Prophylactic and Early Thyroidectomy in RET Germline Mutation Carriers in Pediatric and Adult Population: Long-Term Outcomes of a Series of 63 Patients
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Clinical, Genetic and Pathological Findings According to the Age at Surgery
3.2. Clinical, Genetic and Pathological Findings According to the ATA Risk Mutational Profile
3.3. Surgical Morbidity According to Age at Surgery and Extent of Surgery
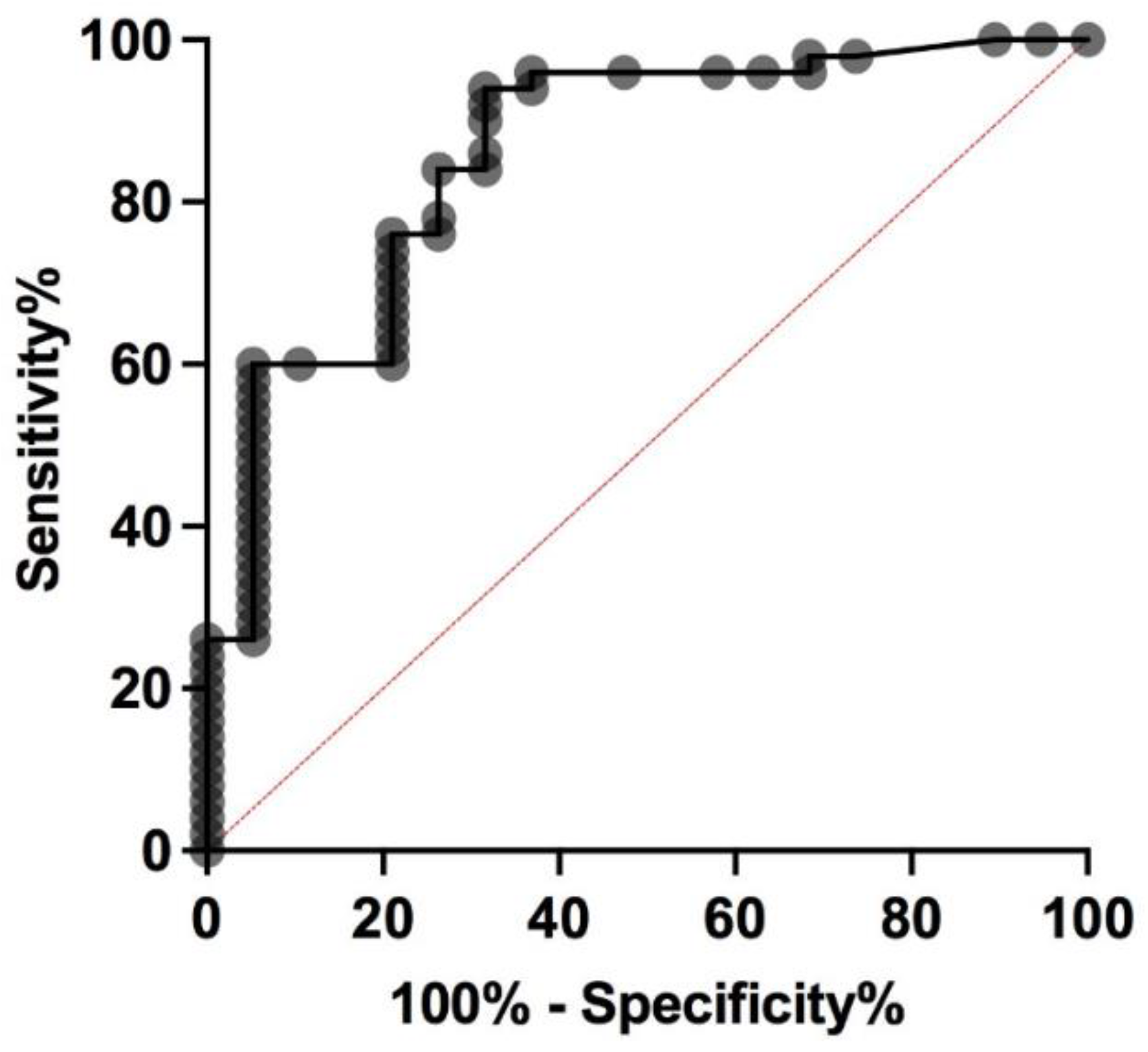
3.4. Predictive Factors of MTC
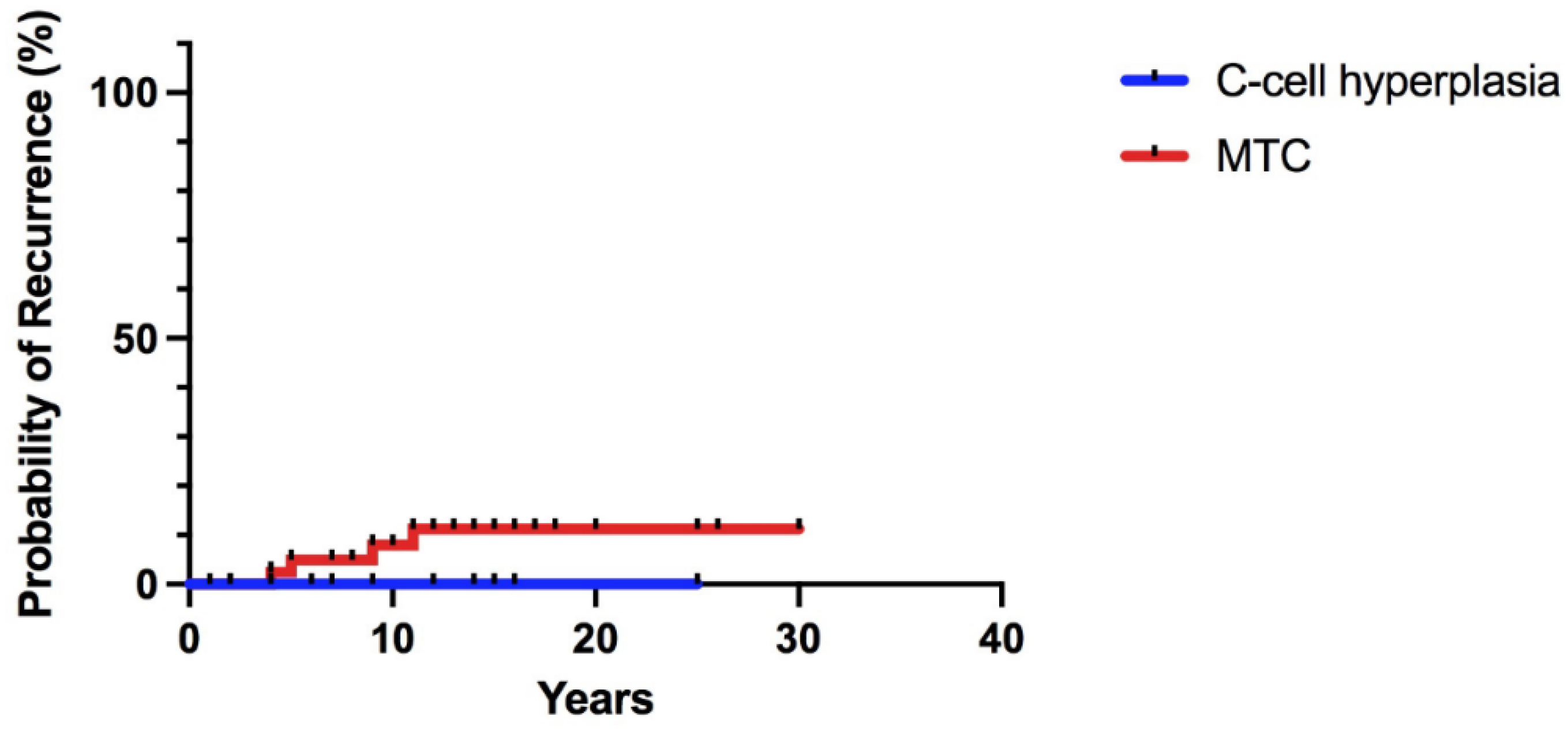
3.5. Follow-Up
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wells, S.A.; Asa, S.L.; Dralle, H.; Elisei, R.; Evans, D.B.; Gagel, R.F.; Lee, N.; Machens, A.; Moley, J.F.; Pacini, F.; et al. Revised American thyroid association guidelines for the management of medullary thyroid carcinoma. Thyroid 2015, 25, 567–610. [Google Scholar] [CrossRef] [PubMed]
- Machens, A.; Dralle, H. Therapeutic effectiveness of screening for multiple endocrine neoplasia type 2A. J. Clin. Endocrinol. Metab. 2015, 100, 2539–2545. [Google Scholar] [CrossRef] [PubMed]
- Pelizzo, M.R.; Torresan, F.; Boschin, I.M.; Nacamulli, D.; Pennelli, G.; Barollo, S.; Rubello, D.; Mian, C. Early, prophylactic thyroidectomy in hereditary medullary thyroid carcinoma: A 26-year monoinstitutional experience. Am. J. Clin. Oncol. Cancer Clin. Trials. 2015, 38, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Elisei, R.; Romei, C.; Renzini, G.; Bottici, V.; Cosci, B.; Molinaro, E.; Agate, L.; Cappagli, V.; Miccoli, P.; Berti, P.; et al. The timing of total thyroidectomy in RET gene mutation carriers could be personalized and safely planned on the basis of serum calcitonin: 18 Years experience at one single center. J. Clin. Endocrinol. Metab. 