Surgical Aspects of Intrahepatic Cholangiocarcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Preoperative Workup
3. Imaging
4. Staging
5. Staging Laparoscopy
6. Indications for Surgery
7. Surgery
7.1. Tumor Resection and Margins
7.2. Future Liver Remnant
7.3. Transplantation
7.4. Lymphadenectomy
7.5. Minimally Invasive Surgery
8. Recurrence
9. Neoadjuvant and Adjuvant Therapy
10. Targeted and Immune-Based Therapy
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Beal, E.W.; Tumin, D.; Moris, D.; Zhang, X.F.; Chakedis, J.; Dilhoff, M.; Schmidt, C.M.; Pawlik, T.M. Cohort contributions to trends in the incidence and mortality of intrahepatic cholangiocarcinoma. Hepatobiliary Surg. Nutr. 2018, 7, 270–276. [Google Scholar] [CrossRef]
- Wu, L.; Tsilimigras, D.I.; Paredes, A.Z.; Mehta, R.; Hyer, J.M.; Merath, K.; Sahara, K.; Bagante, F.; Beal, E.W.; Shen, F.; et al. Trends in the Incidence, Treatment and Outcomes of Patients with Intrahepatic Cholangiocarcinoma in the USA: Facility Type is Associated with Margin Status, Use of Lymphadenectomy and Overall Survival. World J. Surg. 2019, 43, 1777–1787. [Google Scholar] [CrossRef]
- Florio, A.A.; Ferlay, J.; Znaor, A.; Ruggieri, D.; Alvarez, C.S.; Laversanne, M.; Bray, F.; McGlynn, K.A.; Petrick, J.L. Global trends in intrahepatic and extrahepatic cholangiocarcinoma incidence from 1993 to 2012. Cancer 2020, 126, 2666–2678. [Google Scholar] [CrossRef]
- Brown, K.M.; Parmar, A.D.; Geller, D.A. Intrahepatic cholangiocarcinoma. Surg. Oncol. Clin. N. Am. 2014, 23, 231–246. [Google Scholar] [CrossRef]
- Van Dyke, A.L.; Shiels, M.S.; Jones, G.S.; Pfeiffer, R.M.; Petrick, J.L.; Beebe-Dimmer, J.L.; Koshiol, J. Biliary tract cancer incidence and trends in the United States by demographic group, 1999–2013. Cancer 2019, 125, 1489–1498. [Google Scholar] [CrossRef]
- Geramizadeh, B. Precursor Lesions of Cholangiocarcinoma: A Clinicopathologic Review. Clin. Pathol. 2020, 13, 2632010x20925045. [Google Scholar] [CrossRef]
- Ohtsuka, M.; Shimizu, H.; Kato, A.; Yoshitomi, H.; Furukawa, K.; Tsuyuguchi, T.; Sakai, Y.; Yokosuka, O.; Miyazaki, M. Intraductal papillary neoplasms of the bile duct. Int. J. Hepatol. 2014, 2014, 459091. [Google Scholar] [CrossRef]
- Klöppel, G.; Adsay, V.; Konukiewitz, B.; Kleeff, J.; Schlitter, A.M.; Esposito, I. Precancerous lesions of the biliary tree. Best Pract. Res. Clin. Gastroenterol. 2013, 27, 285–297. [Google Scholar] [CrossRef]
- Wan, X.S.; Xu, Y.Y.; Qian, J.Y.; Yang, X.B.; Wang, A.Q.; He, L.; Zhao, H.T.; Sang, X.T. Intraductal papillary neoplasm of the bile duct. World J. Gastroenterol. 2013, 19, 8595–8604. [Google Scholar] [CrossRef]
- Khan, S.A.; Tavolari, S.; Brandi, G. Cholangiocarcinoma: Epidemiology and risk factors. Liver Int. 2019, 39 (Suppl. 1), 19–31. [Google Scholar] [CrossRef]
