Simple Summary
A review of the molecular and cellular features of the major cancer syndromes associated with radiosensitivity revealed the importance of the ATM protein, either as an impaired kinase in the nucleus or as a complex in the cytoplasm, with the mutated protein responsible for the syndrome.
Abstract
There are a number of genetic syndromes associated with both high cancer risk and clinical radiosensitivity. However, the link between these two notions remains unknown. Particularly, some cancer syndromes are caused by mutations in genes involved in DNA damage signaling and repair. How are the DNA sequence errors propagated and amplified to cause cell transformation? Conversely, some cancer syndromes are caused by mutations in genes involved in cell cycle checkpoint control. How is misrepaired DNA damage produced? Lastly, certain genes, considered as tumor suppressors, are not involved in DNA damage signaling and repair or in cell cycle checkpoint control. The mechanistic model based on radiation-induced nucleoshuttling of the ATM kinase (RIANS), a major actor of the response to ionizing radiation, may help in providing a unified explanation of the link between cancer proneness and radiosensitivity. In the frame of this model, a given protein may ensure its own specific function but may also play additional biological role(s) as an ATM phosphorylation substrate in cytoplasm. It appears that the mutated proteins that cause the major cancer and radiosensitivity syndromes are all ATM phosphorylation substrates, and they generally localize in the cytoplasm when mutated. The relevance of the RIANS model is discussed by considering different categories of the cancer syndromes.
1. Introduction
1.1. The Syndromes Combining Cancer Proneness and Radiosensitivity
To date, radiotherapy (RT) remains as one of the major tools for cancer treatment to control and eradicate cancer cells. Radiation oncologists have to face a double challenge: kill the tumor and spare the healthy tissues. After more than one century of anti-cancer RT, a considerable amount of data has been accumulated to better understand the molecular mechanisms underlying the cellular response of tumors and healthy tissues to ionizing radiation (IR) but also to some chemotherapy agents that mimic IR [1,2,3,4,5]. Particularly, in 5 to 20% of RT-treated cancer cases, some adverse tissue reactions, ranging from radiation-induced (RI) blushes without consequence to fatal reactions, can occur during or after RT, which oblige clinicians to modify or stop the scheduled treatment [6,7,8]. The prediction and prevention of such adverse reactions of exposed tissue, called radiosensitivity reactions or radiotoxicities, is a big challenge in radiotherapy and in radiobiology [9]. Hence, cancer proneness can be associated with a large spectrum of RI adverse reactions, therefore defining some “syndromes” that link both cancer proneness and radiosensitivity. These particular syndromes raise the question of the interplay between the cellular transformation process leading to cancer and an abnormal response to IR leading to tissue injuries [4,5].
1.2. Interplay between DNA Damage Repair and Signaling and Cell Cycle Checkpoint Arrests
While carcinogenesis and the cellular transformation process have been mainly associated with a lack of control of cell cycle checkpoint arrests enabling cellular proliferation, radiosensitivity has been generally linked to dysfunctions in chromosomal and DNA damage signaling and repair pathways [9,10,11]. However, such interplay between cancer and radiosensitivity remains unclear. For example, some mutations of the LIG4 and XP genes that cause cancer and radiosensitivity syndromes have been shown to directly impact DNA damage signaling and repair but not on the cell cycle checkpoint arrest control. Conversely, some mutations of the p53 or CHK2 genes that cause some other cancer and radiosensitivity syndromes have been shown to directly impact the cell cycle checkpoint arrest controls but not DNA damage signaling and repair [4,5]. Lastly, the mutations of NF1 or TSC genes, whose biological function directly impacts DNA damage signaling and repair and cell cycle checkpoint arrest controls, have been associated with cancer and radiosensitivity [12,13]. Hence, these examples reveal not only that the molecular bases of cancer proneness remain to be more documented, but also their link with radiosensitivity is still poorly understood.
1.3. The ATM Protein as the Crossroads of Cancer and Radiosensitivity
IR generally causes three major types of DNA damage: base damage (BD), single-strand breaks (SSB) and DNA double-strand breaks (DSB). Throughout the natural selection, cells are equipped with various pathways to manage BD, SSB and DSB specifically [4,14]. Each type of DNA damage triggers a specific ordered succession of enzymatic steps, frequently operating via a cascade of phosphorylations directed by upstream kinases: DNA damage is firstly recognized several minutes post-stress, is repaired for a few hours, cell cycle checkpoint arrests are activated some hours post-stress, and then cellular death is triggered several hours thereafter together with the initiation of cell transformation or aging processes (Figure 1) [15]. Any lack or impairment of the most upstream molecular events occurring immediately after the DNA damage formation results in the most severe biological effects and may condition the next molecular and cellular steps [15]. Such an RI cascade of phosphorylations reveals that RI DNA damage formation, recognition and repair occur systematically before cell cycle checkpoint arrests, suggesting that cells do not arrest their cycle to repair their DNA damage since the great majority of them are already recognized and/or repaired [10,16].
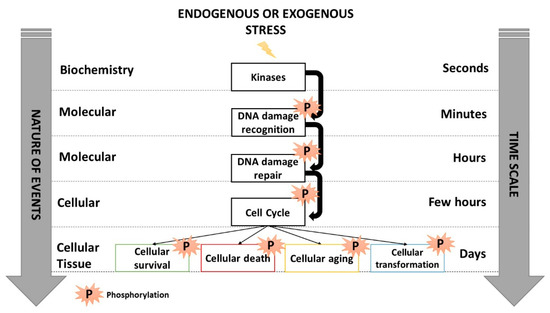
Figure 1.
Schematic view of the cascades of phosphorylations occurring in response to IR activated by kinases that trigger DNA damage recognition, repair, cell cycle arrests and cellular death. Inspired from [15].
Among the RI DNA damage, DSB appears to be the key damage of cell lethality [17,18]. Among the kinases involved in both DSB signaling and repair and cell cycle checkpoint arrest control, the ATM kinase is considered as a major actor of the response to IR, since it phosphorylates numerous protein substrates belonging to the RI cascade of phosphorylations evoked above [15,19]. More recently, we have documented the role of the RI ATM nucleoshuttling (RIANS) in the individual response to IR and showed that the RIANS may serve as a reliable marker of radiosensitivity, whatever the nature and the dose of IR [8,18,20]. Briefly, after exposure to IR, the cytoplasmic ATM dimers dissociate as ATM monomers and diffuse in the nucleus. Once in the nucleus, ATM monomers phosphorylate the X variant of the H2A histone protein (γH2AX), which triggers the recognition of the RI DSB by the non-homologous end-joining (NHEJ) pathway, the most predominant DSB signaling and repair pathway in humans [8,18,20]. However, the RIANS can be delayed by the overexpression of some cytoplasmic substrates of ATM (called X-proteins). These X-proteins hold SQ and TQ domains that are specifically phosphorylated by ATM after exposure to IR [19]. The X-proteins sequestrate the RI ATM monomers, decrease their flux in the nucleus and therefore affect the nuclear ATM kinase activity and the number of DSB recognized by NHEJ [20,21,22]. Hence, an overexpression of cytoplasmic X-proteins and a delayed RIANS may cause radiosensitivity because some DSB are not recognized or repaired, and cancer because of DSB non-recognized by NHEJ may be misrepaired [4]. In the RIANS model, the mutated protein responsible for each syndrome associated with delayed RIANS may elicit two impaired functions: one in the nucleus or cytoplasm, as a single mutated protein, and the other, as an overexpressed cytoplasmic ATM substrate. These two impaired functions may explain impaired DSB signaling and repair defects on one hand and lack of cell cycle checkpoint arrest on the other hand [20].
The RIANS model has been validated in a subset of cancer and radiosensitivity syndromes [8,13,23,24]. This review aims to better identify and understand the molecular and cellular features of the major cancer syndromes associated with radiosensitivity by successively examining the syndromes related to impairments of DNA damage signaling and repair (category 1), impairments of cell cycle checkpoint arrest control (category 2), and the other syndromes for which impairments of both DNA damage signaling and repair and cell cycle checkpoint arrest control are not obvious (category 3) (Figure 2). For each syndrome described, the potential role of the ATM kinase and the RIANS, the presence of SQ/TQ domains potentially phosphorylated by ATM, and the cytoplasmic forms of the mutated proteins will be discussed to provide a novel and unified mechanistic model for both cancer susceptibility and radiosensitivity (Figure 2).
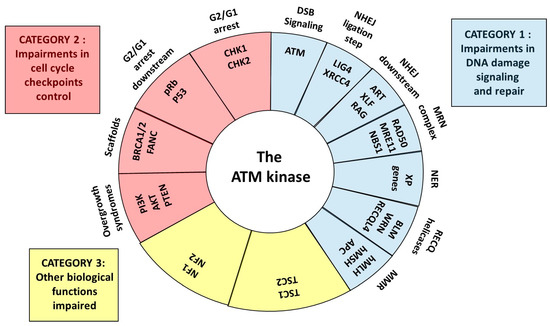
Figure 2.
Schematic view of the central role of ATM as a kinase and partner for the proteins whose mutations cause cancer and radiosensitivity syndromes. Three categories can be defined: syndromes due to some impairments in DNA damage signaling and repair, some impairments in cell cycle checkpoint control and to other impaired biological functions.
2. Diseases of DNA Damage Repair and Signaling
2.1. Mutations of the ATM and ATR Kinases
The phosphatidylinositol-3 (PI3) kinases are the components of a large family of enzymes involved in various cellular functions such as cell proliferation, survival and signaling of DNA damage, through their capacity to phosphorylate their substrates [25]. Among them, the ATM and ATR kinases are the two major actors [15,26,27].
The ATM mutations play a major role in DNA break recognition, repair and signaling but also in the cell cycle checkpoint control. Homozygous ATM mutations cause ataxia telangiectasia (AT), the human syndrome associated with the highest radiosensitivity [1,28,29]. AT was described for the first time by Syllaba and Hennen in 1926 and by Madame Louis Bar in 1941 [30,31]. AT is characterized by cerebellar ataxia with severe prognosis oculocutaneous telangiectasia, a deficient synthesis of immunoglobulins IgA, IgE and IgG2 and a strong predisposition to certain cancers, notably leukemias and lymphomas [32,33,34]. AT is also associated with a spontaneous reorganization of chromosomes (10% of metaphases elicit aberrations on chromosomes 7 and 14) [35]. In the United States and Great Britain, the incidence of AT is estimated to be about 1/100,000 [36]. AT cell lines are systematically characterized by hyper-radiosensitivity [28]. Other radiobiological features of AT cells include numerous chromosomal aberrations, lack of control of the G1 cycle and inhibition of DNA synthesis (called radio-resistant synthesis). No hypersensitivity to UV has been observed in AT patients [32,37,38,39,40]. The ATM gene has been cloned and sequenced in 1995 [29,41]. As evoked above, the ATM kinase preferentially phosphorylates the SQ/TQ domains in response to IR [19]. Most of the homozygous ATM mutations lead to complete inactivation of the protein [42]. For example, cells from AT patients either do not show γH2AX foci or show a small number of tiny γH2AX foci, suggesting an absent or impaired RIANS [18].
About 15% of AT patients, with so-called variants, suffer from mutations that lead to a less severe pathology, less marked clinical signs, and a life expectancy of 50–60 years (vs. less than 30 years for “classical” AT patients). Such mutations do not concern the kinase domain of ATM [43,44]. Heterozygous carriers (ATH) represent about 1% of the whole population and elicit an increased risk of breast cancer [45], although this epidemiological feature is still debated [46,47,48]. ATH cells may be not more radiosensitive than cells from radioresistant, apparently healthy individuals, although such a hypothesis remains to be confirmed [49,50,51,52]. Altogether, this short review shows that homozygous “classical” and variant ATM mutations are associated with both impaired DNA damage signaling and repair and a lack of cell cycle checkpoint control.
The ATR kinase also belongs to the PI3K family and elicits an ATM-like structure with the same affinity for the SQ/TQ domains as ATM. However, ATR is activated by UV, SSB and BD rather than IR and DSB, such as ATM [15,27,53]. ATR may be more essential than ATM for cell viability since only punctual mutations of ATR have been observed: such ATR mutations cause Seckel syndrome (or microcephalic primordial dwarfism (SCKL)) [54,55,56]. SCKL is associated with severe microcephaly, bird-headed dwarfism, growth and mental retardation. However, literature data suggest that SCKL is not associated with a strong radiosensitivity such as that observed in AT cells [56]. Regarding the susceptibility to cancer, it seems that it is not a major feature of SCKL, although some cases of leukemias have been reported [56]. Lastly, it has been shown that mutations of the pericentrin PCNT gene cause a SCKL-like syndrome, suggesting a possible interaction between the PCNT and ATR proteins. PCNT mutations have been associated with high cancer risk. However, the level of radiosensitivity potentially associated with PCNT mutations needs to be documented [57]. Hence, the literature data suggest that further investigations are needed to better document that SCKL and SCKL-like syndromes can be considered as cancer and radiosensitivity syndromes.
2.2. Non-Homologous End-Joining Diseases
2.2.1. Mutations of Ku and DNA-PKcs Genes
As said above, the non-homologous end-joining (NHEJ) pathway is the most predominant DSB repair pathway in G0/G1 mammalian cells [58,59]. Historically, as opposed to homologous recombination (HR), another DSB repair pathway, the term “non-homologous” has been added to “end-joining” to give “non-homologous end-joining” [16]. As explained in another review, this term has produced confusion since the notion of strand homology has no sense in the G0/G1 phase (what would “homologous” end-joining mean?) [4].
The end-joining consists in a ligation of both broken DNA ends. The Ku80 and Ku70 proteins bind to DNA to form the Ku heterodimer. The Ku heterodimer slides on DNA and stops at the broken ends: a third protein, DNA-PKcs, is then recruited and the trimeric complex, named DNA-PK, and acts as a serine-threonine kinase [60]. When activated as kinases (generally after an oxidative stress), ATM and DNA-PK can phosphorylate certain protein substrates such as γH2AX, which triggers the formation of nuclear γH2AX foci at the DSB sites. As evoked above, the formation of nuclear γH2AX foci is considered as the earliest recognition step of DSB managed by NHEJ [61,62]. After this step, ligase IV (LIG4) and XRCC4 proteins are recruited at the DSB sites, and broken DNA ends are joined. This is the ligation step [60]. The biological role of the DNA-PK components (Ku70, Ku80, DNA-PKcs) is so crucial for cell viability that no human syndrome is caused by their mutations. However, in rodents, some Ku and DNA-PKs mutants exist, and they are characterized by a severe defect of DSB repair and hyper-radiosensitivity [63]. In humans, only the glial tumor line MO59J shows a mutation of DNA-PKs [64,65], and only one patient has been identified with a DNA-PK mutation that does not concern the kinase domain [66,67]. In some cases of lupus erythematosus, an autoimmune disease, the expression of Ku proteins is generally low. However, no systematic link between lupus and radiosensitivity has been established yet, probably because the Ku protein is so abundant that a decreased expression does not significantly affect its role in the response to IR [68]. Hence, considering their importance in cell viability, no cancer and radiosensitivity syndrome associated with mutations of the early actors of NHEJ (namely the components of the DNA-PK complex) have been described yet.
2.2.2. Mutations of the LIG4 and XRCC4 Genes
In 2003, from the case of a patient suffering from a lymphoma who succumbed to its radio-chemotherapeutic treatment [69,70], O’Driscoll et al. have defined a human syndrome associated with LIG4 mutations, characterized by high radiosensitivity, immunodeficiency, strong pancytopenia, growth retardation and dysmorphic facial features [54]. To date, in addition to this historical case, about forty cases of patients holding LIG4 mutations have been described [71,72].
As evoked above, the XRCC4 protein forms a complex with LIG4. XRCC4 mutations may cause microcephalic primordial dwarfism associated with cardiomyopathy but not with immunodeficiency nor predisposition to any malignancy [73,74]. About 15 cases of XRCC4 mutations have been reported in humans. The first was described in 2014 [75].
In the frame of the RIANS model, can the LIG4 and XRCC4 proteins serve as X-proteins? The LIG4 and XRCC4 proteins hold seven and one SQ/TQ domain, respectively, but no ATM phosphorylated form of these two proteins has been described yet. Some cytoplasmic forms of LIG4 have been reported in response to specific viral infections [76] and the LIG4 protein has been shown to regulate the nuclear localization of XRCC4 [77,78], suggesting that XRCC4 may potentially interact with ATM in the cytoplasm in the case of specific mutations of LIG4. However, considering the limited number of LIG4 and XRCC4 patients, further experiments are needed to verify such hypotheses.
