Intracranial Hemorrhage in Patients with Anticoagulant Therapy Undergoing Stereotactic Radiosurgery for Brain Metastases: A Bi-Institutional Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
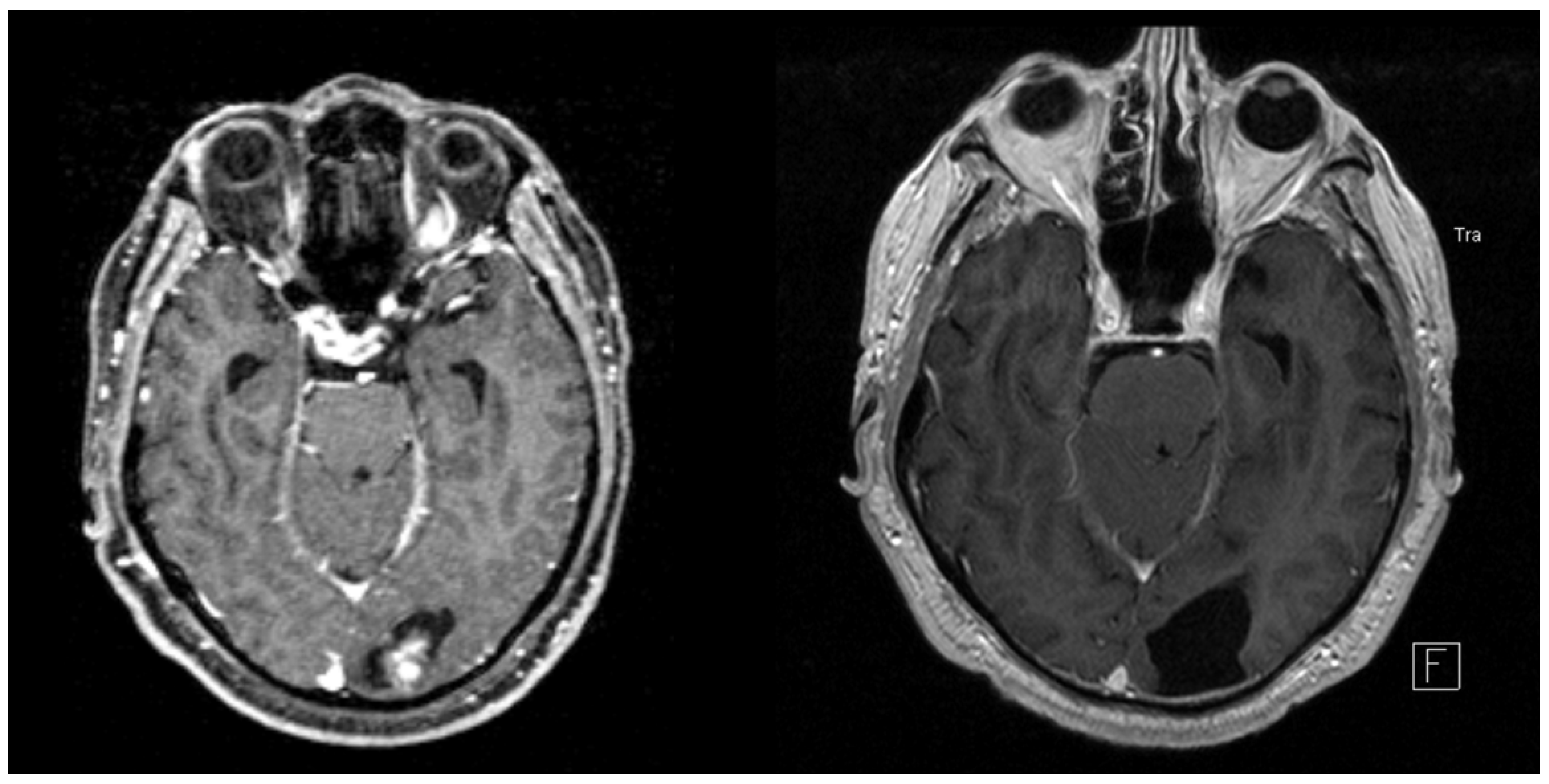
3.1. Patient and Treatment Characteristics
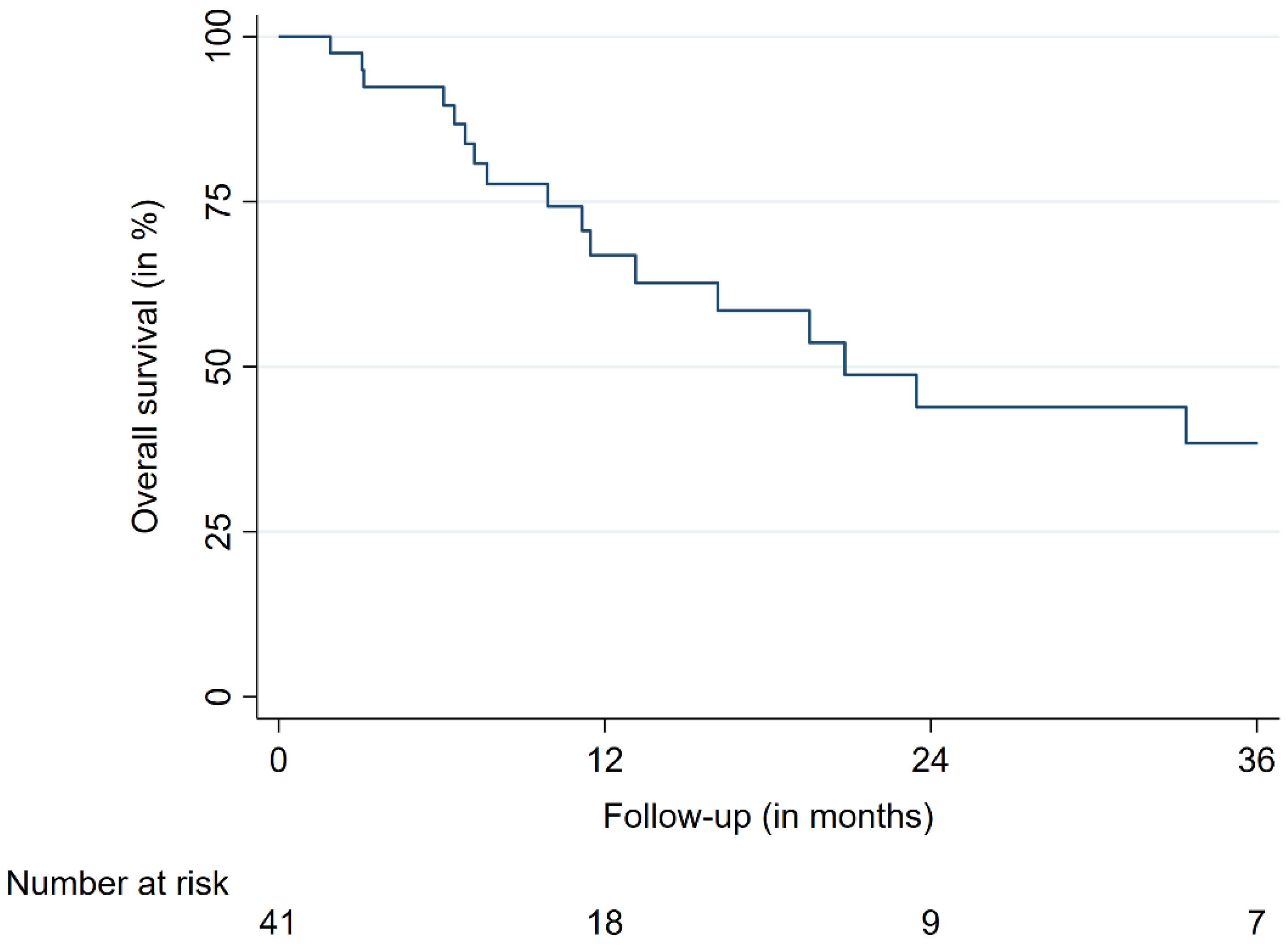
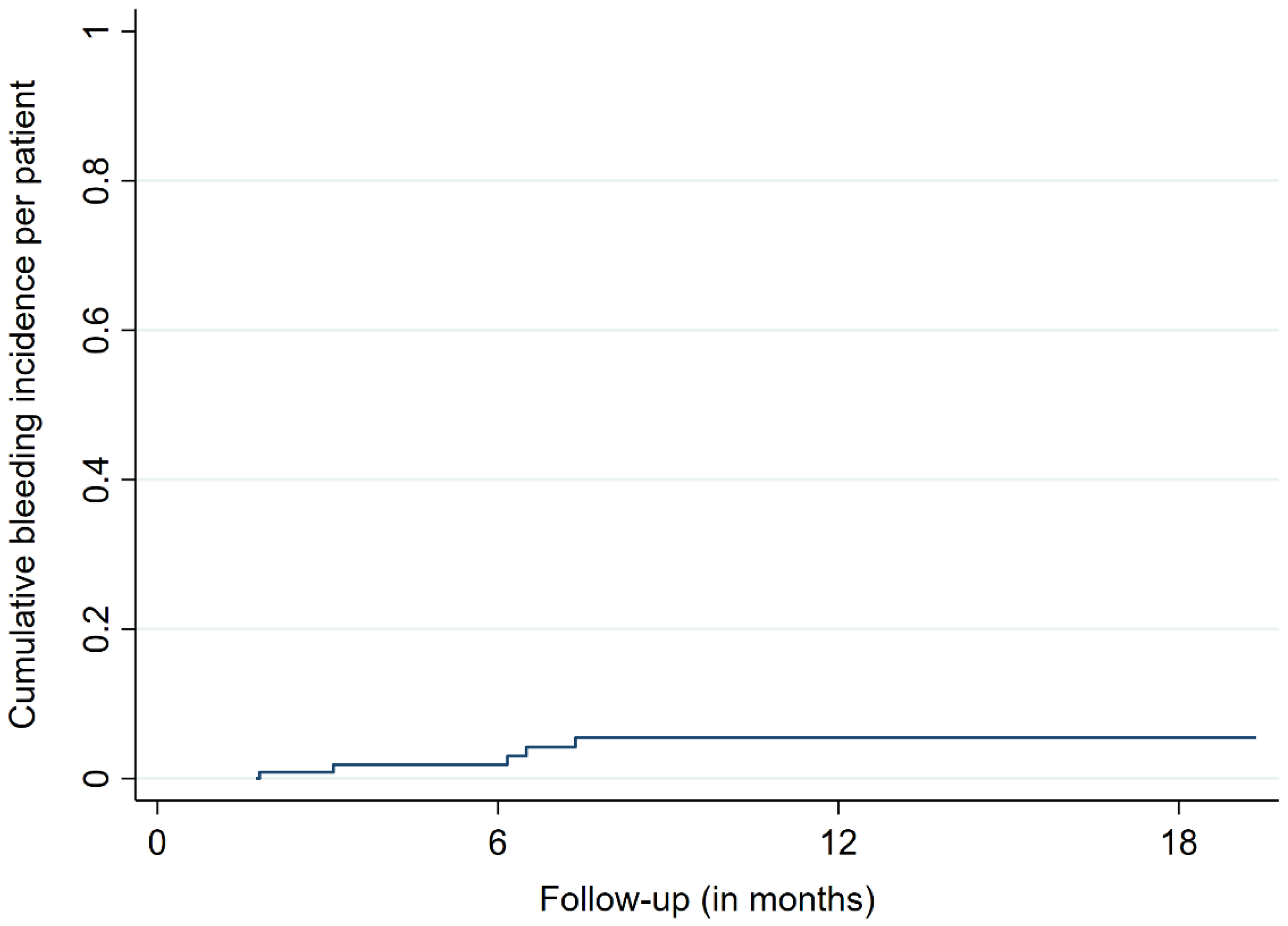
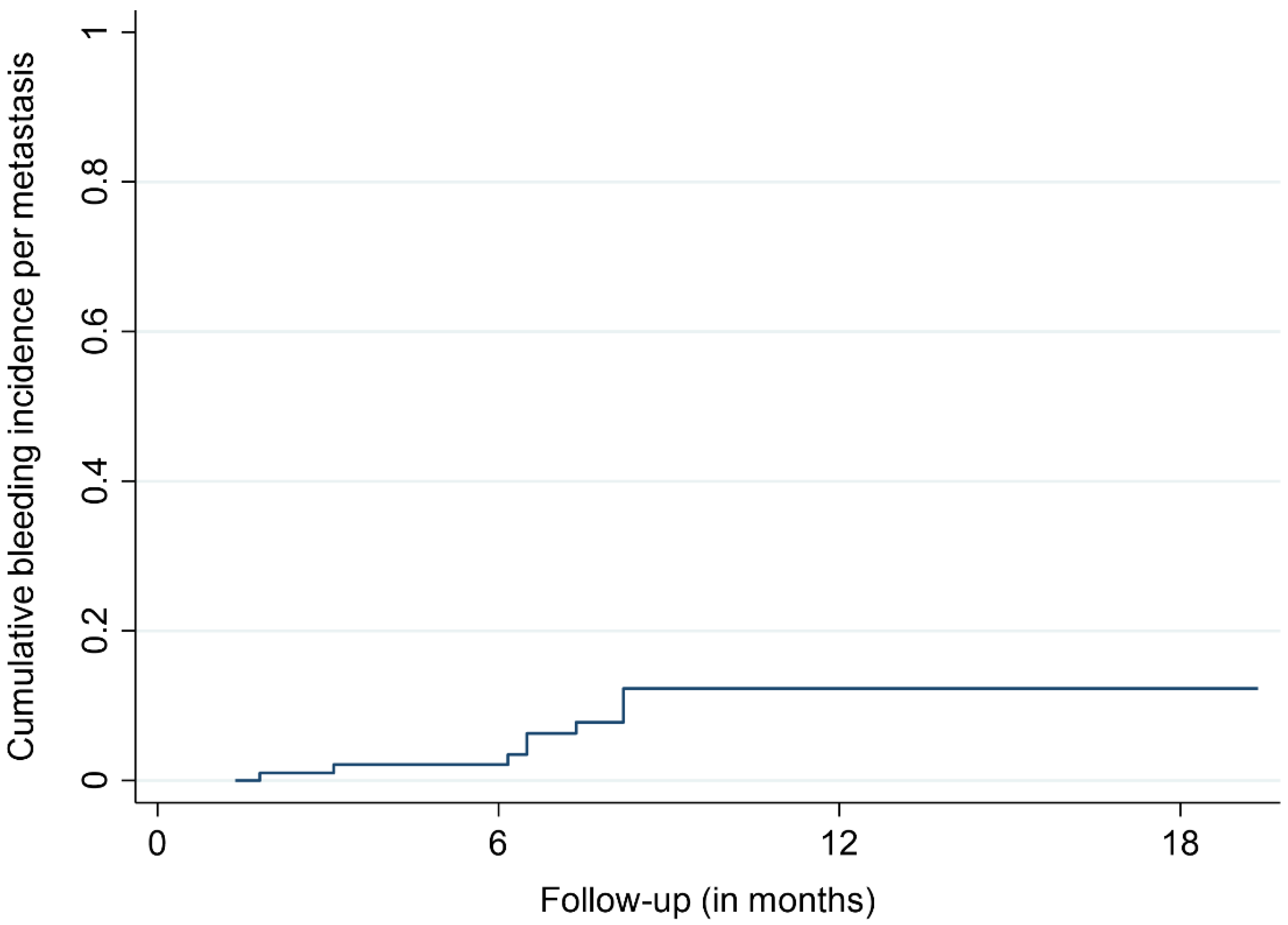
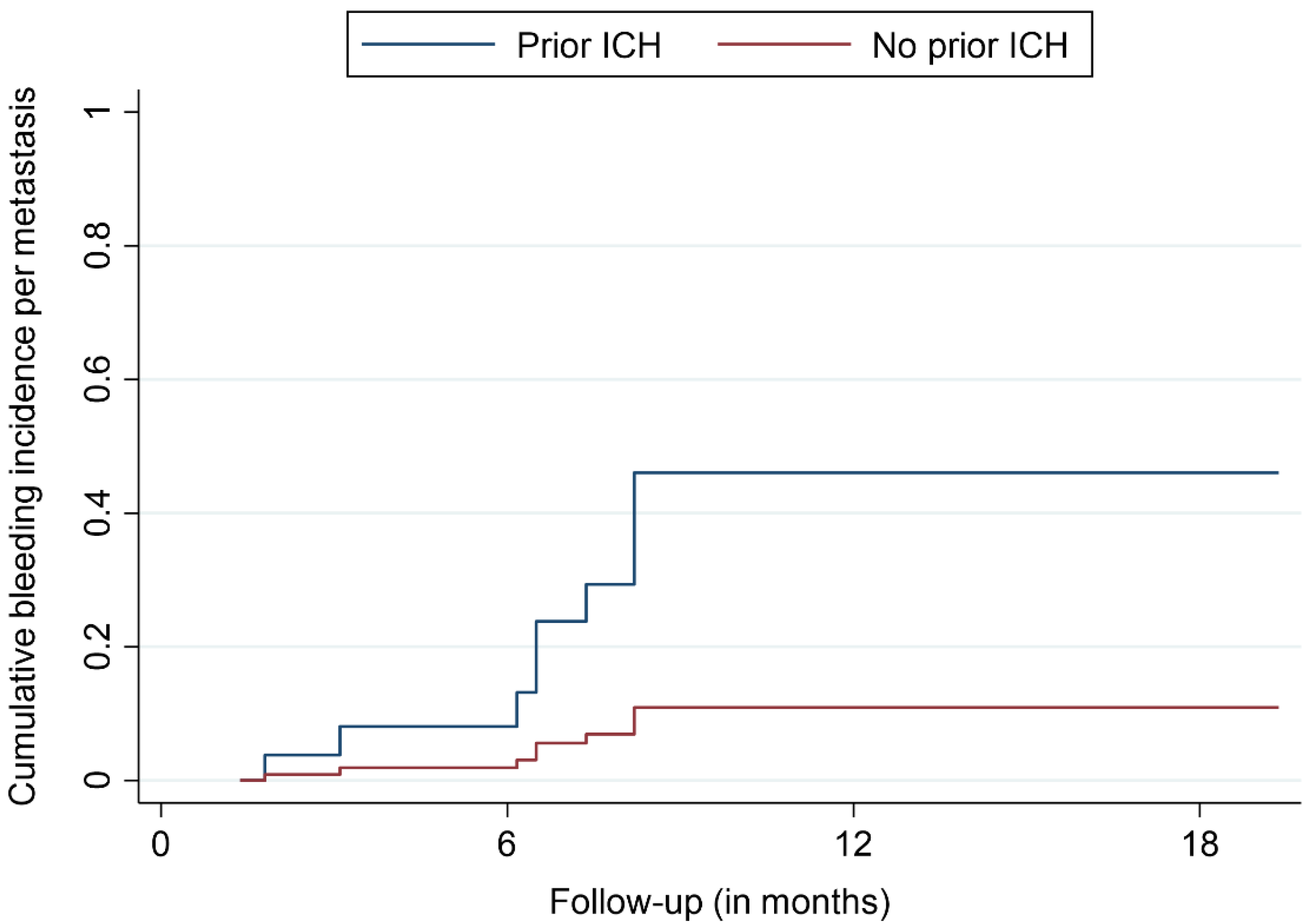
3.2. Treatment Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Achrol, A.S.; Rennert, R.C.; Anders, C.; Soffietti, R.; Ahluwalia, M.S.; Nayak, L.; Peters, S.; Arvold, N.D.; Harsh, G.R.; Steeg, P.S.; et al. Brain metastases. Nat. Rev. Dis. Primers 2019, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Nayak, L.; Lee, E.Q.; Wen, P.Y. Epidemiology of brain metastases. Curr. Oncol. Rep. 2012, 14, 48–54. [Google Scholar] [CrossRef]
- Barnholtz-Sloan, J.S.; Sloan, A.E.; Davis, F.G.; Vigneau, F.D.; Lai, P.; Sawaya, R.E. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J. Clin. Oncol. 2004, 22, 2865–2872. [Google Scholar] [CrossRef] [PubMed]
- Tabouret, E.; Chinot, O.; Metellus, P.; Tallet, A.; Viens, P.; Gonçalves, A. Recent trends in epidemiology of brain metastases: An overview. Anticancer Res. 2012, 32, 4655–4662. [Google Scholar] [PubMed]
- Langer, C.J.; Mehta, M.P. Current management of brain metastases, with a focus on systemic options. J. Clin. Oncol. 2005, 23, 6207–6219. [Google Scholar] [CrossRef] [PubMed]
- Soffietti, R.; Abacioglu, U.; Baumert, B.; Combs, S.E.; Kinhult, S.; Kros, J.M.; Marosi, C.; Metellus, P.; Radbruch, A.; Villa Freixa, S.S.; et al. Diagnosis and treatment of brain metastases from solid tumors: Guidelines from the European Association of Neuro-Oncology (EANO). Neuro. Oncol. 2017, 19, 162–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aiyama, H.; Yamamoto, M.; Kawabe, T.; Watanabe, S.; Koiso, T.; Sato, Y.; Higuchi, Y.; Ishikawa, E.; Yamamoto, T.; Matsumura, A.; et al. Complications after stereotactic radiosurgery for brain metastases: Incidences, correlating factors, treatments and outcomes. Radiother. Oncol. 2018, 129, 364–369. [Google Scholar] [CrossRef]
