Survival and Prognostic Factors of Ultra-Central Tumors Treated with Stereotactic Body Radiotherapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Treatment Schedules
2.3. Follow-Up
2.4. Endpoints
2.5. Evaluation of Prognostic Factors
2.6. Statistical Analysis
3. Results
3.1. Patient, Treatment and Tumor Characteristics
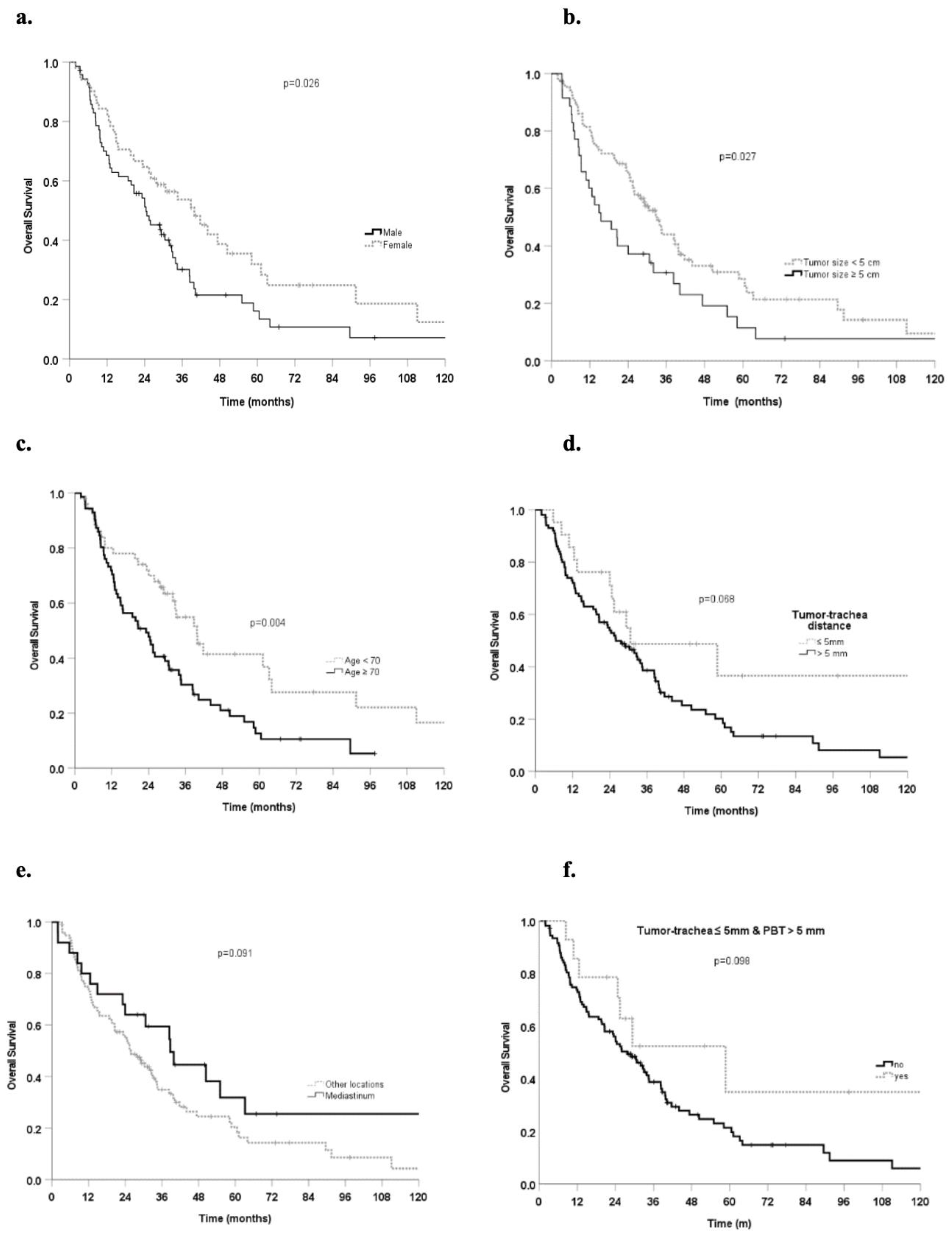
3.2. Survival and Local Control
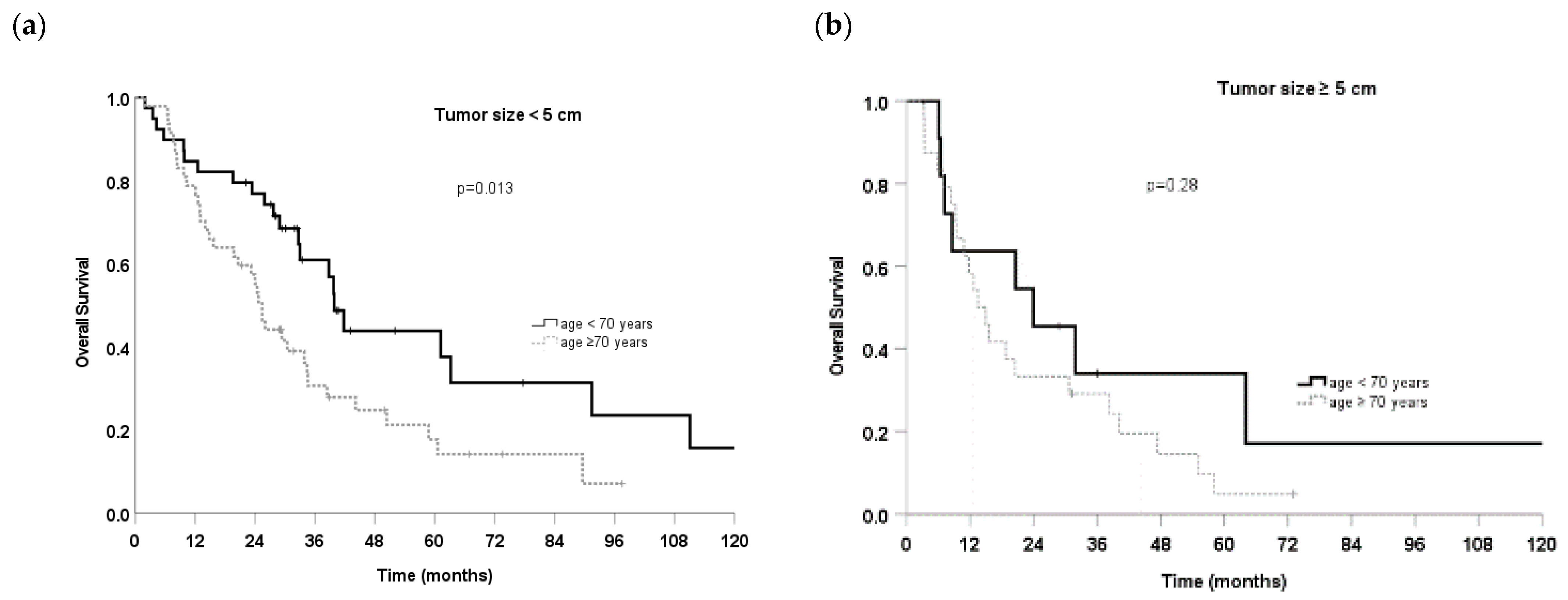
3.3. Prognostic Factors
3.4. Acute and Late Toxicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Timmerman, R.; Paulus, R.; Galvin, J.; Michalski, J.; Straube, W.; Bradley, J.; Fakiris, A.; Bezjak, A.; Videtic, G.; Johnstone, D.; et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010, 303, 1070–1076. [Google Scholar] [CrossRef]
- Videtic, G.M.M.; Donington, J.; Giuliani, M.; Heinzerling, J.; Karas, T.Z.; Kelsey, C.R.; Lally, B.E.; Latzka, K.; Lo, S.S.; Moghanaki, D.; et al. Stereotactic body radiation therapy for early-stage non-small cell lung cancer: Executive summary of an ASTRO evidence-based guideline. Pract. Radiat. Oncol. 2017, 7, 295–301. [Google Scholar] [CrossRef]
- Lodeweges, J.E.; Klinkenberg, T.J.; Ubbels, J.F.; Groen, H.J.M.; Langendijk, J.A.; Widder, J. Long-Term outcome of surgery or stereotactic radiotherapy for lung Oligometastases. J. Thorac. Oncol. 2017, 12, 1442–1445. [Google Scholar] [CrossRef]
- Onishi, H.; Araki, T.; Shirato, H.; Nagata, Y.; Hiraoka, M.; Gomi, K.; Yamashita, T.; Niibe, Y.; Karasawa, K.; Hayakawa, K.; et al. Stereotactic hypofractionated high-dose irradiation for stage I nonsmall cell lung carcinoma: Clinical outcomes in 245 subjects in a Japanese multiinstitutional study. Cancer 2004, 101, 1623–1631. [Google Scholar] [CrossRef]
- Guckenberger, M.; Andratschke, N.; Dieckmann, K.; Hoogeman, M.S.; Hoyer, M.; Hurkmans, C.; Tanadini-Lang, S.; Lartigau, E.; Méndez Romero, A.; Senan, S.; et al. ESTRO ACROP consensus guideline on implementation and practice of stereotactic body radiotherapy for peripherally located early stage non-small cell lung cancer. Radiother. Oncol. 2017, 124, 11–17. [Google Scholar] [CrossRef]