2.2.3. Mutations of Art, XLF, 53BP1, RAG1 and RAG2 Genes
The Artemis protein, encoded by the Art/DCLRE1C gene, is a DNA exo/endonuclease that acts with DNA-PK to prepare broken DNA ends for ligation [79,80]. Such a step is important, not only for DSB repair via NHEJ but also for the V(D)J recombination process, required for immunoglobulins production. The hypomorphic (homozygous or compound heterozygous) mutations of Art cause Artemis syndrome, a human severe combined immunodeficiency associated with a moderate radiosensitivity but a variable predisposition to lymphoma [81,82,83,84,85]. With 10 SQ/TQ domains, the Artemis protein was found to be a major ATM phosphorylation substrate in response to IR that may serve as a regulator of the G2/M cell cycle checkpoint [86]. However, the subcellular localization of the Artemis protein when mutated needs to be documented.
Another important but non-essential NHEJ protein, XLF/Cernunnos, has been identified. Similar to Artemis, XLF/Cernunnos also acts downstream DNA-PK [87,88,89]. Hypomorphic (homozygous or compound heterozygous) mutations of this gene cause Cernunnos syndrome that is associated with mental retardation, microcephaly, strong lymphopenia, and severe immunodeficiency [90]. XLF interacts with XRCC4-LIG4 complex, which stimulates the LIG4 ligase activity by helping to align broken DNA ends. To date, only five Cernunnos syndrome patients have been identified. Similar to Artemis, Cernunnos syndrome is associated with moderate radiosensitivity and a variable predisposition to lymphoma [87,88,89,90]. With four SQ/TQ domains, the XLF/Cernunnos protein was also found to be an ATM phosphorylation substrate in response to IR [91]. The Akt kinase was shown to phosphorylate XLF, which triggers its dissociation from the LIG4-XRCC4 complex and its cytoplasmic relocalization [92]. Further investigations are needed to identify the role of ATM in the cascade of phosphorylation between Akt, XLF, XRCC4 and LIG4.
The 53BP1 protein has been hypothesized to act as a NHEJ protein, although its role is still poorly understood. The 53BP1 protein forms nuclear foci after irradiation, such as many repair proteins cited in this review [93]. The absence of 53BP1 nuclear foci in a patient who showed strong immunodeficiency, dysmorphic aspects, intellectual difficulties and radiosensitivity comparable to Artemis syndrome has been reported. This case was at the origin of the definition of the RIDDLE syndrome (radiosensitivity, immunodeficiency, dysmorphic features and learning difficulties). However, further investigations have documented the molecular features of RIDDLE syndrome that appeared to be caused not by mutations of 53BP1 but by mutations of the ubiquitin-ligase RNF168 [93,94,95]. Hence, while loss of 53BP1 function has been observed in some tumors, no syndrome associated with 53BP1 mutations has been defined yet [93,94,95].
Finally, in this list of syndromes associated with NHEJ impairments, Omenn syndrome (OS) was found to be caused by mutations of the RAG1 and RAG2 genes but also by certain mutations of Artemis [96,97]. OS is a severe immunodeficiency syndrome associated with erythroderma, hepatocellular carcinoma, splenomegaly, lymphadenopathy, and some alopecia. OS patients have a low or total absence of B lymphocytes. The few radiobiological studies about OS suggest that the radiosensitivity of OS is similar to that associated with Artemis and Cernunnos syndromes [98,99]. Phosphorylation of RAG1 or RAG2 by ATM has been described [100] but the subcellular localization of the mutated RAG1 and RAG2 proteins needs to be better documented before considering RAG proteins as potential X-proteins.
2.2.4. NHEJ Impairments: Immunodeficiency Rather Than Radiosensitivity?
Unlike with the early actors of the NHEJ pathway, the mutations of the downstream NHEJ actors such as Art, XLF, RAG1, RAG2 cause a moderate radiosensitivity rather than hyper-radiosensitivity, probably because their functions are redundant during the NHEJ process. When cancer proneness was also reported, all the proteins concerned were found to be phosphorylation substrates of ATM. Since the NHEJ actors are required for a normal V(D)J recombination process, unlike for DSB repair, the major clinical features of the NHEJ impairments described above is severe immunodeficiency rather than severe radiosensitivity. Furthermore, considering the importance of the role of NHEJ actors in lymphocytes, the NHEJ impairments are generally associated with a high risk of leukemia/lymphoma rather than any other cancer type. Most of the mechanistic NHEJ models proposed in the literature do not integrate ATM kinase, while this protein acts upstream the NHEJ actors that serve as phosphorylation substrates, and ATM mutations lead to the lack of recognition of a great majority of RI DSB [4,5,16].
3. Recombination Repair Diseases
3.1. Mutations of the RAD51 and RAD52 Genes
In general, recombination consists in replacing the damaged DNA sequence either by the identical sequence of the homologous chromosome (homologous recombination, HR) [101] or by a sequence taken randomly (illegitimate or non-homologous recombination, NHR), the most frequent ones being the AGCT (AluI) sequences [102,103,104]. While a functional HR is required for meiosis and mitosis, and more generally, in proliferating organisms (e.g., bacteria, yeasts), HR is nearly absent from quiescent cells [101]. Although many studies suggest that the RAD51-RAD52 multimeric complex is essential for the recognition of DSB by HR, the subsequent DNA strand exchange process is still poorly understood. Similar to the Ku heterodimer, the RAD52 protein, as a multimeric ring, was hypothesized to slide along the DNA and stop at the DSB sites. At the DSB sites, RAD51 may associate with RAD52, and its phosphorylation by tyrosine kinases may activate both its nuclease function and its change of shape as a filament [101,102,103,104]. While the RecA protein is considered as the human RAD51 homolog and is essential for the exchange of DNA strands in bacteria, the RecA sequence is however much smaller than that of RAD51 and does not hold any endonuclease site such as RAD51 [105]. Furthermore, resolvases and the complex process of DNA strand exchange (Holliday junctions) remain misunderstood in humans [106]. As evoked for the upstream NHEJ proteins, the mutations of the upstream HR actors lead to the absence of viability, and no corresponding human syndrome exists. Lastly, yeast strains carrying Rad52 mutations are among the most hyper-radiosensitive ones, confirming the importance of HR for proliferating organisms and the differences existing between micro-organisms and mammalians [107].
3.1.1. The Hyper-Recombination Process—At the Origin of Carcinogenesis?
Many proteins other than RAD51 and RAD52 are involved in the recombination process and ensure the stability of the genome via multiprotein complexes. This is particularly the case of scaffold proteins such as BRCA1, BRCA2, and FANC, whose mutations combine cancer predisposition and lack of control of the cell cycle checkpoints (the consequences of their mutations will be discussed in the next chapter). In addition, the mutations of these proteins cause a lack of control of recombination (also called hyper-recombination). The hyper-recombination process is responsible for the production of additional and misrepaired DNA breaks [108,109,110]. In other terms, hyper-recombination results in an accumulation of errors and numerous spontaneous breaks, notably through the impairment of nucleases function [108,109,110]. Hyper-recombination is a common feature of all the syndromes associated with a high risk of cancer. In the frame of the model of carcinogenesis proposed by Weinberg, hyper-recombination and the resulting gene mutations may serve as the endogenous initiation step required for cell transformation and tumorigenesis [111].
3.1.2. Mutations of the RAD50-MRE11-NBS1 Complex
The RAD50, MRE11 and NBS1 proteins form a complex involved during the several steps of DNA damage response from DNA damage recognition to assembly repair complexes, and mutations of proteins constituting this complex are associated with neurological syndromes with tumorigenic potential. Furthermore, the MRE11 endonuclease activity was shown to strongly depend on the integrity of the RAD50-MRE11-NBS1 complex [112,113,114].
The mutations of NBS1 cause Nijmegen’s syndrome (NBS), first described in the 1980s [115]. NBS was long considered as a variant form of the AT syndrome, but with a lower radiosensitivity [116,117]. Microcephaly, small stature, mental retardation, high lymphoma susceptibility and immunodeficiency are the main clinical manifestations of NBS [115]. NBS patients elicit neither ataxia nor telangiectasia [116]. NBS1-mutated cells are characterized by chromosomal instability, and their radiosensitivity may be considered as hyper-radiosensitivity even if it is systematically lower than that observed in AT cells [4,116,117,118]. NBS1-mutated cells show a lack of cycle arrest in G1 [116]. Two groups of complementation V1 (Berlin syndrome) and V2 (Nijmegen’s syndrome) were described with the same absence of cycle arrest in G1 [119,120]. However, some studies have reported that there is only one NBS1 gene and that it is located on chromosome 8 [121,122,123]. The NBS1 protein (also called nibrin) may serve as a phosphorylation substrate of ATM with seven SQ/TQ domains [124]. As a scaffold for the RAD50-MRE11-NBS1 complex, in NBS cells, the NBS1 protein is absent in NBS cells, and MRE11 and RAD50 are cytoplasmic, suggesting that these two proteins may serve as X-proteins in the case of mutations of the NBS1 gene [125] (see also below).
Mutations of MRE11 have been initially identified in three patients whose fibroblasts have been found radiosensitive and deficient in DNA damage repair. The radiosensitivity associated with mutations of MRE11 is lower than with NBS1 mutations [126]. Historically, the first identified family with homozygous mutations of MRE11 showed similar clinical features to AT but with less pronounced intensity. The associated syndrome has been therefore called Ataxia–Telangiectasia-Like Disorder (ATLD) [126]. After identifying other families, ATLD is now considered as a neurological syndrome with radiosensitivity comparable to NBS but associated neither with immunodeficiency nor with high susceptibility to cancer [127,128,129]. With seven SQ/TQ domains, MRE11 is a phosphorylated substrate of ATM that is localized in both the cytoplasm and nucleus and provides nuclear foci after exposure to IR in an ATM-dependent manner. We have shown that cancer syndromes are generally associated with the formation of MRE11 foci early (some minutes) after irradiation while aging syndromes are generally associated with the formation of late foci (24 h) after irradiation [5].
Finally, the case of a patient showing a mutation of RAD50 has been described in 2009 with microcephaly, mental retardation, a bird face, and a small stature [130]. This RAD50-mutated patient developed a malignant lymphoma at the age of 23 without severe immunodeficiency. The cells from this patient elicited radiosensitivity similar to those observed with NBS. Such a syndrome has been called Nijmegen Breakage Syndrome Like-Disorder (NBSLD) [130]. With seven SQ/TQ domains, the RAD50 protein is phosphorylated by ATM at Ser-635 that plays an important adaptor role in signaling for cell cycle control and DNA repair [131,132]. However, in the cells from the single NBSLD case, the MRE11 protein predominantly appeared cytoplasmic, and the RAD50 protein was absent [130].
Altogether, this brief review about the mutations of the RAD50-MRE11-NBS1 complex reveals that all the components of the complex are some ATM phosphorylation substrates, and the mutation of one component may lead to the cytoplasmic localization of at least one of the other components of the RAD50-MRE11-NBS1 and to at least the possibility of sequestrating ATM and preventing rapid RIANS.
3.1.3. Mutations of the Nucleotide and Base Excision Repair Genes
The single-strand annealing (SSA) pathway has been defined only in vitro with very specific sequences (short DNA sequences in which some reporter genes have been placed very close (few hundred bases) to each other) [133,134]: in such a limited and specific DNA sequence system, even a random phenomenon can lead to a faithful repair. Hence, caution must be taken about any in vivo extrapolation of the SSA phenomenon since coding sequences are generally much more spaced and the genome is much longer than the sequences used for the investigations about SSA. Lastly, few proteins have been considered specific to SSA, which relativizes again the existence of such a repair pathway in vivo, especially in response to IR [4].
It has been shown that ligase III, PARP, XRCC1 are the major proteins involved in the base excision resynthesis (BER) [135,136]. However, no genetic syndrome has been associated with mutations of the BER proteins in humans. Such a situation is similar to that discussed above with the Ku heterodimer and with the RAD51 and RAD52 proteins: XRCC1, PARP, ligase III, and DNA polymerase β are proteins required for DNA damage recognition (here BD recognition). They may be so abundant and essential for survival that their mutations systematically lead to a loss of viability: the homozygous mutations of these three genes are lethal at the embryonic state. However, the literature regularly reports diseases associated with BER defects or impairments, but these are generally either the syndromes already mentioned whose mutated genes are not directly involved in BER, or else polymorphisms [136]. Lastly, PARP inhibitors are currently used in the treatments of BRCA1/2-mutated tumors in which DNA breaks accumulate until cell killing [137].
Unlike SSA and BER, the nucleotide excision resynthesis (NER) pathway involves many proteins acting from BD recognition to final DNA sequences polymerization, whose mutations may cause human syndromes associated with cancer, radiosensitivity or even photosensitivity. Particularly, the mutations of some XP genes involved in the transcription factor II H (TFIIH) complex, essential for a functional NER, can cause xeroderma pigmentosum syndrome (XP). XP is dispatched in several groups of complementation [138,139]. Some XP proteins are involved in the NER as endonucleases, helicases, or oriented polymerases. This is notably the case of XP-A to XP-G XP-G, whose mutations are linked to photosensitivity, neurodegeneration and/or brain or skin cancer [139,140,141]. In addition, some complementation groups such as XPD may be also associated with moderate radiosensitivity, suggesting some role in DSB repair and signaling [23,142]. All the mutations of XP-A to XP-G genes may cause misrepaired BD, SSB and/or DSB [4]. All the XP-A to XP-G genes hold SQ/TQ domains and show cytoplasmic forms when mutated, suggesting that ATM may phosphorylate and interact with them in the cytoplasm. For example, some specific mutations of XPD were shown to sequestrate ATM in the cytoplasm after exposure to IR and lead to cancer proneness and radiosensitivity [23].
Often necessary during or after the DNA strands exchange process, the RECQ helicases are inextricably linked to the maintenance of genome integrity. The RECQ family contains three proteins identified in humans, including WRN, BLM, RECQL4 [143,144]. Mutations of BLM, WRN and RECQL4 cause Bloom (BLM), Werner (WRN) and Rothmund–Thompson (RTS) syndromes, respectively. The BLM, WRN and RECQL4 proteins show nuclease domains, which support their involvement in hyper-recombination processes [145,146,147]. In addition to growth disorders and accelerated aging, these three syndromes have in common a strong predisposition to sarcoma. With regard to aging and predisposition to early senescence, it can be hypothesized that an instable helicase–endonuclease complex would be responsible of the generation of spontaneous breaks that may promote a senescence phenomenon [145,146,147]. All these syndromes are associated with significant but moderate radiosensitivity [1].
The WRN and BLM proteins hold SQ/TQ domains and have been shown to be phosphorylated by ATM [148,149]. The WRN and BLM cells show cytoplasmic forms [150,151]. With regard to RECQL4 cells, we recently pointed out the existence of the SQ/TQ domain, the cytoplasmic forms of some mutated RECQL4 proteins, and the delay of RIANS.
4. Mutations of Mismatch Repair Genes
Human non-polyposis hereditary colon cancers (HNPCC) syndromes are often grouped under the name of Lynch syndrome (LS), even though some physicians distinguish both [152,153,154]. HNPCC syndromes are caused by mutations of DNA mismatch repair genes (MMR) [155]. MMR is a DNA damage repair and signaling pathway that manages erroneous insertions or deletions of bases during DNA replication (S phase) and recombination (mitosis) [156]. Such a pathway is therefore particularly active in proliferating cells and tissues. By highlighting the biases raised by the extrapolation from micro-organisms to mammalians evoked above, it is noteworthy that many MMR genes found in yeasts do not exist in humans. Mutations of the MMR hMLH1, hMSH2, hMSH6 and hPMS2 genes are responsible for many forms of HNPCC [152,153,154,155]. HNPCC show a high susceptibility to colon cancer but also to endometrial, ovarian, stomach, small intestine, liver, upper urinary tract, brain, and skin cancers [153,157]. HNPCC are generally characterized by chemosensitivity and moderate radiosensitivity. However, some cases of severe radiosensitivity have been reported but always in the context of Turcot syndrome (TS), a pathology often associated with the mutation of hMSH2 [158]. Intestinal pathologies generally associated with mutations of the APC gene may also be associated with MMR gene mutations: familial recto-colic polyposis and attenuated forms of familial recto-colic polyposis, Gardner syndrome (GS) and Turcot syndrome [154,158]. The last two syndromes are associated with other tumors such as osteomas, fibroids, lipomas and thyroid or adrenal tumors for GS and central nervous system tumors for TS [154,158]. Although polyposis is generally associated with mutations of the APC gene, mutations of hMSH2 can also cause TS as mentioned above. Both GS and TS are associated with significant but moderate radiosensitivity. However, the biological functions of the APC gene are still unknown, but some studies have reported a role of APC in the regulation of mitosis microtubules, which cannot explain the radiosensitivity observed in quiescent cells derived from these syndromes [1,158].
The hMLH1, hMSH2, hMSH6, hPMS2 and APC proteins hold SQ/TQ domains, show cytoplasmic localization when mutated and can interact with ATM [159,160].
5. Diseases of Cell Cycle Checkpoint Control
5.1. A Lack of Control of the Cell Cycle Checkpoint, Another Requirement for Carcinogenesis?
In the frame of the most current models of carcinogenesis, including the initiation/promotion/progression model, one mutation in one cell or even one mutation in several cells does not necessarily lead to cell transformation and cancer [111]. An amplification of the number of cells holding mutations is therefore required to ensure the formation of a pre-tumor. Such amplification may occur when the cell cycle checkpoints are impaired. Let us review the major diseases of the cell cycle checkpoint control.
