Smart Polymeric Micelles for Anticancer Hydrophobic Drugs
Simple Summary
Abstract
1. Introduction
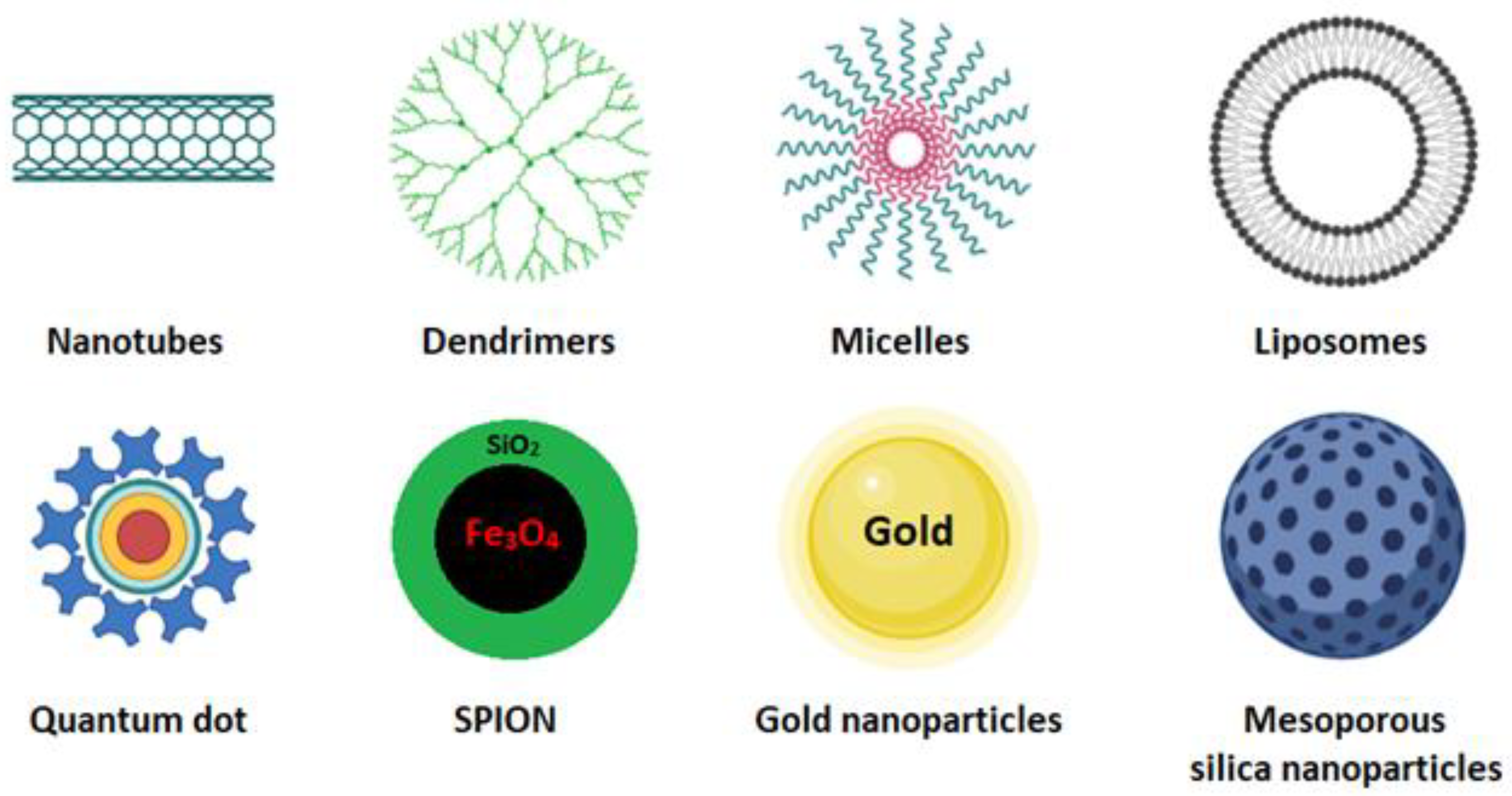
1.1. Polymeric Micelles
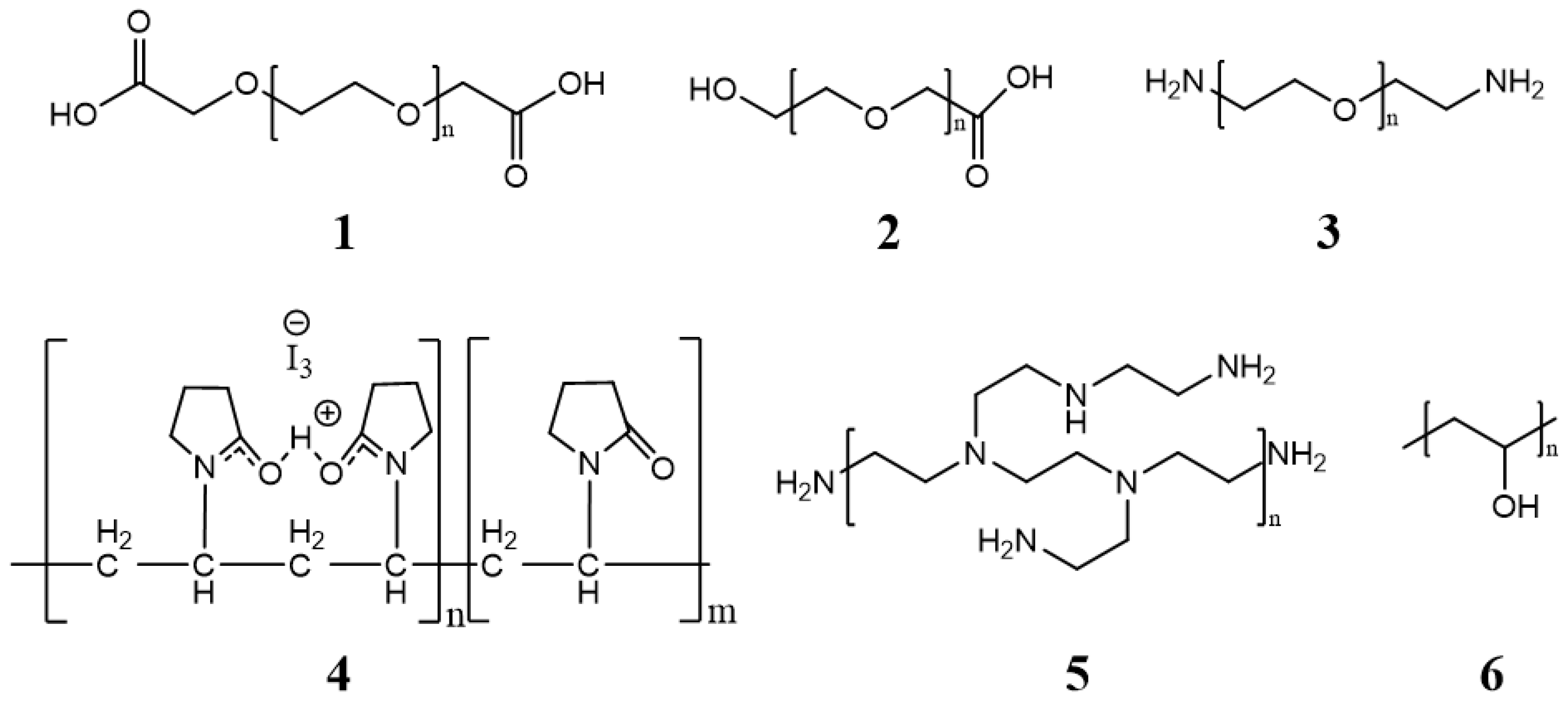
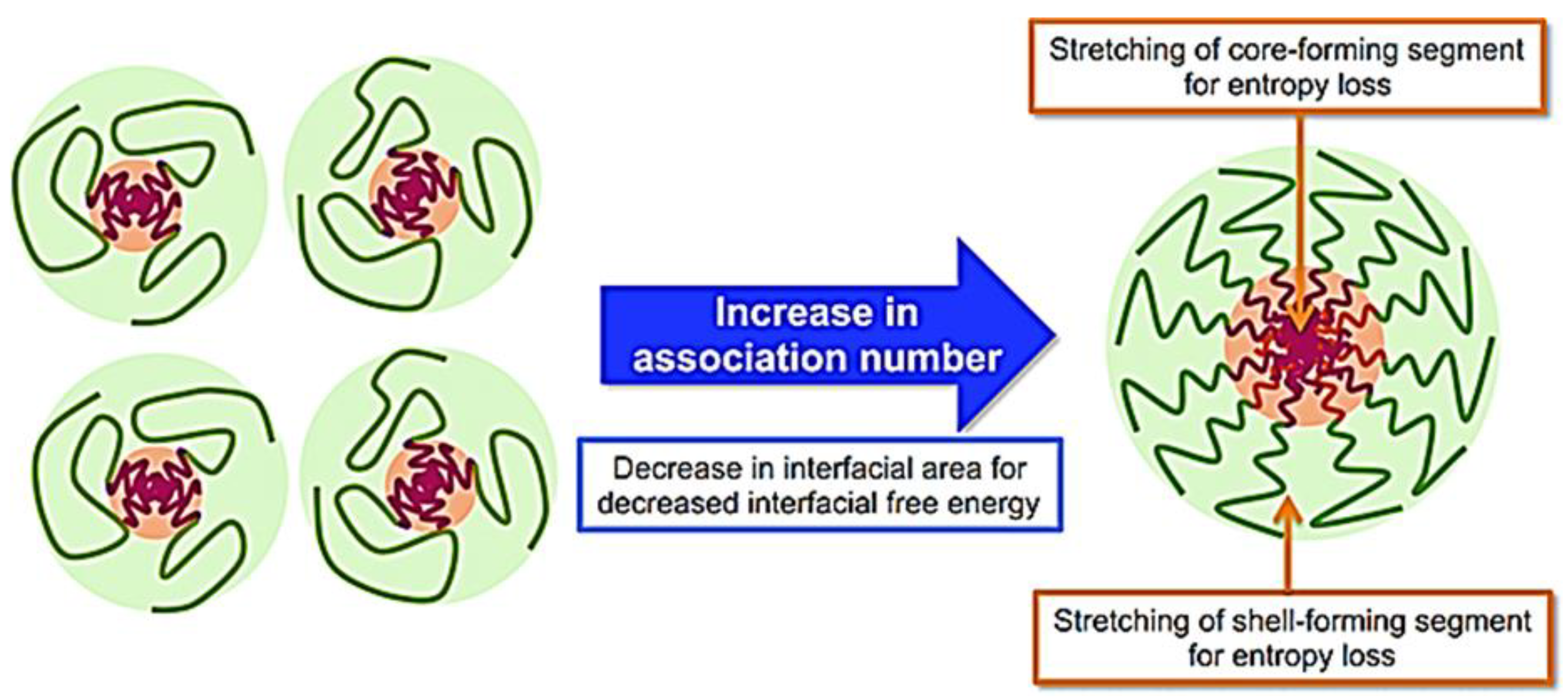
1.2. Micelle Synthesis
1.3. Size
1.4. Surface Charge
1.5. Shape
2. Endogenous Stimuli
2.1. pH-Responsive Polymeric Micelles
2.2. Redox-Responsive Polymeric Micelles
2.3. Dual pH/Redox-Responsive Polymeric Micelles
2.4. Enzyme Sensitive
3. Exogenous Stimuli
3.1. Thermo-Responsive
3.2. Ultrasound
3.3. Light-Responsive
3.4. Magnetic Field-Responsive
3.5. Electric Field-Responsive
4. Micelle–Lipid Nanocapsules
5. Computational Approaches to Design Polymeric Micelles
6. Current Status and Future Prospects
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, Y.; Wang, W.; Yang, J.; Zhou, C.; Sun, J. pH-sensitive polymeric micelles triggered drug release for extracellular and intracellular drug targeting delivery. Asian J. Pharm. Sci. 2013, 8, 159–167. [Google Scholar] [CrossRef]
- Mishra, V.; Singh, M.; Nayak, P.; Sriram, P.; Suttee, A. Carbon Nanotubes as Emerging Nanocarriers in Drug Delivery: An Overview. Int. J. Pharm. Qual. Assur. 2020, 11, 373–378. [Google Scholar] [CrossRef]
- Yousefi, M.; Narmani, A.; Jafari, S.M. Dendrimers as efficient nanocarriers for the protection and delivery of bioactive phytochemicals. Adv. Colloid Interface Sci. 2020, 278, 102125. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.L.C.d.S.L.; Zerillo, L.; Cruz, L.J.; Schomann, T.; Chan, A.B.; de Carvalho, T.G.; Souza, S.V.d.P.; Araújo, A.A.; de Geus-Oei, L.F.; de Araújo Júnior, R.F. Maximizing the potency of oxaliplatin coated nanoparticles with folic acid for modulating tumor progression in colorectal cancer. Mater. Sci. Eng. C 2020, 120, 111678. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Huang, Z.; Wan, F.; Sun, Y. Carbon quantum dots-stabilized Pickering emulsion to prepare NIR light-responsive PLGA drug delivery system. Mater. Today Commun. 2020, 23, 100951. [Google Scholar] [CrossRef]
- de Oliveira, J.K.; Ueda-Nakamura, T.; Corrêa, A.G.; Petrilli, R.; Lopez, R.F.V.; Nakamura, C.V.; Auzely-Velty, R. Liposome-based nanocarrier loaded with a new quinoxaline derivative for the treatment of cutaneous leishmaniasis. Mater. Sci. Eng. C 2020, 110, 110720. [Google Scholar] [CrossRef]
- Barriga, H.M.G.; Holme, M.N.; Stevens, M.M. Cubosomes: The Next Generation of Smart Lipid Nanoparticles? Angew. Chem. Int. Ed. 2019, 58, 2958–2978. [Google Scholar] [CrossRef]
- Elhasany, K.A.; Khattab, S.N.; Bekhit, A.A.; Ragab, D.M.; Abdulkader, M.A.; Zaky, A.; Helmy, M.W.; Ashour, H.M.A.; Teleb, M.; Haiba, N.S.; et al. Combination of magnetic targeting with synergistic inhibition of NF-κB and glutathione via micellar drug nanomedicine enhances its anti-tumor efficacy. Eur. J. Pharm. Biopharm. 2020, 155, 162–176. [Google Scholar] [CrossRef]
- Souza, F.R.; Fornasier, F.; Carvalho, A.S.; Silva, B.M.; Lima, M.C.; Pimentel, A.S. Polymer-coated gold nanoparticles and polymeric nanoparticles as nanocarrier of the BP100 antimicrobial peptide through a lung surfactant model. J. Mol. Liq. 2020, 314, 113661. [Google Scholar] [CrossRef]
- Hossen, S.; Hossain, M.K.; Basher, M.K.; Mia, M.N.H.; Rahman, M.T.; Uddin, M.J. Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: A review. J. Adv. Res. 2019, 15, 1–18. [Google Scholar] [CrossRef]
- Hejmady, S.; Pradhan, R.; Alexander, A.; Agrawal, M.; Singhvi, G.; Gorain, B.; Tiwari, S.; Kesharwani, P.; Dubey, S.K. Recent advances in targeted nanomedicine as promising antitumor therapeutics. Drug Discov. Today 2020, 25, 2227–2244. [Google Scholar] [CrossRef] [PubMed]
- Brighenti, R.; Cosma, M.P. Swelling mechanism in smart polymers responsive to mechano-chemical stimuli. J. Mech. Phys. Solids 2020, 143, 104011. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Z.; Peng, Y.; Ding, J.; Zhou, W. A smart pH-sensitive delivery system for enhanced anticancer efficacy via paclitaxel endosomal escape. Front. Pharmacol. 2019, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Han, J.; Zhao, F.; Pan, X.; Tian, B.; Ding, X.; Zhang, J. Redox-sensitive micelles based on retinoic acid modified chitosan conjugate for intracellular drug delivery and smart drug release in cancer therapy. Carbohydr. Polym. 2019, 215, 8–19. [Google Scholar] [CrossRef]
- Barve, A.; Jain, A.; Liu, H.; Zhao, Z.; Cheng, K. Enzyme-responsive polymeric micelles of cabazitaxel for prostate cancer targeted therapy. Acta Biomater. 2020, 113, 501–511. [Google Scholar] [CrossRef]
- Xu, L.; Wang, H.; Chu, Z.; Cai, L.; Shi, H.; Zhu, C.; Pan, D.; Pan, J.; Fei, X.; Lei, Y. Temperature-Responsive Multilayer Films of Micelle-Based Composites for Controlled Release of a Third-Generation EGFR Inhibitor. ACS Appl. Polym. Mater. 2020, 2, 741–750. [Google Scholar] [CrossRef]
