Improving the Effect of Cancer Cells Irradiation with X-rays and High-Energy Protons Using Bimetallic Palladium-Platinum Nanoparticles with Various Nanostructures
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Synthesis of Bimetallic PdPt NPs
2.2.1. PdPt Nano-Alloy Synthesis (PdPt I NPs)
2.2.2. PdPt Core-Shell Synthesis (PdPt II NPs)
2.3. TEM Characterization
2.4. Zeta Potential Measurements
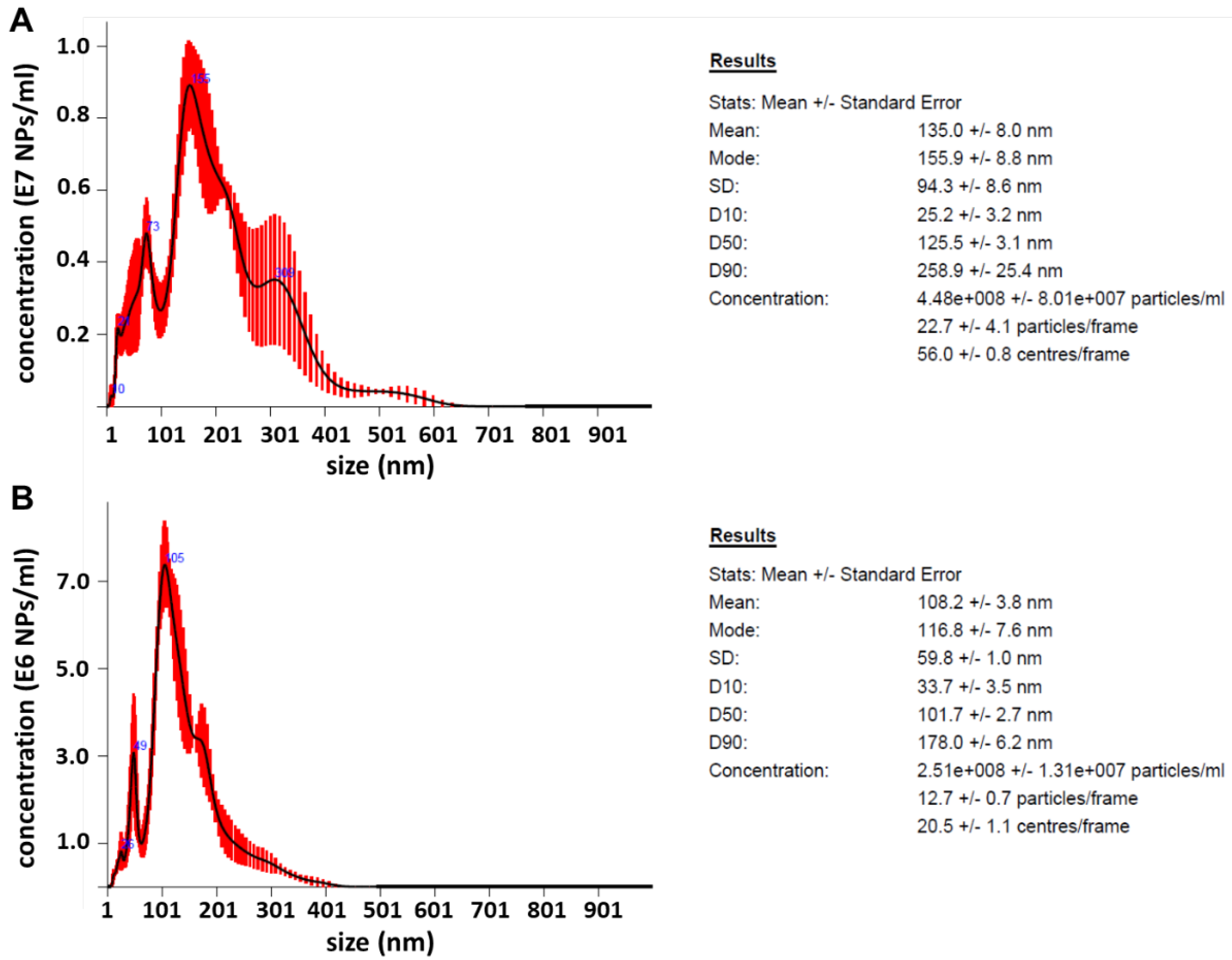
2.5. Nanoparticle Tracking Analysis (NTA)
2.6. Irradiation Simulation Protocols
2.6.1. X-ray Irradiation
2.6.2. Proton Irradiation
2.7. Cell Culture
2.8. MTS Assay
2.9. Annexin V-Binding Assay
2.10. Statistical Analysis
3. Results and Discussion
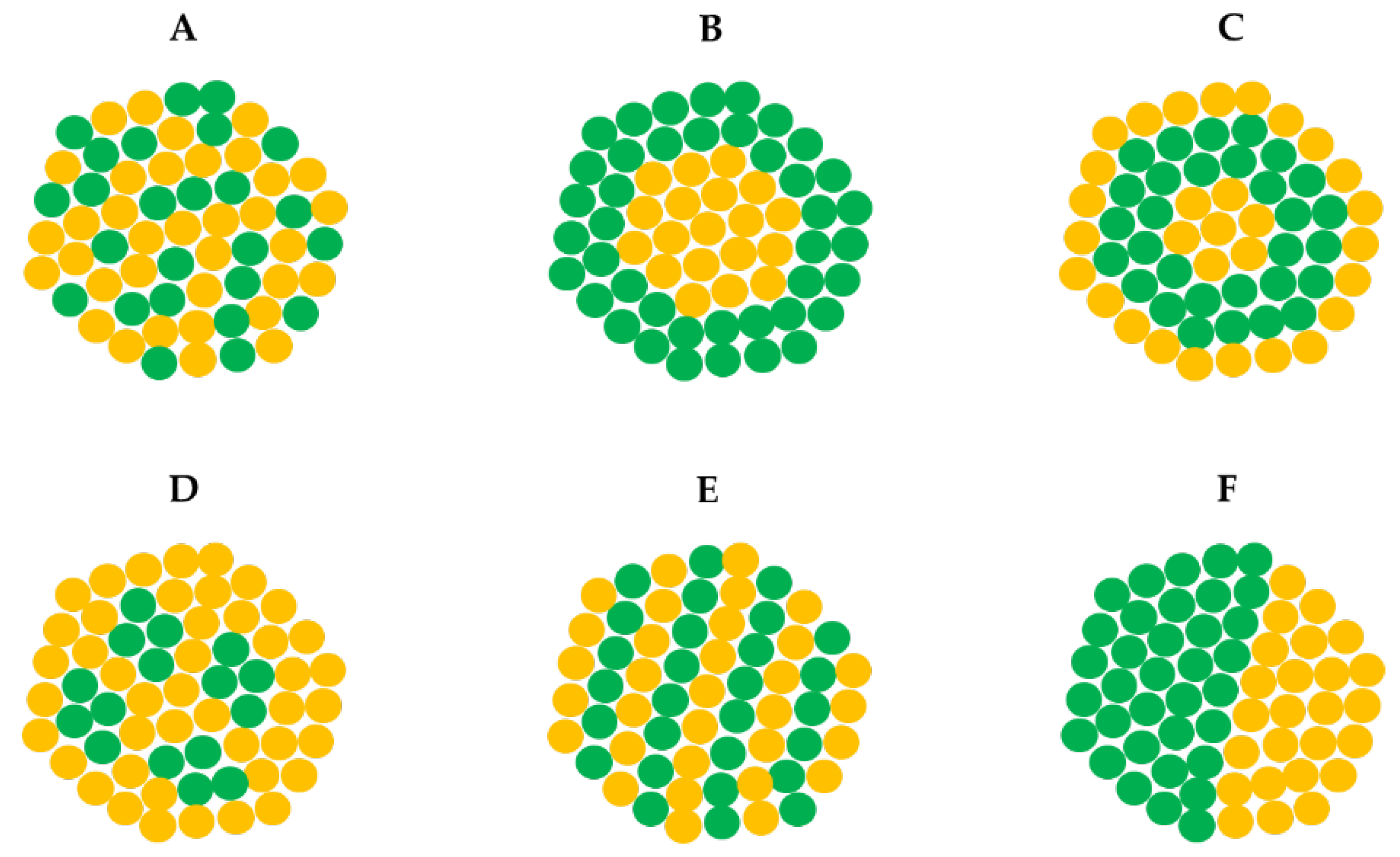
3.1. Mechanism of PdPt NPs Synthesis
3.2. Morphology, Structure, Chemical Composition, Zeta Potential and NTA of PdPt NPs
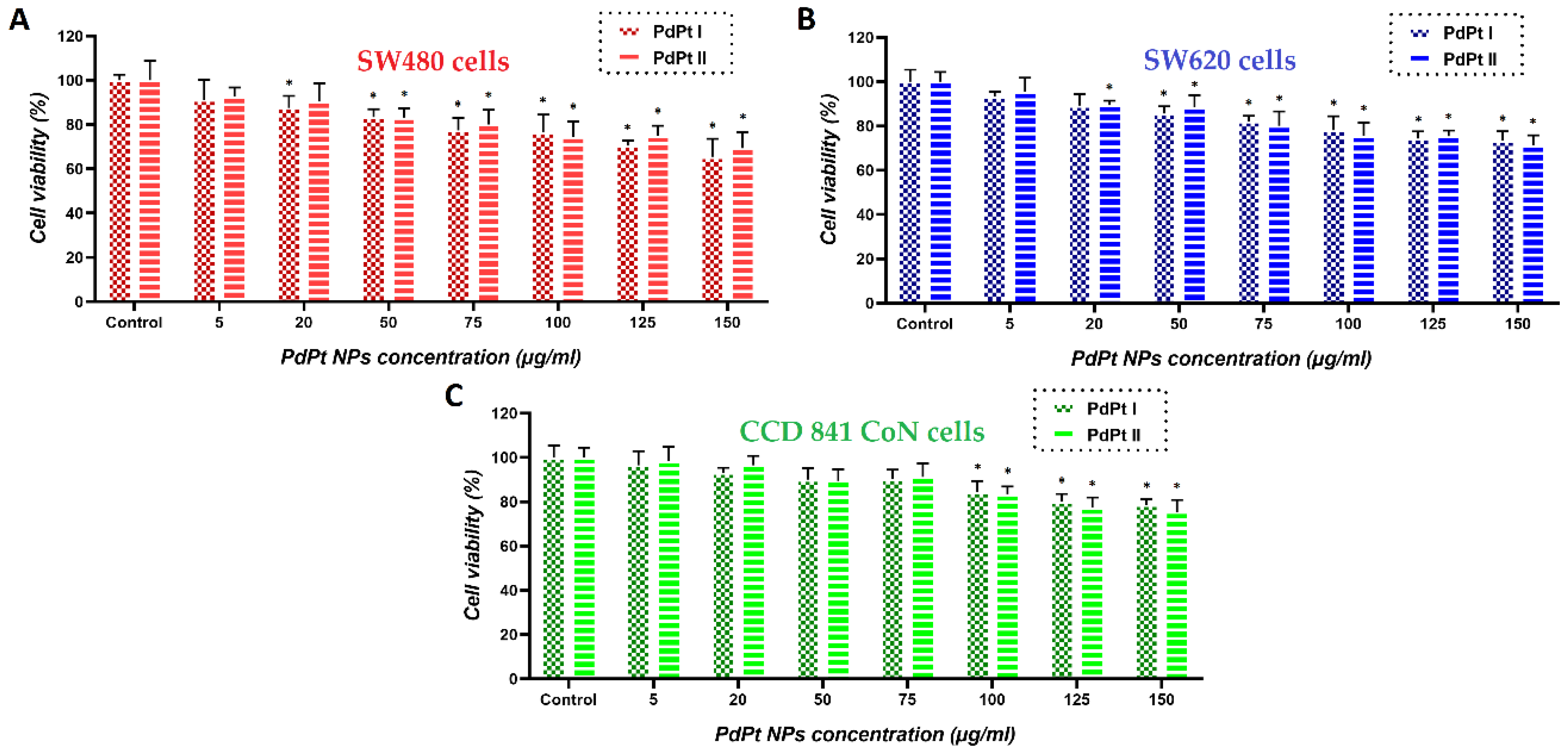
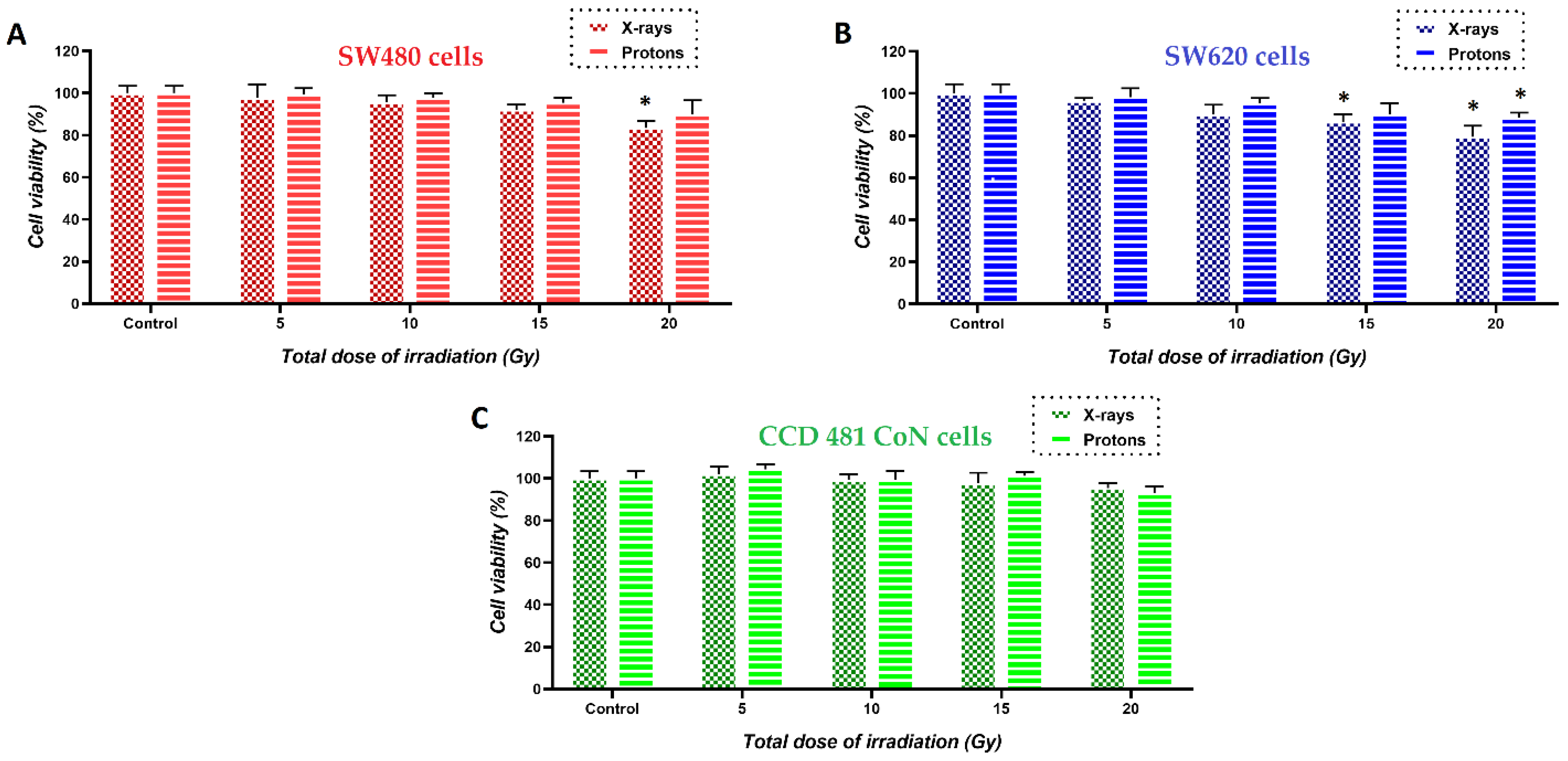
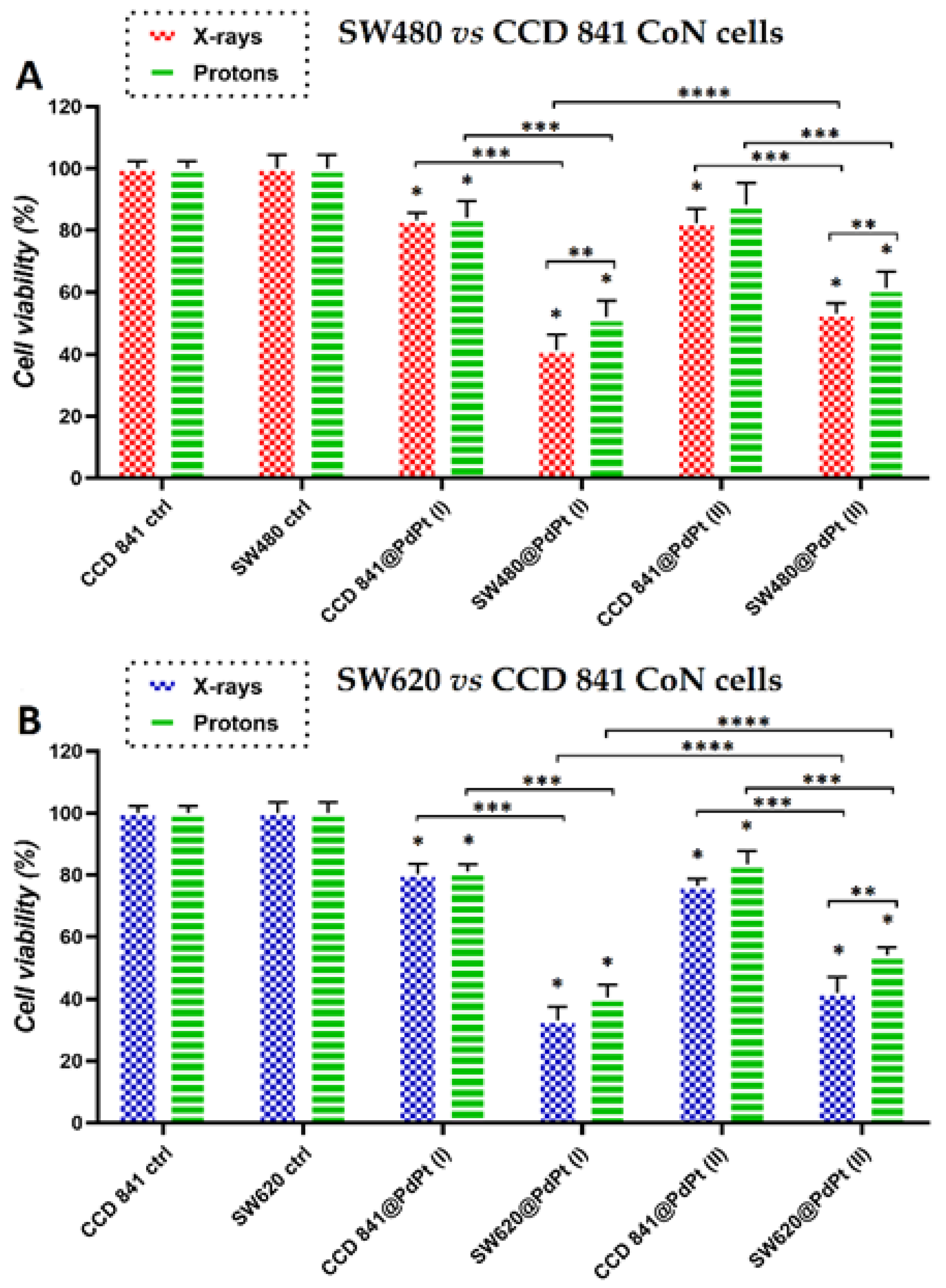
3.3. Changes in Viability of Colon Cancer and Normal Cells Induced by the Treatment with PdPt NPs Only, or Irradiation with X-ray/Protons in the Presence of PdPt NPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of multidrug resistance in cancer chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef] [PubMed]
- Galaeaz, C.; Totis, C.; Bisio, A. Radiation resistance: A matter of transcription factors. Front. Oncol. 2021, 11, 662840. [Google Scholar]
- Esfahani, K.; Roudaia, L.; Buhlaiga, N.; Del Rincon, S.V.; Papneja, N.; Miller, W.H., Jr. A review of cancer immunotherapy: From the past, to the present, to the future. Curr. Oncol. 2020, 27, S87–S97. [Google Scholar] [CrossRef] [PubMed]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.-W. Cancer and radiation therapy: Current advances and future directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef]