- Ghia, A.J.; Tward, J.D.; Anker, C.J.; Boucher, K.M.; Jensen, R.L.; Shrieve, D.C. Radiosurgery for melanoma brain metastases: The impact of hemorrhage on local control. J. Radiosurg. SBRT 2014, 3, 43–50. [Google Scholar]
- Kalfas, F.; Ronchini, N.; Godowicz, T.T.; Cavazzani, P.; Severi, P. Peritumoral and intratumoral hemorrhage after stereotactic radiosurgery for renal cell carcinoma metastasis to the brain. J. Radiosurg. SBRT 2011, 1, 163–168. [Google Scholar]
- Yomo, S.; Hayashi, M. Fatal tumoral hemorrhage after stereotactic radiosurgery for metastatic brain tumors: Report of three cases and review of literature. Acta Neurochir. 2012, 154, 1685–1690. [Google Scholar] [CrossRef]
- Redmond, A.J.; Diluna, M.L.; Hebert, R.; Moliterno, J.A.; Desai, R.; Knisely, J.P.; Chiang, V.L. Gamma Knife surgery for the treatment of melanoma metastases: The effect of intratumoral hemorrhage on survival. J. Neurosurg. 2008, 109, 99–105. [Google Scholar] [CrossRef] [Green Version]
- Sloan, L.; Lin, D.D.M.; Shen, C.; Redmond, K.J.; Bettegowda, C.; Grimm, J.; Link, K.; Lim, M.; Kleinberg, L.R. Rate of Intralesional Hemorrhage in Hypofractionated Stereotactic Radiosurgery for Brain Metastases in Patients with Metastatic Melanoma Treated with Concurrent Immunotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, E109. [Google Scholar] [CrossRef] [Green Version]
- Entezami, P.; Riccio, A.; Kenning, T.J. Intratumoral Hemorrhage within Petrous Meningioma. World Neurosurg. 2018, 117, 246–248. [Google Scholar] [CrossRef]