- García-Couce, J.; Schomann, T.; Chung, C.K.; Que, I.; Jorquera-Cordero, C.; Fuentes, G.; Almirall, A.; Chan, A.; Cruz, L.J. Thermosensitive Injectable Hydrogels for Intra-Articular Delivery of Etanercept for the Treatment of Osteoarthritis. Gels 2022, 8, 488. [Google Scholar] [CrossRef]
- García-Couce, J.; Tomás, M.; Fuentes, G.; Que, I.; Almirall, A.; Cruz, L.J. Chitosan/Pluronic F127 Thermosensitive Hydrogel as an Injectable Dexamethasone Delivery Carrier. Gels 2022, 8, 44. [Google Scholar] [CrossRef]
- Liang, B.; Wang, Z.; Xia, H. High intensity focused ultrasound responsive release behavior of metallo-supramolecular block PPG-PEG copolymer micelles. Ultrason. Sonochemistry 2020, 68, 105217. [Google Scholar] [CrossRef]
- Jora, M.Z.; Sabadini, E.; Raghavan, S.R. Light-Triggered Rheological Changes in a System of Cationic Wormlike Micelles Formulated with a Photoacid Generator. Langmuir 2020, 36, 13408–13414. [Google Scholar] [CrossRef]
- Zhao, Y.; Tavares, A.C.; Gauthier, M.A. Nano-engineered electro-responsive drug delivery systems. J. Mater. Chem. B 2016, 4, 3019–3030. [Google Scholar] [CrossRef] [PubMed]
- Kapare, H.S.; Metkar, S.R. Micellar Drug Delivery System: A Review. Pharm. Reson. 2020, 2, 21–26. [Google Scholar]
- Ahmad, Z.; Shah, A.; Siddiq, M.; Kraatz, H.B. Polymeric micelles as drug delivery vehicles. RSC Adv. 2014, 4, 17028–17038. [Google Scholar] [CrossRef]
- Deshmukh, A.S.; Chauhan, P.N.; Noolvi, M.N.; Chaturvedi, K.; Ganguly, K.; Shukla, S.S.; Nadagouda, M.N.; Aminabhavi, T.M. Polymeric micelles: Basic research to clinical practice. Int. J. Pharm. 2017, 532, 249–268. [Google Scholar] [CrossRef]
- Chiappetta, D.A.; Sosnik, A. Poly(ethylene oxide)-poly(propylene oxide) block copolymer micelles as drug delivery agents: Improved hydrosolubility, stability and bioavailability of drugs. Eur. J. Pharm. Biopharm. 2007, 66, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Jin, G.; Kang, L.; Chen, L.; Gao, Z.; Huang, W. Smart polymeric nanoparticles with pH-responsive and PEG-detachable properties for co-delivering paclitaxel and survivin siRNA to enhance antitumor outcomes. Int. J. Nanomed. 2018, 13, 2405–2426. [Google Scholar] [CrossRef]
- Liu, Y.; Van Steenbergen, M.J.; Zhong, Z.; Oliveira, S.; Hennink, W.E.; Van Nostrum, C.F. Dithiolane-Crosslinked Poly(ϵ-caprolactone)-Based Micelles: Impact of Monomer Sequence, Nature of Monomer, and Reducing Agent on the Dynamic Crosslinking Properties. Macromolecules 2020, 53, 7009–7024. [Google Scholar] [CrossRef]
- Wang, P.; Liu, W.; Liu, S.; Yang, R.; Pu, Y.; Zhang, W.; Wang, X.; Liu, X.; Ren, Y.; Chi, B. pH-responsive nanomicelles of poly(ethylene glycol)-poly(ε-caprolactone)-poly(L-histidine) for targeted drug delivery. J. Biomater. Sci. Polym. Edition 2020, 31, 277–292. [Google Scholar] [CrossRef]
- Johnson, R.P.; Uthaman, S.; Augustine, R.; Zhang, Y.; Jin, H.; Choi, C.I.; Park, I.K.; Kim, I. Glutathione and endosomal pH-responsive hybrid vesicles fabricated by zwitterionic polymer block poly(L-aspartic acid) as a smart anticancer delivery platform. React. Funct. Polym. 2017, 119, 47–56. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, K.; Kong, X.; Yin, J. Poly(l-glutamic acid)-based micellar hydrogel with improved mechanical performance and proteins loading. J. Polym. Sci. Part B: Polym. Phys. 2019, 57, 1115–1125. [Google Scholar] [CrossRef]
- Pham, D.T.; Chokamonsirikun, A.; Phattaravorakarn, V.; Tiyaboonchai, W. Polymeric micelles for pulmonary drug delivery: A comprehensive review. J. Mater. Sci. 2021, 56, 2016–2036. [Google Scholar] [CrossRef]
- Cai, Y.; Xu, Z.; Shuai, Q.; Zhu, F.; Xu, J.; Gao, X.; Sun, X. Tumor-targeting peptide functionalized PEG-PLA micelles for efficient drug delivery. Biomater. Sci. 2020, 8, 2274–2282. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, X.; Zhang, J.; Ma, J.; Chi, L.; Qiu, N.; Li, Y. Synthesis of a biodegradable branched copolymer mPEG-b-PLGA-g-OCol and its pH-sensitive micelle. Mater. Sci. Eng. C 2020, 108, 110455. [Google Scholar] [CrossRef] [PubMed]
- Franco, P.; De Marco, I. The use of poly(N-vinyl pyrrolidone) in the delivery of drugs: A review. Polymers 2020, 12, 1114. [Google Scholar] [CrossRef] [PubMed]
- Umemoto, Y.; Uchida, S.; Yoshida, T.; Shimada, K.; Kojima, H.; Takagi, A.; Tanaka, S.; Kashiwagura, Y.; Namiki, N. An effective polyvinyl alcohol for the solubilization of poorly water-soluble drugs in solid dispersion formulations. J. Drug Deliv. Sci. Technol. 2020, 55, 101401. [Google Scholar] [CrossRef]
- Zeinali, M.; Abbaspour-Ravasjani, S.; Ghorbani, M.; Babazadeh, A.; Soltanfam, T.; Santos, A.C.; Hamishehkar, H.; Hamblin, M.R. Nanovehicles for co-delivery of anticancer agents. Drug Discov. Today 2020, 25, 1416–1430. [Google Scholar] [CrossRef]
- Cabral, H.; Miyata, K.; Osada, K.; Kataoka, K. Block Copolymer Micelles in Nanomedicine Applications. Chem. Rev. 2018, 118, 6844–6892. [Google Scholar] [CrossRef]