- Vanderwaeren, L.; Dok, R.; Verstrepen, K.; Nuyts, S. Clinical progress in proton radiotherapy: Biological unknowns. Cancers 2021, 13, 604. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Fukumitsu, N.; Ishikawa, H.; Nakai, K.; Sakurai, H. A critical review of radiation therapy: From particle beam therapy (proton, carbon, and BNCT) to beyond. J. Pers. Med. 2021, 11, 825. [Google Scholar] [CrossRef]
- Cramer, G.; Simone, C.B.; Busch, T.M.; Cengel, K.A. Adjuvant, neoadjuvant, and definitive radiation therapy for malignant pleural mesothelioma. J. Thorac. Dis. 2018, 10, S2565–S2573. [Google Scholar] [CrossRef]
- Lutz, S.T.; Jones, J.; Chow, E. Role of radiation therapy in palliative care of the patient with cancer. J. Clin. Oncol. 2014, 32, 2913–2919. [Google Scholar] [CrossRef]
- Liew, H.; Mein, S.; Debus, J.; Dokic, I.; Mairani, A. Modeling direct and indirect action on cell survival after photon irradiation under normoxia and hypoxia. Int. J. Mol. Sci. 2020, 21, 3471. [Google Scholar] [CrossRef]
- Srinivas, U.S.; Tan, B.W.Q.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA damage response in cancer. Redox Biol. 2019, 25, 101084. [Google Scholar] [CrossRef]
- Klębowski, B.; Depciuch, J.; Parlińska-Wojtan, M.; Baran, J. Applications of noble metal-based nanoparticles in medicine. Int. J. Mol. Sci. 2018, 19, 4031. [Google Scholar] [CrossRef] [PubMed]
- Butterworth, K.T.; McMahon, S.J.; Currell, F.J.; Prise, K.M. Physical basis and biological mechanisms of gold nanoparticle radiosensitization. Nanoscale 2012, 4, 4830. [Google Scholar] [CrossRef]
- Kempson, I. Mechanisms of nanoparticle radiosensitization. Wiley Interdiscipl. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1656. [Google Scholar] [CrossRef] [PubMed]
- Pagacova, E.; Stefancikowa, L.; Schmidt-Kaler, F.; Hildenbrand, G.; Vicar, T.; Depes, D.; Lee, J.-H.; Bestvater, F.; Lacombe, S.; Porcel, E.; et al. Challenges and contradictions of metal nano-particle applications for radio-sensitivity enhancement in cancer therapy. Int. J. Mol. Sci. 2019, 20, 588. [Google Scholar] [CrossRef]