5.2. Overgrowth Syndromes
The PI3K kinase is composed of an 85 kDa regulatory subunit and a 110 kDa catalytic subunit. Among the numerous variants of these subunits, the p110α one, called PI3KCA, has been shown to be involved in the control of cellular proliferation. Some somatic mosaic mutations of the PI3KCA gene are associated with overgrowth syndromes called PI3KCA-related overgrowth spectrum (PROS) syndromes [161,162,163,164,165]. Some cases of cancers have been reported in the PROS patients [164,165]. All these syndromes are associated with overgrowth malformations in skin, vasculature, bones or brain tissues due to somatic mosaic heterozygous mutations leading to overactivity of the PI3K kinase [161,162,163,164,165]. ATM and PI3K kinases have been shown to interact and activate in response to genotoxic stress [161,166,167]. Recently, we have shown that PROS syndromes are associated with radiosensitivity and radiosusceptibility: some mutations of the PI3KCA gene lead to the over-expression of cytoplasmic forms that, as substrates of ATM, result in a delayed RIANS [161].
Proteus syndrome, which does not belong to the PROS syndromes family, is characterized by tissue overgrowth, and hyperplasia of multiple tissues may also be associated with high susceptibility to the development of tumors. Proteus syndrome is caused by somatic activating mutations of the AKT1 gene, which triggers activation of the PI3K-AKT pathway [168]. The pleckstrin homology domain of AKT binds to the cellular membrane via its affinity for PI molecules phosphorylated by PI3K, which stimulates cell proliferation and invasiveness. Cytoplasmic ATM was found to be an upstream activator of AKT1, and both proteins are involved in a common pathway with PI3K that promotes cell proliferation when altered or hyperactivated [169,170].
The PTEN protein is a phosphatase responsible of the dephosphorylation of PI molecules, which inhibits the PI3K-AKT signaling pathway described above [171,172]. In the case of PTEN mutations, the PI3K-AKT signaling pathway is activated, which stimulates cell proliferation and invasiveness [171,172,173,174,175]. Inherited mutations of the PTEN gene notably cause Cowden disease associated with a high risk of developing breast cancer [176]. The PTEN-mutated cells were found to be sensitive to radiation [177]. ATM is known to phosphorylate PTEN in the cytoplasm, which triggers its translocation from the cytoplasm to the nucleus in response to oxidative stress [178].
5.3. Mutations of the CHK1 and CHK2 Genes
Checkpoint kinases 1 and 2, namely CHK1 and CHK2, are serine/threonine kinases that coordinate cell cycle response to genotoxic stress and are generally activated by the phosphorylation of both ATM and ATR kinases [15,179,180]
CHK1 is particularly required for G2/M arrest in response to IR [15,180]. However, while CHK1 gene overexpression (as its non-phosphorylated form) has been reported in several tumor models, no germline CHK1 mutation has been detected in any cancer syndrome. Conversely, heritable mutations within the CHK1 C-terminal regulatory domain have been recently shown to cause female infertility in humans through the blockage of oocytes in their first mitosis [180].
Unlike CHK1, CHK2 phosphorylated by ATM is required for ensuring G1 arrest in response to IR [15,179]. Again, unlike CHK1, several CHK2 mutations have been observed in different types of cancers, including prostate, colon, lung, thyroid and mainly breast cancers. Hence, to date, CHK2 is considered as a tumor suppressor gene, a phosphorylation substrate of ATM [181]. Overexpression and cytoplasmic localization of CHK2 was observed in a large subset of tumors and are associated with genomic instability and high levels of DNA damage [182,183].
5.4. Mutations of the BRCA1, BRCA2, FANC Genes
While the BRCA1 and BRCA2 proteins have almost the same acronyms, they do not share identical sequences or domains [184]. The BRCA1 protein is a large protein (220 kDa), and two major structural domains have been identified: a RING domain in its N-terminal region, and a tandem of two BRCT (BRCA1 C-terminal) domains [185,186]. This last domain is found in many proteins involved in DNA damage repair (e.g., LIG4) and cell cycle checkpoint control (e.g., p53) [187]. Furthermore, BRCA1 holds 13 SQ and 3 TQ domains that are specifically phosphorylated by ATM and ATR kinases [19]. The phosphorylation of BRCA1 by ATM in response to IR leads to the formation of nuclear foci [188,189]. Except for the three identified domains, RING, BRCT and the SQ/TQ cluster, no other functional sites such as kinase, ligase, nuclease, etc., have been identified in the BRCA1 sequence [190]. The BRCA1 protein is localized both in the nucleus and cytoplasm and acts as a scaffold protein for a multitude of ATM phosphorylation substrates [188,189]. Homozygous mutations of BRCA1 are not viable in humans. Heterozygous mutations of BRCA1 lead to inherited breast cancer syndromes but are also seen in about 18% of ovarian cancers and represent a significant factor of risk for other cancers such as prostate cancers [191,192,193,194]. Mutations in the C-terminal BRCT domains of the BRCA1 protein result in cytoplasmic mislocalization [195,196,197].
BRCA2 has a molecular weight of 384 kDa and is a protein larger than BRCA1. BRCA2 contains BRC domains different from the BRCT domains observed in the BRCA1 sequence. Similar to BRCA1, the BRCA2 protein serves as a scaffold and has many protein partners, including the ATM kinase [191]. Similar to BRCA1, homozygous mutations of BRCA2 do not exist in humans since they cause lethality at the embryonic state. Heterozygous mutations of BRCA2 are the cause of inherited ovarian cancers and male breast cancers [110,190,198].
The BRCA1 and BRCA2 proteins are essential for the action of RAD51 and RAD52 in active HR in the G2/M phase [189]. Interactions between BRCA1 and MRE11 have also been described, reinforcing the hypothesis that BRCA1 may participate in both HR and NHR processes [199,200]. Most BRCA1 and BRCA2 mutations confer a moderate radiosensitivity in G1 comparable to that observed in the case of MRE11 mutations [201]. Some mutations of BRCA1 and BRCA2 are also associated with high chemosensitivities, particularly to alkylating agents such as cis-platinum [201,202]. However, some studies about the RI cascade of phosphorylations of ATM substrates show that BRCA1 and BRCA2 should be considered more as cell cycle checkpoint proteins than as DNA repair proteins since the kinetics of RI phosphorylation of BRCA1/2 proteins are slower than the molecular events involved in DNA damage recognition and repair [15].
The Fanconi anemia (FA) complementation group (FANC) gathers 14 FANC proteins that are involved in post-replication repair and in cell cycle checkpoint control [203,204]. Most of them interact with BRCA1/2 proteins and ATM (FANCD2 and BRCA2 are the same protein) [110,205]. Furthermore, such as the BRCA1/2 proteins, the FANC proteins have no active domain for ensuring a specific enzymatic function. Mutations of the FANC proteins cause Fanconi anemia (FA) syndrome that was first described by the Swiss pediatrician Guido Fanconi in 1927 [206,207,208]. FA is one of the major hereditary syndromes of spinal cord failure. It is often associated with congenital malformations (including microcephaly), growth defects (small size), skin disorders (café-au-lait spots) and generally progresses to aplasia or leukemia. The predisposition to FA-related cancer is not limited to lymphocytes, but also extends to breast cancer. Rather characterized by their chemosensitivity, cells from FA patients show low but significant radiosensitivity [206,209,210,211]. Although the FANC proteins hold numerous SQ/TQ domains, the phosphorylation of FANC by ATM has been described only for FANCD2/BRCA2 [191,209]. In addition, since nearly all the FANC proteins show cytoplasmic forms, whether mutated or not, mutations of the FANC gene may lead to a sequestration of ATM by FANC proteins. However, further experiments are needed to confirm this hypothesis.
5.5. Mutations of the RB1 and P53 Genes
The pRB protein, a product of the RB1 gene, acts as a negative regulator of the cell cycle, notably by blocking cells in G1 phase through its non-phosphorylated form. In proliferating cells, the cyclin-dependent kinase complexes phosphorylate pRB, which liberates the E2F transcription factor and favors the transition to S phase [212,213]. Heterozygous germline RB1 mutations cause retinoblastoma syndrome (RB), a rare pediatric disease associated with tumors in retina cells [214,215,216,217]. The pRB protein is also a phosphorylation substrate of the ATM kinase, and we have shown that skin fibroblasts from RB patients elicit moderate but significant radiosensitivity associated with delayed RIANS caused by the cytoplasmic overexpression of some mutated pRB proteins [24].
The p53 protein is the most documented of the human transcription actors and is involved in the cell cycle checkpoint and in some specific cellular death pathways [218,219,220]. Again, from studies about the RI cascade of phosphorylations of ATM substrates, p53 cannot be considered as a DNA damage repair protein since its activation appears later than the DNA damage recognition and repair step [15]. Similar to the pRB and BRCA1/2 proteins, p53 is phosphorylated by the ATM kinase in response to IR [15]. While homozygous mutations of the p53 gene are lethal in the embryonic state, heterozygous mutations of p53 cause Li-Fraumeni syndrome (LFS), associated with predisposition to rhabdomyosarcoma, but also to multiple cancers such as in muscles, breast, bones, and blood cancers [221,222]. Similarly to pRB, we have shown that skin fibroblasts from LFS patients elicit moderate but significant radiosensitivity associated with delayed RIANS caused by the cytoplasmic overexpression of some mutated p53 proteins [222].
6. Cancer Syndromes and the RI ATM Nucleoshuttling Model
Some cancer syndromes may be caused by mutations of genes whose protein products are directly involved either in DNA repair and signaling or in cell cycle checkpoint control. Such diseases therefore raise the question of the molecular mechanisms of carcinogenesis. Let us review the molecular and clinical features of the major ones.
6.1. Mutations of the NF1, NF2, TSC1 and TSC2 Genes
The NF1 gene encodes for the neurofibromin 1 protein, a GTPase-activating protein involved in neural development. Furthermore, neurofibromin was reported to modulate the Ras-dependent oncogenic pathways, which may favor abnormal proliferation [223]. However, considering individual specificities, the NF1 gene cannot be considered as directly involved in cell cycle checkpoint control such as CHK1 or CHK2. The heterozygous mutations of neurofibromin cause neurofibromatosis type 1 (NF1) syndrome associated with benign tumors along the peripheral and optic nerves and malignant tumors such as neurofibrosarcomas, astrocytomas, and rhabdomyosarcomas [224,225,226,227,228]. A study has shown that the neurofibromin 1 protein was a substrate of ATM kinase and that cells from NF1 patients elicit a moderate but significant radiosensitivity associated with delayed RIANS caused by the cytoplasmic overexpression of the mutated neurofibromin [13].
The NF2 gene encodes for the neurofibromin 2 protein (also called schwannomin or moesin–ezrin–radixin (merlin) protein), which is a cytoskeletal protein. Neurofibromin 2 is considered as a scaffold protein linking transmembrane receptors, actors of cell adhesion, small GTPases, mTOR- and PI3K/AKT-dependent pathways proteins [223,229]. Loss of function mutations or deletions in NF2 genes cause neurofibromatosis type 2 (NF2) syndrome associated with a multiple-tumor-forming disease of the nervous system and notably schwannomas, meningiomas and ependymomas [229,230]. Even if the clinical consequences of the mutations of NF2 gene strongly suggest that it is a tumor suppressor gene, the biological role of the merlin is not documented enough to consider it as directly involved in DNA damage repair and signaling and/or in the cell cycle checkpoint control. Preliminary experiments in our lab reveal that fibroblasts from NF2 patients show a moderate but significant radiosensitivity (N.F., personal communication). Although the merlin protein holds one TQ domain, no ATM phosphorylation of the merlin protein has been described yet. Conversely, the merlin protein was shown to be cytoplasmic in G0/G1 and mitosis but nuclear in S phase, through the mediation of its phosphorylation by AKT1 in serine 518, hence describing a cell-cycle-dependent nucleoshuttling [231]. Further experiments are needed to examine whether ATM and merlin proteins interact in cytoplasm in cells from NF2 patients.
The TSC1 and TSC2 genes encode for the hamartin [232,233] and the tuberin [232,234] proteins, respectively, that interact with a very large multiprotein complex called the tuberous sclerosis complex (TSC). Both proteins are involved in the regulation of cell growth control and the activity of the target of rapamycin (TOR) complex 1 (TORC1). However, their direct role in cell proliferation remains to be more documented [235,236,237,238,239]. Heterozygous mutations of either of the two TSC1 and TSC2 genes cause the TSC syndrome [240,241]. TSC is associated with high incidence of angiofibroma, astrocytoma, renal angiomyolipoma and lymphangioleiomyomatosis [232,242]. Recently, it was reported that fibroblasts from TSC patients show a moderate but significant radiosensitivity and that TSC and ATM dynamically interact in response to IR. In cells from TSC patients, hamartin was found overexpressed in cytoplasm and complexed with ATM, therefore causing a delayed RIANS [12].
6.2. Requirement of Both Impaired DNA Damage and Cell Cycle Checkpoints
Literature data and this review suggest that carcinogenesis and cell transformation require both misrepaired DNA damage that generates DNA sequence errors and impaired cell cycle checkpoint control that facilitates cell proliferation and therefore errors propagation. These two steps are consistent with the hypothesis of initiation and promotion steps proposed by several oncologists [111]. As evoked above, the cancer and radiosensitivity syndromes described in this review may be therefore classified into three categories (Figure 2):
Category 1: the cancer syndromes caused by mutations of genes directly involved in DNA damage recognition, repair and signaling pathway. Considering the importance of DNA damage recognition and repair in cell viability, these syndromes are generally very rare (prevalence of about 1/100,000), recessive and caused by hypomorphic mutations leading to a partial loss of the function of the gene. However, two subcategories can be considered according to the importance of the mutated gene and the associated radiosensitivity quantified by the cell survival at 2 Gy (SF2). Radiosensitivity can be either extreme (10% < SF2 < 30%) such as LIG4 syndrome or moderate (30% < SF2 < 60%) such as Artemis syndrome [4,5]. All these gene products are substrates of ATM and may localize in cytoplasm when mutated. The gene mutations may explain misrepaired DNA damage. However, how can the resulting sequence errors be propagated through impaired cell cycle checkpoints?
Category 2: the cancer syndromes caused by mutations of the genes directly involved in the cell cycle checkpoint control. These syndromes are more frequent than the first category (prevalence is higher than 1/40,000), are generally dominant and are caused by heterozygous mutations. One of the most representative examples of such syndromes is Li-Fraumeni syndrome (heterozygous p53 mutations). Their associated radiosensitivity is moderate but significant (30% < SF2 < 60%) [4,5]. Again, all these gene products are substrates of ATM and may localize in the cytoplasm when mutated. The gene mutations may explain the impairment of the cell cycle checkpoints that lead to the propagation of errors. However, how can the misrepaired DNA damage be generated with such gene mutations?
Category 3: the cancer syndromes caused by mutations of genes that are directly involved either in DNA damage recognition, repair and signaling pathway or in the cell cycle checkpoint control. The gene mutations that cause these syndromes may be homozygous, heterozygous or de novo, which explains why their prevalence can be lower than the syndromes from Category 2. One of the most representative examples of such syndromes are the PROS syndromes. Their associated radiosensitivity is moderate but significant (30% < SF2 < 60%) [4,5]. Again, all these gene products are substrates of ATM and may localize in the cytoplasm when mutated [12,13]. How can the misrepaired DNA damage be generated and propagated with such gene mutations?
Altogether, these categories raise the question of gene mutations that would lead to two concomitant but distinct molecular and cellular consequences, one in DNA damage recognition and repair and the other in cell cycle checkpoint control. Throughout the RI ATM nucleoshuttling (RIANS) model that is now very documented [20], let us propose a mechanistic model to integrate these three categories of syndromes in a unique explanation.
6.3. The ATM Nucleoshuttling Model: A Possible Explanation for Carcinogenesis?
While the upstream action of the ATM protein in the predominant DNA damage repair and signaling pathways, notably in NHEJ, suggests a nuclear sublocalization, some authors have reported that ATM is also a cytoplasmic protein [22,243,244,245]. By analyzing hundreds of fibroblast cell lines derived from cancer patients eliciting a large spectrum of post-radiotherapy radiosensitivity, it appears that IR triggers a drastic change in the subcellular localization of ATM. As evoked above, in quiescent cells, after exposure to IR, the cytoplasmic ATM dimers dissociate as ATM monomers and diffuse in the nucleus. Once in the nucleus, ATM monomers phosphorylate H2AX histone protein (γH2AX), which triggers the activation of NHEJ [20,21,22]. However, in the nucleus, ATM monomers also phosphorylate some other ATM substrates such as MRE11 (which limits the nuclease activity of the RAD50–MRE11–NBS1 complex evoked above) and CHK1 and CHK2 proteins (which trigger cell cycle arrest in G2/M and G1, respectively) [15,20]. Nevertheless, in cells that show moderate but significant radiosensitivity, RIANS is delayed by the overexpression of cytoplasmic substrates of ATM, which sequestrate the RI ATM monomers in the cytoplasm. Such a model was confirmed by both immunoblots and proximity ligation assays [20,21,246]. These ATM substrates, called X-proteins, are generally the proteins mutated specifically in the syndrome considered [5,8,18,20,21] (Figure 3). Hence, the RIANS model integrates the hypothesis that two distinct biological functions may be impaired as discussed in Section 1.3. A mutation of an X-protein may lead to: (1) the impairment of its intrinsic biological function as a single protein, and (2) the impairment of some biological functions caused by the X-protein as a cytoplasmic ATM substrate.
