Scabertopin Derived from Elephantopus scaber L. Mediates Necroptosis by Inducing Reactive Oxygen Species Production in Bladder Cancer In Vitro
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Main Reagents
2.2. Material Preparation
2.3. Infrared Spectroscopy
2.4. UV Absorption Assay
2.5. Cell Lines and Culture
2.6. CCK-8 Assay
2.7. LIVE/DEAD Cell Activity Assay
2.8. Scanning and Transmission Electron Microscopy
2.9. Measurement of Reactive Oxygen Species
2.10. Mitochondrial Membrane Potential Assay
2.11. GSH Assay
2.12. Wound Healing Assay
2.13. Transwell Assay
2.14. Cell cycle Assays
2.15. Cell Apoptosis Analysis
2.16. Western Blot Analysis
2.17. Statistical Analysis
3. Results
3.1. Bladder Cancer Cells Are Sensitive to Scabertopin
3.2. Increased Mitochondrial ROS Levels in J82 Cells Treated with Scabertopin
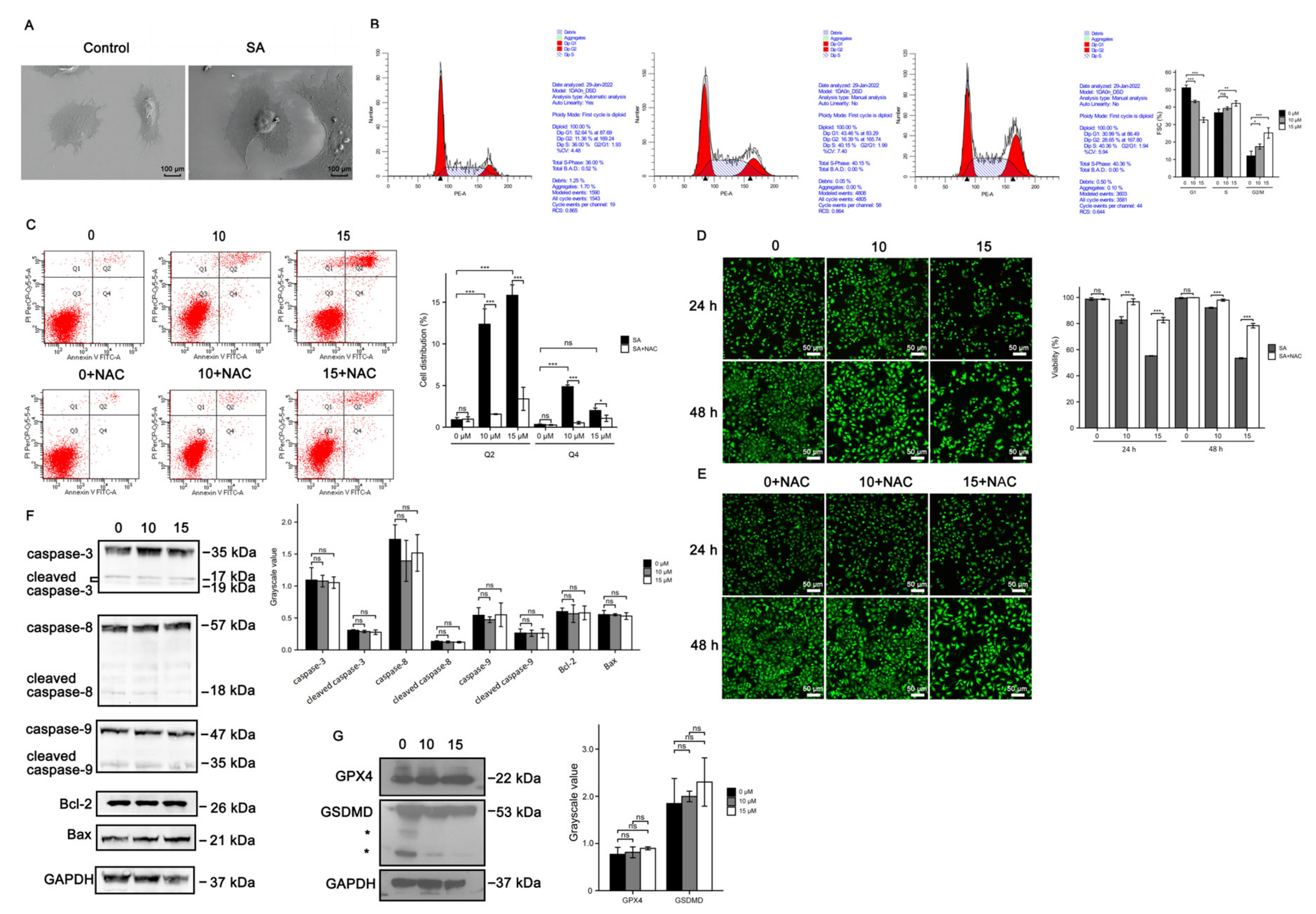
3.3. Cell Death Induced by Scabertopin Treatment Was Not Apoptosis and Ferroptosis
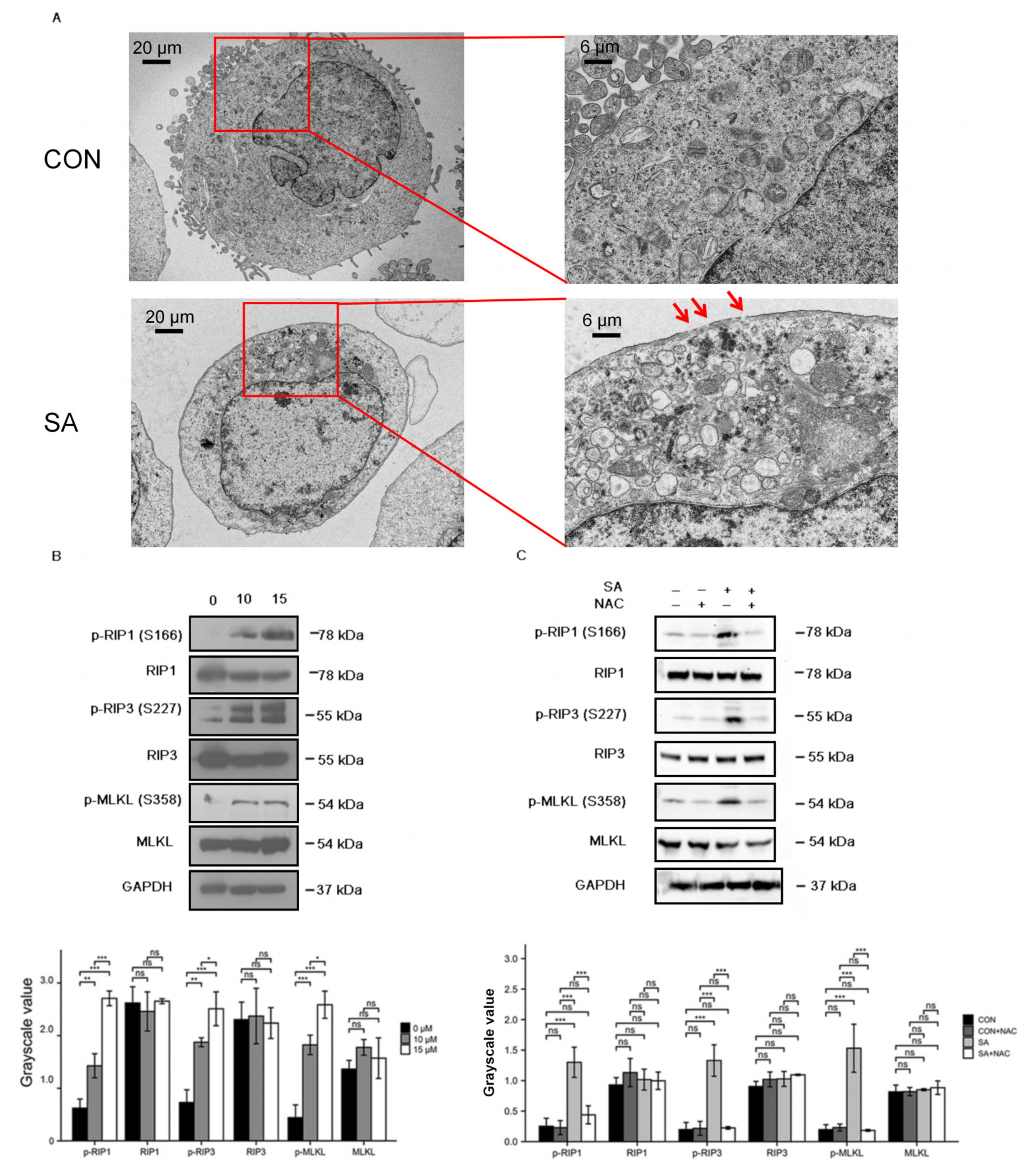
3.4. Necroptosis Is a Type of Cell Death Induced by Scabertopin and can Be Inhibited by NAC
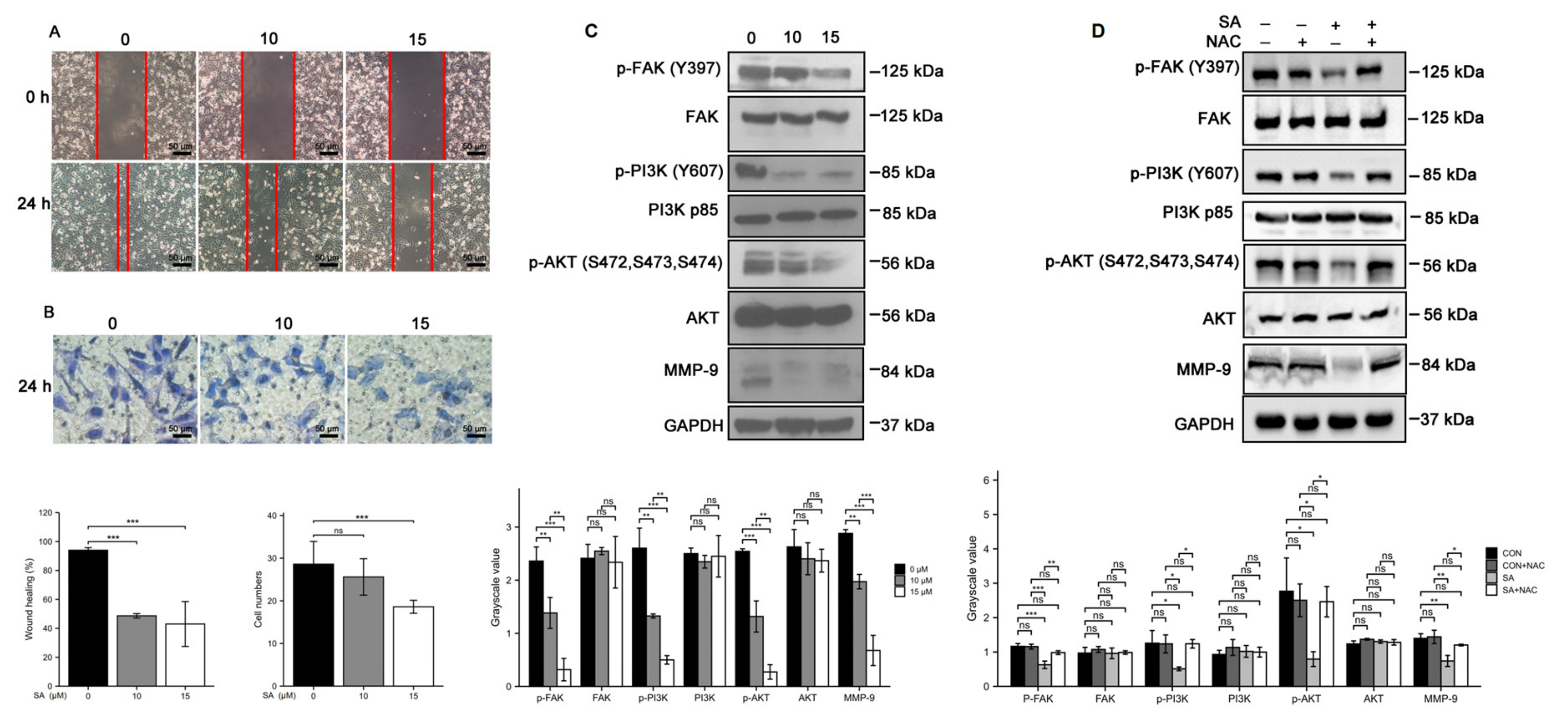
3.5. Scabertopin Inhibits the Migration and Invasion of Bladder Cancer Cells
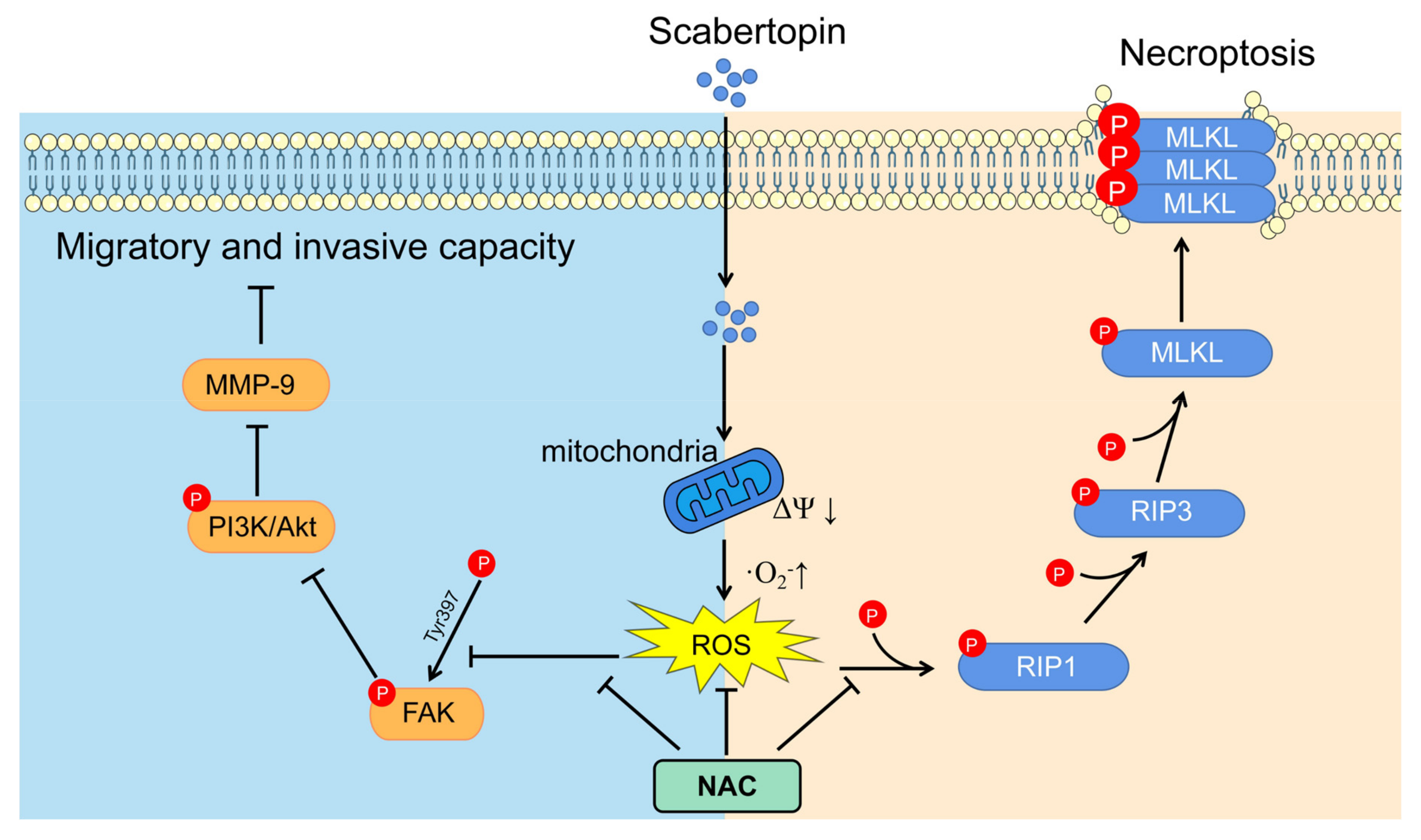
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA A Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA A Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zheng, R.; Baade, P.D.; Zhang, S.; Zeng, H.; Bray, F.; Jemal, A.; Xue, Q.Y.; Jie, H. Cancer statistics in China, 2015. CA A Cancer J. Clin. 2016, 66, 115–132. [Google Scholar] [CrossRef] [PubMed]
- Saluja, M.; Gilling, P. Intravesical bacillus Calmette-Guérin instillation in non-muscle-invasive bladder cancer: A review. Int. J. Urol. 2017, 25, 18–24. [Google Scholar] [CrossRef]
- Motzer, R.J.; Jonasch, E.; Boyle, S.; Carlo, M.I.; Motter, A.D. NCCN Guidelines Insights: Kidney Cancer, Version 1.2021. J. Natl. Compr. Cancer Netw. 2020, 18, 1160–1170. [Google Scholar] [CrossRef]
- Campodonico, A.; Crocellà, A.; Fresu, I. Influence of glucose and saline solutions on biliary secretion, in relation to hepatic elimination of biligrafin. Experimental study on cholecystectomized dogs. IL Fegato 1965, 11, 219–225. [Google Scholar]
