Prospective Investigation of 18FDG-PET/MRI with Intravoxel Incoherent Motion Diffusion-Weighted Imaging to Assess Survival in Patients with Oropharyngeal or Hypopharyngeal Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. 18F-FDG PET/MRI
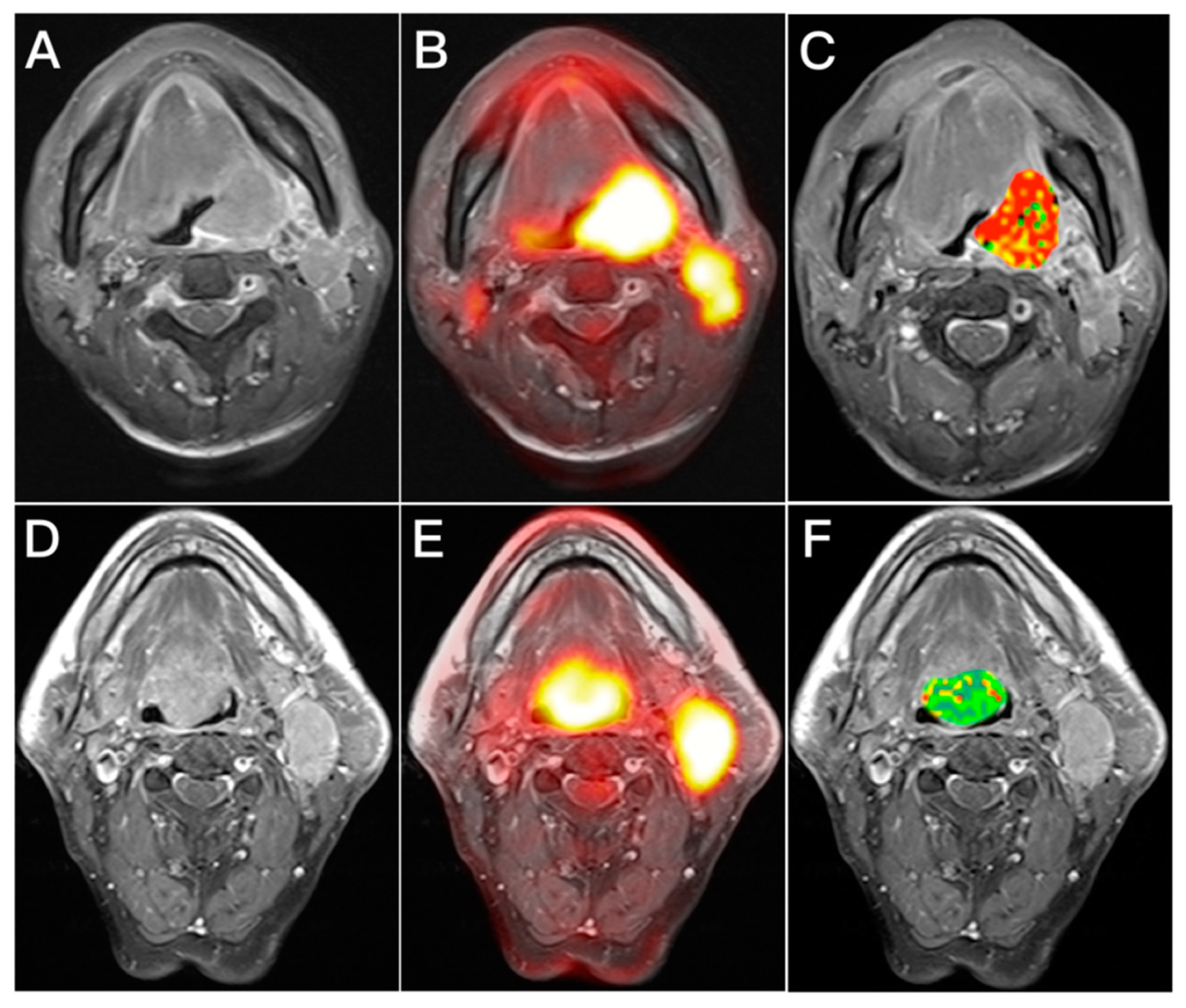
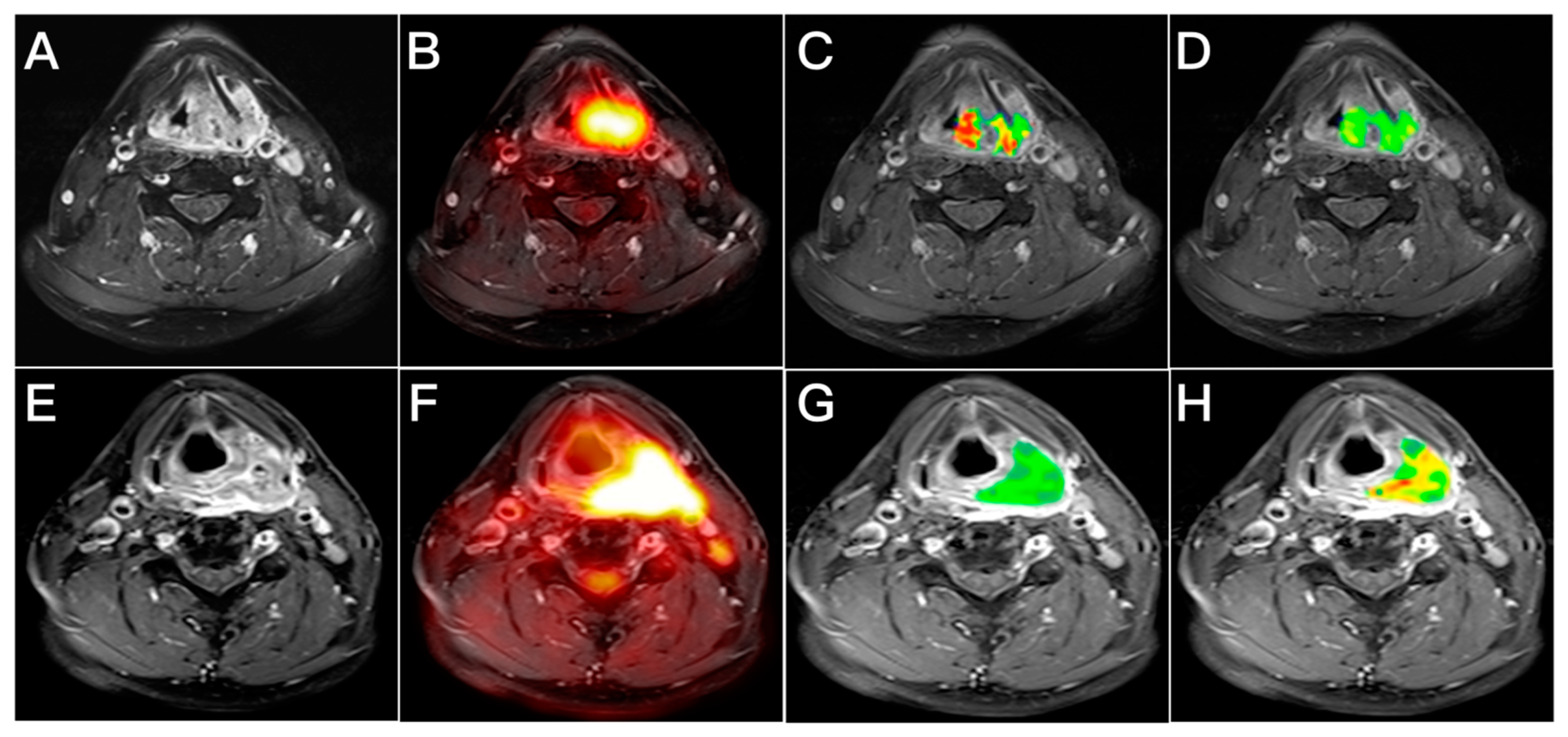
2.3. Analysis of Image
2.4. Treatment and Follow-Up
2.5. Statistical Analysis
3. Results
3.1. Univariate and Multivariable Predictors of Survival Outcomes
3.2. Performance of Multiparametric Prognostic Models Comprising IVIM PET/MRI Biomarkers
3.3. Correlation between Imaging Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chow, L.Q.M. Head and Neck Cancer. N. Engl. J. Med. 2020, 382, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Argiris, A.; Karamouzis, M.V.; Raben, D.; Ferris, R.L. Head and neck cancer. Lancet 2008, 371, 1695–1709. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.H.; Liao, C.T.; Lin, C.Y.; Chan, S.C.; Lin, Y.C.; Yen, T.C.; Chang, J.T.; Ko, S.F.; Fan, K.H.; Wang, H.M.; et al. Dynamic contrast-enhanced MRI, diffusion-weighted MRI and (18)F-FDG PET/CT for the prediction of survival in oropharyngeal or hypopharyngeal squamous cell carcinoma treated with chemoradiation. Eur. Radiol. 2016, 26, 4162–4172. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.C.; Yeh, C.H.; Yen, T.C.; Ng, S.H.; Chang, J.T.; Lin, C.Y.; Yen-Ming, T.; Fan, K.H.; Huang, B.S.; Hsu, C.L.; et al. Clinical utility of simultaneous whole-body (18)F-FDG PET/MRI as a single-step imaging modality in the staging of primary nasopharyngeal carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1297–1308. [Google Scholar] [CrossRef] [PubMed]
- Pace, L.; Nicolai, E.; Cavaliere, C.; Basso, L.; Garbino, N.; Spinato, G.; Salvatore, M. Prognostic value of 18F-FDG PET/MRI in patients with advanced oropharyngeal and hypopharyngeal squamous cell carcinoma. Ann. Nucl. Med. 2021, 35, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.H.; Chan, S.C.; Lin, C.Y.; Yen, T.C.; Chang, J.T.; Ko, S.F.; Fan, K.H.; Wang, H.M.; Liao, C.T.; Ng, S.H. Comparison of (18)F-FDG PET/MRI, MRI, and (18)F-FDG PET/CT for the detection of synchronous cancers and distant metastases in patients with oropharyngeal and hypopharyngeal squamous cell carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 94–104. [Google Scholar] [CrossRef]
