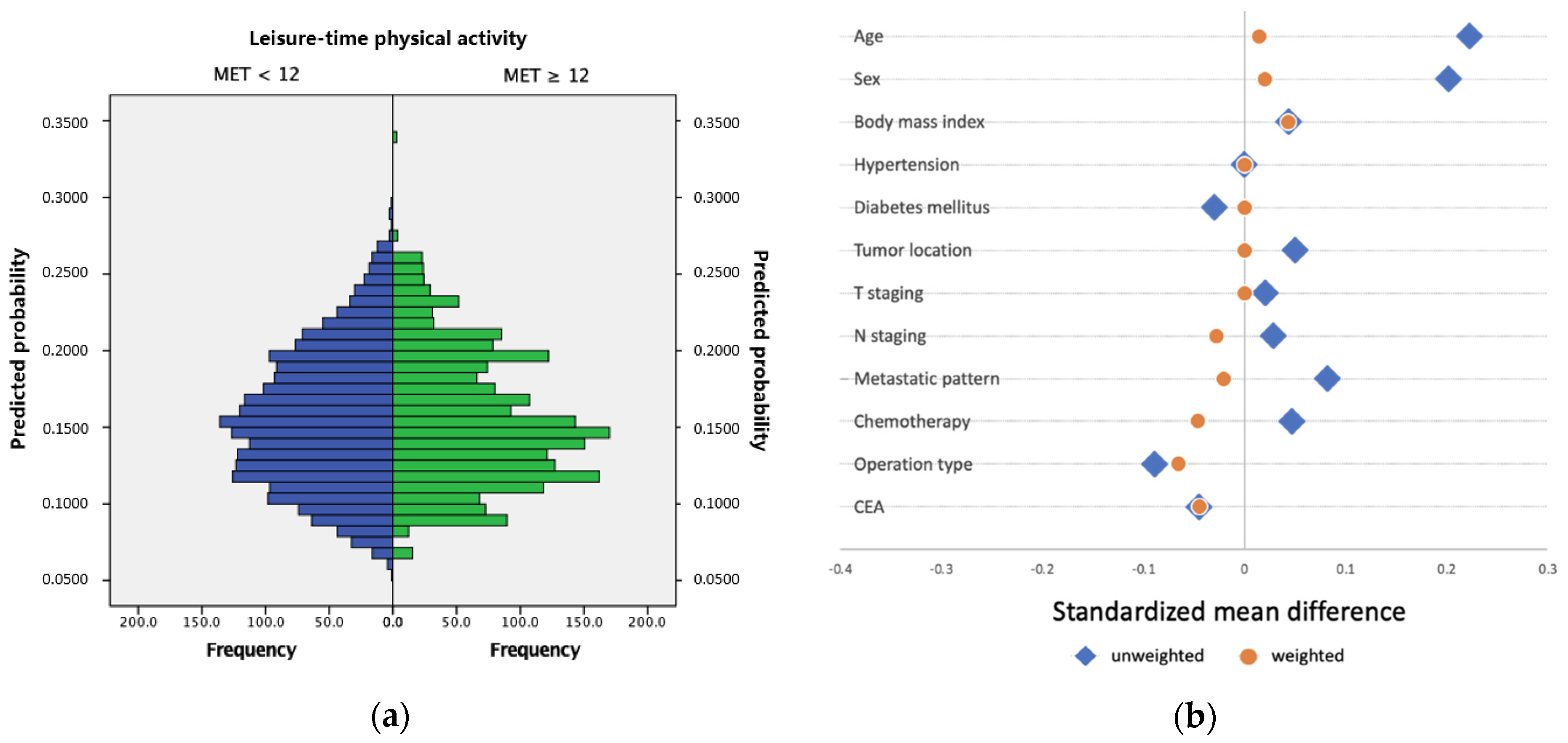
| Variables | Overall n = 2185 | MET < 12 n = 1845 | MET ≥ 12 n = 340 | p Value | IPTW-Adjusted p Value |
|---|---|---|---|---|---|
| Age, mean ± SD * | 60.59 ± 13.941 | 60.11 ± 14.161 | 63.2 ± 12.374 | <0.001 | 0.612 |
| Sex, n (%) * | <0.001 | 0.483 | |||
| Male | 1220 (55.8) | 1001 (54.3) | 219 (64.4) | ||
| Female | 965 (44.2) | 844 (45.7) | 121 (35.6) | ||
| BMI, kg/m2, n (%) | 0.464 | 0.154 | |||
| <25 | 1421 (69.9) | 1205 (70.2) | 216 (68.1) | ||
| ≥25 | 613 (30.1) | 512 (29.8) | 101 (31.9) | ||
| missing | 151 | ||||
| Underlying illness, n (%) * | |||||
| Hypertension * | 0.893 | 0.981 | |||
| Yes | 574 (26.3) | 486 (26.3) | 88 (25.9) | ||
| No | 1611 (73.7) | 1359 (73.7) | 252 (74.1) | ||
| Diabetes mellitus * | 0.484 | 0.823 | |||
| Yes | 287 (13.1) | 247 (13.4) | 40 (11.8) | ||
| No | 1898 (86.9) | 1598 (86.6) | 300 (88.2) | ||
| Tumor location, n (%) * | 0.723 | 0.876 | |||
| Right-sided colon | 603 (27.6) | 504 (27.3) | 99 (29.1) | ||
| Left-sided colon | 774 (35.4) | 653 (35.4) | 121 (35.6) | ||
| Rectum | 808 (37.0) | 688 (37.3) | 120 (35.3) | ||
| T staging, n (%) * | 0.762 | 0.804 | |||
| non-T4 | 855 (39.1) | 725 (39.3) | 130 (38.2) | ||
| T4 | 1330 (60.9) | 1120 (60.7) | 210 (61.8) | ||
| N staging, n (%) * | 0.456 | 0.226 | |||
| N0 | 325 (14.9) | 270 (14.6) | 55 (16.2) | ||
| N+ | 1860 (85.1) | 1575 (85.4) | 285 (83.8) | ||
| Metastasis, n (%) * | 0.227 | 0.555 | |||
| Single organ | 1329 (60.8) | 1112 (60.3) | 217 (63.8) | ||
| Multiple organs | 856 (39.2) | 733 (39.7) | 123 (36.2) | ||
| Chemotherapy, n (%) * | 0.582 | 0.382 | |||
| Yes | 1654 (75.7) | 1392 (75.4) | 262 (77.1) | ||
| No | 531 (24.3) | 453 (24.6) | 78 (22.9) | ||
| Operation type, n (%) | 0.867 | 0.217 | |||
| Right colectomy | 518 (23.7) | 432 (23.4) | 86 (25.3) | ||
| Left hemicolectomy | 90 (4.1) | 74 (4.0) | 16 (4.7) | ||
| Anterior resection | 1225 (56.1) | 1034 (56.0) | 191 (56.2) | ||
| APR | 79 (3.6) | 68 (3.7) | 11 (3.2) | ||
| Segmental resection | 59 (2.7) | 50 (2.7) | 9 (2.6) | ||
| Subtotal or total | 71 (3.2) | 61 (3.3) | 10 (2.9) | ||