- Safe, S.; Kasiappan, R. Natural Products as Mechanism-based Anticancer Agents: Sp Transcription Factors as Targets. Phytother. Res. 2016, 30, 1723–1732. [Google Scholar] [CrossRef]
- Xu, G.; Liang, Q.; Gong, Z.; Yu, W.; He, S.; Xi, L. Antitumor activities of the four sesquiterpene lactones from Elephantopus scaber L. Exp. Oncol. 2006, 28, 106–109. [Google Scholar]
- Anitha, V.T.; Marimuthuantonisamy, J.; Jeeva, S. Anti-bacterial studies on Hemigraphis colorata (Blume) H.G. Hallier and Elephantopus scaber L. Asian Pac. J. Trop. Med. 2012, 5, 52–57. [Google Scholar] [CrossRef]
- Tahir, M.; Amara, M.; Hamed, G.; Muhammad, K.; Ma, T. Deoxyelephantopin and Isodeoxyelephantopin as Potential Anticancer Agents with Effects on Multiple Signaling Pathways. Molecules 2017, 22, 1013. [Google Scholar]
- Su, M.; Chung, H.Y.; Li, Y. Deoxyelephantopin from Elephantopus scaber L. induces cell-cycle arrest and apoptosis in the human nasopharyngeal cancer CNE cells. Biochem. Biophys. Res. Commun. 2011, 411, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.R.; Tan, Z.; Wang, Y.; Xu, M.L.; Yu, G.; Li, Y.; He, Q.Y. Quantitative proteomics characterization on the antitumor effects of isodeoxyelephantopin against nasopharyngeal carcinoma. Proteomics 2013, 13, 3222–3232. [Google Scholar] [CrossRef] [PubMed]
- Beeran, A.A.; Maliyakkal, N.; Rao, M.C.; Udupa, N. The enriched fraction of Elephantopus scaber Triggers apoptosis and inhibits multi-drug resistance transporters in human epithelial cancer cells. Pharmacogn. Mag. 2015, 11, 257–268. [Google Scholar] [PubMed]
- Huang, C.C.; Lo, C.P.; Chiu, C.Y.; Shyur, L.F. Deoxyelephantopin, a novel multifunctional agent, suppresses mammary tumour growth and lung metastasis and doubles survival time in mice. Br. J. Pharmacol. 2010, 159, 856–871. [Google Scholar] [CrossRef]
- Hong, L.; Chen, J.; Wu, F.; Wu, F.; Xia, Y. Isodeoxyelephantopin Inactivates Thioredoxin Reductase 1 and Activates ROS-Mediated JNK Signaling Pathway to Exacerbate Cisplatin Effectiveness in Human Colon Cancer Cells. Front. Cell Dev. Biol. 2020, 8, 580517. [Google Scholar] [CrossRef] [PubMed]
