High-Resolution, High b-Value Computed Diffusion-Weighted Imaging Improves Detection of Pancreatic Ductal Adenocarcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
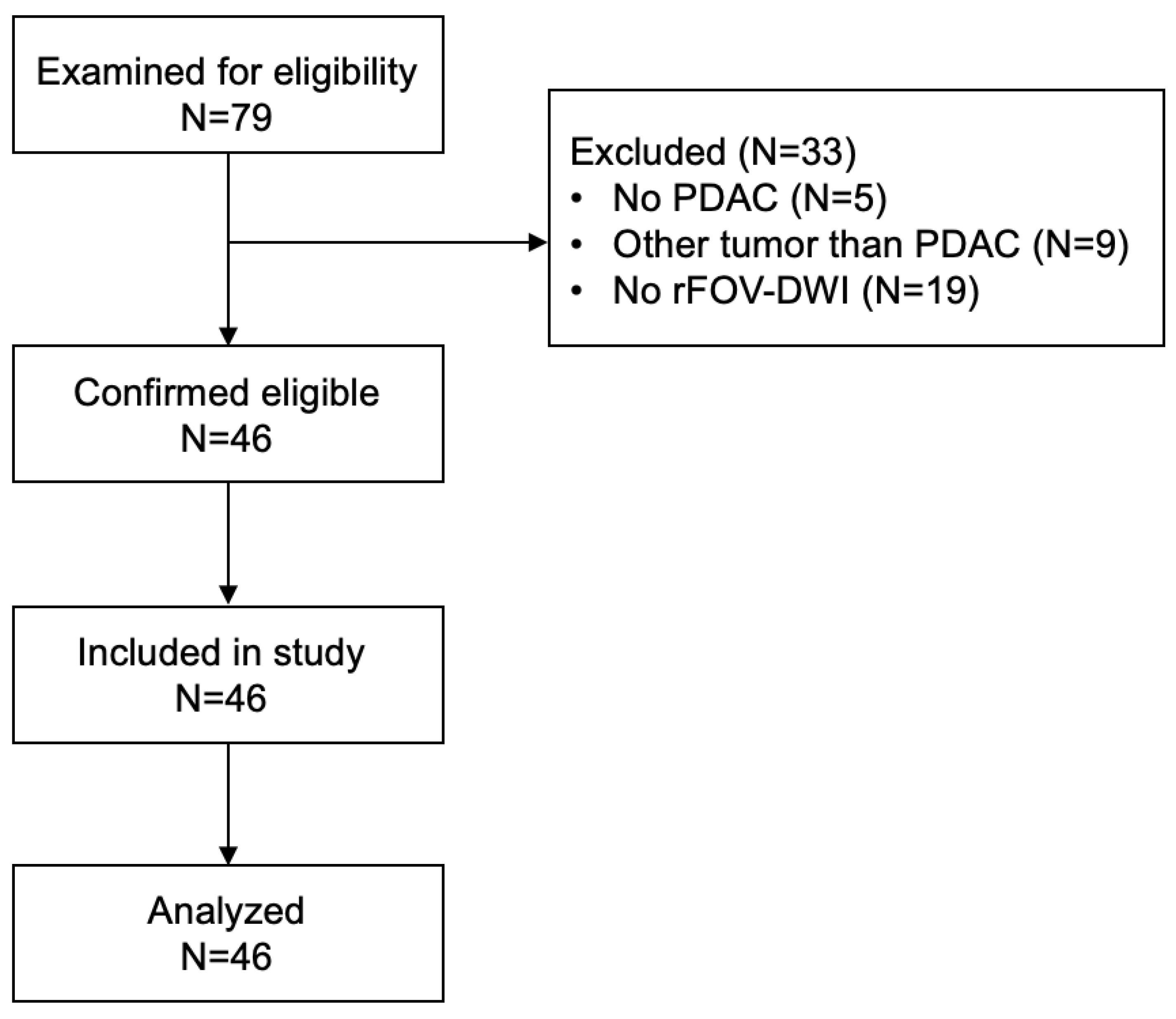
2.1. Patient Cohort and Study Design
2.2. Data Acquisition and Postprocessing
2.3. Image Analysis
2.4. Qualitative Analysis
2.5. Quantitative Analysis
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Qualitative Parameters
3.2.1. Image Quality
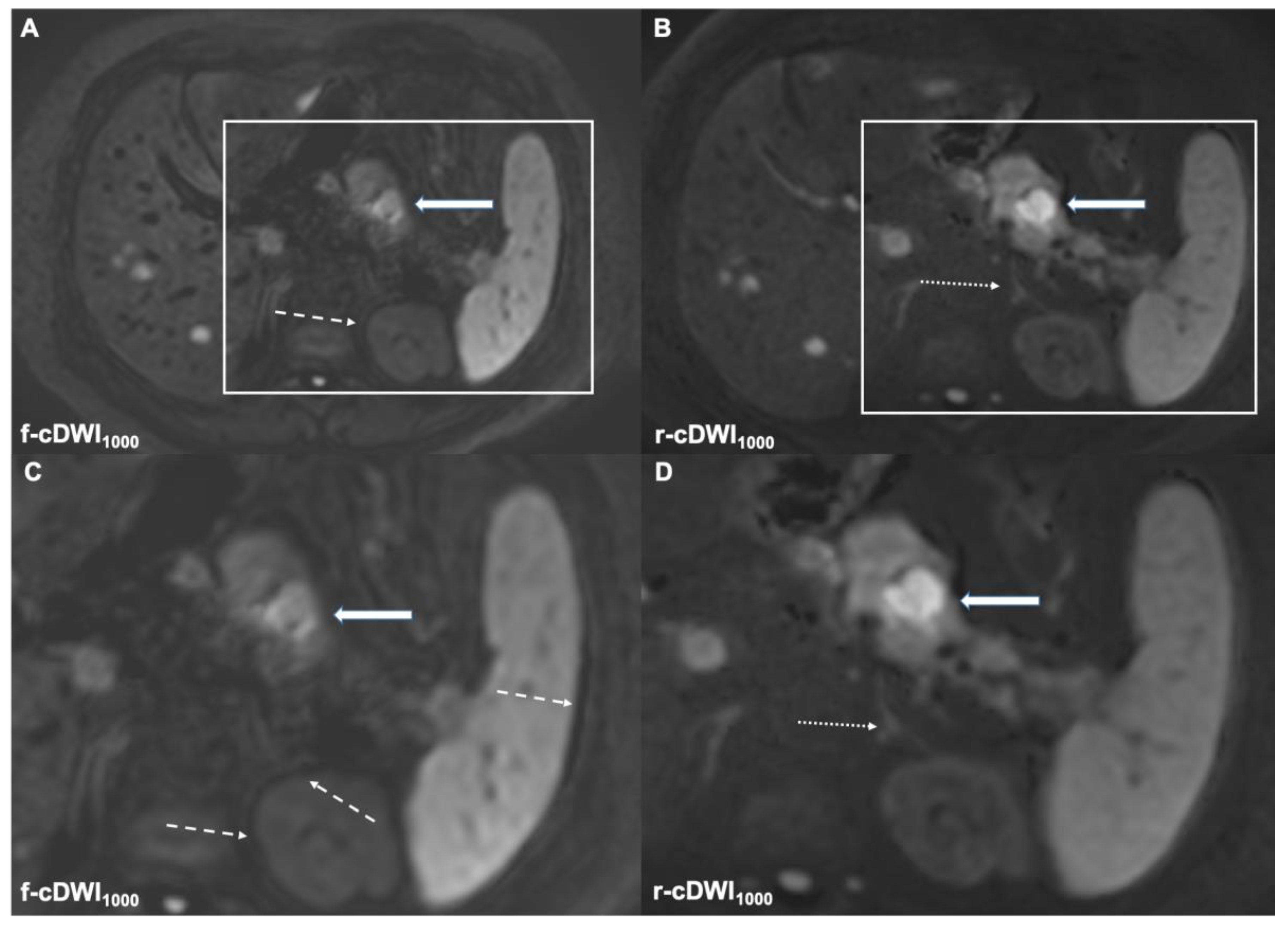
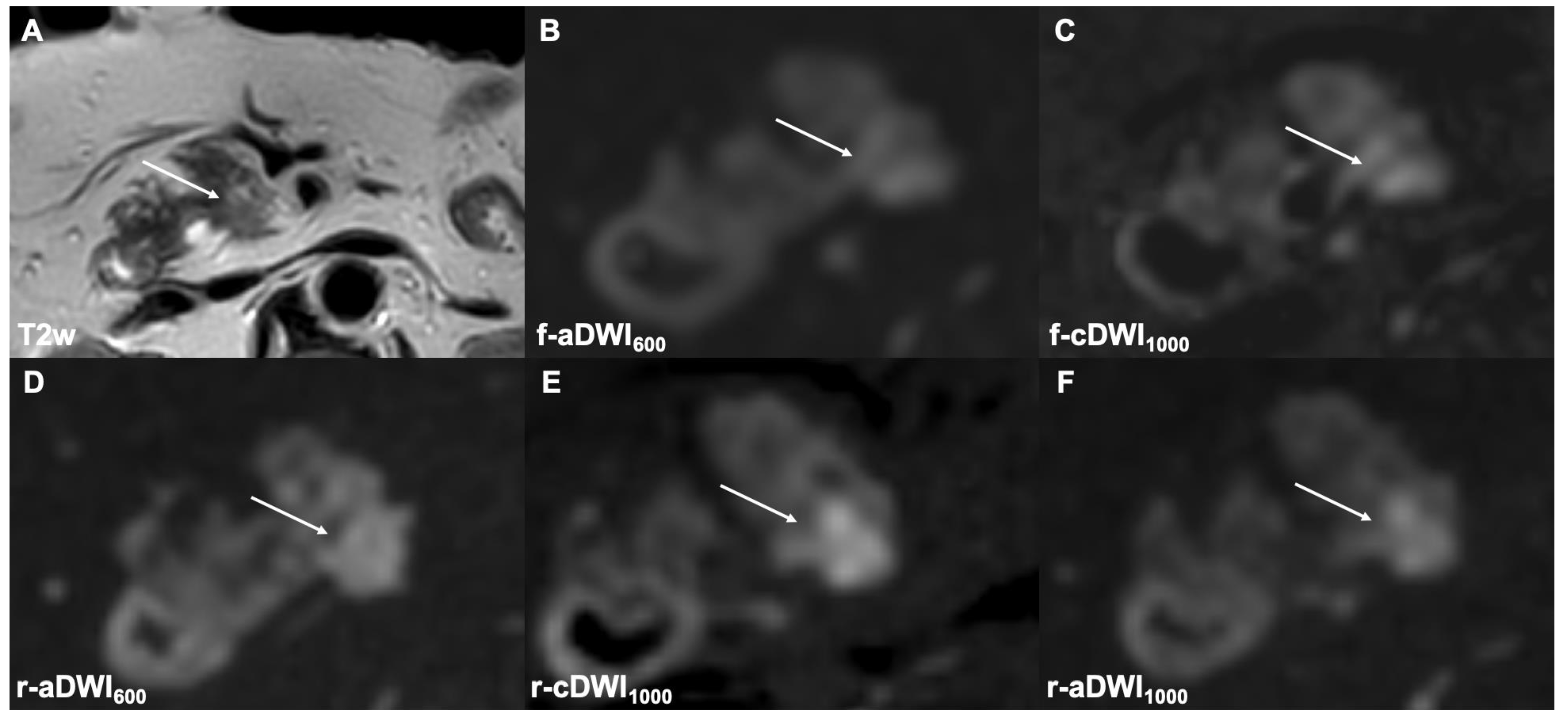
3.2.2. Lesion Detection
3.2.3. DWI Type
3.3. Quantitative Parameters
3.3.1. aSNR
3.3.2. aCNR
3.3.3. CR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
Appendix A
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahib, L.; Wehner, M.R.; Matrisian, L.M.; Nead, K.T. Estimated Projection of US Cancer Incidence and Death to 2040. JAMA Netw. Open 2021, 4, e214708. [Google Scholar] [CrossRef]
- Singhi, A.D.; Koay, E.J.; Chari, S.T.; Maitra, A. Early Detection of Pancreatic Cancer: Opportunities and Challenges. Gastroenterology 2019, 156, 2024–2040. [Google Scholar] [CrossRef] [Green Version]
- Toft, J.; Hadden, W.J.; Laurence, J.M.; Lam, V.; Yuen, L.; Janssen, A.; Pleass, H. Imaging modalities in the diagnosis of pancreatic adenocarcinoma: A systematic review and meta-analysis of sensitivity, specificity and diagnostic accuracy. Eur. J. Radiol. 2017, 92, 17–23. [Google Scholar] [CrossRef]
- Raman, S.P.; Horton, K.M.; Fishman, E.K. Multimodality imaging of pancreatic cancer-computed tomography, magnetic resonance imaging, and positron emission tomography. Cancer J. 2012, 18, 511–522. [Google Scholar] [CrossRef]