- Kim, H.U.; Lim, K.H. Description of Temperature Dependence of Critical Micelle Concentration. Bull. Korean Chem. Soc. 2003, 24, 1449–1454. [Google Scholar] [CrossRef]
- Sanchez-Moreno, P.; Ortega-Vinuesa, J.L.; Peula-Garcia, J.M.; Marchal, J.A.; Boulaiz, H. Smart Drug-Delivery Systems for Cancer Nanotherapy. Curr. Drug Targets 2016, 19, 339–359. [Google Scholar] [CrossRef]
- Aliabadi, H.M.; Lavasanifar, A. Polymeric micelles for drug delivery. Expert Opin. Drug Deliv. 2006, 3, 139–162. [Google Scholar] [CrossRef]
- Yang, L.; Wu, X.; Liu, F.; Duan, Y.; Li, S. Novel biodegradable polylactide/poly (ethylene glycol) micelles prepared by direct dissolution method for controlled delivery of anticancer drugs. Pharm. Res. 2009, 26, 2332–2342. [Google Scholar] [CrossRef] [PubMed]
- Muthu, M.S.; Avinash Kulkarni, S.; Liu, Y.; Feng, S.-S. Development of docetaxel-loaded vitamin E TPGS micelles: Formulation optimization, effects on brain cancer cells and biodistribution in rats. Nanomedicine 2012, 7, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Varshosaz, J.; Hassanzadeh, F.; Sadeghi-Aliabadi, H.; Larian, Z.; Rostami, M. Synthesis of Pluronic® F127-poly (methyl vinyl ether-alt-maleic acid) copolymer and production of its micelles for doxorubicin delivery in breast cancer. Chem. Eng. J. 2014, 240, 133–146. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, D.; Park, E.; Jang, S.-y.; Cheon, S.Y.; Han, S.; Koo, H. Rhamnolipid-coated W/O/W double emulsion nanoparticles for efficient delivery of doxorubicin/erlotinib and combination chemotherapy. J. Nanobiotechnol. 2021, 19, 411. [Google Scholar] [CrossRef] [PubMed]
- Schoubben, A.; Ricci, M.; Giovagnoli, S. Meeting the unmet: From traditional to cutting-edge techniques for poly lactide and poly lactide-co-glycolide microparticle manufacturing. J. Pharm. Investig. 2019, 49, 381–404. [Google Scholar] [CrossRef]
- Ai, X.; Zhong, L.; Niu, H.; He, Z. Thin-film hydration preparation method and stability test of DOX-loaded disulfide-linked polyethylene glycol 5000-lysine-di-tocopherol succinate nanomicelles. Asian J. Pharm. Sci. 2014, 9, 244–250. [Google Scholar] [CrossRef]
- Chatziathanasiadou, M.V.; Mavromoustakos, T.; Tzakos, A.G. Unveiling the thermodynamic aspects of drug-cyclodextrin interactions through isothermal titration calorimetry. In Supramolecules in Drug Discovery and Drug Delivery; Springer: Berlin/Heidelberg, Germany, 2021; pp. 187–198. [Google Scholar]
- Muley, P.; Kumar, S.; El Kourati, F.; Kesharwani, S.S.; Tummala, H. Hydrophobically modified inulin as an amphiphilic carbohydrate polymer for micellar delivery of paclitaxel for intravenous route. Int. J. Pharm. 2016, 500, 32–41. [Google Scholar] [CrossRef]
- Yu, J.; Deng, H.; Xie, F.; Chen, W.; Zhu, B.; Xu, Q. The potential of pH-responsive PEG-hyperbranched polyacylhydrazone micelles for cancer therapy. Biomaterials 2014, 35, 3132–3144. [Google Scholar] [CrossRef]
- Bao, Y.; Deng, Q.; Li, Y.; Zhou, S. Engineering docetaxel-loaded micelles for non-small cell lung cancer: A comparative study of microfluidic and bulk nanoparticle preparation. RSC Adv. 2018, 8, 31950–31966. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Y.; Zhao, Y.; Liu, H.; Zhao, Y.; Li, X.; Lin, Q. Biodegradable micelles for NIR/GSH-triggered chemophototherapy of cancer. Nanomaterials 2019, 9, 91. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Wang, N.; Liu, H.; Gou, J.; He, H.; Zhang, Y.; Yin, T.; Wang, Y.; Tang, X. Preparation of mPEG-b-PLA/TM-2 micelle lyophilized products by mixed Lyoprotectors and antitumor effect in vivo. AAPS PharmSciTech 2021, 22, 38. [Google Scholar] [CrossRef] [PubMed]
- Ojha, T.; Hu, Q.; Colombo, C.; Wit, J.; van Geijn, M.; van Steenbergen, M.J.; Bagheri, M.; Königs-Werner, H.; Buhl, E.M.; Bansal, R. Lyophilization stabilizes clinical-stage core-crosslinked polymeric micelles to overcome cold chain supply challenges. Biotechnol. J. 2021, 16, 2000212. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Zhou, Y. Multimicelle aggregate mechanism for spherical multimolecular micelles: From theories, characteristics and properties to applications. Mater. Chem. Front. 2019, 3, 1994–2009. [Google Scholar] [CrossRef]
- Crothers, M.; Zhou, Z.; Ricardo, N.M.P.S.; Yang, Z.; Taboada, P.; Chaibundit, C.; Attwood, D.; Booth, C. Solubilisation in aqueous micellar solutions of block copoly(oxyalkylene)s. Int. J. Pharm. 2005, 293, 91–100. [Google Scholar] [CrossRef]
- Aswal, V.K.; Goyal, P.S. Counterions in the growth of ionic micelles in aqueous electrolyte solutions: A small-angle neutron scattering study. Phys. Rev. E 2000, 61, 2947–2953. [Google Scholar] [CrossRef]
- Kanazawa, T.; Morisaki, K.; Suzuki, S.; Takashima, Y. Prolongation of life in rats with malignant glioma by intranasal siRNA/drug codelivery to the brain with cell-penetrating peptide-modified micelles. Mol. Pharm. 2014, 11, 1471–1478. [Google Scholar] [CrossRef] [PubMed]