- Lindberg, K.; Grozman, V.; Karlsson, K.; Lindberg, S.; Lax, I.; Wersäll, P.; Persson, G.F.; Josipovic, M.; Khalil, A.A.; Moeller, D.S.; et al. The HILUS-Trial-a Prospective Nordic Multicenter Phase 2 Study of Ultracentral Lung Tumors Treated with Stereotactic Body Radiotherapy. J. Thorac. Oncol. 2021, 16, 1200–1210. [Google Scholar] [CrossRef]
- Bezjak, A.; Paulus, R.; Gaspar, L.E.; Timmerman, R.D.; Straube, W.L.; Ryan, W.F.; Garces, Y.I.; Pu, A.T.; Singh, A.K.; Videtic, G.M.; et al. Safety and Efficacy of a Five-Fraction Stereotactic Body Radiotherapy Schedule for Centrally Located Non-Small-Cell Lung Cancer: NRG Oncology/RTOG 0813 Trial. J. Clin. Oncol. 2019, 37, 1316–1325. [Google Scholar] [CrossRef]
- Haseltine, J.M.; Rimner, A.; Gelblum, D.Y.; Modh, A.; Rosenzweig, K.E.; Jackson, A.; Yorke, E.D.; Wu, A.J. Fatal complications after stereotactic body radiation therapy for central lung tumors abutting the proximal bronchial tree. Pract. Radiat. Oncol. 2016, 6, e27–e33. [Google Scholar] [CrossRef]
- Tekatli, H.; Haasbeek, N.; Dahele, M.; De Haan, P.; Verbakel, W.; Bongers, E.; Hashemi, S.; Nossent, E.; Spoelstra, F.; de Langen, A.J.; et al. Outcomes of hypofractionated high-dose radiotherapy in poor-risk patients with ‘‘ultracentral” non-small cell lung cancer. J. Thorac. Oncol. 2016, 11, 1081–1089. [Google Scholar] [CrossRef]
- Senthi, S.; Haasbeek, C.J.; Slotman, B.J.; Senan, S. Outcomes of stereotactic ablative radiotherapy for central lung tumours: A systematic review. Radiother. Oncol 2013, 106, 276–282. [Google Scholar] [CrossRef]
- Timmerman, R.; McGarry, R.; Yiannoutsos, C.; Papiez, L.; Tudor, K.; DeLuca, J.; Ewing, M.; Abdulrahman, R.; DesRosiers, C.; Williams, M.; et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J. Clin. Oncol. 2006, 24, 4833–4839. [Google Scholar] [CrossRef]
- Guckenberger, M.; Heilman, K.; Wulf, J.; Mueller, G.; Beckmann, G.; Flentje, M. Pulmonary injury and tumor response after stereotactic body radiotherapy (SBRT): Results of a serial follow-up CT study. Radiother. Oncol. 2007, 85, 435–442. [Google Scholar] [CrossRef]
- Schanne, D.H.; Nestle, U.; Allgäuer, M.; Andratschke, N.; Appold, S.; Dieckmann, U.; Ernst, I.; Ganswindt, U.; Grosu, A.L.; Holy, R.; et al. Stereotactic body radiotherapy for centrally located stage I NSCLC: A multicenter analysis. Strahlenther. Onkol. 2015, 191, 125–132. [Google Scholar] [CrossRef]