2012, 97, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Machens, A.; Elwerr, M.; Thanh, P.N.; Lorenz, K.; Schneider, R.; Dralle, H. Impact of central node dissection on postoperative morbidity in pediatric patients with suspected or proven thyroid cancer. Surgery 2016, 160, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Torresan, F.; Cavedon, E.; Mian, C.; Iacobone, M. Long-Term Outcome After Surgery for Medullary Thyroid Carcinoma: A Single-Center Experience. World J. Surg. 2018, 42, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Torresan, F.; Mian, C.; Cavedon, E.; Iacobone, M. Cure and survival of sporadic medullary thyroid carcinoma following systematic preoperative calcitonin screening. Langenbeck’s Arch. Surg. 2019, 404, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.N.; Yip, L.; Lubitz, C.C.; Grubbs, E.G.; Miller, B.S.; Shen, W.; Angelos, P.; Chen, H.; Doherty, G.M.; Fahey, T.J.; et al. The American association of endocrine surgeons guidelines for the definitive surgical management of thyroid disease in adults. Ann. Surg. 2020, 271, E21–E93. [Google Scholar] [CrossRef] [PubMed]
- Innella, G.; Rossi, C.; Romagnoli, M.; Repaci, A.; Bianchi, D.; Cantarini, M.E.; Martorana, D.; Godino, L.; Pession, A.; Percesepe, A.; et al. Results and clinical interpretation of germline RET analysis in a series of patients with medullary thyroid carcinoma: The challenge of the variants of uncertain significance. Cancers 2020, 12, 3268. [Google Scholar] [CrossRef] [PubMed]
- Rohmer, V.; Vidal-Trecan, G.; Bourdelot, A.; Niccoli, P.; Murat, A.; Wemeau, J.L.; Borson-Chazot, F.; Schvartz, C.; Tabarin, A.; Chabre, O.; et al. Prognostic factors of disease-free survival after thyroidectomy in 170 young patients with a RET germline mutation: A multicenter study of the Groupe Français d’Etude des Tumeurs Endocrines. J. Clin. Endocrinol. Metab. 2011, 96, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Niccoli-Sire, P.; Murat, A.; Rohmer, V.; Gibelin, H.; Chabrier, G.; Conte-Devolx, B.; Visset, J.; Ronceray, J.; Jaeck, D.; Henry, J.F.; et al. When should thyroidectomy be performed in familial medullary thyroid carcinoma gene carriers with non-cysteine RET mutations? Surgery 2003, 134, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Machens, A.; Niccoli-Sire, P.; Hoegel, J.; Frank-Raue, K.; van Vroonhoven, T.J.; Roeher, H.-D.; Wahl, R.A.; Lamesch, P.; Raue, F.; Conte-Devolx, B.; et al. Early Malignant Progression of Hereditary Medullary Thyroid Cancer. N. Engl. J. Med. 2003, 349, 1517–1525. [Google Scholar] [CrossRef] [PubMed]
