Real-Time Split-Dose PET/CT-Guided Ablation Improves Colorectal Liver Metastasis Detection and Ablation Zone Margin Assessments without the Need for Repeated Contrast Injection
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Study Design and Participants
3. Study Method
PET and CT-Acquisition Protocols
4. Split-Dose PET
5. Image Analysis
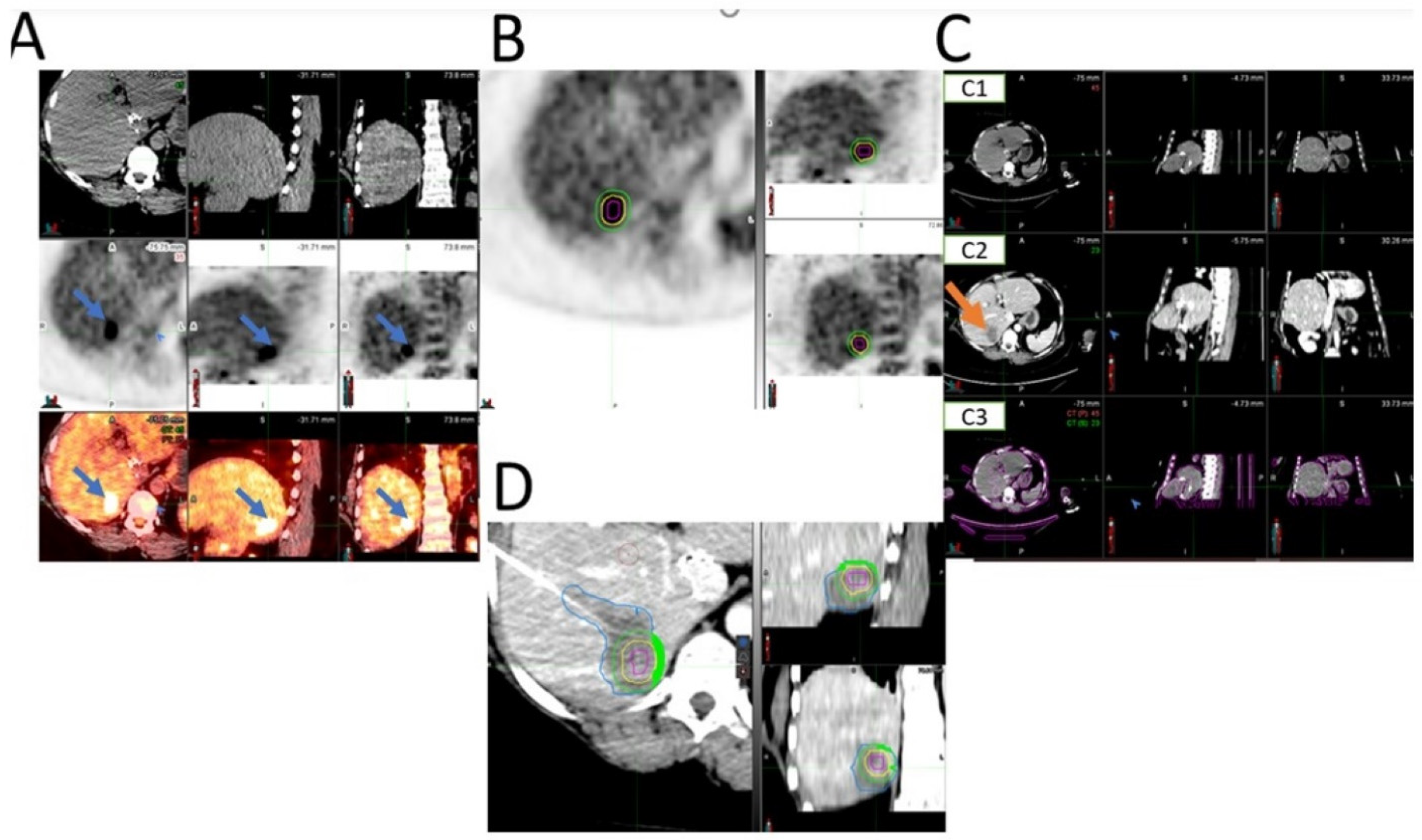
6. Statistics
7. Results
8. Discussion
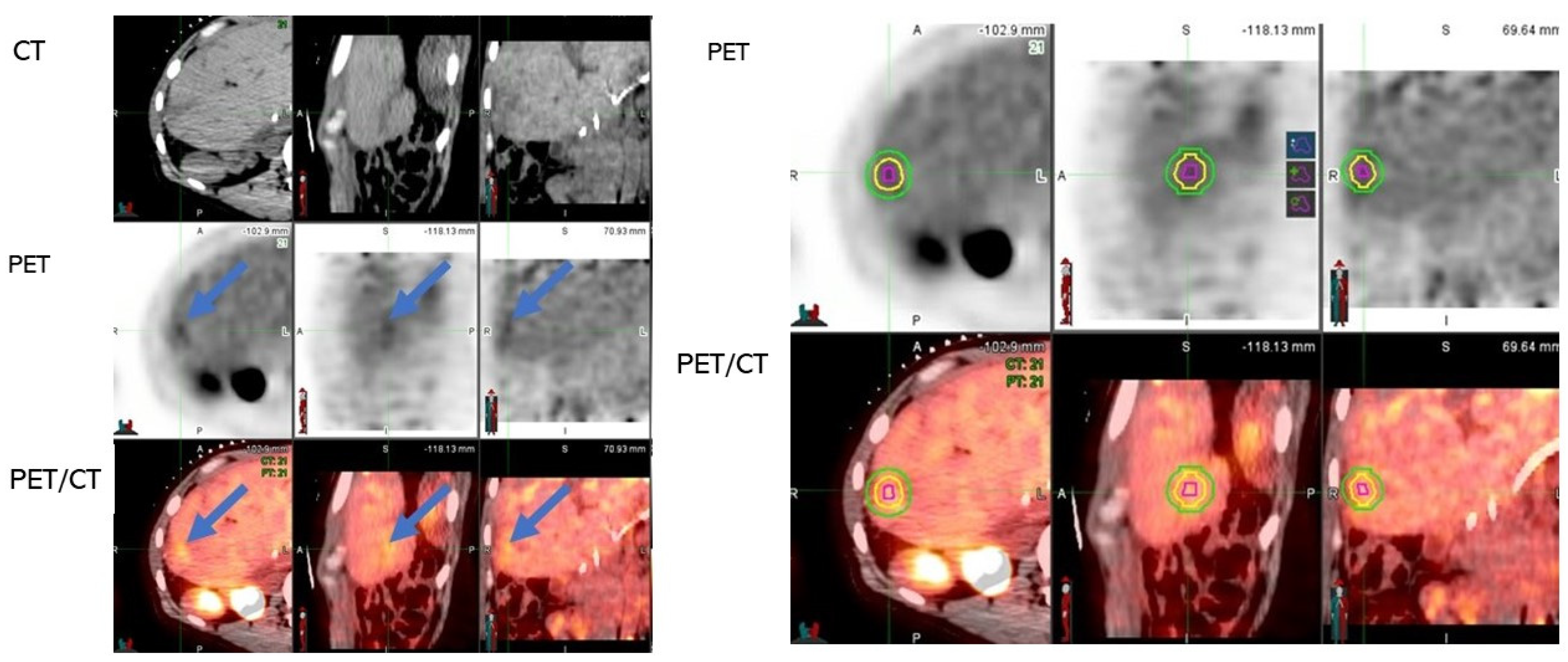
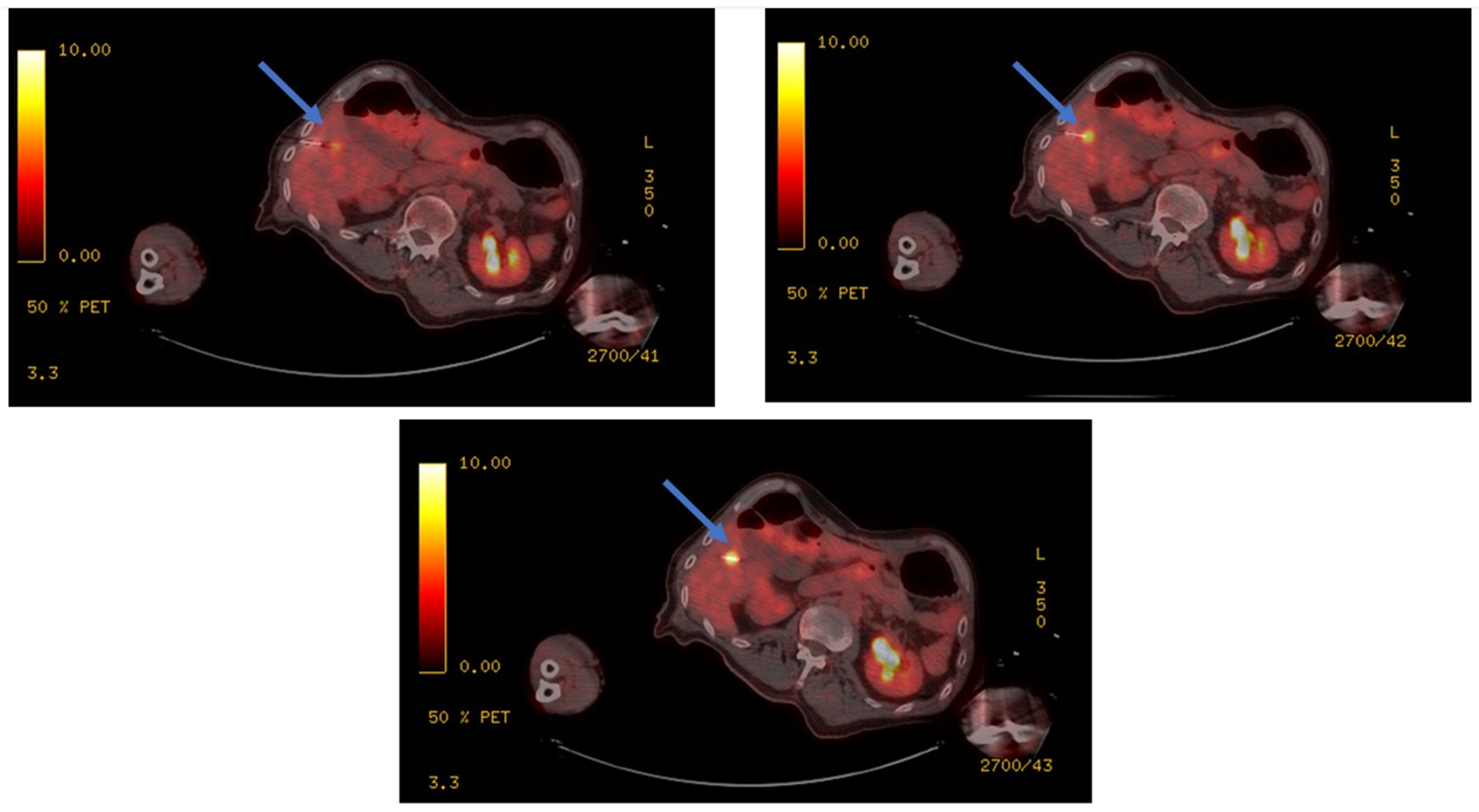
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
References
- Edwards, B.K.; Ward, E.; Kohler, B.A.; Eheman, C.; Zauber, A.G.; Anderson, R.N.; Jemal, A.; Schymura, M.; Lansdorp-Vogelaar, I.; Seeff, L.; et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer 2010, 116, 544–573. [Google Scholar] [CrossRef] [PubMed]
- van der Geest, L.G.; Lam-Boer, J.; Koopman, M.; Verhoef, C.; Elferink, M.A.; de Wilt, J.H. Nationwide trends in incidence, treatment and survival of colorectal cancer patients with synchronous metastases. Clin. Exp. Metastasis 2015, 32, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Renehan, A.G.; Egger, M.; Saunders, M.P.; O’Dwyer, S.T. Impact on survival of intensive follow up after curative resection for colorectal cancer: Systematic review and meta-analysis of randomised trials. BMJ 2002, 324, 813. [Google Scholar] [CrossRef] [PubMed]
- Camacho, J.C.; Petre, E.N.; Sofocleous, C.T. Thermal Ablation of Metastatic Colon Cancer to the Liver. Semin. Interv. Radiol. 2019, 36, 310–318. [Google Scholar] [CrossRef]
- Solbiati, L.; Ahmed, M.; Cova, L.; Ierace, T.; Brioschi, M.; Goldberg, S.N. Small Liver Colorectal Metastases Treated with Percutaneous Radiofrequency Ablation: Local Response Rate and Long-term Survival with up to 10-year Follow-up. Radiology 2012, 265, 958–968. [Google Scholar] [CrossRef]