- King, A.D.; Thoeny, H.C. Functional MRI for the prediction of treatment response in head and neck squamous cell carcinoma: Potential and limitations. Cancer Imaging 2016, 16, 23. [Google Scholar] [CrossRef]
- Zheng, D.; Yue, Q.; Ren, W.; Liu, M.; Zhang, X.; Lin, H.; Lai, G.; Chen, W.; Chan, Q.; Chen, Y. Early responses assessment of neoadjuvant chemotherapy in nasopharyngeal carcinoma by serial dynamic contrast-enhanced MR imaging. Magn. Reson. Imaging 2017, 35, 125–131. [Google Scholar] [CrossRef]
- Kim, S.; Loevner, L.A.; Quon, H.; Kilger, A.; Sherman, E.; Weinstein, G.; Chalian, A.; Poptani, H. Prediction of response to chemoradiation therapy in squamous cell carcinomas of the head and neck using dynamic contrast-enhanced MR imaging. AJNR Am. J. Neuroradiol. 2010, 31, 262–268. [Google Scholar] [CrossRef]
- Hatakenaka, M.; Nakamura, K.; Yabuuchi, H.; Shioyama, Y.; Matsuo, Y.; Ohnishi, K.; Sunami, S.; Kamitani, T.; Setoguchi, T.; Yoshiura, T.; et al. Pretreatment apparent diffusion coefficient of the primary lesion correlates with local failure in head-and-neck cancer treated with chemoradiotherapy or radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 339–345. [Google Scholar] [CrossRef]
- Zahra, M.A.; Hollingsworth, K.G.; Sala, E.; Lomas, D.J.; Tan, L.T. Dynamic contrast-enhanced MRI as a predictor of tumour response to radiotherapy. Lancet Oncol. 2007, 8, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.C.; Ng, S.H.; Yeh, C.H.; Chang, K.P. Multiparametric positron emission tomography/magnetic resonance imaging in nasopharyngeal carcinoma: Correlations between magnetic resonance imaging functional parameters and (18)F-fluorodeoxyglucose positron emission tomography imaging biomarkers and their predictive value for treatment failure. Tzu Chi Med. J. 2021, 33, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Chen, Y.; Liu, X.; Chen, Y.; Xu, L.; Ren, W.; Chen, W.; Chan, Q. Early response to chemoradiotherapy for nasopharyngeal carcinoma treatment: Value of dynamic contrast-enhanced 3.0 T MRI. J. Magn. Reson. Imaging 2015, 41, 1528–1540. [Google Scholar] [CrossRef] [PubMed]
- Le Bihan, D.; Breton, E.; Lallemand, D.; Aubin, M.L.; Vignaud, J.; Laval-Jeantet, M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 1988, 168, 497–505. [Google Scholar] [CrossRef]
