Prostate-Specific Membrane Antigen Targeted Pet/CT Imaging in Patients with Colon, Gastric and Pancreatic Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Data Acquisition and Image Reconstruction
2.3. Quantitative Image Analysis
2.4. Immunohistochemistry
2.5. Statistical Analysis
3. Results
3.1. Quantitative Analysis of PET/CT Scans
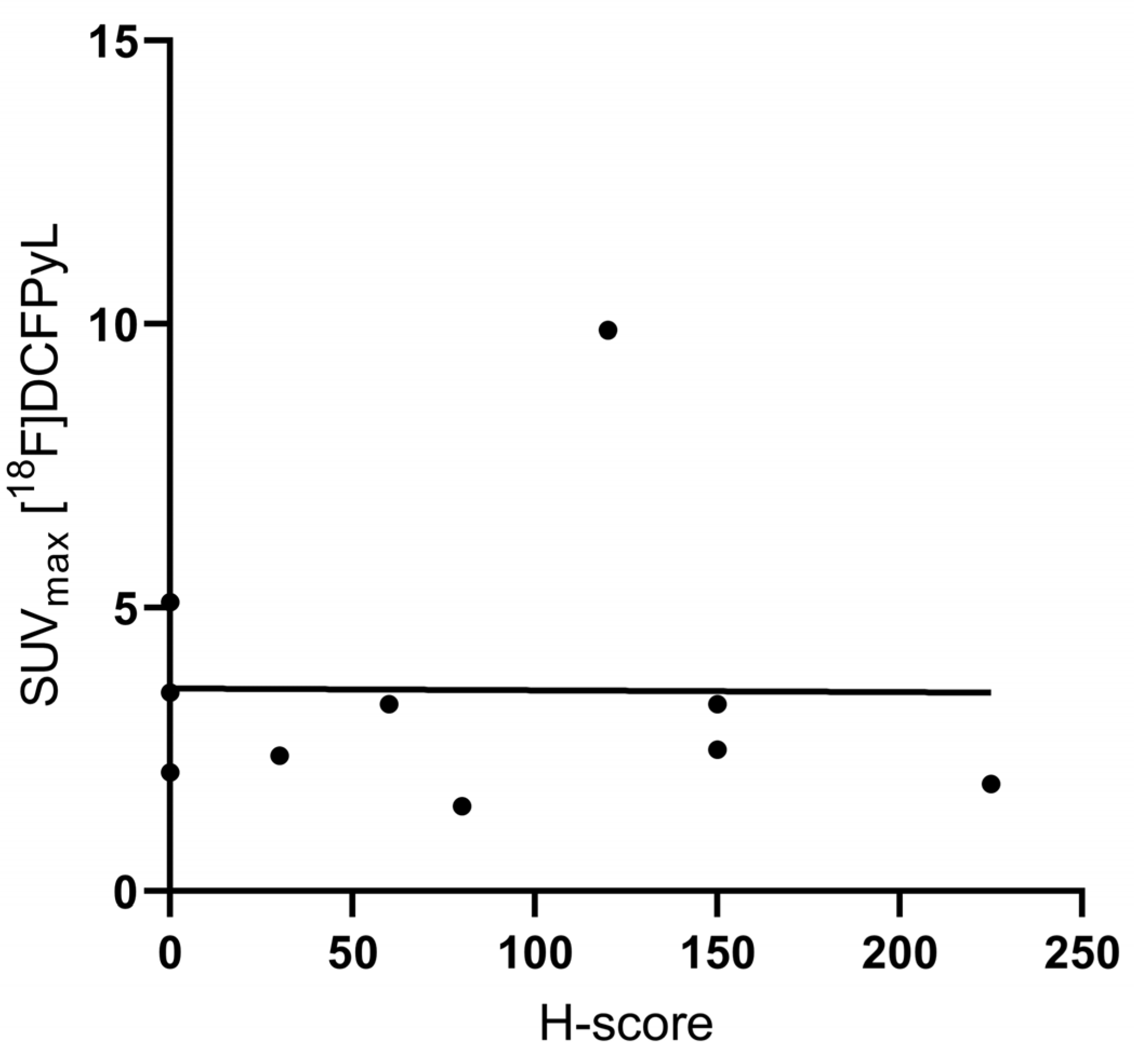
3.2. Immunohistochemical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2020; Available online: https://gco.iarc.fr/today (accessed on 9 February 2022).