| Hartmann operation | 143 (6.5) | 126 (6.8) | 17 (5.0) | ||
| CEA (ng/mL), n (%) | 0.459 | 0.191 | |||
| <5 | 574 (26.9) | 479 (26.6) | 95 (28.6) | ||
| ≥5 | 1560 (73.1) | 1323 (73.4) | 237 (71.4) | ||
| missing |
| Variables | MET < 12 n = 1845 | MET ≥ 12 n = 340 | IPTW-Adjusted p Value | OR (95% CI) |
|---|---|---|---|---|
| Postoperative morbidities, n (%) | ||||
| Yes | 345 (18.7) | 36 (10.6) | <0.001 | 0.532 (0.448–0.632) |
| No | 1500 (81.3) | 304 (89.4) | ||
| Type of complications, n (%) | ||||
| Wound infection | 65 (3.5) | 5 (1.5) | <0.001 | 0.377 (0.247–0.578) |
| Lung | 24 (1.3) | 2 (0.6) | 0.043 | 0.498 (0.262–0.949) |
| Cardiovascular | 8 (0.4) | 1 (0.3) | 0.607 | 0.668 (0.237–1.88) |
| Urinary tract | 57 (3.1) | 10 (2.9) | 1.0 | 1.004 (0.713–1.412) |
| Gastrointestinal | 77 (4.2) | 6 (1.8) | <0.001 | 0.383 (0.259–0.565) |
| Intraabdominal | 21 (1.1) | 2 (0.6) | 0.072 | 0.519 (0.265–1.017) |
| Anastomosis | 34 (1.8) | 3 (0.9) | 0.062 | 0.607 (0.368–1.003) |
| Others | 37 (2.0) | 4 (1.2) | 0.026 | 0.555 (0.336–0.918) |
| Postoperative mortality, n (%) | ||||
| Yes | 44 (2.4) | 2 (0.6) | <0.001 | 0.304 (0.173–0.533) |
| No | 1801 (97.6) | 338 (99.4) | ||
| Modified Clavien–Dindo classification | ||||
| Low grade (I/II) | 236 (12.8) | 25 (7.4) | <0.001 | 0.53 (0.432–0.65) |
| High grade (III/IV/V) | 109 (5.9) | 11 (3.2) | 0.001 | 0.619 (0.466–0.821) |
| Postoperative hospital stay, (day) | ||||
| Mean (±SD) | 12.75 (11.463) | 10.98 (6.116) | <0.001 | |
| Median | 10.0 | 9.0 |
| Variables | IPTW-Adjusted | ||
|---|---|---|---|
| Hazard Ratio | 95% CI | p Value | |
| Age | |||
| <65 | 0.847 | 0.611–1.175 | 0.098 |
| ≥65 | 1 | ||
| Gender | |||
| female | 1.028 | 0.847–1.248 | 0.319 |
| male | 1 | ||
| BMI, kg/m2 | |||
| <25 | 1.024 | 0.605–1.735 | 0.668 |
| ≥25 | 1 | ||
| Hypertension | |||
| No | 1.022 | 0.056–18.806 | 0.939 |
| Yes | 1 | ||
| Diabetes mellitus | |||
| No | 1.029 | 0.239–4.437 | 0.846 |
| Yes | 1 | ||
| Tumor location | |||
| Right-sided colon | 0.971 | 0.617–1.528 | 0.563 |
| Left-sided colon | 0.772 | 0.691–0.861 | 0.021 |
| Rectum | 1 | ||
| T staging | |||
| non-T4 | 0.734 | 0.586–0.919 | 0.036 |