- Guo, B.; Ouyang, F.; Ouyang, L.; Huang, X.; Guo, T.; Lin, S.; Liu, Z.; Zhang, R.; Yang, S.M.; Chen, H.; et al. Intravoxel Incoherent Motion Magnetic Resonance Imaging for Prediction of Induction Chemotherapy Response in Locally Advanced Hypopharyngeal Carcinoma: Comparison With Model-Free Dynamic Contrast-Enhanced Magnetic Resonance Imaging. J. Magn. Reson. Imaging 2021, 54, 91–100. [Google Scholar] [CrossRef]
- Qamar, S.; King, A.D.; Ai, Q.H.; So, T.Y.; Mo, F.K.F.; Chen, W.; Poon, D.M.C.; Tong, M.; Ma, B.B.; Hui, E.P.; et al. Pre-treatment intravoxel incoherent motion diffusion-weighted imaging predicts treatment outcome in nasopharyngeal carcinoma. Eur. J. Radiol. 2020, 129, 109127. [Google Scholar] [CrossRef]
- Hauser, T.; Essig, M.; Jensen, A.; Laun, F.B.; Munter, M.; Maier-Hein, K.H.; Stieltjes, B. Prediction of treatment response in head and neck carcinomas using IVIM-DWI: Evaluation of lymph node metastasis. Eur. J. Radiol. 2014, 83, 783–787. [Google Scholar] [CrossRef]
- Hauser, T.; Essig, M.; Jensen, A.; Gerigk, L.; Laun, F.B.; Munter, M.; Simon, D.; Stieltjes, B. Characterization and therapy monitoring of head and neck carcinomas using diffusion-imaging-based intravoxel incoherent motion parameters-preliminary results. Neuroradiology 2013, 55, 527–536. [Google Scholar] [CrossRef]
- Zhao, D.W.; Fan, W.J.; Meng, L.L.; Luo, Y.R.; Wei, J.; Liu, K.; Liu, G.; Li, J.F.; Zang, X.; Li, M.; et al. Comparison of the pre-treatment functional MRI metrics’ efficacy in predicting Locoregionally advanced nasopharyngeal carcinoma response to induction chemotherapy. Cancer Imaging 2021, 21, 59. [Google Scholar] [CrossRef]
- Martens, R.M.; Koopman, T.; Lavini, C.; Brug, T.V.; Zwezerijnen, G.J.C.; Marcus, J.T.; Vergeer, M.R.; Leemans, C.R.; Bree, R.; Graaf, P.; et al. Early Response Prediction of Multiparametric Functional MRI and (18)F-FDG-PET in Patients with Head and Neck Squamous Cell Carcinoma Treated with (Chemo)Radiation. Cancers 2022, 14, 216. [Google Scholar] [CrossRef]
- Chan, S.C.; Hsu, C.L.; Yen, T.C.; Ng, S.H.; Liao, C.T.; Wang, H.M. The role of 18F-FDG PET/CT metabolic tumour volume in predicting survival in patients with metastatic nasopharyngeal carcinoma. Oral Oncol. 2013, 49, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Lim, R.; Eaton, A.; Lee, N.Y.; Setton, J.; Ohri, N.; Rao, S.; Wong, R.; Fury, M.; Schoder, H. 18F-FDG PET/CT Metabolic Tumor Volume and Total Lesion Glycolysis Predict Outcome in Oropharyngeal Squamous Cell Carcinoma. J. Nucl. Med. 2012, 53, 1506–1513. [Google Scholar] [CrossRef] [PubMed]