- Shady, W.; Petre, E.N.; Gonen, M.; Erinjeri, J.P.; Brown, K.T.; Covey, A.M.; Alago, W.; Durack, J.; Maybody, M.; Brody, L.A.; et al. Percutaneous Radiofrequency Ablation of Colorectal Cancer Liver Metastases: Factors Affecting Outcomes—A 10-year Experience at a Single Center. Radiology 2016, 278, 601–611. [Google Scholar] [CrossRef]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Arain, M.A.; Chen, Y.J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Farkas, L.; et al. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 329–359. [Google Scholar] [CrossRef]
- Kaye, E.A.; Cornelis, F.H.; Petre, E.N.; Tyagi, N.; Shady, W.; Shi, W.; Zhang, Z.; Solomon, S.B.; Sofocleous, C.T.; Durack, J.C. Volumetric 3D assessment of ablation zones after thermal ablation of colorectal liver metastases to improve prediction of local tumor progression. Eur. Radiol. 2019, 29, 2698–2705. [Google Scholar] [CrossRef]
- Shady, W.; Petre, E.N.; Vakiani, E.; Ziv, E.; Gonen, M.; Brown, K.T.; Kemeny, N.E.; Solomon, S.B.; Solit, D.B.; Sofocleous, C.T. Kras mutation is a marker of worse oncologic outcomes after percutaneous radiofrequency ablation of colorectal liver metastases. Oncotarget 2017, 8, 66117–66127. [Google Scholar] [CrossRef]
- Kurilova, I.; Bendet, A.; Petre, E.N.; Boas, F.E.; Kaye, E.; Gonen, M.; Covey, A.; Brody, L.A.; Brown, K.T.; Kemeny, N.E.; et al. Factors Associated With Local Tumor Control and Complications after Thermal Ablation of Colorectal Cancer Liver Metastases: A 15-year Retrospective Cohort Study. Clin. Color. Cancer 2021, 20, e82–e95. [Google Scholar] [CrossRef]