- Messina, C.; Bignone, R.; Bruno, A.; Bruno, A.; Bruno, F.; Calandri, M.; Caruso, D.; Coppolino, P.; Robertis, R.D.; Gentili, F.; et al. Diffusion-Weighted Imaging in Oncology: An Update. Cancers 2020, 12, 1493. [Google Scholar] [CrossRef] [PubMed]
- Bammer, R. Basic principles of diffusion-weighted imaging. Eur. J. Radiol. 2003, 45, 169–184. [Google Scholar] [CrossRef]
- Barral, M.; Taouli, B.; Guiu, B.; Koh, D.M.; Luciani, A.; Manfredi, R.; Vilgrain, V.; Hoeffel, C.; Kanematsu, M.; Soyer, P. Diffusion-weighted MR imaging of the pancreas: Current status and recommendations. Radiology 2015, 274, 45–63. [Google Scholar] [CrossRef] [Green Version]
- Kaissis, G.; Ziegelmayer, S.; Lohöfer, F.; Algül, H.; Eiber, M.; Weichert, W.; Schmid, R.; Friess, H.; Rummeny, E.; Ankerst, D.; et al. A machine learning model for the prediction of survival and tumor subtype in pancreatic ductal adenocarcinoma from preoperative diffusion-weighted imaging. Eur. Radiol. Exp. 2019, 3, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukukura, Y.; Takumi, K.; Kamimura, K.; Shindo, T.; Kumagae, Y.; Tateyama, A.; Nakajo, M. Pancreatic adenocarcinoma: Variability of diffusion-weighted MR imaging findings. Radiology 2012, 263, 732–740. [Google Scholar] [CrossRef]
- Crinò, S.F.; Larghi, A.; Bernardoni, L.; Parisi, A.; Frulloni, L.; Gabbrielli, A.; Parcesepe, P.; Scarpa, A.; Manfrin, E. Touch imprint cytology on endoscopic ultrasound fine-needle biopsy provides comparable sample quality and diagnostic yield to standard endoscopic ultrasound fine-needle aspiration specimens in the evaluation of solid pancreatic lesions. Cytopathology 2019, 30, 179–186. [Google Scholar] [CrossRef]