- Cunningham, D.; Allum, W.H.; Stenning, S.P.; Thomposon, J.N.; Van de Velde, C.J.H.; Nicolson, M.; Scarffe, J.H.; Lofts, F.J.; Falk, S.J.; Iveson, T.J.; et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N. Engl. J. Med. 2006, 355, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Shim, K.-N.; Kim, S.-E.; Jung, H.-K.; Jung, S.-A.; Yoo, K. The Clinical Value of 18F-Fluorodeoxyglucose Uptake on Positron Emission Tomography/Computed Tomography for Predicting Regional Lymph Node Metastasis and Non-curative Surgery in Primary Gastric Carcinoma. Korean J. Gastroenterol. 2014, 64, 340–347. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Seevaratnam, R.; Cardoso, R.; McGregor, C.; Lourenco, L.; Mahar, A.; Sutradhar, R.; Law, C.; Paszat, L.; Coburn, N. How useful is preoperative imaging for tumor, node, metastasis (TNM) staging of gastric cancer? A meta-analysis. Gastric Cancer 2012, 15, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, J.-Q. Imaging in assessing hepatic and peritoneal metastases of gastric cancer: A systematic review. BMC Gastroenterol. 2011, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Gertsen, E.C.; Brenkman, H.J.F.; van Hillegersberg, R.; van Sandick, J.; van Berge Henegouwen, M.I.; Gisbertz, S.S.; Luyer, M.D.P.; Nieuwenhuijzen, G.A.P.; van Lanschot, J.J.B.; Lagarde, S.M.; et al. 18F-Fludeoxyglucose-Positron Emission Tomography/Computed Tomography and Laparoscopy for Staging of Locally Advanced Gastric Cancer: A Multicenter Prospective Dutch Cohort Study (PLASTIC). JAMA Surg. 2021, 156, e215340. [Google Scholar] [CrossRef]