- Keil, S.; Bruners, P.; Schiffl, K.; Sedlmair, M.; Mühlenbruch, G.; Günther, R.W.; Das, M.; Mahnken, A.H. Radiofrequency Ablation of Liver Metastases—Software-Assisted Evaluation of the Ablation Zone in MDCT: Tumor-Free Follow-up Versus Local Recurrent Disease. Cardiovasc. Interv. Radiol. 2010, 33, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Shady, W.; Petre, E.N.; Do, K.G.; Gonen, M.; Yarmohammadi, H.; Brown, K.T.; Kemeny, N.E.; D’Angelica, M.; Kingham, P.T.; Solomon, S.B.; et al. Percutaneous Microwave versus Radiofrequency Ablation of Colorectal Liver Metastases: Ablation with Clear Margins (A0) Provides the Best Local Tumor Control. J. Vasc. Interv. Radiol. 2018, 29, 268–275.e1. [Google Scholar] [CrossRef] [PubMed]
- Ruers, T.; Van Coevorden, F.; Punt, C.J.A.; Pierie, J.-P.E.N.; Borel-Rinkes, I.; Ledermann, J.A.; Poston, G.; Bechstein, W.; Lentz, M.-A.; Mauer, M.; et al. Local Treatment of Unresectable Colorectal Liver Metastases: Results of a Randomized Phase II Trial. Gynecol. Oncol. 2017, 109, djx015. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Kim, J.H.; Yang, S.G.; Park, S.H.; Choi, H.-K.; Chun, S.-Y.; Kim, P.N.; Park, J.; Lee, M. A Single-Center Retrospective Analysis of Periprocedural Variables Affecting Local Tumor Progression after Radiofrequency Ablation of Colorectal Cancer Liver Metastases. Radiology 2021, 298, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Laimer, G.; Jaschke, N.; Schullian, P.; Putzer, D.; Eberle, G.; Solbiati, M.; Solbiati, L.; Goldberg, S.N.; Bale, R. Volumetric assessment of the periablational safety margin after thermal ablation of colorectal liver metastases. Eur. Radiol. 2021, 31, 6489–6499. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sofocleous, C.T.; Erinjeri, J.P.; Petre, E.N.; Gonen, M.; Do, K.G.; Brown, K.T.; Covey, A.M.; Brody, L.A.; Alago, W.; et al. Margin Size is an Independent Predictor of Local Tumor Progression After Ablation of Colon Cancer Liver Metastases. Cardiovasc. Interv. Radiol. 2013, 36, 166–175. [Google Scholar] [CrossRef]