- Fukukura, Y.; Kumagae, Y.; Hakamada, H.; Shindo, T.; Takumi, K.; Kamimura, K.; Nakajo, M.; Umanodan, A.; Yoshiura, T. Computed diffusion-weighted MR imaging for visualization of pancreatic adenocarcinoma: Comparison with acquired diffusion-weighted imaging. Eur. J. Radiol. 2017, 95, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, S.; Kromrey, M.L.; Motosugi, U.; Onishi, H. Optimal target b-value on computed diffusion-weighted magnetic resonance imaging for visualization of pancreatic ductal adenocarcinoma and focal autoimmune pancreatitis. Abdom. Radiol. 2021, 46, 636–646. [Google Scholar] [CrossRef]
- Dietrich, O.; Biffar, A.; Baur-Melnyk, A.; Reiser, M.F. Technical aspects of MR diffusion imaging of the body. Eur. J. Radiol. 2010, 76, 314–322. [Google Scholar] [CrossRef]
- Akagi, M.; Nakamura, Y.; Higaki, T.; Matsubara, Y.; Terada, H.; Honda, Y.; Tatsugami, F.; Baba, Y.; Iida, M.; Awai, K. Preliminary Results of High-Precision Computed Diffusion Weighted Imaging for the Diagnosis of Hepatocellular Carcinoma at 3 Tesla. J. Comput. Assist. Tomogr. 2018, 42, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Rosenkrantz, A.B.; Chandarana, H.; Hindman, N.; Deng, F.M.; Babb, J.S.; Taneja, S.S.; Geppert, C. Computed diffusion-weighted imaging of the prostate at 3 T: Impact on image quality and tumour detection. Eur. Radiol. 2013, 23, 3170–3177. [Google Scholar] [CrossRef]
- Tamura, T.; Takasu, M.; Higaki, T.; Yokomachi, K.; Akiyama, Y.; Sumida, H.; Nagata, Y.; Awai, K. How to Improve the Conspicuity of Breast Tumors on Computed High b-value Diffusion-weighted Imaging. Magn. Reson. Med. Sci. MRMS Off. J. Jpn. Soc. Magn. Reson. Med. 2019, 18, 119–125. [Google Scholar] [CrossRef]
- Gatidis, S.; Schmidt, H.; Martirosian, P.; Nikolaou, K.; Schwenzer, N.F. Apparent diffusion coefficient-dependent voxelwise computed diffusion-weighted imaging: An approach for improving SNR and reducing T2 shine-through effects. J. Magn. Reson. Imaging JMRI 2016, 43, 824–832. [Google Scholar] [CrossRef]