- Smyth, E.; Schöder, H.; Strong, V.E.; Capanu, M.; Kelsen, D.P.; Coit, D.G.; Shah, M.A. A prospective evaluation of the utility of 2-deoxy-2-[18F]fluoro-D-glucose positron emission tomography and computed tomography in staging locally advanced gastric cancer. Cancer 2012, 118, 5481–5488. [Google Scholar] [CrossRef] [PubMed]
- Gerritsen, A.; Dutch Pancreatic Cancer Group; Molenaar, I.Q.; Bollen, T.L.; Nio, C.Y.; Dijkgraaf, M.G.; Van Santvoort, H.C.; Offerhaus, G.J.; Brosens, L.A.; Biermann, K.; et al. Preoperative Characteristics of Patients with Presumed Pancreatic Cancer but Ultimately Benign Disease: A Multicenter Series of 344 Pancreatoduodenectomies. Ann. Surg. Oncol. 2014, 21, 3999–4006. [Google Scholar] [CrossRef]
- Tummers, W.S.; Groen, J.V.; Mulder, B.G.S.; Farina-Sarasqueta, A.; Morreau, J.; Putter, H.; Van De Velde, C.J.; Vahrmeijer, A.L.; Bonsing, B.A.; Mieog, J.S.; et al. Impact of resection margin status on recurrence and survival in pancreatic cancer surgery. Br. J. Surg. 2019, 106, 1055–1065. [Google Scholar] [CrossRef]
- Haffner, M.C.; Kronberger, I.E.; Ross, J.S.; Sheehan, C.E.; Zitt, M.; Mühlmann, G.; Öfner, D.; Zelger, B.; Ensinger, C.; Yang, X.J.; et al. Prostate-specific membrane antigen expression in the neovasculature of gastric and colorectal cancers. Hum. Pathol. 2009, 40, 1754–1761. [Google Scholar] [CrossRef]
- Ren, H.; Zhang, H.; Wang, X.; Liu, J.; Yuan, Z.; Hao, J. Prostate-specific membrane antigen as a marker of pancreatic cancer cells. Med Oncol. 2014, 31, 1–6. [Google Scholar] [CrossRef]
- Vuijk, F.A.; de Muynck, L.D.A.N.; Franken, L.C.; Busch, O.R.; Wilmink, J.W.; Besselink, M.G.; Bonsing, B.A.; Bhairosingh, S.S.; Kuppen, P.J.K.; Mieog, J.S.D.; et al. Molecular targets for diagnostic and intraoperative imaging of pancreatic ductal adenocarcinoma after neoadjuvant FOLFIRINOX treatment. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-T.; Fong, W.; Thomas, P. Rectal Carcinoma on 68Ga-PSMA PET/CT. Clin. Nucl. Med. 2016, 41, e167–e168. [Google Scholar] [CrossRef] [PubMed]