- Dehdashti, A.R.; Kiehl, T.R.; Guha, A. Vestibular Schwannomas presenting with haemorrhage: Clinical presentation and histopathological evaluation of an unusual entity. Br. J. Neurosurg. 2009, 23, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Franco-Vidal, V.; Songu, M.; Blanchet, H.; Barreau, X.; Darrouzet, V. Intracochlear hemorrhage after gamma knife radiosurgery. Otol. Neurotol. 2007, 28, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Izawa, M.; Chernov, M.; Hayashi, M.; Kubota, Y.; Kasuya, H.; Hori, T. Fatal intratumoral hemorrhage immediately after gamma knife radiosurgery for brain metastases: Case report. Minim. Invasive Neurosurg. 2006, 49, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Nordal, R.A.; Wong, C.S. Molecular targets in radiation-induced blood-brain barrier disruption. Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 279–287. [Google Scholar] [CrossRef]
- Murphy, E.S.; Xie, H.; Merchant, T.E.; Yu, J.S.; Chao, S.T.; Suh, J.H. Review of cranial radiotherapy-induced vasculopathy. J. Neurooncol. 2015, 122, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Toyoda, S.; Muramatsu, M.; Shimizu, T.; Kojima, T.; Taki, W. Spontaneous haemorrhage into metastatic brain tumours after stereotactic radiosurgery using a linear accelerator. J. Neurol. Neurosurg. Psychiatry 2003, 74, 908–912. [Google Scholar] [CrossRef] [Green Version]
- Motozaki, T.; Ban, S.; Yamamoto, T.; Hamasaki, M. Peritumoral hemorrhage after radiosurgery for metastatic brain tumor: A case report. No Shinkei Geka 1994, 22, 789–793. [Google Scholar]
- Steiner, T.; Rosand, J.; Diringer, M. Intracerebral hemorrhage associated with oral anticoagulant therapy: Current practices and unresolved questions. Stroke 2006, 37, 256–262. [Google Scholar] [CrossRef] [Green Version]
- Masotti, L.; Di Napoli, M.; Godoy, D.A.; Rafanelli, D.; Liumbruno, G.; Koumpouros, N.; Landini, G.; Pampana, A.; Cappelli, R.; Poli, D.; et al. The practical management of intracerebral hemorrhage associated with oral anticoagulant therapy. Int. J. Stroke 2011, 6, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Mantia, C.; Zwicker, J.I. Anticoagulation in the Setting of Primary and Metastatic