- Duijm, M.; Schillemans, W.; Aerts, J.G.; Heijmen, B.; Nuyttens, J.J. Dose and volume of the irradiated main bronchi and related side effects in the treatment of central lung tumors with stereotactic radiotherapy. Semin. Radiat. Oncol. 2016, 26, 140–148. [Google Scholar] [CrossRef]
- Corradetti, M.N.; Haas, A.R.; Rengan, R. Central-airway necrosis after stereotacticbody-radiation therapy. N. Engl. J. Med. 2012, 366, 2327–2329. [Google Scholar] [CrossRef]
- Chaudhuri, A.A.; Tang, C.; Binkley, M.S.; Jin, M.; Wynne, J.F.; von Eyben, R.; Hara, W.Y.; Trakul, N.; Loo, B.W., Jr.; Diehn, M. Stereotactic ablative radiotherapy (SABR) for treatment of central and ultra-central lung tumors. Lung Cancer 2015, 89, 50–56. [Google Scholar] [CrossRef]
- Guillaume, E.; Tanguy, R.; Ayadi, M.; Claude, L.; Sotton, S.; Moncharmont, C.; Magné, N.; Martel-Lafay, I. Toxicity and efficacy of stereotactic body radiotherapy for Ultra-central lung tumours: A single institution real life experience. Br. J. Radiol. 2021, 95, 20210533. [Google Scholar] [CrossRef]
- Farrugia, M.; Ma, S.; Hennon, M.; Nwogu, C.; Dexter, E.; Picone, A.; Demmy, T.; Yendamuri, S.; Yu, H.; Fung-Kee-Fung, S.; et al. Exceeding Radiation Dose to Volume Parameters for the Proximal Airways with Stereotactic Body Radiation Therapy Is More Likely for Ultracentral Lung Tumors and Associated with Worse Outcome. Cancers 2021, 13, 3463. [Google Scholar] [CrossRef]
- Lodeweges, J.E.; van Rossum, P.S.N.; Bartels, M.M.T.J.; van Lindert, A.S.R.; Pomp, J.; Peters, M.; Verhoeff, J.J.C. Ultra-central lung tumors: Safety and efficacy of protracted stereotactic body radiotherapy. Acta Oncol. 2021, 60, 1061–1068. [Google Scholar] [CrossRef]
- Mihai, A.; Armstrong, P.; Hickey, D.; Milano, M.; Dunne, M.; Healy, K.; Thirion, P.; Heron, D.; ElBeltagi, N.; Armstrong, J. Late Toxicity and Long-Term Local Control in Patients With Ultra-Central Lung Tumours Treated by Intensity-Modulated Radiotherapy-Based Stereotactic Ablative Body Radiotherapy With Homogenous Dose Prescription. Clin. Oncol. 2021, 33, 627–637. [Google Scholar] [CrossRef]