- Pak, K.; Cheon, G.J.; Nam, H.Y.; Kim, S.J.; Kang, K.W.; Chung, J.K.; Kim, E.E.; Lee, D.S. Prognostic value of metabolic tumor volume and total lesion glycolysis in head and neck cancer: A systematic review and meta-analysis. J. Nucl. Med. 2014, 55, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Kedves, A.; Toth, Z.; Emri, M.; Fabian, K.; Sipos, D.; Freihat, O.; Tollar, J.; Cselik, Z.; Lakosi, F.; Bajzik, G.; et al. Predictive Value of Diffusion, Glucose Metabolism Parameters of PET/MR in Patients With Head and Neck Squamous Cell Carcinoma Treated With Chemoradiotherapy. Front. Oncol. 2020, 10, 1484. [Google Scholar] [CrossRef]
- Kim, Y.I.; Cheon, G.J.; Kang, S.Y.; Paeng, J.C.; Kang, K.W.; Lee, D.S.; Chung, J.K. Prognostic value of simultaneous (18)F-FDG PET/MRI using a combination of metabolo-volumetric parameters and apparent diffusion coefficient in treated head and neck cancer. EJNMMI Res. 2018, 8, 2. [Google Scholar] [CrossRef]
- Chung, M.K.; Jeong, H.S.; Park, S.G.; Jang, J.Y.; Son, Y.I.; Choi, J.Y.; Hyun, S.H.; Park, K.; Ahn, M.J.; Ahn, Y.C.; et al. Metabolic tumor volume of [18F]-fluorodeoxyglucose positron emission tomography/computed tomography predicts short-term outcome to radiotherapy with or without chemotherapy in pharyngeal cancer. Clin. Cancer Res. 2009, 15, 5861–5868. [Google Scholar] [CrossRef]
- Cheng, N.M.; Fang, Y.H.; Chang, J.T.; Huang, C.G.; Tsan, D.L.; Ng, S.H.; Wang, H.M.; Lin, C.Y.; Liao, C.T.; Yen, T.C. Textural features of pretreatment 18F-FDG PET/CT images: Prognostic significance in patients with advanced T-stage oropharyngeal squamous cell carcinoma. J. Nucl. Med. 2013, 54, 1703–1709. [Google Scholar] [CrossRef]
- Tofts, P.S.; Berkowitz, B.; Schnall, M.D. Quantitative analysis of dynamic Gd-DTPA enhancement in breast tumors using a permeability model. Magn. Reson. Med. 1995, 33, 564–568. [Google Scholar] [CrossRef]
- Wang, H.M.; Wang, C.S.; Chen, J.S.; Chen, I.H.; Liao, C.T.; Chang, T.C. Cisplatin, tegafur, and leucovorin: A moderately effective and minimally toxic outpatient neoadjuvant chemotherapy for locally advanced squamous cell carcinoma of the head and neck. Cancer 2002, 94, 2989–2995. [Google Scholar] [CrossRef]