- Rizvi, S.; Khan, S.A.; Hallemeier, C.L.; Kelley, R.K.; Gores, G.J. Cholangiocarcinoma—Evolving concepts and therapeutic strategies. Nat. Rev. Clin. Oncol. 2018, 15, 95–111. [Google Scholar] [CrossRef]
- Bertuccio, P.; Malvezzi, M.; Carioli, G.; Hashim, D.; Boffetta, P.; El-Serag, H.B.; La Vecchia, C.; Negri, E. Global trends in mortality from intrahepatic and extrahepatic cholangiocarcinoma. J. Hepatol. 2019, 71, 104–114. [Google Scholar] [CrossRef]
- Patel, T. Worldwide trends in mortality from biliary tract malignancies. BMC Cancer 2002, 2, 10. [Google Scholar] [CrossRef]
- Khan, S.A.; Taylor-Robinson, S.D.; Toledano, M.B.; Beck, A.; Elliott, P.; Thomas, H.C. Changing international trends in mortality rates for liver, biliary and pancreatic tumours. J. Hepatol. 2002, 37, 806–813. [Google Scholar] [CrossRef]
- Zhang, X.F.; Beal, E.W.; Bagante, F.; Chakedis, J.; Weiss, M.; Popescu, I.; Marques, H.P.; Aldrighetti, L.; Maithel, S.K.; Pulitano, C.; et al. Early versus late recurrence of intrahepatic cholangiocarcinoma after resection with curative intent. Br. J. Surg. 2018, 105, 848–856. [Google Scholar] [CrossRef]
- Dhanasekaran, R.; Hemming, A.W.; Zendejas, I.; George, T.; Nelson, D.R.; Soldevila-Pico, C.; Firpi, R.J.; Morelli, G.; Clark, V.; Cabrera, R. Treatment outcomes and prognostic factors of intrahepatic cholangiocarcinoma. Oncol. Rep. 2013, 29, 1259–1267. [Google Scholar] [CrossRef]
- Coon, C.; Berger, N.; Eastwood, D.; Tsai, S.; Christians, K.; Mogal, H.; Clarke, C.; Gamblin, T.C. Primary Liver Cancer: An NCDB Analysis of Overall Survival and Margins After Hepatectomy. Ann. Surg. Oncol. 2020, 27, 1156–1163. [Google Scholar] [CrossRef]
- Lee, G.C.; Gamblin, T.C.; Fong, Z.V.; Ferrone, C.R.; Goyal, L.; Lillemoe, K.D.; Blaszkowsky, L.S.; Tanabe, K.K.; Qadan, M. Facility Type is Associated with Margin Status and Overall Survival of Patients with Resected Intrahepatic Cholangiocarcinoma. Ann. Surg. Oncol. 2019, 26, 4091–4099. [Google Scholar] [CrossRef]
- El-Diwany, R.; Pawlik, T.M.; Ejaz, A. Intrahepatic Cholangiocarcinoma. Surg. Oncol. Clin. N. Am. 2019, 28, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef] [PubMed]
- Beal, E.W.; Cloyd, J.M.; Pawlik, T.M. Surgical Treatment of Intrahepatic Cholangiocarcinoma: Current and Emerging Principles. J. Clin. Med. 2020, 10, 104. [Google Scholar] [CrossRef]
- Liu, J.; Ren, W.X.; Shu, J. Multimodal molecular imaging evaluation for early diagnosis and prognosis of cholangiocarcinoma. Insights Imaging 2022, 13, 10. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Hwang, S.; Lee, Y.J.; Kim, K.H.; Ahn, C.S.; Moon, D.B.; Ha, T.Y.; Song, G.W.; Jung, D.H.; Lee, S.G. Prognostic comparison of the 7th and 8th editions of the American Joint Committee on Cancer staging system for intrahepatic cholangiocarcinoma. J. Hepato-Biliary-Pancreat. Sci. 2018, 25, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.J.; Chun, Y.S. Intrahepatic cholangiocarcinoma: The AJCC/UICC 8th edition updates. Chin. Clin. Oncol. 