- Morozov, K.V.; Kolyvanowa, M.A.; Kartseva, M.E.; Shishmakova, E.M.; Dementeva, O.V.; Isagulieva, A.K.; Salpagarov, M.H.; Belousov, A.V.; Rudoy, V.M.; Shtil, A.A.; et al. Radiosensitization by gold nanoparticles: Impact of the size, dose rate and photon energy. Nanomaterials 2020, 10, 952. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Wu, F.-G.; Zhang, X.; Jiang, Y.-W.; Jia, H.-R.; Wang, H.-Y.; Li, Y.-H.; Liu, P.; Gu, N.; Chen, Z. Shape-dependent radiosensitization effect of gold nanostructures in cancer radiotherapy: Comparison of gold nanoparticles, nanospikes, and nanorods. ACS Appl. Mater. Interfaces 2017, 9, 13017–13048. [Google Scholar] [CrossRef]
- Depciuch, J.; Stec, M.; Maximenko, A.; Drzymała, E.; Pawlyta, M.; Baran, J.; Parlinska-Wojtan, M. Synthesis method-dependent photothermal effects of colloidal solutions of platinum nanoparticles used in photothermal anticancer therapy. Appl. Organometal. Chem. 2020, 34, e5401. [Google Scholar] [CrossRef]
- Paul, P.; Chatterjee, S.; Pramanik, A.; Karmakar, P.; Bhattacharya, S.C.; Kumar, G.S. Thionine conjugated gold nanoparticles triggers apoptotic activity towards HepG2 cancer cell line. ACS Biomater. Sci. Eng. 2018, 4, 635–646. [Google Scholar] [CrossRef]
- Ahmed, S.; Baijal, G.; Somashekar, R.; Iyer, S.; Nayak, V. One pot synthesis of PEGylated bimetallic gold-silver nanoparticles for imaging and radiosensitization of oral cancers. Int. J. Nanomed. 2021, 16, 7103–7121. [Google Scholar] [CrossRef]
- Klębowski, B.; Stec, M.; Depciuch, J.; Gałuszka, A.; Pajor-Swierzy, A.; Baran, J.; Parlinska-Wojtan, M. Gold-decorated platinum and palladium nanoparticles as modern nanocomplexes to improve the effectiveness of simulated anticancer proton therapy. Pharmaceutics 2021, 13, 1726. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Wang, M.; Gonzalez-Lepera, C.; Mawlawi, M.; Cho, S.H. Development of bimetallic (Zn@Au) nanoparticles as potential PET-imageable radiosensitizers. Med. Phys. 2016, 43, 4775. