Cancer Immunotherapy: The Checkpoint between Chronic Colitis and Colorectal Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Inflammatory Bowel Disease
Mouse Models
3. Inflammatory Mediators in Inflammatory Bowel Disease
3.1. Immune Cells in Inflammatory Bowel Disease
3.2. Cytokines and Chemokines
3.3. Toll-Like Receptors
3.4. The Nuclear Factor Kappa B
3.5. Matrix Metalloproteinases
3.6. Cyclooxygenase-2
3.7. Myeloid Differentiation Primary Response 88
4. Inflammation and Cancer
4.1. Colitis Associated Cancer/Colorectal Cancer
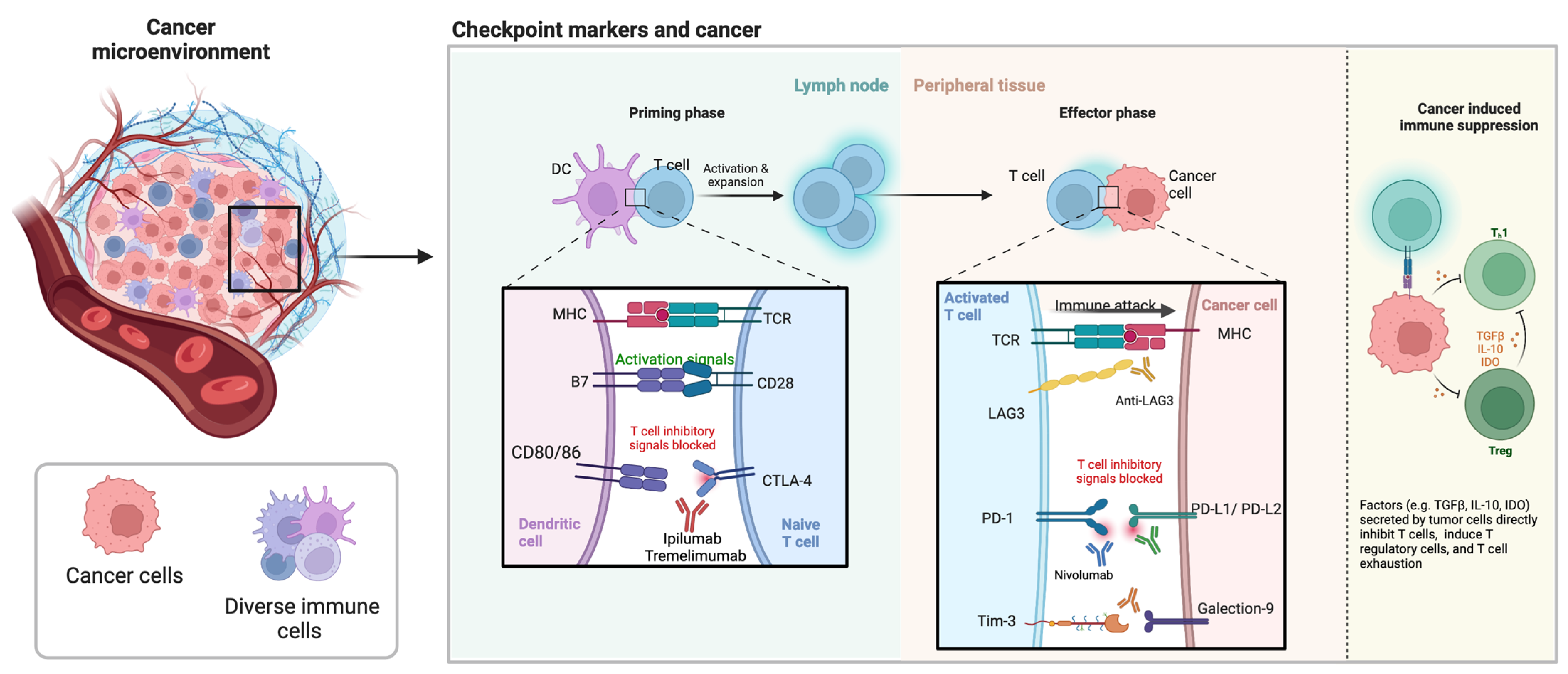
4.2. Cancer Microenvironment
5. Checkpoint Markers
5.1. Role of Checkpoint Inhibitors
5.2. Stimulatory Checkpoint Molecules
6. Challenges and Gaps
7. Conclusions and Future Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Matricon, J.; Barnich, N.; Ardid, D. Immunopathogenesis of inflammatory bowel disease. Self/Nonself 2010, 1, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, B.A.; Gokhale, R.; Cho, J.H. Clinical aspects and pathophysiology of inflammatory bowel disease. Clin. Microbiol. Rev. 2002, 15, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Triantafillidis, J.K.; Merikas, E.; Georgopoulos, F. Current and emerging drugs for the treatment of inflammatory bowel disease. Drug Des. Dev. Ther. 2011, 5, 185–210. [Google Scholar] [CrossRef] [PubMed]
- Axelrad, J.E.; Lichtiger, S.; Yajnik, V. Inflammatory bowel disease and cancer: The role of inflammation, immunosuppression, and cancer treatment. World J. Gastroenterol. 2016, 22, 4794–4801. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef]
- McCarthy, E.F. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop. J. 2006, 26, 154–158. [Google Scholar]
- Říhová, B.; Šťastný, M. History of Immuno-therapy—From Coley Toxins to Check-points of the Immune Reaction. Klin. Onkol. 2015, 28 (Suppl. S4), 4S8-14. [Google Scholar] [CrossRef]
- Farkona, S.; Diamandis, E.P.; Blasutig, I.M. Cancer immunotherapy: The beginning of the end of cancer? BMC Med. 2016, 14, 73. [Google Scholar] [CrossRef]
- Agarwal, M.B. Is cancer chemotherapy dying? Asian J. Transfus. Sci. 2016, 10, S1–S7. [Google Scholar] [CrossRef]
- Apostolopoulos, V.; Lofthouse, S.A.; Popovski, V.; Chelvanayagam, G.; Sandrin, M.S.; McKenzie, I.F. Peptide mimics of a tumor antigen induce functional cytotoxic T cells. Nat. Biotechnol. 1998, 16, 276–280. [Google Scholar] [CrossRef]
- Apostolopoulos, V.; McKenzie, I.F.; Pietersz, G.A. Breast cancer immunotherapy: Current status and future prospects. Immunol. Cell Biol. 1996, 74, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Pietersz, G.A.; Pouniotis, D.S.; Apostolopoulos, V. Design of peptide-based vaccines for cancer. Curr. Med. Chem. 2006, 13, 1591–1607. [Google Scholar] [CrossRef] [PubMed]
- Cebon, J. Perspective: Cancer vaccines in the era of immune checkpoint blockade. Mamm. Genome 2018, 29, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Korman, A.J.; Peggs, K.S.; Allison, J.P. Checkpoint blockade in cancer immunotherapy. Adv. Immunol. 2006, 90, 297–339. [Google Scholar] [CrossRef] [PubMed]
- Barriga, V.; Kuol, N.; Nurgali, K.; Apostolopoulos, V. The Complex Interaction between the Tumor Micro-Environment and Immune Checkpoints in Breast Cancer. Cancers 2019, 11, 1205. [Google Scholar] [CrossRef] [PubMed]
- Gun, S.Y.; Lee, S.W.L.; Sieow, J.L.; Wong, S.C. Targeting immune cells for cancer therapy. Redox Biol. 2019, 25, 101174. [Google Scholar] [CrossRef]
- Koury, J.; Lucero, M.; Cato, C.; Chang, L.; Geiger, J.; Henry, D.; Hernandez, J.; Hung, F.; Kaur, P.; Teskey, G.; et al. Immunotherapies: Exploiting the Immune System for Cancer Treatment. J. Immunol. Res. 2018, 2018, 9585614. [Google Scholar] [CrossRef]
- Kuol, N.; Stojanovska, L.; Nurgali, K.; Apostolopoulos, V. The mechanisms tumor cells utilize to evade the host’s immune system. Maturitas 2017, 105, 8–15. [Google Scholar] [CrossRef]
- Kuol, N.; Stojanovska, L.; Nurgali, K.; Apostolopoulos, V. PD-1/PD-L1 in disease. Immunotherapy 2018, 10, 149–160. [Google Scholar] [CrossRef]
- Kuol, N.; Yan, X.; Barriga, V.; Karakkat, J.; Vassilaros, S.; Fyssas, I.; Tsimpanis, A.; Fraser, S.; Nurgali, K.; Apostolopoulos, V. Pilot Study: Immune Checkpoints Polymorphisms in Greek Primary Breast Cancer Patients. Biomedicines 2022, 10, 1827. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, J. Current status and future directions of cancer immunotherapy. J. Cancer 2018, 9, 1773–1781. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M. Managing symptoms of irritable bowel syndrome in patients with inflammatory bowel disease. Gut 2011, 60, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, D.C.; Carding, S.R. Inflammatory bowel disease: Cause and immunobiology. Lancet 2007, 369, 1627–1640. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.A.R.; Rodrigues, B.; Ayrizono, M.D.L.S.; Leal, R.F. The Immunological Basis of Inflammatory Bowel Disease. Gastroenterol. Res. Pract. 2016, 2016, 2097274. [Google Scholar] [CrossRef] [PubMed]
- Love, B.L. Management of patients with inflammatory bowel disease: Current and future treatments. Clin. Pharm. 2019, 10. [Google Scholar]
- Pithadia, A.B.; Jain, S. Treatment of inflammatory bowel disease (IBD). Pharmacol. Rep. 2011, 63, 629–642. [Google Scholar] [CrossRef]
- Van der Woude, C.J.; Shitrit, A.B.-G. Pregnancy, psychiatry and IBD: Multidisciplinary care is crucial. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 265–266. [Google Scholar] [CrossRef]