- Honary, S.; Zahir, F. Effect of zeta potential on the properties of nano-drug delivery systems-a review (Part 1). Trop. J. Pharm. Res. 2013, 12, 255–264. [Google Scholar] [CrossRef]
- Zhou, D.-h.; Zhang, J.; Zhang, G.; Gan, Z.-h. Effect of surface charge of polymeric micelles on in vitro cellular uptake. Chin. J. Polym. Sci. 2013, 31, 1299–1309. [Google Scholar] [CrossRef]
- Kalinova, R.; Dimitrov, I. Triblock Copolymer Micelles with Tunable Surface Charge as Drug Nanocarriers: Synthesis and Physico-Chemical Characterization. Nanomaterials 2022, 12, 434. [Google Scholar] [CrossRef]
- Xiao, K.; Li, Y.; Luo, J.; Lee, J.S.; Xiao, W.; Gonik, A.M.; Agarwal, R.G.; Lam, K.S. The effect of surface charge on in vivo biodistribution of PEG-oligocholic acid based micellar nanoparticles. Biomaterials 2011, 32, 3435–3446. [Google Scholar] [CrossRef]
- Kang, X.; Kang, W.; Yang, H.; Hou, X.; Zhu, T.; Wang, P.; Li, M.; Jiang, H.; Zhang, M. pH-Responsive aggregates transition from spherical micelles to WLMs induced by hydrotropes based on the dynamic imine bond. Soft Matter 2020, 16, 9705–9711. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Ren, J.; Li, J.; Leng, J.; Qu, Y.; Lin, C.; Shi, D. Magnetothermally responsive star-block copolymeric micelles for controlled drug delivery and enhanced thermo-chemotherapy. Nanoscale 2015, 7, 9655–9663. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Zheng, N.; Wang, Z. Tetraphenylsilane-Cored Star-Shaped Polymer Micelles with pH/Redox Dual Response and Active Targeting Function for Drug-Controlled Release. Biomacromolecules 2019, 20, 4602–4610. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Yin, X.; Yin, X.; Chen, A.; Zhao, L.; Zhang, G.; Liao, W.; Huang, X.; Li, J.; Zhang, C.Y. Dual ph/redox-responsive mixed polymeric micelles for anticancer drug delivery and controlled release. Pharmaceutics 2019, 11, 176. [Google Scholar] [CrossRef]
- Qi, P.; Wu, X.; Liu, L.; Yu, H.; Song, S. Hydrazone-containing triblock copolymeric micelles for pH-controlled drug delivery. Front. Pharmacol. 2018, 9, 12. [Google Scholar] [CrossRef]
- Sonawane, S.J.; Kalhapure, R.S.; Govender, T. Hydrazone linkages in pH responsive drug delivery systems. Eur. J. Pharm. Sci. 2017, 99, 45–65. [Google Scholar] [CrossRef]
- Long, Y.B.; Gu, W.X.; Pang, C.; Ma, J.; Gao, H. Construction of coumarin-based cross-linked micelles with pH responsive hydrazone bond and tumor targeting moiety. J. Mater. Chem. B 2016, 4, 1480–1488. [Google Scholar] [CrossRef]
- Zhai, Y.; Zhou, X.; Jia, L.; Ma, C.; Song, R.; Deng, Y.; Hu, X.; Sun, W. Acetal-linked paclitaxel polymeric prodrug based on functionalized mPEG-PCL diblock polymer for pH-Triggered drug delivery. Polymers 2017, 9, 698. [Google Scholar] [CrossRef]
- Huang, D.; Zhuang, Y.; Shen, H.; Yang, F.; Wang, X.; Wu, D. Acetal-linked PEGylated paclitaxel prodrugs forming free-paclitaxel-loaded pH-responsive micelles with high drug loading capacity and improved drug delivery. Mater. Sci. Eng. C 2018, 82, 60–68. [Google Scholar] [CrossRef]
- Zhou, S.; Fu, S.; Wang, H.; Deng, Y.; Zhou, X.; Sun, W.; Zhai, Y. Acetal-linked polymeric prodrug micelles based on aliphatic polycarbonates for paclitaxel delivery: Preparation, characterization, in vitro release and anti-proliferation effects. J. Biomater. Sci. Polym. Ed. 2020, 31, 2007–2023. [Google Scholar] [CrossRef]
- Smyth, P.; Gibson, T.J.; Irvine, G.; Black, G.; Lavery, D.; Semsarilar, M.; Scott, C.J.; Themistou, E. pH-Responsive benzaldehyde-functionalized PEG-based polymeric nanoparticles for drug delivery: Effect of preparation method on morphology, dye encapsulation and attachment. Eur. Polym. J. 2020, 124, 109471. [Google Scholar] [CrossRef]
- Hsu, C.W.; Hsieh, M.H.; Xiao, M.C.; Chou, Y.H.; Wang, T.H.; Chiang, W.H. pH-responsive polymeric micelles self-assembled from benzoic-imine-containing alkyl-modified PEGylated chitosan for delivery of amphiphilic drugs. Int. J. Biol. Macromol. 2020, 163, 1106–1116. [Google Scholar] [CrossRef] [PubMed]
- Thambi, T.; Deepagan, V.G.; Yoo, C.K.; Park, J.H. Synthesis and physicochemical characterization of amphiphilic block copolymers bearing acid-sensitive orthoester linkage as the drug carrier. Polymer 2011, 52, 4753–4759. [Google Scholar] [CrossRef]
- Deirram, N.; Zhang, C.; Kermaniyan, S.S.; Johnston, A.P.R.; Such, G.K. pH-Responsive Polymer Nanoparticles for Drug Delivery. Macromol. Rapid Commun. 2019, 40, 1800917. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Dong, D.; Li, P.; Wang, D.; Mu, H.; Niu, H.; Duan, J. Novel pH-sensitive polysialic acid based polymeric micelles for triggered intracellular release of hydrophobic drug. Carbohydr. Polym. 2016, 139, 75–81. [Google Scholar] [CrossRef]
- Weng, J.; Huang, Z.; Pu, X.; Chen, X.; Yin, G.; Tian, Y.; Song, Y. Preparation of polyethylene glycol-polyacrylic acid block copolymer micelles with pH/hypoxic dual-responsive for tumor chemoradiotherapy. Colloids Surf. B 2020, 191, 110943. [Google Scholar] [CrossRef]