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, B.S.; Rao, A.G.; Sankarshan, B.M.; Vicas, C.S.; Namratha, K.; Umesh, T.K.; Someshekar, R.; Byrappa, K. Evaluation of gold, silver and silver-gold (bimetallic) nanoparticles as radiosensitizers for radiation therapy in cancer treatment. Cancer Oncol. Res. 2016, 4, 42–51. [Google Scholar] [CrossRef]
- Garza-Cervantes, J.A.; Chavez-Reyes, A.; Castillo, E.C.; Garcia-Rivas, G.; Ortega-Rivera, O.A.; Salinas, E.; Ortiz-Martinez, M.; Gomez-Flores, S.L.; Pena-Martinez, J.A.; Pepi-Molina, A.; et al. Synergistic antimicrobial effects of silver/transition-metal combinatorial treatments. Sci. Rep. 2017, 7, 903. [Google Scholar] [CrossRef]
- Arora, N.; Thangavelu, K.; Karanikolos, G.N. Bimetallic nanoparticles for antimicrobial applications. Front. Chem. 2020, 8, 412. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Ahmad, F.; Koivisto, J.T.; Kellomaki, M. Green synthesis of controlled size gold and silver nanoparticles using antioxidant as capping and reducing agent. Colloid Interface Sci. Commun. 2020, 39, 100322. [Google Scholar] [CrossRef]
- Nemcekova, K.; Scitkova, V.; Sochr, J.; Gemeiner, P.; Labuda, J. Gallic acid-coated silver nanoparticles as perspective drug nanocarriers: Bioanalytical study. Anal. Bioanal. Chem. 2022, 414, 5493–5505. [Google Scholar] [CrossRef]
- Park, J.; Cha, S.-H.; Cho, S. Green synthesis of gold and silver nanoparticles using gallic acid: Catalytic activity and conversion yield toward the 4-nitrphenol reduction reaction. J. Nanopart. Res. 2016, 18, 166. [Google Scholar] [CrossRef]
- Zhang, G.; Zheng, H.; Shen, M.; Wang, L.; Wang, X. Green synthesis and characterization of Au@Pt core-shell bimetallic nanoparticles using gallic acid. J. Phys. Chem. Solids 2015, 81, 79–87. [Google Scholar] [CrossRef]
- Cao, M.; Lin, J.; Yang, H.; Cao, R. Facile synthesis of palladium nanoparticles with high chemical activity using cucurbit[6]uril as protecting agent. Chem. Commun. 2010, 46, 5088–5090. [Google Scholar] [CrossRef]