- Morris, L.F.; Waguespack, S.G.; Edeiken-Monroe, B.S.; Lee, J.E.; Rich, T.A.; Ying, A.K.; Warneke, C.L.; Evans, D.B.; Perrier, N.D.; Grubbs, E.G. Ultrasonography should not guide the timing of thyroidectomy in pediatric patients diagnosed with multiple endocrine neoplasia syndrome 2a through genetic screening. Ann. Surg. Oncol. 2013, 20, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Shepet, K.; Alhefdhi, A.; Lai, N.; Mazeh, H.; Sippel, R.; Chen, H. Hereditary medullary thyroid cancer: Age-appropriate thyroidectomy improves disease-free survival. Ann. Surg. Oncol. 2013, 20, 1451–1455. [Google Scholar] [CrossRef] [PubMed]
- Censi, S.; Di Stefano, M.; Repaci, A.; Benvenuti, T.; Manso, J.; Pagotto, U.; Iacobone, M.; Barollo, S.; Bertazza, L.; Galuppini, F.; et al. Basal and Calcium-Stimulated Procalcitonin for the Diagnosis of Medullary Thyroid Cancers: Lights and Shadows. Front Endocrinol. (Lausanne) 2021, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Brauckhoff, M.; Machens, A.; Lorenz, K.; Bjøro, T.; Varhaug, J.E.; Dralle, H. Surgical curability of medullary thyroid cancer in multiple endocrine neoplasia 2b: A changing perspective. Ann. Surg. 2014, 259, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Moley, J.F.; Skinner, M.; Gillanders, W.E.; Lairmore, T.C.; Rowland, K.J.; Traugott, A.L.; Jin, L.X.; Wells, S.A. Management of the parathyroid glands during preventive thyroidectomy in patients with multiple endocrine neoplasia type 2. Ann. Surg. 2015, 262, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Machens, A.; Elwerr, M.; Lorenz, K.; Weber, F.; Dralle, H. Long-term outcome of prophylactic thyroidectomy in children carrying RET germline mutations. Br. J. Surg. 2018, 105, e150–e157. [Google Scholar] [CrossRef] [PubMed]