- Shahriari, M.; Torchilin, V.P.; Taghdisi, S.M.; Abnous, K.; Ramezani, M.; Alibolandi, M. “smart” self-assembled structures: Toward intelligent dual responsive drug delivery systems. Biomater. Sci. 2020, 8, 5787–5803. [Google Scholar] [CrossRef]
- Liao, J.; Peng, H.; Liu, C.; Li, D.; Yin, Y.; Lu, B.; Zheng, H.; Wang, Q. Dual pH-responsive-charge-reversal micelle platform for enhanced anticancer therapy. Mater. Sci. Eng. C 2021, 118, 111527. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, L.; Yang, T.H.; Wu, H. Stimuli-responsive polymeric micelles for drug delivery and cancer therapy. Int. J. Nanomed. 2018, 13, 2921–2942. [Google Scholar] [CrossRef]
- Kang, Y.; Lu, L.; Lan, J.; Ding, Y.; Yang, J.; Zhang, Y.; Zhao, Y.; Zhang, T.; Ho, R.J.Y. Redox-responsive polymeric micelles formed by conjugating gambogic acid with bioreducible poly(amido amine)s for the co-delivery of docetaxel and MMP-9 shRNA. Acta Biomater. 2018, 68, 137–153. [Google Scholar] [CrossRef]
- Shi, L.; Jin, Y.; Du, W.; Lai, S.; Shen, Y.; Zhou, R. Diselenide-containing nonionic gemini polymeric micelles as a smart redox-responsive carrier for potential programmable drug release. Polymer 2020, 198, 122551. [Google Scholar] [CrossRef]
- Birhan, Y.S.; Hailemeskel, B.Z.; Mekonnen, T.W.; Hanurry, E.Y.; Darge, H.F.; Andrgie, A.T.; Chou, H.Y.; Lai, J.Y.; Hsiue, G.H.; Tsai, H.C. Fabrication of redox-responsive Bi(mPEG-PLGA)-Se2 micelles for doxorubicin delivery. Int. J. Pharm. 2019, 567, 118486. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhuang, W.; Ma, B.; Su, X.; Yu, T.; Li, G.; Hu, Y.; Wang, Y. Redox-Responsive Biomimetic Polymeric Micelle for Simultaneous Anticancer Drug Delivery and Aggregation-Induced Emission Active Imaging. Bioconjugate Chem. 2018, 29, 1897–1910. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Lou, B.; Cheng, J.; Zhao, P.; Lin, C.; Wen, X. Redox-responsive amphipathic dextran nanomicelles for solid tumor therapy. J. Biomed. Nanotechnol. 2016, 12, 2083–2096. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Wang, Y.; Zhang, T.; Pu, X.; Zong, L.; Zhu, H.; Zhao, L.; Feng, B. Redox-responsive disulfide bond-bridged mPEG-PBLA prodrug micelles for enhanced paclitaxel biosafety and antitumor efficacy. Front. Oncol. 2019, 9, 823. [Google Scholar] [CrossRef]
- Zhang, X.; Ren, X.; Tang, J.; Wang, J.; Zhang, X.; He, P.; Yao, C.; Bian, W.; Sun, L. Hyaluronic acid reduction-sensitive polymeric micelles achieving co-delivery of tumor-targeting paclitaxel/apatinib effectively reverse cancer multidrug resistance. Drug Deliv. 2020, 27, 825–835. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, Z.; Cao, Z.; Zhou, W.; Zhang, Y.; Chen, Q.; Lu, Y.; Chen, X.; Guo, Q.; Li, C.; et al. Endogenous albumin-mediated delivery of redox-responsive paclitaxel-loaded micelles for targeted cancer therapy. Biomaterials 2018, 183, 243–257. [Google Scholar] [CrossRef]
- Hoang, Q.T.; Lee, D.; Choi, D.G.; Kim, Y.-C.; Shim, M.S. Efficient and selective cancer therapy using pro-oxidant drug-loaded reactive oxygen species (ROS)-responsive polypeptide micelles. J. Ind. Eng. Chem. 2021, 95, 101–108. [Google Scholar] [CrossRef]
- Pei, P.; Sun, C.; Tao, W.; Li, J.; Yang, X.; Wang, J. ROS-sensitive thioketal-linked polyphosphoester-doxorubicin conjugate for precise phototriggered locoregional chemotherapy. Biomaterials 2019, 188, 74–82. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, P.; He, J.; Dong, S.; Li, P.; Zhang, C.Y.; Ma, T. TME-Responsive Polyprodrug Micelles for Multistage Delivery of Doxorubicin with Improved Cancer Therapeutic Efficacy in Rodents. Adv. Healthc. Mater. 2020, 9, 2000387. [Google Scholar] [CrossRef]
- Huang, Y.; Yan, J.; Peng, S.; Tang, Z.; Tan, C.; Ling, J.; Lin, W.; Lin, X.; Zu, X.; Yi, G. pH/reduction dual-stimuli-responsive cross-linked micelles based on multi-functional amphiphilic star copolymer: Synthesis and controlled anti-cancer drug release. Polymers 2020, 12, 82. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.L.; Huang, X.S.; Chen, H.Y.; Huang, Y.C.; Liao, Z.X.; Wang, L.F. ROP and ATRP fabricated redox sensitive micelles based on PCL-SS-PMAA diblock copolymers to co-deliver PTX and CDDP for lung cancer therapy. Colloids Surf. B Biointerfaces 2020, 198, 111443. [Google Scholar] [CrossRef] [PubMed]
- Sang, X.; Yang, Q.; Shi, G.; Zhang, L.; Wang, D.; Ni, C. Preparation of pH/redox dual responsive polymeric micelles with enhanced stability and drug controlled release. Mater. Sci. Eng. C 2018, 91, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Pham, S.H.; Choi, Y.; Choi, J. Stimuli-responsive nanomaterials for application in antitumor therapy and drug delivery. Pharmaceutics 2020, 12, 630. [Google Scholar] [CrossRef]
- Yang, F.; Xu, J.; Fu, M.; Ji, J.; Chi, L.; Zhai, G. Development of stimuli-responsive intelligent polymer micelles for the delivery of doxorubicin. J. Drug Target. 2020, 28, 993–1011. [Google Scholar] [CrossRef]