2018, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Spolverato, G.; Bagante, F.; Weiss, M.; Alexandrescu, S.; Marques, H.P.; Aldrighetti, L.; Maithel, S.K.; Pulitano, C.; Bauer, T.W.; Shen, F.; et al. Comparative performances of the 7th and the 8th editions of the American Joint Committee on Cancer staging systems for intrahepatic cholangiocarcinoma. J. Surg. Oncol. 2017, 115, 696–703. [Google Scholar] [CrossRef]
- Kim, Y.; Moris, D.P.; Zhang, X.F.; Bagante, F.; Spolverato, G.; Schmidt, C.; Dilhoff, M.; Pawlik, T.M. Evaluation of the 8th edition American Joint Commission on Cancer (AJCC) staging system for patients with intrahepatic cholangiocarcinoma: A surveillance, epidemiology, and end results (SEER) analysis. J. Surg. Oncol. 2017, 116, 643–650. [Google Scholar] [CrossRef]
- Endo, I.; Gonen, M.; Yopp, A.C.; Dalal, K.M.; Zhou, Q.; Klimstra, D.; D’Angelica, M.; DeMatteo, R.P.; Fong, Y.; Schwartz, L.; et al. Intrahepatic cholangiocarcinoma: Rising frequency, improved survival, and determinants of outcome after resection. Ann. Surg. 2008, 248, 84–96. [Google Scholar] [CrossRef]
- Tan, J.C.; Coburn, N.G.; Baxter, N.N.; Kiss, A.; Law, C.H. Surgical management of intrahepatic cholangiocarcinoma—A population-based study. Ann. Surg. Oncol. 2008, 15, 600–608. [Google Scholar] [CrossRef]
- Franken, L.C.; Coelen, R.J.S.; Roos, E.; Verheij, J.; Phoa, S.S.; Besselink, M.G.; Busch, O.R.C.; van Gulik, T.M. Staging Laparoscopy in Patients with Intrahepatic Cholangiocarcinoma: Is It Still Useful? Visc. Med. 2020, 36, 501–505. [Google Scholar] [CrossRef]
- Weber, S.M.; Ribero, D.; O’Reilly, E.M.; Kokudo, N.; Miyazaki, M.; Pawlik, T.M. Intrahepatic cholangiocarcinoma: Expert consensus statement. HPB 2015, 17, 669–680. [Google Scholar] [CrossRef]
- Hewitt, D.B.; Brown, Z.J.; Pawlik, T.M. Surgical management of intrahepatic cholangiocarcinoma. Expert Rev. Anticancer Ther. 2022, 22, 27–38. [Google Scholar] [CrossRef]
- van Vugt, J.L.A.; Gaspersz, M.P.; Coelen, R.J.S.; Vugts, J.; Labeur, T.A.; de Jonge, J.; Polak, W.G.; Busch, O.R.C.; Besselink, M.G.; JNM, I.J.; et al. The prognostic value of portal vein and hepatic artery involvement in patients with perihilar cholangiocarcinoma. HPB 2018, 20, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Moustafa, M.; Linecker, M.; Lurje, G.; Capobianco, I.; Baumgart, J.; Ratti, F.; Rauchfuss, F.; Balci, D.; Fernandes, E.; et al. ALPPS for Locally Advanced Intrahepatic Cholangiocarcinoma: Did Aggressive Surgery Lead to the Oncological Benefit? An International Multi-center Study. Ann. Surg. Oncol. 2020, 27, 1372–1384. [Google Scholar] [CrossRef]