- Breen, W.G.; Jeans, E.B.; Gergelis, K.R.; Garces, Y.I.; Park, S.S.; Merrell, K.W.; Peikert, T.D.; Mansfield, A.S.; Wigle, D.A.; Harmsen, W.S. Ablative radiotherapy for ultracentral lung cancers: Dosimetric, geometric, and volumetric predictors of outcomes and toxicity. Radiother. Oncol. 2021, 158, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Regnery, S.; Eichkorn, T.; Weykamp, F.; Held, T.; Weusthof, K.; Dinges, L.-A.; El-Shafie, R.A.; Winter, H.; Thomas, M.; Debus, J.; et al. Safety and Efficacy of Stereotactic Body Radiotherapy in Ultracentral Lung Tumors Using a Risk-optimized Fractionation Scheme. Clin. Lung Cancer 2020, 22, 332–340.e3. [Google Scholar] [CrossRef] [PubMed]
- Loi, M.; Franceschini, D.; Dominici, L.; Franzese, C.; Chiola, I.; Comito, T.; Marzo, M.; Reggiori, G.; Mancosu, P.; Tomatis, S.; et al. Stereotactic radiotherapy for ultra-central lung oligometastases in non-small-cell lung cancer. Cancers 2020, 12, 885. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Khawandanh, E.; Thomas, S.; Zhang, S.; Dunne, E.M.; Liu, M.; Schellenberg, D. Outcomes of stereotactic body radiotherapy 60 Gy in 8 fractions when prioritizing organs at risk for central and ultracentral lung tumors. Radiat. Oncol. 2020, 15, 61. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Duijm, M.; Oomen-de Hoop, E.; Aerts, J.G.; Verhoef, C.; Hoogeman, M.; Nuyttens, J.J. Factors affecting local control of pulmonary oligometastases treated with stereotactic body radiotherapy. Acta Oncol. 2018, 57, 1031–1037. [Google Scholar] [CrossRef]
- van der Voort van Zyp, N.C.; Prévost, J.B.; Hoogeman, M.S.; Praag, J.; van der Holt, B.; Levendag, P.C.; van Klaveren, R.J.; Pattynama, P.; Nuyttens, J.J. Stereotactic radiotherapy with real-time tumor tracking for non-small cell lung cancer: Clinical outcome. Radiother. Oncol. 2009, 91, 296–300. [Google Scholar] [CrossRef]
- Nuyttens, J.J.; van de Pol, M. The CyberKnife radiosurgery system for lung cancer. Expert Rev. Med. Devices 2012, 9, 465–475. [Google Scholar] [CrossRef]
- Hoogeman, M.; Prévost, J.B.; Nuyttens, J.; Pöll, J.; Levendag, P.; Heijmen, B. Clinical accuracy of the respiratory tumor tracking system of the cyberknife: Assessment by analysis of log files. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 297–303. [Google Scholar] [CrossRef]