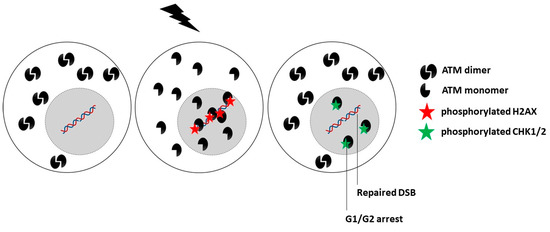
Figure 3.
Schematic representation of the RIANS model in radioresistant cells. A rapid RIANS leads to the ATM-dependent recognition of all the DSB induced by IR via the NHEJ pathway, their complete repair, and phosphorylation of the CHK proteins that induce G1/G2 arrest.
Hence, the RIANS model permits to revisit the three categories of cancer syndromes defined above by providing a relevant unified model.
Category 1: Two subcategories can be considered. In the first one, mutations of crucial genes directly involved in the DNA damage recognition, repair and signaling that do not result in an embryonic state (such as ATM and LIG4 mutations) produce a level of misrepaired DNA breaks and genomic instability that is so high that it also produces mutations in some other genes involved in the cell cycle checkpoint control. The spontaneous (p14; q11) chromosome exchanges currently observed in ATM-mutated cells are a representative example of cytogenetic events that may concern more than one gene [32,33,247]. In other terms, in this category of cancer syndromes, the initiation and promotion steps may occur concomitantly [4,18]. This is notably the case of ATM- and LIG4-mutations (Figure 4A and Table 1). In the second subcategory, the mutations of less crucial DNA damage recognition and repair genes may result in high cytoplasmic expression of the gene products (as X-proteins). Such expression of X-proteins may lead to delayed RIANS, which limits DSB recognition through incomplete γH2AX phosphorylation and G2/M and G1 arrests through incomplete CHK1 and CHK2 phosphorylation. Hence, in this subcategory, the mutated protein, as an impaired contributor of DNA damage recognition and repair, favors errors formation, while the mutated protein, as an X-protein, delays the RIANS, which favors their propagation via impaired phosphorylation of cell cycle checkpoint control proteins. This is notably the case of some XPD mutations (Figure 4B, Table 1).
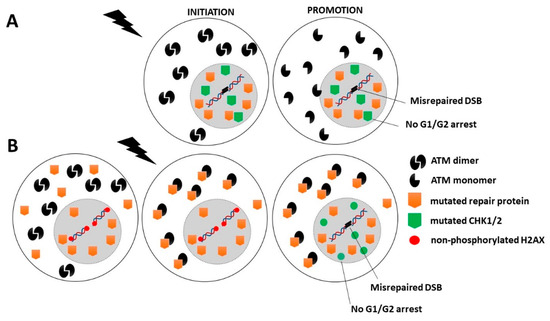
Figure 4.
Schematic representation of the RIANS model in cells from Category 1 cancer syndromes. (A) First subcategory: Mutated genes are crucial for DNA damage recognition and repair. In this case, the genomic instability is so high that some spontaneous mutations may also affect cell cycle checkpoint control. (B) Second subcategory: Mutated genes are involved in DNA damage recognition and repair but are not crucial. The encoded proteins are X-proteins. The RIANS is delayed by the cytoplasmic overexpression of X-proteins, and few ATM monomers enter in the nucleus. Consequently, CHK proteins are not phosphorylated, and therefore G1/G2 arrests are impaired.

Table 1.
Molecular and cellular features of the major syndromes associated with both cancer and individual radiosensitivity.
Category 2: Mutations of genes directly involved in the cell cycle checkpoint control are generally heterozygous mutations and are associated with a high expression of cytoplasmic forms of the protein. This is notably the case of PI3KCa [161], p53 [201,222], or pRB proteins [24]. These abundant cytoplasmic X-proteins contribute to sequestrate the ATM monomers and therefore lead to a decrease in the flux of ATM monomers entering in the nucleus. Consequently, less DSB are recognized by NHEJ, and the number of misrepaired DSB increases. Hence, in this category, the mutated protein, as an X-protein, delays the RIANS, which favors error formation, while the mutated protein, as an impaired contributor to cell cycle checkpoint control, favors their propagation (Figure 5A and Table 1).
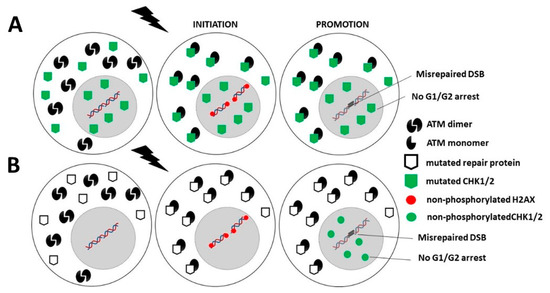
Figure 5.
Schematic representation of the RIANS model in cells from Category 2 and 3 cancer syndromes. (A). Category 2: Mutated genes are involved in cell cycle checkpoint control. The encoded proteins are X-proteins. The RIANS is delayed by the high cytoplasmic expression of X-proteins, and few ATM monomers enter into the nucleus. Consequently, few DSB are recognized by NHEJ and therefore may be misrepaired. (B). Category 3: Mutated genes are directly involved either in DNA damage recognition and repair or in the cell cycle checkpoint control. The encoded proteins are X-proteins. The RIANS is therefore delayed by the cytoplasmic overexpression of X-proteins, and few ATM monomers may enter into the nucleus. Consequently, few DSB are recognized by NHEJ and therefore may be misrepaired. Furthermore, CHK proteins are not phosphorylated, and therefore, G1/G2 arrests are impaired.
Category 3: Mutated genes that are directly involved either in DNA damage recognition and repair or in the cell cycle checkpoint control may also be associated with high cancer proneness if they are X-proteins. Their heterozygous mutations are responsible for their high expression in the cytoplasm. Thus, as described above, the resulting sequestration of RI ATM monomers contributes to reducing the flux of ATM monomers in the nucleus, to decreasing the DSB recognition by NHEJ and to increasing the number of misrepaired DSB. In addition, a delayed RIANS may also decrease the phosphorylation of CHK1 and CHK2 by ATM in the nucleus, which may impair the G2 and G1 arrests, respectively (Figure 5B, Table 1).
6.4. What Are the Limits of the Validity of the RIANS Model?
All the genes whose mutations that cause cancer and radiosensitivity syndromes show SQ/TQ domains: they have been identified or considered as phosphorylation ATM substrates and may present cytoplasmic forms, at least, when mutated, suggesting that the RIANS model may unify the three categories of syndromes defined above. However, the validity of our model should be discussed in three specific conditions of irradiation, at least: the influence of cell cycle, the effect of linear energy transfer (LET) and the influence of low dose, which all may act in cancer proneness and radiosensitivity.
In the frame of the RIANS model, all the DNA damage is supposed to be induced in the G0/G1 phase. In this specific cell cycle phase, the NHEJ repair pathway is predominant. In these conditions, the propagation of errors is mostly conditioned to the impairment of G1/S arrest to reach the G2/M phase. By contrast, the RIANS model is not relevant for the G2/M cells since ATM nucleoshuttling does not exist in this phase. Hence, the model proposed here may be completed with mechanisms describing the fate of the DNA damage when induced during the G2/M phase. In this phase, the HR repair pathway is predominant. More specifically, the role of HR to manage the DNA breaks generated by IR in the G2/M phase should be integrated into the RIANS model together with the HR/NHEJ balance that may be dependent on ATM but also on ATM substrates such as MRE11 or BRCA1 [49].
LET is an important factor influencing both cancer induction and radiosensitivity. The RIANS model has been shown to be relevant whatever the LET value. Particularly, the spatial and density distribution of the energy depositions that occur after irradiation is specific to each particle or rays. Notably, the spatial and density distribution of the energy deposition conditions the level of oxidative stress that causes RI ATM monomerization in the cytoplasm and RI DNA breaks in the nucleus. Hence, the ratio between the number of ATM monomers and that of RI DSB directly depends on the LET value and therefore on the corresponding clinical consequences [248]. However, further experiments are needed to verify whether RI cancer risk and radiosensitivity observed clinically may be predicted by in vitro experiments performed with RIANS biomarkers.
The fact that low doses of IR (namely lower than 0.5 Gy) may cause cancer is an important question that has been debated for several decades [249]. The RIANS model has been validated at low doses and provides a biological explanation for hormesis and hypersensitivity to low doses phenomena [250,251]. Furthermore, the RIANS biomarkers have already been validated with two scenarios of CT exams [252,253]. The ratio between the number of ATM monomers and that of RI DSB evoked above with the LET is also an important feature to predict the risk of low doses. For example, while the number of RI DSB is directly proportional to the dose, the number of ATM monomers depends on the amount of X-proteins present in the cytoplasm and therefore on the individual factor. Again, the model of cancer proneness and radiosensitivity proposed in this review must be verified and documented in different irradiation conditions involving low doses to better evaluate the risk of RI cancer and toxicity for each of the genetic syndromes reviewed here.
7. Conclusions
This review aimed to survey the major cancer and radiosensitivity syndromes. One of the first conclusions is that the proteins whose mutations are responsible for these syndromes are all phosphorylation substrates of ATM and may present cytoplasmic forms when mutated. The ATM kinase therefore appears at the crossroads of the molecular and cellular bases of cancer proneness and radiosensitivity.
The impairment of the ATM-dependent DNA damage recognition, signaling and repair may cause misrepaired DNA damage that may be either endogenous (e.g., spontaneous DNA breaks) or exogenous (e.g., from the environment). Their formation can be considered as an initiation step in carcinogenesis.
The impairment of the ATM-dependent cell cycle checkpoint control: impaired G2/M and/or G1 arrests may contribute to the propagation of the errors described above. This step can be considered as a promotion step in carcinogenesis.
While these two requirements involve two different functions that should be both impaired, our review shows that the major cancer and radiosensitivity syndromes are caused by one mutation in a single gene. In the frame of the RIANS model, a given protein may ensure its own intrinsic function but may also play additional biological role(s) as a cytoplasmic ATM substrate (called X-protein). Hence, whether spontaneously or after oxidative stress (such as exposure to IR), the flux of ATM monomers from the cytoplasm to the nucleus may be delayed by X-proteins, which may cause impairments of both recognition and repair of DSB via NHEJ and in cell cycle checkpoint control via a limited phosphorylation of CHK1 and/or CHK2 proteins. Such a model appears relevant for the three categories of the major cancer and radiosensitivity syndromes. However, further experiments are needed to better document and consolidate such hypotheses, especially in specific conditions of irradiation.
Author Contributions
The authors of this manuscript have contributed in the following manners: Conceptualization, N.F. and M.B.; data acquisition and methodology, L.E.N., E.B., M.L.F., E.L.R., J.A.-C.; J.R.-V., A.G., L.S., data analysis writing and original draft preparation, L.E.N., E.B., M.L.F. and N.F.; writing and editing, L.E.N., J.A.-C., M.B. and N.F.; project administration and funding acquisition, N.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Commissariat General à l’Investissement (Programmes Investissement d’avenir—INDIRA project), and the National Space Agency (CNES) (ICARE project).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Deschavanne, P.J.; Fertil, B. A review of human cell radiosensitivity in vitro. Int. J. Radiat. Oncol. Biol. Phys. 1996, 34, 251–266. [Google Scholar] [CrossRef] [PubMed]
- Arlett, C.F.; Harcourt, S.A. Survey of radiosensitivity in a variety of human cell strains. Cancer Res. 1980, 40, 926–932. [Google Scholar] [PubMed]
- Weichselbaum, R.R.; Nove, J.; Little, J.B. X-ray sensitivity of fifty-three human diploid fibroblast cell strains from patients with characterized genetic disorders. Cancer Res. 1980, 40, 920–925. [Google Scholar]
- Foray, N.; Bourguignon, M.; Hamada, N. Individual response to ionizing radiation. Mutat. Res. Rev. 2016, 770, 369–386. [Google Scholar] [CrossRef] [PubMed]
- El-Nachef, L.; Al-Choboq, J.; Restier-Verlet, J.; Granzotto, A.; Berthel, E.; Sonzogni, L.; Ferlazzo, M.L.; Bouchet, A.; Leblond, P.; Combemale, P.; et al. Human Radiosensitivity and Radiosusceptibility: What Are the Differences? Int. J. Mol. Sci. 2021, 22, 7158. [Google Scholar] [CrossRef]
- National Institutes of Health; National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0; US Department of Health and Human Sciences: Washington, DC, USA, 2010.