- Harder, F.N.; Kamal, O.; Kaissis, G.A.; Heid, I.; Lohöfer, F.K.; McTavish, S.; Van, A.T.; Katemann, C.; Peeters, J.M.; Karampinos, D.C.; et al. Qualitative and Quantitative Comparison of Respiratory Triggered Reduced Field-of-View (FOV) Versus Full FOV Diffusion Weighted Imaging (DWI) in Pancreatic Pathologies. Acad. Radiol. 2020, 28, S234–S243. [Google Scholar] [CrossRef]
- Ma, C.; Li, Y.J.; Pan, C.S.; Wang, H.; Wang, J.; Chen, S.Y.; Lu, J.P. High resolution diffusion weighted magnetic resonance imaging of the pancreas using reduced field of view single-shot echo-planar imaging at 3 T. Magn. Reson. Imaging 2014, 32, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.; Lee, J.H.; Baek, H.J.; Ha, J.Y.; Ryu, K.H.; Park, S.E.; Moon, J.I.; Gho, S.M.; Wakayama, T. Clinical Feasibility of Reduced Field-of-View Diffusion-Weighted Magnetic Resonance Imaging with Computed Diffusion-Weighted Imaging Technique in Breast Cancer Patients. Diagnostics 2020, 10, 538. [Google Scholar] [CrossRef]
- Ning, P.; Shi, D.; Sonn, G.A.; Vasanawala, S.S.; Loening, A.M.; Ghanouni, P.; Obara, P.; Shin, L.K.; Fan, R.E.; Hargreaves, B.A.; et al. The impact of computed high b-value images on the diagnostic accuracy of DWI for prostate cancer: A receiver operating characteristics analysis. Sci. Rep. 2018, 8, 3409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukukura, Y.; Shindo, T.; Hakamada, H.; Takumi, K.; Umanodan, T.; Nakajo, M.; Kamimura, K.; Umanodan, A.; Ideue, J.; Yoshiura, T. Diffusion-weighted MR imaging of the pancreas: Optimizing b-value for visualization of pancreatic adenocarcinoma. Eur. Radiol. 2016, 26, 3419–3427. [Google Scholar] [CrossRef]