- van der Voort van Zyp, N.C.; Hoogeman, M.S.; van de Water, S.; Levendag, P.C.; van der Holt, B.; Heijmen, B.J.; Nuyttens, J.J. Clinical introduction of Monte Carlo treatment planning: A different prescription dose for non-small cell lung cancer according to tumor location and size. Radiother. Oncol. 2010, 96, 55–60. [Google Scholar] [CrossRef]
- Chen, H.; Laba, J.M.; Zayed, S.; Boldt, R.G.; Palma, D.A.; Louie, A.V. Safety and Effectiveness of Stereotactic Ablative Radiotherapy for Ultra-Central Lung Lesions: A Systematic Review. J. Thorac. Oncol. 2019, 14, 1332–1342. [Google Scholar] [CrossRef]
- Unger, K.; Ju, A.; Oermann, E.; Suy, S.; Yu, X.; Vahdat, S.; Subramaniam, D.; Harter, K.W.; Collins, S.P.; Dritschilo, A. CyberKnife for hilar lung tumors: Report of clinical response and toxicity. J. Hematol. Oncol. 2010, 3, 39. [Google Scholar] [CrossRef] [PubMed]
- Duijm, M.; Oomen-de Hoop, E.; van Voort van der Zyp, N.; van de Vaart, P.; Tekatli, H.; Hoogeman, M.; Senan, S.; Nuyttens, J. The development and external validation of an overall survival nomogram in medically inoperable centrally located early-stage non-small cell lung carcinoma. Radiother. Oncol. 2021, 156, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.H.; Poon, I.; Erler, D.; Zhang, L.; Cheung, P. The safety and effectiveness of stereotactic body radiotherapy for central versus ultracentral lung tumors. Radiother. Oncol. 2018, 129, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Murrell, D.H.; Laba, J.M.; Erickson, A.; Millman, B.; Palma, D.A.; Louie, A.V. Stereotactic ablative radiotherapy for ultra-central lung tumors:prioritize target coverage or organs at risk? Radiat. Oncol. 2018, 13, 57. [Google Scholar] [CrossRef]
- Nguyen, K.N.B.; Hause, D.J.; Novak, J.; Monjazeb, A.M.; Daly, M.E. Tumor control and toxicity after SBRT for ultracentral, central, and paramediastinal lung tumors. Pract. Radiat. Oncol. 2019, 9, e196–e202. [Google Scholar] [CrossRef]
- Yang, D.; Cui, J.; Zhao, J.; You, J.; Yu, R.; Yu, H.; Jiang, L.; Li, D.; Xu, B.; Shi, A. Stereotactic ablative radiotherapy of 60 Gy in eight fractions is safe for ultracentral non-small cell lung cancer. Thorac. Cancer 2020, 11, 754–761. [Google Scholar]