- Neuendorf, R.; Harding, A.; Stello, N.; Hanes, D.; Wahbeh, H. Depression and anxiety in patients with Inflammatory Bowel Disease: A systematic review. J. Psychosom. Res. 2016, 87, 70–80. [Google Scholar] [CrossRef]
- Davidson, M.; Rashidi, N.; Nurgali, K.; Apostolopoulos, V. The Role of Tryptophan Metabolites in Neuropsychiatric Disorders. Int. J. Mol. Sci. 2022, 23, 9968. [Google Scholar] [CrossRef]
- Papageorgiou, M.; Raza, A.; Fraser, S.; Nurgali, K.; Apostolopoulos, V. Methamphetamine and its immune-modulating effects. Maturitas 2019, 121, 13–21. [Google Scholar] [CrossRef]
- Prakash, M.D.; Tangalakis, K.; Antonipillai, J.; Stojanovska, L.; Nurgali, K.; Apostolopoulos, V. Methamphetamine: Effects on the brain, gut and immune system. Pharmacol. Res. 2017, 120, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Bai, D.; Zhao, Y.; Zhu, Q.; Zhou, Y.; Li, Z.; Lu, N. LL202 ameliorates colitis against oxidative stress of macrophage by activation of the Nrf2/HO-1 pathway. J. Cell. Physiol. 2019, 234, 10625–10639. [Google Scholar] [CrossRef]
- Kiesler, P.; Fuss, I.J.; Strober, W. Experimental Models of Inflammatory Bowel Diseases. Cell. Mol. Gastroenterol. Hepatol. 2015, 1, 154–170. [Google Scholar] [CrossRef] [PubMed]
- Mirabelli, P.; Coppola, L.; Salvatore, M. Cancer Cell Lines Are Useful Model Systems for Medical Research. Cancers 2019, 11, 1098. [Google Scholar] [CrossRef] [PubMed]
- Wirtz, S.; Neufert, C.; Weigmann, B.; Neurath, M.F. Chemically induced mouse models of intestinal inflammation. Nat. Protoc. 2007, 2, 541–546. [Google Scholar] [CrossRef]
- Morampudi, V.; Bhinder, G.; Wu, X.; Dai, C.; Sham, H.P.; Vallance, B.A.; Jacobson, K. DNBS/TNBS colitis models: Providing insights into inflammatory bowel disease and effects of dietary fat. J. Vis. Exp. JoVE 2014, 84, e51297. [Google Scholar] [CrossRef]
- Barone, M.; Chain, F.; Sokol, H.; Brigidi, P.; Bermudez-Humaran, L.; Langella, P.; Martin, R. A versatile new model of chemically induced chronic colitis using an outbred murine strain. Front. Microbiol. 2018, 9, 565. [Google Scholar] [CrossRef]
- Eissa, N.; Kermarrec, L.; Hussein, H.; Bernstein, C.N.; Ghia, J.E. Appropriateness of reference genes for normalizing messenger RNA in mouse 2,4-dinitrobenzene sulfonic acid (DNBS)-induced colitis using quantitative real time PCR. Sci. Rep. 2017, 7, 42427. [Google Scholar] [CrossRef]
- Peritore, A.F.; D’Amico, R.; Cordaro, M.; Siracusa, R.; Fusco, R.; Gugliandolo, E.; Genovese, T.; Crupi, R.; Di Paola, R.; Cuzzocrea, S.; et al. PEA/Polydatin: Anti-Inflammatory and Antioxidant Approach to Counteract DNBS-Induced Colitis. Antioxidants 2021, 10, 464. [Google Scholar] [CrossRef]
- Andrade, A.W.L.; Guerra, G.C.B.; de Souza Araújo, D.F.; de Araújo Júnior, R.F.; de Araújo, A.A.; de Carvalho, T.G.; Fernandes, J.M.; Diez-Echave, P.; Hidalgo-García, L.; Rodriguez-Cabezas, M.E.; et al. Anti-Inflammatory and Chemopreventive Effects of Bryophyllum pinnatum (Lamarck) Leaf Extract in Experimental Colitis Models in Rodents. Front. Pharmacol. 2020, 11, 998. [Google Scholar] [CrossRef]
- Eri, R.D.; Adams, R.J.; Tran, T.V.; Tong, H.; Das, I.; Roche, D.K.; Oancea, I.; Png, C.W.; Jeffery, P.L.; Radford-Smith, G.L.; et al. An intestinal epithelial defect conferring ER stress results in inflammation involving both innate and adaptive immunity. Mucosal Immunol. 2011, 4, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.A.; Robinson, A.M.; Jovanovska, V.; Eri, R.; Nurgali, K. Alterations in the distal colon innervation in Winnie mouse model of spontaneous chronic colitis. Cell Tissue Res. 2015, 362, 497–512. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Hasnain, S.Z.; Tong, H.; Das, I.; Chen, A.C.-H.; Oancea, I.; Proctor, M.; Florin, T.H.; Eri, R.D.; McGuckin, M.A. Neutralizing IL-23 is superior to blocking IL-17 in suppressing intestinal inflammation in a spontaneous murine colitis model. Inflamm. Bowel Dis. 2015, 21, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.D.; Luster, A.D. The role of tissue resident cells in neutrophil recruitment. Trends Immunol. 2015, 36, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Selders, G.S.; Fetz, A.E.; Radic, M.Z.; Bowlin, G.L. An overview of the role of neutrophils in innate immunity, inflammation and host-biomaterial integration. Regen. Biomater. 2017, 4, 55–68. [Google Scholar] [CrossRef]
- Krystel-Whittemore, M.; Dileepan, K.N.; Wood, J.G. Mast Cell: A Multi-Functional Master Cell. Front. Immunol. 2016, 6, 620. [Google Scholar] [CrossRef]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Et Biophys. Acta 2014, 1843, 2563–2582. [Google Scholar] [CrossRef]
- Morán, G.A.G.; Parra-Medina, R.; Cardona, A.G.; Quintero-Ronderos, P.; Rodríguez, É.G. Cytokines, chemokines and growth factors. In Autoimmunity: From Bench to Bedside [Internet]; El Rosario University Press: Bogotá, Colombia, 2013. [Google Scholar]
- Vatankhah, A.; Halász, J.; Piurkó, V.; Barbai, T.; Rásó, E.; Tímár, J. Characterization of the inflammatory cell infiltrate and expression of costimulatory molecules in chronic echinococcus granulosus infection of the human liver. BMC Infect. Dis. 2015, 15, 530. [Google Scholar] [CrossRef]
- Chapman, C.G.; Pekow, J. The emerging role of miRNAs in inflammatory bowel disease: A review. Ther. Adv. Gastroenterol. 2015, 8, 4–22. [Google Scholar] [CrossRef]
- Fisher, K.; Lin, J. MicroRNA in inflammatory bowel disease: Translational research and clinical implication. World J. Gastroenterol. 2015, 21, 12274–12282. [Google Scholar] [CrossRef]