Brain Tumors. Cancer Treat Res. 2019, 179, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Kattelus, H.; Kesäniemi, Y.A.; Huikuri, H.; Ukkola, O. Cancer increases the risk of atrial fibrillation during long-term follow-up (OPERA study). PLoS ONE 2018, 13, e0205454. [Google Scholar] [CrossRef] [PubMed]
- Guzzetti, S.; Costantino, G.; Vernocchi, A.; Sada, S.; Fundarò, C. First diagnosis of colorectal or breast cancer and prevalence of atrial fibrillation. Intern. Emerg. Med. 2008, 3, 227–231. [Google Scholar] [CrossRef]
- Erichsen, R.; Christiansen, C.F.; Mehnert, F.; Weiss, N.S.; Baron, J.A.; Sørensen, H.T. Colorectal cancer and risk of atrial fibrillation and flutter: A population-based case–control study. Intern. Emerg. Med. 2012, 7, 431–438. [Google Scholar] [CrossRef]
- Chu, G.; Versteeg, H.H.; Verschoor, A.J.; Trines, S.A.; Hemels, M.E.W.; Ay, C.; Huisman, M.V.; Klok, F.A. Atrial fibrillation and cancer—An unexplored field in cardiovascular oncology. Blood Rev. 2019, 35, 59–67. [Google Scholar] [CrossRef]
- Key, N.S.; Khorana, A.A.; Kuderer, N.M.; Bohlke, K.; Lee, A.Y.Y.; Arcelus, J.I.; Wong, S.L.; Balaban, E.P.; Flowers, C.R.; Francis, C.W.; et al. Venous Thromboembolism Prophylaxis and Treatment in Patients With Cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2020, 38, 496–520. [Google Scholar] [CrossRef]
- Ay, C.; Pabinger, I.; Cohen, A.T. Cancer-associated venous thromboembolism: Burden, mechanisms, and management. Thromb. Haemost. 2017, 117, 219–230. [Google Scholar] [CrossRef] [Green Version]
- Alvarado, G.; Noor, R.; Bassett, R.; Papadopoulos, N.E.; Kim, K.B.; Hwu, W.-J.; Bedikian, A.; Patel, S.; Hwu, P.; Davies, M.A. Risk of intracranial hemorrhage with anticoagulation therapy in melanoma patients with brain metastases. Melanoma Res. 2012, 22, 310–315. [Google Scholar] [CrossRef]
