Effect of Sarcopenia on Survival and Health-Related Quality of Life in Patients with Hepatocellular Carcinoma after Hepatectomy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cohort and Clinical Data
2.2. Assessment of Sarcopenia
2.3. Outcome measures
2.4. Statistical Analysis
3. Results
3.1. Sarcopenia
3.2. The Surgical Approach
3.3. Subgroup Analysis
3.4. Interaction of Sarcopenia with the Surgical Approach
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Santilli, V.; Bernetti, A.; Mangone, M.; Paoloni, M. Clinical definition of sarcopenia. Clin. Cases Miner Bone Metab. 2014, 11, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Ganapathy, A.; Nieves, J.W. Nutrition and Sarcopenia-What Do We Know? Nutrients 2020, 12, 1755. [Google Scholar] [CrossRef] [PubMed]
- Hanna, L.; Nguo, K.; Furness, K.; Porter, J.; Huggins, C.E. Association between skeletal muscle mass and quality of life in adults with cancer: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2022, 13, 839–857. [Google Scholar] [CrossRef]
- Fujiwara, N.; Nakagawa, H.; Kudo, Y.; Tateishi, R.; Taguri, M.; Watadani, T.; Nakagomi, R.; Kondo, M.; Nakatsuka, T.; Minami, T.; et al. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J. Hepatol. 2015, 63, 131–140. [Google Scholar] [CrossRef]
- Chang, K.V.; Chen, J.D.; Wu, W.T.; Huang, K.C.; Hsu, C.T.; Han, D.S. Association between Loss of Skeletal Muscle Mass and Mortality and Tumor Recurrence in Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Liver Cancer 2018, 7, 90–103. [Google Scholar] [CrossRef]
- Yang, J.; Chen, K.; Zheng, C.; Chen, K.; Lin, J.; Meng, Q.; Chen, Z.; Deng, L.; Yu, H.; Deng, T.; et al. Impact of sarcopenia on outcomes of patients undergoing liver resection for hepatocellular carcinoma. J. Cachexia Sarcopenia Muscle 2022, 13, 2383–2392. [Google Scholar] [CrossRef]
- Marasco, G.; Serenari, M.; Renzulli, M.; Alemanni, L.V.; Rossini, B.; Pettinari, I.; Dajti, E.; Ravaioli, F.; Golfieri, R.; Cescon, M.; et al. Clinical impact of sarcopenia assessment in patients with hepatocellular carcinoma undergoing treatments. J. Gastroenterol. 2020, 55, 927–943. [Google Scholar] [CrossRef]
- Tandon, P.; Ney, M.; Irwin, I.; Ma, M.M.; Gramlich, L.; Bain, V.G.; Esfandiari, N.; Baracos, V.; Montano-Loza, A.J.; Myers, R.P. Severe muscle depletion in patients on the liver transplant wait list: Its prevalence and independent prognostic value. Liver Transpl. 2012, 18, 1209–1216. [Google Scholar] [CrossRef]
- Harimoto, N.; Shirabe, K.; Yamashita, Y.I.; Ikegami, T.; Yoshizumi, T.; Soejima, Y.; Ikeda, T.; Maehara, Y.; Nishie, A.; Yamanaka, T. Sarcopenia as a predictor of prognosis in patients following hepatectomy for hepatocellular carcinoma. Br. J.Surg. 2013, 100, 1523–1530. [Google Scholar] [CrossRef]
- Nishikawa, H.; Nishijima, N.; Enomoto, H.; Sakamoto, A.; Nasu, A.; Komekado, H.; Nishimura, T.; Kita, R.; Kimura, T.; Iijima, H.; et al. Prognostic significance of sarcopenia in patients with hepatocellular carcinoma undergoing sorafenib therapy. Oncol. Lett. 2017, 14, 1637–1647. [Google Scholar] [CrossRef] [PubMed]
- Yuri, Y.; Nishikawa, H.; Enomoto, H.; Ishii, A.; Iwata, Y.; Miyamoto, Y.; Ishii, N.; Hasegawa, K.; Nakano, C.; Nishimura, T.; et al. Implication of Psoas Muscle Index on Survival for Hepatocellular Carcinoma Undergoing Radiofrequency Ablation Therapy. J. Cancer 2017, 8, 1507–1516. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 601. [Google Scholar] [CrossRef] [PubMed]
- Estimated Number of New Cases in 2020, World, both Sexes, All Ages. Available online: https://gco.iarc.fr/today/online-analysis-table?v=2020&mode=cancer&mode_population=continents&population=900&populations=900&key=asr&sex=0&cancer=39&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&group_cancer=1&include_nmsc=0&include_nmsc_other=1 (accessed on 1 November 2022).