- Hangaard, L.; Jochumsen, M.R.; Vendelbo, M.H.; Bouchelouche, K. Metastases from Colorectal Cancer Avid on 68Ga-PSMA PET/CT. Clin. Nucl. Med. 2017, 42, 532–533. [Google Scholar] [CrossRef] [PubMed]
- Stoykow, C.; Huber-Schumacher, S.; Almanasreh, N.; Jilg, C.; Ruf, J. Strong PSMA Radioligand Uptake by Rectal Carcinoma: Who Put the ‘S’ in PSMA? Clin. Nucl. Med. 2017, 42, 225–226. [Google Scholar] [CrossRef] [PubMed]
- Krishnaraju, V.S.; Kumar, R.; Mittal, B.R.; Sharma, V.; Singh, H.; Nada, R.; Bal, A.; Rohilla, M.; Singh, H.; Rana, S.S. Differentiating benign and malignant pancreatic masses: Ga-68 PSMA PET/CT as a new diagnostic avenue. Eur. Radiol. 2021, 31, 2199–2208. [Google Scholar] [CrossRef]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177–PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef]
- Jansen, B.H.E.; Yaqub, M.; Voortman, J.; Cysouw, M.C.F.; Windshorst, A.D.; Schuit, R.C.; Kramer, G.M.; van den Eertwegh, A.J.M.; Schwarte, L.A.; Hendrikse, N.H.; et al. Simplified Methods for Quantification of 18F-DCFPyL Uptake in Patients with Prostate Cancer. J. Nucl. Med. 2019, 60, 1730–1735. [Google Scholar] [CrossRef]
- Wondergem, M.; van der Zant, F.M.; Knol, R.J.J.; Lazarenko, S.V.; Pruim, J.; de Jong, I. 18F-DCFPyL PET/CT in the Detection of Prostate Cancer at 60 and 120 Minutes: Detection Rate, Image Quality, Activity Kinetics, and Biodistribution. J. Nucl. Med. 2017, 58, 1797–1804. [Google Scholar] [CrossRef]