- Donato, J.; Campigotto, F.; Uhlmann, E.J.; Coletti, E.; Neuberg, D.; Weber, G.M.; Zwicker, J.I. Intracranial hemorrhage in patients with brain metastases treated with therapeutic enoxaparin: A matched cohort study. Blood 2015, 126, 494–499. [Google Scholar] [CrossRef] [Green Version]
- Kondziolka, D.; Bernstein, M.; Resch, L.; Tator, C.H.; Fleming, J.F.; Vanderlinden, R.G.; Schutz, H. Significance of hemorrhage into brain tumors: Clinicopathological study. J. Neurosurg. 1987, 67, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Wowra, B.; Muacevic, A.; Tonn, J.C. CyberKnife radiosurgery for brain metastases. Prog. Neurol. Surg. 2012, 25, 201–209. [Google Scholar] [CrossRef]
- Zwicker, J.I.; Karp Leaf, R.; Carrier, M. A meta-analysis of intracranial hemorrhage in patients with brain tumors receiving therapeutic anticoagulation. J. Thromb. Haemost. 2016, 14, 1736–1740. [Google Scholar] [CrossRef] [PubMed]
- Schiff, D.; DeAngelis, L.M. Therapy of venous thromboembolism in patients with brain metastases. Cancer 1994, 73, 493–498. [Google Scholar] [CrossRef]
- Horstman, H.; Gruhl, J.; Smith, L.; Ganti, A.K.; Shonka, N.A. Safety of long-term anticoagulation in patients with brain metastases. Med. Oncol. 2018, 35, 43. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.J.; Green, D.L.; Shah, G.L. Therapeutic Anticoagulation in Patients with Primary Brain Tumors or Secondary Brain Metastasis. Oncologist 2018, 23, 468–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burth, S.; Ohmann, M.; Kronsteiner, D.; Kieser, M.; Löw, S.; Riedemann, L.; Laible, M.; Berberich, A.; Drüschler, K.; Rizos, T.; et al. Prophylactic anticoagulation in patients with glioblastoma or brain metastases and atrial fibrillation: An increased risk for intracranial hemorrhage? J. Neurooncol. 2021, 152, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Wood, P.; Boyer, G.; Mehanna, E.; Cagney, D.; Lamba, N.; Catalano, P.; Connors, J.M.; Hsu, L.; Mendu, M.; Tanguturi, S.; et al. Intracerebral haemorrhage in patients with brain metastases receiving therapeutic anticoagulation. J. Neurol. Neurosurg. Psychiatry 2021, 92, 655–661. [Google Scholar] [CrossRef]