- Reich, H.; McGlynn, F.; DeCaprio, J.; Budin, R. Laparoscopic excision of benign liver lesions. Obstet. Gynecol. 1991, 78, 956–958. [Google Scholar] [PubMed]
- Vanounou, T.; Steel, J.L.; Nguyen, K.T.; Tsung, A.; Marsh, J.W.; Geller, D.A.; Gamblin, T.C. Comparing the clinical and economic impact of laparoscopic versus open liver resection. Ann. Surg. Oncol. 2010, 17, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Ikeda, T.; Kurihara, T.; Yoshida, Y.; Takeishi, K.; Itoh, S.; Harimoto, N.; Kawanaka, H.; Shirabe, K.; Maehara, Y. Long-term favorable surgical results of laparoscopic hepatic resection for hepatocellular carcinoma in patients with cirrhosis: A single-center experience over a 10-year period. J. Am. Coll. Surg. 2014, 219, 1117–1123. [Google Scholar] [CrossRef]
- Hu, B.S.; Chen, K.; Tan, H.M.; Ding, X.M.; Tan, J.W. Comparison of laparoscopic vs open liver lobectomy (segmentectomy) for hepatocellular carcinoma. World J. Gastroenterol. 2011, 17, 4725–4728. [Google Scholar] [CrossRef]
- Dowson, H.M.; Ballard, K.; Gage, H.; Jackson, D.; Williams, P.; Rockall, T.A. Quality of life in the first 6 weeks following laparoscopic and open colorectal surgery. Value Health 2013, 16, 367–372. [Google Scholar] [CrossRef][Green Version]
- Pędziwiatr, M.; Pisarska, M.; Major, P.; Grochowska, A.; Matłok, M.; Przęczek, K.; Stefura, T.; Budzyński, A.; Kłęk, S. Laparoscopic colorectal cancer surgery combined with enhanced recovery after surgery protocol (ERAS) reduces the negative impact of sarcopenia on short-term outcomes. Eur. J. Surg. Oncol. 2016, 42, 779–787. [Google Scholar] [CrossRef]
- Zhang, F.M.; Ma, B.W.; Huang, Y.Y.; Chen, W.Z.; Chen, J.J.; Dong, Q.T.; Chen, W.S.; Chen, X.L.; Shen, X.; Yu, Z.; et al. Laparoscopic colorectal cancer surgery reduces the adverse impacts of sarcopenia on postoperative outcomes: A propensity score-matched analysis. Surg. Endosc. 2020, 34, 4582–4592. [Google Scholar] [CrossRef]
- General Office of National Health Commission. Standard for diagnosis and treatment of primary liver cancer (2022 edition). J. Clin. Hepatol. 2022, 38, 288–303. [Google Scholar] [CrossRef]
- Association, H.a.P.S.P.C.o.C.R.H. Chinese expert consensus on laparoscopic hepatectomy for hepatocellular carcinoma (2020 edtion). Chin. J. Dig. Surg. 2020, 19, 1119–1134. [Google Scholar] [CrossRef]
- Dodds, R.M.; Syddall, H.E.; Cooper, R.; Benzeval, M.; Deary, I.J.; Dennison, E.M.; Der, G.; Gale, C.R.; Inskip, H.M.; Jagger, C.; et al. Grip strength across the life course: Normative data from twelve British studies. PLoS ONE 2014, 9, e113637. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.J.; Rikli, R.E.; Beam, W.C. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res. Q. Exerc. Sport 1999, 70, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Studenski, S.; Perera, S.; Patel, K.; Rosano, C.; Faulkner, K.; Inzitari, M.; Brach, J.; Chandler, J.; Cawthon, P.; Connor, E.B.; et al. Gait speed and survival in older adults. JAMA 2011, 305, 50–58. [Google Scholar] [CrossRef]
