Assessment of Functional and Nutritional Status and Skeletal Muscle Mass for the Prognosis of Critically Ill Solid Cancer Patients
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients Cohort
2.2. Data Collection
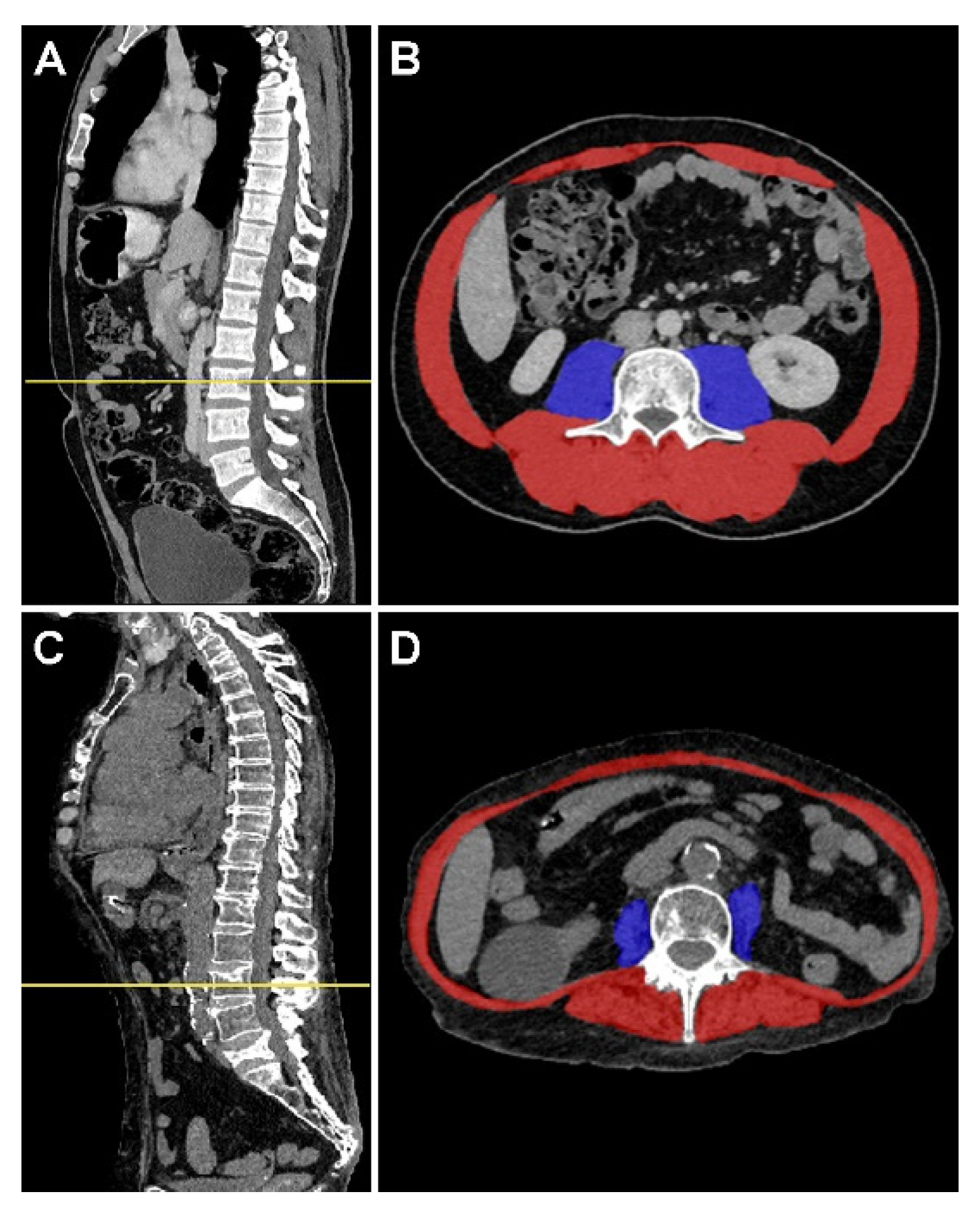
2.3. Muscle Mass Assessment
2.4. Statistical Analysis
2.5. Ethics Approval
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vincent, J.-L.; Marshall, J.C.; Ñamendys-Silva, S.A.; François, B.; Martin-Loeches, I.; Lipman, J.; Reinhart, K.; Antonelli, M.; Pickkers, P.; Njimi, H.; et al. Assessment of the Worldwide Burden of Critical Illness: The Intensive Care Over Nations (ICON) Audit. Lancet Respir. Med. 2014, 2, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Vigneron, C.; Charpentier, J.; Valade, S.; Alexandre, J.; Chelabi, S.; Palmieri, L.-J.; Franck, N.; Laurence, V.; Mira, J.-P.; Jamme, M.; et al. Patterns of ICU Admissions and Outcomes in Patients with Solid Malignancies over the Revolution of Cancer Treatment. Ann. Intensive Care 2021, 11, 182. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro-Vornhagen, A.; Böll, B.; Kochanek, M.; Azoulay, É.; von Bergwelt-Baildon, M.S. Critical Care of Patients with Cancer. CA Cancer J. Clin. 2016, 66, 496–517. [Google Scholar] [CrossRef] [PubMed]
- Schellongowski, P.; Sperr, W.R.; Wohlfarth, P.; Knoebl, P.; Rabitsch, W.; Watzke, H.H.; Staudinger, T. Critically ill Patients with Cancer: Chances and Limitations of Intensive Care Medicine-a Narrative Review. ESMO Open 2016, 1, e000018. [Google Scholar] [CrossRef]
- Zampieri, F.G.; Romano, T.G.; Salluh, J.I.F.; Taniguchi, L.U.; Mendes, P.V.; Nassar, A.P.; Costa, R.; Viana, W.N.; Maia, M.O.; Lima, M.F.A.; et al. Trends in Clinical Profiles, Organ Support Use and Outcomes of Patients with Cancer Requiring Unplanned ICU Admission: A Multicenter Cohort Study. Intensive Care Med. 2021, 47, 170–179. [Google Scholar] [CrossRef] [PubMed]
- For the ORCHESTRA Study Investigators; Zampieri, F.G.; Iwashyna, T.J.; Viglianti, E.M.; Taniguchi, L.U.; Viana, W.N.; Costa, R.; Corrêa, T.D.; Moreira, C.E.N.; Maia, M.O.; et al. Association of Frailty with Short-Term Outcomes, Organ Support and Resource Use in Critically ill Patients. Intensive Care Med. 2018, 44, 1512–1520. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, L.E.; Pisani, M.A.; Murphy, T.E.; Gahbauer, E.A.; Leo-Summers, L.S.; Gill, T.M. The Association of Frailty with Post-ICU Disability, Nursing Home Admission, and Mortality. Chest 2018, 153, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- Muscedere, J.; Waters, B.; Varambally, A.; Bagshaw, S.M.; Boyd, J.G.; Maslove, D.; Sibley, S.; Rockwood, K. The Impact of Frailty on Intensive Care Unit Outcomes: A Systematic Review and Meta-Analysis. Intensive Care Med. 2017, 43, 1105–1122. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, C.; Rizos, M.; Galani, E.; Rellos, K.; Skarlos, D.V.; Michalopoulos, A. Performance Status (PS): A Simple Predictor of Short-Term Outcome of Cancer Patients with Solid Tumors Admitted to the Intensive Care Unit (ICU). Anticancer. Res. 2007, 27, 2945–2948. [Google Scholar] [PubMed]