- Schaefer, J.S.; Attumi, T.; Opekun, A.R.; Abraham, B.; Hou, J.; Shelby, H.; Graham, D.Y.; Streckfus, C.; Klein, J.R. MicroRNA signatures differentiate Crohn’s disease from ulcerative colitis. BMC Immunol. 2015, 16, 5. [Google Scholar] [CrossRef] [PubMed]
- Olaru, A.V.; Yamanaka, S.; Vazquez, C.; Mori, Y.; Cheng, Y.; Abraham, J.M.; Bayless, T.M.; Harpaz, N.; Selaru, F.M.; Meltzer, S.J. MicroRNA-224 negatively regulates p21 expression during late neoplastic progression in inflammatory bowel disease. Inflamm. Bowel Dis. 2013, 19, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, J. microRNAs as therapeutic targets in intestinal diseases. ExRNA 2019, 1, 1–12. [Google Scholar] [CrossRef]
- Dalal, S.; Kwon, J. The Role of MicroRNA in Inflammatory Bowel Disease. Gastroenterol. Hepatol. 2010, 6, 714–722. [Google Scholar]
- Xu, X.M.; Zhang, H.J. MiRNAs as new molecular insights into inflammatory bowel disease: Crucial regulators in autoimmunity and inflammation. World J. Gastroenterol. 2016, 22, 2206–2218. [Google Scholar] [CrossRef]
- Moein, S.; Vaghari-Tabari, M.; Qujeq, D.; Majidinia, M.; Nabavi, S.M.; Yousefi, B. MiRNAs and inflammatory bowel disease: An interesting new story. J. Cell. Physiol. 2019, 234, 3277–3293. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Sheng, K.C.; Day, S.; Wright, M.D.; Stojanovska, L.; Apostolopoulos, V. Enhanced Dendritic Cell-Mediated Antigen-Specific CD4+ T Cell Responses: IFN-Gamma Aids TLR Stimulation. J. Drug Deliv. 2013, 2013, 516749. [Google Scholar] [CrossRef]
- Sheng, K.C.; Pietersz, G.A.; Tang, C.K.; Ramsland, P.A.; Apostolopoulos, V. Reactive oxygen species level defines two functionally distinctive stages of inflammatory dendritic cell development from mouse bone marrow. J. Immunol. 2010, 184, 2863–2872. [Google Scholar] [CrossRef]
- Sheng, K.C.; Wright, M.D.; Apostolopoulos, V. Inflammatory mediators hold the key to dendritic cell suppression and tumor progression. Curr. Med. Chem. 2011, 18, 5507–5518. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Morgillo, F.; Dallio, M.; Della Corte, C.M.; Gravina, A.G.; Viscardi, G.; Loguercio, C.; Ciardiello, F.; Federico, A. Carcinogenesis as a Result of Multiple Inflammatory and Oxidative Hits: A Comprehensive Review from Tumor Microenvironment to Gut Microbiota. Neoplasia 2018, 20, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Moss, S.F.; Blaser, M.J. Mechanisms of Disease: Inflammation and the origins of cancer. Nat. Clin. Pract. Oncol. 2005, 2, 90–97. [Google Scholar] [CrossRef]
- Morrison, W.B. Inflammation and Cancer: A Comparative View. J. Vet. Intern. Med. 2012, 26, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Xiao, B.; Laroui, H.; Ayyadurai, S.; Viennois, E.; Charania, M.A.; Zhang, Y.; Merlin, D. Mannosylated bioreducible nanoparticle-mediated macrophage-specific TNF-α RNA interference for IBD therapy. Biomaterials 2013, 34, 7471–7482. [Google Scholar] [CrossRef] [PubMed]
- Filippone, R.T.; Sahakian, L.; Apostolopoulos, V.; Nurgali, K. Eosinophils in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2019, 25, 1140–1151. [Google Scholar] [CrossRef] [PubMed]
- Stavely, R.; Rahman, A.A.; Sahakian, L.; Prakash, M.D.; Robinson, A.M.; Hassanzadeganroudsari, M.; Filippone, R.T.; Fraser, S.; Eri, R.; Bornstein, J.C.; et al. Divergent Adaptations in Autonomic Nerve Activity and Neuroimmune Signaling Associated with the Severity of Inflammation in Chronic Colitis. Inflamm. Bowel Dis. 2022, 28, 1229–1243. [Google Scholar] [CrossRef]
- Filippone, R.T.; Dargahi, N.; Eri, R.; Uranga, J.A.; Bornstein, J.C.; Apostolopoulos, V.; Nurgali, K. Potent CCR3 Receptor Antagonist, SB328437, Suppresses Colonic Eosinophil Chemotaxis and Inflammation in the Winnie Murine Model of Spontaneous Chronic Colitis. Int. J. Mol. Sci. 2022, 23, 7780. [Google Scholar] [CrossRef]
- Filippone, R.T.; Robinson, A.M.; Jovanovska, V.; Stavely, R.; Apostolopoulos, V.; Bornstein, J.C.; Nurgali, K. Targeting eotaxin-1 and CCR3 receptor alleviates enteric neuropathy and colonic dysfunction in TNBS-induced colitis in guinea pigs. Neurogastroenterol. Motil. 2018, 30, e13391. [Google Scholar] [CrossRef]
- Lu, Q.; Yang, M.F.; Liang, Y.J.; Xu, J.; Xu, H.M.; Nie, Y.Q.; Wang, L.S.; Yao, J.; Li, D.F. Immunology of Inflammatory Bowel Disease: Molecular Mechanisms and Therapeutics. J. Inflamm. Res. 2022, 15, 1825–1844. [Google Scholar] [CrossRef] [PubMed]
- Gelfo, V.; Romaniello, D.; Mazzeschi, M.; Sgarzi, M.; Grilli, G.; Morselli, A.; Manzan, B.; Rihawi, K.; Lauriola, M. Roles of IL-1 in Cancer: From Tumor Progression to Resistance to Targeted Therapies. Int. J. Mol. Sci. 2020, 21, 6009. [Google Scholar] [CrossRef] [PubMed]
- Kuol, N.; Stojanovska, L.; Apostolopoulos, V.; Nurgali, K. Role of the Nervous System in Tumor Angiogenesis. Cancer Microenviron. 2018, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kuol, N.; Stojanovska, L.; Apostolopoulos, V.; Nurgali, K. Role of the nervous system in cancer metastasis. J. Exp. Clin. Cancer Res. 2018, 37, 5. [Google Scholar] [CrossRef]
- Kuol, N.; Stojanovska, L.; Apostolopoulos, V.; Nurgali, K. Crosstalk between cancer and the neuro-immune system. J. Neuroimmunol. 2018, 315, 15–23. [Google Scholar] [CrossRef]
- Wang, X.; Lin, Y. Tumor necrosis factor and cancer, buddies or foes? Acta Pharmacol. Sin. 2008, 29, 1275–1288. [Google Scholar] [CrossRef]