- Cox, J.D.; Stetz, J.; Pajak, T.F. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 1341–1346. [Google Scholar] [CrossRef]
- Granzotto, A.; Benadjaoud, M.A.; Vogin, G.; Devic, C.; Ferlazzo, M.L.; Bodgi, L.; Pereira, S.; Sonzogni, L.; Forcheron, F.; Viau, M.; et al. Influence of Nucleoshuttling of the ATM Protein in the Healthy Tissues Response to Radiation Therapy: Toward a Molecular Classification of Human Radiosensitivity. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 450–460. [Google Scholar] [CrossRef]
- Averbeck, D.; Candeias, S.; Chandna, S.; Foray, N.; Friedl, A.A.; Haghdoost, S.; Jeggo, P.A.; Lumniczky, K.; Paris, F.; Quintens, R.; et al. Establishing mechanisms affecting the individual response to ionizing radiation. Int. J. Radiat. Biol. 2020, 96, 297–323. [Google Scholar] [CrossRef]
- Iliakis, G. The role of DNA double strand breaks in ionizing radiation-induced killing of eukaryotic cells. BioEssays News Rev. Mol. Cell. Dev. Biol. 1991, 13, 641–648. [Google Scholar]
- Cornforth, M.N.; Bedford, J.S. High-resolution measurement of breaks in prematurely condensed chromosomes by differential staining. Chromosoma 1983, 88, 315–318. [Google Scholar] [CrossRef]
- Ferlazzo, M.L.; Bach-Tobdji, M.K.E.; Djerad, A.; Sonzogni, L.; Burlet, S.F.; Devic, C.; Granzotto, A.; Bodgi, L.; Djeffal-Kerrar, A.; Foray, N. Radiobiological characterization of tuberous sclerosis: A delay in the nucleo-shuttling of ATM may be responsible for radiosensitivity. Mol. Neurobiol. 2017, 55, 4973–4983. [Google Scholar] [CrossRef] [PubMed]
- Combemale, P.; Sonzogni, L.; Devic, C.; Bencokova, Z.; Ferlazzo, M.L.; Granzotto, A.; Burlet, S.F.; Pinson, S.; Amini-Adle, M.; Al-Choboq, J.; et al. Individual Response to Radiation of Individuals with Neurofibromatosis Type I: Role of the ATM Protein and Influence of Statins and Bisphosphonates. Mol. Neurobiol. 2022, 59, 556–573. [Google Scholar] [CrossRef] [PubMed]
- Goodhead, D.T.; Thacker, J.; Cox, R. Weiss Lecture. Effects of radiations of different qualities on cells: Molecular mechanisms of damage and repair. Int. J. Radiat. Biol. 1993, 63, 543–556. [Google Scholar] [CrossRef]
- Foray, N.; Marot, D.; Gabriel, A.; Randrianarison, V.; Carr, A.M.; Perricaudet, M.; Ashworth, A.; Jeggo, P. A subset of ATM- and ATR-dependent phosphorylation events requires the BRCA1 protein. EMBO J. 2003, 22, 2860–2871. [Google Scholar] [CrossRef] [PubMed]
- Berthel, E.; Ferlazzo, M.L.; Devic, C.; Bourguignon, M.; Foray, N. What does the History of Research on the Repair of DNA Double-Strand Breaks Tell Us?—A Comprehensive Review of Human Radiosensitivity. Int. J. Mol. Sci. 2019, 20, 5339. [Google Scholar] [CrossRef] [PubMed]
- Steel, G. Basic Clinical Radiobiology, 3rd ed.; Arnold Publishers: London, UK, 1993; p. 262. [Google Scholar]
- Le Reun, E.; Bodgi, L.; Granzotto, A.; Sonzogni, L.; Ferlazzo, M.L.; Al-Choboq, J.; El-Nachef, L.; Restier-Verlet, J.; Berthel, E.; Devic, C.; et al. Quantitative correlations between radiosensitivity biomarkers show that the ATM protein kinase is strongly involved in the radiotoxicities observed after radiotherapy. Int. J. Mol. Sci. 2022, 23, 10434. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.T.; Lim, D.S.; Canman, C.E.; Kastan, M.B. Substrate specificities and identification of putative substrates of ATM kinase family members. J. Biol. Chem. 1999, 274, 37538–37543. [Google Scholar] [CrossRef] [PubMed]
- Berthel, E.; Foray, N.; Ferlazzo, M.L. The Nucleoshuttling of the ATM Protein: A Unified Model to Describe the Individual Response to High- and Low-Dose of Radiation? Cancers 2019, 11, 7. [Google Scholar] [CrossRef]
- Bodgi, L.; Foray, N. The nucleo-shuttling of the ATM protein as a basis for a novel theory of radiation response: Resolution of the linear-quadratic model. Int. J. Radiat. Biol. 2016, 92, 117–131. [Google Scholar] [CrossRef]
- Paull, T.T. Mechanisms of ATM Activation. Annu. Rev. Biochem. 2015, 84, 711–738. [Google Scholar] [CrossRef]
- Ferlazzo, M.; Berthel, E.; Granzotto, A.; Devic, C.; Sonzogni, L.; Bachelet, J.T.; Pereira, S.; Bourguignon, M.; Sarasin, A.; Mezzina, M.; et al. Some mutations in the xeroderma pigmentosum D gene may lead to moderate but significant radiosensitivity associated with a delayed radiation-induced ATM nuclear localization. Int. J. Radiat. Biol. 2020, 96, 394–410. [Google Scholar] [CrossRef] [PubMed]
- Moulay Lakhdar, I.; Ferlazzo, M.L.; Al Choboq, J.; Berthel, E.; Sonzogni, L.; Devic, C.; Granzotto, A.; Thariat, J.; Foray, N. Fibroblasts from Retinoblastoma Patients Show Radiosensitivity Linked to Abnormal Localization of the ATM Protein. Curr. Eye Res. 2020, 46, 546–557. [Google Scholar] [CrossRef] [PubMed]
- Okkenhaug, K. Signaling by the phosphoinositide 3-kinase family in immune cells. Annu. Rev. Immunol. 2013, 31, 675–704. [Google Scholar] [CrossRef] [PubMed]
- Lobrich, M.; Jeggo, P.A. The two edges of the ATM sword: Co-operation between repair and checkpoint functions. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2005, 76, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.M.; Zhang, X. Roles of ATM and ATR in DNA double strand breaks and replication stress. Prog. Biophys. Mol. Biol. 2021, 163, 109–119. [Google Scholar] [CrossRef]
- Taylor, A.M.; Harnden, D.G.; Arlett, C.F.; Harcourt, S.A.; Lehmann, A.R.; Stevens, S.; Bridges, B.A. Ataxia telangiectasia: A human mutation with abnormal radiation sensitivity. Nature 1975, 258, 427–429. [Google Scholar] [CrossRef]
- Savitsky, K.; Bar-Shira, A.; Gilad, S.; Rotman, G.; Ziv, Y.; Vanagaite, L.; Tagle, D.A.; Smith, S.; Uziel, T.; Sfez, S.; et al. A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science 1995, 268, 1749–1753. [Google Scholar] [CrossRef]
- Syllaba, L.; Hennen, K. Contribution à l’indépendance de l’athétose double idiopathique et congénitale. Atteinte familiale, syndrôme dystrophique, signe du réseau vasculaire conjonctival, intégrité psychique. Rev. Neurol. 1926, 5, 541–562. [Google Scholar]
- Louis-Bar, M. Sur un syndrôme progressif comprenant des telangiectasies capillaires cutanées et conjonctivales symétriques, à disposition naevoïde et des troubles cérébelleux. Confin. Neurol. 1941, 4, 32–43. [Google Scholar]
- McKinnon, P.J. Ataxia-telangiectasia: An inherited disorder of ionizing-radiation sensitivity in man. Progress in the elucidation of the underlying biochemical defect. Hum. Genet. 1987, 75, 197–208. [Google Scholar] [CrossRef]
- Bundey, S. Clinical and genetic features of ataxia-telangiectasia. Int. J. Radiat. Biol. 1994, 66, S23–S29. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; Wang, Y.L. Ataxia-telangiectasia complicated with Hodgkin’s lymphoma: A case report. World J. Clin. Cases 2020, 8, 2387–2391. [Google Scholar] [CrossRef] [PubMed]
- Aurias, A. Ataxia-télangiectasie: Aspects cliniques épidémiologiques et génétiques. Med. Sci. 1994, 10, 957–961. [Google Scholar]
- Jorgensen, T.J.; Shiloh, Y. The ATM gene and the radiobiology of ataxia-telangiectasia. Int. J. Radiat. Biol. 1996, 69, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.M.; Byrd, P.J.; McConville, C.M.; Thacker, S. Genetic and cellular features of ataxia telangiectasia. Int. J. Radiat. Biol. 1994, 65, 65–70. [Google Scholar] [CrossRef]
- Painter, R.B.; Young, B.R. Radiosensitivity in ataxia-telangiectasia: A new explanation. Proc. Natl. Acad. Sci. USA 1980, 77, 7315–7317. [Google Scholar] [CrossRef]
- Houldsworth, J.; Lavin, M.F. Effect of ionizing radiation on DNA synthesis in ataxia telangiectasia cells. Nucleic Acids Res. 1980, 8, 3709–3720. [Google Scholar] [CrossRef]
- Rothblum-Oviatt, C.; Wright, J.; Lefton-Greif, M.A.; McGrath-Morrow, S.A.; Crawford, T.O.; Lederman, H.M. Ataxia telangiectasia: A review. Orphanet J. Rare Dis. 2016, 11, 159. [Google Scholar] [CrossRef]
- Savitsky, K.; Sfez, S.; Tagle, D.A.; Ziv, Y.; Sartiel, A.; Collins, F.S.; Shiloh, Y.; Rotman, G. The complete sequence of the coding region of the ATM gene reveals similarity to cell cycle regulators in different species. Hum. Mol. Genet. 1995, 4, 2025–2032. [Google Scholar] [CrossRef]
- Gilad, S.; Khosravi, R.; Shkedy, D.; Uziel, T.; Ziv, Y.; Savitsky, K.; Rotman, G.; Smith, S.; Chessa, L.; Jorgensen, T.J.; et al. Predominance of null mutations in ataxia-telangiectasia. Hum. Mol. Genet. 1996, 5, 433–439. [Google Scholar] [CrossRef]
- Taylor, A.M.; Metcalfe, J.A.; Thick, J.; Mak, Y.F. Leukemia and lymphoma in ataxia telangiectasia. Blood 1996, 87, 423–438. [Google Scholar] [CrossRef] [PubMed]
- McConville, C.M.; Stankovic, T.; Byrd, P.J.; McGuire, G.M.; Yao, Q.Y.; Lennox, G.G.; Taylor, M.R. Mutations associated with variant phenotypes in ataxia-telangiectasia. Am. J. Hum. Genet. 1996, 59, 320–330. [Google Scholar] [PubMed]
- Swift, M.; Morrell, D.; Massey, R.B.; Chase, C.L. Incidence of cancer in 161 families affected by ataxia-telangiectasia. N. Engl. J. Med. 1991, 325, 1831–1836. [Google Scholar] [CrossRef] [PubMed]
- Vorechovsky, I.; Rasio, D.; Luo, L.; Monaco, C.; Hammarstrom, L.; Webster, A.D.; Zaloudik, J.; Barbanti-Brodani, G.; James, M.; Russo, G.; et al. The ATM gene and susceptibility to breast cancer: Analysis of 38 breast tumors reveals no evidence for mutation. Cancer Res. 1996, 56, 2726–2732. [Google Scholar] [PubMed]
- Jerzak, K.J.; Mancuso, T.; Eisen, A. Ataxia-telangiectasia gene (ATM) mutation heterozygosity in breast cancer: A narrative review. Curr. Oncol. 2018, 25, e176–e180. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.; Duedal, S.; Kirner, J.; McGuffog, L.; Last, J.; Reiman, A.; Byrd, P.; Taylor, M.; Easton, D.F. Cancer risks and mortality in heterozygous ATM mutation carriers. J. Natl. Cancer Inst. 2005, 97, 813–822. [Google Scholar] [CrossRef]
- Joubert, A.; Zimmerman, K.M.; Bencokova, Z.; Gastaldo, J.; Rénier, W.; Chavaudra, N.; Favaudon, V.; Arlett, C.; Foray, N. DNA double-strand break repair defects in syndromes associated with acute radiation response: At least two different assays to predict intrinsic radiosensitivity? Int. J. Radiat. Biol. 2008, 84, 107–125. [Google Scholar] [CrossRef]
- DahlBerg, W.K.; Little, J.B. Response of dermal fibroblast cultures from patients with unusually severe responses to radiotherapy and from ataxia telangiectasia heterozygotes to fractionated radiation. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 1995, 1, 785–790. [Google Scholar]
- Kato, T.A.; Nagasawa, H.; Weil, M.M.; Little, J.B.; Bedford, J.S. Levels of gamma-H2AX Foci after low-dose-rate irradiation reveal a DNA DSB rejoining defect in cells from human ATM heterozygotes in two at families and in another apparently normal individual. Radiat. Res. 2006, 166, 443–453. [Google Scholar] [CrossRef]
- Tchirkov, A.; Bay, J.O.; Pernin, D.; Bignon, Y.J.; Rio, P.; Grancho, M.; Kwiatkowski, F.; Giollant, M.; Malet, P.; Verrelle, P. Detection of heterozygous carriers of the ataxia-telangiectasia (ATM) gene by G2 phase chromosomal radiosensitivity of peripheral blood lymphocytes. Hum. Genet. 1997, 101, 312–316. [Google Scholar] [CrossRef]
- Traven, A.; Heierhorst, J. SQ/TQ cluster domains: Concentrated ATM/ATR kinase phosphorylation site regions in DNA-damage-response proteins. BioEssays News Rev. Mol. Cell. Dev. Biol. 2005, 27, 397–407. [Google Scholar] [CrossRef] [PubMed]
- O’Driscoll, M.; Gennery, A.R.; Seidel, J.; Concannon, P.; Jeggo, P.A. An overview of three new disorders associated with genetic instability: LIG4 syndrome, RS-SCID and ATR-Seckel syndrome. DNA Repair 2004, 3, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- O’Driscoll, M.; Jeggo, P.A. Clinical impact of ATR checkpoint signalling failure in humans. Cell Cycle 2003, 2, 194–195. [Google Scholar] [CrossRef] [PubMed]
- O’Driscoll, M.; Ruiz-Perez, V.L.; Woods, C.G.; Jeggo, P.A.; Goodship, J.A. A splicing mutation affecting expression of ataxia-telangiectasia and Rad3-related protein (ATR) results in Seckel syndrome. Nat. Genet. 2003, 33, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Griffith, E.; Walker, S.; Martin, C.A.; Vagnarelli, P.; Stiff, T.; Vernay, B.; Al Sanna, N.; Saggar, A.; Hamel, B.; Earnshaw, W.C.; et al. Mutations in pericentrin cause Seckel syndrome with defective ATR-dependent DNA damage signaling. Nat. Genet. 2008, 40, 232–236. [Google Scholar] [CrossRef]
- Collis, S.J.; DeWeese, T.L.; Jeggo, P.A.; Parker, A.R. The life and death of DNA-PK. Oncogene 2005, 24, 949–961. [Google Scholar] [CrossRef]
- Jackson, S.P.; Jeggo, P.A. DNA double-strand break repair and V(D)J recombination: Involvement of DNA-PK. Trends Biochem. Sci. 1995, 20, 412–415. [Google Scholar] [CrossRef]
- Pastwa, E.; Blasiak, J. Non-homologous DNA end joining. Acta Biochim. Pol. 2003, 50, 891–908. [Google Scholar] [CrossRef]
- Rothkamm, K.; Lobrich, M. Evidence for a lack of DNA double-strand break repair in human cells exposed to very low x-ray doses. Proc. Natl. Acad. Sci. USA 2003, 100, 5057–5062. [Google Scholar] [CrossRef]
- Rogakou, E.P.; Pilch, D.R.; Orr, A.H.; Ivanova, V.S.; Bonner, W.M. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 1998, 273, 5858–5868. [Google Scholar] [CrossRef]
- Kemp, L.M.; Sedgwick, S.G.; Jeggo, P.A. X-ray sensitive mutants of Chinese hamster ovary cells defective in double-strand break rejoining. Mutat. Res. 1984, 132, 189–196. [Google Scholar] [CrossRef]
- Allalunis-Turner, M.J.; Barron, G.M.; Day, R.S., 3rd; Dobler, K.D.; Mirzayans, R. Isolation of two cell lines from a human malignant glioma specimen differing in sensitivity to radiation and chemotherapeutic drugs. Radiat. Res. 1993, 134, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Lees-Miller, S.P.; Godbout, R.; Chan, D.W.; Weinfeld, M.; Day, R.S., 3rd; Barron, G.M.; Allalunis-Turner, J. Absence of p350 subunit of DNA-activated protein kinase from a radiosensitive human cell line. Science 1995, 267, 1183–1185. [Google Scholar] [CrossRef] [PubMed]
- Van der Burg, M.; Ijspeert, H.; Verkaik, N.S.; Turul, T.; Wiegant, W.W.; Morotomi-Yano, K.; Mari, P.O.; Tezcan, I.; Chen, D.J.; Zdzienicka, M.Z.; et al. A DNA-PKcs mutation in a radiosensitive T-B- SCID patient inhibits Artemis activation and nonhomologous end-joining. J. Clin. Investig. 2009, 119, 91–98. [Google Scholar] [CrossRef]