- Oh, T.K.; Lee, J.; Lee, Y.J.; Hwang, J.-W.; Do, S.-H.; Jeon, Y.-T.; Song, I.-A. Association between Modified Body Mass Index and 30-Day and 1-Year Mortality after Intensive Care Unit Admission: A Retrospective Cohort Study. J. Clin. Med. 2018, 7, 81. [Google Scholar] [CrossRef]
- Chandrasinghe, P.C.; Ediriweera, D.S.; Kumarage, S.K.; Deen, K.I. Pre-Operative Hypoalbuminaemia Predicts Poor Overall Survival in Rectal Cancer: A Retrospective Cohort Analysis. BMC Clin. Pathol. 2013, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, Y.; Yuan, Y.; Chen, Y.; Kong, W.; Chen, H.; Zhang, J.; Huang, Y. Preoperative Serum Pre-Albumin as an Independent Prognostic Indicator in Patients with Localized Upper Tract Urothelial Carcinoma after Radical Nephroureterectomy. Oncotarget 2016, 8, 36772–36779. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, Y.; Oya, R.; Takemoto, N.; Inohara, H. Predictive Impact of Sarcopenia in Solid Cancers Treated with Immune Checkpoint Inhibitors: A Meta-analysis. J. Cachexia Sarcopenia Muscle 2021, 12, 1122–1135. [Google Scholar] [CrossRef] [PubMed]
- Martini, K.; Chassagnon, G.; Fournel, L.; Prieto, M.; Hoang-Thi, T.-N.; Halm, N.; Bobbio, A.; Revel, M.-P.; Alifano, M. Sarcopenia as Independent Risk Factor of Postpneumonectomy Respiratory Failure, ARDS and Mortality. Lung Cancer 2020, 149, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.M.; Power, D.G.; Daly, L.; Cushen, S.J.; Bhuachalla, Ē.N.; Prado, C.M. Cancer-Associated Malnutrition, Cachexia and Sarcopenia: The Skeleton in the Hospital Closet 40 Years Later. Proc. Nutr. Soc. 2016, 75, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Shachar, S.S.; Williams, G.R.; Muss, H.B.; Nishijima, T.F. Prognostic Value of Sarcopenia in Adults with Solid Tumours: A Meta-Analysis and Systematic Review. Eur. J. Cancer 2016, 57, 58–67. [Google Scholar] [CrossRef]
- Vigneron, C.; Charpentier, J.; Wislez, M.; Mira, J.-P.; Lefebvre, A.; Fournel, L.; Jamme, M.; Pène, F. Short-Term and Long-Term Outcomes of Patients with Lung Cancer and Life-Threatening Complications. Chest 2021, 160, 1560–1564. [Google Scholar] [CrossRef]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonça, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-Related Organ Failure Assessment) Score to Describe Organ Dysfunction/Failure. On Behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Laousy, O.; Chassagnon, G.; Oyallon, E.; Paragios, N.; Revel, M.-P.; Vakalopoulou, M. Deep Reinforcement Learning for L3 Slice Localization in Sarcopenia Assessment. In Machine Learning in Medical Imaging; Lian, C., Cao, X., Rekik, I., Xu, X., Yan, P., Eds.; Lecture Notes in Computer Science; Springer International Publishing: Cham, Switzerland, 2021; Volume 12966, pp. 317–326. ISBN 978-3-030-87588-6. [Google Scholar]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and Classification of Cancer Cachexia: An International Consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Zampieri, F.G.; Bozza, F.A.; Moralez, G.M.; Mazza, D.D.S.; Scotti, A.V.; Santino, M.S.; Ribeiro, R.A.B.; Rodrigues Filho, E.M.; Cabral, M.M.; Maia, M.O.; et al. The Effects of Performance Status One Week before Hospital Admission on the Outcomes of Critically ill Patients. Intensive Care Med. 2017, 43, 39–47. [Google Scholar] [CrossRef]