- Kabeer, F.A.; Rajalekshmi, D.S.; Nair, M.S.; Prathapan, R. Molecular mechanisms of anticancer activity of deoxyelephantopin in cancer cells. Integr. Med. Res. 2017, 6, 190–206. [Google Scholar] [CrossRef]
- Mehmood, T.; Maryam, A.; Zhang, H.; Li, Y.; Khan, M.; Ma, T. Deoxyelephantopin induces apoptosis in HepG2 cells via oxidative stress, NF-κB inhibition and mitochondrial dysfunction. BioFactors 2017, 43, 63–72. [Google Scholar] [CrossRef]
- Geetha, B.S.; Nair, M.S.; Latha, P.G.; Remani, P. Sesquiterpene Lactones Isolated from Elephantopus scaber L. Inhibits Human Lymphocyte Proliferation and the Growth of Tumour Cell Lines and Induces Apoptosis In Vitro. J. Biomed. Biotechnol. 2012, 2012, 721285. [Google Scholar] [CrossRef]
- Koe, X.F.; Lim, E.L.; Seah, T.C.; Amanah, A.; Wahab, H.A.; Adenan, M.I.; Sulaiman, S.F.; Tan, M.L. Evaluation of in vitro cytochrome P450 induction and inhibition activity of deoxyelephantopin, a sesquiterpene lactone from Elephantopus scaber L. Food Chem. Toxicol. 2013, 60, 98–108. [Google Scholar] [CrossRef]
- Galluzzi, L.; Kroemer, G. Necroptosis: A Specialized Pathway of Programmed Necrosis. Cell 2009, 135, 1161–1163. [Google Scholar] [CrossRef]
- Berghe, T.V.; Linkermann, A.; Jouan-Lanhouet, S.; Walczak, H.; Vandenabeele, P. Regulated necrosis: The expanding network of non-apoptotic cell death pathways. Nat. Rev. Mol. Cell Biol. 2014, 15, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Wang, F.; Guo, Q.; Li, M.; Wang, L.; Zhang, Z.; Jiang, S.; Jin, H.; Chen, A.; Tan, S.; et al. Curcumol induces RIPK1/RIPK3 complex-dependent necroptosis via JNK1/2-ROS signaling in hepatic stellate cells. Redox Biol. 2018, 19, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Y.; Gao, H.; Hou, Y.; Lu, J.J.; Feng, Y.; Xu, Q.; Liu, B.; Chen, X. Induction of an MLKL mediated non-canonical necroptosis through reactive oxygen species by tanshinol A in lung cancer cells—ScienceDirect. Biochem. Pharmacol. 2019, 171, 113684. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, W.; Ren, J.; Huang, D.; He, W.T.; Song, Y.; Chao, Y. Translocation of mixed lineage kinase domain-like protein to plasma membrane leads to necrotic cell death. Cell Res. 2014, 24, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sun, L.; Su, L.; Rizo, J.; Liu, L.; Wang, L.F.; Wang, F.S.; Wang, X. Mixed Lineage Kinase Domain-like Protein MLKL Causes Necrotic Membrane Disruption upon Phosphorylation by RIP3. Mol. Cell 2014, 54, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Dondelinger, Y.; Declercq, W.; Montessuit, S.; Roelandt, R.; Goncalves, A.; Bruggeman, I.; Hulpiau, P.; Weber, K.; Sehon, C.A.; Marquis, R.W.; et al. MLKL comprises plasma membrane integrity by binding to phosphatidylinositol phosphates. Cell Rep. 2014, 7, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.K.; Chang, W.T.; Lin, I.L.; Chen, Y.F.; Chiu, C.C. The Role of Necroptosis in ROS-Mediated Cancer Therapies and Its Promising Applications. Cancers 2020, 12, 2185. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, S.; Zhao, S.; Yang, Z.; Zhong, C.; Chen, X.; Cai, Q.; Yang, Z.; Huang, D.; Wu, R.; et al. RIP1 autophosphorylation is promoted by mitochondrial ROS and is essential for RIP3 recruitment into necrosome. Nat. Commun. 2017, 8, 14329. [Google Scholar] [CrossRef]
- Chauhan, A.K.; Min, K.J.; Kwon, T.K. RIP1-dependent reactive oxygen species production executes artesunate-induced cell death in renal carcinoma Caki cells. Mol. Cell. Biochem. 2017, 435, 15–24. [Google Scholar] [CrossRef]
- Wang, K.J.; Meng, X.Y.; Chen, J.F.; Wang, K.Y.; Ma, Q. Emodin Induced Necroptosis and Inhibited Glycolysis in the Renal Cancer Cells by Enhancing ROS. Oxid. Med. Cell. Longev. 2021, 2021, 8840590. [Google Scholar] [CrossRef]
- Pak, V.; Ezeriņa, D.; Lyublinskaya, O.; Pedre, B.; Tyurin-Kuzmin, P.; Mishina, N.; Thauvin, M.; Young, D.; Wahni, K.; Martínez Gache, S.; et al. Ultrasensitive Genetically Encoded Indicator for Hydrogen Peroxide Identifies Roles for the Oxidant in Cell Migration and Mitochondrial Function. Cell Metab. 2020, 31, 642–653.E6. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.; Chen, M.; Gao, Y.; Huang, D.; Cao, H.; Peng, Y.; Guo, N.; Zhang, S. Regulated Cell Death in Urinary Malignancies. Front. Cell Dev. Biol. 2021, 9, 789004. [Google Scholar] [CrossRef] [PubMed]