- Malmstrom, T.K.; Miller, D.K.; Simonsick, E.M.; Ferrucci, L.; Morley, J.E. SARC-F: A symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J. Cachexia Sarcopenia Muscle 2016, 7, 28–36. [Google Scholar] [CrossRef]
- Cesari, M.; Pahor, M.; Bartali, B.; Cherubini, A.; Penninx, B.W.; Williams, G.R.; Atkinson, H.; Martin, A.; Guralnik, J.M.; Ferrucci, L. Antioxidants and physical performance in elderly persons: The Invecchiare in Chianti (InCHIANTI) study. Am. J. Clin. Nutr. 2004, 79, 289–294. [Google Scholar] [CrossRef]
- Baines, S.; Powers, J.; Brown, W.J. How does the health and well-being of young Australian vegetarian and semi-vegetarian women compare with non-vegetarians? Public Health Nutr. 2007, 10, 436–442. [Google Scholar] [CrossRef]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; de Haes, J.C.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J. Natl. Cancer Instig. 1993, 85, 365–376. [Google Scholar] [CrossRef]
- Fayers, P.M.; Aaronson, N.K.; Bjordal, K.; Groenvold, M.; Curran, D.; Bottomley, A.; on behalf of the EORTC Quality of Life Group. The EORTC QLQ-C30 Scoring Manual, 3rd ed.; European Organisation for Research and Treatment of Cancer: Brussels, Belgium, 2001. [Google Scholar]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef]
- Marasco, G.; Dajti, E.; Serenari, M.; Alemanni, L.V.; Ravaioli, F.; Ravaioli, M.; Vestito, A.; Vara, G.; Festi, D.; Golfieri, R.; et al. Sarcopenia Predicts Major Complications after Resection for Primary Hepatocellular Carcinoma in Compensated Cirrhosis. Cancers 2022, 14, 1935. [Google Scholar] [CrossRef] [PubMed]
- De Buyser, S.L.; Petrovic, M.; Taes, Y.E.; Toye, K.R.; Kaufman, J.M.; Lapauw, B.; Goemaere, S. Validation of the FNIH sarcopenia criteria and SOF frailty index as predictors of long-term mortality in ambulatory older men. Age Ageing 2016, 45, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Granito, A.; Forgione, A.; Marinelli, S.; Renzulli, M.; Ielasi, L.; Sansone, V.; Benevento, F.; Piscaglia, F.; Tovoli, F. Experience with regorafenib in the treatment of hepatocellular carcinoma. Therap. Adv. Gastroenterol. 2021, 14, 17562848211016959. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Takai, K.; Miwa, T.; Taguchi, D.; Hanai, T.; Suetsugu, A.; Shiraki, M.; Shimizu, M. Rapid Depletions of Subcutaneous Fat Mass and Skeletal Muscle Mass Predict Worse Survival in Patients with Hepatocellular Carcinoma Treated with Sorafenib. Cancers 2019, 11, 1206. [Google Scholar] [CrossRef]
- Loosen, S.; Schulze-Hagen, M.; Bruners, P.; Tacke, F.; Trautwein, C.; Kuhl, C.; Luedde, T.; Roderburg, C. Sarcopenia Is a Negative Prognostic Factor in Patients Undergoing Transarterial Chemoembolization (TACE) for Hepatic Malignancies. Cancers 2019, 11, 1503. [Google Scholar] [CrossRef]