- Park, C.-M.; Koh, Y.; Jeon, K.; Na, S.; Lim, C.-M.; Choi, W.-I.; Lee, Y.-J.; Kim, S.C.; Chon, G.R.; Kim, J.H.; et al. Impact of Eastern Cooperative Oncology Group Performance Status on Hospital Mortality in Critically ill Patients. J. Crit. Care 2014, 29, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Greenlee, H.; Unger, J.M.; LeBlanc, M.; Ramsey, S.; Hershman, D.L. Association between Body Mass Index and Cancer Survival in a Pooled Analysis of 22 Clinical Trials. Cancer Epidemiol. Biomark. Prev. 2017, 26, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Padkins, M.; Breen, T.; Anavekar, N.; Barsness, G.; Kashani, K.; Jentzer, J.C. Association Between Albumin Level and Mortality Among Cardiac Intensive Care Unit Patients. J. Intensive Care Med. 2020, 36, 1475–1482. [Google Scholar] [CrossRef]
- Covinsky, K.E.; Covinsky, M.H.; Palmer, R.M.; Sehgal, A.R. Serum Albumin Concentration and Clinical Assessments of Nutritional Status in Hospitalized Older People: Different Sides of Different Coins? J. Am. Geriatr. Soc. 2002, 50, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.L.; Oh, E.S.; Lee, R.W.; Finucane, T.E. Serum Albumin and Prealbumin in Calorically Restricted, Nondiseased Individuals: A Systematic Review. Am. J. Med. 2015, 128, e1–e1023. [Google Scholar] [CrossRef] [PubMed]
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.I.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef]
- Wang, J.W.; Williams, M. Exploring Definitions of Radiological Sarcopenia in Cancer: A Protocol for a Scoping Review. BMJ Open 2021, 11, e053076. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European Consensus on Definition and Diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Raynard, B.; Pigneur, F.; Di Palma, M.; Deluche, E.; Goldwasser, F. The Prevalence of CT-Defined Low Skeletal Muscle Mass in Patients with Metastatic Cancer: A Cross-Sectional Multicenter French Study (the SCAN Study). Support. Care Cancer 2022, 30, 3119–3129. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.C.; Booth, M.; Ghita, G.; Wang, Z.; Gardner, A.; Hawkins, R.B.; Darden, D.B.; Leeuwenburgh, C.; Moldawer, L.L.; Moore, F.A.; et al. The Impact of Sarcopenia and Acute Muscle Mass Loss on Long-term Outcomes in Critically ill Patients with Intra-abdominal Sepsis. J. Cachexia Sarcopenia Muscle 2021, 12, 1203–1213. [Google Scholar] [CrossRef]
- Marquardt, J.P.; Roeland, E.J.; Van Seventer, E.E.; Best, T.D.; Horick, N.K.; Nipp, R.D.; Fintelmann, F.J. Percentile-based Averaging and Skeletal Muscle Gauge Improve Body Composition Analysis: Validation at Multiple Vertebral Levels. J. Cachexia Sarcopenia Muscle 2022, 13, 190–202. [Google Scholar] [CrossRef]