- Calandri, M.; Yamashita, S.; Gazzera, C.; Fonio, P.; Veltri, A.; Bustreo, S.; Sheth, R.A.; Yevich, S.M.; Vauthey, J.-N.; Odisio, B.C. Ablation of colorectal liver metastasis: Interaction of ablation margins and RAS mutation profiling on local tumour progression-free survival. Eur. Radiol. 2018, 28, 2727–2734. [Google Scholar] [CrossRef]
- Puijk, R.S.; Nieuwenhuizen, S.; Bemd, B.A.V.D.; Ruarus, A.H.; Geboers, B.; Vroomen, L.G.; Muglia, R.; de Jong, M.C.; de Vries, J.J.; Scheffer, H.J.; et al. Transcatheter CT Hepatic Arteriography Compared with Conventional CT Fluoroscopy Guidance in Percutaneous Thermal Ablation to Treat Colorectal Liver Metastases: A Single-Center Comparative Analysis of 2 Historical Cohorts. J. Vasc. Interv. Radiol. 2020, 31, 1772–1783. [Google Scholar] [CrossRef]
- Kong, G.; Jackson, C.; Koh, D.M.; Lewington, V.; Sharma, B.; Brown, G.; Cunningham, D.; Cook, G.J.R. The use of 18F-FDG PET/CT in colorectal liver metastases—Comparison with CT and liver MRI. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1323–1329. [Google Scholar] [CrossRef]
- Ryan, E.R.; Sofocleous, C.T.; Schöder, H.; Carrasquillo, J.A.; Nehmeh, S.; Larson, S.; Thornton, R.; Siegelbaum, R.H.; Erinjeri, J.P.; Solomon, S.B. Split-Dose Technique for FDG PET/CT–guided Percutaneous Ablation: A Method to Facilitate Lesion Targeting and to Provide Immediate Assessment of Treatment Effectiveness. Radiology 2013, 268, 288–295. [Google Scholar] [CrossRef]