- Quinten, C.; Coens, C.; Mauer, M.; Comte, S.; Sprangers, M.A.; Cleeland, C.; Osoba, D.; Bjordal, K.; Bottomley, A. Baseline quality of life as a prognostic indicator of survival: A meta-analysis of individual patient data from EORTC clinical trials. Lancet Oncol. 2009, 10, 865–871. [Google Scholar] [CrossRef]
- Diouf, M.; Filleron, T.; Barbare, J.C.; Fin, L.; Picard, C.; Bouché, O.; Dahan, L.; Paoletti, X.; Bonnetain, F. The added value of quality of life (QoL) for prognosis of overall survival in patients with palliative hepatocellular carcinoma. J. Hepatol. 2013, 58, 509–521. [Google Scholar] [CrossRef]
- Yeo, W.; Mo, F.K.; Koh, J.; Chan, A.T.; Leung, T.; Hui, P.; Chan, L.; Tang, A.; Lee, J.J.; Mok, T.S.; et al. Quality of life is predictive of survival in patients with unresectable hepatocellular carcinoma. Ann. Oncol. 2006, 17, 1083–1089. [Google Scholar] [CrossRef]
- Sternby Eilard, M.; Hagström, H.; Mortensen, K.E.; Wilsgaard, T.; Vagnildhaug, O.M.; Dajani, O.; Stål, P.; Rizell, M. Quality of life as a prognostic factor for survival in hepatocellular carcinoma. Liver Int. 2018, 38, 885–894. [Google Scholar] [CrossRef]
- Secord, A.A.; Coleman, R.L.; Havrilesky, L.J.; Abernethy, A.P.; Samsa, G.P.; Cella, D. Patient-reported outcomes as end points and outcome indicators in solid tumours. Nat. Rev. Clin. Oncol. 2015, 12, 358–370. [Google Scholar] [CrossRef]
- Vogel, A.; Qin, S.; Kudo, M.; Su, Y.; Hudgens, S.; Yamashita, T.; Yoon, J.H.; Fartoux, L.; Simon, K.; López, C.; et al. Lenvatinib versus sorafenib for first-line treatment of unresectable hepatocellular carcinoma: Patient-reported outcomes from a randomised, open-label, non-inferiority, phase 3 trial. Lancet Gastroenterol. Hepatol. 2021, 6, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Galle, P.R.; Finn, R.S.; Qin, S.; Ikeda, M.; Zhu, A.X.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.; et al. Patient-reported outcomes with atezolizumab plus bevacizumab versus sorafenib in patients with unresectable hepatocellular carcinoma (IMbrave150): An open-label, randomised, phase 3 trial. Lancet Oncol. 2021, 22, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ortega, A.J.; Piñar-Gutiérrez, A.; Serrano-Aguayo, P.; González-Navarro, I.; Remón-Ruíz, P.J.; Pereira-Cunill, J.L.; García-Luna, P.P. Perioperative Nutritional Support: A Review of Current Literature. Nutrients 2022, 14, 1601. [Google Scholar] [CrossRef] [PubMed]
- Bruun, L.I.; Bosaeus, I.; Bergstad, I.; Nygaard, K. Prevalence of malnutrition in surgical patients: Evaluation of nutritional support and documentation. Clin. Nutr. 1999, 18, 141–147. [Google Scholar] [CrossRef]
- Verde, Z.; Giaquinta, A.; Sainz, C.M.; Ondina, M.D.; Araque, A.F. Bone Mineral Metabolism Status, Quality of Life, and Muscle Strength in Older People. Nutrients 2019, 11, 2748. [Google Scholar] [CrossRef] [PubMed]