- Jullien, M.; Tessoulin, B.; Ghesquières, H.; Oberic, L.; Morschhauser, F.; Tilly, H.; Ribrag, V.; Lamy, T.; Thieblemont, C.; Villemagne, B.; et al. Deep-Learning Assessed Muscular Hypodensity Independently Predicts Mortality in DLBCL Patients Younger Than 60 Years. Cancers 2021, 13, 4503. [Google Scholar] [CrossRef] [PubMed]
- Thibault, R.; Makhlouf, A.-M.; Mulliez, A.; Gonzalez, M.C.; Kekstas, G.; Kozjek, N.R.; Preiser, J.-C.; Rozalen, I.C.; Dadet, S.; Krznaric, Z.; et al. Fat-Free Mass at Admission Predicts 28-Day Mortality in Intensive Care Unit Patients: The International Prospective Observational Study Phase Angle Project. Intensive Care Med. 2016, 42, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Moisey, L.L.; Mourtzakis, M.; Cotton, B.A.; Premji, T.; Heyland, D.K.; Wade, C.E.; Bulger, E.; Kozar, R.A. Skeletal Muscle Predicts Ventilator-Free Days, ICU-Free Days, and Mortality in Elderly ICU Patients. Crit. Care 2013, 17, R206. [Google Scholar] [CrossRef]
- Jaitovich, A.; Khan, M.M.H.S.; Itty, R.; Chieng, H.C.; Dumas, C.L.; Nadendla, P.; Fantauzzi, J.P.; Yucel, R.M.; Feustel, P.J.; Judson, M.A. ICU Admission Muscle and Fat Mass, Survival, and Disability at Discharge. Chest 2019, 155, 322–330. [Google Scholar] [CrossRef]
- Kou, H.-W.; Yeh, C.-H.; Tsai, H.-I.; Hsu, C.-C.; Hsieh, Y.-C.; Chen, W.-T.; Cheng, H.-T.; Yu, M.-C.; Lee, C.-W. Sarcopenia Is an Effective Predictor of Difficult-to-Wean and Mortality among Critically ill Surgical Patients. PLoS ONE 2019, 14, e0220699. [Google Scholar] [CrossRef]
- Weijs, P.J.; Looijaard, W.G.; Dekker, I.M.; Stapel, S.N.; Girbes, A.R.; Straaten, H.M.O.; Beishuizen, A. Low Skeletal Muscle Area Is a Risk Factor for Mortality in Mechanically Ventilated Critically ill Patients. Crit. Care 2014, 18, R12. [Google Scholar] [CrossRef] [PubMed]
- Thackeray, M.; Mohebbi, M.; Orford, N.; Kotowicz, M.A.; Pasco, J.A. Lean Mass as a Risk Factor for Intensive Care Unit Admission: An Observational Study. Crit. Care 2021, 25, 364. [Google Scholar] [CrossRef] [PubMed]
- Elfassy, M.D.; Ferreyro, B.L.; Rozenberg, D.; Sklar, M.C.; Mathur, S.; Detsky, M.E.; Goligher, E.C.; Mehta, S.; Prica, A.; Thyagu, S.; et al. Association of Thoracic Computed Tomographic Measurements and Outcomes in Patients with Hematologic Malignancies Requiring Mechanical Ventilation. Ann. Am. Thorac. Soc. 2021, 18, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Jaitovich, A.; Dumas, C.L.; Itty, R.; Chieng, H.C.; Khan, M.M.H.S.; Naqvi, A.; Fantauzzi, J.; Hall, J.B.; Feustel, P.J.; Judson, M.A. ICU Admission Body Composition: Skeletal Muscle, Bone, and Fat Effects on Mortality and Disability at Hospital Discharge—A Prospective, Cohort Study. Crit. Care 2020, 24, 566. [Google Scholar] [CrossRef]
- Looijaard, W.G.P.M.; Dekker, I.M.; Stapel, S.N.; Girbes, A.R.J.; Twisk, J.W.R.; Oudemans-van Straaten, H.M.; Weijs, P.J.M. Skeletal Muscle Quality as Assessed by CT-Derived Skeletal Muscle Density Is Associated with 6-Month Mortality in Mechanically Ventilated Critically ill Patients. Crit. Care 2016, 20, 386. [Google Scholar] [CrossRef] [PubMed]