- Aguilar-Cazares, D.; Chavez-Dominguez, R.; Carlos-Reyes, A.; Lopez-Camarillo, C.; Hernadez de la Cruz, O.N.; Lopez-Gonzalez, J.S. Contribution of Angiogenesis to Inflammation and Cancer. Front. Oncol. 2019, 9, 1399. [Google Scholar] [CrossRef]
- Chow, M.T.; Luster, A.D. Chemokines in cancer. Cancer Immunol. Res. 2014, 2, 1125–1131. [Google Scholar] [CrossRef]
- Landskron, G.; De la Fuente, M.; Thuwajit, P.; Thuwajit, C.; Hermoso, M.A. Chronic Inflammation and Cytokines in the Tumor Microenvironment. J. Immunol. Res. 2014, 2014, 149185. [Google Scholar] [CrossRef]
- Sanchez-Munoz, F.; Dominguez-Lopez, A.; Yamamoto-Furusho, J.-K. Role of cytokines in inflammatory bowel disease. World J. Gastroenterol. 2008, 14, 4280–4288. [Google Scholar] [CrossRef]
- Strober, W.; Fuss, I.J. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology 2011, 140, 1756–1767. [Google Scholar] [CrossRef] [PubMed]
- Carty, E.; Rampton, D.S. Evaluation of new therapies for inflammatory bowel disease. Br. J. Clin. Pharmacol. 2003, 56, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Biasi, F.; Leonarduzzi, G.; Oteiza, P.I.; Poli, G. Inflammatory bowel disease: Mechanisms, redox considerations, and therapeutic targets. Antioxid. Redox Signal. 2013, 19, 1711–1747. [Google Scholar] [CrossRef] [PubMed]
- Argollo, M.; Fiorino, G.; Hindryckx, P.; Peyrin-Biroulet, L.; Danese, S. Novel therapeutic targets for inflammatory bowel disease. J. Autoimmun. 2017, 85, 103–116. [Google Scholar] [CrossRef]
- Mogensen, T.H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 2009, 22, 240–273. [Google Scholar] [CrossRef]
- Jang, J.-H.; Shin, H.W.; Lee, J.M.; Lee, H.-W.; Kim, E.-C.; Park, S.H. An Overview of Pathogen Recognition Receptors for Innate Immunity in Dental Pulp. Mediat. Inflamm. 2015, 2015, 794143. [Google Scholar] [CrossRef]
- Szebeni, B.; Veres, G.; Dezsõfi, A.; Rusai, K.; Vannay, A.; Mraz, M.; Majorova, E.; Arató, A. Increased expression of Toll-like receptor (TLR) 2 and TLR4 in the colonic mucosa of children with inflammatory bowel disease. Clin. Exp. Immunol. 2008, 151, 34–41. [Google Scholar] [CrossRef]
- Oliveira-Nascimento, L.; Massari, P.; Wetzler, L.M. The Role of TLR2 in Infection and Immunity. Front. Immunol. 2012, 3, 79. [Google Scholar] [CrossRef]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Prakash, M.D.; Miller, S.; Randall-Demllo, S.; Nurgali, K. Mesenchymal Stem Cell Treatment of Inflammation-Induced Cancer. Inflamm. Bowel Dis. 2016, 22, 2694–2703. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Atreya, I.; Atreya, R.; Neurath, M.F. NF-kappaB in inflammatory bowel disease. J. Intern. Med. 2008, 263, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Visse, R.; Nagase, H. Matrix Metalloproteinases and Tissue Inhibitors of Metalloproteinases. Circ. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Barbara, G.; Buurman, W.; Ockhuizen, T.; Schulzke, J.-D.; Serino, M.; Tilg, H.; Watson, A.; Wells, J.M. Intestinal permeability—A new target for disease prevention and therapy. BMC Gastroenterol. 2014, 14, 189. [Google Scholar] [CrossRef]
- Al-Sadi, R.; Youssef, M.; Rawat, M.; Guo, S.; Dokladny, K.; Haque, M.; Watterson, M.D.; Ma, T.Y. MMP-9-induced increase in intestinal epithelial tight permeability is mediated by p38 kinase signaling pathway activation of MLCK gene. Am. J. Physiol. 2019, 316, G278–G290. [Google Scholar] [CrossRef]
- Kuper, H.; Adami, H.O.; Trichopoulos, D. Infections as a major preventable cause of human cancer. J. Intern. Med. 2000, 248, 171–183. [Google Scholar] [CrossRef]
- Wahl, L.M.; Kleinman, H.K. Tumor-associated macrophages as targets for cancer therapy. J. Natl. Cancer Inst. 1998, 90, 1583–1584. [Google Scholar] [CrossRef]
- Desai, S.J.; Prickril, B.; Rasooly, A. Mechanisms of Phytonutrient Modulation of Cyclooxygenase-2 (COX-2) and Inflammation Related to Cancer. Nutr. Cancer 2018, 70, 350–375. [Google Scholar] [CrossRef]
- El Miedany, Y.; Youssef, S.; Ahmed, I.; El Gaafary, M. The gastrointestinal safety and effect on disease activity of etoricoxib, a selective cox-2 inhibitor in inflammatory bowel diseases. Am. J. Gastroenterol. 2006, 101, 311–317. [Google Scholar] [CrossRef]
- Dudhgaonkar, S.P.; Tandan, S.K.; Kumar, D.; Raviprakash, V.; Kataria, M. Influence of simultaneous inhibition of cyclooxygenase-2 and inducible nitric oxide synthase in experimental colitis in rats. Inflammopharmacology 2007, 15, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Deguine, J.; Barton, G.M. MyD88: A central player in innate immune signaling. F1000prime Rep. 2014, 6, 97. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Chen, Z.; Geng, T.; Wang, M.; Yi, S.; Liu, K.; Zhou, W.; Gao, J.; Song, W.; Tang, H. Deleting MyD88 signaling in myeloid cells promotes development of adenocarcinomas of the colon. Cancer Lett. 2018, 433, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Araki, A.; Kanai, T.; Ishikura, T.; Makita, S.; Uraushihara, K.; Iiyama, R.; Totsuka, T.; Takeda, K.; Akira, S.; Watanabe, M. MyD88-deficient mice develop severe intestinal inflammation in dextran sodium sulfate colitis. J. Gastroenterol. 2005, 40, 16–23. [Google Scholar] [CrossRef]
- Bhinder, G.; Stahl, M.; Sham, H.P.; Crowley, S.M.; Morampudi, V.; Dalwadi, U.; Ma, C.; Jacobson, K.; Vallance, B.A. Intestinal epithelium-specific MyD88 signaling impacts host susceptibility to infectious colitis by promoting protective goblet cell and antimicrobial responses. Infect. Immun. 2014, 82, 3753–3763. [Google Scholar] [CrossRef]