- Cornelis, F.H.; Petre, E.N.; Vakiani, E.; Klimstra, D.; Durack, J.C.; Gonen, M.; Osborne, J.; Solomon, S.B.; Sofocleous, C.T. Immediate Postablation 18F-FDG Injection and Corresponding SUV Are Surrogate Biomarkers of Local Tumor Progression After Thermal Ablation of Colorectal Carcinoma Liver Metastases. J. Nucl. Med. 2018, 59, 1360–1365. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, F.; Storchios, V.; Violari, E.; Sofocleous, C.T.; Schoder, H.; Durack, J.C.; Siegelbaum, R.H.; Maybody, M.; Humm, J.; Solomon, S.B. 18F-FDG PET/CT is an Immediate Imaging Biomarker of Treatment Success after Liver Metastasis Ablation. J. Nucl. Med. 2016, 57, 1052–1057. [Google Scholar] [CrossRef] [PubMed]
- Lai, D.T.M.; Fulham, M.; Stephen, M.S.; Chu, K.-M.; Solomon, M.; Thompson, J.F.; Sheldon, D.M.; Storey, D.W. The Role of Whole-Body Positron Emission Tomography With [18F]Fluorodeoxyglucose in Identifying Operable Colorectal Cancer Metastases to the Liver. Arch. Surg. 1996, 131, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Bipat, S.; van Leeuwen, M.S.; Comans, E.F.; Pijl, M.E.; Bossuyt, P.M.; Zwinderman, A.H.; Stoker, J. Colorectal liver metastases: CT, MR imaging, and PET for diagnosis—Meta-analysis. Radiology 2005, 237, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Floriani, I.; Torri, V.; Rulli, E.; Garavaglia, D.; Compagnoni, A.; Salvolini, L.; Giovagnoni, A. Performance of imaging modalities in diagnosis of liver metastases from colorectal cancer: A systematic review and meta-analysis. J. Magn. Reson. Imaging 2010, 31, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Mulier, S.; Ruers, T.; Jamart, J.; Michel, L.; Marchal, G.; Ni, Y. Radiofrequency ablation versus resection for resectable colorectal liver metastases: Time for a randomized trial? Dig. Surg. 2008, 25, 445–460. [Google Scholar] [CrossRef] [PubMed]