- Buettner, S.; Ten Cate, D.W.G.; Bagante, F.; Alexandrescu, S.; Marques, H.P.; Lamelas, J.; Aldrighetti, L.; Gamblin, T.C.; Maithel, S.K.; Pulitano, C.; et al. Survival after Resection of Multiple Tumor Foci of Intrahepatic Cholangiocarcinoma. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract 2019, 23, 2239–2246. [Google Scholar] [CrossRef]
- Benson, A.B.; D’Angelica, M.I.; Abbott, D.E.; Abrams, T.A.; Alberts, S.R.; Anaya, D.A.; Anders, R.; Are, C.; Brown, D.; Chang, D.T.; et al. Guidelines Insights: Hepatobiliary Cancers, Version 2.2019. J. Natl. Compr. Cancer Netw. 2019, 17, 302–310. [Google Scholar] [CrossRef]
- Bridgewater, J.; Galle, P.R.; Khan, S.A.; Llovet, J.M.; Park, J.W.; Patel, T.; Pawlik, T.M.; Gores, G.J. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J. Hepatol. 2014, 60, 1268–1289. [Google Scholar] [CrossRef]
- Spolverato, G.; Yakoob, M.Y.; Kim, Y.; Alexandrescu, S.; Marques, H.P.; Lamelas, J.; Aldrighetti, L.; Gamblin, T.C.; Maithel, S.K.; Pulitano, C.; et al. The Impact of Surgical Margin Status on Long-Term Outcome After Resection for Intrahepatic Cholangiocarcinoma. Ann. Surg. Oncol. 2015, 22, 4020–4028. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lin, L.; Lin, Z.; Chen, Y.; Huang, Q.; Ding, L.; Lou, J.; Zheng, S.; Bi, X.; Wang, J.; et al. Impact of surgical margin width on long-term outcomes for intrahepatic cholangiocarcinoma: A multicenter study. BMC Cancer 2021, 21, 840. [Google Scholar] [CrossRef] [PubMed]
- Regimbeau, J.M.; Kianmanesh, R.; Farges, O.; Dondero, F.; Sauvanet, A.; Belghiti, J. Extent of liver resection influences the outcome in patients with cirrhosis and small hepatocellular carcinoma. Surgery 2002, 131, 311–317. [Google Scholar] [CrossRef]
- Li, B.; Song, J.L.; Aierken, Y.; Chen, Y.; Zheng, J.L.; Yang, J.Y. Nonanatomic resection is not inferior to anatomic resection for primary intrahepatic cholangiocarcinoma: A propensity score analysis. Sci. Rep. 2018, 8, 17799. [Google Scholar] [CrossRef]
- Si, A.; Li, J.; Yang, Z.; Xia, Y.; Yang, T.; Lei, Z.; Cheng, Z.; Pawlik, T.M.; Lau, W.Y.; Shen, F. Impact of Anatomical Versus Non-anatomical Liver Resection on Short- and Long-Term Outcomes for Patients with Intrahepatic Cholangiocarcinoma. Ann. Surg. Oncol. 2019, 26, 1841–1850. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Bagante, F.; Chakedis, J.; Moris, D.; Beal, E.W.; Weiss, M.; Popescu, I.; Marques, H.P.; Aldrighetti, L.; Maithel, S.K.; et al. Perioperative and Long-Term Outcome for Intrahepatic Cholangiocarcinoma: Impact of Major Versus Minor Hepatectomy. J. Gastrointest. Surg. 2017, 21, 1841–1850. [Google Scholar] [CrossRef]
- Rahbari, N.N.; Garden, O.J.; Padbury, R.; Brooke-Smith, M.; Crawford, M.; Adam, R.; Koch, M.; Makuuchi, M.; Dematteo, R.P.; Christophi, C.; et al. Posthepatectomy liver failure: A definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 2011, 149, 713–724. [Google Scholar] [CrossRef]