- Uemura, K.; Doi, T.; Lee, S.; Shimada, H. Sarcopenia and Low Serum Albumin Level Synergistically Increase the Risk of Incident Disability in Older Adults. J. Am. Med. Dir. Assoc. 2019, 20, 90–93. [Google Scholar] [CrossRef]
- Colloca, G.; Di Capua, B.; Bellieni, A.; Cesari, M.; Marzetti, E.; Valentini, V.; Calvani, R. Muscoloskeletal aging, sarcopenia and cancer. J. Geriatr. Oncol. 2019, 10, 504–509. [Google Scholar] [CrossRef]
- Renzulli, M.; Golfieri, R. Proposal of a new diagnostic algorithm for hepatocellular carcinoma based on the Japanese guidelines but adapted to the Western world for patients under surveillance for chronic liver disease. J. Gastroenterol. Hepatol. 2016, 31, 69–80. [Google Scholar] [CrossRef]
- Chie, W.C.; Blazeby, J.M.; Hsiao, C.F.; Chiu, H.C.; Poon, R.T.; Mikoshiba, N.; Al-Kadhimi, G.; Heaton, N.; Calara, J.; Collins, P.; et al. International cross-cultural field validation of an Eucropean Organization for Research and Treatment of Cancer questionnaire module for patients with primary liver cancer, the European Organization for Research and Treatment of Cancer quality-of-life questionnaire HCC18. Hepatology 2012, 55, 1122–1129. [Google Scholar] [CrossRef]
- EuroQol Group. EuroQol--a new facility for the measurement of health-related quality of life. Health Policy 1990, 16, 199–208. [Google Scholar] [CrossRef]

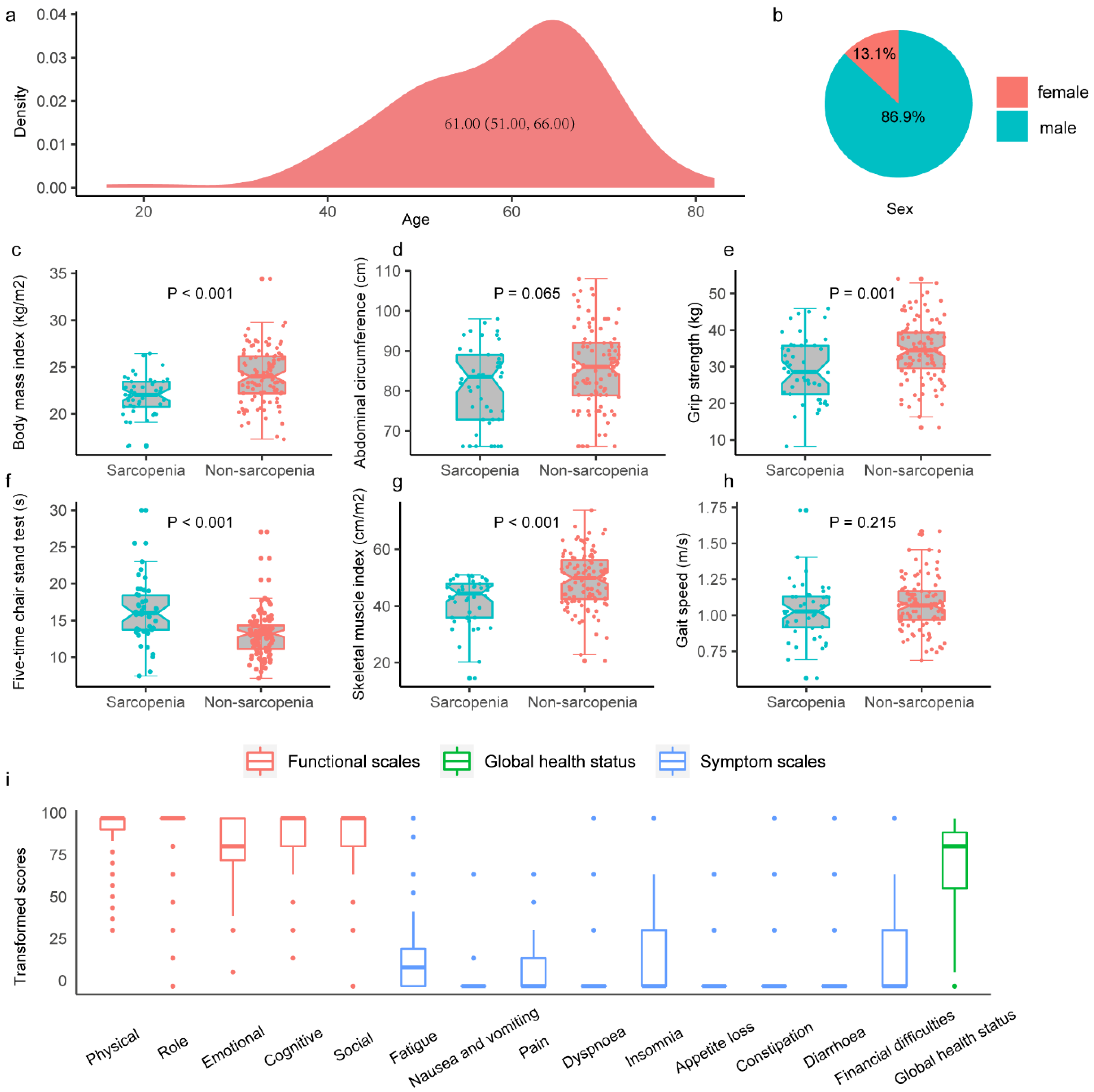
| Characteristics | Total Population N = 153 | Patients with Sarcopenia N = 45 | Patients without Sarcopenia N = 108 | p-value |
|---|---|---|---|---|
| Age, n (%) | 0.311 | |||
| 16–60 | 76 (49.7) | 19 (42.2) | 57 (52.8) | |
| 61–82 | 77 (50.3) | 26 (57.8) | 51 (47.2) | |
| Sex, n (%) | 0.210 | |||
| Female | 20 (13.1) | 3 (6.7) | 17 (15.7) | |
| Male | 133 (86.9) | 42 (93.3) | 91 (84.3) | |
| Body mass index, n (%) | <0.001 | |||
| <25 kg/m2 | 109 (71.2) | 42 (93.3) | 67 (62.0) | |
| ≥25 kg/m2 | 44 (28.8) | 3 (6.7) | 41 (38.0) | |
| Abdominal circumference, cm | 85.0 (76.0,91.0) | 83.5 (72.8,89.0) | 86.00 (78.9,92.0) | 0.065 |
| Grip strength test, kg | 32.89 (8.60) | 29.38 (8.83) | 34.35 (8.11) | 0.001 |
| Chair stand test, s | 13.6 (11.6, 15.9) | 16.0 (13.3, 18.4) | 13.2 (11.2, 14.3) | <0.001 |
| Skeletal muscle index, cm/m2 | 47.04 (9.95) | 41.84 (8.45) | 49.20 (9.75) | <0.001 |
| Gait speed, m/s | 1.07 (0.96, 1.16) | 1.03 (0.92, 1.13) | 1.07 (0.97, 1.17) | 0.215 |
| High blood pressure, n (%) | 50 (32.7) | 14 (31.1) | 36 (33.3) | 0.938 |
| Diabetes, n (%) | 33 (21.6) | 13 (28.9) | 20 (18.5) | 0.228 |
| Heart disease, n (%) | 7 (4.6) | 3 (6.7) | 4 (3.7) | 0.708 |
| Hepatitis B, n (%) | 91 (59.5) | 23 (51.1) | 68 (63.0) | 0.238 |
| Physical activity, n (%) | 99 (64.7) | 29 (64.4) | 70 (64.8) | 1.000 |
| SARC-F questionnaire, n (%) | 0.154 | |||
| ≥4 | 151 (98.7) | 43 (95.6) | 108 (100) | |
| <4 | 2 (1.3) | 2 (4.4) | 0 (0.0) | |
| Sleep time, n (%) | 0.737 | |||
| ≥8 h | 35 (22.9) | 9 (20.0) | 26 (24.1) | |