- Devivaraprasad, R.; Ramesh, R.; Naresh, N.; Kar, T.; Singh, R.K.; Neergat, M. Oxygen reduction reaction and peroxide generation on shape-controlled and polycrystalline platinum nanoparticles in acidic and alkaline electrolytes. Langmuir 2014, 30, 8995–9006. [Google Scholar] [CrossRef] [PubMed]
- Sikora, A.; Bartczak, D.; Geissler, D.; Kestens, V.; Roebben, G.; Ramaye, Y.; Varga, Z.; Palmai, M.; Shard, A.G.; Goenaga-Infante, H.; et al. A systematic comparison of different techniques to determine the zeta potential of silica nanoparticles in biological medium. Anal. Methods 2015, 7, 9835–9843. [Google Scholar] [CrossRef]
- Hao, G.; Xu, Z.P.; Li, L. Manipulating extracellular tumour pH: An effective target for cancer therapy. RSC Adv. 2018, 8, 22182. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, M.K.; Pedersen, J.N.; Marie, R. Size and surface charge characterization of nanoparticles with a salt gradient. Nat. Commun. 2020, 11, 2337. [Google Scholar] [CrossRef]
- Kutwin, M.; Sawosz, E.; Jaworski, S.; Wierzbicki, M.; Strojny, B.; Grodzik, M.; Sosnowska, M.E.; Trzaskowski, M.; Chwalibog, A. Nanocomplexes of graphene oxide and platinum nanoparticles against colorectal cancer Colo205, HT-29, HTC-116, SW480, liver cancer HepG2, human breast cancer MCF-7, and adenocarcinoma LNCaP and human cervical Hela B cell lines. Materials 2019, 12, 909. [Google Scholar] [CrossRef]
- Luo, F.; Li, J.; Wu, S.; Chen, M.; Zhong, X.; Liu, K. Comparative profiling between primary colorectal carcinomas and metastases identifies heterogeneity on drug resistance. Oncotarget 2016, 7, 63937–63949. [Google Scholar] [CrossRef]
- Tarkanyi, F.; Hermanne, A.; Ditroi, F.; Takacs, S.; Spahn, I.; Spellerberg, S. Activation cross-section measurements of proton induced reactions on cerium. Nucl. Instrum. Methods Phys. Res. B Nucl. Instrum. Meth. B 2017, 412, 46–53. [Google Scholar] [CrossRef][Green Version]