- Dixon, M.; Cruz, J.; Sarwani, N.; Gusani, N. The Future Liver Remnant: Definition, Evaluation, and Management. Am. Surg. 2021, 87, 276–286. [Google Scholar] [CrossRef]
- Kishi, Y.; Vauthey, J.N. Issues to be considered to address the future liver remnant prior to major hepatectomy. Surg. Today 2021, 51, 472–484. [Google Scholar] [CrossRef]
- Ribero, D.; Chun, Y.S.; Vauthey, J.N. Standardized liver volumetry for portal vein embolization. Semin. Interv. Radiol. 2008, 25, 104–109. [Google Scholar] [CrossRef]
- Azoulay, D.; Castaing, D.; Krissat, J.; Smail, A.; Hargreaves, G.M.; Lemoine, A.; Emile, J.F.; Bismuth, H. Percutaneous portal vein embolization increases the feasibility and safety of major liver resection for hepatocellular carcinoma in injured liver. Ann. Surg. 2000, 232, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Cassese, G.; Han, H.S.; Al Farai, A.; Guiu, B.; Troisi, R.I.; Panaro, F. Future remnant liver optimization: Preoperative assessment, volume augmentation procedures and management of PVE failure. Minerva Surg. 2022, 77, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Deshayes, E.; Piron, L.; Bouvier, A.; Lapuyade, B.; Lermite, E.; Vervueren, L.; Laurent, C.; Pinaquy, J.B.; Chevallier, P.; Dohan, A.; et al. Study protocol of the HYPER-LIV01 trial: A multicenter phase II, prospective and randomized study comparing simultaneous portal and hepatic vein embolization to portal vein embolization for hypertrophy of the future liver remnant before major hepatectomy for colo-rectal liver metastases. BMC Cancer 2020, 20, 574. [Google Scholar] [CrossRef]
- Müller, P.C.; Linecker, M.; Kirimker, E.O.; Oberkofler, C.E.; Clavien, P.-A.; Balci, D.; Petrowsky, H. Induction of liver hypertrophy for extended liver surgery and partial liver transplantation: State of the art of parenchyma augmentation–assisted liver surgery. Langenbeck’s Arch. Surg. 2021, 406, 2201–2215. [Google Scholar] [CrossRef] [PubMed]
- Goldaracena, N.; Gorgen, A.; Sapisochin, G. Current status of liver transplantation for cholangiocarcinoma. Liver Transplant. 2018, 24, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Sapisochin, G.; Rodríguez de Lope, C.; Gastaca, M.; Ortiz de Urbina, J.; Suarez, M.A.; Santoyo, J.; Castroagudín, J.F.; Varo, E.; López-Andujar, R.; Palacios, F.; et al. “Very early” intrahepatic cholangiocarcinoma in cirrhotic patients: Should liver transplantation be reconsidered in these patients? Am. J. Transplant. 2014, 14, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Twohig, P.; Peeraphatdit, T.B.; Mukherjee, S. Current status of liver transplantation for cholangiocarcinoma. World J. Gastrointest. Surg. 2022, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Lv, G.; Dong, J. Liver Transplantation for Intrahepatic Cholangiocarcinoma: What Are New Insights and What Should We Follow? Front. Oncol. 