- Francescone, R.; Hou, V.; Grivennikov, S.I. Cytokines, IBD, and Colitis-associated Cancer. Inflamm. Bowel Dis. 2015, 21, 409–418. [Google Scholar] [CrossRef]
- Grivennikov, S.I. Inflammation and colorectal cancer: Colitis-associated neoplasia. Semin. Immunopathol. 2013, 35, 229–244. [Google Scholar] [CrossRef]
- Song, G.; Lu, Y.; Yu, Z.; Xu, L.; Liu, J.; Chen, K.; Zhang, P. The inhibitory effect of polysaccharide from Rhizopus nigricans on colitis-associated colorectal cancer. Biomed. Pharmacother. 2019, 112, 108593. [Google Scholar] [CrossRef]
- Dienstmann, R.; Vermeulen, L.; Guinney, J.; Kopetz, S.; Tejpar, S.; Tabernero, J. Consensus molecular subtypes and the evolution of precision medicine in colorectal cancer. Nat. Rev. Cancer 2017, 17, 79. [Google Scholar] [CrossRef]
- Khalil, A.; Rubin, D.T.; Haider, H.I.; Meckel, K.; Bissonnette, M.; Talisila, N.; Siva, S.; Li, Y.C.; Deng, Z.; Pekow, J.; et al. IBD-associated colon cancers differ in DNA methylation and gene expression profiles compared to sporadic colon cancers. J. Crohn’s Colitis 2019, 13, 884–893. [Google Scholar] [CrossRef]
- Itzkowitz, S.H.; Yio, X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: The role of inflammation. Am. J. Physiol. Liver Physiol. 2004, 287, G7–G17. [Google Scholar] [CrossRef] [PubMed]
- McQuade, R.M.; Stojanovska, V.; Abalo, R.; Bornstein, J.C.; Nurgali, K. Chemotherapy-Induced Constipation and Diarrhea: Pathophysiology, Current and Emerging Treatments. Front. Pharmacol. 2016, 7, 414. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.E.R.; Borthwick, A.; Rigg, A.; Leary, A.; Assersohn, L.; Last, K.; Tan, S.; Milan, S.; Tait, D.; Smith, I.E. Mortality within 30 days of chemotherapy: A clinical governance benchmarking issue for oncology patients. Br. J. Cancer 2006, 95, 1632–1636. [Google Scholar] [CrossRef] [PubMed]
- Currey, N.; Jahan, Z.; Caldon, C.E.; Tran, P.N.; Benthani, F.; De Lacavalerie, P.; Roden, D.L.; Gloss, B.S.; Campos, C.; Bean, E.G.; et al. Mouse Model of ‘Mutated in Colorectal Cancer’Gene Deletion Reveals Novel Pathways in Inflammation and Cancer. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 819–839. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, T.L. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008, 27, 5904–5912. [Google Scholar] [CrossRef]
- Apostolopoulos, V. Cancer Vaccines: Research and Applications. Cancers 2019, 11, 1041. [Google Scholar] [CrossRef] [PubMed]
- Baghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun. Signal. 2020, 18, 1–19. [Google Scholar] [CrossRef]
- Kong, Y.Y.; Fuchsberger, M.; Xiang, S.D.; Apostolopoulos, V.; Plebanski, M. Myeloid derived suppressor cells and their role in diseases. Curr. Med. Chem. 2013, 20, 1437–1444. [Google Scholar] [CrossRef]
- Apostolopoulos, V.; McKenzie, I.F.C. Cellular Mucins: Targets for Immunotherapy. Crit. Rev. Immunol. 2017, 37, 421–437. [Google Scholar] [CrossRef]
- Ephraim, R.; Fraser, S.; Nurgali, K.; Apostolopoulos, V. Checkpoint Markers and Tumor Microenvironment: What Do We Know? Cancers 2022, 14, 3788. [Google Scholar] [CrossRef]
- Deshpande, R.P.; Sharma, S.; Watabe, K. The Confounders of Cancer Immunotherapy: Roles of Lifestyle, Metabolic Disorders and Sociological Factors. Cancers 2020, 12, 2983. [Google Scholar] [CrossRef] [PubMed]
- Lemiale, V.; Meert, A.-P.; Vincent, F.; Darmon, M.; Bauer, P.R.; Van de Louw, A.; Azoulay, E.; Groupe de Recherche en Reanimation Respiratoire du patient d’Onco-Hématologie (Grrr-OH). Severe toxicity from checkpoint protein inhibitors: What intensive care physicians need to know? Ann. Intensive Care 2019, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Sari-Ak, D.; Bagga, T. Siglecs as Therapeutic Targets in Cancer. Biology 2021, 10, 1178. [Google Scholar] [CrossRef] [PubMed]
- Borst, J.; Ahrends, T.; Bąbała, N.; Melief, C.J.M.; Kastenmüller, W. CD4+ T cell help in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2018, 18, 635–647. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef]
- Yousuf, H.; Mekki, R.; Khan, K.; Hussain, A. Pembrolizumab-Induced Sarcoid-Like Reaction in a Patient with Lung Cancer. Cureus 2020, 12, e12395. [Google Scholar] [CrossRef]
- Rotte, A. Combination of CTLA-4 and PD-1 blockers for treatment of cancer. J. Exp. Clin. Cancer Res. 2019, 38, 1–12. [Google Scholar] [CrossRef]
- Ganesh, K.; Stadler, Z.K.; Cercek, A.; Mendelsohn, R.B.; Shia, J.; Segal, N.H.; Diaz, L.A. Immunotherapy in colorectal cancer: Rationale, challenges and potential. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 361–375. [Google Scholar] [CrossRef]
- Rowshanravan, B.; Halliday, N.; Sansom, D.M. CTLA-4: A moving target in immunotherapy. Blood 2018, 131, 58–67. [Google Scholar] [CrossRef]
- Kolar, P.; Knieke, K.; Hegel, J.K.; Quandt, D.; Burmester, G.R.; Hoff, H.; Brunner-Weinzierl, M.C. CTLA-4 (CD152) controls homeostasis and suppressive capacity of regulatory T cells in mice. Arthritis Rheum. 2009, 60, 123–132. [Google Scholar] [CrossRef]
- Buchbinder, E.I.; Desai, A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am. J. Clin. Oncol. 2016, 39, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Qiu, H.; Tang, W.; Wang, Y.; Lan, B.; Chen, Y. CTLA4 tagging polymorphisms and risk of colorectal cancer: A case-control study involving 2306 subjects. OncoTargets Ther. 2018, 11, 4609–4619. [Google Scholar] [CrossRef] [PubMed]