| <8 h | 118 (77.1) | 36 (80.0) | 82 (75.9) | |
| Diet, n (%) | 0.527 | |||
| Semi-vegetarian | 58 (37.9) | 16 (35.6) | 42 (38.9) | |
| Meat-eater | 63 (41.2) | 17 (37.8) | 46 (42.6) | |
| Vegetarian | 32 (20.9) | 12 (26.7) | 20 (18.5) | |
| Smoking history, n (%) | 97 (63.4) | 32 (71.1) | 65 (60.2) | 0.274 |
| Drinking history, n (%) | 86 (56.2) | 27 (60.0) | 59 (54.6) | 0.666 |
| Medical insurance, n (%) | 148 (96.7) | 45 (100.0) | 103 (95.4) | 0.333 |
| Employment, n (%) | 65 (42.5) | 14 (31.1) | 51 (47.2) | 0.097 |
| Education, years | 0.097 | |||
| 1–6 | 88 (57.5) | 31 (68.9) | 57 (52.8) | |
| 6+ | 65 (42.5) | 14 (31.1) | 51 (47.2) | |
| Annual income, yuan | 0.341 | |||
| 0–49,999 | 120 (78.4) | 38 (84.4) | 82 (75.9) | |
| 50,000~ | 33 (21.6) | 7 (15.6) | 26 (24.1) | |
| TNM, n (%) | 0.569 | |||
| I | 95 (62.1) | 30 (66.7) | 65 (60.2) | |
| II-IV | 58 (37.9) | 15 (33.3) | 43 (39.8) | |
| BCLC, n (%) | 0.729 | |||
| 0-A | 104 (68) | 32 (71.1) | 72 (66.7) | |
| B-C | 49 (32.0) | 13 (28.9) | 36 (33.3) | |
| Tumor size, n (%) | 0.256 | |||
| 0.1 cm–5.0 cm | 126 (82.4) | 40 (88.9) | 86 (79.6) | |
| ≥5.1 cm | 27 (17.6) | 5 (11.1) | 22 (20.4) | |
| Surgical approach, n (%) | 1.000 | |||
| Laparoscopy | 77 (50.3) | 23 (51.1) | 54 (50.0) | |
| Open | 76 (49.7) | 22 (48.9) | 54 (50.0) | |
| Transfuse blood, n (%) | 0.446 | |||
| No | 77 (50.3) | 20 (44.4) | 57 (52.8) | |
| Yes | 76 (49.7) | 25 (55.6) | 51 (47.2) | |
| Degree of differentiation, n (%) | 0.286 | |||
| Low | 41 (26.8) | 16 (35.6) | 25 (23.1) | |
| Middle | 92 (60.1) | 24 (53.3) | 68 (63.0) | |
| High | 20 (13.1) | 5 (11.1) | 15 (13.9) | |
| AFP, n (%) | 0.530 | |||
| ≤400 ng/mL | 130 (85.0) | 40 (88.9) | 90 (83.3) | |
| >400 ng/mL | 23 (15.0) | 5 (11.1) | 18 (16.7) | |
| Albumin, n (%) | 0.185 | |||
| ≥40 g/L | 79 (51.6) | 19 (42.2) | 60 (55.6) | |
| <40 g/L | 74 (48.4) | 26 (57.8) | 48 (44.4) | |
| Costs, (yuan) | 54189 (22598) | 61132 (31947) | 51297 (16645) | 0.014 |
| Postoperative stay, (day) | 12.35 (5.45) | 14.04 (7.51) | 11.65 (4.17) | 0.013 |
| Complication, n (%) | 0.162 | |||
| No | 74 (48.4) | 17 (37.8) | 57 (52.8) | |
| I-II | 29 (19.0) | 12 (26.7) | 17 (15.7) | |
| III-IV | 50 (32.7) | 16 (35.6) | 34 (31.5) | |
| 90-day readmission, n (%) | 0.449 | |||
| No | 118 (77.1) | 37 (82.2) | 81 (75.0) | |
| Yes | 35 (22.9) | 8 (17.8) | 27 (25.0) | |
| 180-day readmission, n (%) | 0.339 | |||
| No | 109 (71.2) | 35 (77.8) | 74 (68.5) | |
| Yes | 44 (28.8) | 10 (22.2) | 34 (31.5) | |
| Died within 30 days, n (%) | NA | |||
| No | 153 (100.0) | 45 (100.0) | 108 (100.0) | |
| Yes | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Died within 90 days, n (%) | 0.650 | |||
| No | 152 (100.0) | 44 (97.8) | 108 (100.0) | |
| Yes | 1 (0.7) | 1 (2.2) | 0 (0.0) | |
| Died within 1 year, n (%) | 5 (3.3) | 4 (8.9) | 1 (0.9) | 0.043 |
| HRQOL questionnaire, n (%) | 129 (84.3) | 34 (75.6) | 95 (88.0) | 0.093 |