- Gaensler, E.H.; Dillon, W.P.; Edwards, M.S.; Larson, D.A.; Rosenau, W.; Wilson, C.B. Radiation-induced telangiectasia in the brain simulates cryptic vascular malformations at MR imaging. Radiology 1994, 193, 629–636. [Google Scholar] [CrossRef]
- Keezer, M.R.; Del Maestro, R. Radiation-Induced Cavernous Hemangiomas: Case Report and Literature Review. Can. J. Neurol. Sci./J. Can. Des. Sci. Neurol. 2009, 36, 303–310. [Google Scholar] [CrossRef] [Green Version]
- Seiger, K.; Pendharkar, A.; Samghabadi, P.; Chang, S.; Cho, N.; Choi, C.; Wang, C.; Gephart, M.; Soltys, S. Cavernous malformations are rare sequelae of stereotactic radiosurgery for brain metastases. Acta Neurochir. 2019, 161, 43–48. [Google Scholar] [CrossRef]
- Koike, S.; Aida, N.; Hata, M.; Fujita, K.; Ozawa, Y.; Inoue, T. Asymptomatic Radiation-induced Telangiectasia in Children after Cranial Irradiation: Frequency, Latency, and Dose Relation. Radiology 2004, 230, 93–99. [Google Scholar] [CrossRef]
- Poussaint, T.Y.; Siffert, J.; Barnes, P.D.; Pomeroy, S.L.; Goumnerova, L.C.; Anthony, D.C.; Sallan, S.E.; Tarbell, N.J. Hemorrhagic vasculopathy after treatment of central nervous system neoplasia in childhood: Diagnosis and follow-up. AJNR Am. J. Neuroradiol. 1995, 16, 693–699. [Google Scholar]
- Perry, J.R. Thromboembolic disease in patients with high-grade glioma. Neuro-Oncology 2012, 14 (Suppl. 4), iv73–iv80. [Google Scholar] [CrossRef]
- Norden, A.D.; Bartolomeo, J.; Tanaka, S.; Drappatz, J.; Ciampa, A.S.; Doherty, L.M.; Lafrankie, D.C.; Ruland, S.; Quant, E.C.; Beroukhim, R.; et al. Safety of concurrent bevacizumab therapy and anticoagulation in glioma patients. J. Neurooncol. 2012, 106, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Khoury, M.N.; Missios, S.; Edwin, N.; Sakruti, S.; Barnett, G.; Stevens, G.; Peereboom, D.M.; Khorana, A.A.; Ahluwalia, M.S. Intracranial hemorrhage in setting of glioblastoma with venous thromboembolism. Neurooncol. Pract. 