- Ayav, A.; Germain, A.; Marchal, F.; Tierris, I.; Laurent, V.; Bazin, C.; Yuan, Y.; Robert, L.; Brunaud, L.; Bresler, L. Radiofrequency ablation of unresectable liver tumors: Factors associated with incomplete ablation or local recurrence. Am. J. Surg. 2010, 200, 435–439. [Google Scholar] [CrossRef]
- Mulier, S.; Ni, Y.; Jamart, J.; Ruers, T.; Marchal, G.; Michel, L. Local recurrence after hepatic radiofrequency coagulation: Multivariate meta-analysis and review of contributing factors. Ann. Surg. 2005, 242, 158. [Google Scholar] [CrossRef]
- Sandu, R.-M.; Paolucci, I.; Ruiter, S.J.S.; Sznitman, R.; de Jong, K.P.; Freedman, J.; Weber, S.; Tinguely, P. Volumetric Quantitative Ablation Margins for Assessment of Ablation Completeness in Thermal Ablation of Liver Tumors. Front. Oncol. 2021, 11, 623098. [Google Scholar] [CrossRef]
- Sotirchos, V.S.; Petrovic, L.M.; Gönen, M.; Klimstra, D.S.; Do, R.K.G.; Petre, E.N.; Garcia, A.R.; Barlas, A.; Erinjeri, J.P.; Brown, K.T.; et al. Colorectal Cancer Liver Metastases: Biopsy of the Ablation Zone and Margins Can be Used to Predict Oncologic Outcome. Radiology 2016, 280, 949–959. [Google Scholar] [CrossRef]
- Vasiniotis Kamarinos, N.; Vakiani, E.; Gonen, M.; Kemeny, N.E.; Sigel, C.; Saltz, L.B.; Brown, K.T.; Covey, A.M.; Erinjeri, J.P.; Brody, L.A.; et al. Biopsy and Margins Optimize Outcomes after Thermal Ablation of Colorectal Liver Metastases. Cancers 2022, 14, 693. [Google Scholar] [CrossRef] [PubMed]
- Casadaban, L.C.; Catalano, P.J.; Lee, L.K.; Hyun, H.; Tuncali, K.; Gerbaudo, V.H.; Shyn, P.B. Assessing ablation margins of FDG-avid liver tumors during PET/CT-guided thermal ablation procedures: A retrospective study. Eur. J. Nucl. Med. 2021, 48, 2914–2924. [Google Scholar] [CrossRef] [PubMed]