2021, 11, 841694. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferro, V.; Gorgen, A.; Roayaie, S.; Droz Dit Busset, M.; Sapisochin, G. Liver resection and transplantation for intrahepatic cholangiocarcinoma. J. Hepatol. 2020, 72, 364–377. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Han, D.H.; Choi, G.H.; Choi, J.S.; Kim, K.S. Extent of Lymph Node Dissection for Accurate Staging in Intrahepatic Cholangiocarcinoma. J. Gastrointest. Surg. 2022, 26, 70–76. [Google Scholar] [CrossRef]
- Zhang, X.F.; Xue, F.; Dong, D.H.; Weiss, M.; Popescu, I.; Marques, H.P.; Aldrighetti, L.; Maithel, S.K.; Pulitano, C.; Bauer, T.W.; et al. Number and Station of Lymph Node Metastasis After Curative-intent Resection of Intrahepatic Cholangiocarcinoma Impact Prognosis. Ann. Surg. 2021, 274, e1187–e1195. [Google Scholar] [CrossRef]
- Hu, H.; Xu, G.; Du, S.; Luo, Z.; Zhao, H.; Cai, J. The role of lymph node dissection in intrahepatic cholangiocarcinoma: A multicenter retrospective study. BMC Surg. 2021, 21, 359. [Google Scholar] [CrossRef]
- Jolissaint, J.S.; Soares, K.C.; Seier, K.P.; Kundra, R.; Gönen, M.; Shin, P.J.; Boerner, T.; Sigel, C.; Madupuri, R.; Vakiani, E.; et al. Intrahepatic Cholangiocarcinoma with Lymph Node Metastasis: Treatment-Related Outcomes and the Role of Tumor Genomics in Patient Selection. Clin. Cancer Res. 2021, 27, 4101–4108. [Google Scholar] [CrossRef]
- Patrone, R.; Izzo, F.; Palaia, R.; Granata, V.; Nasti, G.; Ottaiano, A.; Pasta, G.; Belli, A. Minimally invasive surgical treatment of intrahepatic cholangiocarcinoma: A systematic review. World J. Gastrointest. Oncol. 2021, 13, 2203–2215. [Google Scholar] [CrossRef]
- Jinhuan, Y.; Yi, W.; Yuanwen, Z.; Delin, M.; Xiaotian, C.; Yan, W.; Liming, D.; Haitao, Y.; Lijun, W.; Tuo, D.; et al. Laparoscopic Versus Open Surgery for Early-Stage Intrahepatic Cholangiocarcinoma After Mastering the Learning Curve: A Multicenter Data-Based Matched Study. Front. Oncol. 2022, 11, 5527. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, F.; Eberhard, J.; Rückert, F.; Schmelzle, M.; Lehwald-Tywuschik, N.; Fichtner-Feigl, S.; Gaedcke, J.; Oldhafer, K.J.; Oldhafer, F.; Diener, M.; et al. Repeated resection for recurrent intrahepatic cholangiocarcinoma: A retrospective German multicentre study. Liver Int. 2021, 41, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Takasaki, K.; Otsubo, T.; Katsuragawa, H.; Katagiri, S. Recurrence after surgical resection of intrahepatic cholangiocarcinoma. J. Hepato-Biliary-Pancreat. Surg. 2001, 8, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Guglielmi, A.; Ruzzenente, A.; Campagnaro, T.; Pachera, S.; Valdegamberi, A.; Nicoli, P.; Cappellani, A.; Malfermoni, G.; Iacono, C. Intrahepatic cholangiocarcinoma: Prognostic factors after surgical resection. World J. Surg. 2009, 33, 1247–1254. [Google Scholar] [CrossRef] [PubMed]