- Kang, L.; Chen, W.; Petrick, N.A.; Gallas, B.D. Comparing two correlated C indices with right-censored survival outcome: A one-shot nonparametric approach. Stat. Med. 2015, 34, 685–703. [Google Scholar] [CrossRef]
- Ko, C.C.; Yeh, L.R.; Kuo, Y.T.; Chen, J.H. Imaging biomarkers for evaluating tumor response: RECIST and beyond. Biomark Res. 2021, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Suh, C.H.; Kim, H.S.; Lee, S.S.; Kim, N.; Yoon, H.M.; Choi, C.G.; Kim, S.J. Atypical imaging features of primary central nervous system lymphoma that mimics glioblastoma: Utility of intravoxel incoherent motion MR imaging. Radiology 2014, 272, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Martens, R.M.; Koopman, T.; Lavini, C.; Ali, M.; Peeters, C.F.W.; Noij, D.P.; Zwezerijnen, G.; Marcus, J.T.; Vergeer, M.R.; Leemans, C.R.; et al. Multiparametric functional MRI and (18)F-FDG-PET for survival prediction in patients with head and neck squamous cell carcinoma treated with (chemo)radiation. Eur. Radiol. 2021, 31, 616–628. [Google Scholar] [CrossRef] [PubMed]
- Gasparini, G.; Weidner, N.; Maluta, S.; Pozza, F.; Boracchi, P.; Mezzetti, M.; Testolin, A.; Bevilacqua, P. Intratumoral microvessel density and p53 protein: Correlation with metastasis in head-and-neck squamous-cell carcinoma. Int. J. Cancer 1993, 55, 739–744. [Google Scholar] [CrossRef]
- Lemke, A.; Laun, F.B.; Simon, D.; Stieltjes, B.; Schad, L.R. An in vivo verification of the intravoxel incoherent motion effect in diffusion-weighted imaging of the abdomen. Magn. Reson. Med. 2010, 64, 1580–1585. [Google Scholar] [CrossRef]
- Seol, Y.M.; Kwon, B.R.; Song, M.K.; Choi, Y.J.; Shin, H.J.; Chung, J.S.; Cho, G.J.; Lee, J.C.; Lee, B.J.; Wang, S.G.; et al. Measurement of tumor volume by PET to evaluate prognosis in patients with head and neck cancer treated by chemo-radiation therapy. Acta Oncol. 2010, 49, 201–208. [Google Scholar] [CrossRef]
- Xie, P.; Yue, J.B.; Fu, Z.; Feng, R.; Yu, J.M. Prognostic value of 18F-FDG PET/CT before and after radiotherapy for locally advanced nasopharyngeal carcinoma. Ann. Oncol. 2010, 21, 1078–1082. [Google Scholar] [CrossRef]

| Contrast | Region | Sequence | TR | TE | ST | FOV | VS | PAT | T |
|---|---|---|---|---|---|---|---|---|---|
| Pre-contrast | Whole body | Dixon VIBE AC | 3.6 | 1.23 | 500 | 4.1 × 2.6 × 3.1 | 2 | 01:35 | |