- Van der Burg, M.; van Dongen, J.J.; van Gent, D.C. DNA-PKcs deficiency in human: Long predicted, finally found. Curr. Opin. Allergy Clin. Immunol. 2009, 9, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.; Dynan, W.S. Autoantibodies against DNA double-strand break repair proteins. Front. Biosci. 2001, 6, D1412–D1422. [Google Scholar] [CrossRef] [PubMed]
- Badie, C.; Iliakis, G.; Foray, N.; Alsbeih, G.; Pantellias, G.E.; Okayasu, R.; Cheong, N.; Russell, N.S.; Begg, A.C.; Arlett, C.F.; et al. Defective repair of DNA double-strand breaks and chromosome damage in fibroblasts from a radiosensitive leukemia patient. Cancer Res. 1995, 55, 1232–1234. [Google Scholar]
- O’Driscoll, M.; Cerosaletti, K.M.; Girard, P.M.; Dai, Y.; Stumm, M.; Kysela, B.; Hirsch, B.; Gennery, A.; Palmer, S.E.; Seidel, J.; et al. DNA ligase IV mutations identified in patients exhibiting developmental delay and immunodeficiency. Mol. Cell 2001, 8, 1175–1185. [Google Scholar] [CrossRef]
- Huang, M.; Dong, G.; Lu, X.; Xiao, F.; Zhou, Q.; Zhang, S. DNA ligase IV dificiency with elevated serum IgG levels suspected to have myelodysplastic syndrome: A case report. BMC Pediatr. 2022, 22, 588. [Google Scholar] [CrossRef]
- Gerasimou, P.; Koumas, L.; Miltiadous, A.; Kyprianou, I.; Chi, J.; Gavrielidou, R.; Socratous, E.; Loizou, L.; Papachhristodoulou, E.; Karaoli, E.; et al. The rare DNA ligase IV syndrome: A case report. Hum. Pathol. Case Rep. 2020, 22, 200442. [Google Scholar] [CrossRef]
- Fredette, M.E.; Lombardi, K.C.; Duker, A.L.; Buck, C.O.; Phornphutkul, C.; Bober, M.B.; Quintos, J.B. Novel Xrcc4 Mutations in an Infant with Microcephalic Primordial Dwarfism, Dilated Cardiomyopathy, Subclinical Hypothyroidism, and Early Death: Expanding the Phenotype of Xrcc4 Mutations. AACE Clin. Case Rep. 2020, 6, e1–e4. [Google Scholar] [CrossRef] [PubMed]
- Bee, L.; Nasca, A.; Zanolini, A.; Cendron, F.; d’Adamo, P.; Costa, R.; Lamperti, C.; Celotti, L.; Ghezzi, D.; Zeviani, M. A nonsense mutation of human XRCC4 is associated with adult-onset progressive encephalocardiomyopathy. EMBO Mol. Med. 2015, 7, 918–929. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, R.; Faqeih, E.; Ansari, S.; Abdel-Salam, G.; Al-Hassnan, Z.N.; Al-Shidi, T.; Alomar, R.; Sogaty, S.; Alkuraya, F.S. Genomic analysis of primordial dwarfism reveals novel disease genes. Genome Res. 2014, 24, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Luteijn, R.D.; Drexler, I.; Smith, G.L.; Lebbink, R.J.; Wiertz, E. Mutagenic repair of double-stranded DNA breaks in vaccinia virus genomes requires cellular DNA ligase IV activity in the cytosol. J. Gen. Virol. 2018, 99, 790–804. [Google Scholar] [CrossRef] [PubMed]
- Francis, D.B.; Kozlov, M.; Chavez, J.; Chu, J.; Malu, S.; Hanna, M.; Cortes, P. DNA Ligase IV regulates XRCC4 nuclear localization. DNA Repair 2014, 21, 36–42. [Google Scholar] [CrossRef]
- Fukuchi, M.; Wanotayan, R.; Liu, S.; Imamichi, S.; Sharma, M.K.; Matsumoto, Y. Lysine 271 but not lysine 210 of XRCC4 is required for the nuclear localization of XRCC4 and DNA ligase IV. Biochem. Biophys. Res. Commun. 2015, 461, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Li, S.; Zhang, X.; Wang, L.C.; Niewolik, D.; Schwarz, K.; Legerski, R.J.; Zandi, E.; Lieber, M.R. DNA-PKcs regulates a single-stranded DNA endonuclease activity of Artemis. DNA Repair 2010, 9, 429–437. [Google Scholar] [CrossRef]
- Goodarzi, A.A.; Yu, Y.; Riballo, E.; Douglas, P.; Walker, S.A.; Ye, R.; Harer, C.; Marchetti, C.; Morrice, N.; Jeggo, P.A.; et al. DNA-PK autophosphorylation facilitates Artemis endonuclease activity. EMBO J. 2006, 25, 3880–3889. [Google Scholar] [CrossRef]
- Moshous, D.; Callebaut, I.; de Chasseval, R.; Corneo, B.; Cavazzana-Calvo, M.; Le Deist, F.; Tezcan, I.; Sanal, O.; Bertrand, Y.; Philippe, N.; et al. Artemis, a novel DNA double-strand break repair/V(D)J recombination protein, is mutated in human severe combined immune deficiency. Cell 2001, 105, 177–186. [Google Scholar] [CrossRef]
- Riballo, E.; Kuhne, M.; Rief, N.; Doherty, A.; Smith, G.C.; Recio, M.J.; Reis, C.; Dahm, K.; Fricke, A.; Krempler, A.; et al. A pathway of double-strand break rejoining dependent upon ATM, Artemis, and proteins locating to gamma-H2AX foci. Mol. Cell 2004, 16, 715–724. [Google Scholar] [CrossRef]
- Wang, J.; Pluth, J.M.; Cooper, P.K.; Cowan, M.J.; Chen, D.J.; Yannone, S.M. Artemis deficiency confers a DNA double-strand break repair defect and Artemis phosphorylation status is altered by DNA damage and cell cycle progression. DNA Repair 2005, 4, 556–570. [Google Scholar] [CrossRef] [PubMed]
- Beucher, A.; Birraux, J.; Tchouandong, L.; Barton, O.; Shibata, A.; Conrad, S.; Goodarzi, A.A.; Krempler, A.; Jeggo, P.A.; Lobrich, M. ATM and Artemis promote homologous recombination of radiation-induced DNA double-strand breaks in G2. EMBO J. 2009, 28, 3413–3427. [Google Scholar] [CrossRef] [PubMed]
- Volk, T.; Pannicke, U.; Reisli, I.; Bulashevska, A.; Ritter, J.; Bjorkman, A.; Schaffer, A.A.; Fliegauf, M.; Sayar, E.H.; Salzer, U.; et al. DCLRE1C (ARTEMIS) mutations causing phenotypes ranging from atypical severe combined immunodeficiency to mere antibody deficiency. Hum. Mol. Genet. 2015, 24, 7361–7372. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.; Zhang, X.; Zheng, S.; Legerski, R.J. Artemis links ATM to G2/M checkpoint recovery via regulation of Cdk1-cyclin B. Mol. Cell. Biol. 2007, 27, 2625–2635. [Google Scholar] [CrossRef]
- Ahnesorg, P.; Smith, P.; Jackson, S.P. XLF Interacts with the XRCC4-DNA Ligase IV Complex to Promote DNA Nonhomologous End-Joining. Cell 2006, 124, 301–313. [Google Scholar] [CrossRef]
- Li, Y.; Chirgadze, D.Y.; Bolanos-Garcia, V.M.; Sibanda, B.L.; Davies, O.R.; Ahnesorg, P.; Jackson, S.P.; Blundell, T.L. Crystal structure of human XLF/Cernunnos reveals unexpected differences from XRCC4 with implications for NHEJ. EMBO J. 2008, 27, 290–300. [Google Scholar] [CrossRef]
- Riballo, E.; Woodbine, L.; Stiff, T.; Walker, S.A.; Goodarzi, A.A.; Jeggo, P.A. XLF-Cernunnos promotes DNA ligase IV-XRCC4 re-adenylation following ligation. Nucleic Acids Res. 2009, 37, 482–492. [Google Scholar] [CrossRef]
- Menon, V.; Povirk, L.F. XLF/Cernunnos: An important but puzzling participant in the nonhomologous end joining DNA repair pathway. DNA Repair 2017, 58, 29–37. [Google Scholar] [CrossRef]
- Yu, Y.; Mahaney, B.L.; Yano, K.; Ye, R.; Fang, S.; Douglas, P.; Chen, D.J.; Lees-Miller, S.P. DNA-PK and ATM phosphorylation sites in XLF/Cernunnos are not required for repair of DNA double strand breaks. DNA Repair 2008, 7, 1680–1692. [Google Scholar] [CrossRef]
- Liu, P.; Gan, W.; Guo, C.; Xie, A.; Gao, D.; Guo, J.; Zhang, J.; Willis, N.; Su, A.; Asara, J.M.; et al. Akt-mediated phosphorylation of XLF impairs non-homologous end-joining DNA repair. Mol. Cell 2015, 57, 648–661. [Google Scholar] [CrossRef]
- Stewart, G.S. Solving the RIDDLE of 53BP1 recruitment to sites of damage. Cell Cycle 2009, 8, 1532–1538. [Google Scholar] [CrossRef] [PubMed]
- Stewart, G.S.; Stankovic, T.; Byrd, P.J.; Wechsler, T.; Miller, E.S.; Huissoon, A.; Drayson, M.T.; West, S.C.; Elledge, S.J.; Taylor, A.M. RIDDLE immunodeficiency syndrome is linked to defects in 53BP1-mediated DNA damage signaling. Proc. Natl. Acad. Sci. USA 2007, 104, 16910–16915. [Google Scholar] [CrossRef] [PubMed]
- FitzGerald, J.E.; Grenon, M.; Lowndes, N.F. 53BP1: Function and mechanisms of focal recruitment. Biochem. Soc. Trans. 2009, 37, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Santagata, S.; Villa, A.; Sobacchi, C.; Cortes, P.; Vezzoni, P. The genetic and biochemical basis of Omenn syndrome. Immunol. Rev. 2000, 178, 64–74. [Google Scholar] [CrossRef]
- Villa, A.; Santagata, S.; Bozzi, F.; Imberti, L.; Notarangelo, L.D. Omenn syndrome: A disorder of Rag1 and Rag2 genes. J. Clin. Immunol. 1999, 19, 87–97. [Google Scholar] [CrossRef]
- De Villartay, J.P. V(D)J recombination deficiencies. Adv. Exp. Med. Biol. 2009, 650, 46–58. [Google Scholar]
- Sobacchi, C.; Marrella, V.; Rucci, F.; Vezzoni, P.; Villa, A. RAG-dependent primary immunodeficiencies. Hum. Mutat. 2006, 27, 1174–1184. [Google Scholar] [CrossRef]
- Gapud, E.J.; Lee, B.S.; Mahowald, G.K.; Bassing, C.H.; Sleckman, B.P. Repair of chromosomal RAG-mediated DNA breaks by mutant RAG proteins lacking phosphatidylinositol 3-like kinase consensus phosphorylation sites. J. Immunol. 2011, 187, 1826–1834. [Google Scholar] [CrossRef]
- Dudas, A.; Chovanec, M. DNA double-strand break repair by homologous recombination. Mutat. Res. 2004, 566, 131–167. [Google Scholar] [CrossRef]
- Stary, A. La recombinaison illégitime dans les cellules de mammifères. Med. Sci. 1994, 10, 986–994. [Google Scholar]
- Duesberg, P.H.; Zhou, R.P.; Goodrich, D. Cancer genes by illegitimate recombination. Ann. N. Y. Acad. Sci. 1989, 567, 259–273. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, S.D.; Bierne, H.; d’Alencon, E.; Vilette, D.; Petranovic, M.; Noirot, P.; Michel, B. Mechanisms of illegitimate recombination. Gene 1993, 135, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Kong, H.; Nei, M.; Ma, H. Origins and evolution of the recA/RAD51 gene family: Evidence for ancient gene duplication and endosymbiotic gene transfer. Proc. Natl. Acad. Sci. USA 2006, 103, 10328–10333. [Google Scholar] [CrossRef]
- Symington, L.S.; Holloman, W.K. Resolving resolvases: The final act? Mol. Cell 2008, 32, 603–604. [Google Scholar] [CrossRef] [PubMed]
- Dardalhon, M.; Nohturfft, A.; Meniel, V.; Averbeck, D. Repair of DNA double-strand breaks induced in Saccharomyces cerevisiae using different gamma-ray dose-rates: A pulsed-field gel electrophoresis analysis. Int. J. Radiat. Biol. 1994, 65, 307–314. [Google Scholar] [CrossRef]
- Yamagata, K.; Kato, J.; Shimamoto, A.; Goto, M.; Furuichi, Y.; Ikeda, H. Bloom’s and Werner’s syndrome genes suppress hyperrecombination in yeast sgs1 mutant: Implication for genomic instability in human diseases. Proc. Natl. Acad. Sci. USA 1998, 95, 8733–8738. [Google Scholar] [CrossRef]
- Cousineau, I.; Belmaaza, A. BRCA1 haploinsufficiency, but not heterozygosity for a BRCA1-truncating mutation, deregulates homologous recombination. Cell Cycle 2007, 6, 962–971. [Google Scholar] [CrossRef]
- Howlett, N.G.; Taniguchi, T.; Olson, S.; Cox, B.; Waisfisz, Q.; de Die-Smulders, C.; Persky, N.; Grompe, M.; Joenje, H.; Pals, G.; et al. Biallelic inactivation of BRCA2 in Fanconi anemia. Science 2002, 297, 606–609. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Dong, Z.; Zhong, Q.; Chen, P.L. The Nijmegen breakage syndrome protein is essential for Mre11 phosphorylation upon DNA damage. J. Biol. Chem. 1999, 274, 19513–19516. [Google Scholar] [CrossRef]
- Trujillo, K.M.; Yuan, S.S.; Lee, E.Y.; Sung, P. Nuclease activities in a complex of human recombination and DNA repair factors Rad50, Mre11, and p95. J. Biol. Chem. 1998, 273, 21447–21450. [Google Scholar] [CrossRef] [PubMed]
- Maser, R.S.; Monsen, K.J.; Nelms, B.E.; Petrini, J.H. hMre11 and hRad50 nuclear foci are induced during the normal cellular response to DNA double-strand breaks. Mol. Cell. Biol. 1997, 17, 6087–6096. [Google Scholar] [CrossRef] [PubMed]
- Weemaes, C.M.; Hustinx, T.W.; Scheres, J.M.; van Munster, P.J.; Bakkeren, J.A.; Taalman, R.D. A new chromosomal instability disorder: The Nijmegen breakage syndrome. Acta Paediatr. Scand. 1981, 70, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Taalman, R.D.; Hustinx, T.W.; Weemaes, C.M.; Seemanova, E.; Schmidt, A.; Passarge, E.; Scheres, J.M. Further delineation of the Nijmegen breakage syndrome. Am. J. Med. Genet. 1989, 32, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Taalman, R.D.; Jaspers, N.G.; Scheres, J.M.; de Wit, J.; Hustinx, T.W. Hypersensitivity to ionizing radiation, in vitro, in a new chromosomal breakage disorder, the Nijmegen Breakage Syndrome. Mutat. Res. 1983, 112, 23–32. [Google Scholar] [CrossRef]
- Antoccia, A.; Ricordy, R.; Maraschio, P.; Prudente, S.; Tanzarella, C. Chromosomal sensitivity to clastogenic agents and cell cycle perturbations in Nijmegen breakage syndrome lymphoblastoid cell lines. Int. J. Radiat. Biol. 1997, 71, 41–49. [Google Scholar] [CrossRef]
- Jaspers, N.G.; Gatti, R.A.; Baan, C.; Linssen, P.C.; Bootsma, D. Genetic complementation analysis of ataxia telangiectasia and Nijmegen breakage syndrome: A survey of 50 patients. Cytogenet. Genome Res. 1988, 49, 259–263. [Google Scholar] [CrossRef]
- Wegner, R.D.; Metzger, M.; Hanefeld, F.; Jaspers, N.G.; Baan, C.; Magdorf, K.; Kunze, J.; Sperling, K. A new chromosomal instability disorder confirmed by complementation studies. Clin. Genet. 1988, 33, 20–32. [Google Scholar] [CrossRef]
- Stumm, M.; Gatti, R.A.; Reis, A.; Udar, N.; Chrzanowska, K.; Seemanova, E.; Sperling, K.; Wegner, R.D. The ataxia-telangiectasia-variant genes 1 and 2 are distinct from the ataxia-telangiectasia gene on chromosome 11q23.1. Am. J. Hum. Genet. 1995, 57, 960–962. [Google Scholar]
- Komatsu, K.; Matsuura, S.; Tauchi, H.; Endo, S.; Kodama, S.; Smeets, D.; Weemaes, C.; Oshimura, M. The gene for Nijmegen breakage syndrome (V2) is not located on chromosome 11. Am. J. Hum. Genet. 1996, 58, 885–888. [Google Scholar]
- Matsuura, S.; Royba, E.; Akutsu, S.N.; Yanagihara, H.; Ochiai, H.; Kudo, Y.; Tashiro, S.; Miyamoto, T. Analysis of individual differences in radiosensitivity using genome editing. Ann. ICRP 2016, 45, S290–S296. [Google Scholar] [CrossRef] [PubMed]
- Warren, C.; Pavletich, N.P. Structure of the human ATM kinase and mechanism of Nbs1 binding. Elife 2022, 11, e74218. [Google Scholar] [CrossRef] [PubMed]
- Desai-Mehta, A.; Cerosaletti, K.M.; Concannon, P. Distinct functional domains of nibrin mediate Mre11 binding, focus formation, and nuclear localization. Mol. Cell. Biol. 2001, 21, 2184–2191. [Google Scholar] [CrossRef] [PubMed]
- Stewart, G.S.; Maser, R.S.; Stankovic, T.; Bressan, D.A.; Kaplan, M.I.; Jaspers, N.G.; Raams, A.; Byrd, P.J.; Petrini, J.H.; Taylor, A.M. The DNA double-strand break repair gene hMRE11 is mutated in individuals with an ataxia-telangiectasia-like disorder. Cell 1999, 99, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Alsbeih, G.; Al-Hadyan, K.; Al-Harbi, N. Assessment of carriers’ frequency of a novel MRE11 mutation responsible for the rare ataxia telangiectasia-like disorder. Genet. Test. 2008, 12, 387–389. [Google Scholar] [CrossRef]
