High Serum Levels of IL-6 Predict Poor Responses in Patients Treated with Pembrolizumab plus Axitinib for Advanced Renal Cell Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Treatments
2.2. Sample Collection and Measurement of Serum IL-6
2.3. Cytokine Secretion Assays and Flow Cytometry
2.4. Analysis of a Public Database
2.5. Statistical Analysis
3. Results
3.1. Potential Effects of IL-6 on Tumor Immunity
3.2. Patient Demography and Disease Outcome
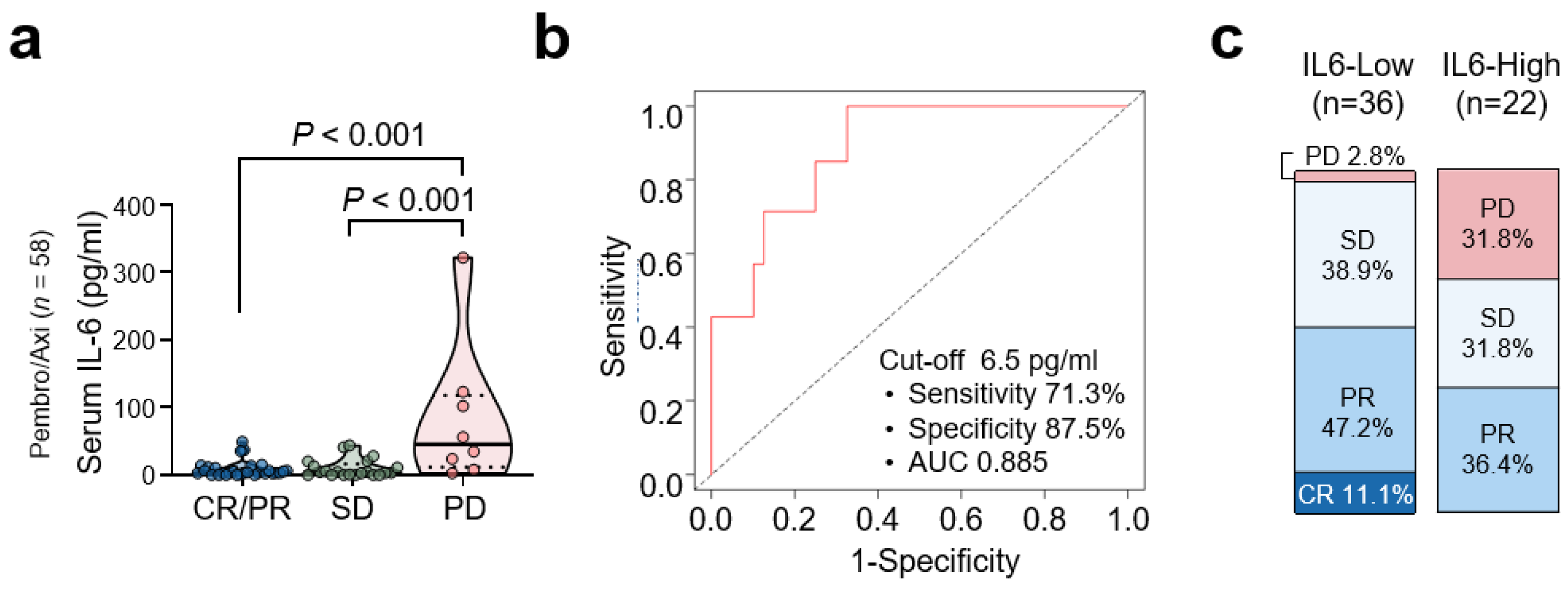
3.3. Baseline Serum IL-6 Levels and Clinical Response to Pembrolizumab/Axitinib
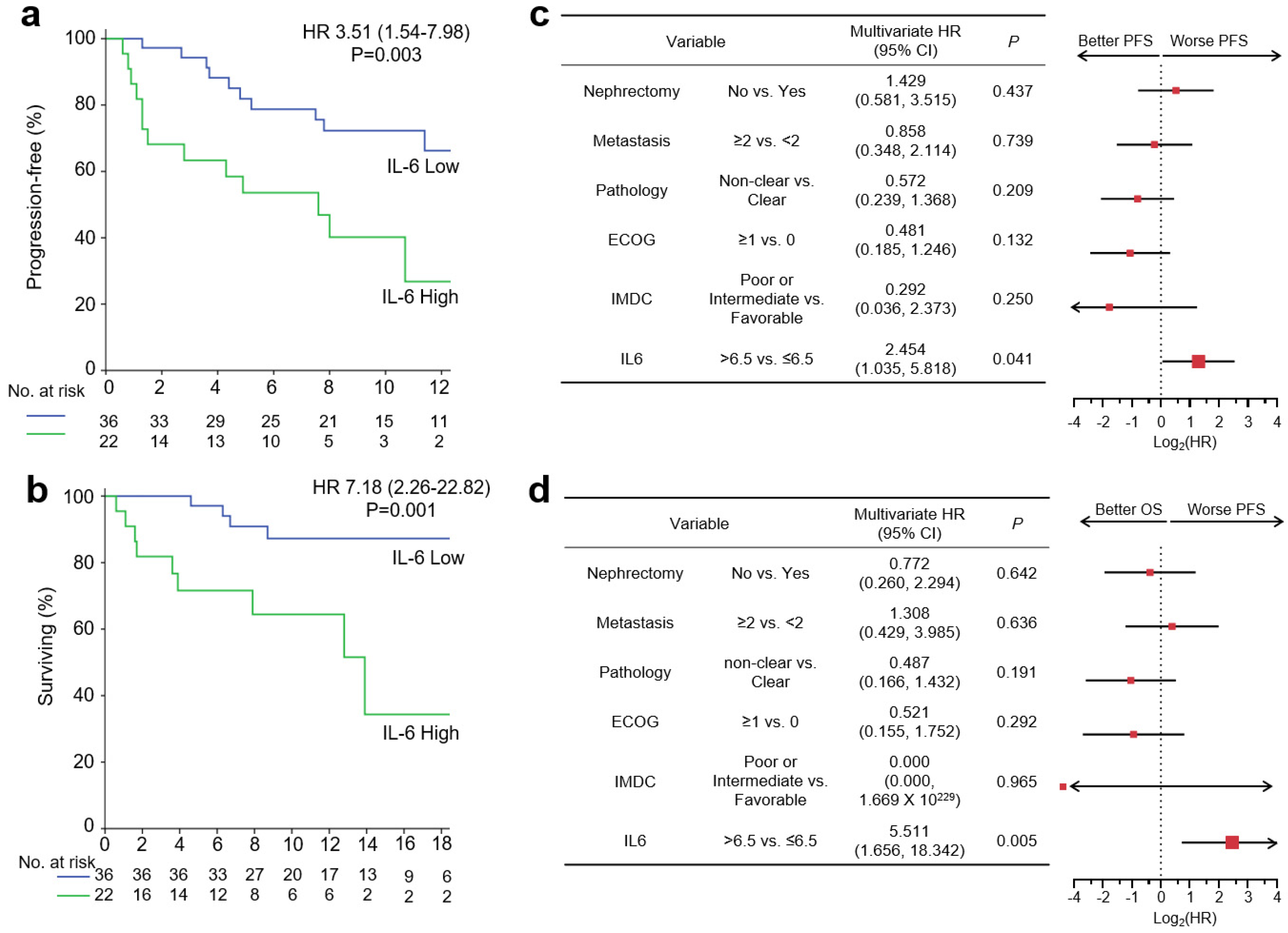
3.4. Baseline Serum IL-6 Levels and Survival Outcome with Pembro/Axi Therapy
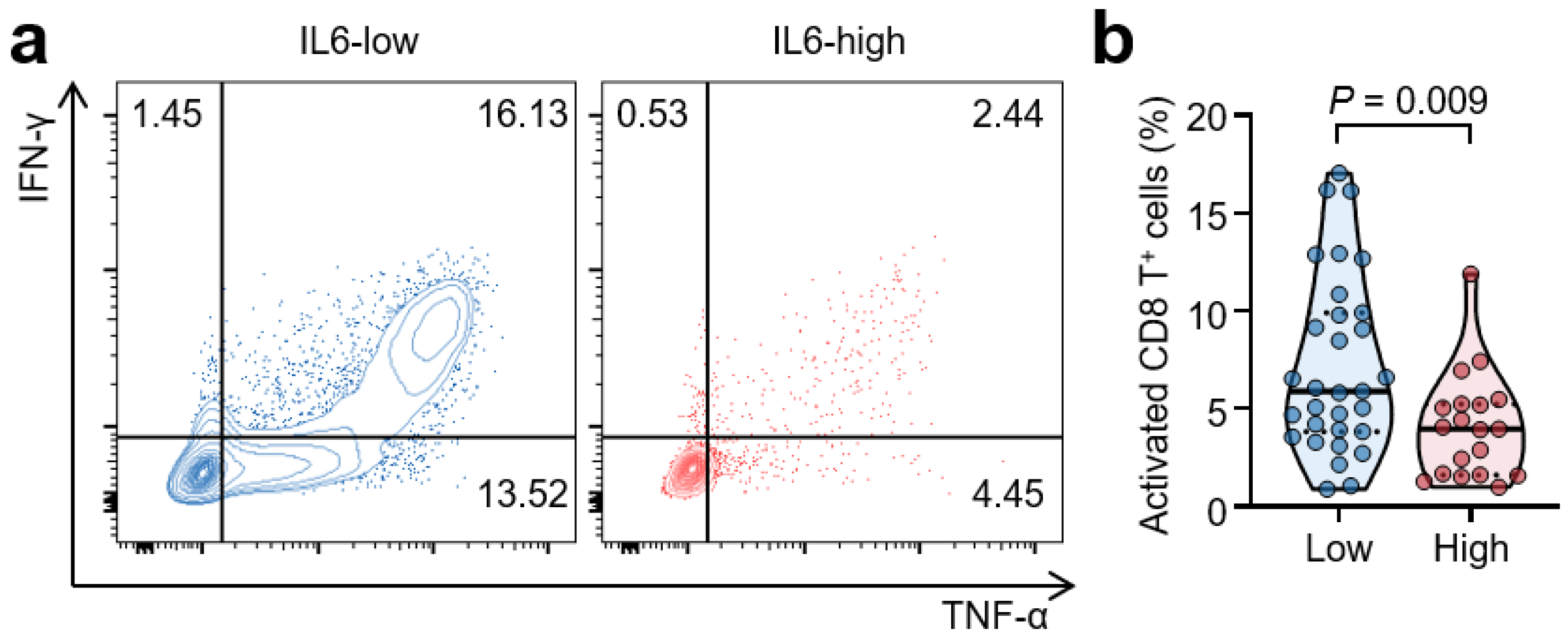
3.5. High Serum IL-6 Levels Are Associated with Reduced T-Cell Responses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Padala, S.A.; Barsouk, A.; Thandra, K.C.; Saginala, K.; Mohammed, A.; Vakiti, A.; Rawla, P.; Barsouk, A. Epidemiology of renal cell carcinoma. World J. Oncol. 2020, 11, 79. [Google Scholar] [CrossRef] [PubMed]
- Osawa, T.; Takeuchi, A.; Kojima, T.; Shinohara, N.; Eto, M.; Nishiyama, H. Overview of current and future systemic therapy for metastatic renal cell carcinoma. Jpn. J. Clin. Oncol. 2019, 49, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.H.; Kim, H.S.; Kim, C.; Ahn, J.R.; Chon, H.J.; Shin, S.-J.; Ahn, J.-B.; Chung, H.C.; Rha, S.Y. Treatment outcomes of sunitinib treatment in advanced renal cell carcinoma patients: A single cancer center experience in Korea. Cancer Res. Treat. 2009, 41, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Rini, B.I.; Plimack, E.R.; Stus, V.; Gafanov, R.; Hawkins, R.; Nosov, D.; Pouliot, F.; Alekseev, B.; Soulières, D.; Melichar, B. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 2019, 380, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Plimack, E.R.; Soulières, D.; Waddell, T.; Stus, V.; Gafanov, R.; Nosov, D.; Pouliot, F.; Melichar, B.; Vynnychenko, I. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): Extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol. 2020, 21, 1563–1573. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Stein, J.E.; Rimm, D.L.; Wang, D.W.; Bell, J.M.; Johnson, D.B.; Sosman, J.A.; Schalper, K.A.; Anders, R.A.; Wang, H. Comparison of biomarker modalities for predicting response to PD-1/PD-L1 checkpoint blockade: A systematic review and meta-analysis. JAMA Oncol. 2019, 5, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- An, H.J.; Chon, H.J.; Kim, C. Peripheral blood-based biomarkers for immune checkpoint inhibitors. Int. J. Mol. Sci. 2021, 22, 9414. [Google Scholar] [CrossRef]
- Kim, C.W.; Chon, H.J.; Kim, C. Combination Immunotherapies to Overcome Intrinsic Resistance to Checkpoint Blockade in Microsatellite Stable Colorectal Cancer. Cancers 2021, 13, 4906. [Google Scholar] [CrossRef]
- Schumacher, N.; Schmidt, S.; Schwarz, J.; Dohr, D.; Lokau, J.; Scheller, J.; Garbers, C.; Chalaris, A.; Rose-John, S.; Rabe, B. Circulating soluble IL-6R but not ADAM17 activation drives mononuclear cell migration in tissue inflammation. J. Immunol. 2016, 197, 3705–3715. [Google Scholar] [CrossRef]
- Lippitz, B.E.; Harris, R.A. Cytokine patterns in cancer patients: A review of the correlation between interleukin 6 and prognosis. Oncoimmunology 2016, 5, e1093722. [Google Scholar] [CrossRef]
- Knüpfer, H.; Preiss, R. Serum interleukin-6 levels in colorectal cancer patients—A summary of published results. Int. J. Color. Dis. 2010, 25, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, H.; Fujieda, K.; Hirayama, M.; Ikeda, T.; Yuno, A.; Matsumura, K.; Fukuma, D.; Araki, K.; Mizuta, H.; Nakayama, H. Soluble IL6R Expressed by Myeloid Cells Reduces Tumor-Specific Th1 Differentiation and Drives Tumor ProgressionMyeloid Cell–Derived sIL6R Dampens Antitumor Th1 Responses. Cancer Res. 2017, 77, 2279–2291. [Google Scholar] [CrossRef] [PubMed]