- Läubli, H.; Varki, A. Sialic acid-binding immunoglobulin-like lectins (Siglecs) detect self-associated molecular patterns to regulate immune responses. Cell Mol. Life Sci. 2020, 77, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Caselles, T.; Martínez-Esparza, M.; Pérez-Oliva, A.B.; Quintanilla-Cecconi, A.M.; García-Alonso, A.; Alvarez-López, D.M.R.; García-Peñarrubia, P. A study of CD33 (SIGLEC-3) antigen expression and function on activated human T and NK cells: Two isoforms of CD33 are generated by alternative splicing. J. Leukoc. Biol. 2006, 79, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Kelm, S.; Ravindran, R. 3.24—Siglecs. In Comprehensive Glycoscience; Kamerling, H., Ed.; Elsevier: Oxford, UK, 2007; pp. 523–538. [Google Scholar]
- Wei, Y.; Chhiba, K.D.; Zhang, F.; Ye, X.; Wang, L.; Zhang, L.; Robida, P.A.; Moreno-Vinasco, L.; Schnaar, R.L.; Roers, A.; et al. Mast Cell-Specific Expression of Human Siglec-8 in Conditional Knock-in Mice. Int. J. Mol. Sci. 2018, 20, 19. [Google Scholar] [CrossRef]
- Kano, G.; Almanan, M.; Bochner, B.S.; Zimmermann, N. Mechanism of Siglec-8-mediated cell death in IL-5-activated eosinophils: Role for reactive oxygen species-enhanced MEK/ERK activation. J. Allergy Clin. Immunol. 2013, 132, 437–445. [Google Scholar] [CrossRef]
- Graeter, S.; Simon, H.-U.; von Gunten, S. Granulocyte death mediated by specific antibodies in intravenous immunoglobulin (IVIG). Pharmacol. Res. 2019, 56, 103156. [Google Scholar] [CrossRef]
- Delputte, P.L.; Van Gorp, H.; Favoreel, H.W.; Hoebeke, I.; Delrue, I.; Dewerchin, H.; Verdonck, F.; Verhasselt, B.; Cox, E.; Nauwynck, H.J. Porcine sialoadhesin (CD169/Siglec-1) is an endocytic receptor that allows targeted delivery of toxins and antigens to macrophages. PLoS ONE 2011, 6, e16827. [Google Scholar] [CrossRef]
- O’Neill, A.S.; van den Berg, T.K.; Mullen, G.E. Sialoadhesin—A macrophage-restricted marker of immunoregulation and inflammation. Immunology 2013, 138, 198–207. [Google Scholar] [CrossRef]
- Ren, X.; Ji, Y.; Jiang, X.; Qi, X. Down-regulation of siglec-2 (CD22) predicts worse overall survival from HBV-related early-stage hepatocellular carcinoma: A preliminary analysis from Gene Expression Omnibus. Biosci. Rep. 2018, 38, BSR20181423. [Google Scholar] [CrossRef]
- Tsai, T.Y.; Huang, M.T.; Sung, P.S.; Peng, C.Y.; Tao, M.H.; Yang, H.I.; Chang, W.C.; Yang, A.S.; Yu, C.M.; Lin, Y.P.; et al. SIGLEC-3 (CD33) serves as an immune checkpoint receptor for HBV infection. J. Clin. Invest. 2021, 131, e141965. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Shaper, N.L.; Itonori, S.; Heffer-Lauc, M.; Sheikh, K.A.; Schnaar, R.L. Myelin-associated glycoprotein (Siglec-4) expression is progressively and selectively decreased in the brains of mice lacking complex gangliosides. Glycobiology 2004, 14, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Erickson-Miller, C.L.; Freeman, S.D.; Hopson, C.B.; D’Alessio, K.J.; Fischer, E.I.; Kikly, K.K.; Abrahamson, J.A.; Holmes, S.D.; King, A.G. Characterization of Siglec-5 (CD170) expression and functional activity of anti-Siglec-5 antibodies on human phagocytes. Exp. Hematol. 2003, 31, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Blokhuis, B.R.J.; Diks, M.A.P.; Keshavarzian, A.; Garssen, J.; Redegeld, F.A. Functional Inhibitory Siglec-6 Is Upregulated in Human Colorectal Cancer-Associated Mast Cells. Front. Immunol. 2018, 9, 2138. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Yu, C.; Rodrigues, E.; Shi, Y.; Chen, H.; Wang, P.; Chapla, D.G.; Gao, T.; Zhuang, R.; Moremen, K.W.; et al. Modulation of Siglec-7 Signaling Via In Situ-Created High-Affinity cis-Ligands. ACS Cent. Sci. 2021, 7, 1338–1346. [Google Scholar] [CrossRef] [PubMed]
- Trebo, A.; Ditsch, N.; Degenhardt, T.; Kuhn, C.; Rahmeh, M.; Schmoeckel, E.; Mayr, D.; Czogalla, B.; Kolben, T.; Meister, S.; et al. First Evidence for a Role of Siglec-8 in Breast Cancer. Int. J. Mol. Sci. 2021, 22, 2000. [Google Scholar] [CrossRef]
- Choi, H.; Ho, M.; Adeniji, O.S.; Giron, L.; Bordoloi, D.; Kulkarni, A.J.; Puchalt, A.P.; Abdel-Mohsen, M.; Muthumani, K. Development of Siglec-9 Blocking Antibody to Enhance Anti-Tumor Immunity. Front. Oncol. 2021, 11, 778989. [Google Scholar] [CrossRef]
- Barkal, A.A.; Brewer, R.E.; Markovic, M.; Kowarsky, M.; Barkal, S.A.; Zaro, B.W.; Krishnan, V.; Hatakeyama, J.; Dorigo, O.; Barkal, L.J.; et al. CD24 signalling through macrophage Siglec-10 is a target for cancer immunotherapy. Nature 2019, 572, 392–396. [Google Scholar] [CrossRef]
- Fraschilla, I.; Pillai, S. Viewing Siglecs through the lens of tumor immunology. Immunol. Rev. 2017, 276, 178–191. [Google Scholar] [CrossRef]
- Mitra, N.; Banda, K.; Altheide, T.K.; Schaffer, L.; Johnson-Pais, T.L.; Beuten, J.; Leach, R.J.; Angata, T.; Varki, N.; Varki, A. SIGLEC12, a human-specific segregating (pseudo)gene, encodes a signaling molecule expressed in prostate carcinomas. J. Biol. Chem. 2011, 286, 23003–23011. [Google Scholar] [CrossRef]
- Huang, P.J.; Low, P.Y.; Wang, I.; Hsu, S.D.; Angata, T. Soluble Siglec-14 glycan-recognition protein is generated by alternative splicing and suppresses myeloid inflammatory responses. J. Biol. Chem. 2018, 293, 19645–19658. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, J.; Liu, L.N.; Flies, D.B.; Nie, X.; Toki, M.; Zhang, J.; Song, C.; Zarr, M.; Zhou, X.; et al. Siglec-15 as an immune suppressor and potential target for normalization cancer immunotherapy. Nat. Med. 2019, 25, 656–666. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yu, M.; Guo, L.; Zhang, B.; Liu, S.; Zhang, W.; Zhou, B.; Yan, J.; Ma, Q.; Yang, Z.; et al. Tumor Derived SIGLEC Family Genes May Play Roles in Tumor Genesis, Progression, and Immune Microenvironment Regulation. Front. Oncol. 2020, 10, 586820. [Google Scholar] [CrossRef] [PubMed]