- Prete, F.P.; Abdel-Aziz, T.; Morkane, C.; Brain, C.; Kurzawinski, T.R.; Hindmarsh, P.; Dattani, M.; Spoudeas, H.; Amin, R.; Watkinson, J.; et al. Prophylactic thyroidectomy in children with multiple endocrine neoplasia type 2. Br. J. Surg. 2018, 105, 1319–1327. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | n = (%) | |
|---|---|---|
| Age (years) | <18 | 21 (33%) |
| ≥18 | 42 (67%) | |
| Sex (F/M ratio) | 1.6 | |
| Pre-op Ct levels | 1.02 (1.8) | |
| RET mutation | MOD-risk | 48 (76%) |
| H-risk | 15 (24%) | |
| Surgery | TT | 27 (43%) |
| TT + CNR | 36 (57%) | |
| Pathology | CCH | 19 (30%) |
| MTC | 44 (70%) | |
| Size MTC (mm) | 4 (3.5) | |
| Characteristics | Pediatric | Adults | p-Value | |
|---|---|---|---|---|
| (n = 21) | (n = 42) | |||
| Age at surgery (yrs) | 12 (6.5) | 31 (21) | <0.0001 | |
| Gender | Female | 12 (57%) | 27 (64%) | 0.58 |
| Male | 9 (43%) | 15 (36%) | ||
| Pre-op Ct levels | 0.6 (0.8) | 1.15 (4.62) | 0.04 | |
| Pre-op Ct levels | <1 | 14 (67%) | 17 (40%) | 0.06 |
| ≥1 | 7 (33%) | 25 (60%) | ||
| RET mutation | MOD-risk | 14 (67%) | 34 (81%) | 0.23 |
| H-risk | 7 (33%) | 8 (19%) | ||
| Surgery | TT | 13 (62%) | 14 (33%) | 0.057 |
| TT + CNR | 8 (38%) | 28 (67%) | ||
| Pathology | CCH | 11 (52%) | 8 (19%) | 0.009 |
| MTC | 10 (48%) | 34 (81%) | ||
| Size MTC (mm) | 2 (2.5) | 5 (4) | 0.0016 | |
| Characteristics | All Patients | MOD-Risk | HIGH-Risk | p-Value | |
|---|---|---|---|---|---|
| n = 63 | (n = 48) | (n = 15) | |||
| Sex ratio (Female/Male) | 39/24 | 31/17 | 8/7 | 0.54 | |
| Age at surgery | <18 | 21 | 14 (29%) | 7 (47%) | 0.23 |
| ≥18 | 42 | 34 (71%) | 8 (53%) | ||
| Pre-op Ct levels | <1 | 31 | 26 (54%) | 5 (33%) | 0.23 |
| ≥1 | 32 | 22 (46%) | 10 (66.7%) | ||
| Surgery | TT | 27 | 23 (48%) | 4 (27%) | 0.23 |
| TT + CNR | 36 | 25 (52%) | 11 (73%) | ||
| Pathology | CCH | 19 | 18 (37.5%) | 1 (7%) | 0.026 |
| MTC | 44 | 30 (62.5%) | 14 (93%) | ||
| Characteristics | Pediatric | Adults | p-Value | |
|---|---|---|---|---|
| (n = 21) | (n = 42) | |||
| Overall Morbidity | yes | 7 (33%) | 13 (31%) | >0.99 |
| no | 14 (67%) | 29 (69%) | ||
| Hypoparathyroidism | Transient | 7 (33%) | 11 (26%) | 0.57 |
| Permanent | 0 | 4 (10%) | 0.29 | |