- Hoejberg, L.; Bastholt, L.; Schmidt, H. Interleukin-6 and melanoma. Melanoma Res. 2012, 22, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Rossi, J.-F.; Lu, Z.-Y.; Jourdan, M.; Klein, B. Interleukin-6 as a Therapeutic TargetAnti-IL6 Therapy. Clin. Cancer Res. 2015, 21, 1248–1257. [Google Scholar] [CrossRef] [PubMed]
- Negrier, S.; Perol, D.; Menetrier-Caux, C.; Escudier, B.; Pallardy, M.; Ravaud, A.; Douillard, J.-Y.; Chevreau, C.; Lasset, C.; Blay, J.-Y. Interleukin-6, interleukin-10, and vascular endothelial growth factor in metastatic renal cell carcinoma: Prognostic value of interleukin-6—From the Groupe Francais d’Immunotherapie. J. Clin. Oncol. 2004, 22, 2371–2378. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y. Prognostic role of interleukin-6 in renal cell carcinoma: A meta-analysis. Clin. Transl. Oncol. 2020, 22, 835–843. [Google Scholar] [CrossRef]
- Gudbrandsdottir, G.; Aarstad, H.H.; Bostad, L.; Hjelle, K.M.; Aarstad, H.J.; Bruserud, Ø.; Tvedt, T.H.A.; Beisland, C. Serum levels of the IL-6 family of cytokines predict prognosis in renal cell carcinoma (RCC). Cancer Immunol. Immunother. 2021, 70, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.T.; Liu, Y.; Zurita, A.J.; Lin, Y.; Baker-Neblett, K.L.; Martin, A.-M.; Figlin, R.A.; Hutson, T.E.; Sternberg, C.N.; Amado, R.G. Prognostic or predictive plasma cytokines and angiogenic factors for patients treated with pazopanib for metastatic renal-cell cancer: A retrospective analysis of phase 2 and phase 3 trials. Lancet Oncol. 2012, 13, 827–837. [Google Scholar] [CrossRef]
- Tsukamoto, H.; Fujieda, K.; Senju, S.; Ikeda, T.; Oshiumi, H.; Nishimura, Y. Immune-suppressive effects of interleukin-6 on T-cell-mediated anti-tumor immunity. Cancer Sci. 2018, 109, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Veglia, F.; Perego, M.; Gabrilovich, D. Myeloid-derived suppressor cells coming of age. Nat. Immunol. 2018, 19, 108–119. [Google Scholar] [CrossRef]