- Tokunaga, K.; Arizono, S.; Shimizu, H.; Fujimoto, K.; Kurata, M.; Minamiguchi, S.; Isoda, H.; Togashi, K. Optimizing b-values for accurate depiction of pancreatic cancer with tumor-associated pancreatitis on computed diffusion-weighted imaging. Clin. Imaging 2020, 61, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Higaki, T.; Nakamura, Y.; Tatsugami, F.; Kaichi, Y.; Akagi, M.; Akiyama, Y.; Baba, Y.; Iida, M.; Awai, K. Introduction to the Technical Aspects of Computed Diffusion-weighted Imaging for Radiologists. Radiogr. Rev. Publ. Radiol. Soc. N. Am. Inc. 2018, 38, 1131–1144. [Google Scholar] [CrossRef]
- Bittencourt, L.K.; Attenberger, U.I.; Lima, D.; Strecker, R.; de Oliveira, A.; Schoenberg, S.O.; Gasparetto, E.L.; Hausmann, D. Feasibility study of computed vs measured high b-value (1400 s/mm²) diffusion-weighted MR images of the prostate. World J. Radiol. 2014, 6, 374–380. [Google Scholar] [CrossRef]
- Grant, K.B.; Agarwal, H.K.; Shih, J.H.; Bernardo, M.; Pang, Y.; Daar, D.; Merino, M.J.; Wood, B.J.; Pinto, P.A.; Choyke, P.L.; et al. Comparison of calculated and acquired high b value diffusion-weighted imaging in prostate cancer. Abdom. Imaging 2015, 40, 578–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saritas, E.U.; Cunningham, C.H.; Lee, J.H.; Han, E.T.; Nishimura, D.G. DWI of the spinal cord with reduced FOV single-shot EPI. Magn. Reson. Med. 2008, 60, 468–473. [Google Scholar] [CrossRef]
- Wilm, B.J.; Svensson, J.; Henning, A.; Pruessmann, K.P.; Boesiger, P.; Kollias, S.S. Reduced field-of-view MRI using outer volume suppression for spinal cord diffusion imaging. Magn. Reson. Med. 2007, 57, 625–630. [Google Scholar] [CrossRef]
- Zhang, Y.; Wells, S.A.; Triche, B.L.; Kelcz, F.; Hernando, D. Stimulated-echo diffusion-weighted imaging with moderate b values for the detection of prostate cancer. Eur. Radiol. 2020, 30, 3236–3244. [Google Scholar] [CrossRef]