2016, 3, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Porfidia, A.; Giordano, M.; Sturiale, C.L.; D’Arrigo, S.; Donadini, M.P.; Olivi, A.; Ageno, W.; Pola, R. Risk of intracranial bleeding in patients with primary brain cancer receiving therapeutic anticoagulation for venous thromboembolism: A meta-analysis. Brain Behav. 2020, 10, e01638. [Google Scholar] [CrossRef]
- Carney, B.J.; Uhlmann, E.J.; Puligandla, M.; Mantia, C.; Weber, G.M.; Neuberg, D.S.; Zwicker, J.I. Intracranial hemorrhage with direct oral anticoagulants in patients with brain tumors. J. Thromb. Haemost. 2019, 17, 72–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leader, A.; Hamulyák, E.N.; Carney, B.J.; Avrahami, M.; Knip, J.J.; Rozenblatt, S.; Beenen, L.F.M.; Yust-Katz, S.; Icht, O.; Coppens, M.; et al. Intracranial hemorrhage with direct oral anticoagulants in patients with brain metastases. Blood Adv. 2020, 4, 6291–6297. [Google Scholar] [CrossRef] [PubMed]
| Parameter | All Patients (n = 41, with n = 97 BM) |
|---|---|
| Age (y), median (range) | 69.0 (32.6–84.4) |
| Sex, n (%) | |
| Male | 23 (56) |
| Female | 18 (44) |
| Performance status, n (%) | |
| ECOG 0 | 20 (49) |
| ECOG 1 | 16 (39) |
| ECOG 2 | 5 (12) |
| Reason for ACT, n (%) | |
| Pulmonary embolism | 17 (41) |
| Atrial fibrillation | 14 (34) |
| Deep-vein thrombosis | 3 (7) |
| ACT, n (%) | |
| Phenprocoumon | 15 (37) |
| Novel oral anticoagulants | 13 (32) |
| Low-molecular-weight heparin | 8 (20) |
| Number of treated BM, n (%) | |
| 1 | 25 (61) |
| 2 | 6 (15) |
| ≥3 | 10 (24) |
| Tumor entity, n (%) | |
| Lung (NSCLC and SCLC) | 20 (49) |
| Malignant melanoma | 6 (15) |
| Renal cell | 5 (12) |
| Breast | 3 (7) |
| Colorectal | 2 (5) |
| Other | 5 (12) |
| BM with prior surgery, n (%) | 3 (3) |
| BM with prior WBRT, n (%) | 12 (12) |
| BM with prior SRS, n (%) | 6 (6) |
| BM with prior conventional radiotherapy, n (%) | 2 (2) |