- Millimouno, F.M.; Dong, J.; Yang, L.; Li, J.; Li, X. Targeting apoptosis pathways in cancer and perspectives with natural compounds from mother nature. Cancer Prev. Res. 2014, 7, 1081. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Dong, W. ROS and ROS-mediated cellular signaling. Oxid. Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef] [PubMed]
- De Sá Junior, P.L.; Dias, C.D.A.; Saj, P.A.; Moreira, F.P.M.; Doria, J.S.O.; Pinheiro, A.R.; Kleber, F.A. The Roles of ROS in Cancer Heterogeneity and Therapy. Oxid. Med. Cell. Longev. 2017, 2017, 2467940. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Ding, C.; Rasul, A.; Fei, Y.; Ma, T. Isoalantolactone Induces Reactive Oxygen Species Mediated Apoptosis in Pancreatic Carcinoma PANC-1 Cells. Int. J. Biol. Sci. 2012, 8, 533–547. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.M.; Jeong, K.; Lim, S. FAK Family Kinases in Vascular Diseases. Int. J. Mol. Sci. 2020, 21, 3630. [Google Scholar] [CrossRef]
- Mclean, G.W.; Carragher, N.O.; Avizienyte, E.; Evans, J.; Brunton, V.G.; Frame, M.C. The role of focal-adhesion kinase in cancer—a new therapeutic opportunity. Nat. Rev. Cancer 2005, 5, 505–515. [Google Scholar] [CrossRef]
- Lai, I.R.; Chu, P.Y.; Lin, H.S.; Liou, J.Y.; Jan, Y.J.; Lee, J.C.; Shen, T.L. Phosphorylation of Focal Adhesion Kinase at Tyr397 in Gastric Carcinomas and its Clinical Significance. Am. J. Pathol. 2010, 177, 1629–1637. [Google Scholar] [CrossRef]
- Jang, H.; Hong, O.; Youn, H.; Kim, M.; Kim, C.; Jung, S.; Kim, J. 15d-PGJ2 inhibits NF-κB and AP-1-mediated MMP-9 expression and invasion of breast cancer cell by means of a heme oxygenase-1-dependent mechanism. BMB Rep. 2020, 53, 212–217. [Google Scholar] [CrossRef]
- Yang, W.; Stockwell, B. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem. Biol. 2008, 15, 234–245. [Google Scholar] [CrossRef]
- Yang, W.; Stockwell, B. Ferroptosis: Death by Lipid Peroxidation. Trends Cell Biol. 2016, 26, 165–176. [Google Scholar] [CrossRef] [PubMed]
- D’Herde, K.; Krysko, D. Ferroptosis: Oxidized PEs trigger death. Nat. Chem. Biol. 2017, 13, 4–5. [Google Scholar] [CrossRef] [PubMed]