| Vocal cord paresis | Transient | 1 (5%) | 3 (7%) | >0.99 |
| Permanent | 1 (5%) | 1 (2%) | >0.99 | |
| Characteristics | All Patients | Univariate Analysis | Multivariate Analysis | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CCH | MTC | p-Value | Odds Ratio | 95% CI | p-Value | ||||
| n = 19 | n = 44 | ||||||||
| Age at surgery | <18 | 21 | 11 (58%) | 10 (23%) | 0.01 | 7.51 | 1.72–32.8 | 0.007 | |
| ≥18 | 42 | 8 (42%) | 34 (77%) | ||||||
| Gender | Female | 39 | 12 (63%) | 27 (61%) | 0.89 | ||||
| Male | 24 | 7 (37%) | 17 (39%) | ||||||
| Pre-op Ct levels | <1 | 31 | 15 (79%) | 16 (36%) | 0.002 | 5.02 | 1.22–20.6 | 0.02 | |
| ≥1 | 32 | 4 (21%) | 28 (64%) | ||||||
| RET mutational risk | MOD | 48 | 18 (95%) | 30 (68%) | 0.026 | 18.5 | 1.63–209 | 0.01 | |
| H | 15 | 1 (5%) | 14 (32%) | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torresan, F.; Censi, S.; Pennelli, G.; Galuppini, F.; Mian, C.; Iacobone, M. Prophylactic and Early Thyroidectomy in RET Germline Mutation Carriers in Pediatric and Adult Population: Long-Term Outcomes of a Series of 63 Patients. Cancers 2022, 14, 6226. https://doi.org/10.3390/cancers14246226
Torresan F, Censi S, Pennelli G, Galuppini F, Mian C, Iacobone M. Prophylactic and Early Thyroidectomy in RET Germline Mutation Carriers in Pediatric and Adult Population: Long-Term Outcomes of a Series of 63 Patients. Cancers. 2022; 14(24):6226. https://doi.org/10.3390/cancers14246226
Chicago/Turabian StyleTorresan, Francesca, Simona Censi, Gianmaria Pennelli, Francesca Galuppini, Caterina Mian, and Maurizio Iacobone. 2022. "Prophylactic and Early Thyroidectomy in RET Germline Mutation Carriers in Pediatric and Adult Population: Long-Term Outcomes of a Series of 63 Patients" Cancers 14, no. 24: 6226. https://doi.org/10.3390/cancers14246226
APA StyleTorresan, F., Censi, S., Pennelli, G., Galuppini, F., Mian, C., & Iacobone, M. (2022). Prophylactic and Early Thyroidectomy in RET Germline Mutation Carriers in Pediatric and Adult Population: Long-Term Outcomes of a Series of 63 Patients. Cancers, 14(24), 6226. https://doi.org/10.3390/cancers14246226