| Physical functioning | 93.80 (13.54) | 91.37 (15.44) | 94.67 (12.78) | 0.225 |
| Role functioning | 94.06 (16.77) | 92.65 (17.01) | 94.56 (16.74) | 0.570 |
| Emotional functioning | 83.79 (16.38) | 85.78 (15.29) | 83.07 (16.78) | 0.409 |
| Cognitive functioning | 90.83 (16.00) | 93.14 (14.86) | 90.00 (16.38) | 0.328 |
| Social functioning | 89.53 (19.88) | 84.31 (22.82) | 91.40 (18.49) | 0.074 |
| Fatigue | 15.68 (19.47) | 17.97 (21.19) | 14.85 (18.87) | 0.425 |
| Nausea and vomiting | 0.90 (6.36) | 0.00 (0.00) | 1.23 (7.39) | 0.336 |
| Pain | 8.01 (12.52) | 4.41 (9.45) | 9.30 (13.25) | 0.050 |
| Dyspnea | 6.72 (16.86) | 8.82 (22.19) | 5.96 (14.57) | 0.398 |
| Insomnia | 19.90 (31.05) | 20.59 (33.85) | 19.65 (30.17) | 0.880 |
| Appetite loss | 3.88 (14.21) | 3.92 (13.64) | 3.86 (14.48) | 0.983 |
| Constipation | 8.53 (20.95) | 10.78 (24.23) | 7.72 (18.48) | 0.466 |
| Diarrhea | 3.62 (15.16) | 7.84 (21.80) | 2.11 (11.72) | 0.058 |
| Financial difficulties | 25.06 (34.62) | 25.49 (31.84) | 24.91 (35.72) | 0.934 |
| Global health status | 72.09 (25.76) | 68.87 (28.15) | 73.25 (24.91) | 0.398 |
| Characteristics | β (95%CI) | p-Value |
|---|---|---|
| Physical functioning | −1.02 (−11.57, 9.54) | 0.849 |
| Role functioning | −3.59 (−17.06, 9.88) | 0.599 |
| Emotional functioning | −2.45 (−15.34, 10.43) | 0.707 |
| Cognitive functioning | 0.85 (−11.98, 13.68) | 0.896 |
| Social functioning | −5.77 (−20.86, 9.32) | 0.450 |
| Fatigue | 11.49 (−3.55, 26.53) | 0.133 |
| Nausea and vomiting | −1.21 (−6.11, 3.69) | 0.625 |
| Pain | 3.10 (−6.60, 12.80) | 0.528 |
| Dyspnea | 12.63 (−0.29, 25.55) | 0.055 |
| Insomnia | −2.76 (−28.22, 22.70) | 0.830 |
| Appetite loss | 2.25 (−8.90, 13.41) | 0.690 |
| Constipation | −10.56 (−27.01, 5.90) | 0.207 |
| Diarrhea | 17.82 (6.18, 29.47) | 0.003 |
| Financial difficulties | 3.43 (−22.57, 29.44) | 0.794 |
| Global health status | −23.08 (−43.22, −2.94) | 0.025 |
| Characteristics | Univariate Analysis p-Value | Multivariate Analysis | |
|---|---|---|---|
| β (95%CI) | p-Value | ||
| Age | |||
| 16–60 | Reference | ||
| 61–82 | 0.074 | ||
| Sex | |||
| Female | Reference | ||
| Male | 0.888 | ||
| Physical activity | |||
| No | Reference | ||
| Yes | 0.826 | ||
| Sleep time | |||
| ≥8 h | Reference | ||
| <8 h | 0.912 | ||
| Diet | |||
| Semi-vegetarian | Reference | ||
| Meat-eater | 0.228 | ||
| Vegetarian | 0.723 | ||
| Smoking history | |||
| No | Reference | ||
| Yes | 0.410 | ||
| Drinking history | |||
| No | Reference | ||
| Yes | 0.751 | ||
| Hypertension | |||
| No | Reference | ||
| Yes | 0.221 | ||
| Diabetes | |||
| No | Reference | ||
| Yes | 0.118 | ||
| Medical insurance | |||
| No | Reference | ||
| Yes | 0.173 | ||
| Employment | |||
| No | Reference | ||
| Yes | 0.203 | ||
| Education, years | |||
| 1–6 | Reference | ||
| 6+ | 0.064 | ||
| Annual income, yuan | |||
| 0–49,999 | Reference | ||