- Wan, D.; Zhu, Q.; Zhang, J.; Chen, X.; Li, F.; Liu, Y.; Pan, J. Intracellular and extracellular enzymatic responsive micelle for intelligent therapy of cancer. Nano Res. 2022. [Google Scholar] [CrossRef]
- Tagami, T.; Ando, Y.; Ozeki, T. Fabrication of liposomal doxorubicin exhibiting ultrasensitivity against phospholipase A2 for efficient pulmonary drug delivery to lung cancers. Int. J. Pharm. 2017, 517, 35–41. [Google Scholar] [CrossRef]
- Can, A.; Zhang, Q.; Rudolph, T.; Schacher, F.H.; Gohy, J.F.; Schubert, U.S.; Hoogenboom, R. Schizophrenic thermoresponsive block copolymer micelles based on LCST and UCST behavior in ethanol-water mixtures. Eur. Polym. J. 2015, 69, 460–471. [Google Scholar] [CrossRef]
- Lu, A.; Petit, E.; Li, S.; Wang, Y.; Su, F.; Monge, S. Novel thermo-responsive micelles prepared from amphiphilic hydroxypropyl methyl cellulose-block-JEFFAMINE copolymers. Int. J. Biol. Macromol. 2019, 135, 38–45. [Google Scholar] [CrossRef]
- Kikuchi, A.; Okano, T. Pulsatile drug release control using hydrogels. Adv. Drug Deliv. Rev. 2002, 54, 53–77. [Google Scholar] [CrossRef]
- Lee, J.H.; Yeo, Y. Controlled drug release from pharmaceutical nanocarriers. Chem. Eng. Sci. 2015, 125, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.E.; Yokoyama, M.; Okano, T. Inner core segment design for drug delivery control of thermo-responsive polymeric micelles. J. Control. Release 2000, 65, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Sanoj Rejinold, N.; Muthunarayanan, M.; Divyarani, V.V.; Sreerekha, P.R.; Chennazhi, K.P.; Nair, S.V.; Tamura, H.; Jayakumar, R. Curcumin-loaded biocompatible thermoresponsive polymeric nanoparticles for cancer drug delivery. J. Colloid Interface Sci. 2011, 360, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Zhao, Y.; Tong, R. Ultrasound-mediated polymeric micelle drug delivery. Adv. Exp. Med. Biol. 2016, 880, 365–384. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; He, Q.; Zhao, W.; Luo, J.; Gao, W. Tumor-homing, pH- and ultrasound-responsive polypeptide-doxorubicin nanoconjugates overcome doxorubicin resistance in cancer therapy. J. Control. Release 2017, 264, 66–75. [Google Scholar] [CrossRef]
- Wu, P.; Jia, Y.; Qu, F.; Sun, Y.; Wang, P.; Zhang, K.; Xu, C.; Liu, Q.; Wang, X. Ultrasound-Responsive Polymeric Micelles for Sonoporation-Assisted Site-Specific Therapeutic Action. ACS Appl. Mater. Interfaces 2017, 9, 25706–25716. [Google Scholar] [CrossRef]
- Zhang, H.; Xia, H.; Wang, J.; Li, Y. High intensity focused ultrasound-responsive release behavior of PLA-b-PEG copolymer micelles. J. Control. Release 2009, 139, 31–39. [Google Scholar] [CrossRef]
- Ilhami, F.B.; Peng, K.C.; Chang, Y.S.; Alemayehu, Y.A.; Tsai, H.C.; Lai, J.Y.; Chiao, Y.H.; Kao, C.Y.; Cheng, C.C. Photo-responsive supramolecular micelles for controlled drug release and improved chemotherapy. Int. J. Mol. Sci. 2021, 22, 154. [Google Scholar] [CrossRef]
- Doi, N.; Yamauchi, Y.; Ikegami, R.; Kuzuya, M.; Sasai, Y.; Kondo, S.i. Photo-responsive polymer micelles from o-nitrobenzyl ester-based amphiphilic block copolymers synthesized by mechanochemical solid-state copolymerization. Polym. J. 2020, 52, 1375–1385. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, W.; Gao, C. Shape Transformation of Light-Responsive Pyrene-Containing Micelles and Their Influence on Cytoviability. Biomacromolecules 2015, 16, 2276–2281. [Google Scholar] [CrossRef]
- Ma, K.; Wei, X.; Liu, J.; Chen, D.; Zhao, X.; Shen, J.; Lu, H.; Jia, P. Near-Infrared-Light-Responsive Nanocomposites of Cell Membrane Mimetic Copolymers and Upconverting Nanoparticles for On-Demand Drug Release. ACS Appl. Nano Mater. 2020, 3, 8294–8303. [Google Scholar] [CrossRef]
- Yap, J.E.; Zhang, L.; Lovegrove, J.T.; Beves, J.E.; Stenzel, M.H. Visible Light—Responsive Drug Delivery Nanoparticle via Donor–Acceptor Stenhouse Adducts (DASA). Macromol. Rapid Commun. 2020, 41, 2000236. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Tang, Z.; Zhou, Y.; Zhou, X.; Jin, Y.; Li, D.; Yang, Y.; Zhou, S. Magnetic micelles as a potential platform for dual targeted drug delivery in cancer therapy. Int. J. Pharm. 2012, 429, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.; Zhang, Y.; Feng, Z.; Yang, J.; Tian, Q.; Yao, X.; Zhao, X.; Tan, H.; Chen, Y. Synthesis and application of a series of amphipathic chitosan derivatives and the corresponding magnetic nanoparticle-embedded polymeric micelles. Carbohydr. Polym. 2019, 223, 114966. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.D.; Maghsoodi, F.; Panahandeh, F.; Yazdian-Robati, R.; Reisi-Vanani, A.; Tafaghodi, M. Doxorubicin delivery via magnetic nanomicelles comprising from reduction-responsive poly(ethylene glycol)-b-poly(ε-caprolactone) (PEG-SS-PCL) and loaded with superparamagnetic iron oxide (SPIO) nanoparticles: Preparation, characterization and simulation. Mater. Sci. Eng. C 2018, 92, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Pourjavadi, A.; Mazaheri Tehrani, Z.; Dastanpour, L. Smart magnetic self-assembled micelle: An effective nanocarrier for thermo-triggered paclitaxel delivery. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 741–749. [Google Scholar] [CrossRef]