- Spolverato, G.; Kim, Y.; Alexandrescu, S.; Marques, H.P.; Lamelas, J.; Aldrighetti, L.; Clark Gamblin, T.; Maithel, S.K.; Pulitano, C.; Bauer, T.W.; et al. Management and Outcomes of Patients with Recurrent Intrahepatic Cholangiocarcinoma Following Previous Curative-Intent Surgical Resection. Ann. Surg. Oncol. 2016, 23, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.S.; Zhang, X.F.; Weiss, M.; Popescu, I.; Marques, H.P.; Aldrighetti, L.; Maithel, S.K.; Pulitano, C.; Bauer, T.W.; Shen, F.; et al. Recurrence Patterns and Timing Courses Following Curative-Intent Resection for Intrahepatic Cholangiocarcinoma. Ann. Surg. Oncol. 2019, 26, 2549–2557. [Google Scholar] [CrossRef] [PubMed]
- Bekki, Y.; Von Ahrens, D.; Takahashi, H.; Schwartz, M.; Gunasekaran, G. Recurrent Intrahepatic Cholangiocarcinoma—Review. Front. Oncol. 2021, 11, 776863. [Google Scholar] [CrossRef]
- Li, Q.; Chen, C.; Su, J.; Qiu, Y.; Wu, H.; Song, T.; Mao, X.; He, Y.; Cheng, Z.; Li, J.; et al. Recurrence and prognosis in intrahepatic cholangiocarcinoma patients with different etiology after radical resection: A multi-institutional study. BMC Cancer 2022, 22, 329. [Google Scholar] [CrossRef]
- Kojima, T.; Umeda, Y.; Fuji, T.; Niguma, T.; Sato, D.; Endo, Y.; Sui, K.; Inagaki, M.; Oishi, M.; Ota, T.; et al. Efficacy of surgical management for recurrent intrahepatic cholangiocarcinoma: A multi-institutional study by the Okayama Study Group of HBP surgery. PLoS ONE 2020, 15, e0238392. [Google Scholar] [CrossRef]
- Primrose, J.N.; Fox, R.P.; Palmer, D.H.; Malik, H.Z.; Prasad, R.; Mirza, D.; Anthony, A.; Corrie, P.; Falk, S.; Finch-Jones, M.; et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): A randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019, 20, 663–673. [Google Scholar] [CrossRef]
- Edeline, J.; Benabdelghani, M.; Bertaut, A.; Watelet, J.; Hammel, P.; Joly, J.P.; Boudjema, K.; Fartoux, L.; Bouhier-Leporrier, K.; Jouve, J.L.; et al. Gemcitabine and Oxaliplatin Chemotherapy or Surveillance in Resected Biliary Tract Cancer (PRODIGE 12-ACCORD 18-UNICANCER GI): A Randomized Phase III Study. J. Clin. Oncol. 2019, 37, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.-F.; Liu, W.-R.; Shi, Y.-H. Adjuvant chemotherapy for intrahepatic cholangiocarcinoma: Far from a clinical consensus. Hepatobiliary Surg. Nutr. 2021, 10, 887–889. [Google Scholar] [CrossRef] [PubMed]
- Kamarajah, S.; Giovinazzo, F.; Roberts, K.J.; Punia, P.; Sutcliffe, R.P.; Marudanayagam, R.; Chatzizacharias, N.; Isaac, J.; Mirza, D.F.; Muiesan, P.; et al. The role of down staging treatment in the management of locally advanced intrahepatic cholangiocarcinoma: Review of literature and pooled analysis. Ann. Hepato-Biliary-Pancreat. Surg. 2020, 24, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Nault, J.C.; Villanueva, A. Biomarkers for Hepatobiliary Cancers. Hepatology 2021, 73 (Suppl. 1), 115–127. [Google Scholar] [CrossRef]