- Liu, C.; Yang, L.; Xu, H.; Zheng, S.; Wang, Z.; Wang, S.; Yang, Y.; Zhang, S.; Feng, X.; Sun, N. Systematic analysis of IL-6 as a predictive biomarker and desensitizer of immunotherapy responses in patients with non-small cell lung cancer. BMC Med. 2022, 20, 187. [Google Scholar]
- Zheng, Z.; Zheng, X.; Zhu, Y.; Yao, Z.; Zhao, W.; Zhu, Y.; Sun, F.; Mu, X.; Wang, Y.; He, W. IL-6 promotes the proliferation and immunosuppressive function of myeloid-derived suppressor cells via the MAPK signaling pathway in bladder cancer. BioMed Res. Int. 2021, 2021, 5535578. [Google Scholar] [CrossRef] [PubMed]
- Bent, E.H.; Millán-Barea, L.R.; Zhuang, I.; Goulet, D.R.; Fröse, J.; Hemann, M.T. Microenvironmental IL-6 inhibits anti-cancer immune responses generated by cytotoxic chemotherapy. Nat. Commun. 2021, 12, 6218. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, W.S.; Lee, H.J.; Yang, H.; Lee, S.J.; Kong, S.J.; Je, S.; Yang, H.-J.; Jung, J.; Cheon, J. Deep learning model enables the discovery of a novel immunotherapeutic agent regulating the kynurenine pathway. Oncoimmunology 2021, 10, 2005280. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Fu, J.; Zeng, Z.; Cohen, D.; Li, J.; Chen, Q.; Li, B.; Liu, X.S. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 2020, 48, W509–W514. [Google Scholar] [CrossRef]
- Walter, M.; Liang, S.; Ghosh, S.; Hornsby, P.; Li, R. Interleukin 6 secreted from adipose stromal cells promotes migration and invasion of breast cancer cells. Oncogene 2009, 28, 2745–2755. [Google Scholar] [CrossRef]
- Taniguchi, K.; Karin, M. IL-6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin. Immunol. 2014, 26, 54–74. [Google Scholar]
- Naka, T.; Nishimoto, N.; Kishimoto, T. The paradigm of IL-6: From basic science to medicine. Arthritis Res. Ther. 2002, 4, S233–S242. [Google Scholar] [CrossRef]