- Wargo, C.J.; Moore, J.; Gore, J.C. A comparison and evaluation of reduced-FOV methods for multi-slice 7T human imaging. Magn. Reson. Imaging 2013, 31, 1349–1359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blackledge, M.D.; Leach, M.O.; Collins, D.J.; Koh, D.M. Computed diffusion-weighted MR imaging may improve tumor detection. Radiology 2011, 261, 573–581. [Google Scholar] [CrossRef] [PubMed]


| Category | Variable | n |
|---|---|---|
| Sex | Male | 29 (63%) |
| Female | 17 (37%) | |
| Age (years) | Mean ± SD | 68.9 ± 11.3 |
| Tumor size | cT1 | 5 (10%) |
| cT2 | 15 (33%) | |
| cT3 | 10 (22%) | |
| cT4 | 16 (35%) | |
| Nodal status | cN0 | 24 (52%) |
| cN1 | 12 (26%) | |
| cN2 | 10 (22%) | |
| Metastasis | cM0 | 22 (48%) |
| cM1 | 24 (52%) | |
| CA19-9 (U/mL) | Median | 197 |
| IQR | 1025 | |
| CEA (ng/mL) | Median | 3.61 |
| IQR | 5.88 | |
| Tumor location | Head | 30 (65%) |
| Body | 8 (17%) | |
| Tail | 8 (17%) |
| Category | f-aDWI600 vs. f-cDWI1000 | r-aDWI600 vs. r-cDWI1000 | f-cDWI1000 vs. r-cDWI1000 | r-aDWI1000 vs. r-cDWI1000 |
|---|---|---|---|---|
| Image quality | <0.00001 | 0.00222 | <0.00001 | 0.0096 |
| Lesion detection | 0.00022 | <0.00001 | <0.00001 | 0.041 |
| DWI type | 0.277 | 0.008 | 0.006 | 0.41 |
| Category | f-aFOVDWI600 | f-cFOVDWI1000 | r-aFOVDWI600 | r-cFOVDWI1000 | r-aFOVDWI1000 |
|---|---|---|---|---|---|
| Image quality | 2.98 ± 0.61 | 2.15 ± 0.69 | 3.52 ± 0.62 | 3.26 ± 0.6 | 3.5 ± 0.58 |
| Lesion detection | 2.34 ± 0.93 | 2.78 ± 0.88 | 2.7 ± 0.91 | 3.67 ± 0.7 | 3.47 ± 0.68 |
| DWI: type 1 | 10 | 15 | 15 | 29 | 23 |
| DWI: type 2 | 21 | 20 | 22 | 11 | 17 |
| DWI: type 3 | 14 | 7 | 7 | 1 | 2 |
| DWI: type 4 | 1 | 4 | 2 | 5 | 4 |