| BM with prior bleeding, n (%) | 8 (8) |
| Follow-up (months), median (mean, range) | 8.2 (15.5, 1.7–77.5) |
| BM with ICH during follow-up, n (%) | 9 (9) |
| BM size (cc), median (mean, range) | 0.47 (0.02–10.28) |
| Prescription dose (Gy), median (range) | 20 (16–22) |
| Maximum tumor dose (Gy), median (range) | 29.2 (22.8–35.0) |
| Mean tumor dose (Gy), median (range) | 24.8 (18.9–28.8) |
| Minimum tumor dose (Gy), median (range) | 19.5 (12.5–30.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ehret, F.; Kaul, D.; Mose, L.; Budach, V.; Vajkoczy, P.; Fürweger, C.; Haidenberger, A.; Muacevic, A.; Mehrhof, F.; Kufeld, M. Intracranial Hemorrhage in Patients with Anticoagulant Therapy Undergoing Stereotactic Radiosurgery for Brain Metastases: A Bi-Institutional Analysis. Cancers 2022, 14, 465. https://doi.org/10.3390/cancers14030465
Ehret F, Kaul D, Mose L, Budach V, Vajkoczy P, Fürweger C, Haidenberger A, Muacevic A, Mehrhof F, Kufeld M. Intracranial Hemorrhage in Patients with Anticoagulant Therapy Undergoing Stereotactic Radiosurgery for Brain Metastases: A Bi-Institutional Analysis. Cancers. 2022; 14(3):465. https://doi.org/10.3390/cancers14030465
Chicago/Turabian StyleEhret, Felix, David Kaul, Lucas Mose, Volker Budach, Peter Vajkoczy, Christoph Fürweger, Alfred Haidenberger, Alexander Muacevic, Felix Mehrhof, and Markus Kufeld. 2022. "Intracranial Hemorrhage in Patients with Anticoagulant Therapy Undergoing Stereotactic Radiosurgery for Brain Metastases: A Bi-Institutional Analysis" Cancers 14, no. 3: 465. https://doi.org/10.3390/cancers14030465
APA StyleEhret, F., Kaul, D., Mose, L., Budach, V., Vajkoczy, P., Fürweger, C., Haidenberger, A., Muacevic, A., Mehrhof, F., & Kufeld, M. (2022). Intracranial Hemorrhage in Patients with Anticoagulant Therapy Undergoing Stereotactic Radiosurgery for Brain Metastases: A Bi-Institutional Analysis. Cancers, 14(3), 465. https://doi.org/10.3390/cancers14030465