- Wu, J.; Gao, F.; Wang, C.; Qin, M.; Han, F.; Xu, T.; Hu, Z.; Long, Y.; He, X.; Deng, X. IL-6 and IL-8 secreted by tumour cells impair the function of NK cells via the STAT3 pathway in oesophageal squamous cell carcinoma. J. Exp. Clin. Cancer Res. 2019, 38, 321. [Google Scholar] [CrossRef]
- Pilskog, M.; Nilsen, G.H.; Beisland, C.; Straume, O. Elevated plasma interleukin 6 predicts poor response in patients treated with sunitinib for metastatic clear cell renal cell carcinoma. Cancer Treat. Res. Commun. 2019, 19, 100127. [Google Scholar] [CrossRef]
- Mizuno, R.; Kimura, G.; Fukasawa, S.; Ueda, T.; Kondo, T.; Hara, H.; Shoji, S.; Kanao, K.; Nakazawa, H.; Tanabe, K. Angiogenic, inflammatory and immunologic markers in predicting response to sunitinib in metastatic renal cell carcinoma. Cancer Sci. 2017, 108, 1858–1863. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Number of Patients, n (% of Total) |
|---|---|
| Age | |
| ≥65 | 23 (39.7) |
| Male | 42 (72.4) |
| ECOG performance status | |
| 0 | 22 (37.9) |
| 1 | 29 (50.0) |
| 2 | 7 (12.1) |
| IMDC prognostic risk | |
| Favorable | 11 (19.0) |
| Intermediate | 29 (50.0) |
| Poor | 18 (31.0) |
| Pathology | |
| Clear cell | 41 (70.7) |
| Non-clear cell | 17 (29.3) |
| No. of organs with metastases | |
| 1 | 20 (34.5) |
| ≥2 | 38 (65.5) |
| Sites of metastasis | |
| Lung | 47 (81.0) |
| Lymph node | 17 (29.3) |
| Bone | 21 (36.2) |
| Liver | 6 (10.3) |
| Previous nephrectomy | 30 (51.7) |
| Characteristic | IL-6 Low (n = 36) | IL-6 High (n = 22) | p-Value |
|---|---|---|---|
| Age | |||
| Median (range), y | 61 (39–82) | 59 (47–83) | 0.572 |
| ≥65, n (%) | 13 (36.1) | 10 (45.5) | 0.480 |
| Male, n (%) | 28 (77.8) | 14 (63.6) | 0.242 |
| ECOG performance status | 0.316 | ||
| 0 | 16 (44.4) | 6 (27.3) | |
| 1 | 17 (47.2) | 12 (54.5) | |
| 2 | 3 (8.3) | 4 (18.2) | |
| IMDC prognostic risk, n (%) | 0.009 | ||
| Favorable | 9 (25.0) | 2 (9.1) | |