| Whole body | Ax T2 HASTE | 1000 | 84 | 6 | 380 | 1.5 × 1.2 × 6.0 | 2 | 03:00 | |
| Whole body | Cor STIR | 1000 | 51 | 6 | 450 | 2.5 × 1.8 × 6.0 | 3 | 03:55 | |
| Whole body | Sag STIR | 3400 | 57 | 4 | 260 | 1.4 × 1.0 × 4.0 | 2 | 04:25 | |
| Whole body | Sag T1 | 450 | 9.8 | 4 | 280 | 1.5 × 1.1 × 4.0 | 2 | 04:06 | |
| Head Neck | Dixon VIBE AC | 3.6 | 1.23 | 500 | 4.1 × 2.6 × 3.1 | 2 | 00:19 | ||
| Head Neck | Cor T1 TSE | 528 | 12 | 4 | 300 | 1.2 × 0.9 × 4.0 | 2 | 01:28 | |
| Head Neck | Cor T2 TSE FS | 4300 | 83 | 4 | 300 | 1.1 × 0.9 × 4.0 | 2 | 02:45 | |
| Head Neck | Ax T1 TSE | 580 | 11 | 4 | 200 | 0.9 × 0.8 × 4.0 | 2 | 01:32 | |
| Head Neck | Ax T2 TSE FS | 5730 | 87 | 4 | 200 | 0.8 × 0.6 × 4.0 | 2 | 03:11 | |
| Head Neck | Ax IVIM (10 b-values) | 2900 | 79 | 4 | 240 | 2.0 × 2.0 × 5.0 | 2 | 05:34 | |
| Post-contrast | Head Neck | Ax DCE MRI | 3.73 | 1.16 | 5 | 256 | 2.0 × 2.0 × 5.0 | 2 | 05:04 |
| Head Neck | Cor T1 TSE FS | 679 | 11 | 4 | 300 | 1.2 × 0.9 × 4.0 | 2 | 01:53 | |
| Head Neck | Ax T1 TSE FS | 520 | 9.7 | 4 | 200 | 0.8 × 0.6 × 4.0 | 2 | 02:14 | |
| Whole body | Ax T1 VIBE FS | 4.56 | 1.95 | 3 | 400 | 1.9 × 1.4 × 3.0 | 2 | 01:12 |
| Variable | Number of Patients (%) |
|---|---|
| Age (years), mean ± SD | 60 ± 10 |
| Sex | |
| Male | 134 (93) |
| Female | 10 (7) |
| Tumor site | |
| Oropharynx | 70 (49) |
| Hypopharynx | 74 (51) |
| Tumor stage | |
| I | 7 (5) |
| II | 21 (15) |
| III | 30 (20) |
| IVa-b | 86 (60) |
| T classification | |
| T1 | 6 (4) |
| T2 | 37 (26) |
| T3 | 18 (12) |
| T4 | 83 (58) |
| N classification | |
| N0 | 30 (21) |
| N1 | 12 (8) |
| N2 | 87 (60) |
| N3 | 15 (11) |
| Hemoglobin (g/dL), mean ± SD | 14 ± 1.9 |
| Smoking | |
| Yes | 120 (83) |
| No | 24 (17) |
| Alcohol drinking | |
| Yes | 120 (83) |
| No | 24 (17) |
| Expression of p16 | |
| Positive | 15 |
| Negative | 67 |
| Unavailable | 62 |
| Variable | Number of Patients | Overall Survival | Recurrence-Free Survival | ||
|---|---|---|---|---|---|
| 3-Year OS (Number of Events) | p-Value | 3-Year RFS (Number of Events) | p-Value | ||
| Age (years) | 0.427 | 0.108 | |||
| ≤60 | 76 | 53.5 (39) | 44.8 (40) | ||
| >60 | 68 | 58.6 (28) | 57.5 (26) | ||
| Sex | 0.144 | 0.481 | |||
| Male | 134 | 54.0 (65) | 60.0 (4) | ||
| Female | 10 | 80.0 (2) | 50.0 (62) | ||
| Tumor site | 0.756 | 0.834 | |||
| Oropharynx | 70 | 56.3 (34) | 51.2 (33) | ||