- Boellaard, R.; Delgado-Bolton, R.; Oyen, W.J.G.; Giammarile, F.; Tatsch, K.; Eschner, W.; Verzijlbergen, F.J.; Barrington, S.F.; Pike, L.C.; Weber, W.A.; et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: Version 2.0. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 328–354. [Google Scholar] [CrossRef]
- Nioche, C.; Orlhac, F.; Boughdad, S.; Reuzé, S.; Goya-Outi, J.; Robert, C.; Pellot-Barakat, C.; Soussan, M.; Frouin, F.; Buvat, I. LIFEx: A Freeware for Radiomic Feature Calculation in Multimodality Imaging to Accelerate Advances in the Characterization of Tumor Heterogeneity. Cancer Res. 2018, 78, 4786–4789. [Google Scholar] [CrossRef]
- Schmuck, S.; von Klot, C.A.; Henkenberens, C.; Sohns, J.M.; Christiansen, H.; Wester, H.-J.; Ross, T.L.; Bengel, F.M.; Derlin, T. Initial Experience with Volumetric 68Ga-PSMA I&T PET/CT for Assessment of Whole-Body Tumor Burden as a Quantitative Imaging Biomarker in Patients with Prostate Cancer. J. Nucl. Med. 2017, 58, 1962–1968. [Google Scholar] [CrossRef] [PubMed]
- Jansen, B.H.E.; Cysouw, M.C.F.; Vis, A.N.; van Moorselaar, R.J.A.; Voortman, J.; Bodar, Y.J.L.; Schober, P.R.; Hendrikse, N.H.; Hoekstra, O.S.; Boellaard, R.; et al. Repeatability of Quantitative 18F-DCFPyL PET/CT Measurements in Metastatic Prostate Cancer. J. Nucl. Med. 2020, 61, 1320–1325. [Google Scholar] [CrossRef] [PubMed]
- Jung, I.; Gurzu, S.; Raica, M.; Cîmpean, A.M.; Szentirmay, Z. The differences between the endothelial area marked with CD31 and CD105 in colorectal carcinomas by computer-assisted morphometrical analysis. Rom. J. Morphol. Embryol. 2009, 50, 239–243. [Google Scholar] [PubMed]
- Hirsch, F.R.; Varella-Garcia, M.; Bunn, P.A., Jr.; Di Maria, M.V.; Veve, R.; Bremnes, R.M.; Barón, A.E.; Zeng, C.; Franklin, W.A. Epidermal Growth Factor Receptor in Non–Small-Cell Lung Carcinomas: Correlation Between Gene Copy Number and Protein Expression and Impact on Prognosis. J. Clin. Oncol. 2003, 21, 3798–3807. [Google Scholar] [CrossRef] [PubMed]