- Shyn, P.B.; Casadaban, L.C.; Sainani, N.I.; Sadow, C.A.; Bunch, P.M.; Levesque, V.M.; Kim, C.K.; Gerbaudo, V.H.; Silverman, S.G. Intraprocedural Ablation Margin Assessment by Using Ammonia Perfusion PET during FDG PET/CT–guided Liver Tumor Ablation: A Pilot Study. Radiology 2018, 288, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Faber, R.A.; Burghout, K.S.; Bijlstra, O.D.; Hendriks, P.; van Erp, G.C.; Broersen, A.; Dijkstra, J.; Vahrmeijer, A.L.; Burgmans, M.C.; Mieog, J.S.D. Three-dimensional quantitative margin assessment in patients with colorectal liver metastases treated with percutaneous thermal ablation using semi-automatic rigid MRI/CECT-CECT co-registration. Eur. J. Radiol. 2022, 156, 110552. [Google Scholar] [CrossRef] [PubMed]
- Ruiter, S.J.S.; Tinguely, P.; Paolucci, I.; Engstrand, J.; Candinas, D.; Weber, S.; de Haas, R.J.; de Jong, K.P.; Freedman, J. 3D Quantitative Ablation Margins for Prediction of Ablation Site Recurrence After Stereotactic Image-Guided Microwave Ablation of Colorectal Liver Metastases: A Multicenter Study. Front. Oncol. 2021, 11, 110552. [Google Scholar] [CrossRef] [PubMed]
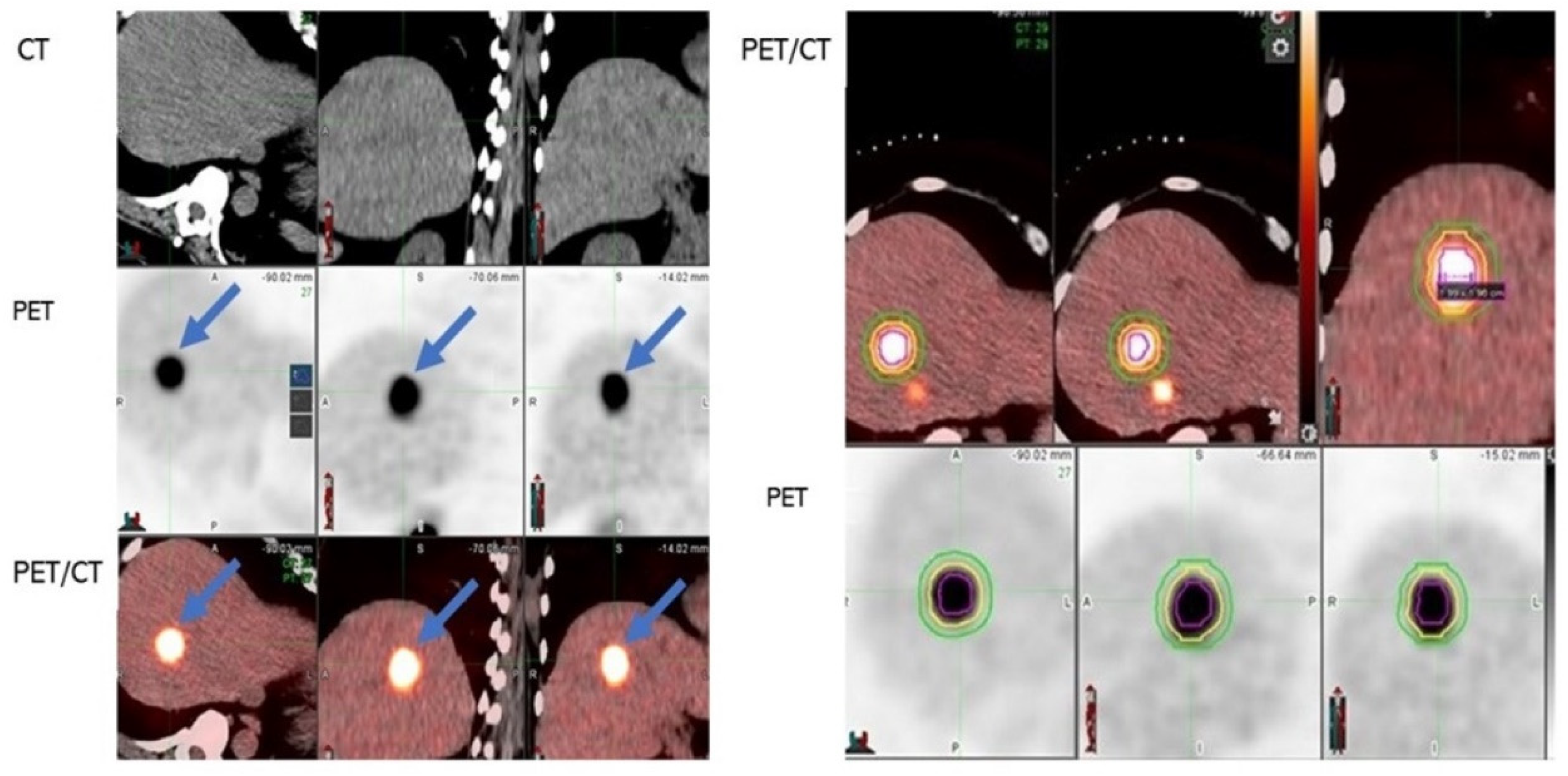


| Number of Participants/CLMs: 125/190 |
|---|
|
|
|
|
|
|
|
|
| Frequency | Percent | ||
|---|---|---|---|
| CT | Non-detectable or poor conspicuity | 61 | 32.1 |
| Detectable | 129 | 67.9 | |
| Total | 190 | 100.0 | |
| PET/CT | Non-detectable or low FDG avidity | 4 | 2.1 |
| Detectable | 186 | 97.9 | |
| Total | 190 | 100.0 | |
| Symmetric Measures | |||
|---|---|---|---|
| Value | Approximate Significance | ||
| Nominal by Nominal | Phi | 0.135 | 0.063 |