- Wu, T.; Jiang, X.; Zhang, X.; Wu, B.; Xu, B.; Liu, X.; Zheng, L.; Wang, Y. Intrahepatic Cholangiocarcinoma: State of the Art of FGFR Inhibitors. Cancer Control 2021, 28, 1073274821989314. [Google Scholar] [CrossRef]
- Borad, M.J.; Champion, M.D.; Egan, J.B.; Liang, W.S.; Fonseca, R.; Bryce, A.H.; McCullough, A.E.; Barrett, M.T.; Hunt, K.; Patel, M.D.; et al. Integrated genomic characterization reveals novel, therapeutically relevant drug targets in FGFR and EGFR pathways in sporadic intrahepatic cholangiocarcinoma. PLoS Genet. 2014, 10, e1004135. [Google Scholar] [CrossRef]
- Acher, A.W.; Paro, A.; Elfadaly, A.; Tsilimigras, D.; Pawlik, T.M. Intrahepatic Cholangiocarcinoma: A Summative Review of Biomarkers and Targeted Therapies. Cancers 2021, 13, 5169. [Google Scholar] [CrossRef]
- Makawita, S.; Abou-Alfa, G.K.; Roychowdhury, S.; Sadeghi, S.; Borbath, I.; Goyal, L.; Cohn, A.; Lamarca, A.; Oh, D.-Y.; Macarulla, T.; et al. Infigratinib in patients with advanced cholangiocarcinoma with FGFR2 gene fusions/translocations: The PROOF 301 trial. Future Oncol. 2020, 16, 2375–2384. [Google Scholar] [CrossRef]
- Lombardi, P.; Marino, D.; Fenocchio, E.; Chilà, G.; Aglietta, M.; Leone, F. Emerging molecular target antagonists for the treatment of biliary tract cancer. Expert Opin. Emerg. Drugs 2018, 23, 63–75. [Google Scholar] [CrossRef]
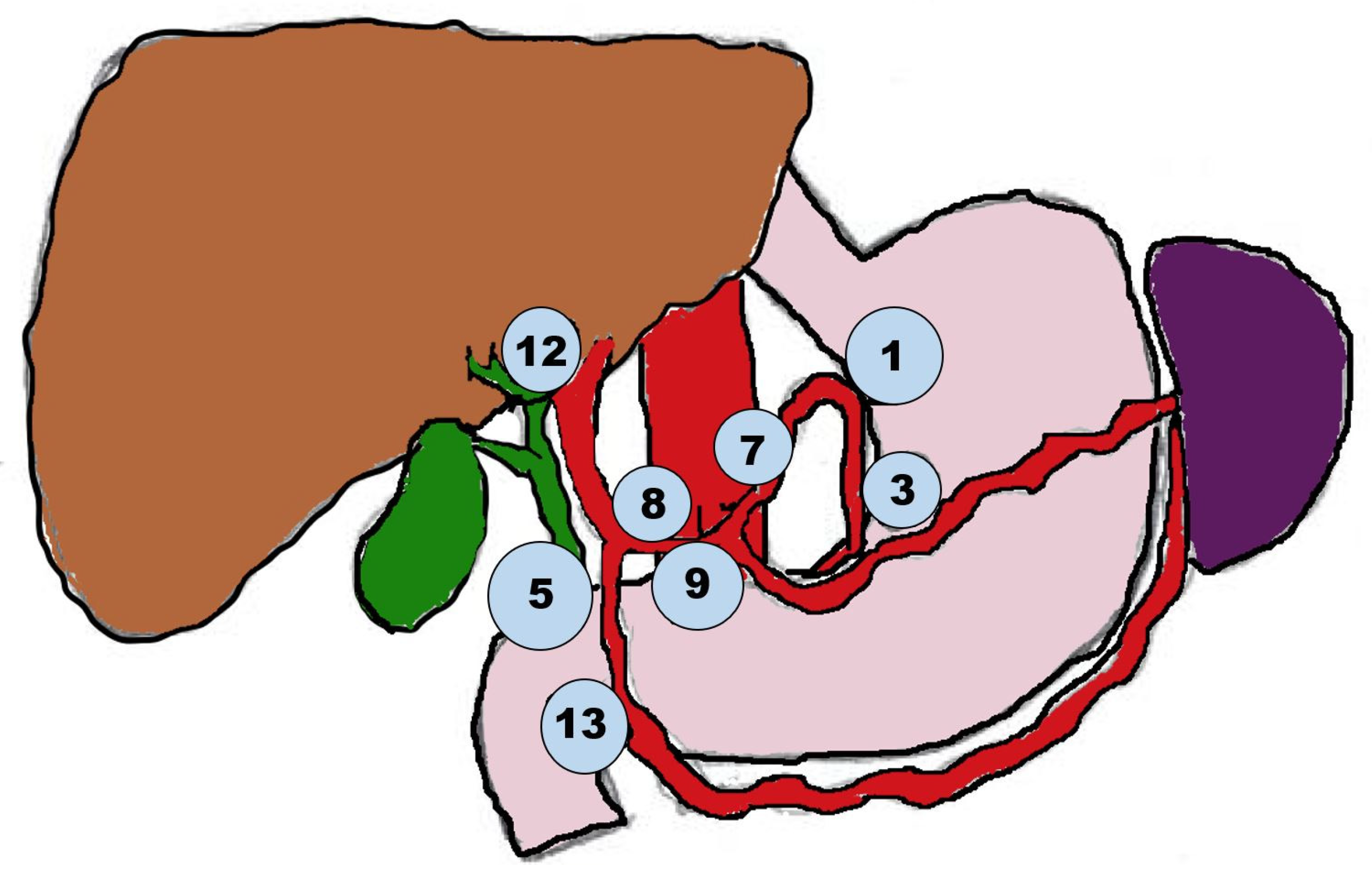
| T1 | Solitary Tumor without Vascular Invasion |
|---|---|
| T1a | Solitary tumor ≤ 5 cm without vascular invasion |
| T1b | Solitary tumor > 5 cm without vascular invasion |
| T2 | Solitary tumor with intrahepatic vascular invasion or multipletumors, with or without vascular invasion |
| T3 | Tumor perforating the visceral peritoneum |
| T4 | Tumor involving local extrahepatic structures by direct invasion |
| N0 | No regional lymph node metastasis |
| N1 | Regional lymph node metastasis present |
| M1 | Distant metastasis |
| IA | T1aN0M0 |
| IB | T1bN0M0 |
| II | T2N0M0 |
| IIIA | T3N0M0 |
| IIIB | T4N0M0, TAnyN1M0 |
| IV | TAnyNAnyM1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kupietzky, A.; Ariche, A. Surgical Aspects of Intrahepatic Cholangiocarcinoma. Cancers 2022, 14, 6265. https://doi.org/10.3390/cancers14246265
Kupietzky A, Ariche A. Surgical Aspects of Intrahepatic Cholangiocarcinoma. Cancers. 2022; 14(24):6265. https://doi.org/10.3390/cancers14246265
Chicago/Turabian StyleKupietzky, Amram, and Arie Ariche. 2022. "Surgical Aspects of Intrahepatic Cholangiocarcinoma" Cancers 14, no. 24: 6265. https://doi.org/10.3390/cancers14246265
APA StyleKupietzky, A., & Ariche, A. (2022). Surgical Aspects of Intrahepatic Cholangiocarcinoma. Cancers, 14(24), 6265. https://doi.org/10.3390/cancers14246265