- John, T.; Liu, G.; Tsao, M.-S. Overview of molecular testing in non-small-cell lung cancer: Mutational analysis, gene copy number, protein expression and other biomarkers of EGFR for the prediction of response to tyrosine kinase inhibitors. Oncogene 2009, 28, S14–S23. [Google Scholar] [CrossRef]
- Arçay, A.; Eiber, M.; Langbein, T. Incidental Finding of Colon Carcinoma Related to High Uptake in 18F-PSMA-1007 PET. Clin. Nucl. Med. 2020, 45, 561–562. [Google Scholar] [CrossRef]
- Cuda, T.J.; Riddell, A.D.; Liu, C.; Whitehall, V.L.; Borowsky, J.; Wyld, D.K.; Burge, M.E.; Ahern, E.; Griffin, A.; Lyons, N.J.; et al. PET Imaging Quantifying 68Ga-PSMA-11 Uptake in Metastatic Colorectal Cancer. J. Nucl. Med. 2020, 61, 1576–1579. [Google Scholar] [CrossRef]
- Ferreira, G.; Iravani, A.; Hofman, M.S.; Hicks, R.J. Intra-individual comparison of 68Ga-PSMA-11 and 18F-DCFPyL normal-organ biodistribution. Cancer Imaging 2019, 19, 23. [Google Scholar] [CrossRef]
- Man, K.D.; Laeken, N.V.; Schelfhout, V.; Fendler, W.P.; Lambert, B.; Kersemans, K.; Piron, S.; Lumen, N.; Decaestecker, K.; Fonteyne, V.; et al. 18F-PSMA-11 Versus 68Ga-PSMA-11 Positron Emission Tomography/Computed Tomography for Staging and Biochemical Recurrence of Prostate Cancer: A Prospective Double-blind Randomised Cross-over Trial. Eur. Urol. 2022, 82, 501–509. [Google Scholar] [CrossRef]
- Giesel, F.L.; Will, L.; Lawal, I.; Lengana, T.; Kratochwil, C.; Vorster, M.; Neels, O.; Reyneke, F.; Harberkon, U.; Kopka, K.; et al. Intraindividual Comparison of 18F-PSMA-1007 and 18F-DCFPyL PET/CT in the Prospective Evaluation of Patients with Newly Diagnosed Prostate Carcinoma: A Pilot Study. J. Nucl. Med. 2018, 59, 1076–1080. [Google Scholar] [CrossRef]