- Fernet, M.; Gribaa, M.; Salih, M.A.; Seidahmed, M.Z.; Hall, J.; Koenig, M. Identification and functional consequences of a novel MRE11 mutation affecting 10 Saudi Arabian patients with the ataxia telangiectasia-like disorder. Hum. Mol. Genet. 2005, 14, 307–318. [Google Scholar] [CrossRef]
- Delia, D.; Piane, M.; Buscemi, G.; Savio, C.; Palmeri, S.; Lulli, P.; Carlessi, L.; Fontanella, E.; Chessa, L. MRE11 mutations and impaired ATM-dependent responses in an Italian family with ataxia-telangiectasia-like disorder. Hum. Mol. Genet. 2004, 13, 2155–2163. [Google Scholar] [CrossRef]
- Waltes, R.; Kalb, R.; Gatei, M.; Kijas, A.W.; Stumm, M.; Sobeck, A.; Wieland, B.; Varon, R.; Lerenthal, Y.; Lavin, M.F.; et al. Human RAD50 deficiency in a Nijmegen breakage syndrome-like disorder. Am. J. Hum. Genet. 2009, 84, 605–616. [Google Scholar] [CrossRef]
- Gatei, M.; Jakob, B.; Chen, P.; Kijas, A.W.; Becherel, O.J.; Gueven, N.; Birrell, G.; Lee, J.H.; Paull, T.T.; Lerenthal, Y.; et al. ATM protein-dependent phosphorylation of Rad50 protein regulates DNA repair and cell cycle control. J. Biol. Chem. 2011, 286, 31542–31556. [Google Scholar] [CrossRef]
- Gatei, M.; Young, D.; Cerosaletti, K.M.; Desai-Mehta, A.; Spring, K.; Kozlov, S.; Lavin, M.F.; Gatti, R.A.; Concannon, P.; Khanna, K. ATM-dependent phosphorylation of nibrin in response to radiation exposure. Nat. Genet. 2000, 25, 115–119. [Google Scholar] [CrossRef]
- Frankenberg-Schwager, M.; Becker, M.; Garg, I.; Pralle, E.; Wolf, H.; Frankenberg, D. The role of nonhomologous DNA end joining, conservative homologous recombination, and single-strand annealing in the cell cycle-dependent repair of DNA double-strand breaks induced by H(2)O(2) in mammalian cells. Radiat. Res. 2008, 170, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Wang, S.; Jiao, B.; Yuan, D.; Dai, D.; Wang, L.; Xu, K.; Wang, X. Gene cloning and seamless site-directed mutagenesis using single-strand annealing (SSA). Appl. Microbiol. Biotechnol. 2018, 102, 10119–10126. [Google Scholar] [CrossRef] [PubMed]
- Maynard, S.; Schurman, S.H.; Harboe, C.; de Souza-Pinto, N.C.; Bohr, V.A. Base excision repair of oxidative DNA damage and association with cancer and aging. Carcinogenesis 2009, 30, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.M., 3rd; Bohr, V.A. The mechanics of base excision repair, and its relationship to aging and disease. DNA Repair 2007, 6, 544–559. [Google Scholar] [CrossRef] [PubMed]
- Tew, W.P.; Lacchetti, C.; Kohn, E.C. Poly(ADP-Ribose) Polymerase Inhibitors in the Management of Ovarian Cancer: ASCO Guideline Rapid Recommendation Update. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2022, 40, 3878–3881. [Google Scholar] [CrossRef] [PubMed]
- Petit, C.; Sancar, A. Nucleotide excision repair: From E. coli to man. Biochimie 1999, 81, 15–25. [Google Scholar] [CrossRef]
- Lambert, W.C.; Gagna, C.E.; Lambert, M.W. Xeroderma pigmentosum: Its overlap with trichothiodystrophy, Cockayne syndrome and other progeroid syndromes. Adv. Exp. Med. Biol. 2008, 637, 128–137. [Google Scholar]
- Lehmann, A.R. DNA repair-deficient diseases, xeroderma pigmentosum, Cockayne syndrome and trichothiodystrophy. Biochimie 2003, 85, 1101–1111. [Google Scholar] [CrossRef]
- Kraemer, K.H.; Patronas, N.J.; Schiffmann, R.; Brooks, B.P.; Tamura, D.; DiGiovanna, J.J. Xeroderma pigmentosum, trichothiodystrophy and Cockayne syndrome: A complex genotype-phenotype relationship. Neuroscience 2007, 145, 1388–1396. [Google Scholar] [CrossRef]
- Lehmann, A.R. The xeroderma pigmentosum group D (XPD) gene: One gene, two functions, three diseases. Genes Dev. 2001, 15, 15–23. [Google Scholar] [CrossRef]
- Chu, W.K.; Hickson, I.D. RecQ helicases: Multifunctional genome caretakers. Nat. Rev. Cancer 2009, 9, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, K.J.; Woo, L.L.; Ellis, N.A. Homologous recombination and maintenance of genome integrity: Cancer and aging through the prism of human RecQ helicases. Mech. Ageing Dev. 2008, 129, 425–440. [Google Scholar] [CrossRef] [PubMed]
- Muftuoglu, M.; Oshima, J.; von Kobbe, C.; Cheng, W.H.; Leistritz, D.F.; Bohr, V.A. The clinical characteristics of Werner syndrome: Molecular and biochemical diagnosis. Hum. Genet. 2008, 124, 369–377. [Google Scholar] [CrossRef]
- Amor-Gueret, M. Bloom syndrome, genomic instability and cancer: The SOS-like hypothesis. Cancer Lett. 2006, 236, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ozgenc, A.; Loeb, L.A. Werner Syndrome, aging and cancer. Genome Dyn. 2006, 1, 206–217. [Google Scholar]
- Pichierri, P.; Rosselli, F.; Franchitto, A. Werner’s syndrome protein is phosphorylated in an ATR/ATM-dependent manner following replication arrest and DNA damage induced during the S phase of the cell cycle. Oncogene 2003, 22, 1491–1500. [Google Scholar] [CrossRef]
- Ababou, M.; Dutertre, S.; Lecluse, Y.; Onclercq, R.; Chatton, B.; Amor-Gueret, M. ATM-dependent phosphorylation and accumulation of endogenous BLM protein in response to ionizing radiation. Oncogene 2000, 19, 5955–5963. [Google Scholar] [CrossRef]
- Matsumoto, T.; Shimamoto, A.; Goto, M.; Furuichi, Y. Impaired nuclear localization of defective DNA helicases in Werner’s syndrome. Nat. Genet. 1997, 16, 335–336. [Google Scholar] [CrossRef]
- Kaneko, H.; Orii, K.O.; Matsui, E.; Shimozawa, N.; Fukao, T.; Matsumoto, T.; Shimamoto, A.; Furuichi, Y.; Hayakawa, S.; Kasahara, K.; et al. BLM (the causative gene of Bloom syndrome) protein translocation into the nucleus by a nuclear localization signal. Biochem. Biophys. Res. Commun. 1997, 240, 348–353. [Google Scholar] [CrossRef]
- Bellizzi, A.M.; Frankel, W.L. Colorectal cancer due to deficiency in DNA mismatch repair function: A review. Adv. Anat. Pathol. 2009, 16, 405–417. [Google Scholar] [CrossRef]
- Lynch, P.M. The hMSH2 and hMLH1 genes in hereditary nonpolyposis colorectal cancer. Surg. Oncol. Clin. 2009, 18, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Foulkes, W.D. A tale of four syndromes: Familial adenomatous polyposis, Gardner syndrome, attenuated APC and Turcot syndrome. QJM 1995, 88, 853–863. [Google Scholar] [PubMed]
- Zhang, Y.; Rohde, L.H.; Wu, H. Involvement of nucleotide excision and mismatch repair mechanisms in double strand break repair. Curr. Genom. 2009, 10, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Li, G.M. Mechanisms and functions of DNA mismatch repair. Cell Res. 2008, 18, 85–98. [Google Scholar] [CrossRef]
- Biller, L.H.; Syngal, S.; Yurgelun, M.B. Recent advances in Lynch syndrome. Fam. Cancer 2019, 18, 211–219. [Google Scholar] [CrossRef]
- Lebrun, C.; Olschwang, S.; Jeannin, S.; Vandenbos, F.; Sobol, H.; Frenay, M. Turcot syndrome confirmed with molecular analysis. Eur. J. Neurol. 2007, 14, 470–472. [Google Scholar] [CrossRef]
- Neufeld, K.L. Nuclear APC. Adv. Exp. Med. Biol. 2009, 656, 13–29. [Google Scholar]
- Yan, S.; Sorrell, M.; Berman, Z. Functional interplay between ATM/ATR-mediated DNA damage response and DNA repair pathways in oxidative stress. Cell. Mol. Life Sci. 2014, 71, 3951–3967. [Google Scholar] [CrossRef]
- Bachelet, J.T.; Granzotto, A.; Ferlazzo, M.; Sonzogni, L.; Berthel, E.; Devic, D.; Foray, N. First Radiobiological Characterization of Skin and Bone Cells from A Patient Suffering from the PI3KCA-Related Overgrowth Spectrum (PROS) Syndrome. Arch. Med. Clin. Case Rep. 2020, 4, 1052–1066. [Google Scholar] [CrossRef]
- Keppler-Noreuil, K.M.; Rios, J.J.; Parker, V.E.; Semple, R.K.; Lindhurst, M.J.; Sapp, J.C.; Alomari, A.; Ezaki, M.; Dobyns, W.; Biesecker, L.G. PIK3CA-related overgrowth spectrum (PROS): Diagnostic and testing eligibility criteria, differential diagnosis, and evaluation. Am. J. Med. Genet. A 2015, 167, 287–295. [Google Scholar] [CrossRef]
- Martinez-Lopez, A.; Blasco-Morente, G.; Perez-Lopez, I.; Herrera-Garcia, J.D.; Luque-Valenzuela, M.; Sanchez-Cano, D.; Lopez-Gutierrez, J.C.; Ruiz-Villaverde, R.; Tercedor-Sanchez, J. CLOVES syndrome: Review of a PIK3CA-related overgrowth spectrum (PROS). Clin. Genet. 2017, 91, 14–21. [Google Scholar] [CrossRef]
- Venot, Q.; Canaud, G. PIK3CA-related overgrowth syndrome (PROS). Nephrol. Ther. 2017, 13 (Suppl. 1), S155–S156. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, B.; Henderson-Jackson, E.; Cruse, C.W.; Brohl, A.S. PIK3CA-Related Overgrowth Syndrome (PROS) and Angiosarcoma: A Case Report. Eplasty 2020, 20, ic6. [Google Scholar]
- Toulamy, M.; Rodemann, H.P. Phosphatidylinositol 3-kinase/Akt signaling as a key mediator of tumor cell responsiveness to radiation. Semin. Cancer Biol. 2015, 35, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Irarrazabal, C.E.; Burg, M.B.; Ward, S.G.; Ferraris, J.D. Phosphatidylinositol 3-kinase mediates activation of ATM by high NaCl and by ionizing radiation: Role in osmoprotective transcriptional regulation. Proc. Natl. Acad. Sci. USA 2006, 103, 8882–8887. [Google Scholar] [CrossRef] [PubMed]
- Keppler-Noreuil, K.M.; Parker, V.E.; Darling, T.N.; Martinez-Agosto, J.A. Somatic overgrowth disorders of the PI3K/AKT/mTOR pathway & therapeutic strategies. Am. J. Med. Genet. C Semin. Med. Genet. 2016, 172, 402–421. [Google Scholar] [PubMed]
- Halaby, M.J.; Hibma, J.C.; He, J.; Yang, D.Q. ATM protein kinase mediates full activation of Akt and regulates glucose transporter 4 translocation by insulin in muscle cells. Cell. Signal. 2008, 20, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Ersahin, T.; Tuncbag, N.; Cetin-Atalay, R. The PI3K/AKT/mTOR interactive pathway. Mol. Biosyst. 2015, 11, 1946–1954. [Google Scholar] [CrossRef] [PubMed]
- Biesecker, L.G.; Happle, R.; Mulliken, J.B.; Weksberg, R.; Graham, J.M., Jr.; Viljoen, D.L.; Cohen, M.M., Jr. Proteus syndrome: Diagnostic criteria, differential diagnosis, and patient evaluation. Am. J. Med. Genet. 1999, 84, 389–395. [Google Scholar] [CrossRef]
- Cohen, M.M., Jr. Proteus syndrome review: Molecular, clinical, and pathologic features. Clin. Genet. 2014, 85, 111–119. [Google Scholar] [CrossRef]
- Eng, C.; Thiele, H.; Zhou, X.P.; Gorlin, R.J.; Hennekam, R.C.; Winter, R.M. PTEN mutations and proteus syndrome. Lancet 2001, 358, 2079–2080. [Google Scholar] [CrossRef] [PubMed]
- Ram, S.P.; Noor, A.R. Neonatal proteus syndrome. Am. J. Med. Genet. 1993, 47, 303. [Google Scholar] [CrossRef] [PubMed]
- Sapp, J.C.; Buser, A.; Burton-Akright, J.; Keppler-Noreuil, K.M.; Biesecker, L.G. A dyadic genotype-phenotype approach to diagnostic criteria for Proteus syndrome. Am. J. Med. Genet. C Semin. Med. Genet. 2019, 181, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Magana, M.; Landeta-Sa, A.P.; Lopez-Flores, Y. Cowden Disease: A Review. Am. J. Dermatopathol. 2022, 44, 705–717. [Google Scholar] [CrossRef] [PubMed]
- Hanna, C.; Dunne, V.L.; Walker, S.M.; Butterworth, K.T.; McCabe, N.; Waugh, D.J.J.; Kennedy, R.D.; Prise, K.M. ATM Kinase Inhibition Preferentially Sensitises PTEN-Deficient Prostate Tumour Cells to Ionising Radiation. Cancers 2020, 13, 79. [Google Scholar] [CrossRef]
- Chen, J.H.; Zhang, P.; Chen, W.D.; Li, D.D.; Wu, X.Q.; Deng, R.; Jiao, L.; Li, X.; Ji, J.; Feng, G.K.; et al. ATM-mediated PTEN phosphorylation promotes PTEN nuclear translocation and autophagy in response to DNA-damaging agents in cancer cells. Autophagy 2015, 11, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Mustofa, M.K.; Tanoue, Y.; Tateishi, C.; Vaziri, C.; Tateishi, S. Roles of Chk2/CHEK2 in guarding against environmentally induced DNA damage and replication-stress. Environ. Mol. Mutagen. 2020, 61, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, D.A. When more is less: Heritable gain-of-function chk1 mutations impair human fertility. FEBS J. 2022. [Google Scholar] [CrossRef]
- Kleiblova, P.; Stolarova, L.; Krizova, K.; Lhota, F.; Hojny, J.; Zemankova, P.; Havranek, O.; Vocka, M.; Cerna, M.; Lhotova, K.; et al. Identification of deleterious germline CHEK2 mutations and their association with breast and ovarian cancer. Int. J. Cancer 2019, 145, 1782–1797. [Google Scholar] [CrossRef]
- Cai, Z.; Chehab, N.H.; Pavletich, N.P. Structure and activation mechanism of the CHK2 DNA damage checkpoint kinase. Mol. Cell 2009, 35, 818–829. [Google Scholar] [CrossRef]
- Staalesen, V.; Falck, J.; Geisler, S.; Bartkova, J.; Borresen-Dale, A.L.; Lukas, J.; Lillehaug, J.R.; Bartek, J.; Lonning, P.E. Alternative splicing and mutation status of CHEK2 in stage III breast cancer. Oncogene 2004, 23, 8535–8544. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.E.; Ngo, B.; Modrek, A.S.; Lee, W.H. Targeting tumor suppressor networks for cancer therapeutics. Curr. Drug Targets 2014, 15, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.C.; Songyang, Z. BRCT domains: Phosphopeptide binding and signaling modules. Front Biosci. 2008, 13, 5905–5915. [Google Scholar] [CrossRef]
- Silver, D.P.; Livingston, D.M. Mechanisms of BRCA1 tumor suppression. Cancer Discov. 2012, 2, 679–684. [Google Scholar] [CrossRef]
- Koonin, E.V.; Altschul, S.F.; Bork, P. BRCA1 protein products … Functional motifs. Nat. Genet. 1996, 13, 266–268. [Google Scholar] [CrossRef] [PubMed]
- Scully, R.; Chen, J.; Ochs, R.L.; Keegan, K.; Hoekstra, M.; Feunteun, J.; Livingston, D.M. Dynamic changes of BRCA1 subnuclear location and phosphorylation state are initiated by DNA damage. Cell 1997, 90, 425–435. [Google Scholar] [CrossRef]
- Scully, R.; Chen, J.; Plug, A.; Xiao, Y.; Weaver, D.; Feunteun, J.; Ashley, T.; Livingston, D.M. Association of BRCA1 with Rad51 in mitotic and meiotic cells. Cell 1997, 88, 265–275. [Google Scholar] [CrossRef]
- Clark, S.L.; Rodriguez, A.M.; Snyder, R.R.; Hankins, G.D.; Boehning, D. Structure-Function Of The Tumor Suppressor BRCA1. Comput. Struct. Biotechnol. J. 2012, 1, e201204005. [Google Scholar] [CrossRef]
- Scully, R.; Puget, N. BRCA1 and BRCA2 in hereditary breast cancer. Biochimie 2002, 84, 95–102. [Google Scholar] [CrossRef]
- Wang, F.; Fang, Q.; Ge, Z.; Yu, N.; Xu, S.; Fan, X. Common BRCA1 and BRCA2 mutations in breast cancer families: A meta-analysis from systematic review. Mol. Biol. Rep. 2012, 39, 2109–2118. [Google Scholar] [CrossRef]