- Wang, C.; Rimner, A.; Gelblum, D.Y.; Flynn, J.; Jackson, A.; Yorke, E.; Wu, A.J. Analysis of toxic effects with antiangiogenic agents plus stereotactic body radiation in Ultracentral lung tumors. JAMA Oncol. 2019, 5, 737. [Google Scholar] [CrossRef]
- Wang, C.; Rimner, A.; Gelblum, D.Y.; Dick-Godfrey, R.; McKnight, D.; Torres, D.; Flynn, J.; Zhang, Z.; Sidiqi, B.; Jackson, A.; et al. Analysis of pneumonitis and esophageal injury after stereotactic body radiation therapy for ultra-central lung tumors. Lung Cancer 2020, 147, 45–48. [Google Scholar] [CrossRef]
- Rosenberg, S.A.; Mak, R.; Kotecha, R.; Loo, B.W., Jr.; Senan, S. The Nordic-HILUS Trial: Ultracentral Lung Stereotactic Ablative Radiotherapy and a Narrow Therapeutic Window. J. Thorac. Oncol. 2021, 16, e79–e80. [Google Scholar] [CrossRef]
| Study, Year | N of Patients | Definition of Ultra-Central |
|---|---|---|
| Guillaume, 2021 [17] | 74 | PTV overlapping trachea, right and left main bronchi, intermediate bronchus, lobar bronchi, esophagus, heart. |
| Lindberg, 2021 [6] | 65 | 1-cm zone around the PBT. |
| Farrugia, 2021 [18] | 43 | GTV abutting the PBT, trachea, mediastinum, aorta, or spinal cord. |
| Lodeweges, 2021 [19] | 72 | PTV abutting or overlapping the main bronchi, trachea and/or esophagus |
| Mihai, 2021 [20] | 57 | GTV abutting or involving trachea, main or lobar bronchi. |
| Breen, 2021 [21] | 110 | GTV directly touching the PBT or trachea. PTV overlapping the trachea or mainstem bronchi.GTV within 1 cm of the PBT. |
| Regnery, 2021 [22] | 51 | Overlap of the PTV with the PBT |
| Loi, 2020 [23] | 109 | PTV overlapping with central bronchial tree, esophagus, pulmonary vein, or pulmonary artery. |
| Zhao, 2020 [24] | 98 | PTV overlapping with PBT, esophagus, pulmonary vein or pulmonary artery. |
| Characteristics | n | Percentage | ||
|---|---|---|---|---|
| Age (median, range) | 72 years | (34–91) | ||
| <70 years | 51 | 42% | ||
| ≥70 years | 71 | 58% | ||
| Gender | ||||
| Male | 71 | 58% | ||
| Female | 51 | 42% | ||
| Comorbidity index | ||||
| CCI (median, range) | 1 | (0–8) | ||
| Score 0 | 27 | 22% | ||
| Score 1–2 | 56 | 46% | ||
| Score ≥ 3 | 39 | 32% | ||
| CIRS (median, range) | 3 | (0–16) | ||
| Score 0–4 | 90 | 74% | ||
| Score ≥ 5 | 32 | 26% | ||
| Tumor-PBT distance | ||||
| ≤5 mm | 92 | 73% | ||