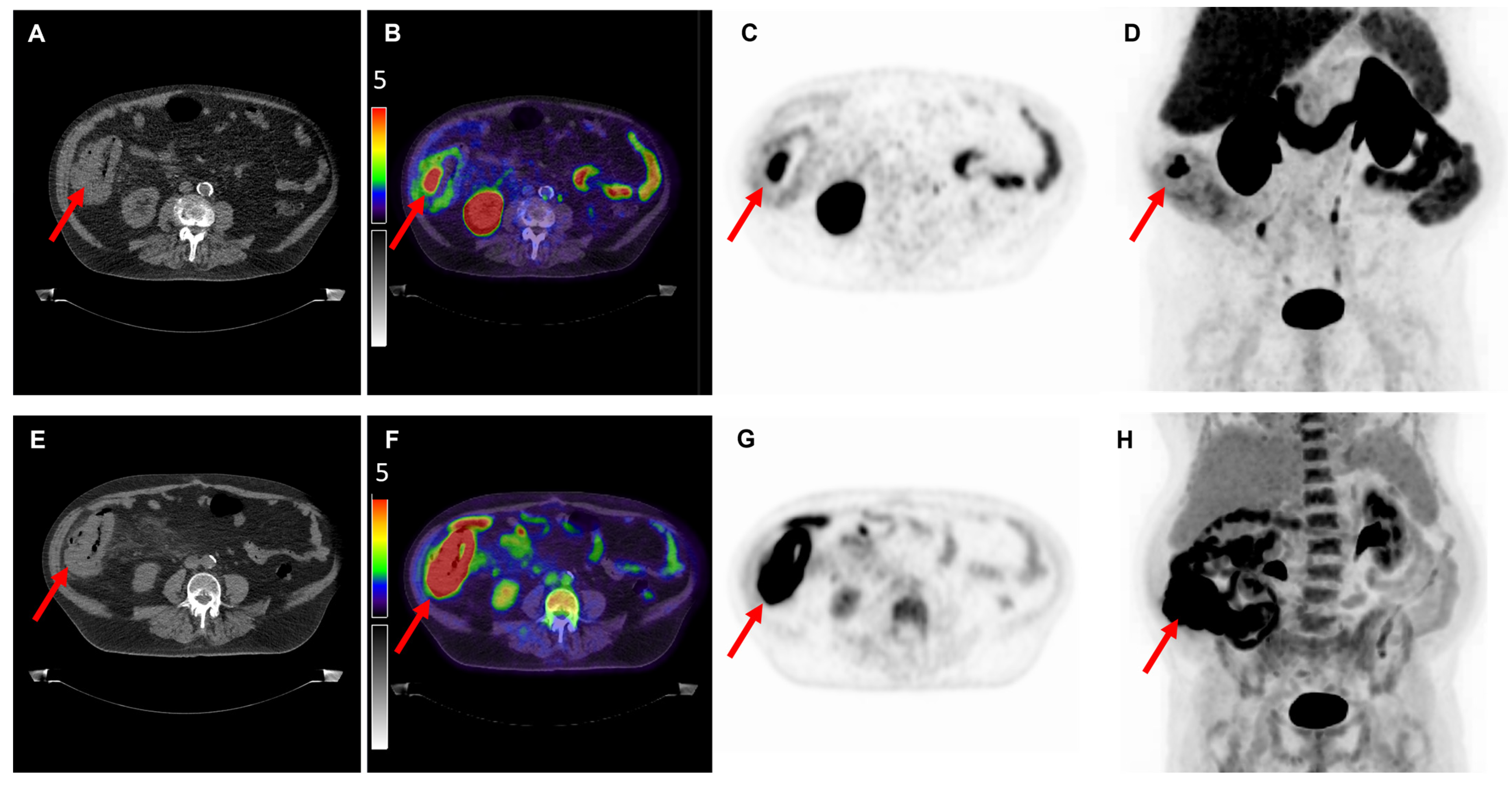

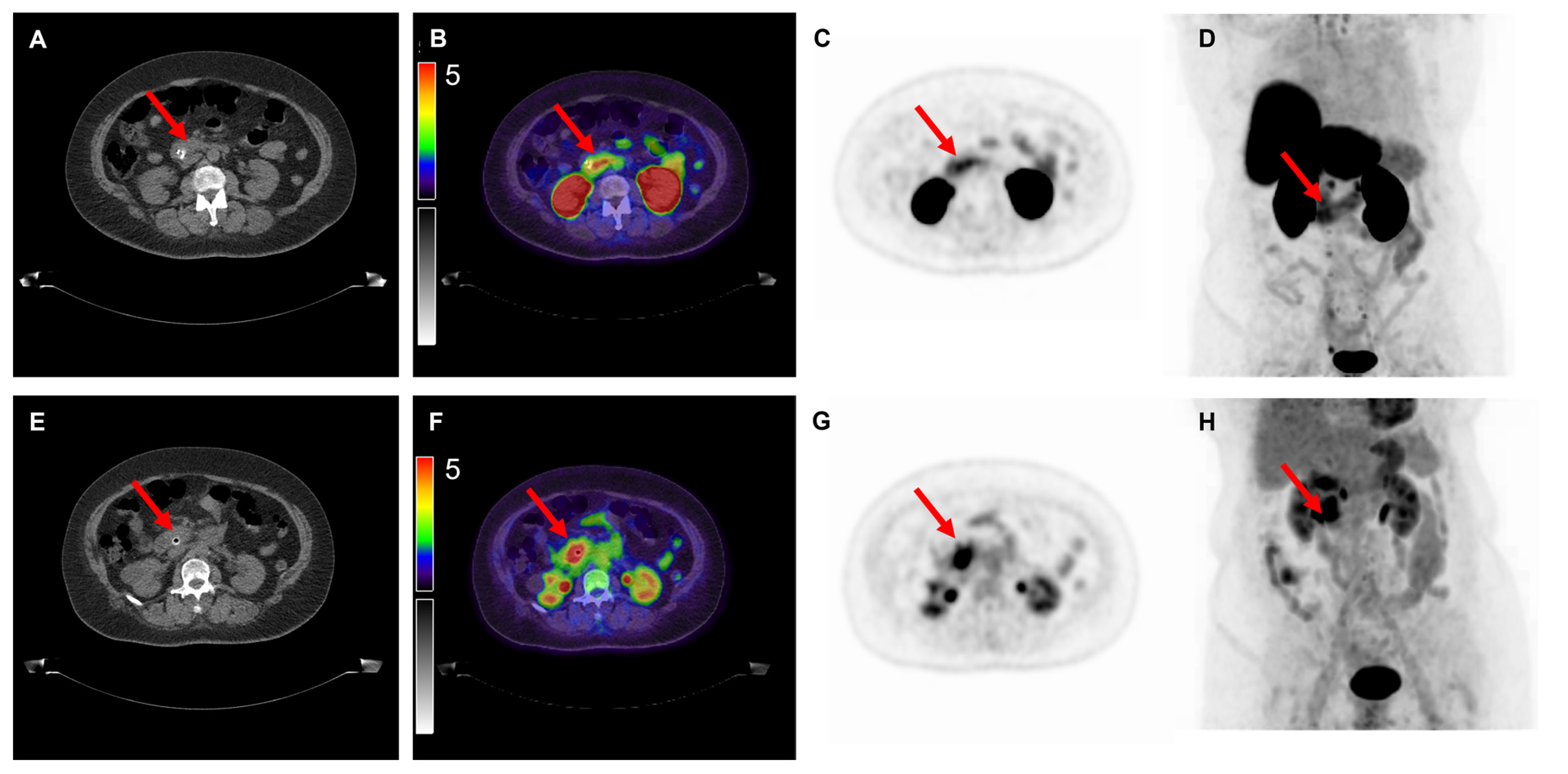
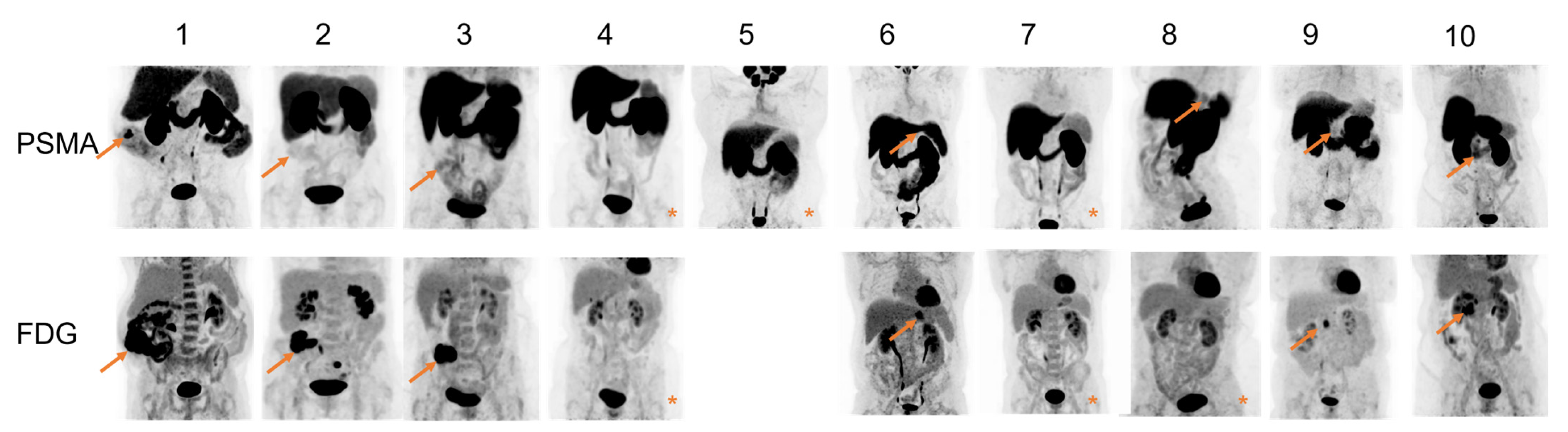

| No Figure | Age | Tumor Location | Tumor Differentiation | cTNM Stage * | pTNM Stage | Max Diameter (mm) ** | SUVmax [18F]DCFPyL | SUVmax [18F]FDG | TBR [18F]DCFPyL | TBR [18F]FDG | H-Score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 72 | Colon adenocarcinoma | Well/moderate | cT3/4N1M0 | pT3N0M0 | 180 | 9.9 | 45.5 | 7.3 | 20.4 | 120 |
| 2 | 68 | Colon adenocarcinoma | Well/moderate | cT4N2M0 | pT4N0M0 | 80 | 1.9 | 29.1 | 2.3 | 15.6 | 225 |
| 3 | 73 | Colon adenocarcinoma | Poor | cT4N0M0 | pT4N0M0 | 50 | 3.3 | 22.5 | 2.4 | 14.1 | 60 |
| 4 | 58 | Colon adenocarcinoma | Well/moderate | cTxN0M0 | pT4N0M0 | 15 | 1.5 | 4.5 | 1.2 | 1.7 | 80 |
| 5 | 38 | Signet ring cell gastric carcinoma | Poor | cT2-3N0M0 | ypT3N0M0 | 42 | 3.5 | n.a. | 1.9 | n.a. | 0 |
| 6 | 71 | Tubular gastric adenocarcinoma | Moderate | cT4N1M0 | ypT3N1M0 | 25 | 2.5 | 7.8 | 2.3 | 2.9 | 150 |
| 7 | 50 | Tubular gastric adenocarcinoma | Poor | cT3N0M0 | ypT4N1M0 | 45 | 2.1 | 4.4 | 1.4 | 1.6 | 0 |
| 8 | 70 | PDAC | Well | cTxN0M0 | pT2N1M0 | 22 | 3.3 | 3.6 | 2.0 | 1.3 | 150 |
| 9 | 76 | PDAC | Moderate | cTxN0M0 | pT2N1M0 | 28 | 2.4 | 6.8 | 2.0 | 4.3 | 30 |
| 10 | 63 | PDAC | Well | cTxN2M0 | pT2N2M0 | 35 | 5.1 | 10.1 | 2.8 | 3.9 | 0 |
| [18F]DCFPyL | [18F]FDG | |
|---|---|---|
| SUVmax | 9.9 | 45.5 |
| SUVmean | 6.4 | 28.4 |
| SUVmin | 4.5 | 22.8 |
| SUVpeak | 8.4 | 41.0 |
| TBR | 7.3 | 20.4 |
| TVDCFPyL/MTV (cm3) | 13.6 | 59.4 |