| T4 | 1 | ||
| N staging | |||
| N0 | 0.62 | 0.305–1.26 | 0.074 |
| N+ | 1 | ||
| Metastasis | |||
| Single organ | 0.718 | 0.09–5.747 | 0.293 |
| Multiple organs | 1 | ||
| Chemotherapy | |||
| No | 2.579 | 0.753–8.837 | 0.065 |
| Yes | 1 | ||
| Primary and metastatic tumor resection | |||
| R0 resection | 0.455 | 0.319–0.649 | 0.023 |
| Non-R0 resection | 1 | ||
| CEA (ng/mL) | |||
| <5 | 0.802 | 0.446–1.441 | 0.131 |
| ≥5 | 1 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, C.-C.; Lai, I.-L.; Huang, S.-H.; Tsai, W.-S.; Hsieh, P.-S.; Yeh, C.-Y.; Chiang, S.-F.; Hung, H.-Y.; You, J.-F. Association of Preoperative Physical Activity with Short- and Long-Term Outcomes in Patients Undergoing Palliative Resection for Metastatic Colorectal Cancer: An Inverse Probability of Treatment Weighting Analysis. Cancers 2022, 14, 489. https://doi.org/10.3390/cancers14030489
Cheng C-C, Lai I-L, Huang S-H, Tsai W-S, Hsieh P-S, Yeh C-Y, Chiang S-F, Hung H-Y, You J-F. Association of Preoperative Physical Activity with Short- and Long-Term Outcomes in Patients Undergoing Palliative Resection for Metastatic Colorectal Cancer: An Inverse Probability of Treatment Weighting Analysis. Cancers. 2022; 14(3):489. https://doi.org/10.3390/cancers14030489
Chicago/Turabian StyleCheng, Ching-Chung, I-Li Lai, Shu-Huan Huang, Wen-Sy Tsai, Pao-Shiu Hsieh, Chien-Yuh Yeh, Sum-Fu Chiang, Hsin-Yuan Hung, and Jeng-Fu You. 2022. "Association of Preoperative Physical Activity with Short- and Long-Term Outcomes in Patients Undergoing Palliative Resection for Metastatic Colorectal Cancer: An Inverse Probability of Treatment Weighting Analysis" Cancers 14, no. 3: 489. https://doi.org/10.3390/cancers14030489
APA StyleCheng, C.-C., Lai, I.-L., Huang, S.-H., Tsai, W.-S., Hsieh, P.-S., Yeh, C.-Y., Chiang, S.-F., Hung, H.-Y., & You, J.-F. (2022). Association of Preoperative Physical Activity with Short- and Long-Term Outcomes in Patients Undergoing Palliative Resection for Metastatic Colorectal Cancer: An Inverse Probability of Treatment Weighting Analysis. Cancers, 14(3), 489. https://doi.org/10.3390/cancers14030489