| Intermediate | 21 (58.3) | 8 (36.4) | |
| Poor | 6 (16.7) | 12 (54.5) | |
| Pathology, n (%) | 0.356 | ||
| Clear cell | 27 (75.0) | 14 (63.6) | |
| Non-clear cell | 9 (25.0) | 8 (36.4) | |
| No. of organs with metastases, n (%) | 0.366 | ||
| 0, 1 | 14 (38.9) | 6 (27.3) | |
| ≥2 | 22 (61.1) | 16 (72.7) | |
| Sites of metastasis, n (%) | |||
| Lung | 28 (77.8) | 19 (86.4) | 0.418 |
| Lymph node | 7 (19.4) | 10 (45.5) | 0.035 |
| Bone | 9 (25.0) | 12 (54.5) | 0.023 |
| Liver | 4 (11.1) | 2 (9.1) | 0.806 |
| Previous nephrectomy, n (%) | 28 (53.8) | 2 (33.3) | 0.018 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sang, Y.B.; Yang, H.; Lee, W.S.; Lee, S.J.; Kim, S.-G.; Cheon, J.; Kang, B.; Kim, C.W.; Chon, H.J.; Kim, C. High Serum Levels of IL-6 Predict Poor Responses in Patients Treated with Pembrolizumab plus Axitinib for Advanced Renal Cell Carcinoma. Cancers 2022, 14, 5985. https://doi.org/10.3390/cancers14235985
Sang YB, Yang H, Lee WS, Lee SJ, Kim S-G, Cheon J, Kang B, Kim CW, Chon HJ, Kim C. High Serum Levels of IL-6 Predict Poor Responses in Patients Treated with Pembrolizumab plus Axitinib for Advanced Renal Cell Carcinoma. Cancers. 2022; 14(23):5985. https://doi.org/10.3390/cancers14235985
Chicago/Turabian StyleSang, Yun Beom, Hannah Yang, Won Suk Lee, Seung Joon Lee, Seul-Gi Kim, Jaekyung Cheon, Beodeul Kang, Chang Woo Kim, Hong Jae Chon, and Chan Kim. 2022. "High Serum Levels of IL-6 Predict Poor Responses in Patients Treated with Pembrolizumab plus Axitinib for Advanced Renal Cell Carcinoma" Cancers 14, no. 23: 5985. https://doi.org/10.3390/cancers14235985
APA StyleSang, Y. B., Yang, H., Lee, W. S., Lee, S. J., Kim, S.-G., Cheon, J., Kang, B., Kim, C. W., Chon, H. J., & Kim, C. (2022). High Serum Levels of IL-6 Predict Poor Responses in Patients Treated with Pembrolizumab plus Axitinib for Advanced Renal Cell Carcinoma. Cancers, 14(23), 5985. https://doi.org/10.3390/cancers14235985