- Yang, T.; Niu, D.; Chen, J.; He, J.; Yang, S.; Jia, X.; Hao, J.; Zhao, W.; Li, Y. Biodegradable organosilica magnetic micelles for magnetically targeted MRI and GSH-triggered tumor chemotherapy. Biomater. Sci. 2019, 7, 2951–2960. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.L.; Choi, H.J. Stimuli-responsive polymers and colloids under electric and magnetic fields. Polymers 2014, 6, 2803–2818. [Google Scholar] [CrossRef]
- Peng, L.; Feng, A.; Zhang, H.; Wang, H.; Jian, C.; Liu, B.; Gao, W.; Yuan, J. Voltage-responsive micelles based on the assembly of two biocompatible homopolymers. Polym. Chem. 2014, 5, 1751–1759. [Google Scholar] [CrossRef]
- Bukhari, S.I.; Imam, S.S.; Ahmad, M.Z.; Vuddanda, P.R.; Alshehri, S.; Mahdi, W.A.; Ahmad, J. Recent progress in lipid nanoparticles for cancer theranostics: Opportunity and challenges. Pharmaceutics 2021, 13, 840. [Google Scholar] [CrossRef]
- Matougui, N.; Boge, L.; Groo, A.C.; Umerska, A.; Ringstad, L.; Bysell, H.; Saulnier, P. Lipid-based nanoformulations for peptide delivery. Int. J. Pharm. 2016, 502, 80–97. [Google Scholar] [CrossRef] [PubMed]
- Gill, K.K.; Kaddoumi, A.; Nazzal, S. PEG-lipid micelles as drug carriers: Physiochemical attributes, formulation principles and biological implication. J. Drug Target. 2015, 23, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Xiao, R.; Zeng, Z.; Xu, L.; Wang, J. Application of poly(ethylene glycol)- distearoylphosphatidylethanolamine (PEG-DSPE) block copolymers and their derivatives as nanomaterials in drug delivery. Int. J. Nanomed. 2012, 7, 4185–4198. [Google Scholar] [CrossRef]
- Wu, H.; Zhu, L.; Torchilin, V.P. PH-sensitive poly(histidine)-PEG/DSPE-PEG co-polymer micelles for cytosolic drug delivery. Biomaterials 2013, 34, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Sai, N.; Dong, X.; Huang, P.; You, L.; Yang, C.; Liu, Y.; Wang, W.; Wu, H.; Yu, Y.; Du, Y.; et al. A novel gel-forming solution based on PEG-DSPE/Solutol HS 15 mixed micelles and gellan gum for ophthalmic delivery of curcumin. Molecules 2020, 25, 81. [Google Scholar] [CrossRef] [PubMed]
- Arias, E.R.; Angarita-Villamizar, V.; Baena, Y.; Parra-Giraldo, C.; Perez, L.D. Phospholipid-conjugated peg-b-pcl copolymers as precursors of micellar vehicles for amphotericin b. Polymers 2021, 13, 1747. [Google Scholar] [CrossRef]
- Pohlmann, A.R.; Fonseca, F.N.; Paese, K.; Detoni, C.B.; Coradini, K.; Beck, R.C.; Guterres, S.S. Poly(ε-caprolactone) microcapsules and nanocapsules in drug delivery. Expert Opin. Drug Deliv. 2013, 10, 623–638. [Google Scholar] [CrossRef]
- Drewes, C.C.; Alves, A.d.C.S.; Hebeda, C.B.; Copetti, I.; Sandri, S.; Uchiyama, M.K.; Araki, K.; Guterres, S.S.; Pohlmann, A.R.; Farsky, S.H. Role of poly(ε-caprolactone) lipid-core nanocapsules on melanoma-neutrophil crosstalk. Int. J. Nanomed. 2017, 12, 7153–7163. [Google Scholar] [CrossRef]
- He, X.; Li, L.; Su, H.; Zhou, D.; Song, H.; Wang, L.; Jiang, X. Poly(ethylene glycol)-block-poly(ε-caprolactone)-and phospholipid-based stealth nanoparticles with enhanced therapeutic efficacy on murine breast cancer by improved intracellular drug delivery. Int. J. Nanomed. 2015, 10, 1791–1804. [Google Scholar] [CrossRef]
- Karami, Z.; Hamidi, M. Cubosomes: Remarkable drug delivery potential. Drug Discov. Today 2016, 21, 789–801. [Google Scholar] [CrossRef]
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic nanoparticles and their targeted delivery applications. Molecules 2020, 25, 2193. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, R.; Gontsarik, M.; Yaghmur, A.; Salentinig, S. pH-Responsive Nano-Self-Assemblies of the Anticancer Drug 2-Hydroxyoleic Acid. Langmuir 2019, 35, 7954–7961. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Meikle, T.G.; Drummond, C.J.; Yang, Y.; Conn, C.E. Comparison of cubosomes and liposomes for the encapsulation and delivery of curcumin. Soft Matter 2021, 17, 3306–3313. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, A.; Xu, Z.; Ingram, N.; Coletta, P.L.; Millner, P.A.; Tyler, A.I.I.; Hughes, T.A. Hyaluronic-Acid-Tagged Cubosomes Deliver Cytotoxics Specifically to CD44-Positive Cancer Cells. Mol. Pharm. 2022, 19, 4601–4611. [Google Scholar] [CrossRef]
- Huynh, L.; Neale, C.; Pomès, R.; Allen, C. Computational approaches to the rational design of nanoemulsions, polymeric micelles, and dendrimers for drug delivery. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 20–36. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Sun, Y.; Wu, H.; Zhu, C.; Wei, G.; Li, J.; Chan, T.; Ouyang, D.; Mao, S. Exploring the effect of hydrophilic and hydrophobic structure of grafted polymeric micelles on drug loading. Int. J. Pharm. 2016, 512, 282–291. [Google Scholar] [CrossRef]