| Hypopharynx | 74 | 55.3 (33) | 50.2 (33) | ||
| Tumor stage | <0.001 | 0.018 | |||
| I-II | 28 | 89.1 (4) | 74.1 (9) | ||
| III-IV | 116 | 47.9 (63) | 44.4 (57) | ||
| T classification | <0.001 | <0.001 | |||
| T1-2 | 43 | 85.8 (7) | 75.9 (12) | ||
| T3-4 | 101 | 43.0 (60) | 38.5 (54) | ||
| N classification | 0.014 | 0.002 | |||
| N0-1 | 42 | 73.7 (11) | 75.1 (10) | ||
| N2-3 | 102 | 48.9 (56) | 41.3 (56) | ||
| Hemoglobin (g/dL) | 0.103 | 0.019 | |||
| ≤13.9 | 72 | 49.4 (38) | 41.4 (39) | ||
| >13.9 | 72 | 62.4 (29) | 59.9 (27) | ||
| Smoking | 0.246 | 0.185 | |||
| No | 24 | 66.7 (8) | 61.6 (8) | ||
| Yes | 120 | 53.8 (59) | 48.7 (58) | ||
| Alcohol consumption | 0.173 | 0.928 | |||
| No | 24 | 66.2 (8) | 49.1 (12) | ||
| Yes | 120 | 53.9 (59) | 51.0 (54) | ||
| Imaging Biomarker | |||||
| SUVmax | 0.001 | 0.130 | |||
| ≤14.2 | 59 | 70.9 (17) | 57.0 (25) | ||
| >14.2 | 85 | 45.5 (50) | 46.2 (41) | ||
| MTV (mL) | <0.001 | 0.001 | |||
| ≤81.6 | 109 | 64.7 (41) | 57.4 (44) | ||
| >81.6 | 35 | 28.6 (26) | 29.1 (22) | ||
| TLG (g/mL × mL) | 0.001 | 0.005 | |||
| ≤464.5 | 109 | 64.0 (42) | 56.4 (45) | ||
| >464.5 | 35 | 31.4 (25) | 32.1 (21) | ||
| Ktrans (10−3 min−1) | 0.350 | 0.034 | |||
| ≤297.8 | 117 | 56.8 (53) | 54.2 (51) | ||
| >297.8 | 27 | 50.9 (14) | 34.2 (15) | ||
| Kep (10−3 min−1) | 0.096 | 0.039 | |||
| ≤241.3 | 110 | 58.7 (48) | 54.7 (48) | ||
| >241.3 | 34 | 46.4 (19) | 35.9 (18) | ||
| Ve (10−3) | 0.993 | 0.007 | |||
| ≤122.3 | 23 | 55.8 (10) | 28.7 (15) | ||
| >122.3 | 121 | 55.8 (57) | 54.9 (51) | ||
| iAUC | 0.780 | 0.024 | |||
| ≤1007.2 | 126 | 55.1 (59) | 47.0 (63) | ||
| >1007.2 | 18 | 61.1 (8) | 79.3 (3) | ||
| ADCmean (10−3 mm2/s) | 0.300 | 0.599 | |||
| ≤1389 | 117 | 57.0 (53) | 49.7 (56) | ||
| >1389 | 27 | 51.6 (14) | 56.3 (10) | ||
| D* (10−3 mm2/s) | 0.012 | 0.093 | |||
| ≤403.8 | 41 | 70.6 (12) | 60.3 (15) | ||
| >403.8 | 103 | 49.9 (55) | 46.9 (51) | ||
| D (10−3 mm2/s) | 0.146 | 0.947 | |||
| ≤1239.9 | 122 | 57.7 (49) | 51.2 (52) | ||
| >1239.9 | 32 | 49.2 (18) | 48.9 (14) | ||
| f (%) | 0.070 | 0.022 | |||
| ≤165.1 | 97 | 49.0 (51) | 43.6 (52) | ||
| >165.1 | 47 | 70.1 (16) | 66.3 (14) | ||
| Variable | Multivariate Analysis | |||
|---|---|---|---|---|
| Overall Survival | Recurrence-Free Survival | |||
| Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value | |
| Tumor stage | ns | ns | ||