- Cavanagh, H.; Rogers, K.M. The role of BRCA1 and BRCA2 mutations in prostate, pancreatic and stomach cancers. Hered. Cancer Clin. Pract. 2015, 13, 16. [Google Scholar] [CrossRef] [PubMed]
- Feunteun, J. A paradox and three egnimas about the role of BRCA1 in breast and ovarian cancers. J. Soc. Biol. 2004, 198, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.A.; Au, W.W.; Henderson, B.R. Cytoplasmic mislocalization of BRCA1 caused by cancer-associated mutations in the BRCT domain. Exp. Cell Res. 2004, 293, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Drikos, I.; Boutou, E.; Kastritis, P.L.; Vorgias, C.E. BRCA1-BRCT Mutations Alter the Subcellular Localization of BRCA1 In Vitro. Anticancer Res. 2021, 41, 2953–2962. [Google Scholar] [CrossRef] [PubMed]
- Vincent, A.; Berthel, E.; Dacheux, E.; Magnard, C.; Venezia, N.L. BRCA1 affects protein phosphatase 6 signalling through its interaction with ANKRD28. Biochem. J. 2016, 473, 949–960. [Google Scholar] [CrossRef] [PubMed]
- Ramus, S.J.; Gayther, S.A. The contribution of BRCA1 and BRCA2 to ovarian cancer. Mol. Oncol. 2009, 3, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Chen, C.F.; Li, S.; Chen, Y.; Wang, C.C.; Xiao, J.; Chen, P.L.; Sharp, Z.D.; Lee, W.H. Association of BRCA1 with the hRad50-hMre11-p95 complex and the DNA damage response. Science 1999, 285, 747–750. [Google Scholar] [CrossRef]
- Mohiuddin, M.; Rahman, M.M.; Sale, J.E.; Pearson, C.E. CtIP-BRCA1 complex and MRE11 maintain replication forks in the presence of chain terminating nucleoside analogs. Nucleic Acids Res. 2019, 47, 2966–2980. [Google Scholar] [CrossRef]
- Foray, N.; Randrianarison, V.; Marot, D.; Perricaudet, M.; Lenoir, G.; Feunteun, J. Gamma-rays-induced death of human cells carrying mutations of BRCA1 or BRCA2. Oncogene 1999, 18, 7334–7342. [Google Scholar] [CrossRef][Green Version]
- Van Haaften, C.; van Eendenburg, J.; Boot, A.; Corver, W.E.; Haans, L.; van Wezel, T.; Trimbos, J.B. Chemosensitivity of BRCA1-Mutated Ovarian Cancer Cells and Established Cytotoxic Agents. Int. J. Gynecol. Cancer 2017, 27, 1571–1578. [Google Scholar] [CrossRef]
- Grompe, M.; D’Andrea, A. Fanconi anemia and DNA repair. Hum. Mol. Genet. 2001, 10, 2253–2259. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Nebert, D.W.; Bruford, E.A.; Thompson, D.C.; Joenje, H.; Vasiliou, V. Update of the human and mouse Fanconi anemia genes. Hum. Genom. 2015, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Andreassen, P.R.; D’Andrea, A.D. Functional interaction of monoubiquitinated FANCD2 and BRCA2/FANCD1 in chromatin. Mol. Cell. Biol. 2004, 24, 5850–5862. [Google Scholar] [CrossRef] [PubMed]
- Alter, B.P. Radiosensitivity in Fanconi’s anemia patients. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2002, 62, 345–347. [Google Scholar] [CrossRef] [PubMed]
- Buchwald, M.; Moustacchi, E. Is Fanconi anemia caused by a defect in the processing of DNA damage? Mutat. Res. 1998, 408, 75–90. [Google Scholar] [CrossRef]
- Lobitz, S.; Velleuer, E. Guido Fanconi (1892–1979): A jack of all trades. Nat. Rev. Cancer 2006, 6, 893–898. [Google Scholar] [CrossRef]
- Papadopoulo, D.; Moustacchi, E. Fanconi anemia: Genes and function(s) revisited. Med. Sci. 2005, 21, 730–736. [Google Scholar]
- Haddy, N.; Andriamboavonjy, T.; Paoletti, C.; Dondon, M.G.; Mousannif, A.; Shamsaldin, A.; Doyon, F.; Labbe, M.; Robert, C.; Avril, M.F.; et al. Thyroid adenomas and carcinomas following radiotherapy for a hemangioma during infancy. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2009, 93, 377–382. [Google Scholar] [CrossRef]
- Fang, C.B.; Wu, H.T.; Zhang, M.L.; Liu, J.; Zhang, G.J. Fanconi Anemia Pathway: Mechanisms of Breast Cancer Predisposition Development and Potential Therapeutic Targets. Front. Cell Dev. Biol. 2020, 8, 160. [Google Scholar] [CrossRef]
- Pickering, M.T.; Kowalik, T.F. Rb inactivation leads to E2F1-mediated DNA double-strand break accumulation. Oncogene 2006, 25, 746–755. [Google Scholar] [CrossRef]
- Yen, A.; Coder, D.; Varvayanis, S. Concentration of RB protein in nucleus vs. cytoplasm is stable as phosphorylation of RB changes during the cell cycle and differentiation. Eur. J. Cell Biol. 1997, 72, 159–165. [Google Scholar]
- Chaussade, A.; Millot, G.; Wells, C.; Brisse, H.; Lae, M.; Savignoni, A.; Desjardins, L.; Dendale, R.; Doz, F.; Aerts, I.; et al. Correlation between RB1germline mutations and second primary malignancies in hereditary retinoblastoma patients treated with external beam radiotherapy. Eur. J. Med. Genet. 2019, 62, 217–223. [Google Scholar] [CrossRef]
- Lin, W.; Cao, J.; Liu, J.; Beshiri, M.L.; Fujiwara, Y.; Francis, J.; Cherniack, A.D.; Geisen, C.; Blair, L.P.; Zou, M.R.; et al. Loss of the retinoblastoma binding protein 2 (RBP2) histone demethylase suppresses tumorigenesis in mice lacking Rb1 or Men1. Proc. Natl. Acad. Sci. USA 2011, 108, 13379–13386. [Google Scholar] [CrossRef]
- Yun, J.; Li, Y.; Xu, C.T.; Pan, B.R. Epidemiology and Rb1 gene of retinoblastoma. Int. J. Ophthalmol. 2011, 4, 103–109. [Google Scholar]
- Zhang, L.; Jia, R.; Zhao, J.; Fan, J.; Zhou, Y.; Han, B.; Song, X.; Wu, L.; Zhang, H.; Song, H.; et al. Novel mutations in the RB1 gene from Chinese families with a history of retinoblastoma. Tumor Biol. 2015, 36, 2409–2420. [Google Scholar] [CrossRef]
- Vousden, K.H.; Lane, D.P. p53 in health and disease. Nat. Rev. Mol. Cell Biol. 2007, 8, 275–283. [Google Scholar] [CrossRef]
- Huang, L.C.; Clarkin, K.C.; Wahl, G.M. Sensitivity and selectivity of the DNA damage sensor responsible for activating p53-dependent G1 arrest. Proc. Natl. Acad. Sci. USA 1996, 93, 4827–4832. [Google Scholar] [CrossRef]
- Kabacik, S.; Ortega-Molina, A.; Efeyan, A.; Finnon, P.; Bouffler, S.; Serrano, M.; Badie, C. A minimally invasive assay for individual assessment of the ATM/CHEK2/p53 pathway activity. Cell Cycle 2011, 10, 1152–1161. [Google Scholar] [CrossRef]
- Le, A.N.; Harton, J.; Desai, H.; Powers, J.; Zelley, K.; Bradbury, A.R.; Nathanson, K.L.; Shah, P.D.; Doucette, A.; Freedman, G.M.; et al. Frequency of radiation-induced malignancies post-adjuvant radiotherapy for breast cancer in patients with Li-Fraumeni syndrome. Breast Cancer Res. Treat. 2020, 181, 181–188. [Google Scholar] [CrossRef]
- Thariat, J.; Chevalier, F.; Orbach, D.; Ollivier, L.; Marcy, P.Y.; Corradini, N.; Beddok, A.; Foray, N.; Bougeard, G. Avoidance or adaptation of radiotherapy in patients with cancer with Li-Fraumeni and heritable TP53-related cancer syndromes. Lancet Oncol. 2021, 22, e562–e574. [Google Scholar] [CrossRef]
- Yohay, K.H. The genetic and molecular pathogenesis of NF1 and NF2. Semin. Pediatr. Neurol. 2006, 13, 21–26. [Google Scholar] [CrossRef]
- Ferner, R.E. Neurofibromatosis 1 and neurofibromatosis 2: A twenty first century perspective. Lancet Neurol. 2007, 6, 340–351. [Google Scholar] [CrossRef]
- Zhu, Y.; Parada, L.F. The molecular and genetic basis of neurological tumours. Nat. Rev. Cancer 2002, 2, 616–626. [Google Scholar] [CrossRef]
- Easton, D.F.; Ponder, M.A.; Huson, S.M.; Ponder, B.A. An analysis of variation in expression of neurofibromatosis (NF) type 1 (NF1): Evidence for modifying genes. Am. J. Hum. Genet. 1993, 53, 305–313. [Google Scholar]
- Sabbagh, A.; Pasmant, E.; Laurendeau, I.; Parfait, B.; Barbarot, S.; Guillot, B.; Combemale, P.; Ferkal, S.; Vidaud, M.; Aubourg, P.; et al. Unravelling the genetic basis of variable clinical expression in neurofibromatosis 1. Hum. Mol. Genet. 2009, 18, 2768–2778. [Google Scholar] [CrossRef]
- Campian, J.; Gutmann, D.H. CNS Tumors in Neurofibromatosis. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 2378–2385. [Google Scholar] [CrossRef]
- Petrilli, A.M.; Fernandez-Valle, C. Role of Merlin/NF2 inactivation in tumor biology. Oncogene 2016, 35, 537–548. [Google Scholar] [CrossRef]
- Bachir, S.; Shah, S.; Shapiro, S.; Koehler, A.; Mahammedi, A.; Samy, R.N.; Zuccarello, M.; Schorry, E.; Sengupta, S. Neurofibromatosis Type 2 (NF2) and the Implications for Vestibular Schwannoma and Meningioma Pathogenesis. Int. J. Mol. Sci. 2021, 22, 690. [Google Scholar] [CrossRef]
- Lu, J.; Zou, J.; Wu, H.; Cai, L. Compensative shuttling of merlin to phosphorylation on serine 518 in vestibular schwannoma. Laryngoscope 2008, 118, 169–174. [Google Scholar] [CrossRef]
- Napolioni, V.; Curatolo, P. Genetics and molecular biology of tuberous sclerosis complex. Curr. Genom. 2008, 9, 475–487. [Google Scholar] [CrossRef]
- Van Slegtenhorst, M.; de Hoogt, R.; Hermans, C.; Nellist, M.; Janssen, B.; Verhoef, S.; Lindhout, D.; van den Ouweland, A.; Halley, D.; Young, J.; et al. Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science 1997, 277, 805–808. [Google Scholar] [CrossRef] [PubMed]
- European Chromosome 16 Tuberous Sclerosis Consortium. Identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell 1993, 75, 1305–1315. [Google Scholar] [CrossRef] [PubMed]
- Hoogeveen-Westerveld, M.; Ekong, R.; Povey, S.; Karbassi, I.; Batish, S.D.; den Dunnen, J.T.; van Eeghen, A.; Thiele, E.; Mayer, K.; Dies, K.; et al. Functional assessment of TSC1 missense variants identified in individuals with tuberous sclerosis complex. Hum. Mutat. 2012, 33, 476–479. [Google Scholar] [CrossRef]
- Hoogeveen-Westerveld, M.; Ekong, R.; Povey, S.; Mayer, K.; Lannoy, N.; Elmslie, F.; Bebin, M.; Dies, K.; Thompson, C.; Sparagana, S.P.; et al. Functional assessment of TSC2 variants identified in individuals with tuberous sclerosis complex. Hum. Mutat. 2013, 34, 167–175. [Google Scholar] [CrossRef]
- Hoogeveen-Westerveld, M.; Wentink, M.; van den Heuvel, D.; Mozaffari, M.; Ekong, R.; Povey, S.; den Dunnen, J.T.; Metcalfe, K.; Vallee, S.; Krueger, S.; et al. Functional assessment of variants in the TSC1 and TSC2 genes identified in individuals with Tuberous Sclerosis Complex. Hum. Mutat. 2011, 32, 424–435. [Google Scholar] [CrossRef]
- Qin, J.; Wang, Z.; Hoogeveen-Westerveld, M.; Shen, G.; Gong, W.; Nellist, M.; Xu, W. Structural Basis of the Interaction between Tuberous Sclerosis Complex 1 (TSC1) and Tre2-Bub2-Cdc16 Domain Family Member 7 (TBC1D7). J. Biol. Chem. 2016, 291, 8591–8601. [Google Scholar] [CrossRef]
- Overwater, I.E.; Swenker, R.; van der Ende, E.L.; Hanemaayer, K.B.; Hoogeveen-Westerveld, M.; van Eeghen, A.M.; Lequin, M.H.; van den Ouweland, A.M.; Moll, H.A.; Nellist, M.; et al. Genotype and brain pathology phenotype in children with tuberous sclerosis complex. Eur. J. Hum. Genet. 2016, 24, 1688–1695. [Google Scholar] [CrossRef]
- Baskin, H.J., Jr. The pathogenesis and imaging of the tuberous sclerosis complex. Pediatr. Radiol. 2008, 38, 936–952. [Google Scholar] [CrossRef]
- Sahin, M.; Henske, E.P.; Manning, B.D.; Ess, K.C.; Bissler, J.J.; Klann, E.; Kwiatkowski, D.J.; Roberds, S.L.; Silva, A.J.; Hillaire-Clarke, C.S.; et al. Advances and Future Directions for Tuberous Sclerosis Complex Research: Recommendations From the 2015 Strategic Planning Conference. Pediatr. Neurol. 2016, 60, 1–12. [Google Scholar] [CrossRef]
- Henske, E.P.; Jozwiak, S.; Kingswood, J.C.; Sampson, J.R.; Thiele, E.A. Tuberous sclerosis complex. Nat. Rev. Dis. Prim. 2016, 2, 16035. [Google Scholar] [CrossRef]
- Oka, A.; Takashima, S. Expression of the ataxia-telangiectasia gene (ATM) product in human cerebellar neurons during development. Neurosci. Lett. 1998, 252, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.S.; Kirsch, D.G.; Canman, C.E.; Ahn, J.H.; Ziv, Y.; Newman, L.S.; Darnell, R.B.; Shiloh, Y.; Kastan, M.B. ATM binds to beta-adaptin in cytoplasmic vesicles. Proc. Natl. Acad. Sci. USA 1998, 95, 10146–10151. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Kozlov, S.; Lavin, M.F.; Person, M.D.; Paull, T.T. ATM activation by oxidative stress. Science 2010, 330, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Al-Choboq, J.; Ferlazzo, M.L.; Sonzogni, L.; Granzotto, A.; El-Nachef, L.; Maalouf, M.; Berthel, E.; Foray, N. Usher Syndrome Belongs to the Genetic Diseases Associated with Radiosensitivity: Influence of the ATM Protein Kinase. Int. J. Mol. Sci. 2022, 23, 1570. [Google Scholar] [CrossRef]
- Meyn, M.S. High spontaneous intrachromosomal recombination rates in ataxia-telangiectasia. Science 1993, 260, 1327–1330. [Google Scholar] [CrossRef]
- Maalouf, M.; Granzotto, A.; Devic, C.; Bodgi, L.; Ferlazzo, M.; Peaucelle, C.; Bajard, M.; Giraud, J.Y.; Balosso, J.; Herault, J.; et al. Influence of Linear Energy Transfer on the Nucleo-shuttling of the ATM Protein: A Novel Biological Interpretation Relevant for Particles and Radiation. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 709–718. [Google Scholar] [CrossRef]
- Brenner, D.J.; Doll, R.; Goodhead, D.T.; Hall, E.J.; Land, C.E.; Little, J.B.; Lubin, J.H.; Preston, D.L.; Preston, R.J.; Puskin, J.S.; et al. Cancer risks attributable to low doses of ionizing radiation: Assessing what we really know. Proc. Natl. Acad. Sci. USA 2003, 100, 13761–13766. [Google Scholar] [CrossRef]
- Devic, C.; Ferlazzo, M.L.; Berthel, E.; Foray, N. Influence of Individual Radiosensitivity on the Hormesis Phenomenon: Toward a Mechanistic Explanation Based on the Nucleoshuttling of ATM Protein. Dose-Response A Publ. Int. Hormesis Soc. 2020, 18, 1559325820913784. [Google Scholar] [CrossRef]
- Devic, C.; Ferlazzo, M.L.; Foray, N. Influence of Individual Radiosensitivity on the Adaptive Response Phenomenon: Toward a Mechanistic Explanation Based on the Nucleo-Shuttling of ATM Protein. Dose-Response A Publ. Int. Hormesis Soc. 2018, 16, 1559325818789836. [Google Scholar] [CrossRef]
- Devic, C.; Bodgi, L.; Sonzogni, L.; Pilleul, F.; Ribot, H.; Charry, C.; Le Moigne, F.; Paul, D.; Carbillet, F.; Munier, M.; et al. Influence of cellular models and individual factor in the biological response to chest CT scan exams. Eur. Radiol. Exp. 2022, 6, 14. [Google Scholar] [CrossRef]
- Devic, C.; Bodgi, L.; Sonzogni, L.; Pilleul, F.; Ribot, H.; de Charry, C.; Le Moigne, F.; Paul, D.; Carbillet, F.; Munier, M.; et al. Influence of cellular models and individual factor in the biological response to head CT scan exams. Eur. Radiol. Exp. 2022, 6, 17. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).