- Newhauser, W.D.; Zhang, R. The physics of proton therapy. Phys. Med. Biol. 2015, 60, R155–R209. [Google Scholar] [CrossRef]
- Mesbahi, A. A review on gold nanoparticles radiosensitization effect in radiation therapy of cancer. Rep. Pract. Oncol. Radiother. 2010, 15, 176–180. [Google Scholar] [CrossRef]
- Miszczyk, J.; Rawojć, K.; Panek, A.; Borkowska, A.; Prasanna, P.G.S.; Ahmed, M.M.; Swakoń, J.; Gałaś, A. Do protons and X-rays induce cell-killing in human peripheral blood lymphocytes by different mechanisms? Clin. Transl. Radiat. Oncol. 2018, 9, 23–29. [Google Scholar] [CrossRef]
- Klębowski, B.; Depciuch, J.; Stec, M.; Krzempek, D.; Komenda, W.; Baran, J.; Parlinska-Wojtan, M. Fancy-shaped gold-platinum nanocauliflowers for improved proton irradiation effect on colon cancer cells. Int. J. Mol. Sci. 2020, 21, 9610. [Google Scholar] [CrossRef]
- Rashid, R.A.; Abidin, S.Z.; Anuar, M.A.K.; Tominaga, T.; Akasaka, H.; Sasaki, R.; Kie, K.; Razak, K.A.; Pham, B.T.T.; Hawkett, B.S.; et al. Radiosensitization effects and ROS generation by high Z metallic nanoparticles on human colon carcinoma cell (HCT116) irradiated under 150 MeV proton beam. OpenNano 2019, 4, 100027. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, J.; Fu, S.; Wu, J. Gold nanoparticles as radiosensitizers in cancer radiotherapy. Int. J. Nanomed. 2020, 15, 9407–9430. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.; Guan, R.; Chen, X.; Song, Y.; Jiang, H.; Zhao, J. In vitro toxicity of different-sized ZnO nanoparticles in Caco-2 cells. Nanoscale Res. Lett. 2013, 8, 496. [Google Scholar] [CrossRef]
- Depciuch, J.; Stec, M.; Klebowski, B.; Maximienko, A.; Drzymała, E.; Baran, J.; Parlinska-Wojtan, M. Size effect of platinum nanoparticles in simulated anticancer photothermal therapy. Photodiagnosis Photodyn. Ther. 2020, 29, 101594. [Google Scholar] [CrossRef] [PubMed]