- Manson, G.; Norwood, J.; Marabelle, A.; Kohrt, H.; Houot, R. Biomarkers associated with checkpoint inhibitors. Ann. Oncol. 2016, 27, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Prendergast, G.C.; Smith, C.; Thomas, S.; Mandik-Nayak, L.; Laury-Kleintop, L.; Metz, R.; Muller, A.J. Indoleamine 2,3-dioxygenase pathways of pathogenic inflammation and immune escape in cancer. Cancer Immunol. Immunother. 2014, 63, 721–735. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Wang, T.; Li, Y.; Huang, Z.; Wang, X.; Zheng, J.; Yang, S.; Fan, Y.; Xiang, R. A highly potent and selective inhibitor Roxyl-WL targeting IDO1 promotes immune response against melanoma. J. Enzym. Inhib. Med. Chem. 2018, 33, 1089–1094. [Google Scholar] [CrossRef]
- Xu, X.; Ren, J.; Ma, Y.; Liu, H.; Rong, Q.; Feng, Y.; Wang, Y.; Cheng, Y.; Ge, R.; Li, Z.; et al. Discovery of cyanopyridine scaffold as novel indoleamine-2,3-dioxygenase 1 (IDO1) inhibitors through virtual screening and preliminary hit optimisation. J. Enzym. Inhib. Med. Chem. 2019, 34, 250–263. [Google Scholar] [CrossRef]
- Mondanelli, G.; Mandarano, M.; Belladonna, M.L.; Suvieri, C.; Pelliccia, C.; Bellezza, G.; Sidoni, A.; Carvalho, A.; Grohmann, U.; Volpi, C. Current Challenges for IDO2 as Target in Cancer Immunotherapy. Front. Immunol. 2021, 12, 679953. [Google Scholar] [CrossRef]
- Hou, N.; Ma, J.; Li, W.; Zhao, L.; Gao, Q.; Mai, L. T-cell immunoglobulin and mucin domain-containing protein-3 and galectin-9 protein expression: Potential prognostic significance in esophageal squamous cell carcinoma for Chinese patients. Oncol. Lett. 2017, 14, 8007–8013. [Google Scholar] [CrossRef]
- Yu, M.; Lu, B.; Liu, Y.; Me, Y.; Wang, L.; Zhang, P. Tim-3 is upregulated in human colorectal carcinoma and associated with tumor progression. Mol. Med. Rep. 2017, 15, 689–695. [Google Scholar] [CrossRef]
- Das, M.; Zhu, C.; Kuchroo, V.K. Tim-3 and its role in regulating anti-tumor immunity. Immunol. Rev. 2017, 276, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.C. Tim-3: An Emerging Target in the Cancer Immunotherapy Landscape. Cancer Immunol. Res. 2014, 2, 393–398. [Google Scholar] [CrossRef]
- Graydon, C.G.; Mohideen, S.; Fowke, K.R. LAG3’s Enigmatic Mechanism of Action. Front. Immunol. 2021, 11, 615317. [Google Scholar] [CrossRef]
- Ruffo, E.; Wu, R.C.; Bruno, T.C.; Workman, C.J.; Vignali, D.A.A. Lymphocyte-activation gene 3 (LAG3): The next immune checkpoint receptor. Semin. Immunol. 2019, 42, 101305. [Google Scholar] [CrossRef]
- Andrews, L.P.; Marciscano, A.E.; Drake, C.G.; Vignali, D.A. LAG3 (CD223) as a cancer immunotherapy target. Immunol. Rev. 2017, 276, 80–96. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.R.; Turnis, M.E.; Goldberg, M.V.; Bankoti, J.; Selby, M.; Nirschl, C.J.; Bettini, M.L.; Gravano, D.M.; Vogel, P.; Liu, C.L.; et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012, 72, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Lythgoe, M.P.; Liu, D.S.K.; Annels, N.E.; Krell, J.; Frampton, A.E. Gene of the month: Lymphocyte-activation gene 3 (LAG-3). J. Clin. Pathol. 2021, 74, 543–547. [Google Scholar] [CrossRef]
- Inoue, S.; Ito, H.; Tsunoda, T.; Murakami, H.; Ebi, M.; Ogasawara, N.; Kasugai, K.; Kasai, K.; Ikeda, H.; Inaguma, S. CD70 expression in tumor-associated fibroblasts predicts worse survival in colorectal cancer patients. Virchows Arch. 2019, 475, 425–434. [Google Scholar] [CrossRef]
- Eastwood, D.; Findlay, L.; Poole, S.; Bird, C.; Wadhwa, M.; Moore, M.; Burns, C.; Thorpe, R.; Stebbings, R. Monoclonal antibody TGN1412 trial failure explained by species differences in CD28 expression on CD4+ effector memory T-cells. Br. J. Pharmacol. 2010, 161, 512–526. [Google Scholar] [CrossRef] [PubMed]
- Schoenberger, S.P.; Toes, R.E.; van der Voort, E.I.; Offringa, R.; Melief, C.J. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature 1998, 393, 480–483. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, B.; Thomas, R. CD40 and dendritic cell function. Crit. Rev. Immunol. 2003, 23, 83–107. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Zhang, L.; Wu, J.; Ma, C.; Mu, C.; Zhang, G.; Chen, W. Expression of CD40/CD40L in colon cancer, and its effect on proliferation and apoptosis of SW48 colon cancer cells. J. Buon 2017, 22, 894–899. [Google Scholar] [PubMed]
- Villarreal, D.O.; Allegrezza, M.J.; Smith, M.A.; Chin, D.; Luistro, L.L.; Snyder, L.A. Targeting of CD122 enhances antitumor immunity by altering the tumor immune environment. Oncotarget 2017, 8, 109151–109160. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Zou, W.; Li, X.; Tan, H.; Yang, X.; Shi, H. Early diagnosis of colorectal cancer by CD11b+ myeloid-derived suppressor cells imaging. J. Nucl. Med. 2014, 55, 1397. [Google Scholar]
- Yu, X.; Guo, C.; Fisher, P.B.; Subjeck, J.R.; Wang, X.-Y. Chapter Nine—Scavenger Receptors: Emerging Roles in Cancer Biology and Immunology. In Advances in Cancer Research; Wang, X.-Y., Fisher, P.B., Eds.; Academic Press: Cambridge, MA, USA, 2015; Volume 128, pp. 309–364. [Google Scholar]
- Wang, C.; Feng, H.; Cheng, X.; Liu, K.; Cai, D.; Zhao, R. Potential Therapeutic Targets of B7 Family in Colorectal Cancer. Front. Immunol. 2020, 11, 681. [Google Scholar] [CrossRef]