| T classification | 4.571 (2.043–10.224) | <0.001 | 2.187 (1.144–4.184) | 0.018 |
| N classification | ns | 2.343 (1.158–3.861) | 0.018 | |
| Hemoglobin | - | - | ns | |
| SUVmax | ns | - | - | |
| MTV | 1.907 (1.149–3.165) | 0.013 | ns | |
| Ktrans | - | - | 2.114 (1.158–3.861) | 0.015 |
| Kep | - | - | ns | |
| Ve | - | - | ns | |
| iAUC | - | - | 0.297 (0.092–0.962) | 0.043 |
| D* | 2.331 (1.243–4.368) | 0.008 | - | - |
| f | - | - | ns | |
| Variable | Overall Survival | Recurrence-Free Survival | ||
|---|---|---|---|---|
| Concordance Index | 95% CI | Concordance Index | 95% CI | |
| Tumor stage | 0.60 | 0.56–0.64 | 0.58 | 0.53–0.62 |
| PET/MRI prognostic model for OS | 0.70 * | 0.64–0.76 | - | - |
| PET/MRI prognostic model for RFS | - | - | 0.68 ** | 0.62–0.74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, S.-C.; Yeh, C.-H.; Ng, S.-H.; Lin, C.-Y.; Wang, J.-H.; Chang, J.T.-C.; Cheng, N.-M.; Chang, K.-P.; Hsieh, J.C.-H. Prospective Investigation of 18FDG-PET/MRI with Intravoxel Incoherent Motion Diffusion-Weighted Imaging to Assess Survival in Patients with Oropharyngeal or Hypopharyngeal Carcinoma. Cancers 2022, 14, 6104. https://doi.org/10.3390/cancers14246104
Chan S-C, Yeh C-H, Ng S-H, Lin C-Y, Wang J-H, Chang JT-C, Cheng N-M, Chang K-P, Hsieh JC-H. Prospective Investigation of 18FDG-PET/MRI with Intravoxel Incoherent Motion Diffusion-Weighted Imaging to Assess Survival in Patients with Oropharyngeal or Hypopharyngeal Carcinoma. Cancers. 2022; 14(24):6104. https://doi.org/10.3390/cancers14246104
Chicago/Turabian StyleChan, Sheng-Chieh, Chih-Hua Yeh, Shu-Hang Ng, Chien-Yu Lin, Jen-Hung Wang, Joseph Tung-Chieh Chang, Nai-Ming Cheng, Kai-Ping Chang, and Jason Chia-Hsun Hsieh. 2022. "Prospective Investigation of 18FDG-PET/MRI with Intravoxel Incoherent Motion Diffusion-Weighted Imaging to Assess Survival in Patients with Oropharyngeal or Hypopharyngeal Carcinoma" Cancers 14, no. 24: 6104. https://doi.org/10.3390/cancers14246104
APA StyleChan, S.-C., Yeh, C.-H., Ng, S.-H., Lin, C.-Y., Wang, J.-H., Chang, J. T.-C., Cheng, N.-M., Chang, K.-P., & Hsieh, J. C.-H. (2022). Prospective Investigation of 18FDG-PET/MRI with Intravoxel Incoherent Motion Diffusion-Weighted Imaging to Assess Survival in Patients with Oropharyngeal or Hypopharyngeal Carcinoma. Cancers, 14(24), 6104. https://doi.org/10.3390/cancers14246104