| >5 mm | 34 | 27% | ||
| Tumor-esophagus distance | ||||
| ≤5 mm | 41 | 33% | ||
| >5 mm | 85 | 67% | ||
| Tumor-trachea distance | ||||
| ≤5 mm | 22 | 17% | ||
| >5 mm | 104 | 83% | ||
| Tumor size (median, range) | 37.5 mm | (8–105) | ||
| ≤5 cm | 90 | 71% | ||
| >5 cm | 36 | 29% | ||
| Tumor location | 0% | |||
| Mediastinum | 28 | 22% | ||
| Lower lobe | 46 | 37% | ||
| Other lobes | 52 | 41% | ||
| Dose fractionation schemes | ||||
| 7 fractions of 7 Gy ** | 25 | 20% | ||
| 7 fractions of 7.5 Gy * | 47 | 37% | ||
| 7 fractions of 8 Gy ** | 8 | 6% | ||
| 6 fractions of 8 Gy ** | 19 | 15% | ||
| 5 fractions of 9 Gy ** | 4 | 3% | ||
| 5 fractions of 10 Gy or 12 Gy ** | 6 | 5% | ||
| 5 fractions of 11 Gy * | 17 | 13% | ||
| Markers | 3 | (0–16) | ||
| yes | 95 | 75% | ||
| no | 31 | 25% | ||
| BED (median, range) | 92 Gy | (83–132 Gy) | ||
| <100 Gy | 91 | 72% | ||
| ≥100 Gy | 35 | 28% | ||
| Prescription isodose (median, range) | 77% | (60–87%) | ||
| SBRT target | ||||
| Primary lung tumors | 68 | 54% | ||
| Lung metastasis | 58 | 46% |
| Covariates | Median OS Months (95%CI) | HR (95%CI) | p Value |
|---|---|---|---|
| SBRT target | |||
| Lung metastasis (54) | 34.4 (26.1–42.8) | 0.69 (0.45–1.1) | 0.089 |
| Primary lung tumors (68) | 20.6 (9.6–31.6) | ||
| Gender | |||
| Female (51) | 39.8 (29.3–53.4) | 0.61 (0.4–0.9) | 0.027 |
| Male (71) | 24.4 (16.4–32.5) | ||
| Age | |||
| ≥70 years (71) | 23.2 (14.0–32.4) | 1.91 (1.2–3.0) | 0.005 |
| <70 years (51) | 39.7 (31.1–48.4) | ||
| Tumor size | |||
| ≥5 cm (36) | 15.5 (6.5–24.6) | 1.64 (1.1–2.5) | 0.028 |
| <5 cm (86) | 33.0 (27.4–38.5) | ||
| Tumor-PBT distance | |||
| >5 mm (32) | 33.8 (22.4–45.3) | 0.68 (0.4–1.1) | 0.126 |
| ≤5 mm (90) | 25.9 (18.9–32.9) | ||
| Tumor-esophagus distance | |||
| >5 mm (84) | 25.9 (20.3–31.4) | 1.36 (0.86–2.15) | 0.182 |
| ≤5 mm (38) | 33.9 (22.8–44.9) | ||
| Tumor-trachea distance | |||
| >5 mm (101) | 26.0 (18.3–33.7) | 1.79 (0.95–3.4) | 0.072 |
| ≤5 mm (21) | 30.7 (0.0–61.7) | ||
| Tumor-trachea ≤ 5 mm &PBT> 5 mm distance | |||
| Yes (14) | 58.8 (18.4–99.1) | 0.53 (0.2–1.14) | 0.104 |
| No (108) | 27.7 (20.3–35.1) | ||
| CCI | |||
| Score ≥ 3 (39) | 25.9 (18.9–32.8) | 1.1 (0.7–1.6) | 0.84 |
| Score < 3 (83) | 30.6 (22.8–38.5) | ||