- Sah, B.; Wu, J.; Vanasse, A.; Pandey, N.K.; Chudal, L.; Huang, Z.; Song, W.; Yu, H.; Ma, L.; Chen, W.; et al. Effects of nanoparticle size and radiation energy on copper-cysteamine nanoparticles for X-ray induced photodynamic therapy. Nanomaterials 2020, 10, 1087. [Google Scholar] [CrossRef]
- Zhang, T.; Ma, L.; Wu, P.; Li, W.; Li, T.; Gu, R.; Dan, X.; Li, Z.; Fan, X.; Xiao, Z. Gallic acid has anticancer activity and enhances the anticancer effects of cisplatin in non-small cell lung cancer A549 cells via the JAK/STAT3 signaling pathway. Oncol. Rep. 2019, 41, 1779–1788. [Google Scholar] [CrossRef]
- Subramanian, A.P.; John, A.A.; Velleyappan, M.V.; Balaji, A.; Jaganathan, S.K.; Supriyanto, E.; Yusof, M. Gallic acid: Prospects and molecular mechanisms of its anticancer activity. RSC Adv. 2015, 5, 35608. [Google Scholar] [CrossRef]
- Vlamidis, Y.; Voliani, V. Bringing again noble metal nanoparticles to the forefront of cancer therapy. Front Bioeng. Biotechnol. 2018, 6, 143. [Google Scholar] [CrossRef]
- Ali, M.R.K.; Rahman, M.A.; Wu, Y.; Han, T.; Peng, X.; Mackey, M.A.; Wang, D.; Shin, H.J.; Chen, Z.G.; Xiao, H.; et al. Efficacy, long-term toxicity, and mechanistic studies of gold nanorods photothermal therapy of cancer in xenograft mice. Proc. Natl. Acad. Sci. USA 2017, 114, E3110–E3118. [Google Scholar] [CrossRef]
- Bohara, R.A.; Thorat, N.D.; Pawar, S.H. Role of functionalization: Strategies to explore potential nano-bio applications of magnetic nanoparticles. RSC Adv. 2016, 6, 43989. [Google Scholar] [CrossRef]
- Camaioni, A.; Massimiani, M.; Lacconi, V.; Magrini, A.; Salustri, A.; Sotiriou, G.A.; Singh, D.; Bitounis, D.; Bocca, B.; Pino, A.; et al. Silica encapsulation of ZnO nanoparticles reduces their toxicity for cumulus cell-oocyte-complex expansion. Part. Fibre Toxicol. 2021, 18, 33. [Google Scholar] [CrossRef] [PubMed]
- Lian, B.; Wei, H.; Pan, R.; Sun, J.; Zhang, B.; Wu, J.; Li, X.; Tian, G. Galactose modified liposomes for effective co-delivery of doxorubicin and combretastatin A4. Int. J. Nanomed. 2021, 16, 457–467. [Google Scholar] [CrossRef] [PubMed]