| Cramer’s V | 0.135 | 0.063 | |
| N of Valid Cases | 190 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zirakchian Zadeh, M.; Yeh, R.; Kunin, H.S.; Kirov, A.S.; Petre, E.N.; Gönen, M.; Silk, M.; Cornelis, F.H.; Soares, K.C.; Ziv, E.; et al. Real-Time Split-Dose PET/CT-Guided Ablation Improves Colorectal Liver Metastasis Detection and Ablation Zone Margin Assessments without the Need for Repeated Contrast Injection. Cancers 2022, 14, 6253. https://doi.org/10.3390/cancers14246253
Zirakchian Zadeh M, Yeh R, Kunin HS, Kirov AS, Petre EN, Gönen M, Silk M, Cornelis FH, Soares KC, Ziv E, et al. Real-Time Split-Dose PET/CT-Guided Ablation Improves Colorectal Liver Metastasis Detection and Ablation Zone Margin Assessments without the Need for Repeated Contrast Injection. Cancers. 2022; 14(24):6253. https://doi.org/10.3390/cancers14246253
Chicago/Turabian StyleZirakchian Zadeh, Mahdi, Randy Yeh, Henry S. Kunin, Assen S. Kirov, Elena N. Petre, Mithat Gönen, Mikhail Silk, Francois H. Cornelis, Kevin C. Soares, Etay Ziv, and et al. 2022. "Real-Time Split-Dose PET/CT-Guided Ablation Improves Colorectal Liver Metastasis Detection and Ablation Zone Margin Assessments without the Need for Repeated Contrast Injection" Cancers 14, no. 24: 6253. https://doi.org/10.3390/cancers14246253
APA StyleZirakchian Zadeh, M., Yeh, R., Kunin, H. S., Kirov, A. S., Petre, E. N., Gönen, M., Silk, M., Cornelis, F. H., Soares, K. C., Ziv, E., Solomon, S. B., Sotirchos, V. S., & Sofocleous, C. T. (2022). Real-Time Split-Dose PET/CT-Guided Ablation Improves Colorectal Liver Metastasis Detection and Ablation Zone Margin Assessments without the Need for Repeated Contrast Injection. Cancers, 14(24), 6253. https://doi.org/10.3390/cancers14246253