| CIRS | |||
| Score ≥ 5 (32) | 14.9 (5.1–24.8) | 1.2 (0.7–1.9) | 0.45 |
| Score < 5 (90) | 32.7 (25.1–40.2) | ||
| Tumor location | |||
| Other lobes (76) | 25.9 (19.4–32.3) | 1.0 (0.7–1.6) | 0.93 |
| Lower lobe (46) | 31.9 (25.9–37.9) | ||
| Mediastinum | 38.8 (26.1–51.5) | 0.63 (0.36–1.08) | 0.094 |
| Other locations | 25.9 (20.1–31.6) | ||
| BED | |||
| ≥100 (31) | 25.4 (22.9–27.9) | 1.1 (0.7–1.7) | 0.69 |
| <100 (91) | 30.5 (22.7–38.2) | ||
| Markers | |||
| no (29) | 30.6 (22.3–39.0) | 0.7 (0.4–1.2) | 0.21 |
| yes (93) | 26 (12.0–40.0) |
| Acute Toxicity | Pain | Dyspnea | Cough | Fatigue | Dysphagia | Highest Toxicity |
| G0 | 113 (93%) | 101 (83%) | 85 (70%) | 80 (66%) | 83 (68%) | 36 (30%) |
| G1 | 8 (7%) | 16 (13%) | 30 (25%) | 35 (29%) | 27 (22%) | 59 (48%) |
| G2 | 1 (1%) | 4 (3%) | 6 (5%) | 6 (5%) | 13 (11%) | 26 (21%) |
| G3 | 0 (0%) | 1 (1%) | 0 (0%) | 1 (1%) | 0 (0%) | 1 (1%) |
| Late toxicity | Pain | Dyspnea | Cough | Fatigue | Dysphagia | Highest toxicity |
| G0 | 115 (94%) | 99 (81%) | 100 (82%) | 103 (84%) | 119 (98%) | 79 (65%) |
| G1 | 2 (2%) | 12 (10%) | 17 (14%) | 13 (11%) | 2 (2%) | 26 (21%) |
| G2 | 4 (3%) | 7 (6%) | 5 (4%) | 6 (5%) | 1 (1%) | 12 (10%) |
| G3 | 1 (1%) | 4 (3%) | 0 (0%) | 0 (0%) | 0 (0%) | 5 (4%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvestrini, V.; Duijm, M.; Loi, M.; Nuyttens, J.J. Survival and Prognostic Factors of Ultra-Central Tumors Treated with Stereotactic Body Radiotherapy. Cancers 2022, 14, 5908. https://doi.org/10.3390/cancers14235908
Salvestrini V, Duijm M, Loi M, Nuyttens JJ. Survival and Prognostic Factors of Ultra-Central Tumors Treated with Stereotactic Body Radiotherapy. Cancers. 2022; 14(23):5908. https://doi.org/10.3390/cancers14235908
Chicago/Turabian StyleSalvestrini, Viola, Marloes Duijm, Mauro Loi, and Joost J. Nuyttens. 2022. "Survival and Prognostic Factors of Ultra-Central Tumors Treated with Stereotactic Body Radiotherapy" Cancers 14, no. 23: 5908. https://doi.org/10.3390/cancers14235908
APA StyleSalvestrini, V., Duijm, M., Loi, M., & Nuyttens, J. J. (2022). Survival and Prognostic Factors of Ultra-Central Tumors Treated with Stereotactic Body Radiotherapy. Cancers, 14(23), 5908. https://doi.org/10.3390/cancers14235908