- Daffrè, E.; Prieto, M.; Martini, K.; Hoang-Thi, T.-N.; Halm, N.; Dermine, H.; Bobbio, A.; Chassagnon, G.; Revel, M.P.; Alifano, M. Total Psoas Area and Total Muscular Parietal Area Affect Long-Term Survival of Patients Undergoing Pneumonectomy for Non-Small Cell Lung Cancer. Cancers 2021, 13, 1888. [Google Scholar] [CrossRef] [PubMed]
- Lach, K.; Peterson, S.J. Nutrition Support for Critically ill Patients with Cancer. Nutr. Clin. Pract. 2017, 32, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Nipp, R.D.; Fuchs, G.; El-Jawahri, A.; Mario, J.; Troschel, F.M.; Greer, J.A.; Gallagher, E.R.; Jackson, V.A.; Kambadakone, A.; Hong, T.S.; et al. Sarcopenia Is Associated with Quality of Life and Depression in Patients with Advanced Cancer. Oncologist 2018, 23, 97–104. [Google Scholar] [CrossRef]
- Shachar, S.S.; Deal, A.M.; Weinberg, M.; Nyrop, K.A.; Williams, G.R.; Nishijima, T.F.; Benbow, J.M.; Muss, H.B. Skeletal Muscle Measures as Predictors of Toxicity, Hospitalization, and Survival in Patients with Metastatic Breast Cancer Receiving Taxane-Based Chemotherapy. Clin. Cancer Res. 2017, 23, 658–665. [Google Scholar] [CrossRef]
| Characteristics | n = 462 Patients |
|---|---|
| Age (years) | 64 (56–72) |
| Male gender | 298 (64.5) |
| Time from cancer diagnosis to ICU admission (days) | 273 (113–731) |
| Type of cancer | |
| Gastrointestinal | 161 (34.8) |
| Lung | 138 (29.9) |
| Others a | 163 (35.3) |
| Stage | |
| Localized | 25 (5.4) |
| Advanced | 90 (19.5) |
| Metastatic | 347 (75.1) |
| Cancer status (available for 458 patients) | |
| Newly diagnosed | 89 (19.3) |
| Partial remission | 128 (27.7) |
| Complete remission | 9 (1.9) |
| In progression | 232 (50.2) |
| SOFA score at ICU admission (points) | 5 (4–7) |
| Organ failure supports | |
| Invasive mechanical ventilation | 151 (32.7) |
| Duration of mechanical ventilation (days) | 3 (2–7) |
| Non-invasive mechanical ventilation | 56 (12.1) |
| Vasopressors/inotropes | 138 (29.9) |
| Renal replacement therapy | 51 (11.0) |
| Outcomes | |
| Decision to forgo life-sustaining therapies | 164 (35.5) |
| ICU mortality | 94 (20.3) |
| Length-of-stay in the ICU (days) | 2 (1–5) |
| Hospital mortality | 109 (23.6) |
| One-year mortality (available for 461 patients) | 346 (73.0) |
| Characteristics | n = 462 Patients |
|---|---|
| At ICU admission | |
| Functional status | |
| Performance status 3–4 (n = 381) | 57 (15.0) |
| Nutritional status | |
| Body mass index (kg/m2) (n = 440) | 23.2 (20.3–26.4) |
| Albumin level (g/L) (n = 392) | 29 (24–34) |
| Prealbumin level (g/L) (n = 272) | 0.14 (0.09–0.19) |
| Morphometric measurements (n = 290) | |
| Muscle area (cm2) | |
| Psoas area | 12.9 (9.6–16.0) |
| Parietal area | 101.4 (84.9–120.7) |
| Total muscle area | 116.8 (95.5–136.1) |
| Skeletal muscle index (SMI) (cm2/m2) | |
| SMI psoas area | 4.4 (3.8–5.9) |
| SMI parietal area | 35.0 (30.0–41.2) |
| SMI total muscle area | 39.3 (33.9–46.8) |