- Chun, B.J.; Lu, J.; Weck, M.; Jang, S.S. Characterization of molecular association of poly(2-oxazoline)s-based micelles with various epoxides and diols via the Flory-Huggins theory: A molecular dynamics simulation approach. Phys. Chem. Chem. Phys. 2015, 17, 29161–29170. [Google Scholar] [CrossRef]
- Rezaeisadat, M.; Bordbar, A.K.; Omidyan, R. Molecular dynamics simulation study of curcumin interaction with nano-micelle of PNIPAAm-b-PEG co-polymer as a smart efficient drug delivery system. J. Mol. Liq. 2021, 332, 115862. [Google Scholar] [CrossRef]
- Zeng, S.; Quan, X.; Zhu, H.; Sun, D.; Miao, Z.; Zhang, L.; Zhou, J. Computer Simulations on a pH-Responsive Anticancer Drug Delivery System Using Zwitterion-Grafted Polyamidoamine Dendrimer Unimolecular Micelles. Langmuir 2021, 37, 1225–1234. [Google Scholar] [CrossRef]


| Methods | Advantage/Disadvantage | Drug-Loading Capacity | Solvents | Types of Drugs | Encapsulated Anticancer Drug | Polymers Used |
|---|---|---|---|---|---|---|
| Direct dissolution | The simplest technique to prepare polymeric micelles. Does not use organic solvents. Low-molecular-weight hydrophilic polymers | Low drug-loading capacity due to water solubility of polymers | Water | Not applicable for most hydrophobic drugs | Paclitaxel [41] | Mostly hydrophilic polymers; PLA-PEG |
| Docetaxel [42] | d-a-tocopheryl PEG1000 succinate (TPGS) | |||||
| Doxorubicin [43] | Pluronic F127/poly (methyl vinyl ether-alt-maleic acid) | |||||
| Oil-in-water emulsification | Easy preparation. Small particles with a narrow size distribution. Not environmentally friendly due to the use of chlorinated organic solvents. | High drug-loading capacity | Organic solvents immiscible in water (CHCl3, EtAc, and CH2Cl2) | Hydrophobic drugs | Doxorubicin and erlotinib [44] | PLGA/pluronic F-127 |
| Triptorelin [45] | PLA/PLGA | |||||
| Thin-film hydration/solvent evaporation | Only applicable for copolymers with high hydrophilic–lipophilic balance (HLB). Feasible for scaling up but very expensive | High drug-loading capacity and encapsulation efficiency | Water-miscible volatile organic solvents (DMF, THF, DMSO, acetonitrile, MeOH, acetone) | Hydrophobic drugs | Doxorubicin [46] | PEG 5000-lysine-di-tocopherol succinate (P5kSSLV) |
| Curcumin [47] | Poly(ethyleneoxide)-b-poly(propylene oxide)-b-poly(ethylene oxide) (PEO-b-PPO-b-PEO/pluronic F-127) | |||||
| Paclitaxel [48] | Inutec SP11 (INT) | |||||
| Dialysis | For highly hydrophobic polymers with long alkyl chains. Difficulty releasing. Easy to remove organic solvents. Not applicable on a large scale due to high water consumption. | High drug-loading capacity | Water-miscible volatile organic solvents (DMF, THF, DMSO, acetonitrile, MeOH, acetone) | Hydrophobic drugs | Docetaxel [49] | PEG/hyperbranched poly(amidoamine) HAPH |
| Docetaxel [50] | PLGA/PEG–maleimide | |||||
| Doxorubicin [51] | PCL-S-S- biodegradable photoluminescent polymer (BPLP) | |||||
| Freeze-drying | High stability and narrow size distribution. Organic-solvent reusability. Thermolabile drug-encapsulation suitability. Limited lyophilize organic solvents and copolymers soluble in them. | High drug-loading capacity | The mixture of water and freeze-dryable organic solvents such as tert-butanol and dimethyl acetamide | Hydrophobic drugs | TM-2 [52] | mPEG/PLA |
| Docetaxel [53] | Thermosensitive methoxy poly(ethylene glycol)-b-poly[N-(2-hydroxypropyl) methacrylamide lactate] (mPEG-bpHPMAmLacn) |
| Type | pH | Acid-Sensitive Chemical Bonds | Degradation Products |
|---|---|---|---|
| Vinyl ester | 4.5–5.0 | 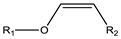 |  |
| Amide | 4.5–6.0 |  | 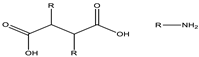 |
| Imine | 6.8 | 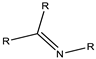 |  |
| Oxime | 4.8–5.0 | 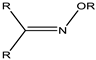 |  |
| Hydrazone | 5.0 |  |  |
| Orthoester | 5.0–6.0 |  |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzmán Rodríguez, A.; Sablón Carrazana, M.; Rodríguez Tanty, C.; Malessy, M.J.A.; Fuentes, G.; Cruz, L.J. Smart Polymeric Micelles for Anticancer Hydrophobic Drugs. Cancers 2023, 15, 4. https://doi.org/10.3390/cancers15010004
Guzmán Rodríguez A, Sablón Carrazana M, Rodríguez Tanty C, Malessy MJA, Fuentes G, Cruz LJ. Smart Polymeric Micelles for Anticancer Hydrophobic Drugs. Cancers. 2023; 15(1):4. https://doi.org/10.3390/cancers15010004
Chicago/Turabian StyleGuzmán Rodríguez, Andy, Marquiza Sablón Carrazana, Chrislayne Rodríguez Tanty, Martijn J. A. Malessy, Gastón Fuentes, and Luis J. Cruz. 2023. "Smart Polymeric Micelles for Anticancer Hydrophobic Drugs" Cancers 15, no. 1: 4. https://doi.org/10.3390/cancers15010004
APA StyleGuzmán Rodríguez, A., Sablón Carrazana, M., Rodríguez Tanty, C., Malessy, M. J. A., Fuentes, G., & Cruz, L. J. (2023). Smart Polymeric Micelles for Anticancer Hydrophobic Drugs. Cancers, 15(1), 4. https://doi.org/10.3390/cancers15010004