| pH | Zeta Potential–PdPt (I) (mV) | Zeta Potential–PdPt (II) (mV) |
|---|---|---|
| 3 | −8 ± 1 | −8 ± 1 |
| 5 | −15 ± 2 | −8 ± 3 |
| 7 | −16 ± 2 | −9 ± 2 |
| 9 | −10 ± 2 | −8 ± 2 |
| 11 | −11 ± 2 | −11 ± 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klebowski, B.; Stec, M.; Depciuch, J.; Panek, A.; Krzempek, D.; Komenda, W.; Gałuszka-Bulaga, A.; Pajor-Swierzy, A.; Baran, J.; Parlinska-Wojtan, M. Improving the Effect of Cancer Cells Irradiation with X-rays and High-Energy Protons Using Bimetallic Palladium-Platinum Nanoparticles with Various Nanostructures. Cancers 2022, 14, 5899. https://doi.org/10.3390/cancers14235899
Klebowski B, Stec M, Depciuch J, Panek A, Krzempek D, Komenda W, Gałuszka-Bulaga A, Pajor-Swierzy A, Baran J, Parlinska-Wojtan M. Improving the Effect of Cancer Cells Irradiation with X-rays and High-Energy Protons Using Bimetallic Palladium-Platinum Nanoparticles with Various Nanostructures. Cancers. 2022; 14(23):5899. https://doi.org/10.3390/cancers14235899
Chicago/Turabian StyleKlebowski, Bartosz, Malgorzata Stec, Joanna Depciuch, Agnieszka Panek, Dawid Krzempek, Wiktor Komenda, Adrianna Gałuszka-Bulaga, Anna Pajor-Swierzy, Jarek Baran, and Magdalena Parlinska-Wojtan. 2022. "Improving the Effect of Cancer Cells Irradiation with X-rays and High-Energy Protons Using Bimetallic Palladium-Platinum Nanoparticles with Various Nanostructures" Cancers 14, no. 23: 5899. https://doi.org/10.3390/cancers14235899
APA StyleKlebowski, B., Stec, M., Depciuch, J., Panek, A., Krzempek, D., Komenda, W., Gałuszka-Bulaga, A., Pajor-Swierzy, A., Baran, J., & Parlinska-Wojtan, M. (2022). Improving the Effect of Cancer Cells Irradiation with X-rays and High-Energy Protons Using Bimetallic Palladium-Platinum Nanoparticles with Various Nanostructures. Cancers, 14(23), 5899. https://doi.org/10.3390/cancers14235899