- Florean, C.; Song, S.; Dicato, M.; Diederich, M. Redox biology of regulated cell death in cancer: A focus on necroptosis and ferroptosis. Free. Radic. Biol. Med. 2019, 134, 177–189. [Google Scholar] [CrossRef]
- Reczek, C.; Chandel, N. ROS-dependent signal transduction. Curr. Opin. Cell Biol. 2015, 33, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Zhang, N.; Zhou, H.; Li, J.; Li, Q.; Zarubin, T.; Lin, S.; Han, J. Distinct roles of basal steady-state and induced H-ferritin in tumor necrosis factor-induced death in L929 cells. Mol. Cell. Biol. 2005, 25, 6673–6681. [Google Scholar] [CrossRef] [PubMed]
- Vandenabeele, P.; Galluzzi, L.; Vanden Berghe, T.; Kroemer, G. Molecular mechanisms of necroptosis: An ordered cellular explosion. Nature reviews. Mol. Cell. Biol. 2010, 11, 700–714. [Google Scholar] [CrossRef]
- Canli, Ö.; Alankuş, Y.; Grootjans, S.; Vegi, N.; Hültner, L.; Hoppe, P.; Schroeder, T.; Vandenabeele, P.; Bornkamm, G.; Greten, F. Glutathione peroxidase 4 prevents necroptosis in mouse erythroid precursors. Blood 2016, 127, 139–148. [Google Scholar] [CrossRef]
- Galadari, S.; Rahman, A.; Pallichankandy, S.; Thayyullathil, F. Reactive oxygen species and cancer paradox: To promote or to suppress? Free. Radic. Biol. Med. 2017, 104, 144–164. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Z.; Li, Q.; Jiao, H.; Chong, D.; Sun, X.; Zhang, P.; Huo, Q.; Liu, H. Shikonin induces necroptosis by reactive oxygen species activation in nasopharyngeal carcinoma cell line CNE-2Z. J. Bioenerg. Biomembr. 2017, 49, 265–272. [Google Scholar] [CrossRef]
- Liu, T.; Sun, X.; Cao, Z. Shikonin-induced necroptosis in nasopharyngeal carcinoma cells via ROS overproduction and upregulation of RIPK1/RIPK3/MLKL expression. OncoTargets Ther. 2019, 12, 2605–2614. [Google Scholar] [CrossRef] [PubMed]
- Huangfu, M.; Wei, R.; Wang, J.; Qin, J.; Yu, D.; Guan, X.; Li, X.; Fu, M.; Liu, H.; Chen, X. Osthole induces necroptosis via ROS overproduction in glioma cells. FEBS Open Bio. 2021, 11, 456–467. [Google Scholar] [CrossRef]