| 50,000~ | 0.248 | ||
| SARC-F | |||
| ≥4 | Reference | ||
| <4 | 0.536 | ||
| Sarcopenia | |||
| No | Reference | ||
| Yes | 0.398 | ||
| Preoperative ascites | |||
| No | Reference | ||
| Yes | 0.812 | ||
| Cirrhosis | |||
| No | Reference | ||
| Yes | 0.580 | ||
| Child cough | |||
| A | Reference | ||
| B | 0.921 | ||
| TNM | |||
| I | Reference | ||
| II-IV | 0.034 | ||
| Tumor size | |||
| 0.1–5.0 cm | Reference | ||
| ≥5.1 cm | 0.556 | ||
| ASA grade | |||
| I–II | Reference | ||
| III | 0.065 | ||
| Blood loss | |||
| ≤400 mL | Reference | ||
| >400 mL | 0.017 | ||
| Transfuse blood | |||
| No | Reference | ||
| Yes | 0.064 | ||
| BCLC | |||
| 0–A | Reference | ||
| B–C | 0.123 | ||
| AFP | |||
| ≤400 ng/mL | Reference | ||
| >400 ng/mL | 0.953 | ||
| Location | |||
| Left liver | Reference | ||
| Right liver | 0.126 | ||
| Both sides | 0.656 | ||
| Lesions | |||
| Solitary | Reference | ||
| Multiple | 0.172 | ||
| Surgery | |||
| Laparoscopy | Reference | ||
| Open | 0.033 | ||
| Degree of differentiation | |||
| Low | Reference | ||
| Middle | 0.987 | ||
| High | 0.132 | ||
| Satellite stove | |||
| No | Reference | ||
| Yes | 0.302 | ||
| Vascular invasion | |||
| No | Reference | ||
| Yes | 0.426 | ||
| Albumin | |||
| ≥40 g/L | Reference | Reference | |
| <40 g/L | 0.003 | −13.29 (−21.78, −4.79) | 0.002 |
| Interaction of sarcopenia and surgical approach | |||
| Sarcopenia open surgery group | Reference | Reference | |
| Sarcopenia laparoscopic surgery group | 0.003 | 26.68 (9.96, 43.39) | 0.002 |
| Non-sarcopenic open surgery group | 0.023 | 16.88 (2.31, 31.45) | 0.024 |
| Non-sarcopenic laparoscopic surgery group | 0.005 | 20.97 (6.36, 35.59) | 0.005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Yang, J.; Yu, H.; Bo, Z.; Chen, K.; Wang, D.; Xie, Y.; Wang, Y.; Chen, G. Effect of Sarcopenia on Survival and Health-Related Quality of Life in Patients with Hepatocellular Carcinoma after Hepatectomy. Cancers 2022, 14, 6144. https://doi.org/10.3390/cancers14246144
Hu J, Yang J, Yu H, Bo Z, Chen K, Wang D, Xie Y, Wang Y, Chen G. Effect of Sarcopenia on Survival and Health-Related Quality of Life in Patients with Hepatocellular Carcinoma after Hepatectomy. Cancers. 2022; 14(24):6144. https://doi.org/10.3390/cancers14246144
Chicago/Turabian StyleHu, Jiawei, Jinhuan Yang, Haitao Yu, Zhiyuan Bo, Kaiwen Chen, Daojie Wang, Yitong Xie, Yi Wang, and Gang Chen. 2022. "Effect of Sarcopenia on Survival and Health-Related Quality of Life in Patients with Hepatocellular Carcinoma after Hepatectomy" Cancers 14, no. 24: 6144. https://doi.org/10.3390/cancers14246144
APA StyleHu, J., Yang, J., Yu, H., Bo, Z., Chen, K., Wang, D., Xie, Y., Wang, Y., & Chen, G. (2022). Effect of Sarcopenia on Survival and Health-Related Quality of Life in Patients with Hepatocellular Carcinoma after Hepatectomy. Cancers, 14(24), 6144. https://doi.org/10.3390/cancers14246144