| Sequence | f-aDWI600 vs. f-cDWI1000 | r-aDWI600 vs. r-cDWI1000 | f-cDWI1000 vs. r-cDWI1000 | r-aDWI1000 vs. r-cDWI1000 |
|---|---|---|---|---|
| aSNR | ||||
| p-value | 0.01778 | 0.00168 | 0.00804 | 0.00438 |
| aCNR | ||||
| p-value | 0.0035 | 0.00138 | 0.02088 | <0.00001 |
| CR (prox/dist) | ||||
| p-value | 0.00028/0.00014 | <0.00001/<0.00001 | <0.0401/<0.00001 | 0.00128/0.0002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harder, F.N.; Jung, E.; McTavish, S.; Van, A.T.; Weiss, K.; Ziegelmayer, S.; Gawlitza, J.; Gouder, P.; Kamal, O.; Makowski, M.R.; et al. High-Resolution, High b-Value Computed Diffusion-Weighted Imaging Improves Detection of Pancreatic Ductal Adenocarcinoma. Cancers 2022, 14, 470. https://doi.org/10.3390/cancers14030470
Harder FN, Jung E, McTavish S, Van AT, Weiss K, Ziegelmayer S, Gawlitza J, Gouder P, Kamal O, Makowski MR, et al. High-Resolution, High b-Value Computed Diffusion-Weighted Imaging Improves Detection of Pancreatic Ductal Adenocarcinoma. Cancers. 2022; 14(3):470. https://doi.org/10.3390/cancers14030470
Chicago/Turabian StyleHarder, Felix N., Eva Jung, Sean McTavish, Anh Tu Van, Kilian Weiss, Sebastian Ziegelmayer, Joshua Gawlitza, Philip Gouder, Omar Kamal, Marcus R. Makowski, and et al. 2022. "High-Resolution, High b-Value Computed Diffusion-Weighted Imaging Improves Detection of Pancreatic Ductal Adenocarcinoma" Cancers 14, no. 3: 470. https://doi.org/10.3390/cancers14030470
APA StyleHarder, F. N., Jung, E., McTavish, S., Van, A. T., Weiss, K., Ziegelmayer, S., Gawlitza, J., Gouder, P., Kamal, O., Makowski, M. R., Lohöfer, F. K., Karampinos, D. C., & Braren, R. F. (2022). High-Resolution, High b-Value Computed Diffusion-Weighted Imaging Improves Detection of Pancreatic Ductal Adenocarcinoma. Cancers, 14(3), 470. https://doi.org/10.3390/cancers14030470