- Su, Z.; Yang, Z.; Xie, L.; DeWitt, J.; Chen, Y. Cancer therapy in the necroptosis era. Cell Death Differ. 2016, 23, 748–756. [Google Scholar] [CrossRef]
- Moriwaki, K.; Bertin, J.; Gough, P.; Orlowski, G.; Chan, F. Differential roles of RIPK1 and RIPK3 in TNF-induced necroptosis and chemotherapeutic agent-induced cell death. Cell Death Dis. 2015, 6, e1636. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Rai, V.; Awasthee, N.; Dhasmana, A.; Rajalaksmi, D.; Nair, M.; Gupta, S. Isodeoxyelephantopin, a Sesquiterpene Lactone Induces ROS Generation, Suppresses NF-κB Activation, Modulates LncRNA Expression and Exhibit Activities Against Breast Cancer. Sci. Rep. 2019, 9, 17980. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zhu, X.; Liu, P.; Liu, Y.; Geng, Y.; Zhang, L. Xanthatin inhibits non-small cell lung cancer proliferation by breaking the redox balance. Drug Dev. Res. 2022, 83, 1176–1189. [Google Scholar] [CrossRef] [PubMed]
- Ghantous, A.; Gali-Muhtasib, H.; Vuorela, H.; Saliba, N.; Darwiche, N. What made sesquiterpene lactones reach cancer clinical trials? Drug Discov. Today 2010, 15, 668–678. [Google Scholar] [CrossRef]
- Woerdenbag, H.; Merfort, I.; Passreiter, C.; Schmidt, T.; Willuhn, G.; van Uden, W.; Pras, N.; Kampinga, H.; Konings, A. Cytotoxicity of flavonoids and sesquiterpene lactones from Arnica species against the GLC4 and the COLO 320 cell lines. Planta Med. 1994, 60, 434–437. [Google Scholar] [CrossRef]
- Armstrong, J. The role of the mitochondrial permeability transition in cell death. Mitochondrion 2006, 6, 225–234. [Google Scholar] [CrossRef]
- Chang, Y.; Kim, C. Molecular Research of Glycolysis. Int. J. Mol. Sci. 2022, 23, 5052. [Google Scholar] [CrossRef]
- Yagoda, N.; von Rechenberg, M.; Zaganjor, E.; Bauer, A.; Yang, W.; Fridman, D.; Wolpaw, A.; Smukste, I.; Peltier, J.; Boniface, J.; et al. RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature 2007, 447, 864–868. [Google Scholar] [CrossRef] [PubMed]
- National Library of Medicine (US), National Center for Biotechnology Information. PubChem Compound Summary for CID 93959111. 2004. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/93959111 (accessed on 10 August 2022).