| TLDCFPyL/TLG | 87.6 | 1686.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vuijk, F.A.; Kleiburg, F.; Noortman, W.A.; Heijmen, L.; Feshtali Shahbazi, S.; van Velden, F.H.P.; Baart, V.M.; Bhairosingh, S.S.; Windhorst, B.D.; Hawinkels, L.J.A.C.; et al. Prostate-Specific Membrane Antigen Targeted Pet/CT Imaging in Patients with Colon, Gastric and Pancreatic Cancer. Cancers 2022, 14, 6209. https://doi.org/10.3390/cancers14246209
Vuijk FA, Kleiburg F, Noortman WA, Heijmen L, Feshtali Shahbazi S, van Velden FHP, Baart VM, Bhairosingh SS, Windhorst BD, Hawinkels LJAC, et al. Prostate-Specific Membrane Antigen Targeted Pet/CT Imaging in Patients with Colon, Gastric and Pancreatic Cancer. Cancers. 2022; 14(24):6209. https://doi.org/10.3390/cancers14246209
Chicago/Turabian StyleVuijk, Floris A., Fleur Kleiburg, Wyanne A. Noortman, Linda Heijmen, Shirin Feshtali Shahbazi, Floris H. P. van Velden, Victor M. Baart, Shadhvi S. Bhairosingh, Bert D. Windhorst, Lukas J. A. C. Hawinkels, and et al. 2022. "Prostate-Specific Membrane Antigen Targeted Pet/CT Imaging in Patients with Colon, Gastric and Pancreatic Cancer" Cancers 14, no. 24: 6209. https://doi.org/10.3390/cancers14246209
APA StyleVuijk, F. A., Kleiburg, F., Noortman, W. A., Heijmen, L., Feshtali Shahbazi, S., van Velden, F. H. P., Baart, V. M., Bhairosingh, S. S., Windhorst, B. D., Hawinkels, L. J. A. C., Dibbets-Schneider, P., Bouwman, N., Crobach, S. A. L. P., Fariña-Sarasqueta, A., Marinelli, A. W. K. S., Oprea-Lager, D. E., Swijnenburg, R.-J., Smit, F., Vahrmeijer, A. L., ... Slingerland, M. (2022). Prostate-Specific Membrane Antigen Targeted Pet/CT Imaging in Patients with Colon, Gastric and Pancreatic Cancer. Cancers, 14(24), 6209. https://doi.org/10.3390/cancers14246209