- Scarpa, M.; Brun, P.; Scarpa, M.; Morgan, S.; Porzionato, A.; Kotsafti, A.; Bortolami, M.; Buda, A.; D’Incà, R.; Macchi, V.; et al. CD80-CD28 signaling controls the progression of inflammatory colorectal carcinogenesis. Oncotarget 2015, 6, 20058–20069. [Google Scholar] [CrossRef]
- Xu, G.; Jiang, L.; Ye, C.; Qin, G.; Luo, Z.; Mo, Y.; Chen, J. The Ratio of CD86+/CD163+ Macrophages Predicts Postoperative Recurrence in Stage II-III Colorectal Cancer. Front. Immunol. 2021, 12, 724429. [Google Scholar] [CrossRef]
- Shi, S.J.; Wang, L.J.; Wang, G.D.; Guo, Z.Y.; Wei, M.; Meng, Y.L.; Yang, A.G.; Wen, W.H. B7-H1 expression is associated with poor prognosis in colorectal carcinoma and regulates the proliferation and invasion of HCT116 colorectal cancer cells. PLoS ONE 2013, 8, e76012. [Google Scholar] [CrossRef]
- Cao, Y.; Cao, T.; Zhao, W.; He, F.; Lu, Y.; Zhang, G.; Hu, H.; Wang, Z. Expression of B7-H2 on CD8(+) T cells in colorectal cancer microenvironment and its clinical significance. Int. Immunopharmacol. 2018, 56, 128–134. [Google Scholar] [CrossRef]
- Zhou, W.T.; Jin, W.L. B7-H3/CD276: An Emerging Cancer Immunotherapy. Front. Immunol. 2021, 12, 701006. [Google Scholar] [CrossRef]
- Zhou, B.; Lu, Y.; Zhao, Z.; Shi, T.; Wu, H.; Chen, W.; Zhang, L.; Zhang, X. B7-H4 expression is upregulated by PKCdelta activation and contributes to PKCdelta-induced cell motility in colorectal cancer. Cancer Cell Int. 2022, 22, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, H.; Chen, Y.; Zhu, J.; Sun, L.; Li, J.; Yao, Z.; Chen, Y.; Zhang, X.; Xia, S.; et al. B7-H5 blockade enhances CD8(+) T-cell-mediated antitumor immunity in colorectal cancer. Cell Death Discov. 2021, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Dong, C. New B7 Family Checkpoints in Human Cancers. Mol. Cancer Ther. 2017, 16, 1203–1211. [Google Scholar] [CrossRef]
- Sun, X.; Zhao, J.; Ma, L.; Sun, X.; Ge, J.; Yu, Y.; Ma, J.; Zhang, M. B7-H6 as an efficient target for T cell-induced cytotoxicity in haematologic malignant cells. Invest. New Drugs 2021, 39, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Cansever, M.; Göktaş, M.A.; Arslan, D.; Patiroğlu, T. Serum levels of soluble HLA-G correlate with disease activity in pediatric patients with Crohn’s disease. Saudi J. Gastroenterol. 2022, 28, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Bortolotti, D.; Gentili, V.; Rotola, A.; Cassai, E.; Rizzo, R.; Di Luca, D. Impact of HLA-G analysis in prevention, diagnosis and treatment of pathological conditions. World J. Methodol. 2014, 4, 11–25. [Google Scholar] [CrossRef]
- Nalejska, E.; Mączyńska, E.; Lewandowska, M.A. Prognostic and predictive biomarkers: Tools in personalized oncology. Mol. Diagn. Ther. 2014, 18, 273–284. [Google Scholar] [CrossRef]
- Kitano, S.; Nakayama, T.; Yamashita, M. Biomarkers for Immune Checkpoint Inhibitors in Melanoma. Front. Oncol. 2018, 8, 270. [Google Scholar] [CrossRef]



| Siglecs | Function | References |
|---|---|---|
| Siglec-1 (CD169)/Sialoadhesin | Cell adhesion, cancer progression | [140,141] |
| Siglec-2 (CD22) | Dampening B cell receptor activation | [142] |
| Siglec-3 (CD33) | Downstream signalling function | [143] |
| Siglec-4 (Myelin Associated Glycoprotein, MAG) | Interaction between MAG and cancer-associated MUC1, stabilizes myelin-axon interactions | [124,144] |
| Siglec-5 (CD170) | Associates with leukocyte counter-receptor P-selectin glycoprotein ligand-1, prevent leukocyte recruitment to sites of inflammation and maintains a pro-cancer environment. | [145] |
| Siglec-6 (CD327) | Immune-inhibitory, inhibitory receptor on mast cells in CRC | [146] |
| Siglec-7 (p75/AIRM1, CD328) | Natural killer (NK) cell-inhibitory receptor bearing immunoreceptor tyrosine-based inhibition (ITIM) motifs | [147] |
| Siglec-8 | A target in allergen-induced inflammation | [148] |
| Siglec-9 (CD329) | Binds to MUC1 with sialylated T-antigen (MUC1 ST) | [149] |
| Siglec-10 | Repress DAMP-mediated innate inflammatory responses | [150] |
| Siglec-11 | Inhibitory function, microglial activities | [124,151] |
| Siglec-12 (Siglec-XII) | Recruit SHP2-related oncogenic pathways | [152] |
| Siglec-13 | Deleted in humans | [124] |
| Siglec-14 | Suppresses myeloid inflammatory responses | [153] |
| Siglec-15 | Upregulated on cancer cells and tumour-infiltrating myeloid cells | [154] |
| Siglec-16 | Expressed on cancer cells | [155] |
| Siglec-17 | Deleted in humans but pseudogene exists | [124] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ephraim, R.; Feehan, J.; Fraser, S.; Nurgali, K.; Apostolopoulos, V. Cancer Immunotherapy: The Checkpoint between Chronic Colitis and Colorectal Cancer. Cancers 2022, 14, 6131. https://doi.org/10.3390/cancers14246131
Ephraim R, Feehan J, Fraser S, Nurgali K, Apostolopoulos V. Cancer Immunotherapy: The Checkpoint between Chronic Colitis and Colorectal Cancer. Cancers. 2022; 14(24):6131. https://doi.org/10.3390/cancers14246131
Chicago/Turabian StyleEphraim, Ramya, Jack Feehan, Sarah Fraser, Kulmira Nurgali, and Vasso Apostolopoulos. 2022. "Cancer Immunotherapy: The Checkpoint between Chronic Colitis and Colorectal Cancer" Cancers 14, no. 24: 6131. https://doi.org/10.3390/cancers14246131
APA StyleEphraim, R., Feehan, J., Fraser, S., Nurgali, K., & Apostolopoulos, V. (2022). Cancer Immunotherapy: The Checkpoint between Chronic Colitis and Colorectal Cancer. Cancers, 14(24), 6131. https://doi.org/10.3390/cancers14246131