- Lipinski, C.; Lombardo, F.; Dominy, B.; Feeney, P. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef] [PubMed]
- D’Alessio, M.; Cerella, C.; De Nicola, M.; Bergamaschi, A.; Magrini, A.; Gualandi, G.; Alfonsi, A.; Ghibelli, L. Apoptotic GSH extrusion is associated with free radical generation. Ann. N. Y. Acad. Sci. 2003, 1010, 449–452. [Google Scholar] [CrossRef]
- Moloney, J.; Cotter, T. ROS signalling in the biology of cancer. Semin. Cell Dev. Biol. 2018, 80, 50–64. [Google Scholar] [CrossRef]
- Kavčič, N.; Pegan, K.; Vandenabeele, P.; Turk, B. Comparative study of the differential cell death protecting effect of various ROS scavengers. Biol. Chem. 2019, 400, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Sun, L.; Pang, C. Synergistic protection of N-acetylcysteine and ascorbic acid 2-phosphate on human mesenchymal stem cells against mitoptosis, necroptosis and apoptosis. Sci. Rep. 2015, 5, 9819. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Nie, Z.; Cao, H.; Huang, D.; Chen, M.; Xiang, Y.; Yu, X.; Zhang, S. Scabertopin Derived from Elephantopus scaber L. Mediates Necroptosis by Inducing Reactive Oxygen Species Production in Bladder Cancer In Vitro. Cancers 2022, 14, 5976. https://doi.org/10.3390/cancers14235976
Gao Y, Nie Z, Cao H, Huang D, Chen M, Xiang Y, Yu X, Zhang S. Scabertopin Derived from Elephantopus scaber L. Mediates Necroptosis by Inducing Reactive Oxygen Species Production in Bladder Cancer In Vitro. Cancers. 2022; 14(23):5976. https://doi.org/10.3390/cancers14235976
Chicago/Turabian StyleGao, Yuanhui, Zhenyu Nie, Hui Cao, Denggao Huang, Mei Chen, Yang Xiang, Xiaolong Yu, and Shufang Zhang. 2022. "Scabertopin Derived from Elephantopus scaber L. Mediates Necroptosis by Inducing Reactive Oxygen Species Production in Bladder Cancer In Vitro" Cancers 14, no. 23: 5976. https://doi.org/10.3390/cancers14235976
APA StyleGao, Y., Nie, Z., Cao, H., Huang, D., Chen, M., Xiang, Y., Yu, X., & Zhang, S. (2022). Scabertopin Derived from Elephantopus scaber L. Mediates Necroptosis by Inducing Reactive Oxygen Species Production in Bladder Cancer In Vitro. Cancers, 14(23), 5976. https://doi.org/10.3390/cancers14235976