| SMI total muscle area < 55 cm2/m2 (180 male patients) | 167 (92.8) |
| SMI total muscle area < 39 cm2/m2 (110 women patients) | 74 (67.3) |
| Six months before ICU admission | |
| Functional status | |
| Performance status 3–4 (n = 275) | 9 (3.3) |
| Nutritional status | |
| Body mass index (kg/m2) (n = 385) | 24.4 (21.6–28.1) |
| Albumin level (g/L) (n = 376) | 37 (33–41) |
| Prealbumin level (g/L) (n = 284) | 0.20 (0.15–0.26) |
| Characteristics | CSH [95% CI] | p |
|---|---|---|
| Whole cohort (n = 462) | ||
| Surgery < 3 months | 0.15 [0.04–0.58] | 0.0006 |
| Specific complications | 2.09 [1.02–4.28] | 0.04 |
| SOFA score at admission | 1.21 [1.11–1.31] a | <0.001 |
| Performance status at admission | 1.74 [1.27–2.39] a | <0.001 |
| Albumin variation within the past 6 months | ||
| Stable | Ref. | - |
| Decrease > 5% | - | - |
| Increase > 5% | 0.38 [0.16–0.87] | 0.02 |
| Invasive mechanical ventilation (n = 151) | ||
| Surgery < 3 months | 0.16 [0.04–0.65] | 0.01 |
| Specific complications | 2.38 [1.03–5.97] | 0.04 |
| SOFA score at admission | 1.07 [1.01–1.15] a | 0.03 |
| SMI psoas area | 0.82 [0.67–0.98] | 0.04 |
| Characteristics | CSH [95% CI] | p |
|---|---|---|
| Type of cancer | ||
| Non-lung cancer | Ref. | - |
| Lung cancer | 1.84 [1.29–2.64] | <0.001 |
| Status in progression | 2.24 [1.57–3.18] | <0.001 |
| Decision to forgo life-sustaining therapy | 2.40 [1.65–3.50] | <0.001 |
| Performance status at admission | 1.34 [1.14–1.59] a | <0.001 |
| Weight variation within the past six months | ||
| Stable | Ref | - |
| Decrease > 10% | 1.33 [1.17–2.99] | 0.01 |
| Increase > 10% | 0.88 [0.31–2.53] | 0.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vigneron, C.; Laousy, O.; Chassagnon, G.; Vakalopoulou, M.; Charpentier, J.; Alexandre, J.; Jamme, M.; Pène, F. Assessment of Functional and Nutritional Status and Skeletal Muscle Mass for the Prognosis of Critically Ill Solid Cancer Patients. Cancers 2022, 14, 5870. https://doi.org/10.3390/cancers14235870
Vigneron C, Laousy O, Chassagnon G, Vakalopoulou M, Charpentier J, Alexandre J, Jamme M, Pène F. Assessment of Functional and Nutritional Status and Skeletal Muscle Mass for the Prognosis of Critically Ill Solid Cancer Patients. Cancers. 2022; 14(23):5870. https://doi.org/10.3390/cancers14235870
Chicago/Turabian StyleVigneron, Clara, Othmane Laousy, Guillaume Chassagnon, Maria Vakalopoulou, Julien Charpentier, Jérôme Alexandre, Matthieu Jamme, and Frédéric Pène. 2022. "Assessment of Functional and Nutritional Status and Skeletal Muscle Mass for the Prognosis of Critically Ill Solid Cancer Patients" Cancers 14, no. 23: 5870. https://doi.org/10.3390/cancers14235870
APA StyleVigneron, C., Laousy, O., Chassagnon, G., Vakalopoulou, M., Charpentier, J., Alexandre, J., Jamme, M., & Pène, F. (2022). Assessment of Functional and Nutritional Status and Skeletal Muscle Mass for the Prognosis of Critically Ill Solid Cancer Patients. Cancers, 14(23), 5870. https://doi.org/10.3390/cancers14235870






